Abstract
Thyroid cancer is the most common endocrine carcinoma with increasing incidence worldwide and anaplastic subtypes are frequently associated with cancer related death. Radioresistance of thyroid cancer often leads to therapy failure and cancer-related death. In this study, we found that melatonin showed potent suppressive roles on NF-κB signaling via inhibition of p65 phosphorylation and generated redox stress in thyroid cancer including the anaplastic subtypes. Our data showed that melatonin significantly decreased cell viability, suppressed cell migration and induced apoptosis in thyroid cancer cell lines in vitro and impaired tumor growth in the subcutaneous mouse model in vivo. By contrast, irradiation of thyroid cancer cells resulted in elevated level of phosphorylated p65, which could be reversed by cotreatment with melatonin. Consequently, melatonin synergized with irradiation to induce cytotoxicity to thyroid cancer, especially in the undifferentiated subgroups. Taken together, our results suggest that melatonin may exert anti-tumor activities against thyroid carcinoma by inhibition of p65 phosphorylation and induction of reactive oxygen species. Radio-sensitization by melatonin may have clinical benefits in thyroid cancer.
Abbreviations: DTC, differentiated thyroid cancer; ATC, anaplastic thyroid carcinoma; NF-κB, Nuclear factor-κB; IκB, inhibitor of κB; ROS, reactive oxygen species; STR, short tandem repeat; H&E, hematoxylin and eosin
Keywords: Melatonin, Thyroid cancer, Radioresistance, p65, Reactive oxygen species
1. Introduction
Thyroid cancer is the most common endocrine malignancy, with a rapidly increasing annual incidence over the world [1], [2]. In 2015, there were approximately 90,000 newly diagnosed cases and 6800 mortality cases in China [3]. Differentiated thyroid cancer (DTC), which includes papillary thyroid carcinoma and follicular thyroid carcinoma, represents the majority of thyroid cancer patients, and generally responds well to surgery combined with therapeutic radioiodine (131I) [4]. On the other hand, poorly differentiated/undifferentiated thyroid cancers including anaplastic thyroid carcinoma (ATC) is lethal and accounts for almost 50% of thyroid cancer related death [5]. ATC constitutes a highly invasive disease with the most frequent metastatic sites including trachea, esophagus, blood vessels and nerves [6], [7] and is often resistant to 131I therapy [8]. Similarly, some DTC cases respond poorly to 131I treatment, which often leads to therapeutic failure and disease recurrence. Limited alternatives in therapeutic strategies and resistance to 131I treatment compose the major challenges to improving survival of thyroid cancer patients [9]. It is therefore urgent to find additional novel therapeutic alternatives for thyroid carcinoma.
Melatonin is a phylogenetically well-preserved compound secreted by the pineal gland, and is reported to have a wide range of biological activities [10], [11]. For instance, the anti-inflammation and anti-tumor effects of melatonin have generated considerable interest in therapeutic application [12], [13], [14]. Moreover, chemo- [15] or radio-sensitization [16] roles of melatonin in human cancers have been reported with minimal side effects. However, the effects and therapeutic potential of melatonin on thyroid carcinoma remain unsolved.
Nuclear factor-κB (NF-κB) is a protein complex that controls transcription of various target genes participating in cell proliferation, cell apoptosis as well as metabolic reprogramming [17]. The p65 protein, also known as RELA, is a REL-associated protein involved in NF-κB hetero-dimerization formation, nuclear translocation and functional activation. Upon phosphorylation, p65 associates with p50 to form a heterodimer, which is then translocated into the nucleus to initiate gene transcription. There is an emerging body of evidence showing that aberrantly activated p65 or NF-κB signaling contributes to tumor development and progression including thyroid carcinoma [18], [19]. Strong correlations between nuclear localization of p65 and clinicopathological parameters of thyroid carcinoma suggested the role of NF-κB activation in tumor growth and aggressiveness [20]. It has also been demonstrated that NF-κB activation induced by chemotherapy or radiotherapy attenuated the therapeutic efficacies [21], [22], whereas inhibition of NF-κB signaling could promote thyroid cancer cell apoptosis and achieve synergistic effects [23], [24], [25]. Moreover, inhibition of the NF-κB pathway is purported to underlie the anti-tumor effect of melatonin [15], [26]. Reactive oxygen species (ROS) are byproducts of cellular metabolism and acts as a double-edged sword during cancer progression [27]. A certain ROS level is essential for cell proliferation and DNA mutation, while excessive redox stress often leads to exhaustion of intracellular anti-oxidant system and cell apoptosis. Pro-oxidant and antioxidant roles of melatonin have both been reported in human cancer [10], [28]. However, effects of melatonin on NF-κB signaling and redox homeostasis in thyroid carcinoma remains to be explored.
This study was designed to explore the anti-tumor activity of melatonin against thyroid carcinoma and whether or not mechanisms of which are associated with the NF-κB pathway and redox modulation. Moreover, we also explored combinational effects of irradiation and melatonin on thyroid tumor in vitro and in vivo.
2. Materials and methods
2.1. Cell lines and cell culture
Stem Cell Bank, Chinese Academy of Sciences (Shanghai, China), kindly provided the human papillary thyroid cancer cells KTC-1 and BCPAP cells. TPC-1 cells were purchased from Cobioer Biosciences Co., LTD. (Nanjing, China). The human anaplastic thyroid cancer cells 8505c and ARO cell lines were purchased from Guangzhou jenniobio Biotechnology Co. Ltd. Cell lines were authenticated by short tandem repeat (STR) DNA profiling and all cell lines were passaged less than one month prior to experimentation. Cells were incubated with maintained in RPMI 1640 (Gibco, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS, Hyclone, Logan, UT, USA) at 37 °C in a humidified atmosphere with 5% carbon dioxide.
2.2. Reagents
Melatonin was obtained from Sigma Aldrich (Sigma-Aldrich, St. Louis, USA) and dissolved in DMSO at 2 M in stock solution. N-acetyl-l-cysteine (NAC) and 2′,7′-dichlorofluorescein diacetate (DCF-DA) was purchased from Life Technologies (Invitrogen, Carlsbad, California, USA) and dissolved in DMSO.
2.3. Western blot analysis
Cells were lysed by using 1× RIPA buffer (Cell Signaling Technology, Danvers, MA, USA) and the protein concentration was determined using BCA Protein Assay kit (Pierce, Rockford, IL, USA) method. Equal amounts of cell protein were subjected to electrophoresis in SDS-PAGE gels and then transferred to PVDF membranes (Millipore) for antibody blotting. Antibodies used in our study were as follows: β-Actin, PARP, cleaved (c)-caspase 3, NF-κB/p65, p-NF-κB/p65, Bcl-xl, MMP9, Cyclin D1, IL-1α and Lamin A (Cell Signaling Technology, Beverly, MA, USA), Ki-67 (Abcam, Cambridge, Massachusetts, USA). Horseradish peroxidase-conjugated IgG (Pierce) was used as the secondary antibody. Expression level of indicated proteins were detected using Bio-Rad Clarity™ western ECL substrate (Bio-Rad Laboratory, CA, USA). For dissociation of nuclear, mitochondrial and cytoplasmic proteins, a specific isolation kit from Keygen (Nanjing, China) was utilized according to manufacturer's instructions.
2.4. Cell proliferation/viability assays
4 × 103 cells were seeded into 96-well plates and cultured with different doses of melatonin for 48 h. Cell viability was assessed by the MTS ([3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt) methods and the absorbance was measured at the wavelength of 490 nm. For irradiation assays, the cells were exposed to irradiation (2 Gy) before viability detection.
2.5. RNA extraction and real-time qPCR
Thyroid cancer cells were either treated with melatonin, irradiation or DMSO control. The total RNA was extracted from thyroid cancer cell lines using TRIZOL reagent (Life Technologies, Grand Island, USA) and dissolved in RNAasefree water. Equal amounts of RNA were converted to cDNA using the Go Script Reverse Transcription (RT) System (Promega, Madison, USA) following the instructions from the manufacturer. To assess mRNA expression, qRT-PCR was conducted using GoTaq PCR Master Mix (Promega, Madison, USA) on a Roche 480 fast real-time system (Applied Biosystems, Foster City, CA). The housekeeping gene GAPDH was used as an endogenous control. Each sample and gene was conducted in triplicate and analyzed with the 2-△△Ct methods. Sequences for primers were listed in Supplementary Table 1.
2.6. Migration and wound closure assays
The indicated cells were treated with melatonin (1 mM) or irradiation before migration assays. The cells were suspended in 100 μl serum-free medium and plated in the upper chamber (24-well insert; pore size, 8 µm; BD Biosciences), while fetal bovine medium was added into the lower chamber. After 24 h incubation, cells that did not migrate through the pores were removed with a cotton swab. The chambers were stained with crystal violet and counted in four random view fields. Wound healing assays were performed according to a previous report [15].
2.7. Cell apoptosis assays and ROS detection
Thyroid carcinoma cells were collected after indicated treatment and apoptosis was analyzed utilizing a Gallio flow cytometer (Beckman Coulter, Miami, USA). The intracellular ROS level was detected with the specific probe DCF-DA according to the manufacturer's instructions.
2.8. X-ray survival assay
Cells cultured in plates were irradiated with X-ray (2 Gy, X-ray irradiation was carried out using X-ray machine for research only), and the cultures were maintained for 24 h before the cells were collected for migration and wound closure assays or for 48 h before flow cytometer analysis. Cells in 10 cm dishes irradiated with X-ray (2 Gy) were collected after 24 h culture for study of protein and mRNA expression alteration.
2.9. Immunohistochemistry
Paraffin-embedded tissue specimens of tumor xenografts were sectioned, incubated at 65 °C for 2 h, deparaffinized in xylene and rehydrated. Antigen retrieval was done in a microwave oven for heating at 95 °C. The sections were then incubated in 3% H2O2 solution for 10 min to block endogenous peroxidase activity and then in normal goat serum for 1 h. The sections were then incubated with antibodies against Ki-67 or p-NF-κB/p65 (active NF-κB/p65, Millipore) at 4 °C overnight. Staining was performed using a kit from Dako (Copenhagen, Denmark).
2.10. Animal study
Female BALB/c nude mice (4–5 weeks old) were purchased from the Beijing Vital River Laboratory Animal Technology (Beijing, China). TPC-1 cells (1 × 106) or 8505c cells (1 × 106) suspended in 100 μl cold PBS were subcutaneously injected to the right armpit of the mice. The mice were randomly assigned into the following different groups: Control, PBS; Melatonin, 25 mg/kg, once per day; irradiation, 2 Gy, twice per week; Combination, irradiation, 2 Gy, twice per week followed by melatonin, 25 mg/kg, once per day. Tumor size was measured every 3 days by means of a Vernier caliper, and tumor volume was estimated according to the following formula: tumor volume = 0.5 × L × W2, where L is the greatest dimension of the tumor and W is the dimension of the tumor in the perpendicular direction. Mice weight was recorded twice weekly. Mice holder was used during radiation. The tumor was well exposed, while other part of mice body was carefully protected using plumbum mould. After treatment for four weeks, the tumors were extracted from sacrificed mice, embedded in paraffin and sectioned. Tumor tissues from the BALB/c nude mice were stained with H&E or immunohistochemically with p-NF-κB/p65 or Ki-67 according to previously reported protocols [29]. All the animal experiments were performed according to the National Institutes of Health animal use guidelines on the use of experimental animals.
2.11. Statistical analysis
Each cell culture experiment was performed in triplicates for at least three times. Unless otherwise indicated, results were shown as mean ± SD of three independent experiments. To compare the statistical differences, SPSS software (version 16.0, Chicago, IL, USA) or Graph Pad Prism software (San Diego, CA, USA) was used by two-tailed Student's t-test or one-way ANOVA as approximate. P value less than 0.05 was considered significant. The Calcusyn Biosoft (Ferguson, MO, USA) was used to calculate combination index of melatonin with irradiation.
3. Results
3.1. Melatonin inhibits proliferation and migration of thyroid cancer cell lines in vitro
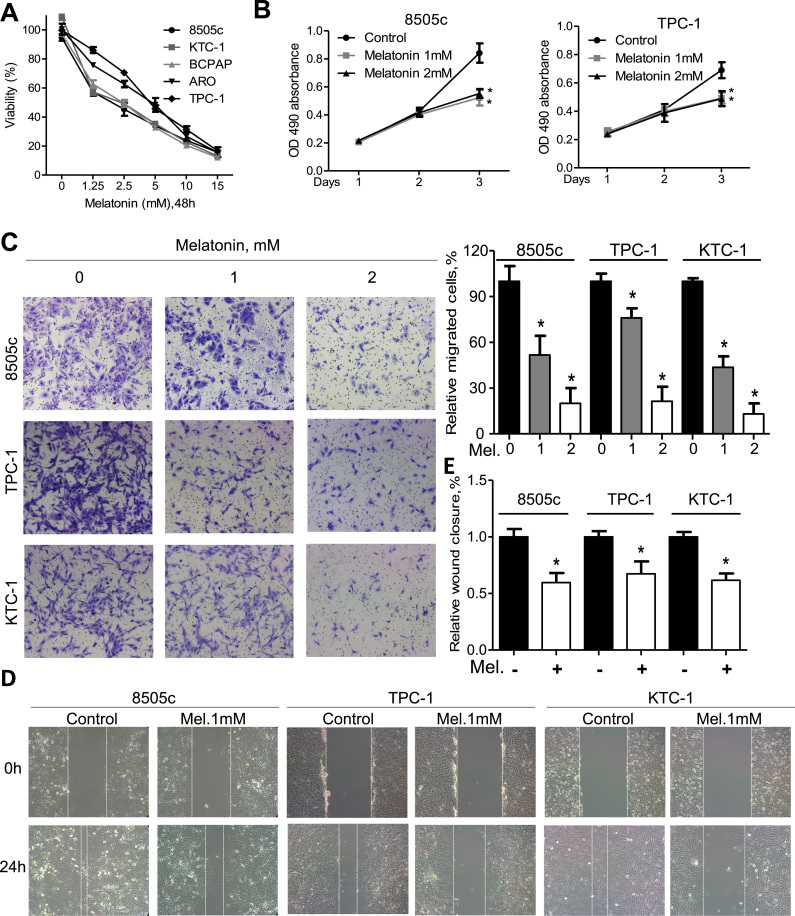
To explore the effects of melatonin on thyroid cancer cells, MTS assay was used. Our data showed that the viability of thyroid cancer cells was less than 10% after treatment with melatonin at 15 mM for 48 h (Fig. 1A). Importantly, melatonin significantly decreased cell viability of 8505c and ARO (two ATC cell lines) to a comparable extent to that of DTC cell lines such as TPC-1, KTC-1 and BCPAP (Fig. 1A). Melatonin at relatively low concentrations suppressed cell viability of 8505c and TPC-1 cells in a time-dependent manner (Fig. 1B). However, melatonin at physiological concentrations (0.01–3 nM) showed no effects on thyroid cancer cells (Supplementary Fig. S1A). Additionally, transwell assays indicated that melatonin was able to impede migration of 8505c, TPC-1, KTC-1 and BCPAP cells to the lower chamber (Fig. 1C and Supplementary Fig. S1B). Further wound closure assays confirmed the anti-migration activity of melatonin (Fig. 1D, E and Supplementary Fig. S1C). These data suggest that melatonin could inhibit proliferation and migration of thyroid cancer cells.
Fig. 1.
Melatonin inhibits proliferation and migration of thyroid cancer cells. (A) Relative cell viability of thyroid cancer cells after treatment with melatonin at indicated concentrations for 48 h. (B) Cell viability of 8505c and TPC-1 cells after treatment with melatonin at 2 mM or 4 mM for three days as measured by OD490 value. (C) Migration assays of 8505c, TPC-1 and KTC-1 cells after treatment with melatonin at indicated concentrations for 24 h. Representative images (left panel) and quantification (right upper panel) were shown. Wound closure assays of 8505c, TPC-1 and KTC-1 cells after treated with melatonin at 1 mM for 24 h. Representative images (D) and quantification (E) were shown. *P<0.05.
3.2. Melatonin induces apoptosis and redox stress of thyroid cancer cell lines
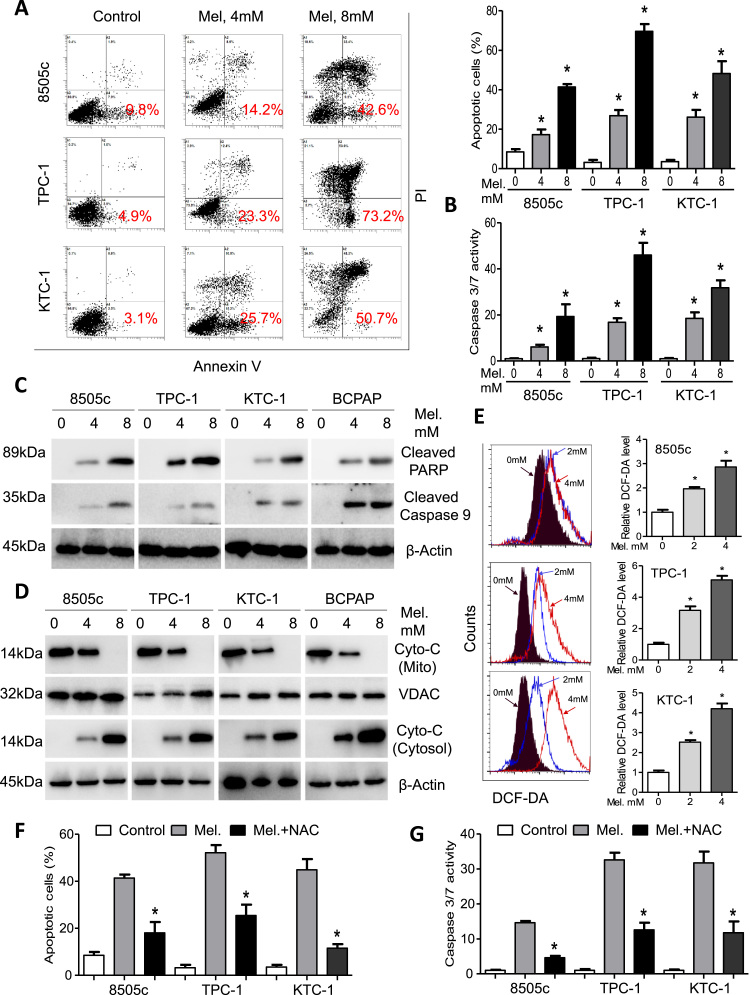
We proceeded to analyze thyroid cancer cell apoptosis after melatonin treatment via flow cytometry. The percentage of apoptotic cells increased in parallel with concentration of melatonin (Fig. 2A and Supplementary Fig. S2A). Percentage of Annexin V positive cells after treatment with melatonin at 8 mM was 42.6%, 73.2%, 50.7% and 43.4% for 8505c, TPC-1, KTC-1 and BCPAP cells, respectively (Fig. 2A and Supplementary Fig. S2A). Moreover, enzymatic activity of caspase 3/7 was significantly increased in 8505c, TPC-1, KTC-1 and BCPAP cells in a dose-dependent manner (Fig. 2B and Supplementary Fig. S2B). Cleavage of PARP and caspase 9 were also upregulated as detected by immunoblotting assays (Fig. 2C). Expression of cytochrome C in the cytosol was significantly increased while that in the mitochondrion was decreased, indicating mitochondrion impairment by melatonin in 8505c, TPC-1, KTC-1 and BCPAP cells (Fig. 2D). Modulation of intracellular ROS in cancer cells by melatonin remains controversial [10], [28]. We performed flow cytometry analysis with DCF-DA as the ROS prober. As shown in Fig. 2E, fluorescence intensity for DCF-DA significantly increased by more than 3-fold after melatonin treatment than control cells. Consistently, apoptotic cells and caspase activity (Figs. 2F and 2G) were reduced by pretreatment with the anti-oxidant NAC, indicating that ROS accumulation may underlie pro-apoptotic activity of melatonin in thyroid cancer cells.
Fig. 2.
Melatonin induces apoptosis and intracellular ROS of thyroid cancer cells. (A) 8505c, TPC-1 and KTC-1 cells were treated with melatonin at the indicated concentrations and cell apoptosis was analyzed by flow cytometry. Representative images (left panel) and quantification (right upper panel) were shown. (B) Relative caspase3/7 activity of 8505c, TPC-1 and KTC-1 cells after treatment with melatonin at the indicated concentrations. (C) Immunoblotting of cleaved PARP and cleaved caspase 9 in 8505c, TPC-1, KTC-1 and BCPAP cells after treated with melatonin at the indicated concentrations. β-Actin was used as a loading control. (D) Western blot analysis of cytochrome C in the mitochondrion and in the cytoplasm. VDAC and β-Actin were used as loading controls. (E) Flow analysis of intracellular ROS level in 8505c, TPC-1 and KTC-1 cells. (F) Percentage of apoptotic cells 8505c, TPC-1 and KTC-1 cells after treated with or without NAC and melatonin at 8 mM for 24 h. (G) Relative caspase3/7 activity of 8505c, TPC-1 and KTC-1 cells after treatment with or without NAC and melatonin at 8 mM for 24 h. *P<0.05.
3.3. Melatonin inhibits NF-κB/p65 signaling in thyroid cancer
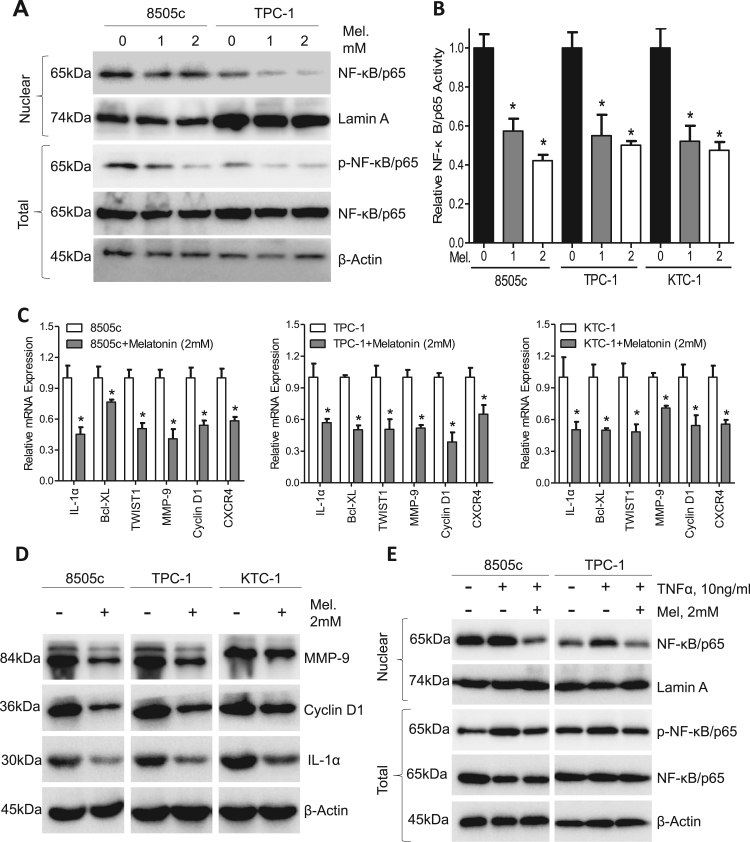
Previous reports have demonstrated that NF-κB signaling blocking suppresses thyroid tumorigenesis [30]. To determine whether melatonin inhibits NF-κB/p65 signaling in thyroid cancer, the total and nuclear portions of 8505c and TPC-1 cells were isolated after treatment with different concentrations of melatonin. Fig. 3A clearly showed that the nuclear expression of NF-κB/p65 and total phosphorylated NF-κB/p65 was significantly suppressed by melatonin in a dose-dependent manner. Phosphorylation and subsequent nuclear translocation of NF-κB/p65 was vital in NF-κB signaling [17]. Concordantly, the NF-κB/p65 DNA binding activity in 8505c, TPC-1 and KTC-1 cells was decreased by approximately 50% after treated with melatonin at 2 mM (Fig. 3B). Well-known NF-κB/p65 response genes including IL-1α, Bcl-xl, TWIST1, MMP9, Cyclin D1 and CXCR4 were downregulated at both the mRNA (Fig. 3C) and protein (Fig. 3D) level after melatonin treatment in 8505c, TPC-1 and KTC-1 cells. TNF-α showed potent capacity to activate NF-κB as is indicated by elevated nuclear NF-κB/p65 expression and total NF-κB/p65 phosphorylation (Fig. 3E). However, these effects could be blocked by co-treatment with melatonin in 8505c and TPC-1 cells (Fig. 3E) as well as in KTC-1 cells (Supplementary Fig. S3A), further demonstrating potent inhibitory roles of melatonin on NF-κB/p65 phosphorylation and nuclear translocation.
Fig. 3.
Melatonin inhibits p65 phosphorylation and nuclear translocation in thyroid carcinoma. (A) Western blot analysis of nuclear NF-κB/p65 and p-NF-κB/p65 in 8505c and TPC-1 cells treated with melatonin for 24 h. (B) ELISA analysis of NF-κB/p65 activity in 8505c, TPC-1 and KTC-1 cells after treatment with melatonin for 24 h. (C) Expression of NF-κB/p65 response genes in 8505c, TPC-1 and KTC-1 cells was detected by qPCR assays. (D) Immunoblotting of IL-1α, Cyclin D1 and MMP9 in 8505c, TPC-1 and KTC-1 cells after treatment with melatonin (2 mM) for 24 h. β-Actin was used as a loading control. (E) Immunoblotting of NF-κB/p65 and p-NF-κB/p65 in 8505c and TPC-1 cells treated with or without TNFα (10 ng/mL) and melatonin (2 mM) for 24 h. β-Actin was used as a loading control. *P < 0.05.
To gain further insights into the roles of NF-κB signaling in mediating the anti-migration activity of melatonin, 8505c and TPC-1 cells were treated with specific NF-κB/p65 siRNA (Supplementary Fig. S3B) before they were subjected to melatonin treatment and subsequent transwell assays. As shown in Supplementary Fig. S3C, melatonin had no effects on cell migration after NF-κB/p65 knockdown by siRNA. These results further suggested that abrogation of the NF-κB/p65 pathway may mediate the oncostatin effects of melatonin in thyroid carcinoma.
3.4. Irradiation activates NF-κB/p65 signaling in thyroid cancer
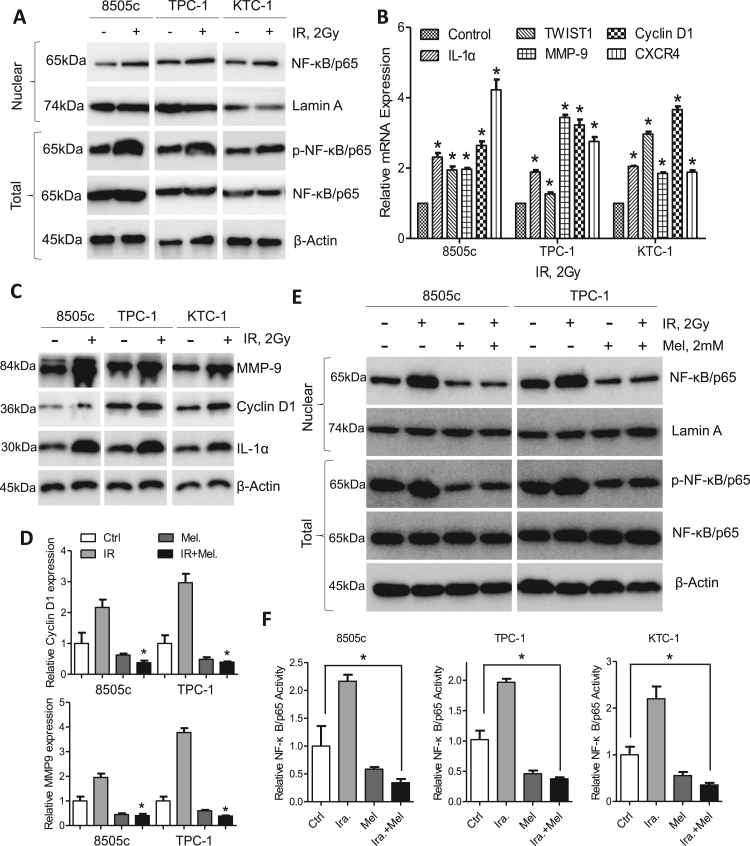
Postoperative radiotherapy has long been the corner stone for thyroid cancer treatment [5]. Intriguingly, treatment of TPC-1 and KTC-1 cells with irradiation resulted in elevated nuclear NF-κB/p65 level and total NF-κB/p65 phosphorylation (Fig. 4A). Consequently, NF-κB/p65 response genes including IL-1α, TWIST1, MMP9, Cyclin D1 and CXCR4 were upregulated at the mRNA and protein levels (Fig. 4B and C). However, elevated Cyclin D1 and MMP9 expression induced by irradiation were reversed by co-treatment with melatonin (Fig. 4D). Examination of NF-κB/p65 expression also indicated that melatonin could inhibit nuclear expression of NF-κB/p65 expression and total phosphorylated NF-κB/p65 in 8505c and TPC-1 cells post irradiation (Fig. 4E). Concordantly, enhanced DNA binding activity of NF-κB induced by irradiation was abrogated by melatonin (Fig. 4F). It was therefore worthwhile to evaluate the combinational effects of melatonin and irradiation in thyroid cancer.
Fig. 4.
Irradiation activates NF-κB/p65 phosphorylation. (A) Western blot analysis of nuclear NF-κB/p65 and p-NF-κB/p65 in 8505c, TPC-1 and KTC-1 cells after irradiation. (B) Expression of NF-κB/p65 response genes in 8505c, TPC-1 and KTC-1 cells after irradiation was detected by qPCR assays. (C) Immunoblotting of IL-1α, Cyclin D1 and MMP9 in 8505c, TPC-1 and KTC-1 cells after irradiation. β-Actin was used as a loading control. (D) qPCR analysis of Cyclin D1 and MMP9 in 8505c and TPC-1 cells after treatment with melatonin, irradiation or both. (E) Western blot analysis of nuclear NF-κB/p65 and p-NF-κB/p65 in 8505c and TPC-1 cells after treated with melatonin, irradiation or both. (F) ELISA analysis of NF-κB/p65 activity in 8505c, TPC-1 and KTC-1 cells after treated with melatonin, irradiation or both. *P<0.05.
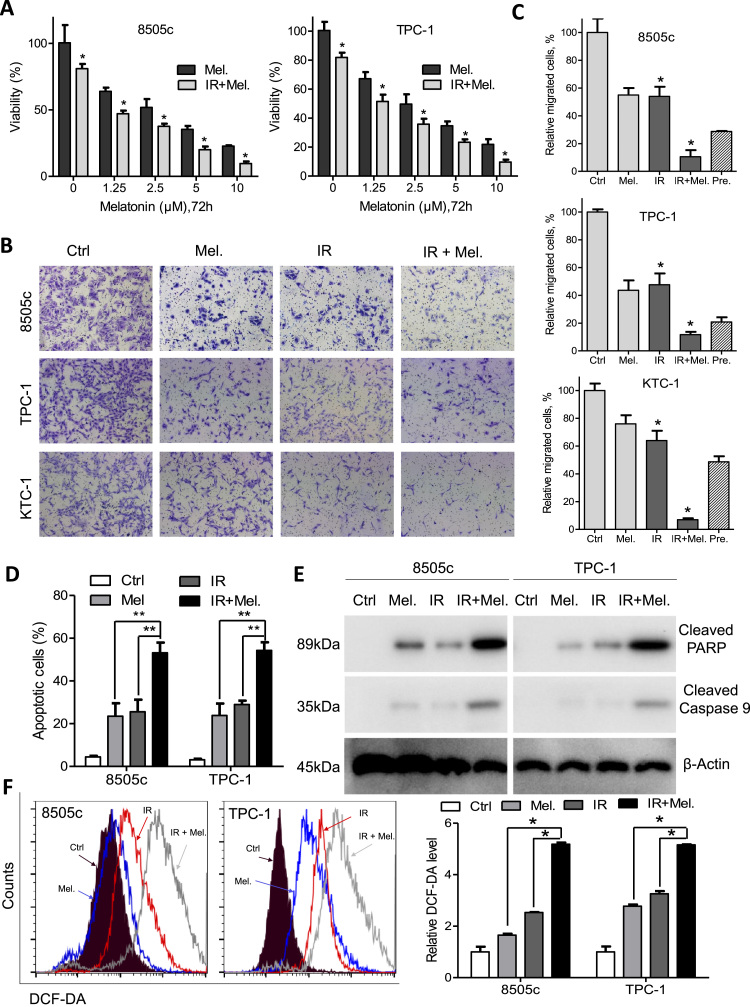
3.5. Melatonin synergizes with irradiation to induce cytotoxicity to thyroid cancer cells
MTS assays showed that combination of irradiation and melatonin significantly decreased cell viability compared with melatonin alone (Fig. 5A). We used the Calcusyn software to calculate combination index and found synergistic effects between irradiation and melatonin in thyroid cancer (Supplementary Fig. S4A). Moreover, combination of irradiation and melatonin significantly suppressed migration of 8505c, TPC-1, KTC-1 and BCPAP cells compared with either agent alone (Fig. 5B, C and Supplementary Fig. S4B). Apoptotic cells as detected by flow cytometry were significantly increased after treatment with irradiation and melatonin than cells treated with either agent alone (Fig. 5D). In line with the flow cytometry results, expression of cleaved PARP and cleaved caspase 9, as well as activity of caspase3/7 was increased after treatment with irradiation and melatonin in 8505c and TPC-1 cells (Fig. 5E, Supplementary Fig. S4C and S4D). Additionally, while intracellular ROS levels were significantly increased by melatonin or irradiation alone, combination of both agents led to synergistic induction of ROS (Fig. 5F). Taken together, melatonin potentiates radiation-induced cytotoxicity in thyroid cancer cells via inhibition of NF-κB/p65 phosphorylation and ROS induction.
Fig. 5.
Melatonin enhances sensitivity of thyroid cancer cells to irradiation in vitro. (A) Relative cell viability of 8505c and TPC-1 cells after treatment with melatonin at the indicated concentrations for 48 h with or without irradiation. (B) Migration assays of 8505c, TPC-1 and KTC-1 cells after treatment with melatonin, irradiation or both. (C) Quantification of migration analysis in 8505c, TPC-1 and KTC-1 cells. When observed value is less than the predicted value, the combination effect is considered as synergistic. (D) Apoptotic cell percentage in 8505c and TPC-1 cells after treated with melatonin, irradiation or both. (E) Immunoblotting of cleaved PARP and cleaved caspase 9 in 8505c and TPC-1 cells after treatment with melatonin, irradiation or both. β-Actin was used as a loading control. (F) Flow analysis of intracellular ROS in 8505c and TPC-1 cells after treatment with melatonin, irradiation or both. Representative images (left panel) and quantification (right panel) were shown. *P<0.05.
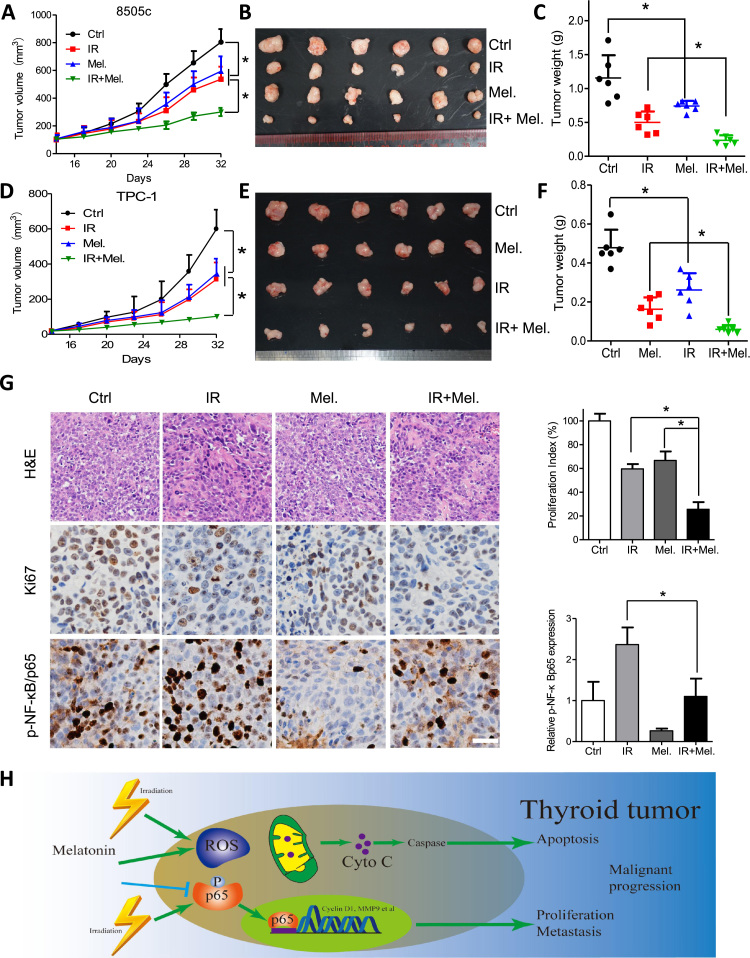
3.6. Combination of melatonin and irradiation suppresses tumor growth of thyroid cancer cells
To further evaluate the anti-tumor activity of melatonin in vivo, subcutaneous xenograft mouse model was used. Tumors of xenografts formed by the APC cell line 8505c cells in the treatment groups grew significantly slower than that in the control group (Fig. 6A and B), as is reflected by the tumor weight (Fig. 6C). Moreover, tumors formed by TPC-1 cells were sensitive to melatonin treatment and achieved successful synergy by combination of melatonin and irradiation (Fig. 6D–F). Also, melatonin or irradiation treatment induced no weight loss in nude mice (Supplementary Fig. S5A and S5B). Moreover, Ki-67 positive cells were significantly decreased by combination treatment, indicating suppressed tumor growth (Fig. 6G). On the other hand, immunohistochemistry staining of phosphorylated NF-κB/p65 in the mice xenografts further demonstrated that melatonin suppressed activated NF-κB induced by irradiation (Fig. 6G). Taken together, melatonin suppresses thyroid cell growth and enhances radiosensitivity via inhibition of NF-κB/p65 phosphorylation in vivo (Fig. 6H).
Fig. 6.
Melatonin suppresses growth of thyroid cancer cells, alone or in combination with irradiation. Growth curve of subcutaneous tumor formed by 8505c (A) or TPC-1 (D) cells in the nude mice after treatment with PBS, melatonin, irradiation or both. (B, E) Dissected tumors were photographed. (C, F) Weight of tumors in the indicated groups were recorded. (G) H&E staining and expression of Ki-67 and p-NF-κB/p65 in dissected samples were shown. (H) Schematic diagram shows that melatonin inhibits p65 phosphorylation and induces ROS in thyroid tumor and enhances sensitivity to irradiation. *P<0.05.
4. Discussion
The burden of thyroid carcinoma has continually increased [1], [3], [31]. Combined total thyroidectomy and postoperative 131I therapy prevails as the corner stone of therapeutic strategy for thyroid cancer patients [5]. Although the overall prognosis for DTC is good, patients refractory to 131I-irradiation could only achieve a 10% 10-year survival rate [9], which is comparable to that of ATC patients [32]. Moreover, the ability of the remaining tumor cells after thyroidectomy to absorb 131I plays vital roles in determination the efficacy of irradiation therapy [33]. The ATC cells could not concentrate 131I during the dedifferentiation process and thus become irradiation resistant. In some cases, even 131I-sensitive DTC lesions become resistant to 131I therapy during the treatment course. Therefore, development of novel anti-tumor methods is urgently needed for thyroid cancer, especially for patients with ATC.
Elevation of basal NF-κB activity in malignant thyroid tissues, which is attributed to the upstream kinase mutation, RET gene rearrangement or PTEN deletion [34] confers survival advantage to thyroid cancer cells and plays a critical role in thyroid carcinogenesis, thus making NF-κB an attractive target for molecular therapy [18], [34]. Several previous reports have shown that inhibition of NF-κB signaling in ATC as well as DTC cells markedly suppressed tumor growth in vitro and in vivo [24], [30], [35]. Based on these studies, we hypothesized that NF-κB–targeted therapy could potentially result in more complete suppression of thyroid cancers. However, off target and side effects has narrowed therapeutic application of previous inhibitors [35]. Clinical trials showed that no effective small molecule compound was available to inhibit the NF-κB cascade selectively. Knowing that melatonin has been reported to inhibit NF-κB signaling, can be easily achieved through dietary supplementation, and has no major side effects, we decided to investigate the effect of this compound in thyroid cancer.
Anti-tumor activity of melatonin, alone or in combination with other chemotherapeutic regimens has been well established in many types of human tumors, such as colorectal [26], esophageal [10], breast [16] and pancreatic [15] cancer. Disruption of oncogenic signaling [10], [15] or modulation of intracellular reactive oxygen species (ROS) [10], [28] underlie the oncostatin roles of melatonin. For instance, inhibition of NF-κB DNA-binding activity by melatonin resulted in reduced metastatic activity of renal cell carcinoma [36], decreased proliferation of colorectal cancer [26] and increased apoptosis in pancreatic cancer [15]. In the current study, we showed for the first time that melatonin at micro molar level in DTC as well as ATC inhibited p65 translocation to the nucleus and decreased the levels of several proteins encoded by NF-κB-dependent genes, which was supported by a previous report [15]. As a result of melatonin treatment, we observed caspases activation and increased apoptosis in vitro and delayed tumor growth in vivo.
Both anti-oxidant and pro-oxidant roles of melatonin have been reported. Melatonin was shown to induce elevated intracellular ROS level in esophageal squamous carcinoma cell lines [10]. By contrast, melatonin was reported to abolish intracellular ROS induced by doxorubicin in A549 lung cancer cells [28]. Our data supported the notion that the anti-tumor activity of melatonin is dependent on its pro-oxidant properties in thyroid cancer, as pretreatment with NAC significantly decreased apoptotic cells induced by melatonin.
Importantly, chemo- or radio-sensitization effects of melatonin have been observed in several cancers [10], [15], [16], [26]. Melatonin induces apoptosis and overcomes gemcitabine resistance in pancreatic cancer by abrogating NF-κB activation [15]. Pretreatment with melatonin sensitizes the human breast cancer cells to the ionizing radiation by decreasing around 50% the activity and expression of proteins involved in the synthesis of estrogens [16]. Moreover, melatonin suppressed proliferation of colorectal cancer cells, and significantly enhanced sensitivity of cancer cells to ursolic acid at micro molar level via inhibition of p65 nuclear translocation [26]. ROS mediates chemo-sensitizing roles of melatonin to 5-Fu in esophageal squamous cell carcinoma [10]. Previous reports showed that aberrantly activated NF-κB signaling induced by irradiation functions as key determinants for radiation efficiency in several malignancies including thyroid cancer [22], [37]. Our results indicated that irradiation activated NF-κB signaling as evidenced by elevated p65 phosphorylation, enhanced NF-κB reporter activity and upregulated Bcl-xl, XIAP and MMP9 expression. However, these effects were reversed by cotreatment with melatonin. Consequently, melatonin could enhance sensitivity of thyroid cancer cells to irradiation via inhibition of NF-κB activation in vitro and in vivo. Previous randomized clinical trials showed that oral supplementation of melatonin (20 mg per day) could enhance the quality of life or minimize the chemoradiation induced sides effects in cancer patients [38], [39]. However, further studies are warranted to explore the effects of melatonin in lowering cancer related recurrence and mortality rate although this naturally occurring agent has shown promising pharmacological potential in preclinical studies [40].
In our present study, we have shown that melatonin significantly decreases clonogenicity and inhibits proliferation of DTC and ATC cell lines as a single agent. Melatonin could sensitize TPC-1 and 8505c cells to irradiation via inhibition of NF-κB through suppression of p65 phosphorylation and induction of ROS in vitro and in vivo. Our data suggest that melatonin-induced radio-sensitization may have clinical benefits for thyroid cancer.
Acknowledgements
This work was primarily supported by National Natural Science Foundation of China (No. 81502697 to P.D.L.; No. 81702767 to Y.L.; No. D81301976 to ZZW), Hubei Provincial Natural Science Foundation of China (No. 2016CFB374 to P.D.L.; No. 2017CFB246 to Y.L.) and Hubei Province Health and Family Planning Scientific Research Project (No. WJ2017Q035 to Y.L.).
Acknowledgments
Conflict of interest
The authors declare no conflict of interests.
Ethics approval
All the animal experiments were performed according to the National Institutes of Health animal use guidelines on the use of experimental animals.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2018.02.025.
Appendix A. Supplementary material
Supplementary material
References
- 1.Kilfoy B.A., Zheng T., Holford T.R., Han X., Ward M.H., Sjodin A. International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control: CCC. 2009;20:525–531. doi: 10.1007/s10552-008-9260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kondo T., Ezzat S., Asa S.L. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat. Rev. Cancer. 2006;6:292–306. doi: 10.1038/nrc1836. [DOI] [PubMed] [Google Scholar]
- 3.Chen W., Zheng R., Baade P.D., Zhang S., Zeng H., Bray F. Cancer statistics in China, 2015. CA: Cancer J. Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Mazzaferri E.L. An overview of the management of papillary and follicular thyroid carcinoma. Thyroid: Off. J. Am. Thyroid Assoc. 1999;9:421–427. doi: 10.1089/thy.1999.9.421. [DOI] [PubMed] [Google Scholar]
- 5.Nagaiah G., Hossain A., Mooney C.J., Parmentier J., Remick S.C. Anaplastic thyroid cancer: a review of epidemiology, pathogenesis, and treatment. J. Oncol. 2011;2011:542358. doi: 10.1155/2011/542358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paunovic I.R., Sipetic S.B., Zoric G.V., Diklic A.D., Savic D.V., Marinkovic J. Survival and prognostic factors of anaplastic thyroid carcinoma. Acta Chir. Belg. 2015;115:62–67. [PubMed] [Google Scholar]
- 7.Nikiforov Y.E., Nikiforova M.N. Molecular genetics and diagnosis of thyroid cancer. Nat. Rev. Endocrinol. 2011;7:569–580. doi: 10.1038/nrendo.2011.142. [DOI] [PubMed] [Google Scholar]
- 8.Pfister D.G., Fagin J.A. Refractory thyroid cancer: a paradigm shift in treatment is not far off. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 2008;26:4701–4704. doi: 10.1200/JCO.2008.17.3682. [DOI] [PubMed] [Google Scholar]
- 9.Durante C., Haddy N., Baudin E., Leboulleux S., Hartl D., Travagli J.P. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J. Clin. Endocrinol. Metab. 2006;91:2892–2899. doi: 10.1210/jc.2005-2838. [DOI] [PubMed] [Google Scholar]
- 10.Lu Y.X., Chen D.L., Wang D.S., Chen L.Z., Mo H.Y., Sheng H. Melatonin enhances sensitivity to fluorouracil in oesophageal squamous cell carcinoma through inhibition of Erk and Akt pathway. Cell Death Dis. 2016;7:e2432. doi: 10.1038/cddis.2016.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su S.C., Hsieh M.J., Yang W.E., Chung W.H., Reiter R.J., Yang S.F. Cancer metastasis: mechanisms of inhibition by melatonin. J. Pineal Res. 2017:62. doi: 10.1111/jpi.12370. [DOI] [PubMed] [Google Scholar]
- 12.Singh M., Jadhav H.R. Melatonin: functions and ligands. Drug Discov. Today. 2014;19:1410–1418. doi: 10.1016/j.drudis.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Dauchy R.T., Xiang S., Mao L., Brimer S., Wren M.A., Yuan L. Circadian and melatonin disruption by exposure to light at night drives intrinsic resistance to tamoxifen therapy in breast cancer. Cancer Res. 2014;74:4099–4110. doi: 10.1158/0008-5472.CAN-13-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farez M.F., Mascanfroni I.D., Mendez-Huergo S.P., Yeste A., Murugaiyan G., Garo L.P. Melatonin contributes to the seasonality of multiple sclerosis relapses. Cell. 2015;162:1338–1352. doi: 10.1016/j.cell.2015.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ju H.Q., Li H., Tian T., Lu Y.X., Bai L., Chen L.Z. Melatonin overcomes gemcitabine resistance in pancreatic ductal adenocarcinoma by abrogating nuclear factor-kappaB activation. J. Pineal Res. 2016;60:27–38. doi: 10.1111/jpi.12285. [DOI] [PubMed] [Google Scholar]
- 16.Alonso-Gonzalez C., Gonzalez A., Martinez-Campa C., Menendez-Menendez J., Gomez-Arozamena J., Garcia-Vidal A. Melatonin enhancement of the radiosensitivity of human breast cancer cells is associated with the modulation of proteins involved in estrogen biosynthesis. Cancer Lett. 2016;370:145–152. doi: 10.1016/j.canlet.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 17.Hoesel B., Schmid J.A. The complexity of NF-kappaB signaling in inflammation and cancer. Mol. Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pacifico F., Leonardi A. Role of NF-kappaB in thyroid cancer. Mol. Cell. Endocrinol. 2010;321:29–35. doi: 10.1016/j.mce.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Ling J., Kang Y., Zhao R., Xia Q., Lee D.F., Chang Z. KrasG12D-induced IKK2/beta/NF-kappaB activation by IL-1alpha and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:105–120. doi: 10.1016/j.ccr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pyo J.S., Kang G., Kim D.H., Chae S.W., Park C., Kim K. Activation of nuclear factor-kappaB contributes to growth and aggressiveness of papillary thyroid carcinoma. Pathol. Res. Pract. 2013;209:228–232. doi: 10.1016/j.prp.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Bu Y., Cai G., Shen Y., Huang C., Zeng X., Cao Y. Targeting NF-kappaB RelA/p65 phosphorylation overcomes RITA resistance. Cancer Lett. 2016;383:261–271. doi: 10.1016/j.canlet.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Lu Y.X., Ju H.Q., Wang F., Chen L.Z., Wu Q.N., Sheng H. Inhibition of the NF-kappaB pathway by nafamostat mesilate suppresses colorectal cancer growth and metastasis. Cancer Lett. 2016;380:87–97. doi: 10.1016/j.canlet.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Meng Z., Lou S., Tan J., Xu K., Jia Q., Zheng W. Nuclear factor-kappa B inhibition can enhance therapeutic efficacy of 131I on the in vivo management of differentiated thyroid cancer. Life Sci. 2012;91:1236–1241. doi: 10.1016/j.lfs.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 24.Starenki D., Namba H., Saenko V., Ohtsuru A., Yamashita S. Inhibition of nuclear factor-kappaB cascade potentiates the effect of a combination treatment of anaplastic thyroid cancer cells. J. Clin. Endocrinol. Metab. 2004;89:410–418. doi: 10.1210/jc.2003-031216. [DOI] [PubMed] [Google Scholar]
- 25.Meng Z., Lou S., Tan J., Xu K., Jia Q., Zheng W. Nuclear factor-kappa B inhibition can enhance apoptosis of differentiated thyroid cancer cells induced by 131I. PLoS One. 2012;7:e33597. doi: 10.1371/journal.pone.0033597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacky B.P., Garay P.E., Dupuy J., Nelson J.B., Cai B., Molina Y. Identification of fibroblast growth factor receptor 3 (FGFR3) as a protein receptor for botulinum neurotoxin serotype A (BoNT/A) PLoS Pathog. 2013;9:e1003369. doi: 10.1371/journal.ppat.1003369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okon I.S., Zou M.H. Mitochondrial ROS and cancer drug resistance: implications for therapy. Pharmacol. Res. 2015;100:170–174. doi: 10.1016/j.phrs.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song N., Kim A.J., Kim H.J., Jee H.J., Kim M., Yoo Y.H. Melatonin suppresses doxorubicin-induced premature senescence of A549 lung cancer cells by ameliorating mitochondrial dysfunction. J. Pineal Res. 2012;53:335–343. doi: 10.1111/j.1600-079X.2012.01003.x. [DOI] [PubMed] [Google Scholar]
- 29.Li P.D., Liu Z., Cheng T.T., Luo W.G., Yao J., Chen J. Redox-dependent modulation of metformin contributes to enhanced sensitivity of esophageal squamous cell carcinoma to cisplatin. Oncotarget. 2017;8:62057–62068. doi: 10.18632/oncotarget.18907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Starenki D.V., Namba H., Saenko V.A., Ohtsuru A., Maeda S., Umezawa K. Induction of thyroid cancer cell apoptosis by a novel nuclear factor kappaB inhibitor, dehydroxymethylepoxyquinomicin. Clin. Cancer Res.: Off. J. Am. Assoc. Cancer Res. 2004;10:6821–6829. doi: 10.1158/1078-0432.CCR-04-0463. [DOI] [PubMed] [Google Scholar]
- 31.Meza R., Chang J.T. Multistage carcinogenesis and the incidence of thyroid cancer in the US by sex, race, stage and histology. BMC Public Health. 2015;15:789. doi: 10.1186/s12889-015-2108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eustatia-Rutten C.F., Corssmit E.P., Biermasz N.R., Pereira A.M., Romijn J.A., Smit J.W. Survival and death causes in differentiated thyroid carcinoma. J. Clin. Endocrinol. Metab. 2006;91:313–319. doi: 10.1210/jc.2005-1322. [DOI] [PubMed] [Google Scholar]
- 33.Sherman E.J., Su Y.B., Lyall A., Schoder H., Fury M.G., Ghossein R.A. Evaluation of romidepsin for clinical activity and radioactive iodine reuptake in radioactive iodine-refractory thyroid carcinoma. Thyroid: Off. J. Am. Thyroid Assoc. 2013;23:593–599. doi: 10.1089/thy.2012.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nikiforov Y.E. Thyroid carcinoma: molecular pathways and therapeutic targets. Mod. Pathol.: Off. J. U. S. Can. Acad. Pathol. Inc. 2008;21(Suppl. 2):S37–S43. doi: 10.1038/modpathol.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bauerle K.T., Schweppe R.E., Haugen B.R. Inhibition of nuclear factor-kappa B differentially affects thyroid cancer cell growth, apoptosis, and invasion. Mol. Cancer. 2010;9:117. doi: 10.1186/1476-4598-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin Y.W., Lee L.M., Lee W.J., Chu C.Y., Tan P., Yang Y.C. Melatonin inhibits MMP-9 transactivation and renal cell carcinoma metastasis by suppressing Akt-MAPKs pathway and NF-kappaB DNA-binding activity. J. Pineal Res. 2016;60:277–290. doi: 10.1111/jpi.12308. [DOI] [PubMed] [Google Scholar]
- 37.Alkalay I., Yaron A., Hatzubai A., Orian A., Ciechanover A., Ben-Neriah Y. Stimulation-dependent I kappa B alpha phosphorylation marks the NF-kappa B inhibitor for degradation via the ubiquitin-proteasome pathway. Proc. Natl. Acad. Sci. USA. 1995;92:10599–10603. doi: 10.1073/pnas.92.23.10599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Onseng K., Johns N.P., Khuayjarernpanishk T., Subongkot S., Priprem A., Hurst C. Beneficial effects of adjuvant melatonin in minimizing oral mucositis complications in head and neck cancer patients receiving concurrent chemoradiation. J. Altern. Complement. Med. 2017;23:957–963. doi: 10.1089/acm.2017.0081. [DOI] [PubMed] [Google Scholar]
- 39.Sookprasert A., Johns N.P., Phunmanee A., Pongthai P., Cheawchanwattana A., Johns J. Melatonin in patients with cancer receiving chemotherapy: a randomized, double-blind, placebo-controlled trial. Anticancer Res. 2014;34:7327–7337. [PubMed] [Google Scholar]
- 40.Tordjman S., Chokron S., Delorme R., Charrier A., Bellissant E., Jaafari N. Melatonin: pharmacology, functions and therapeutic benefits. Curr. Neuropharmacol. 2017;15:434–443. doi: 10.2174/1570159X14666161228122115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material