Abstract
Cases of mucinous ovarian cancer are predominantly resistant to chemotherapies. The present review summarizes current knowledge of the therapeutic potential of targeting the Wingless (WNT) pathway, with particular emphasis on preclinical and clinical studies, for improving the chemoresistance and treatment of mucinous ovarian cancer. A review was conducted of English language literature published between January 2000 and October 2017 that concerned potential signaling pathways associated with the chemoresistance of mucinous ovarian cancer. The literature indicated that aberrant activation of growth factor and WNT signaling pathways is specifically observed in mucinous ovarian cancer. An evolutionarily conserved signaling cascade system including epidermal growth factor/RAS/RAF/mitogen-activated protein kinase kinase/extracellular signal-regulated protein kinase, phosphoinositide 3-kinase/Akt and WNT signaling regulates a variety of cellular functions; their crosstalk mutually enhances signaling activity and induces chemoresistance. Novel antagonists, modulators and inhibitors have been developed for targeting the components of the WNT signaling pathway, namely Frizzled, low-density lipoprotein receptor-related protein 5/6, Dishevelled, casein kinase 1, AXIN, glycogen synthase kinase 3β and β-catenin. Targeted inhibition of WNT signaling represents a rational and promising novel approach to overcome chemoresistance, and several WNT inhibitors are being evaluated in preclinical studies. In conclusion, the WNT receptors and their downstream components may serve as novel therapeutic targets for overcoming chemoresistance in mucinous ovarian cancer.
Keywords: mucinous ovarian cancer, WNT signaling, chemoresistance, therapeutic targets
1. Introduction
Epithelial ovarian cancer comprises a heterogeneous group of tumors that have distinct clinicopathological and molecular features as well as multiple underlying causative genetic mutations (1). High-grade serous ovarian cancer originates de novo from the fallopian tube fimbriae, while clear cell endometrioid tumors arise from endometriosis (1). Mucinous ovarian cancer accounts for approximately 10% of epithelial ovarian cancer, but its tissue origin remains controversial (2). Primary mucinous cancer frequently presents as a large (>10 cm) clinically unilateral tumor similar to benign cystadenoma and borderline tumors (3). Occasional presentation as <10-cm tumor or clinically bilateral tumor may be features that contribute to metastases from other sites including the appendix, colon, stomach, pancreas and biliary tract (3). At baseline, primary mucinous ovarian tumors progress from benign to borderline to invasive cancer in a stepwise manner, all of which generally have a good prognosis (3). Mucinous tumors are more frequently detected in early-stage disease with lower tumor grading compared with high-grade serous cancer; however, patients with advanced disease have poor clinical outcome, possibly due to resistance to taxane and platinum-based conventional chemotherapy (4). An evolutionarily conserved signaling cascade system, including growth factor pathways [epidermal growth factor receptor (EGFR), ERBB and fibroblast growth factor receptor (FGFR)] and Wingless (WNT) signaling pathways, regulates a variety of cellular functions, including chemoresistance (5). The crosstalk between EGFR/KRAS proto-oncogene/B-Raf proto-oncogene (BRAF)/mitogen-activated protein kinase (MAPK), phosphatidylinositol-3 kinase (PI3K)/Akt (also known as protein kinase B) and WNT signaling pathways sustains PI3K/glycogen synthase kinase-3β (GSK3β)/β-catenin signal activation, which is associated with chemoresistance in cancer (6). The WNT receptors and their downstream components are being investigated as potential targets in the development of novel anticancer therapies (5,6).
The present article aimed to summarize the underlying molecular mechanisms of chemoresistance in mucinous ovarian cancer, focusing on the WNT signaling pathway. Novel therapeutics that may target chemoresistant processes from bench to bedside were also discussed. In this regard, a systematic review of the literature using an electronic search of the PubMed database (http://www.ncbi.nlm.nih.gov/pubmed) was conducted. Relevant literature published between January 2000 and October 2017 was searched. The search strategy screened for full-text original research or reviews in peer-reviewed journals with at least one of the key words ‘mucinous ovarian cancer’, ‘chemoresistance’, ‘WNT/Wingless’, ‘EGFR/epidermal growth factor receptor’, ‘FGFR/fibroblast growth factor receptor’, ‘signaling pathway’, ‘inhibitor’ or ‘antagonist’ in their titles or abstracts. English-language publication search results from PubMed and references within the relevant articles were analyzed. To minimize selection bias, screening of the studies was independently performed by two reviewers following agreement on the selection criteria.
2. Potential candidate gene alterations in mucinous ovarian cancer
Previous studies have identified potential gene alterations implicated in the carcinogenesis and progression of mucinous ovarian cancer (2,7–10). Mucinous tumors are likely driven by constitutive signaling activation resulting from mutagenic processes (BRAF and KRAS mutations) and growth factor amplifications (EGFR and MYC proto-oncogene amplifications) (2,8–10). The BRAF and KRAS mutations frequently identified in mucinous ovarian cancer have also been observed in low-grade serous ovarian cancer and serous and mucinous borderline tumors (7). One such activating driver mutation is BRAFV600E, a substitution of glutamic acid for valine in codon 600 in exon 15 (7). BRAF mutations have diagnostic and prognostic value in many tumors including not only mucinous ovarian cancer, but also melanoma (11), colorectal cancer (12), thyroid cancer (13), brain tumors and various other cancers (14). Furthermore, the mutation rate in KRAS for proven pathogenic mutations is 60–70% (7). EGFR triggers cell proliferation through the RAS/RAF/MAPK signaling pathway. Erb-b2 receptor tyrosine kinase 2 (ERBB2; also known as human epidermal growth factor receptor 2, HER2) amplification is relatively common (~20%) in mucinous ovarian cancer and borderline mucinous tumor (2,7–10). Concurrent aberrant ERBB2 and KRAS signaling has been observed in a marked number of cases (~11%), suggesting that acquired ERBB2 amplification is secondary to the emergence of KRAS activating mutation (10). Although oncogenic KRAS driver mutations and ERBB2 amplification are not mutually exclusive (15), KRAS mutations may be mutually exclusive with c-MYC amplification (9).
In addition, some cases of mucinous ovarian tumors may harbor clinically targetable tumor protein p53 (TP53) mutations; indeed TP53 is the most commonly altered gene in high grade-serous ovarian cancer (2,8). TP53 mutations have been observed to be more frequent in mucinous cancer when compared with borderline tumors (57 vs. 12%, respectively) (10). Mucinous ovarian cancer has been associated with homozygous loss of the cyclin dependent kinase inhibitor 2A (CDKN2A) locus (2,8). Infrequent cases of mucinous ovarian cancer also harbored additional mutations, including in ring finger protein 43 (RNF43), WNT and WNT family members, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha, phosphatase and tensin homolog, cadherin 1, E74 like ETS transcription factor 3 (ELF3), AT-rich interaction domain 1A, GNAS complex locus (GNAS), G protein subunit alpha 11 (GNA11), forkhead box L2, FGFR2, serine/threonine kinase 11 (STK11), β-catenin (also known as catenin beta-1, CTNNB1) and SMAD family member 4 (SMAD4) (2,7,10,16). Mutations of these genes are considered to serve roles in the tumorigenesis, progression, aggressive features and clinical outcome of a subset of mucinous ovarian tumors (16). These mutated genes have been identified in a variety of tumor types, including tumors of the gastrointestinal tract, pancreas and endometrium (16). For example, mutations in RNF43, an E3 ubiquitin-protein ligase, have been observed in pancreatic, colorectal and mucinous ovarian cancers (17). Gene mutation data indicated that mucinous-type tumors from different sites have marked similarities to mucinous ovarian cancer (2). Notably, the majority of these mutated genes, including RNF43, ELF3, GNAS, GNA11, STK11, CTNNB1 and SMAD4, may serve a crucial role in regulating WNT signaling (2,7,10). Mutations in key genes and aberrations in the WNT pathway are typically requisites for mucinous-type cancers and often result in increased nuclear β-catenin (2).
The genetic landscape of a variety of benign, borderline and malignant lesions has been gradually characterized. Genetic alterations of the RAS/RAF/MAPK, PI3K/Akt and WNT/β-catenin signaling pathway members have been reported to increase in a stepwise manner from mucinous borderline tumors to mucinous ovarian cancer (18). The crosstalk between the growth factor and WNT signaling pathways may sustain PI3K/GSK3β/β-catenin signal activation, which is associated with chemoresistance in cancer (18).
3. Growth factor and WNT pathways
Growth factor pathways
Somatic mutations or amplifications of the EGFR (also known as ERBB1 or HER1), ERBB2 and FGFR family members have been reported in numerous cancers, including non-small-cell lung cancer (19), metastatic colorectal cancer, glioblastoma (20), head and neck cancer, pancreatic cancer, breast cancer and ovarian cancer (21). EGFR activates the RAS/RAF/MAPK pathway and the PI3K/Akt pathway, which leads to activation of c-Myc and cyclin D1. Members of the ERBB family of receptors serve key roles in chemoresistance (5,22). FGF stimulates SRC proto-oncogene/focal adhesion kinase, SRC/PI3K/Akt, Hedgehog, Notch, transforming growth factor-β and noncanonical WNT signaling cascades and regulates a variety of cellular functions (5,21,22). The nonreceptor tyrosine kinase Src, the downstream target of FGFR, has been reported to serve an important role in chemoresistance in mucinous ovarian cancer (23). Thus, an association between the expression of growth factor pathways and increased resistance to chemotherapy in mucinous ovarian cancer has been implicated.
WNT pathways
The WNT signaling pathways have been classified into canonical [WNT/β-catenin/T-cell factor (TCF)] and two non-canonical [WNT/planar cell polarity (PCP)] and WNT/Ca2+ pathways (24). WNT signaling regulates a variety of cellular functions, including carcinogenesis, proliferation, adhesion, migration, invasion, angiogenesis, progression, survival, epithelial-to-mesenchymal transition (EMT) and chemoresistance (24). Aberrant activation of the WNT signaling pathway has been implicated to serve a key role in the regulation of chemoresistance in mucinous ovarian cancer (25,26).
In the canonical pathway (WNT/β-catenin/TCF), a WNT ligand forms a ternary complex with the seven-pass transmembrane receptor, Frizzled (Fzd) and low-density lipoprotein receptor-related protein 5/6 (LRP5/6), which activates Dvl homolog 1 (Dvl1) (26,27). Activation of Dvl1 dismantles the β-catenin ‘destruction’ complex to which it is associated, composed of Axin1 (AXIN1), adenomatous polyposis coli (APC), GSK3β and casein kinase 1 (CK1), which promotes the recruitment of β-catenin and TCF to the WNT target-gene promoters in the nucleus, to subsequently induce the transcription of target genes, including c-Myc and cyclin D1 (28). Ovarian cancer relapse, metastasis and chemoresistance may occur when the canonical WNT/β-catenin signaling induces the EMT program (29).
Alternatively, WNT ligands of the non-canonical WNT/PCP signaling pathway bind to the co-receptors Fzd and receptor tyrosine kinase like orphan receptor 2 (ROR2) to activate Dvl, which in turn promotes Rac family small GTPase 1/Ras homolog family member A signaling, leading to activation of c-Jun N-terminal kinase (JNK; also known as MAPK8) and Rho-associated coiled-coil containing protein kinase 2 (ROCK2) (30). JNK and ROCK2 are involved in cytoskeletal remodeling, cell motility and metastasis (31). In the non-canonical WNT/Ca2+ pathway, WNT proteins interact within a ternary complex composed of Fzd and ROR2 receptors, with the resultant activation of Dvl leading to increased intracellular Ca2+ levels, which in turn activate calcium/calmodulin dependent protein kinase II gamma and protein kinase C (PKC) (30). Overexpression of PKC has been associated with increased expression of multidrug resistant (MDR) proteins and chemoresistance (30). Collectively, these data suggest that the canonical and non-canonical WNT signaling pathways are important molecular determinants of chemoresistance in mucinous ovarian cancer.
Overall, the growth factor and WNT signaling pathways are an evolutionarily conserved signaling cascade system, and their mutual crosstalk may enhance the processes of carcinogenesis, progression and chemoresistance in mucinous ovarian cancer.
4. Molecular mechanism of WNT signaling implicated in chemoresistance
Overcoming intrinsic and acquired drug resistance is a challenge in the clinical treatment of patients with ovarian cancer (32). A previous review summarized numerous molecular aspects of chemoresistance, including oncogenes (EGFR/PI3K/Akt and WNT), ATP binding cassette (ABC) transporter pumps, EMT and cancer cell stemness (32). Ovarian cancer subtypes are distinct entities, having different responses to chemotherapy (23). Epithelial ovarian cancer is originally classified into two groups based on chemosensitivity. Serous and endometrioid ovarian cancers exhibit a hallmark of chemosensitivity, with higher response rates for taxane/platinum-based regimens. By contrast, mucinous and clear cell cancers are primarily resistant to chemotherapies (23). Patients with advanced-stage mucinous ovarian cancer exhibit higher rates of chemoresistance and have poorer survival outcomes compared with those with advanced-stage high-grade serous ovarian cancer (33).
Studies have been performed to identify the genes that contribute to chemoresistance in mucinous cancer (34,35). Previous genetic pathway enrichment analyses identified that upregulated transcripts in high-grade serous cancer were enriched for cell cycle signaling pathways, such as the TP53/breast cancer gene (BRCA) driver signaling pathway, while mucinous tumors were associated with upregulation of the WNT signaling pathway (2,35). Furthermore, a number of somatic mutations in proto-oncogenes, including in KRAS (36) and BRAF (2), have been identified in mucinous tumors. Overall, the available data indicate that crosstalk between the EGFR/PI3K/Akt and WNT signal pathways is implicated in the chemoresistance of mucinous ovarian cancer (32,36). Therefore, the WNT receptors and their downstream components may serve as novel therapeutic targets for treatment.
5. WNT-related potential candidates for overcoming chemoresistance
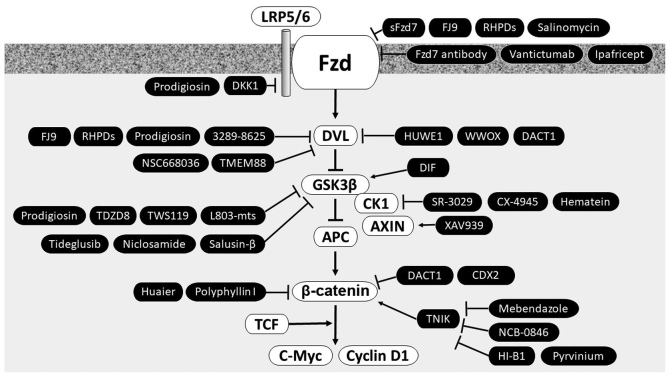
In particular, novel therapeutics that target chemoresistant processes may be useful from bench to bedside in mucinous ovarian cancer. Key components identified in the WNT signaling pathways are potential candidates of chemoresistance. Table I lists the WNT-related candidates and corresponding targeting agents for overcoming chemoresistance. Fig. 1 depicts the WNT pathway antagonists, inhibitors and modulators of particular interest in preclinical/clinical studies and their different mechanisms of action.
Table I.
WNT-related potential candidates for overcoming chemoresistance in mucinous ovarian cancer.
| Target | Therapeutic agent | Summary | (Refs.) |
|---|---|---|---|
| Fzd | sFzd7 | Soluble Fzd7 peptide inhibitor | 39 |
| FJ9 | Small molecule inhibitor | 39 | |
| RHPDs | Small interfering peptides | 39 | |
| Salinomycin | Ionophore antibiotic | 36 | |
| Fzd7 antibody | Anti-FZD | 39 | |
| Vantictumab | Anti-FZD | 41 | |
| Ipafricept | Inhibitory FZD8-Fc fusion protein, also known as OMP-54F28 | 41 | |
| LRP5/6 | Dickkopf1 | Secreted inhibitor of the Wnt/β-catenin pathway | 48 |
| Prodigiosin | Natural red pigment produced by bacterial species | 51 | |
| Dvl | FJ9 | Small molecule inhibitor | 39 |
| RHPDs | Small interfering peptides | 39 | |
| Prodigiosin | Natural red pigment produced by bacterial species | 51 | |
| 3289–8625 | Synthetic compound that binds to and blocks the PDZ domain of Dvl | 38,52 | |
| HUWE1 | E3 ubiquitin protein ligase | 55 | |
| WWOX | Member of the SDR protein family | 56 | |
| NSC668036 | Organic molecule that binds to and blocks the PDZ domain of Dvl | 54 | |
| DACT1 | Induces Dvl degradation | 25,54,57 | |
| Transmembrane protein 88 | Interacts with Dvl | 53 | |
| CK1 | SR-3029 | ATP-competitive CK1 inhibitor | 58 |
| CX-4945 | Selective ATP-competitive CK2 inhibitor | 59 | |
| Hematein (3,4,10,6a-tetrahydroxy-7, 6 adihydroindeno [2,1-c] chroman-9-one) | CK2 inhibitor | 60 | |
| AXIN | XAV939 | Small molecule tankyrase 1 inhibitor | 62 |
| GSK3β | Prodigiosin | Natural red pigment produced by bacterial species | 51 |
| TDZD8 | Small chemical inhibitor | 65 | |
| TWS119 | Small chemical | 65 | |
| L803-mts | Peptide inhibitor | 65 | |
| Tideglusib | Selective and irreversible GSK-3 inhibitor | 64 | |
| Niclosamide | Salicyclamide-derivative anthelmintic drug | 66 | |
| DIF (1-[3-chloro-2,6-dihydroxy-4-methoxyphenyl]hexan-1-one) | Differentiation-inducing factor | 67 | |
| Salusin-β | Parasympathomimetic proatherosclerotic peptide | 68 | |
| β-catenin | Caudal type homeobox 2 | Suppresses the transcriptional activity of the β-catenin-TCF complex and β-catenin nuclear localization | 69 |
| Huaier (TCM Trametes robiniophila Murr) | Reported to inhibit nuclear translocation of β-catenin and transcriptionally downregulate WNT/β-catenin target genes | 72 | |
| Mebendazole | Anthelmintic agent and selective inhibitor of TNIK | 73 | |
| NCB-0846 | Small-molecule TNIK inhibitor | 73 | |
| HI-B1 | Small molecule that directly interacts with β-catenin | 74 | |
| Polyphyllin I (component in the TCM herb Paris polyphylla Smith) | Reported to inhibit nuclear translocation of β-catenin and transcriptionally downregulate WNT/β-catenin target genes | 72 | |
| Pyrvinium | Anthelmintic drug | 75 | |
| Others | TFF1 | Trefoil factor 1 | 76 |
| CXCR4 | CXC chemokine receptor 4 | 77 | |
| FN1 | Fibronectin 1 | 78 | |
| SERPINA1 | Serpin family A member 1 | 79 | |
| Klotho | Co-receptor for FGF23 | 80 | |
| Vemurafenib | Monoclonal antibody against BRAFV600E | 8,10 |
Fzd/FZD, Frizzled; LRP5/6, lipoprotein receptor-related protein 5/6; Dvl, disheveled; SDR, short-chain dehydrogenases/reductases; HUWE1, HECT, UBA and WWE domain-containing 1; WWOX, WW domain-containing oxidoreductase; DACT1, dishevelled-binding antagonist of β-catenin; CK1, casein kinase 1; GSK3β, glycogen synthase kinase 3β; TCM, traditional Chinese medicine; TNIK, Traf2 and Nck-interacting protein kinase; TCF, T-cell factor.
Figure 1.
Schematic diagram depicting the proposed WNT pathway antagonists, inhibitors and modulators. The WNT receptor and its downstream targets are considered as promising therapeutic targets for the treatment of mucinous ovarian cancer. Arrows indicate modulators. LRP5/6, lipoprotein receptor-related protein 5/6; Fzd, Frizzled; sFzd7, soluble Fzd7 peptide; RNF43, ring finger protein 43; DKK, Dickkopf1; DVL, Dishevelled; TMEM88, transmembrane protein 88; HUWE1, HECT, UBA and WWE domain-containing 1; WWOX, WW domain-containing oxidoreductase; DACT1, dishevelled-binding antagonist of β-catenin; GSK3β, glycogen synthase kinase 3β; DIF, differentiation-inducing factor; CK1, casein kinase 1; APC, adenomatous polyposis coli; CDX2, caudal type homeobox 2; TCF, T-cell factor; TNIK, Traf2 and Nck-interacting protein kinase.
WNT ligand interacts with its cognate co-receptors LRP5/6 and Fzd. A number of approaches have been tested to target this interaction, including the use of natural compounds (37), small molecule inhibitors (38) and antibody-based inhibitors (8), with promising results (37). Natural compounds, including curcumin, 3,3-diindolylmethane, and phytoestrogen, have been tested for their capacity to reduce the activity of WNT signaling in cancer cells. Synthetic/small WNT inhibitors, including rofecoxib (cyclooxygenase-2 inhibitor), PRI-724 (β-catenin antagonist) and CWP232291 (synthetic/small WNT inhibitor), and the monoclonal antibody against Fzd receptors, vanituctumab, have been described (37). According to previous literature, several families of secreted antagonists consist of secreted frizzled-related proteins (sFRPs), the Dickkopf (Dkk) protein family, sclerostin (a soluble WNT antagonist), cerberus and WNT inhibitory factor-1 (39,40). However, the majority of these have not been incorporated into clinical practice for the treatment of patients with mucinous ovarian cancer. These WNT inhibitors have been previously reviewed in detail (35,37,41,42).
Fzd inhibitors (sFzd7, FJ9, RHPD, salinomycin, Fzd7 antibody, vantictumab and ipafricept)
Extracellular WNT ligand proteins bind the Fzd receptor family and activate the canonical and non-canonical WNT pathways (39). The sFRP family has been proposed to have inhibitory activity through binding and sequestering WNT ligands (43,44). The expression of sFRPs is downregulated through promoter hypermethylation in cancer (45). Silencing of sFRP expression results in an increase in chemoresistance in ovarian cancer (36). Pharmacological inhibition of Fzd by the soluble extracellular peptide of Fzd (sFzd7) (39), small interfering peptides (RHPDs) (39), a small molecule inhibitor (FJ9) (39), an ionophore antibiotic (salinomycin) (36), anti-Fzd antibody (vantictumab) (41) or ipafricept (FZD8-Fc; also known as OMP-54F28) (41) blocks the signaling ability of the WNT pathway. Generally the WNT antagonists inhibit WNT-induced EMT (46) and potently sensitize cancer cells to taxane and platinum (47). Therefore, targeted inhibition of Fzd represents a rational and promising novel approach for cancer therapy (39). An ongoing clinical trial is evaluating vantictumab and ipafricept (27).
LRP5/6 inhibitors (DKK1 and prodigiosin)
DKK1 and DKK3 are secreted inhibitors of the WNT/β-catenin pathway (48). DKK antagonizes WNT signaling by binding to the WNT co-receptor, LRP5/6 (48). Inactivation of DKK3 has been observed in mucinous ovarian cancer, which may exert a proliferative effect (49). Furthermore, DKK is able to inhibit EMT and ovarian cancer cell metastasis (50).
As a more generally-acting agent, prodigiosin, a natural red pigment produced by bacterial species, particularly strains of Serratia marcescens, has also been reported to promote anticancer activity through inhibition of the WNT signaling by targeting multiple sites of this pathway, including LRP6, Dvl and GSK3β (51).
Dvl inhibitors [3289-8625, transmembrane protein 88 (TMEM88), NSC668036, HECT, UBA and WWE domain-containing 1 (HUWE1), WW domain-containing oxidoreductase (WWOX), dishevelled-binding antagonist of β-catenin (DACT1) and FJ9]
Negative regulators of the downstream targets of WNT signaling are Dvl, APC, CK1, AXIN and GSK3β. Dvl is overexpressed in drug-resistant cancer and its inhibition by its inhibitor (the synthetic compound 3289–8625) or Dvl short hairpin RNA inhibits WNT signaling and increases sensitivity to platinum (38,52). FJ9, a soluble Fzd7 peptide inhibitor, disrupts the interaction between Fzd7 and Dvl (39). TMEM88 inhibits WNT signaling through direct interaction with Dvl (53). The organic molecule NSC668036 that binds to and blocks the PDZ domain of Dvl also inhibits WNT signaling (54). As alternative methods of inhibition, HUWE1 ubiquitylates Dvl to promote its degradation (55); while WWOX, a member of the short-chain dehydrogenases/reductases protein family, prevents nuclear import of Dvl proteins (56). Similar to HUWE1, DACT1 (also known as dapper antagonist of catenin-1) antagonizes WNT/β-catenin signaling by inducing Dvl degradation (54,57). A previous study identified mucinous ovarian cancer to have a higher level of methylation of the DACT1 promoter compared with high-grade serous cancer and normal controls (25). Furthermore, DACT1 overexpression inhibited platinum resistance through inactivation of canonical WNT signaling and suppression of P-glycoprotein expression in mucinous ovarian cancer (25).
CK inhibitors (SR-3029, CX-4945 and hematein)
Canonical WNT signaling induces the disassociation of a complex comprising of Dvl, AXIN and the protein kinases CK1 and CK2 and GSK3β. This WNT signaling may be interrupted by CK inhibitors, including SR-3029 (an ATP-competitive CK1 inhibitor) (58), CX-4945 (a selective ATP-competitive inhibitor of CK2) (59,60) and hematein (a CK2 inhibitor; 3,4,10,6a-tetrahydroxy-7, 6a dihydroindeno [2,1-c] chroman-9-one) (61).
AXIN inhibition with Tankyrase (TNKS) inhibitor (XAV939)
AXIN, the rate-limiting factor for the stability of the β-catenin destruction complex, interacts with APC, β-catenin, GSK3β and protein phosphate 2. Thus, AXIN is considered as a negative regulator of the WNT signaling pathway (62). TNKS, a poly-ADP-ribosyltransferase, promotes WNT signaling by transferring ADP-ribose moieties onto AXIN (63). The TNKS inhibitor XAV939 (a small molecule TNKS1 inhibitor) stabilizes AXIN and subsequently inhibit WNT signaling (62). In this interaction, XAV939 binds the TNKS catalytic poly-ADP-ribose polymerase domain (62).
GSK3β modulators [prodigiosin, TDZD8, TWS119, L803-mts, tideglusib, niclosamide, differentiation-inducing factor (DIF) and salusin-β]
GSK3β is a multifunctional serine/threonine protein kinase. Tideglusib, a potent, selective and irreversible GSK3β inhibitor, exhibits antitumor activity and improves survival in mice (64). Other specific GSK3β inhibitors include the small chemicals TDZD8 (4-benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione) and TWS119 (4,6-disubstituted pyrrolopyrimidine) and the peptide L803-mts [N-myristol-GKEAPPAPPQS(p)P] (65). In addition, niclosamide, a U.S. Food and Drug Administration approved salicyclamide derivative anthelmintic drug used for the treatment of tapeworm infections, binds to GSK3β and inhibits WNT pathway functions (66). DIF [1-(3-chloro-2,6-dihydroxy-4-methoxyphenyl)hexan-1-one], a putative morphogen produced by Dictyostelium discoideum, suppresses the WNT/β-catenin signaling pathway via the activation of GSK3β and subsequently reduces the expression levels of c-Myc and cyclin D1 (67). Meanwhile, salusin-β is an endogenous parasympathomimetic proatherosclerotic peptide, which has been reported to accelerate the proliferation and EMT of ovarian cancer via activation of the WNT/β-catenin pathway through suppression of GSK3β (68); therefore it may also be a therapeutic target in mucinous ovarian cancer.
β-catenin modulators [caudal type homeobox 2 (CDX2), huaier, mebendazole, NCB-0846, HI-B1, polyphyllin I (PPI) and pyrvinium]
The CDX2 gene is a member of the caudal-related homeobox transcription factor gene family. CDX2 may regulate cancer cell proliferation by suppressing transcriptional activity of the β-catenin-TCF complex and β-catenin nuclear localization (69). CDX2 also upregulates MDR1 by binding to its element in the promoter of the MDR1 gene, which may lead to drug resistance in mucinous ovarian cancer (70,71). Huaier (Trametes robiniophila Murr), a traditional Chinese medicine, and PPI, a component in the traditional Chinese medicinal herb Paris polyphylla Smith, have been reported to inhibit the nuclear translocation of β-catenin and to transcriptionally downregulate certain WNT/β-catenin target genes (LRP6, WNT5A and cyclin D1) (72).
Traf2 and Nck-interacting protein kinase (TNIK) mediates WNT signaling through the β-catenin and T-cell factor-4 (TCF-4) complex. A number of small-molecule compounds targeting TNIK, including mebendazole, an anthelmintic agent and selective inhibitor of TNIK (73), NCB-0846, a small-molecule TNIK inhibitor (73) and HI-B1, a small molecule that directly interacts with β-catenin (74), have been demonstrated to exert anti-tumor effects against various cancers. At present, mebendazole is under clinical evaluation (73). In addition, pyrvinium, a potent WNT inhibitor used as an anthelmintic drug, may also sensitize ovarian cancer cells to chemotherapy (75).
Others. Trefoil factor 1 (TFF1)
The secretory protein TFF1 has been identified to be highly expressed in mucinous ovarian cancer, but not serous or any other type of ovarian cancer (76). TFF1 promotes cell proliferation, invasion and chemoresistance through regulating the activation of WNT/β-catenin signaling and the upregulation of Twist expression (76). Notably, TFF1 is considered to serve an oncogenic role in mucinous ovarian cancer (76).
CXC chemokine receptor 4 (CXCR4)
CXCR4 may activate the canonical WNT pathway and upregulate the expression of mesenchymal markers including vimentin and snail family transcriptional repressor 2 transcripts, leading to ovarian cancer cell invasion, metastasis and chemoresistance (77). Thus, CXCR4 may be a novel therapeutic target for the treatment of chemoresistant ovarian cancer.
Fibronectin 1 (FN1)
FN1 is involved in cell attachment, spreading and migration processes including embryogenesis and cancer metastasis (78). FN1 activates the WNT/β-catenin signaling pathway through interaction with integrin-β1 (78). Through this mechanism, FN1 may serve a role in the development of resistance to taxane and platinum (78).
Serpin family A member 1 (SERPINA1)
SERPINA1 is a serine protease inhibitor with targets including elastase, plasmin, thrombin, trypsin, chymotrypsin and plasminogen activator (79). SERPINA1 is regulated by prospero homeobox 1, which may enhance WNT/β-catenin signaling (79). In general, SERPINA1 has been implicated to serve a role in the chemoresistance of ovarian cancers (79).
Klotho
Klotho, a co-receptor for FGF23, may serve as a tumor suppressor, and also inhibit the WNT/β-catenin signaling pathway and reduce expression of c-Myc and cyclin D1 (80). Since Klotho is typically silenced in ovarian cancers (80), this gene is a potential key target for therapy.
Monoclonal antibody against BRAFV600E (vemurafenib)
Frequently mutated genes observed in mucinous ovarian cancer are KRAS, BRAF, CDKN2A and TP53 (2). Targeting the RAS/RAF pathway to treat recurrent or advanced-stage mucinous ovarian cancer may be an effective therapeutic strategy (8,10). Vemurafenib, as a highly selective BRAFV600E inhibitor, may become a model drug for targeted therapy of mucinous ovarian cancer.
6. Combination of cytotoxic agents with therapeutic antibodies or sensitizing agents
Activation of the EGFR or HER/PI3K/Akt cascade is considered to represent a major mechanism of chemoresistance in ovarian cancer (81). Therapeutic antibodies, including cetuximab (EGFR inhibitor), lapatinib (EGFR kinase inhibitor), rituximab (chimeric monoclonal anti-CD20 antibody) and trastuzumab [HER-family receptor tyrosine kinase (HER2) inhibitor] elicit an effective therapeutic response (82). Therefore, addition of EGFR/HER antibodies may improve the therapeutic effect of the conventional cytotoxic agents. Recent developments and the future potential in antibody-based targeting of the EGFR pathway have been previously reviewed in detail (83).
Chemotherapy with taxanes promotes activation of WNT signaling (47). The WNT inhibitors/antagonists, including vantictumab (anti-FZD) and ipafricept (FZD8-Fc), potentiate taxane-mediated cancer cell death. Furthermore, platinum treatment may induce activation of Src kinase (23). Combination therapy of oxaliplatin with dasatinib, a Src inhibitor, has previously exhibited antitumor effects in a mucinous cancer model (23). Additionally, the ABC inhibitors verapamil and elacridar re-sensitized chemoresistant cells to taxanes (84). Therefore, chemotherapeutics may be combined with tyrosine kinase inhibitors or P-glycoprotein inhibitors to enhance cytotoxicity (85).
7. Conclusions
Advanced mucinous ovarian cancer is established to be resistant to taxane- and platinum-based chemotherapy and is associated with poor clinical outcome, and there remains to be a requirement for effective anti-cancer therapies. The present article reviewed the underlying molecular mechanisms of chemoresistance, focusing on growth factor and WNT signaling pathways. Chemoresistance is mediated by the coordinated action of the WNT signaling axis, together with crosstalk from other growth factor receptor pathways, notably EGFR, ERBB2 and FGFR. Research has focused on molecular mechanisms for antagonizing WNT signaling by directly or indirectly targeting Wnt receptors on the cell surface and their downstream components (36). The present review described the characterization of synthetic small molecule inhibitors, small interfering peptides, antibiotics, organic molecules, proteases, protease inhibitors, and monoclonal antibodies that disrupt WNT signaling. Several WNT antagonists, inhibitors or modulators, including Fzd, LRP5/6, Dvl, CK1, AXIN, GSK3β and β-catenin (Table I and Fig. 1), are being evaluated in preclinical/clinical studies. WNT antagonists or blockades may have synergistic effects with platinum and taxane (47). From the published data it may be hypothesized that key components identified in the WNT signaling pathways are potential candidates of chemoresistance. Thus, the WNT inhibitors may provide novel therapeutic benefit in combination with current chemotherapies for mucinous ovarian cancer.
In conclusion, the present review has summarized promising WNT inhibitors for the targeting of chemoresistant processes in mucinous ovarian cancer from bench to bedside.
Acknowledgements
Not applicable.
Funding
This study was supported by a grant from the Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan allocated to the Department of Obstetrics and Gynecology, Nara Medical University (Kashihara, Japan) (awarded to Dr Hiroshi Kobayashi; grant no. 26293361).
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Authors' contributions
EN and SM collected the data for the signaling pathways and underlying mechanism of chemoresistance in mucinous ovarian cancer. KI performed the systematic review and had supervised the study. YY, KO and NK, skillful and dedicated coworkers for many years, made substantial contribution to the conception involved in the study. HK, current leader of the department, contributed to the study design and analysis on many basic research studies. The final version of the manuscript has been read and approved by all authors
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kurman RJ, Shih IeM. The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theory. Am J Surg Pathol. 2010;34:433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryland GL, Hunter SM, Doyle MA, Caramia F, Li J, Rowley SM, Christie M, Allan PE, Stephens AN, Bowtell DD, et al. Australian Ovarian Cancer Study Group Mutational landscape of mucinous ovarian carcinoma and its neoplastic precursors. Genome Med. 2015;7:87. doi: 10.1186/s13073-015-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramalingam P. Morphologic, immunophenotypic, and molecular features of epithelial ovarian cancer. Oncology (Williston Park) 2016;30:166–176. [PubMed] [Google Scholar]
- 4.Harrison ML, Jameson C, Gore ME. Mucinous ovarian cancer. Int J Gynecol Cancer. 2008;18:209–214. doi: 10.1111/j.1525-1438.2007.01022.x. [DOI] [PubMed] [Google Scholar]
- 5.Brasseur K, Gévry N, Asselin E. Chemoresistance and targeted therapies in ovarian and endometrial cancers. Oncotarget. 2017;8:4008–4042. doi: 10.18632/oncotarget.14021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu T, Li C. Convergence between Wnt-β-catenin and EGFR signaling in cancer. Mol Cancer. 2010;9:236. doi: 10.1186/1476-4598-9-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teer JK, Yoder S, Gjyshi A, Nicosia SV, Zhang C, Monteiro ANA. Mutational heterogeneity in non-serous ovarian cancers. Sci Rep. 2017;7:9728. doi: 10.1038/s41598-017-10432-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunter SM, Gorringe KL, Christie M, Rowley SM, Bowtell DD, Campbell IG, Australian Ovarian Cancer Study Group Pre-invasive ovarian mucinous tumors are characterized by CDKN2A and RAS pathway aberrations. Clin Cancer Res. 2012;18:5267–5277. doi: 10.1158/1078-0432.CCR-12-1103. [DOI] [PubMed] [Google Scholar]
- 9.Tafe LJ, Muller KE, Ananda G, Mitchell T, Spotlow V, Patterson SE, Tsongalis GJ, Mockus SM. Molecular genetic analysis of ovarian brenner tumors and associated mucinous epithelial neoplasms: High variant concordance and identification of mutually exclusive RAS driver mutations and MYC amplification. Am J Pathol. 2016;186:671–677. doi: 10.1016/j.ajpath.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mackenzie R, Kommoss S, Winterhoff BJ, Kipp BR, Garcia JJ, Voss J, Halling K, Karnezis A, Senz J, Yang W, et al. Targeted deep sequencing of mucinous ovarian tumors reveals multiple overlapping RAS-pathway activating mutations in borderline and cancerous neoplasms. BMC Cancer. 2015;15:415. doi: 10.1186/s12885-015-1421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ascierto PA, Kirkwood JM, Grob JJ, Simeone E, Grimaldi AM, Maio M, Palmieri G, Testori A, Marincola FM, Mozzillo N. The role of BRAF V600 mutation in melanoma. J Transl Med. 2012;10:85. doi: 10.1186/1479-5876-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santarpia L, Lippman SM, El-Naggar AK. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16:103–119. doi: 10.1517/14728222.2011.645805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen Y, Xing M, Mambo E, Guo Z, Wu G, Trink B, Beller U, Westra WH, Ladenson PW, Sidransky D. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst. 2003;95:625–627. doi: 10.1093/jnci/95.8.625. [DOI] [PubMed] [Google Scholar]
- 14.Obaid NM, Bedard K, Huang WY. Strategies for overcoming resistance in tumours harboring BRAF mutations. Int J Mol Sci. 2017;18:E585. doi: 10.3390/ijms18030585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang KL, Lee MY, Chao WR, Han CP. The status of Her2 amplification and Kras mutations in mucinous ovarian carcinoma. Hum Genomics. 2016;10:40. doi: 10.1186/s40246-016-0096-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mesbah Ardakani N, Giardina T, Amanuel B, Stewart CJ. Molecular profiling reveals a clonal relationship between ovarian mucinous tumors and corresponding mural carcinomatous nodules. Am J Surg Pathol. 2017;41:1261–1266. doi: 10.1097/PAS.0000000000000875. [DOI] [PubMed] [Google Scholar]
- 17.Zou Y, Wang F, Liu FY, Huang MZ, Li W, Yuan XQ, Huang OP, He M. RNF43 mutations are recurrent in Chinese patients with mucinous ovarian carcinoma but absent in other subtypes of ovarian cancer. Gene. 2013;531:112–116. doi: 10.1016/j.gene.2013.08.054. [DOI] [PubMed] [Google Scholar]
- 18.Vereczkey I, Serester O, Dobos J, Gallai M, Szakács O, Szentirmay Z, Tóth E. Molecular characterization of 103 ovarian serous and mucinous tumors. Pathol Oncol Res. 2011;17:551–559. doi: 10.1007/s12253-010-9345-8. [DOI] [PubMed] [Google Scholar]
- 19.Pines G, Köstler WJ, Yarden Y. Oncogenic mutant forms of EGFR: Lessons in signal transduction and targets for cancer therapy. FEBS Lett. 2010;584:2699–2706. doi: 10.1016/j.febslet.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barber TD, Vogelstein B, Kinzler KW, Velculescu VE. Somatic mutations of EGFR in colorectal cancers and glioblastomas. N Engl J Med. 2004;351:2883. doi: 10.1056/NEJM200412303512724. [DOI] [PubMed] [Google Scholar]
- 21.Wee P, Wang Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers (Basel) 2017;9:E52. doi: 10.3390/cancers9050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Momeny M, Zarrinrad G, Moghaddaskho F, Poursheikhani A, Sankanian G, Zaghal A, Mirshahvaladi S, Esmaeili F, Eyvani H, Barghi F, et al. Dacomitinib, a pan-inhibitor of ErbB receptors, suppresses growth and invasive capacity of chemoresistant ovarian carcinoma cells. Sci Rep. 2017;7:4204. doi: 10.1038/s41598-017-04147-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuo K, Nishimura M, Bottsford-Miller JN, Huang J, Komurov K, Armaiz-Pena GN, Shahzad MM, Stone RL, Roh JW, Sanguino AM, et al. Targeting SRC in mucinous ovarian carcinoma. Clin Cancer Res. 2011;17:5367–5378. doi: 10.1158/1078-0432.CCR-10-3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosner A, Miyoshi K, Landesman-Bollag E, Xu X, Seldin DC, Moser AR, MacLeod CL, Shyamala G, Gillgrass AE, Cardiff RD. Pathway pathology: Histological differences between ErbB/Ras and Wnt pathway transgenic mammary tumors. Am J Pathol. 2002;161:1087–1097. doi: 10.1016/S0002-9440(10)64269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li RN, Liu B, Li XM, Hou LS, Mu XL, Wang H, Linghu H. DACT1 overexpression in type I ovarian cancer inhibits malignant expansion and cis-platinum resistance by modulating canonical Wnt signalling and autophagy. Sci Rep. 2017;7:9285. doi: 10.1038/s41598-017-08249-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rask K, Nilsson A, Brännström M, Carlsson P, Hellberg P, Janson PO, Hedin L, Sundfeldt K. Wnt-signalling pathway in ovarian epithelial tumours: Increased expression of beta-catenin and GSK3beta. Br J Cancer. 2003;89:1298–1304. doi: 10.1038/sj.bjc.6601265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katoh M. Canonical and non-canonical WNT signaling in cancer stem cells and their niches: Cellular heterogeneity, omics reprogramming, targeted therapy and tumor plasticity (Review) Int J Oncol. 2017;51:1357–1369. doi: 10.3892/ijo.2017.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barbolina MV, Burkhalter RJ, Stack MS. Diverse mechanisms for activation of Wnt signalling in the ovarian tumour microenvironment. Biochem J. 2011;437:1–12. doi: 10.1042/BJ20110112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang L, Jin Y, Feng S, Zou Y, Xu S, Qiu S, Li L, Zheng J. Role of Wnt/β-catenin, Wnt/c-Jun N-terminal kinase and Wnt/Ca2+ pathways in cisplatin-induced chemoresistance in ovarian cancer. Exp Ther Med. 2016;12:3851–3858. doi: 10.3892/etm.2016.3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohtsu H, Mifune M, Frank GD, Saito S, Inagami T, Kim-Mitsuyama S, Takuwa Y, Sasaki T, Rothstein JD, Suzuki H, et al. Signal-crosstalk between Rho/ROCK and c-Jun NH2-terminal kinase mediates migration of vascular smooth muscle cells stimulated by angiotensin II. Arterioscler Thromb Vasc Biol. 2005;25:1831–1836. doi: 10.1161/01.ATV.0000175749.41799.9b. [DOI] [PubMed] [Google Scholar]
- 32.Zheng HC. The molecular mechanisms of chemoresistance in cancers. Oncotarget. 2017;8:59950–59964. doi: 10.18632/oncotarget.19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karabuk E, Kose MF, Hizli D, Taşkin S, Karadağ B, Turan T, Boran N, Ozfuttu A, Ortaç UF. Comparison of advanced stage mucinous epithelial ovarian cancer and serous epithelial ovarian cancer with regard to chemosensitivity and survival outcome: A matched case-control study. J Gynecol Oncol. 2013;24:160–166. doi: 10.3802/jgo.2013.24.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wamunyokoli FW, Bonome T, Lee JY, Feltmate CM, Welch WR, Radonovich M, Pise-Masison C, Brady J, Hao K, Berkowitz RS, et al. Expression profiling of mucinous tumors of the ovary identifies genes of clinicopathologic importance. Clin Cancer Res. 2006;12:690–700. doi: 10.1158/1078-0432.CCR-05-1110. [DOI] [PubMed] [Google Scholar]
- 35.Stewart DJ. Wnt signaling pathway in non-small cell lung cancer. J Natl Cancer Inst. 2014;106:djt356. doi: 10.1093/jnci/djt356. [DOI] [PubMed] [Google Scholar]
- 36.Garrett AP, Lee KR, Colitti CR, Muto MG, Berkowitz RS, Mok SC. k-ras mutation may be an early event in mucinous ovarian tumorigenesis. Int J Gynecol Pathol. 2001;20:244–251. doi: 10.1097/00004347-200107000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Bahrami A, Amerizadeh F, ShahidSales S, Khazaei M, Ghayour-Mobarhan M, Sadeghnia HR, Maftouh M, Hassanian SM, Avan A. Therapeutic potential of targeting Wnt/β-catenin pathway in treatment of colorectal cancer: Rational and progress. J Cell Biochem. 2017;118:1979–1983. doi: 10.1002/jcb.25903. [DOI] [PubMed] [Google Scholar]
- 38.Luo K, Gu X, Liu J, Zeng G, Peng L, Huang H, Jiang M, Yang P, Li M, Yang Y, et al. Inhibition of disheveled-2 resensitizes cisplatin-resistant lung cancer cells through down-regulating Wnt/β-catenin signaling. Exp Cell Res. 2016;347:105–113. doi: 10.1016/j.yexcr.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 39.King TD, Zhang W, Suto MJ, Li Y. Frizzled7 as an emerging target for cancer therapy. Cell Signal. 2012;24:846–851. doi: 10.1016/j.cellsig.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 41.Le PN, McDermott JD, Jimeno A. Targeting the Wnt pathway in human cancers: Therapeutic targeting with a focus on OMP-54F28. Pharmacol Ther. 2015;146:1–11. doi: 10.1016/j.pharmthera.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pez F, Lopez A, Kim M, Wands JR, Caron de Fromentel C, Merle P. Wnt signaling and hepatocarcinogenesis: Molecular targets for the development of innovative anticancer drugs. J Hepatol. 2013;59:1107–1117. doi: 10.1016/j.jhep.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Saran U, Arfuso F, Zeps N, Dharmarajan A. Secreted frizzled-related protein 4 expression is positively associated with responsiveness to cisplatin of ovarian cancer cell lines in vitro and with lower tumour grade in mucinous ovarian cancers. BMC Cell Biol. 2012;13:25. doi: 10.1186/1471-2121-13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jacob F, Ukegjini K, Nixdorf S, Ford CE, Olivier J, Caduff R, Scurry JP, Guertler R, Hornung D, Mueller R, et al. Loss of secreted frizzled-related protein 4 correlates with an aggressive phenotype and predicts poor outcome in ovarian cancer patients. PLoS One. 2012;7:e31885. doi: 10.1371/journal.pone.0031885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su HY, Lai HC, Lin YW, Liu CY, Chen CK, Chou YC, Lin SP, Lin WC, Lee HY, Yu MH. Epigenetic silencing of SFRP5 is related to malignant phenotype and chemoresistance of ovarian cancer through Wnt signaling pathway. Int J Cancer. 2010;127:555–567. doi: 10.1002/ijc.25083. [DOI] [PubMed] [Google Scholar]
- 46.Bernaudo S, Salem M, Qi X, Zhou W, Zhang C, Yang W, Rosman D, Deng Z, Ye G, Yang B, et al. Cyclin G2 inhibits epithelial-to-mesenchymal transition by disrupting Wnt/β-catenin signaling. Oncogene. 2016;35:4816–4827. doi: 10.1038/onc.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fischer MM, Cancilla B, Yeung VP, Cattaruzza F, Chartier C, Murriel CL, Cain J, Tam R, Cheng CY, Evans JW, et al. WNT antagonists exhibit unique combinatorial antitumor activity with taxanes by potentiating mitotic cell death. Sci Adv. 2017;3:e1700090. doi: 10.1126/sciadv.1700090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Menezes ME, Devine DJ, Shevde LA, Samant RS. Dickkopf1: A tumor suppressor or metastasis promoter? Int J Cancer. 2012;130:1477–1483. doi: 10.1002/ijc.26449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takata A, Terauchi M, Hiramitsu S, Uno M, Wakana K, Kubota T. Dkk-3 induces apoptosis through mitochondrial and Fas death receptor pathways in human mucinous ovarian cancer cells. Int J Gynecol Cancer. 2015;25:372–379. doi: 10.1097/IGC.0000000000000340. [DOI] [PubMed] [Google Scholar]
- 50.Duan H, Yan Z, Chen W, Wu Y, Han J, Guo H, Qiao J. TET1 inhibits EMT of ovarian cancer cells through activating Wnt/β-catenin signaling inhibitors DKK1 and SFRP2. Gynecol Oncol. 2017;147:408–417. doi: 10.1016/j.ygyno.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 51.Wang Z, Li B, Zhou L, Yu S, Su Z, Song J, Sun Q, Sha O, Wang X, Jiang W, et al. Prodigiosin inhibits Wnt/β-catenin signaling and exerts anticancer activity in breast cancer cells; Proc Natl Acad Sci USA; 2016; pp. 13150–13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grandy D, Shan J, Zhang X, Rao S, Akunuru S, Li H, Zhang Y, Alpatov I, Zhang XA, Lang RA, et al. Discovery and characterization of a small molecule inhibitor of the PDZ domain of dishevelled. J Biol Chem. 2009;284:16256–16263. doi: 10.1074/jbc.M109.009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ge YX, Wang CH, Hu FY, Pan LX, Min J, Niu KY, Zhang L, Li J, Xu T. New advances of TMEM88 in cancer initiation and progression, with special emphasis on Wnt signaling pathway. J Cell Physiol. 2018;233:79–87. doi: 10.1002/jcp.25853. [DOI] [PubMed] [Google Scholar]
- 54.Chen X, Deng Y. Simulations of a specific inhibitor of the dishevelled PDZ domain. J Mol Model. 2009;15:91–96. doi: 10.1007/s00894-008-0377-x. [DOI] [PubMed] [Google Scholar]
- 55.de Groot RE, Ganji RS, Bernatik O, Lloyd-Lewis B, Seipel K, Šedová K, Zdráhal Z, Dhople VM, Dale TC, Korswagen HC, Bryja V. Huwe1-mediated ubiquitylation of dishevelled defines a negative feedback loop in the Wnt signaling pathway. Sci Signal. 2014;7:ra26. doi: 10.1126/scisignal.2004985. [DOI] [PubMed] [Google Scholar]
- 56.Bouteille N, Driouch K, Hage PE, Sin S, Formstecher E, Camonis J, Lidereau R, Lallemand F. Inhibition of the Wnt/beta-catenin pathway by the WWOX tumor suppressor protein. Oncogene. 2009;28:2569–2580. doi: 10.1038/onc.2009.120. [DOI] [PubMed] [Google Scholar]
- 57.Yin X, Xiang T, Li L, Su X, Shu X, Luo X, Huang J, Yuan Y, Peng W, Oberst M, et al. DACT1, an antagonist to Wnt/β-catenin signaling, suppresses tumor cell growth and is frequently silenced in breast cancer. Breast Cancer Res. 2013;15:R23. doi: 10.1186/bcr3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosenberg LH, Lafitte M, Quereda V, Grant W, Chen W, Bibian M, Noguchi Y, Fallahi M, Yang C, Chang JC, et al. Therapeutic targeting of casein kinase 1δ in breast cancer. Sci Transl Med. 2015;7:318ra202. doi: 10.1126/scitranslmed.aac8773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zheng Y, McFarland BC, Drygin D, Yu H, Bellis SL, Kim H, Bredel M, Benveniste EN. Targeting protein kinase CK2 suppresses prosurvival signaling pathways and growth of glioblastoma. Clin Cancer Res. 2013;19:6484–6494. doi: 10.1158/1078-0432.CCR-13-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim J, Kim SH. Druggability of the CK2 inhibitor CX-4945 as an anticancer drug and beyond. Arch Pharm Res. 2012;35:1293–1296. doi: 10.1007/s12272-012-0800-9. [DOI] [PubMed] [Google Scholar]
- 61.Hung MS, Xu Z, Lin YC, Mao JH, Yang CT, Chang PJ, Jablons DM, You L. Identification of hematein as a novel inhibitor of protein kinase CK2 from a natural product library. BMC Cancer. 2009;9:135. doi: 10.1186/1471-2407-9-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu X, Luo F, Li J, Zhong X, Liu K. Tankyrase 1 inhibitior XAV939 increases chemosensitivity in colon cancer cell lines via inhibition of the Wnt signaling pathway. Int J Oncol. 2016;48:1333–1340. doi: 10.3892/ijo.2016.3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thorvaldsen TE, Pedersen NM, Wenzel EM, Stenmark H. Differential roles of AXIN1 and AXIN2 in tankyrase inhibitor-induced formation of degradasomes and β-catenin degradation. PLoS One. 2017;12:e0170508. doi: 10.1371/journal.pone.0170508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou A, Lin K, Zhang S, Chen Y, Zhang N, Xue J, Wang Z, Aldape KD, Xie K, Woodgett JR, Huang S. Nuclear GSK3β promotes tumorigenesis by phosphorylating KDM1A and inducing its deubiquitylation by USP22. Nat Cell Biol. 2016;18:954–966. doi: 10.1038/ncb3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun A, Li C, Chen R, Huang Y, Chen Q, Cui X, Liu H, Thrasher JB, Li B. GSK-3β controls autophagy by modulating LKB1-AMPK pathway in prostate cancer cells. Prostate. 2016;76:172–183. doi: 10.1002/pros.23106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahn SY, Yang JH, Kim NH, Lee K, Cha YH, Yun JS, Kang HE, Lee Y, Choi J, Kim HS, Yook JI. Anti-helminthic niclosamide inhibits Ras-driven oncogenic transformation via activation of GSK-3. Oncotarget. 2017;8:31856–31863. doi: 10.18632/oncotarget.16255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jingushi K, Nakamura T, Takahashi-Yanaga F, Matsuzaki E, Watanabe Y, Yoshihara T, Morimoto S, Sasaguri T. Differentiation-inducing factor-1 suppresses the expression of c-Myc in the human cancer cell lines. J Pharmacol Sci. 2013;121:103–109. doi: 10.1254/jphs.12204FP. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Q, Chen WM, Zhang XX, Zhang HX, Wang HC, Zheng FY, Zhu FF. Overexpression of salusin-β is associated with poor prognosis in ovarian cancer. Oncol Rep. 2017;37:1826–1832. doi: 10.3892/or.2017.5429. [DOI] [PubMed] [Google Scholar]
- 69.Guo RJ, Funakoshi S, Lee HH, Kong J, Lynch JP. The intestine-specific transcription factor Cdx2 inhibits beta-catenin/TCF transcriptional activity by disrupting the beta-catenin-TCF protein complex. Carcinogenesis. 2010;31:159–166. doi: 10.1093/carcin/bgp213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koh I, Hinoi T, Sentani K, Hirata E, Nosaka S, Niitsu H, Miguchi M, Adachi T, Yasui W, Ohdan H, Kudo Y. Regulation of multidrug resistance 1 expression by CDX2 in ovarian mucinous adenocarcinoma. Cancer Med. 2016;5:1546–1555. doi: 10.1002/cam4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takakura Y, Hinoi T, Oue N, Sasada T, Kawaguchi Y, Okajima M, Akyol A, Fearon ER, Yasui W, Ohdan H. CDX2 regulates multidrug resistance 1 gene expression in malignant intestinal epithelium. Cancer Res. 2010;70:6767–6778. doi: 10.1158/1538-7445.AM10-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yan X, Lyu T, Jia N, Yu Y, Hua K, Feng W. Huaier aqueous extract inhibits ovarian cancer cell motility via the AKT/GSK3β/β-catenin pathway. PLoS One. 2013;8:e63731. doi: 10.1371/journal.pone.0063731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yamada T, Masuda M. Emergence of TNIK inhibitors in cancer therapeutics. Cancer Sci. 2017;108:818–823. doi: 10.1111/cas.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shin SH, Lim DY, Reddy K, Malakhova M, Liu F, Wang T, Song M, Chen H, Bae KB, Ryu J, et al. A small molecule inhibitor of the β-catenin-TCF4 interaction suppresses colorectal cancer growth in vitro and in vivo. EBioMedicine. 2017;25:22–31. doi: 10.1016/j.ebiom.2017.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang C, Zhang Z, Zhang S, Wang W, Hu P. Targeting of Wnt/β-catenin by anthelmintic drug pyrvinium enhances sensitivity of ovarian cancer cells to chemotherapy. Med Sci Monit. 2017;23:266–275. doi: 10.12659/MSM.901667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao S, Ma Y, Huang X. Trefoil factor 1 elevates the malignant phenotype of mucinous ovarian cancer cell through Wnt/β-catenin signaling. Int J Clin Exp Pathol. 2015;8:10412–10419. [PMC free article] [PubMed] [Google Scholar]
- 77.Wang J, Cai J, Han F, Yang C, Tong Q, Cao T, Wu L, Wang Z. Silencing of CXCR4 blocks progression of ovarian cancer and depresses canonical Wnt signaling pathway. Int J Gynecol Cancer. 2011;21:981–987. doi: 10.1097/IGC.0b013e31821d2543. [DOI] [PubMed] [Google Scholar]
- 78.Gao W, Liu Y, Qin R, Liu D, Feng Q. Silence of fibronectin 1 increases cisplatin sensitivity of non-small cell lung cancer cell line. Biochem Biophys Res Commun. 2016;476:35–41. doi: 10.1016/j.bbrc.2016.05.081. [DOI] [PubMed] [Google Scholar]
- 79.Choi D, Ramu S, Park E, Jung E, Yang S, Jung W, Choi I, Lee S, Kim KE, Seong YJ, et al. Aberrant activation of notch signaling inhibits PROX1 activity to enhance the malignant behavior of thyroid cancer cells. Cancer Res. 2016;76:582–593. doi: 10.1158/0008-5472.CAN-15-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tang X, Wang Y, Fan Z, Ji G, Wang M, Lin J, Huang S, Meltzer SJ. Klotho: A tumor suppressor and modulator of the Wnt/β-catenin pathway in human hepatocellular carcinoma. Lab Invest. 2016;96:197–205. doi: 10.1038/labinvest.2015.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cloven NG, Kyshtoobayeva A, Burger RA, Yu IR, Fruehauf JP. In vitro chemoresistance and biomarker profiles are unique for histologic subtypes of epithelial ovarian cancer. Gynecol Oncol. 2004;92:160–166. doi: 10.1016/j.ygyno.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 82.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147–1157. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 83.Gui T, Shen K. The epidermal growth factor receptor as a therapeutic target in epithelial ovarian cancer. Cancer Epidemiol. 2012;36:490–496. doi: 10.1016/j.canep.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 84.Vaidyanathan A, Sawers L, Gannon AL, Chakravarty P, Scott AL, Bray SE, Ferguson MJ, Smith G. ABCB1 (MDR1) induction defines a common resistance mechanism in paclitaxel- and olaparib-resistant ovarian cancer cells. Br J Cancer. 2016;115:431–441. doi: 10.1038/bjc.2016.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang M, Liu E, Cui Y, Huang Y. Nanotechnology-based combination therapy for overcoming multidrug-resistant cancer. Cancer Biol Med. 2017;14:212–227. doi: 10.20892/j.issn.2095-3941.2017.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.