Abstract
Recent clinical trials with the aim of developing tumor antigen (TA)-specific cancer vaccines against a number of malignancies have focused on the identification of TAs presented by tumor cells and recognized by T cells. In the present study, the TA melanoma antigen family A4 (MAGE-A4) protein was produced using a transgenic (TG) silkworm system. Using in vitro stimulation, it was subsequently determined whether MAGE-A4 protein induced MAGE-A4-specific T cells from peripheral blood mononuclear cells of healthy donors. TG silkworm lines expressing a MAGE-A4 gene under an upstream activating sequence (UAS) were mated with those expressing a yeast transcription activator protein (GAL4) at the middle silk glands (MSGs) and embryos that harbored both the GAL4 and UAS constructs were selected. Recombinant MAGE-A4 protein was extracted from the MSGs of TG silkworms and evaluated using SDS-PAGE and western blot analysis. It was observed that MAGE-A4 produced by the TG silkworm system successfully induced MAGE-A4-specific CD4+ T cell responses. Furthermore, MAGE-A4-specific CD4+ T cells recognized antigen-presenting cells when pulsed with a MAGE-A4+ tumor cell lysate. The present data suggests that recombinant tumor antigen production using the TG silkworm system may be a novel tool in the preparation of cancer vaccines.
Keywords: transgenic silkworms, cancer vaccine, melanoma antigen family A4
Introduction
Recent clinical trials with the aim of developing tumor antigen (TA)-specific cancer vaccines against a number of malignancies have focused on the identification of TAs presented by tumor cells and recognized by T cells (1,2). Although cancer vaccines that aim to enhance antitumor immune responses in cancer patients are an attractive approach, current vaccines remain ineffective in achieving tumor regression (3). Cancer vaccines using TA-derived peptides are a current therapeutic strategy; however, their applications are limited due to difficulties with human leukocyte antigen (HLA) restriction, HLA-peptide binding affinity, immunogenicity and antigenicity. To develop effective and broadly applicable vaccines, a number of approaches are currently being used to design modified peptides with enhanced functional activities, relative to the parental peptide, including multiple epitope peptides or longer peptides covered with multiple epitopes (4). The function of these may be further enhanced by administration with professional antigen-presenting cells (5,6). Cancer vaccines may also be developed through the use of whole recombinant TA proteins as antigens. Using this method, various epitopes are simultaneously presented to T cells, as whole TA proteins contain multiple HLA class I and class II epitopes that are recognized by cluster of differentiation (CD)-4+ and CD8+ T cells, respectively. Furthermore, target patients are not restricted by the type of HLA allele. Many systems now exist for the production of recombinant proteins, and each of these systems have advantages and disadvantages with regard to time, cost and risk of endotoxin contamination (7). A recombinant protein production system using transgenic (TG) silkworms has recently been documented by our group, whereby a yeast transcription activator protein (GAL4) and upstream activating sequence for GAL4 (UAS) system was observed to be an effective technique for transgenic gene expression (8). Using this GAL4/UAS system, it was demonstrated that functional human µ-opioid receptor was expressed in the TG silkworm (9,10). As silk thread produced by silkworms may be used as a surgical suture material, the risk of contamination from endotoxin and allergic reactions is unlikely in patients. Therefore, recombinant TA protein synthesis by a TG silkworm system may be useful in the development of cancer vaccines.
The present study aimed to determine whether proteins obtained from TG silkworms may be used in the development of cancer vaccines. Among the TAs available, melanoma antigen family A4 (MAGE-A4) was selected in the current preliminary study. MAGE-A4 is typically expressed in a number of malignancies, including melanoma, head and neck cancer and lung cancer, while only being expressed in the testis and placenta of normal adult tissues (11–13). In addition, cancer vaccine clinical trials using MAGE-A4 peptides and proteins have been documented (14,15). In the present study, the TA MAGE-A4 protein was produced using a TG silkworm system. Using in vitro stimulation (IVS), it was subsequently determined whether MAGE-A4 protein induced MAGE-A4-specific T cells from peripheral blood mononuclear cells (PBMCs) of healthy donors. Results suggested that TA proteins produced by a TG silkworm system may be useful in the development of cancer vaccines.
Materials and methods
Construction of expression vectors
Plasmids expressing TG silkworm constructs containing the MAGE-A4 gene were prepared as follows: The MAGE-A4 gene (GenBank accession no. AB464618) was amplified from a Flexi ORF Clone pF1KB9825 plasmid (Kazusa DNA Research Institute, Chiba, Japan) using the primers BsmBI_MAGE_U (forward, 5′-GCGTCTCCAGCTATGTCTTCTGAGCAGAAG-3′) and BsmBI_MAGE_His_L (reverse, 5′-GCGTCTCCCTAGTGATGATGATGGTGATGGACTCCCTCTTCCTCC-3′) in order to insert a histidine tag sequence for protein purification and restriction enzyme BsmBI sites (underlined) for gene construction. The MAGE-A4 gene fragment amplified by polymerase chain reaction [1 unit of KOD plus polymerase (Toyobo Co., Ltd., Osaka, Japan)], 5 µM forward and reverse primers, 1 mM MgCl2, 1 mM dNTPs, 0.1 µg template plasmid, and 1X buffer supplied by the manufacturer; Toyobo Co., Ltd., and a temperature program of 94°C for 30 sec, 55°C for 30 sec and 72°C for 90 sec for 15 cycles) using a thermal cycler (C-1000; Bio-Rad Laboratories, Inc., Hercules, CA, USA). The resultant DNA fragment was digested with BsmBI (New England BioLabs, Inc., Ipswich, MA, USA) at 37°C for 2 h. The fragment was then ligated into the BsmBI site of the pBac[SerUAS_Ser1intron_hr5/3×P3-AmCyan_A3-Bla] plasmid (16) by using ligation high version 2 (Toyobo Co., Ltd.) at 16°C for 10 h. The resultant plasmid, pBac[UAS_MAGEA4/3×P3-AmCyan], carrying a MAGE-A4 gene under a UAS promoter and an AmCyan gene as a selection marker, was used to generate TG silkworms.
Generation of TG silkworms
TG silkworms were generated as described previously (16–18). Briefly, the silkworm strain w1-pnd, which is non-diapausing and produces non-pigmented eyes and eggs, was used to generate TG silkworms. The diapausing strain w-1 was also used to mate TG silkworm lines. All strains were maintained at the Transgenic Silkworm Research Unit at National Institute of Agrobiological Sciences (Ibaraki, Japan). Silkworm larvae were reared on an artificial diet (Nosan Corporation, Yokohama, Japan) at 25°C. pBac[UAS_MAGE-A4/3×P3-AmCyan] was injected into embryos at the pre-blastoderm stage with a helper plasmid, pHA3PIG (our collection), to induce expression of a piggyBac transposase gene (17), and resulting generation 0 (G0) adults were mated with other G0 adults. G1 silkworms were screened during the late embryonic stage for expression of the AmCyan gene driven by a 3×P3 neuro-specific promoter in the embryonic compound eyes. The TG silkworm lines obtained were then mated with adults from a Ser1-GAL4 strain carrying a GAL4 gene under the control of a middle silk gland (MSG)-specific sericin1 promoter and a 3×P3-DsRed2 marker gene (Addgene, Inc., Cambridge, MA, USA) (8). F1 embryos harboring GAL4 and UAS constructs were selected based on fluorescence of AmCyan and DsRed2 using fluorescence microscopy (Olympus Corporation, Tokyo, Japan).
Extraction and purification of recombinant MAGE-A4 from MSGs
A pair of MSG (~300 µg) was isolated from one larvae on the sixth day of the 5th instar, then immersed in 1 ml of 20 mM phosphate (pH 7.2) and gently shaken at 4°C for 2 h. The resulting extract was frozen at −80°C for 3 h, and then thawed at 4°C overnight before removal of debris from each extract by filtration. Protein supernatant extracted using a freeze-and-thaw method as described above (10 µg of protein; centrifuged at 2,280 × g for 10 min at 4°C) from MSGs were separated and analyzed by SDS-PAGE using 4–12% gradient gels (NuPAGE Bis-Tris Gels; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer's instructions. The gel was stained with 0.2% Coomassie Brilliant Blue R-250 (Nacalai Tesque, Kyoto, Japan). For western blotting, the gel was transferred onto nylon membranes (Hybond P PVDF; no. 10600023; GE Healthcare Life Sciences, Chalfont, UK), and incubated in blocking buffer (Blocking One; no. 03953-95; Nacalai Tesque, Kyoto, Japan) at room temperature for 1 h. Membrane was incubated with an anti-histidine tag primary antibody (A190-114A, 1:1,000; Bethyl Laboratories, Montgomery, TX, USA) at 4°C overnight. Subsequently, the membrane was washed three times with PBS with Tween-20 (PBST) [8 mM Na2HPO4, 2 mM KH2PO4 (pH 7.4), 150 mM NaCl, 3 mM KCl, 0.05% Tween-20] and incubated with horseradish peroxidase-conjugated anti-rabbit immunoglobulin (Ig)-G secondary antibody (NA934, 1:20,000; GE Healthcare Life Sciences) at room temperature for 1 h. Then, the membrane was washed two times with PBST. Immunoreactive protein bands were detected using ECL Prime reagent (GE Healthcare Life Sciences) and an LAS-3000 Image Analyzer (Fujifilm Image Reader LAS-3000, version 2; Fujifilm Corporation, Tokyo, Japan).
Protein lysates from MSGs were also loaded onto a nickel affinity column (5 ml; GE Healthcare Life Sciences) equilibrated with 20 mM phosphate (pH 7.4) and 500 mM NaCl for purification of recombinant MAGE-A4. After sample loading, the column was washed with 45 ml of 20 mM phosphate (pH 7.4) and 50 mM imidazole, and recombinant MAGE-A4 was eluted with 20 mM phosphate (pH 7.4) and 500 mM imidazole. Each fraction from the column was evaluated using 12.5% SDS-PAGE.
Cell lines
The present study used two human squamous cell carcinoma of the head and neck (SCCHN) cell lines, namely Kuma-1 (MAGE-A4+) and HSC-4 (MAGE-A4−), according to a previously described method (19). Kuma-1 was provided by Dr. Kyogo Itoh at the Kurume University School of Medicine (Kurume, Japan). HSC-4 was purchased from the Japanese Cancer Research Bank (Tokyo, Japan). The SCCHN cell lines were cultured at 37°C in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, L-glutamine (2 mM) and antibiotics (50 U/ml penicillin and 50 µg/ml streptomycin; all from Gibco; Thermo Fisher Scientific, Inc.). Tumor cells were harvested, re-suspended in AIM-V medium (Invitrogen; Thermo Fisher Scientific, Inc.) at 5×106/ml, and lysed by three freeze-thaw cycles. A freeze-thaw cycle consisted of 5 min in liquid nitrogen followed by 5 min at 37°C. Tumor cell lysates of 5×105 cell equivalents/ml were used as a source of antigen in interferon (IFN)-γ ELISA assay, as previously described (20).
Induction of anti-MAGE-A4-specific T cells by IVS
During January 2015 to July 2015, PBMCs were isolated from 5-healthy donors by density gradient centrifugation using a Ficoll-Paque PLUS media (GE Healthcare Life Sciences). Donor cells were obtained in accordance with the regulations by an approved protocol and consent forms of the Institutional Review Board of Gunma University (Maebashi, Japan). Dendritic cells (DC) were generated from PBMCs, as described previously (21). CD4+ and CD8+ T-cells were isolated from non-adherent PBMCs using immunomagnetic beads (CD8 MicroBeads; Miltenyi Biotech GmbH, Bergisch Gladbach, Germany). CD4+ or CD8+ T-cells (5×104) and DCs (1×104) treated by X-ray irradiation (30 Gy) were co-cultured at 37°C for 7 days in the presence of recombinant MAGE-A4 protein (10 µg/ml) in 96-well round-bottomed plates (BD Biosciences, Franklin Lakes, NJ, USA) in a final volume of 0.2 ml/well AIM-V medium supplemented with 5% (v/v) human AB serum (Access Cell Culture LLC, Vista, CA, USA) and 5 ng/ml interleukin (IL)-7 (R&D Systems, Inc., Minneapolis, MN, USA). This solution formed the culture medium (CM). On day 7, responder CD4+ or CD8+ T-cells were re-stimulated with irradiated autologous DCs (1×104) in the presence of MAGE-A4 protein (10 µg/ml) and grown in CM. On day 9, half of the medium volume was replenished with CM containing 10 IU/ml IL-2. Responding cells were screened on day 14 for the production of IFN-γ in the presence of MAGE-A4 protein using an IFN-γ ELISA kit (EHIFNG; Pierce; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. Positive cells were selected and transferred to 24-well plates and cultured at 37°C in CM supplemented with 10 IU/ml IL-2. Cells in culture were subjected to a weekly MAGE-A4 protein re-stimulation (10 µg/ml) with irradiated DCs (1×105) or PBMCs (1×106).
Evaluation of MAGE-A4-specific T-cell responses
Responder T cells (3×104/well) were co-cultured with irradiated DCs (1×104/well) at 37°C in the presence of MAGE-A4 protein (10 µg/ml) in 96-well flat-bottomed plates in a final volume of 200 µl AIM-V medium containing 5% (v/v) human AB serum. Culture supernatants were harvested after 24 h to measure antigen-induced IFN-γ production. The harvested supernatants of the responder T cell medium were frozen and stored at −80°C until IFN-γ concentration was measured. An IFN-γ ELISA kit (EHIFNG; Pierce; Thermo Fisher Scientific, Inc.) was used to quantify the concentration of IFN-γ within the supernatants, according to the manufacturer's instructions, using a detection limit of 2 pg/ml. An antibody blocking assay was also performed to determine the effect on IFN-γ production, whereby irradiated DCs were pre-incubated with anti-HLA-class I antibody (10 µg/ml, no. 560187) or anti-HLA-class II antibody (10 µg/ml; no. 555556) (both from BD Biosciences) at 37°C for 30 min prior to co-culture with responder T cells.
Statistical analysis
Data were expressed as mean ± standard error and analyzed using a Student's t-test. P<0.05 was considered to indicate a statistically significant difference. Statistical analysis was performed using SPSS 22.0 software (IBM SPSS, Armonk, NY, USA).
Results
Generation of TG silkworms expressing MAGE-A4
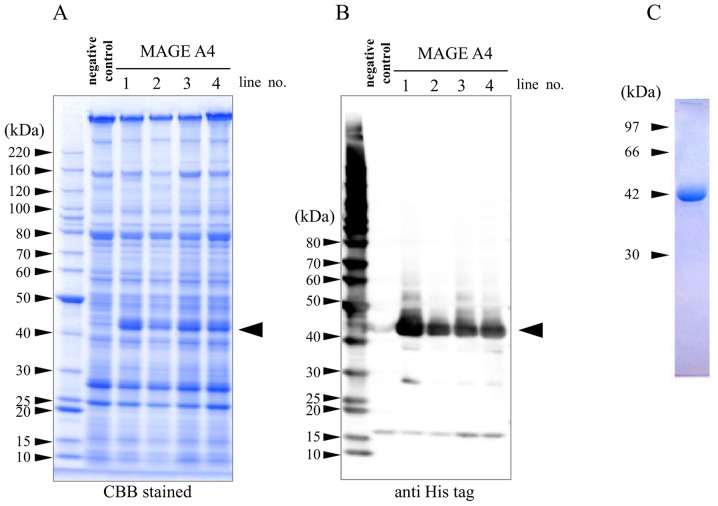
To generate TG silkworm strains expressing MAGE-A4 protein in MSGs, the plasmid pBac[UAS_MAGEA4/3×P3-AmCyan], encoding the MAGE-A4 gene under control of a UAS promoter, was injected into 370 eggs. A total of 171 eggs hatched, and these G0 adults were mated with other G0 adults to generate G1 offspring. A total of 8 broods expressing fluorescent AmCyan were selected after fluorescence screening of G1 offspring, and 4 TG silkworm lines were ultimately established (Table I). All 4 TG silkworm lines were mated with a Ser1-GAL4 strain expressing an MSG-specific GAL4 gene. To confirm the expression of MAGE-A4 protein in selected TG silkworms, extract from the MSGs of silkworms was evaluated by SDS-PAGE (Fig. 1A) and western blot analysis (Fig. 1B). On coomassie brilliant blue-stained gels and immunoblots, specific bands at ~42 kDa were observed in all four lanes of the MAGE-A4 TG lines, but not in the negative control lane. A number of low-molecular-weight bands were also detected on the immunoblots, though were likely derived from degradation products. Purified recombinant MAGE-A4 was confirmed as a single band at ~42 kDa by nickel affinity chromatography and SDS-PAGE analysis (Fig. 1C). The MAGE-A4 fractions were dialyzed with 20 mM phosphate (pH 7.4). A total of 170 µg of MAGE-A4 protein was obtained per TG silkworm after purification.
Table I.
Efficiency of transgenic silkworms production.
| Strain | Injected eggs | Hatched eggs | G1 broods | G1 broods with positive larvae | Established lines |
|---|---|---|---|---|---|
| MAGE-A4 | 370 | 171 | 56 | 8 | 4 |
MAGE-A4, melanoma-associated antigen 4; G1, generation 1.
Figure 1.
Expression and purification of MAGE-A4 in transgenic silkworms. Protein lysates extracted from the middle silk glands of TG silkworms were separated by SDS-PAGE followed by (A) staining with CBB and (B) western blot analysis with an anti-His tag antibody. Black arrow indicates MAGE-A4-specific bands. TG silkworms harboring only the Ser1-GAL4 construct were used as negative controls. Numbers above the gel and western blot images indicate the line number of the MAGE-A4 TG strain. (C) Purified recombinant MAGE-A4 was also analyzed by nickel affinity chromatography and SDS-PAGE and confirmed to be a single band at ~42 kDa. Numbers on the left of each image indicate molecular masses (kDa). MAGE-A4, melanoma-associated antigen 4; TG, transgenic; CBB, Coomassie Brilliant Blue.
Induction of MAGE-A4-specific CD4+ T cell responses
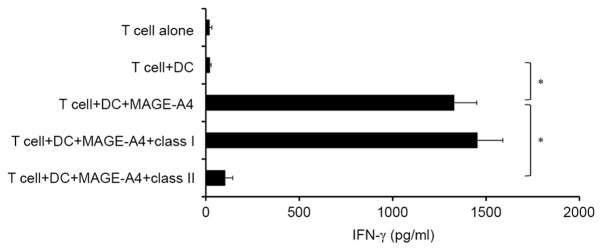
MAGE-A4-specific CD4+ T cells were generated from the PBMCs of healthy donors. After four rounds of IVS, outgrowing CD4+ T cells were assessed for the production of IFN-γ using ELISA. It was observed that CD4+ effector T cells produced IFN-γ in response to MAGE-A4 protein (Fig. 2). In turn, production of IFN-γ in response to MAGE-A4 protein was significantly blocked by anti-HLA class II antibody, but not anti-HLA class I antibody (P<0.05; Fig. 2). However, MAGE-A4-specific CD8+ T cells were not induced to produce IFN-γ using the same culture system (data not shown). Therefore, subsequent experiments were performed using MAGE-A4-specific CD4+ T cells.
Figure 2.
Induction of MAGE-A4-specific CD4+ T cell responses. After 4 rounds of in vitro stimulation with autologous DCs and MAGE-A4 protein, the specificity of outgrowing CD4+ T cells obtained from healthy donors was assessed. The CD4+ T-cell line produced IFN-γ in response to autologous DCs incubated with MAGE-A4 protein, and IFN-γ production by CD4+ T cells was significantly inhibited by anti-HLA class II, but not anti-HLA class I antibody. Data from one representative experiment in three performed are depicted. Each column represents the mean ± standard error of triplicate wells. *P<0.05. MAGE-A4, melanoma-associated antigen 4; CD4, cluster of differentiation 4; DC, dendritic cell; IFN-γ, interferon-γ; HLA, human leukocyte antigen.
To determine the ability of induced MAGE-A4-specific CD4+ T cells to recognize MAGE-A4+ tumor cells, tumor lysates were used as a source of antigen instead of recombinant MAGE-A4 protein. Notably, MAGE-A4-specific CD4+ T cells produced IFN-γ in response to autologous DCs incubated with MAGE-A4+ tumor lysates, but not MAGE-A4-tumor lysates (Fig. 3A). Furthermore, T cell reactivity was significantly inhibited by anti-HLA-class II antibody, but not anti-HLA-class I antibody (P<0.05; Fig. 3B). These results suggest that induced MAGE-A4-specific CD4+ T cells may recognize tumor-derived MAGE-A4 antigen when presented by HLA class II molecules on antigen-presenting DCs.
Figure 3.
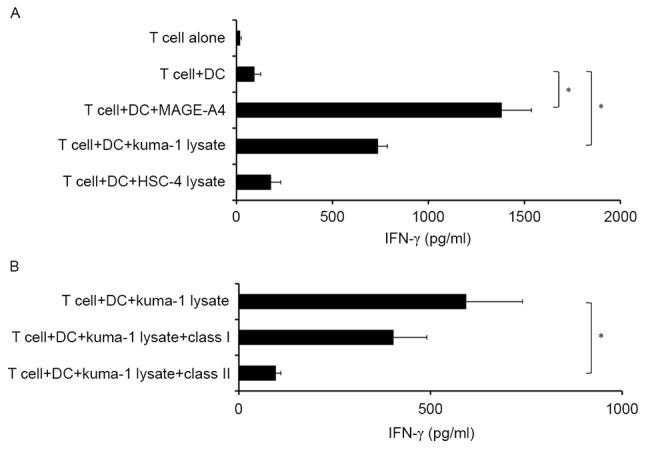
Recognition of MAGE-A4+ tumor cells by CD4+ T cells of healthy donors. (A) A MAGE-A4-specific CD4+ T-cell line recognized autologous DCs incubated with MAGE-A4+ Kuma-1 tumor cell lysate, but not DCs incubated with MAGE-A4− HSC-4 tumor cell lysate. (B) Responses were significantly inhibited by the anti-HLA class II antibody, but not the anti-HLA class I antibody. Data from one representative experiment of two performed are depicted. Each column represents the mean with ± standard error of triplicate wells. *P<0.05. MAGE-A4, melanoma-associated antigen 4; CD4, cluster of differentiation 4; DC, dendritic cell; IFN-γ, interferon-γ; HLA, human leukocyte antigen; Kuma-1 and HSC-4; squamous cell carcinoma of the head and neck cell lines.
Discussion
In the present study, TA with immunogenic properties was successfully produced using a TG silkworm system. Cancer immunotherapy aims to activate and upregulate host immune responses against tumor cells, and a large number of agents and strategies are currently under development or in clinical trials and practice (22). Regarding cancer vaccines, the majority of clinical trials have used peptide-based vaccines; however, they have shown only limited success despite the induction of TA-specific T cell responses in cancer patients (3). Although protein-based vaccines are a high-cost approach when compared to peptide-based vaccines, full-length proteins contain all the potential epitopes capable of stimulating CD4+ and CD8+ T cells, and may be used regardless of the patients' HLA alleles. Many host systems, including bacteria, yeast and insect cells, are now used in the production of recombinant proteins, and the preferred method of production varies according to the purpose of use (7). The TG silkworm system has been used for the expression of recombinant proteins since 2000, and the expression of a number of recombinant proteins in TG silkworms, including green fluorescent protein, human serum albumin, membrane receptors and monoclonal antibodies has been documented (23–25). The TG silkworm system has a number of advantages, including its lower cost, mass breeding capacity, availability of multiple tissues for expression, suitability for large scale production and reduced endotoxin contamination. Regarding the extraction of recombinant proteins from tissues, recombinant MAGE-A4 protein has been extracted from MSGs and silkworm cocoons in our preliminary experiments. As protein extraction from MSGs was more efficient and achieves a higher yield than that from silkworm cocoons, we used MSGs as the source of recombinant MAGE-A4 protein for in vitro study. The MAGE-A4 protein produced using the TG silkworm system successfully induced MAGE-A4-specific CD4+ T cell responses, suggesting that the MAGE-A4 protein is engulfed, processed and presented to CD4+ T cells by antigen-presenting cells in association with HLA class II molecules. Notably, the induced MAGE-A4-specific CD4+ T cells also recognized antigen-presenting cells incubated with a MAGE-A4+ tumor cell lysate. This result indicates that common immunological epitopes exist between the recombinant MAGE-A4 protein produced and MAGE-A4 expressed in tumor cells. However, MAGE-A4-specific CD8+ T cells were not produced in the current experimental system. DCs are capable of presenting exogenous antigens in the context of HLA class I through cross-priming; however, cross-presentation is influenced by many factors, including the DC maturation status, type of DC subset and type of antigen (26,27). Studies are currently ongoing to determine the activities of CD8+ T cells under various conditions including the types of cytokines used and DC activation status.
The MAGE-A4 protein produced in the current study is among a number of cancer-testis antigens that have been identified as cancer therapeutic targets based on their unique expression patterns (28,29). Cesson et al (30) observed that naturally acquired T-cell responses were activated against MAGE-A4 in patients with SCCHN, indicating that vaccines that boost pre-existing MAGE-A4-specific T-cell responses in cancer patients may be a useful therapeutic strategy. A number of clinical trials employing MAGE-A4 as a vaccine in cancer patients have been documented. For instance, Takahashi et al (14) treated patients with colon cancer with pulmonary metastasis with an artificially synthesized long peptide of MAGE-A4 that acted as a helper/killer-hybrid cell epitope. The artificially synthesized long peptide induced MAGE-A4-specific Th1 and Tc1 cells and complement-fixing IgG antibodies. In addition, tumor growth and levels of carcinoembryonic antigen tumor marker were significantly decreased in the final diagnosis (14). More recently, a cancer vaccine clinical trial with the MAGE-A4 protein, derived from M15 Escherichia coli, was conducted in patients with advanced esophageal, stomach or lung cancer (15). Although protein synthesis was not discussed, it was suggested that recombinant proteins manufactured in E. coli may contain residual endotoxins that are harmful to the host (15). Apart from the method of protein synthesis, results of this trial indicated that vaccination with MAGE-A4 protein vaccine was safe and induced CD4+ and/or CD8+ T cell responses in a number of patients. In addition, overall survival rate was longer in patients with tumor cells expressing high levels of MAGE-A4 or HLA class I and exhibiting MAGE-A4-specific immune responses after vaccination (15). Therefore, MAGE-A4 is a potential target for cancer vaccines, and recombinant MAGE-A4 protein may be a potent immunogen, capable of inducing broad T-cell responses in a larger population of cancer patients. A major factor in the design of cancer vaccines is the selection of tumor antigens. Although Cheever et al (31) has documented a prioritized list of cancer vaccine antigens based on predefined and pre-weighted objective criteria, a single antigen cancer vaccine is unlikely to be effective, due to the heterogeneity of antigen expression in tumors and the emergence of antigen loss variants. Therefore, studies aiming to produce other tumor antigens, including wild-type p53 and Wilms tumor 1 (31), using the TG silkworm system are currently ongoing.
Numerous clinical trials using immune checkpoint inhibitors have been conducted and therapeutic benefits were achieved in certain patient populations (32,33). However, as immune checkpoint inhibitors are not intended to target tumor cells themselves, these drugs augment non-specific immune responses against not only tumor cells, but also normal cells, and thus, cause various autoimmune reactions, including colitis, pneumonitis and endocrine disorders as side effects (32,33). In the future, strategies combining the induction and activation of TA-specific T cells with cancer protein-based vaccines and the activation and proliferation of TA-specific T cells with immune checkpoint inhibitors may lead to safer and more effective immunotherapy for the treatment of cancer.
In conclusion, MAGE-A4 produced by the TG silkworm system successfully induced MAGE-A4-specific CD4+ T cell responses. Furthermore, these CD4+ T cells could recognize antigen-presenting cells pulsed with a MAGE-A4+ tumor cell lysate. Thus, recombinant tumor antigen production using the TG silkworm system may be a novel tool in the preparation of cancer vaccines.
Acknowledgements
The present study was supported by the Gunma University Medical Innovation Project to Kazuaki Chikamatsu and Shigeki Takeda and Grants-in-Aid for Scientific Research to Kazuaki Chikamatsu (grant no. 26670736).
References
- 1.Coulie PG, Van den Eynde BJ, van der Bruggen P, Boon T. Tumor antigens recognized by T lymphocytes: At the core of cancer immunotherapy. Nat Rev Cancer. 2014;14:135–146. doi: 10.1038/nrc3670. [DOI] [PubMed] [Google Scholar]
- 2.Hoos A, Parmiani G, Hege K, Sznol M, Loibner H, Eggermont A, Urba W, Blumenstein B, Sacks N, Keilholz U, et al. Cancer vaccine clinical trial working group. A clinical development paradigm for cancer vaccines and related biologics. J Immunother. 2007;30:1–15. doi: 10.1097/01.cji.0000211341.88835.ae. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: Moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pol J, Bloy N, Buqué A, Eggermont A, Cremer I, Sautes-Fridman C, Galon J, Tartour E, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: Peptide-based anticancer vaccines. Oncoimmunology. 2015;4:e974411. doi: 10.4161/2162402X.2014.974411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parmiani G, Castelli C, Dalerba P, Mortarini R, Rivoltini L, Marincola FM, Anichini A. Cancer immunotherapy with peptide-based vaccines: What have we achieved? Where are we going? J Natl Cancer Inst. 2002;94:805–818. doi: 10.1093/jnci/94.11.805. [DOI] [PubMed] [Google Scholar]
- 6.Tagliamonte M, Petrizzo A, Tornesello ML, Buonaguro FM, Buonaguro L. Antigen-specific vaccines for cancer treatment. Hum Vaccin Immunother. 2014;10:3332–3346. doi: 10.4161/21645515.2014.973317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palomares LA, Estrada-Mondaca S, Ramírez OT. Production of recombinant proteins: Challenges and solutions: Methods. Mol Biol. 2004;267:1–52. doi: 10.1385/1-59259-774-2:015. [DOI] [PubMed] [Google Scholar]
- 8.Tatematsu K, Kobayashi I, Uchino K, Sezutsu H, Iizuka T, Yonemura N, Tamura T. Construction of a binary transgenic gene expression system for recombinant protein production in the middle silk gland of the silkworm Bombyx mori. Transgenic Res. 2010;19:473–487. doi: 10.1007/s11248-009-9328-2. [DOI] [PubMed] [Google Scholar]
- 9.Tateno M, Toyooka M, Shikano Y, Takeda S, Kuwabara N, Sezutsu H, Tamura T. Production and characterization of the recombinant human mu-opioid receptor from transgenic silkworms. J Biochme. 2009;145:37–42. doi: 10.1093/jb/mvn147. [DOI] [PubMed] [Google Scholar]
- 10.Nikaido Y, Kurosawa A, Saikawa H, Kuroiwa S, Suzuki C, Kuwabara N, Hoshino H, Obata H, Saito S, Saito T, et al. In vivo and in vitro evaluation of novel μ-opioid receptor agonist compounds. Eur J Pharmacol. 2015;767:193–200. doi: 10.1016/j.ejphar.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 11.Barrow C, Browning J, MacGregor D, Davis ID, Sturrock S, Jungbluth AA, Cebon J. Tumor antigen expression in melanoma varies according to antigen and stage. Clin Cancer Res. 2006;12:764–771. doi: 10.1158/1078-0432.CCR-05-1544. [DOI] [PubMed] [Google Scholar]
- 12.Montoro JR, Mamede RC, Neder Serafini L, Saggioro FP, Figueiredo DL, Silva WA, Jr, Jungbluth AA, Spagnoli GC, Zago MA. Expression of cancer-testis antigens MAGE-A4 and MAGE-C1 in oral squamous cell carcinoma. Head Neck. 2012;34:1123–1128. doi: 10.1002/hed.21880. [DOI] [PubMed] [Google Scholar]
- 13.Shigematsu Y, Hanagiri T, Shiota H, Kuroda K, Baba T, Mizukami M, So T, Ichiki Y, Yasuda M, So T, et al. Clinical significance of cancer/testis antigens expression in patients with non-small cell lung cancer. Lung Cancer. 2010;68:105–110. doi: 10.1016/j.lungcan.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi N, Ohkuri T, Homma S, Ohtake J, Wakita D, Togashi Y, Kitamura H, Todo S, Nishimura T. First clinical trial of cancer vaccine therapy with artificially synthesized helper/killer-hybrid epitope long peptide of MAGE-A4 cancer antigen. Cancer Sci. 2012;103:150–153. doi: 10.1111/j.1349-7006.2011.02106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saito T, Wada H, Yamasaki M, Miyata H, Nishikawa H, Sato E, Kageyama S, Shiku H, Mori M, Doki Y. High expression of MAGE-A4 and MHC class I antigens in tumor cells and induction of MAGE-A4 immune responses are prognostic markers of CHP-MAGE-A4 cancer vaccine. Vaccine. 2014;32:5901–5907. doi: 10.1016/j.vaccine.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Tada M, Tatematsu K, Ishii-Watabe A, Harazono A, Takakura D, Hashii N, Sezutsu H, Kawasaki N. Characterization of anti-CD20 monoclonal antibody produced by transgenic silkworms (Bombyx mori) MAbs. 2015;7:1138–1150. doi: 10.1080/19420862.2015.1078054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamura T, Thibert C, Royer C, Kanda T, Abraham E, Kamba M, Komoto N, Thomas JL, Mauchamp B, Chavancy G, et al. Germline transformation of the silkworm Bombyx mori L. Using a piggyBac transposon derived vector. Nat Biotechnol. 2000;18:81–84. doi: 10.1038/71978. [DOI] [PubMed] [Google Scholar]
- 18.Tatematsu K, Uchino K, Sezutsu H, Tamura T. Effect of ATG initiation codon context motifs on the efficiency of translation of mRNA derived from exogenous genes in the transgenic silkworm, Bombyx mori. SpringerPlus. 2014;3:136. doi: 10.1186/2193-1801-3-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eura M, Ogi K, Chikamatsu K, Lee KD, Nakano K, Masuyama K, Itoh K, Ishikawa T. Expression of the MAGE gene family in human head-and-neck squamous-cell carcinomas. Int J Cancer. 1995;64:304–308. doi: 10.1002/ijc.2910640504. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi S, Kumai T, Matsuda Y, Aoki N, Sato K, Kimura S, Kitada M, Tateno M, Celis E, Kobayashi H. Six-transmembrane epithelial antigen of the prostate and enhancer of zeste homolog 2 as immunotherapeutic targets for lung cancer. J Transl Med. 2011;9:191. doi: 10.1186/1479-5876-9-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chikamatsu K, Nakano K, Storkus WJ, Appella E, Lotze MT, Whiteside TL, DeLeo AB. Generation of anti-p53 cytotoxic T lymphocytes from human peripheral blood using autologous dendritic cells. Clin Cancer Res. 1999;5:1281–1288. [PubMed] [Google Scholar]
- 22.Yang Y. Cancer immunotherapy: Harnessing the immune system to battle cancer. J Clin Invest. 2015;125:3335–3337. doi: 10.1172/JCI83871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imamura M, Nakai J, Inoue S, Quan GX, Kanda T, Tamura T. Targeted gene expression using the GAL4/UAS system in the silkworm Bombyx mori. Genetics. 2003;165:1329–1340. doi: 10.1093/genetics/165.3.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogawa S, Tomita M, Shimizu K, Yoshizato K. Generation of a transgenic silkworm that secretes recombinant proteins in the sericin layer of cocoon: Production of recombinant human serum albumin. J Biotechnol. 2007;128:531–544. doi: 10.1016/j.jbiotec.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 25.Iizuka M, Ogawa S, Takeuchi A, Nakakita S, Kubo Y, Miyawaki Y, Hirabayashi J, Tomita M. Production of a recombinant mouse monoclonal antibody in transgenic silkworm cocoons. FEBS J. 2009;276:5806–5820. doi: 10.1111/j.1742-4658.2009.07262.x. [DOI] [PubMed] [Google Scholar]
- 26.Fehres CM, Unger WWJ, Garcia-Vallejo JJ, van Kooyk Y. Understanding the biology of antigen cross-presentation for the design of vaccines against cancer. Front Immunol. 2014;5:149. doi: 10.3389/fimmu.2014.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gutiérrez-Martínez E, Planès R, Anselmi G, Reynolds M, Menezes S, Adiko AC, Saveanu L, Guermonprez P. Cross-presentation of cell-associated antigens by MHC class I in dendritic cell subsets. Front Immunol. 2015;6:363. doi: 10.3389/fimmu.2015.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fratta E, Coral S, Covre A, Parisi G, Colizzi F, Danielli R, Nicolay HJ, Sigalotti L, Maio M. The biology of cancer testis antigens: Putative function, regulation and therapeutic potential. Mol Oncol. 2011;5:164–182. doi: 10.1016/j.molonc.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gjerstorff MF, Andersen MH, Ditzel HJ. Oncogenic cancer/testis antigens: Prime candidates immunotherapy. Oncotarget. 2015;6:15772–15787. doi: 10.18632/oncotarget.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cesson V, Rivals JP, Escher A, Piotet E, Thielemans K, Posevitz V, Dojcinovic D, Monnier P, Speiser D, Bron L, Romero P. MAGE-A3 and MAGE-A4 specific CD4(+) T cells in head and neck cancer patients: Detection of naturally acquired responses and identification of new epitopes. Cancer Immunol Immunother. 2011;60:23–35. doi: 10.1007/s00262-010-0916-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hasting BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM. The prioritization of cancer antigens: A national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]