Abstract
Climate-change driven increases in water temperature pose challenges for aquatic organisms. Predictions of impacts typically do not account for fine-grained spatiotemporal thermal patterns in rivers. Patches of cooler water could serve as refuges for anadromous species like salmon that migrate during summer. We used high-resolution remotely sensed water temperature data to characterize summer thermal heterogeneity patterns for 11,308 km of 2nd- to 7th-order rivers throughout the Pacific Northwest and northern California (USA). We evaluated (1) water temperature patterns at different spatial resolutions, (2) the frequency, size, and spacing of cool thermal patches suitable for Pacific salmon (i.e., contiguous stretches ≥0.25 km, ≤15°C and ≥2°C cooler than adjacent water), and (3) potential influences of climate change on availability of cool patches. Thermal heterogeneity was nonlinearly related to the spatial resolution of water temperature data, and heterogeneity at fine resolution (<1 km) would have been difficult to quantify without spatially continuous data. Cool patches were generally >2.7 and <13.0 km long, and spacing among patches was generally >5.7 and <49.4 km. Thermal heterogeneity varied among rivers, some of which had long uninterrupted stretches of warm water ≥20°C, and others had many smaller cool patches. Our models predicted little change in future thermal heterogeneity among rivers, but within-river patterns sometimes changed markedly compared to contemporary patterns. These results can inform long-term monitoring programs as well as near-term climate-adaptation strategies.
Keywords: cold-water patch, intermediate scale, connectivity, water temperature, spatial patterns, refugia
Introduction
The availability of cold water for aquatic species is of concern as river temperatures increase (Kaushal et al. 2010; Orr et al. 2015; Whitney et al. 2016). Temperature governs many physiological processes such as metabolic rate and respiratory performance (Angilletta et al. 2002; Brett 1971) and can be a key driver of population and community structure for ectotherms (Breau et al. 2011). Stream animals can survive periods of stressfully warm (or cold) temperatures provided there are adequate and well-connected thermal refuges (Ebersole et al. 2003a; Torgersen et al. 1999). Use of a thermal refuge may reduce metabolic costs and susceptibility to pathogens or toxins (Chiaramonte et al. 2016), and may be especially important to animals at the equatorial limit of their range, those migrating long distances, and those that inhabit marginally suitable habitat. Salmonids evolved in freshwater systems with abundant cold water (Beechie et al. 2012; McCullough et al. 2009); both juvenile and adult salmonids seek refuge in cooler areas when water becomes too warm (Dugdale et al. 2015b; Keefer and Caudill 2015; Petty et al. 2012; Sutton and Soto 2012).
Many studies have evaluated temporal patterns in water temperature and how animals respond to natural and altered thermal regimes (Arismendi et al. 2013; Penaluna et al. 2015; Steel et al. 2012). However, far fewer studies have described spatial patterns in water temperature. Thermal regimes can be diverse at broad spatial scales (e.g., among rivers) (Fullerton et al. 2015; Lisi et al. 2013) and at fine spatial scales (e.g., <1 km) (Dugdale et al. 2015a), but we know little about thermal heterogeneity at intermediate scales (e.g., 10s to 100s of kilometers) that may be important to animals during migration (e.g., cold tributary plumes; Keefer et al. 2009), breeding, and transition among thermally suitable habitats for feeding, growing, and sheltering (Flitcroft et al. 2012; Schlosser 1995). This gap in understanding stems from a paucity of datasets that are simultaneously broad in spatial extent and fine in spatial resolution. It has therefore been difficult to identify the appropriate spatial scale(s) at which water temperature data need to be collected to discern biologically relevant thermal refuges (i.e., those that are sufficiently large and cool to provide respite from hostile environmental conditions, and spaced such that animals can access them during times of need).
Regional climate models suggest that the Pacific Northwest, USA will be warmer, with drier summers and wetter winters, and in many mid-elevation areas, winter precipitation will fall as rain rather than snow (Dalton et al. 2013; Hamlet et al. 2013). Summer stream flows are projected to decrease across most of the region due to decreased snowpack, reduced summer precipitation, and higher evaporation (Jefferson 2011; Tohver et al. 2014). Lower flows will exacerbate stream warming during summer. River temperatures have already increased in parts of the Pacific Northwest (Arismendi et al. 2012; Isaak et al. 2012), and Wu et al. (2012) predicted an increase of 1.68°C in mean annual stream temperatures for 12 Pacific Northwest rivers by the 2080s. Rising air temperature and changes in hydrology were correlated with salmon extinctions in California (Zeug et al. 2011) and Japan (Fukushima et al. 2011), and vulnerability analyses for extant populations have predicted that large portions of freshwater species’ ranges will become unsuitable in the future (Isaak and Rieman 2013; Mantua et al. 2010; Wade et al. 2013). The ability of salmonids to survive and reproduce at water temperatures at or above their thermal tolerances will depend on their ability to adapt to changing conditions (Crozier and Hutchings 2014; Muñoz et al. 2014), and the extent to which patches of cold water can provide refuge from warm water as fish move between habitats needed during different life stages (e.g., breeding, rearing) (Flitcroft et al. 2012; Schlosser 1995).
Managers have defined aspects of thermal requirements for cold-water species, but implementation of regulations required by federal legislation (e.g., the Clean Water and Endangered Species Acts in the USA) has proven challenging (NMFS 2015). Existing federal and state regulations specify that thermal refuges are a part of the broader definition of thermally suitable habitat for cold-water species. In particular, managers aim to understand and implement the concept of “sufficiently distributed” cold water refuges. In addition, conservation and management of thermal habitat in freshwater systems will increasingly require ways to assess and maintain suitable thermal landscapes that are resilient to climate change.
Three questions highlight pressing issues confronting conservation of cold-water systems: (1) What spatial resolutions of water temperature data are sufficient to resolve biologically relevant thermal heterogeneity? (2) Are the frequency, size, and spacing of cold-water patches in rivers adequate to meet the needs of salmonids during the warmest time of year? (3) How might climate change influence patterns of thermal heterogeneity? To address these questions, we used remotely sensed water temperature data to explore longitudinal thermal heterogeneity in rivers throughout the Pacific Northwest. We characterized existing and potential future patterns of summertime thermal heterogeneity in terms of cold-water patches available to Pacific salmon and steelhead (Oncorhynchus spp.) at an intermediate scale.
Methods
Study Area
Our study area encompassed watersheds across Washington, Oregon, Idaho, and northern California (USA). We analyzed water temperature data for 11,308 km of 2nd- through 7th-order portions of streams used by anadromous salmon (Fig. 1). These data were not a random sample of rivers in the region (i.e., surveys were originally commissioned for other projects), but collectively these surveys encompassed the diverse habitat conditions available to salmon. Surveys generally occurred in mid- to high- Strahler order streams (Table S1, Online Resource 1); small tributary habitat was not well represented in the surveys because overhanging vegetation blocks the aerial view of the water. Surveys covered up to 30% of the geographic distribution of many anadromous salmonids but were more representative for mainstem spawners such as some forms of Chinook salmon (O. tshawytscha) than for tributary spawners like coho salmon (O. kisutch) and steelhead (O. mykiss) (Table S2, Online Resource 1). Surveys occurred predominantly in forested areas but also occurred in other natural areas and in developed areas (Table S3, Online Resource 1), and were distributed across four major ecoregions (Table S4, Online Resource 1). Discharge, velocity, elevation, slope, air temperature, and precipitation varied among the surveys (Table S5, Online Resource 1). Precipitation in the region occurs predominantly from October to March and falls as snow or rain, depending mainly on elevation and proximity to the Pacific Ocean. Snowmelt contributes to stream flow from April to September in snowmelt-dominated and transitional watersheds (Hamlet 2010). Human population density in the study area ranged from low (e.g., roadless areas in Idaho) to high (e.g., near major cities). Human influences on processes controlling hydrology and stream temperature include hydropower facilities, water diversion for irrigation, silviculture, and altered riparian vegetation associated with agriculture and urban development.
Fig. 1.
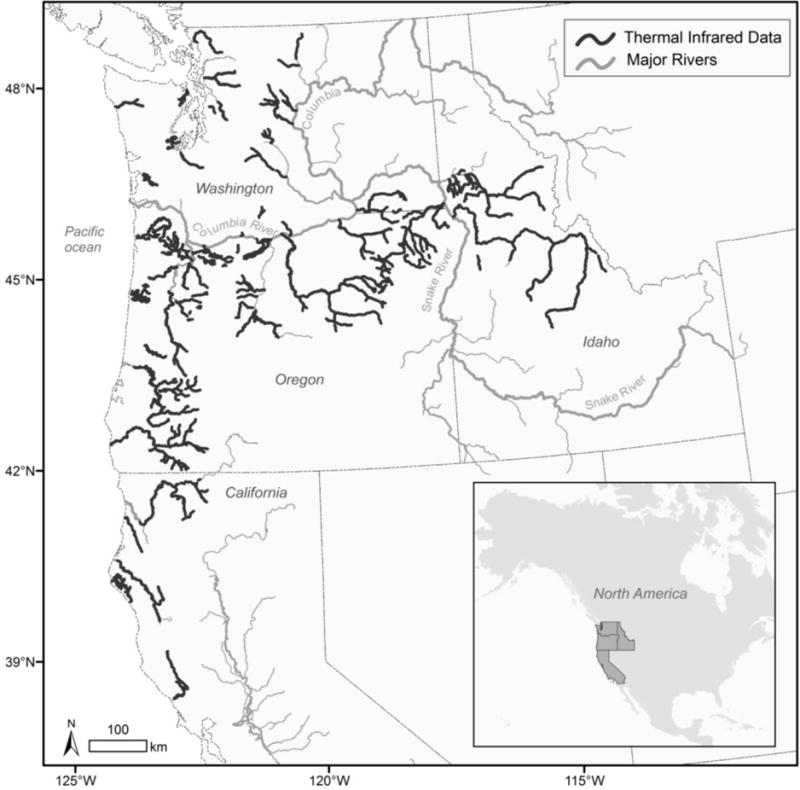
Rivers used by Pacific salmon for which airborne thermal infrared (TIR) surveys of water temperature were available in the western United States
Water Temperature Data
River surface temperature was measured using airborne thermal infrared (TIR) remote sensing (Handcock et al. 2012). Methods for this dataset are further described in Fullerton et al. (2015) and summarized here. All surveys occurred during the afternoon in July or August between 1994 and 2007, when water temperatures were expected to be near the daily and annual maxima and were most stressful for salmonids. Instream thermal sensors indicated that thermal imagery was typically accurate to ±0.5°C (Tables 1 and A4 in Fullerton et al. (2015)). Thermal image data were georeferenced and water temperatures were subsampled from images at approximately 150- to 200-m intervals along the thalweg of each river to create longitudinal profiles of water temperature versus distance from the downstream end of each survey. We included 139 surveys for which at least 20 km were accessible to anadromous fish. This one-dimensional dataset does not capture any potential lateral or vertical variability, but we recognize and discuss below the implications of lateral inputs (e.g., springs, seeps) and deep pools as potential refuges.
Data Analysis
We quantified heterogeneity in thermal habitat at multiple spatial resolutions, characterized spatial metrics of thermal habitat for Pacific salmon and steelhead, and analyzed how these patterns may change in a warming climate. Analyses were conducted in R (R Development Core Team 2015).
Thermal tolerances vary among species, life stages, geographic locations, acclimation histories, and other factors (McCullough et al. 2009). We did not focus on a particular species, nor on a particular life stage. Rather, we classified thermal riverine habitat into three zones considered to be generally optimal, tolerable, or stressful for Pacific salmon and steelhead as a group (references to salmon hereafter reflect this generality). These zones correspond to EPA criteria for 7-day average of daily maxima; see Table 3 in Palmer et al. (2003). The optimal or cool zone encompassed temperatures <15°C, at which most salmonids can thrive. The tolerable zone consisted of water temperatures between 15 and 20°C. The unsuitable or warm zone comprised temperatures >20°C, at which survival and fitness of adults migrating upstream may be decreased and growth and survival of juveniles may be depressed (McCullough et al. 2009; Palmer et al. 2003; Richter and Kolmes 2005).
To describe thermal habitats available to salmon in rivers for which TIR data were available, we partitioned each longitudinal thermal profile into the three thermal zones. Specifically, we recorded each location along the longitudinal profile where temperature crossed a threshold into a new thermal zone. The result was a series of river segments with homogeneous thermal zones. Hereafter, we refer to these zones as cool, tolerable, and warm patches (Fig. 2a). We quantified the spacing, length, number, and temperature range for thermal patches in each river, and summarized the distribution of these metrics pooled across the suite of rivers considered. We also quantified patch density (number of all patches in a given thermal zone divided by the total length in that zone) and total length in each thermal zone.
Fig. 2.
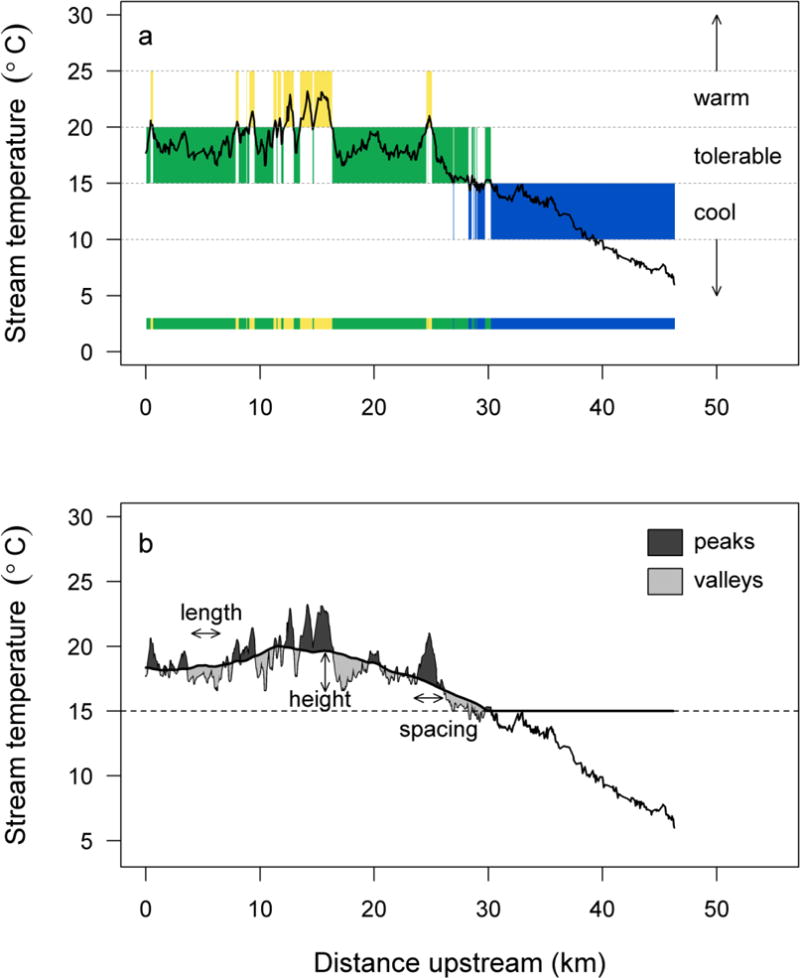
(a) Example of cool, tolerable, and warm thermal zones in a longitudinal stream temperature profile defined by temperature thresholds of 15 and 20°C, for Eightmile Creek, a tributary to the Columbia River in Oregon (USA), surveyed on 3 August 2002. The shaded bar at the bottom provides a longitudinal depiction of the thermal zones. (b) Peaks and valleys within areas that are warmer than 15°C indicate deviations in thermal infrared data from a 10-km moving average (thick black trend line). Annotations illustrate patch length, height, and spacing metrics
Influence of Spatial Grain on Pattern Metrics
We sought to identify whether thermal patches would be missed or mischaracterized at coarser resolutions as compared to the true distribution of thermal patches at finer resolution (i.e., the raw TIR data). We computed mean water temperature at resolutions of 0.25, 0.5, 1, 2, 3, 4, and 5 km using the approach of Welty (2015). For the raw data, and at each scale, we computed metrics summarizing spacing, length, and density of thermal patches as described above. We then evaluated whether the spatial grain of water temperature data influenced our ability to discern thermal patches.
Characterizing Thermal Heterogeneity for Salmon
To assess spatial patterns in thermal habitat that are relevant to salmon, we applied additional criteria that limited our analysis to include only cool patches likely to be important for salmon at an intermediate spatial scale (10s to 100s of kilometers). This restriction in no way suggests that smaller patches are unimportant. We included only patches ≥ 0.25 km long and ≥ 2°C cooler than adjacent water (i.e. short and substantially cooler) or patches ≥ 0.5 km long and ≥ 1°C cooler than adjacent water (i.e., long and marginally cooler). Our intent was to exclude very short patches or short patches with only marginally lower temperatures.
Thermal heterogeneity patterns may differ for species or life stages with different thermal requirements and vary with geographic position and watershed characteristics. To assess how responsive thermal heterogeneity patterns are to such factors, we (1) repeated the analysis using lower temperature thresholds (12 and 18°C) to characterize patterns available to species with cooler thermal requirements; and (2) examined the relationship between cool-patch metrics and position within a river survey, stream order, elevation, and drainage area.
Fish can acclimate to water temperature over time, and previous exposure to a thermal regime can influence how they perceive water temperature as they move through rivers. Areas that are cooler relative to surrounding habitat may represent a reprieve compared to what fish recently experienced in adjacent reaches, even though temperature is warmer than optimal. Thus, we employed a second approach for quantifying thermal heterogeneity in which we identified patches of relatively cooler habitat within warm or moderate zones (i.e., >15°C). We overlaid a smoothed trend (a 10-km moving average) on each longitudinal thermal profile (i.e., raw TIR data), and used the intersection of these lines to identify valleys (areas where TIR data fell below the trend line) and peaks (areas where TIR data exceeded the trend) that may be perceived as thermal refuges or barriers by fish (Fig. 2b; see Online Resource 2 for more details).
We quantified patch characteristics (spacing, length, density, height) for valleys and peaks in each river and summarized metrics across the suite of rivers considered. We applied more restrictive criteria than those used in our threshold-based analysis because valleys and peaks were only quantified within areas considered warmer than optimal. We used length and temperature criteria to identify valleys (peaks) large enough to potentially influence fish behavior. We included only valleys ≥ 0.5 km and ≥ 2°C cooler than the trend line or peaks ≥ 0.5 km and ≥ 2°C warmer than the trend line.
Quantifying Potential Effects of Climate Change
We used two approaches to explore how the size and spatial arrangement of cool patches may change in a warmer climate. For the first approach, we increased the temperature of the entire longitudinal profile by a given amount (Fig. 3a). This approach is supported by the temporal consistency of spatial patterns for surveys that were conducted in both cool and warm years (Fullerton et al. 2015). We represented potential future climate scenarios as increases in water temperature of 0.5, 1, 1.5, 2, 2.5, and 3 °C; no specific time periods were assumed.
Fig. 3.
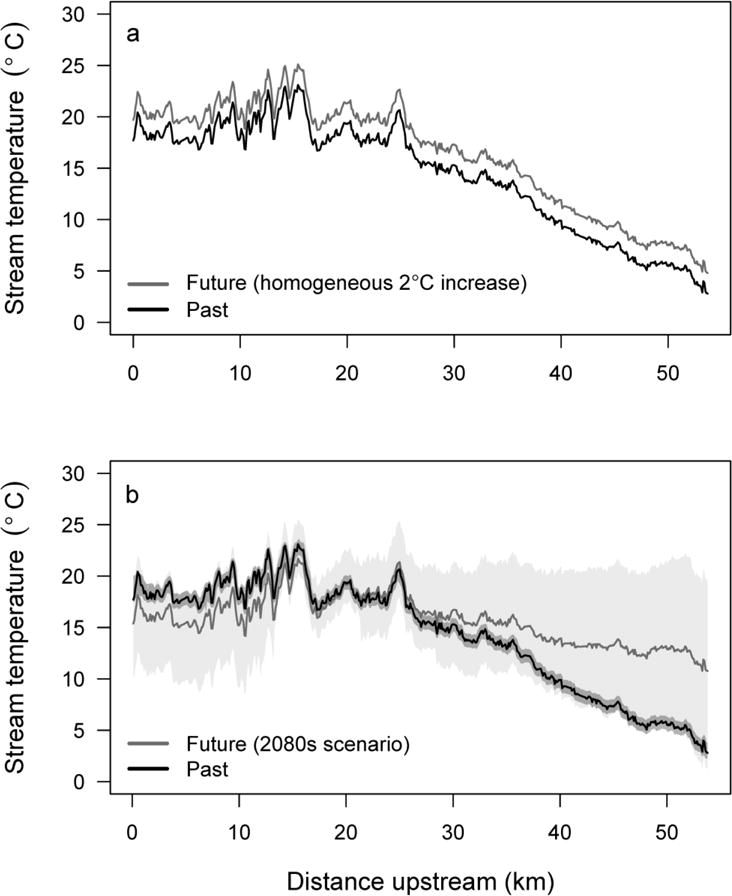
Example of past and potential future longitudinal stream temperature profiles derived from two methods for evaluating effects of climate change in Eightmile Creek, Oregon (USA). (a) past airborne thermal infrared survey on 3 Aug 2002 and a spatially homogenous increase of 2 °C in water temperature, and (b) past airborne thermal infrared survey and a projected future stream temperature profile and 95% prediction intervals for the 2080s (RCP 8.5) generated using a random forest model based on weekly summer air temperature, mean annual precipitation, and the proportion of winter precipitation falling as snow
For the second approach, we used a predictive model based on climate covariates to estimate future water temperature. This approach acknowledges that some rivers may be more sensitive to climate change (Arismendi et al. 2012; Isaak et al. 2012; Luce et al. 2014). For instance, correlations between air and water temperature differ among streams, which may reflect differential contribution of groundwater or other controls on stream temperature over space. Therefore, change may be slower in streams that are groundwater-fed, and faster in snow-fed streams where snowpack is expected to decline. We used a random forest regression (Breiman 2001; Cutler et al. 2007) of water temperature and three climate covariates: (1) maximum weekly air temperature between 16 July and 31 August, (2) mean annual precipitation, and (3) the proportion of precipitation that falls as snow during winter (Dec, Jan, Feb). We also included distance upstream as a covariate. Fitted values capture the trend in longitudinal thermal profiles that was explained by these covariates. Residuals were interpreted as patchiness at fine spatial scales in longitudinal profiles that was not controlled by climate covariates, but rather by processes not included in models, such as local groundwater inputs, anomalies in adjacent landscape features or land use, network geometry or stream geomorphology. We predicted future longitudinal thermal profiles by substituting values of the three climate covariates for the 2080s (2070–2099) based on the Coupled Model Intercomparison Project (CMIP5), representative concentration pathway (RCP) 8.5 (Taylor et al. 2012). We summed predicted future trends and residuals from the fitted model to produce potential future longitudinal profiles (Fig. 3b). Further details about the data sources, modeling, and climate scenarios are available in Online Resource 2.
In each of our two approaches for constructing potential future longitudinal profiles, we computed patch characteristics (spacing, length, and density) relevant to salmon as described earlier (see “Characterizing Thermal Heterogeneity for Salmon”). We compared the resulting patterns to the observed spatial patterns computed from TIR data.
Results
Spatial Resolution and Thermal Patches
Using the raw TIR data, we were able to resolve cool patches at a fine resolution (<0.25 km). Coarsening the spatial resolution of our water temperature data influenced the characteristics of thermal patches. As we increased the grain of water temperature data (i.e., data became more aggregated longitudinally), we were able to resolve fewer of the small patches observed in the raw TIR data. Hence, cool patch length and spacing increased, and patch density decreased (Fig. 4). The same occurred for tolerable and warm patches and for valleys and peaks in the longitudinal stream temperature profile (Fig. S1, Online Resource 1). Notably, the relationships were nonlinear, and changes were particularly pronounced at resolutions <1 km. There was spatial structure below a 1-km resolution that could be observed and quantified in the spatially continuous TIR data that could not be detected if data were sampled or modeled at coarser resolutions.
Fig. 4.
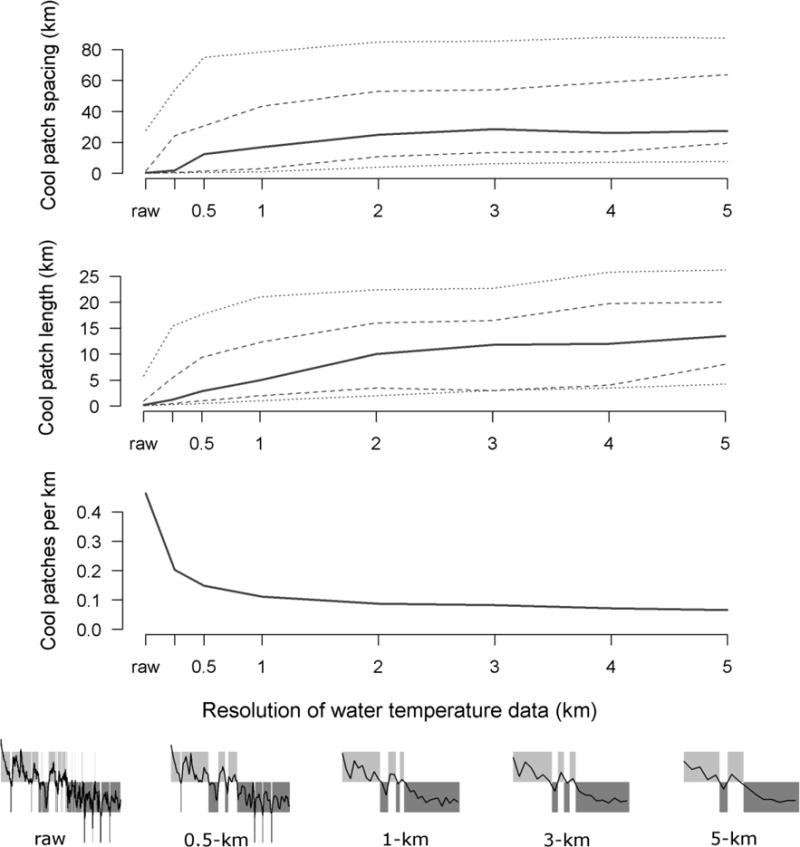
Spacing, length, and density of cool patches (<15°C) as a function of the spatial resolution of the data. X-axis tick marks indicate resolutions we examined: raw is the original thermal infrared survey data, and numeric values indicate data that were aggregated at 0.25-, 0.5-, 1-… and 5-km resolutions. Solid lines are medians, dashed lines are quartiles, and dotted lines are 10th and 90th percentiles. The 5 panels below the bottom panel illustrate the effect of decreasing spatial resolution on the quantification of thermal patches (plotted as in Fig. 2a)
Spatial Heterogeneity in Stream Temperature
The first cool patch was a short distance upstream in some rivers but was far upstream in others (Fig. 5; Fig. S2, Online Resource 1). The distance to the first cool patch encountered in an upstream direction was related to longitudinal position, stream order, and drainage area but not elevation (Fig. S3, Online Resource 1). Cool patches first encountered were longest and coolest in upper portions of river surveys, in lower-order streams, and in streams with smaller drainage areas. However, relationships with descriptors of geographic position were not evident for patches farther upstream than the first patch.
Fig. 5.
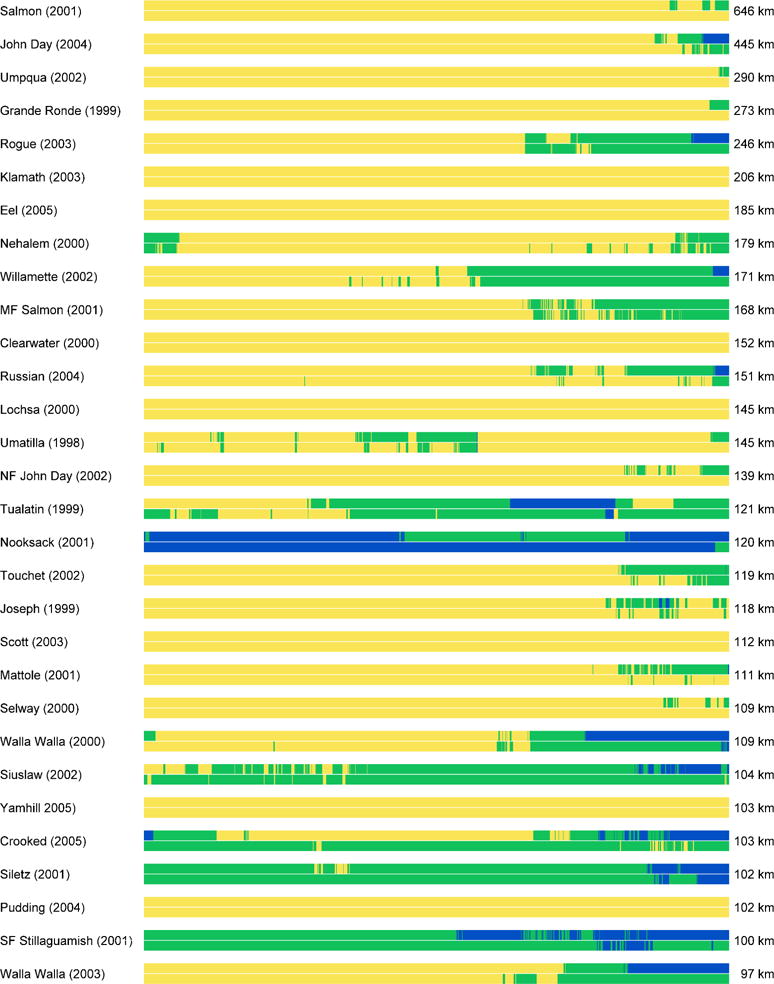
Thermal heterogeneity in longitudinal profiles, depicted as proportional linear distance from the bottom of each survey (left) to the top of each survey (right). For each river, shading denotes patches >20°C (dark gray), 15–20°C (gray), and <15°C (light gray) based on analysis of thermal infrared data surveyed in the year denoted parenthetically (top portion of each bar) and projected thermal heterogeneity for the 2080s based on random forest models (bottom portion of each bar). Patterns are illustrated for the 30 longest surveys and for the parts of the river that are accessible to anadromous fish; results for shorter surveys are provided in Online Resource 1
Water temperature was warm during summer afternoons in many rivers throughout the region: over 50% of the pooled length of surveyed rivers was >20°C (Fig. 6e). Patterns of thermal heterogeneity varied widely within and among rivers. Spacing between cool patches was greater than spacing between tolerable or warm patches. System-wide, the median spacing between cool patches was 21.3 km (interquartile range: 5.7 to 49.4 km; Fig. 6a). However, there was considerable variance, suggesting that cool patches were closely spaced in some rivers but far apart in others. The median spacing between valleys was 7.2 km (interquartile range: 3.5 to 13.7 km; Fig. 6f). The median length of cool patches was 6.4 km (interquartile range: 2.7 to 13.0 km; Fig. 6b). Again, there was considerable variance, wherein some rivers had short cool patches and others had long cool patches. Warm patches were generally longer than cool or tolerable patches. The median length of valleys was 2.1 km (interquartile range: 1.2 to 3.4 km; Fig. 6g). The density of cool and tolerable patches was higher than the density of warm patches (Fig. 6d). Some results differed given species-specific thermal requirements and were sensitive to the temperature bins we chose (i.e., the temperature thresholds delineating each thermal zone) and how close to thresholds individual river segments were. When we used lower temperature thresholds of 12 and 18°C, cool patches were fewer, more distantly spaced and shorter (Fig. 6).
Fig. 6.
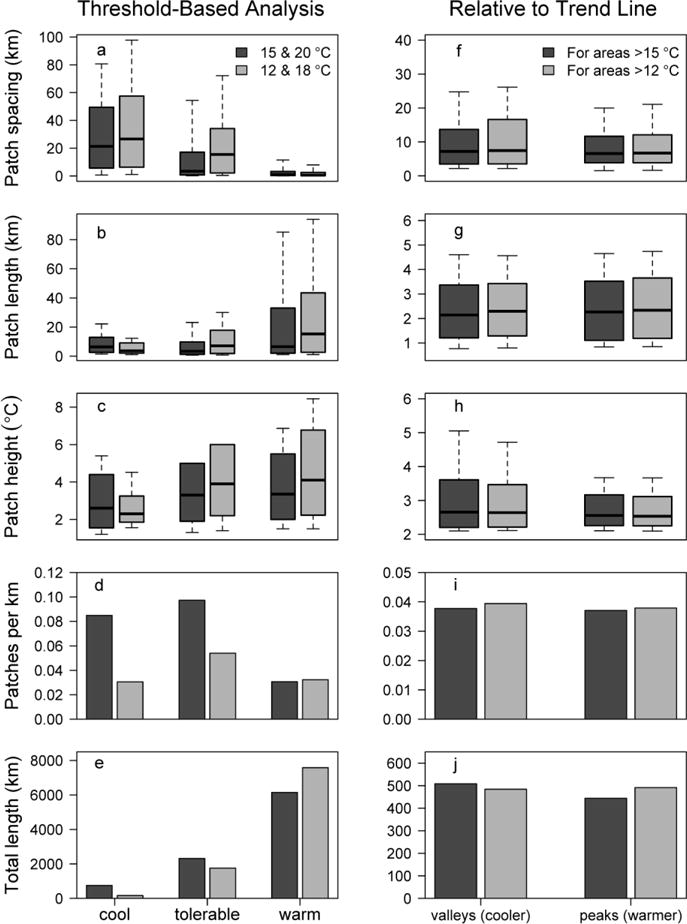
Quantification of thermal patch characteristics using threshold-based (left) and trend line-based (right) methods for rivers surveyed with airborne thermal infrared remote sensing. Calculations of thermal heterogeneity are based on the combined length of streams that were accessible to anadromous fish (11,308 km). (a–e): threshold-based summaries were quantified by partitioning longitudinal profiles into three thermal zones defined by 15 and 20°C (dark bars) or 12 and 18°C thresholds (light bars). (f–j): trend line-based summaries of valleys (relatively cooler) and peaks (relatively warmer) in longitudinal profiles were calculated from a 10-km moving average of portions of the rivers exceeding the cool threshold of 12 or 15°C
Thermal Heterogeneity in a Warmer Climate
When we assumed that future longitudinal profiles would become warmer versions of contemporary profiles, we found that the total amount of warm habitat (>20°C) increased, whereas tolerable and cool habitat decreased (Fig. 7). In warmer climate scenarios, the length of cool patches decreased marginally and the density increased (Fig. 7a). Correspondingly, warm patches became longer and fewer (Fig. 7b). However, the distribution of spacing among patches did not change noticeably because decreases in heterogeneity in downstream sections of river (i.e., as cool patches disappeared) were offset by gains in heterogeneity farther upstream where previously contiguous cool stretches became interspersed with new warm patches. This is illustrated in the Siletz River, Oregon, a coastal watershed where water temperature is a concern and which has threatened populations of coho salmon, steelhead, and coastal cutthroat trout (Fig. 8). In the Siletz River, numerous reaches were projected to warm enough to cross into a new thermal zone in the future when we assumed a spatially homogenous increase of 2°C (Fig. 8a). Cold patches (<15°C) decreased in number and were located farther upstream (Fig. 8b).
Fig. 7.
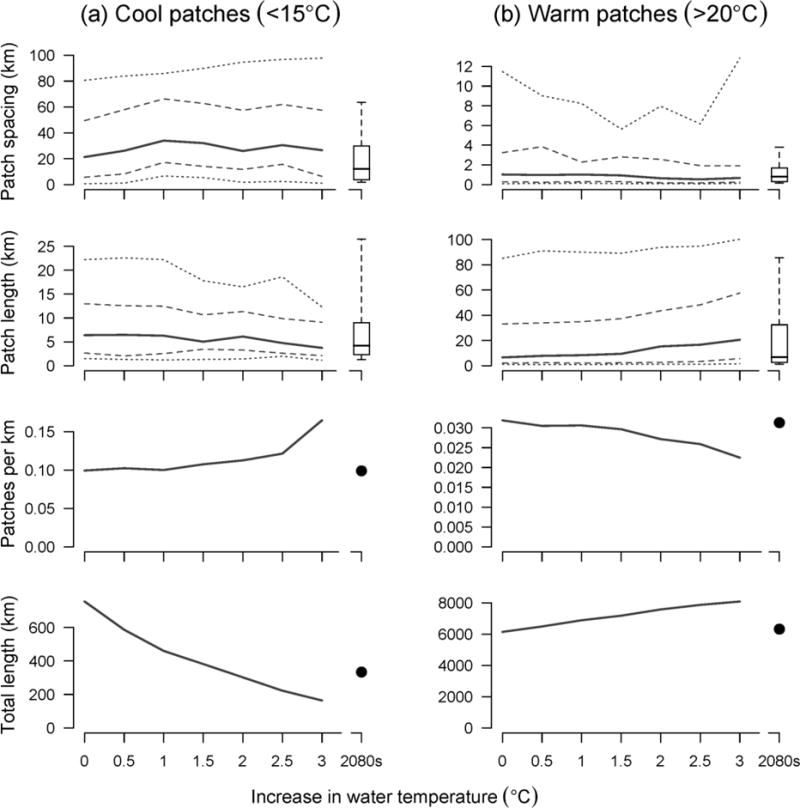
Predicted changes in thermal patch characteristics in (a) cool (<15°C) and (b) warm (>20°C) zones as a function of increases in water temperature (lines) and for the 2080s climate change scenario (boxplots and points). Calculations of thermal heterogeneity are based on the combined length of streams that were accessible to anadromous fish (11,308 km). Solid lines are medians, dashed lines are quartiles, and dotted lines are 10th and 90th percentiles. The values at the far left (0 on the x-axes) summarize the raw thermal infrared data
Fig. 8.
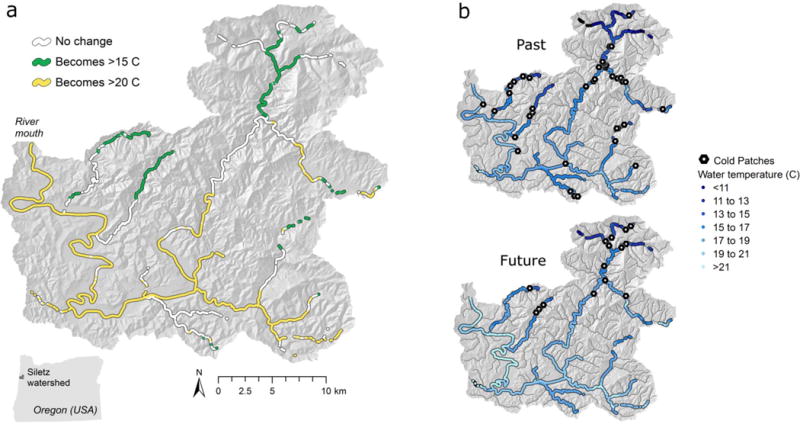
Potential effect of climate change on thermal zones and cold patches in the Siletz River watershed, Oregon (USA) based on thermal infrared data collected on 5–7 August 2001. (a) Reaches where thermal zones would change if water temperature increases by 2°C simultaneously. (b) Distribution of cool (<15°C) patches in the past (August 2001) and if water temperature increases by 2°C in the future
Random forest models fit the data well. The median model mean squared error was 0.109 (95% confidence intervals: 0.011, 1.228), the median pseudo-R2 was 0.970 (95% confidence intervals: 0.592, 0.997), and there were no trends in residuals (Table S6, Online Resource 1). Although prediction intervals were tight for fitted models, we found considerable uncertainty in future stream temperature predictions, which were made by substituting future values of climate covariates into fitted relationships with historical covariates. (Fig. 3b). All four covariates were equally important in the fitted model for the river shown in Fig. 3b (Eightmile Creek, a tributary to the lower Columbia River in Oregon, USA), whereas future stream temperature was predominantly determined by increased summer air temperature and decreased probability of winter precipitation falling as snow (Fig. S4, Online Resource 1). Relationships between climate covariates and water temperature were river-specific, with some longitudinal trends explained largely by air temperature, and others more strongly related to different covariates.
When we used random forest models to project potential stream temperature in the 2080s, the total length of river in cool and tolerable habitat zones decreased and resulted in more warm habitat overall (Fig. 7). Future thermal heterogeneity patterns were similar to contemporary patterns for cool and warm patches (boxplots and dots in Fig. 7) and for valleys and peaks (Fig. S5, Online Resource 1). Some rivers remained uniformly warm (>20°C) with minimal or no heterogeneity, such as the Klamath, Eel, and Clearwater rivers, and others such as the Nooksack, Siuslaw (lower portion), and Crooked rivers cooled in places in the future (Fig. 5). These changes resulted in a loss of heterogeneity for some rivers (e.g., Russian, North Fork John Day, South Fork Stillaguamish) and a gain in heterogeneity for others (e.g., Nehalem, Middle Fork Salmon).
Discussion
We characterized thermal heterogeneity at intermediate spatial scales (e.g., reaches of 10s to 100s of kilometers) and observed substantial spatial variation in summertime river temperature within and among rivers throughout the Pacific Northwest and northern California. Our analyses illustrate the presence of thermal heterogeneity at scales below a 1-km resolution that may be important for cold-water species such as salmonids (Dugdale et al. 2015b; Ebersole et al. 2003b; Petty et al. 2012; Sutton and Soto 2012). We were able to resolve cool patches at densities within the range observed by Dugdale et al. (2015a), who quantified thermal refuges using thermal imagery of similarly sized streams. We suggest that spatial patterns in water temperature assessed at broader resolutions than that of our raw data (i.e., >0.25 km) may not describe important thermal heterogeneity at scales at which fish behaviorally thermoregulate. Cool patches were distributed throughout most rivers. For some rivers, however, salmon would need to move far upstream to reach the first cool patch. Cool patches were generally shorter and warmer toward river mouths and longer and cooler toward their source. We predicted minor changes in spacing, length, and density of cool patches in warmer climate conditions overall, but cool patches were arranged differently, and changes were greater in certain rivers. Our results have implications for freshwater conservation in the context of (1) how cold water refuges are defined and quantified, (2) the adequacy of thermal heterogeneity for coldwater species, and (3) guidance for managing thermal diversity in a changing climate.
Defining Thermal Refuges in Space and Time
Cool patches can be characterized by their size, spacing, density, position within a river, and temporal permanency. Identification and management of cold water refuges is challenging because refuges vary over space and time, and their utility differs among species and life stages. Water quality regulations typically specify simplified metrics for temperature standards (Poole et al. 2004). For instance, the U.S. Environmental Protection Agency defines cold-water refuges as “locations or times at which water temperature is 2°C colder than the surrounding water” (Palmer et al. 2003). This definition provides flexibility in determining what is sufficient given a river’s context and the species living there. However, the challenges have been to match the scale of measurement to the scale of management questions, and to ensure that the metrics used to characterize thermal refuges capture relevant ecological facets (Steel et al. 2017; Steel et al. 2016).
An example of such a facet is the distance between thermal refuges available to adult Chinook salmon as they migrate upstream during August. To define this facet, one would need to know what constitutes a “thermal refuge”. Perhaps for this example, a thermal refuge could be defined as a contiguous area <20°C that is large enough for an adult salmon to use temporarily, as there are fitness consequences for fish migrating above this temperature threshold (Hasler et al. 2012; Hinch et al. 2012; Richter and Kolmes 2005). In our meta-analysis, we used two complementary and generalized definitions of a cool patch that may serve as useful starting points for defining thermal refuges: (1) the contiguous length of river where maximum temperature was below a biologically relevant thermal criterion (e.g., 15°C), and (2) the contiguous length of river within a warm stretch (e.g., > 15°C) where temperature was relatively cooler (e.g., <2°C) than in adjacent stretches. Our inclusion of the second definition may provide useful supplementary information about subtler patterns in thermal habitat than would have been detected using only a thermal criterion approach. Ultimately, analyses such as those presented here will need to be tailored to the particular species and life stage of interest, and should be followed by biological assessments in the field to determine the extent to which fish use refuges of different size and quality.
We have presented one way to resolve spatial patterns of water temperature as a first step in identifying areas of potential management concern at intermediate spatial scales. The data used in our analysis were one-dimensional (i.e., longitudinal patchiness in temperature) and did not capture patterns across the width of the stream where there may be thermal refuges along channel margins (e.g., from seeps, springs, or small tributaries). The rivers surveyed with airborne TIR remote sensing were selected because they were generally well-mixed vertically, but in large slow-moving rivers there may be thermal refuges at depth in larger pools. There may also be thermal refuge at different times of day that are not represented in our data (Dugdale et al. 2013; Ebersole et al. 2015; Wawrzyniak et al. 2016). Our approach of identifying areas of concern at an intermediate scale can guide more comprehensive investigation of thermal habitat at finer spatial scales using remotely sensed data, placement of instream sensors, and field surveys to determine use by animals (Dugdale et al. 2015a; Ebersole et al. 2003b; Monk et al. 2013; Torgersen et al. 1999).
Our spatially continuous stream temperature dataset was uniquely suited for identifying spatial heterogeneity that may be missed between sparsely spaced monitoring sites, and for interpreting coarser-resolution maps of stream temperature generated from spatial statistical techniques or process-based models. When longitudinal thermal profiles were constructed from coarsened data, we detected fewer thermal patches. The strong nonlinear relationships between scale and thermal patch characteristics that we observed indicate that substantial complexity exists at spatial scales <1 km. Spatially explicit water temperature models typically do not make predictions at scales finer than 1 km. Stream network models (Peterson et al. 2013) can make fine-scale predictions from coarse-scale empirical data, but such predictions do not include fine-scale spatial variation unless that variation is closely tied to covariates that are measured at fine spatial scales. Predictions from these models may be useful for some questions (e.g., range shifts over broad spatial scales) but less so for others (e.g., identifying thermal refuges). Without knowledge about fine-scale thermal heterogeneity, managers may assume that a reach is too warm; this misinterpretation could be avoided if higher-resolution data revealed the presence of thermal refuges.
Adequacy of Cold Patches for Coldwater Species During Summer
Evaluating whether cold patches are sufficient for meeting the needs of coldwater species will require consideration of patches that are large enough for animals to use, frequent enough to be encountered, and located in places where, and at times when, they are most needed. Particular species and life stages of mobile stream animals may use refuges in specific ways over time (Brewitt and Danner 2014; Sutton et al. 2007) to optimize growth by moving between warm and cool habitats throughout the day (Armstrong and Schindler 2013; Torgersen et al. 2012).
The sizes of cold-water patches needed by stream biota are not well understood. Our criteria for defining patches adequate for salmon may exclude smaller patches that could benefit salmon or other species. If we characterized cool patches without imposing additional length and temperature criteria, the median patch length would have been 200 m (interquartile range: 90 to 970 m). These cool patches are large enough for temporary use by salmon, which have been observed using areas as small as 0.13 m2 at high densities (Lindeman et al. 2015). The minimum patch size depends on an animal’s age and ecological conditions within the patch, such as density of other animals using the refuge, and whether habitat therein is suitable (Breau et al. 2011; Woolnough et al. 2009). Elevated animal densities can suppress growth and survival for juveniles already stressed by high water temperatures (Crozier et al. 2010; Harvey and Nakamoto 1996) by increasing competition and vulnerability to predators (Keefer et al. 2009; Torgersen et al. 2012). It is unknown whether a group of small, connected cold-water patches is equivalent to a single large patch (e.g., Kingsland 2002; Simberloff and Abele 1982).
The extent to which connectivity among cold-water patches limits animals depends on the species, life stage, and environmental characteristics in a given river at a particular time. Research on stream fish behavior is increasing, but information is needed about why and when fish and other stream organisms seek refuge in cooler habitats. The cool patches that we identified as suitable for salmon were far apart (~10 km). However, animals may be able to use smaller patches as “stepping stones” of tolerable habitat in an otherwise intolerable environment. The median distance between cool patches of any size (i.e., without additional criteria imposed) was 250 m (interquartile range: 100 m to 1.36 km), which is within the swimming capabilities of juvenile and adult salmon. However, these distances may exceed movement capabilities for other stream biota. Salmon can swim long distances and can swim fast enough to move between cool patches, but they may not choose to move through a warm section of river. Migration may be delayed (Keefer and Caudill 2015; Keefer et al. 2009) or may cease at high temperatures (Hasler et al. 2012; Richter and Kolmes 2005). Fish that swim through thermally stressful waters may experience latent sublethal and cumulative effects caused by increased susceptibility to pathogens, toxins, predation or other heat-related stressors (Dietrich et al. 2014; Fenkes et al. 2016; Marcos-Lopez et al. 2010).
Managing Thermal Heterogeneity in a Changing Climate
The diverse thermal patterns that we characterized within and among rivers can be used as a measure of baseline conditions in the Pacific Northwest and northern California to which future patterns can be compared. Important questions remain about how thermal heterogeneity will change and how species will respond in a warmer climate. We predicted that water temperatures will increase overall, and that spatial patterns may be similar to current patterns with minor changes in the number, length, and connectivity of cold patches. However, the arrangement of patches within any given river may change considerably. For instance, a small cool patch between two warm patches may disappear, resulting in a single larger warm patch that could pose a new migration barrier. Conversely, a previously long cool patch may be broken into a series of smaller patches. Although our analysis suggested that the spacing between cool patches would not change appreciably (because these two mechanisms cancel one another), closely spaced cool patches tended to occur farther upstream in the future, as illustrated for the Siletz River (Fig. 8). Changes in thermal heterogeneity cannot uniformly be described as ‘good’ or ‘bad’ for salmon. Losses of heterogeneity associated with cooling (e.g., Nooksack and Siuslaw rivers in Fig. 5) may be beneficial whereas increases in heterogeneity associated with warming of previously cool reaches (e.g., upper Touchet or Russian rivers in Fig. 5) would likely be unfavorable.
Several aspects of our approach for estimating future water temperatures may have under-represented potential changes. Neither of our approaches included hydrologic responses to climate change that may influence thermal heterogeneity patterns. At high discharge, spatial variability may be dampened due to mixing, whereas at low discharge, spatial variability may increase because smaller volumes respond more quickly to radiative forces. In rivers that were historically glacier fed or those that had snowmelt-driven hydrographs, future thermal regimes may change substantially, whereas changes may be slowest in groundwater-fed rivers (Luce et al. 2014; Mayer 2012). In the near future, summer water temperature may decrease as melting glaciers contribute cold water. Eventually, these rivers may warm earlier without the moderating influence of meltwater during spring and summer.
In our random forest models, we observed considerable uncertainty in how the climate covariates will influence stream temperature in the future based on their relationships in the past. One contributing factor is the low spatial resolution of the climate covariate data relative to our high-resolution stream temperature data. The air temperature data we used as covariates may be too coarse to reflect fine-scale air temperature patterns, especially in complex terrains (Minder et al. 2010). We also recognize that our models lacked other potentially influential covariates. For instance, groundwater and subsurface flow pathways abundant in some rivers may buffer them from warming (Arrigoni et al. 2008; Snyder et al. 2015; Tague et al. 2007; Tague et al. 2008). Moreover, there is evidence that rivers vary in their susceptibility to warming (Arismendi et al. 2012; Isaak et al. 2012; Isaak et al. 2016) and that the response by salmonids will vary as well (Isaak et al. 2015).
We did not evaluate fine-scale controls on thermal heterogeneity such as riparian shading, land-use features, or geomorphology. We assumed that fine-scale thermal patterns would be similar in warm and cool years as observed by Fullerton et al. (2015) in rivers where aerial thermal infrared surveys were conducted in sequential years. Models that incorporate the mechanisms driving thermal heterogeneity may be better suited for predicting locations of thermal refuges in places where no empirical data exist, and for future climate conditions.
We recognize that our data are based on surveys that were conducted at a single point in time. Although all rivers were surveyed during afternoons in the heat of summer, temporal differences in climate conditions (air temperature, precipitation) from one day to the next or from one hour to the next may produce different sizes and spatial arrangements of thermal patches at fine scales (Dugdale et al. 2013). Therefore, we cannot predict how much change will occur under extreme heating events, for instance, or during times of extended drought. During such stressful times, the importance of cool patches and how they are spatially distributed may be even greater for salmonids.
Management of the thermal landscape in rivers is based on local conditions, the aquatic ecosystem, and sociopolitical goals. Approaches used to protect, restore, or create cold-water patches (Kurylyk et al. 2015) may need to consider spatiotemporal context explicitly. Managers can locate reaches in a watershed that may be least likely to change in a warmer climate (Fig. 8a). These reaches may represent opportunities for conserving potential climate refugia (Isaak et al. 2015; Keppel et al. 2015; Morelli et al. 2016). Comparison of the distribution of cool patches in past and future scenarios can highlight which refuges may persist into the future as well as areas where more intensive intervention may be necessary (Fig. 8b).
Our study raises several critical uncertainties about cold-water habitat that, if answered, could help resource managers to protect and restore thermal diversity in rivers:
How can thermal refuges best be defined in space and time for different species and life stages?
How might habitat quality or ecological interactions influence the effectiveness of thermal refuges?
How will salmon survival or fitness respond to changes in thermal refuge availability and/or quality?
How can water quality regulations and management strategies accommodate multiple species with different thermal requirements?
What are the tradeoffs between protecting small, connected thermal refuges versus one larger refuge, or between protecting multiple ephemeral refuges versus one persistent refuge?
What are the drivers of thermal patches that will be most influenced by climate change?
How can we improve fine-grain predictions over broad spatial extents?
We believe that these questions will prompt research that will enhance understanding about these important issues. Our approach to characterizing spatial patterns of water temperature could highlight areas of potential management concern that can be used in biological assessments in the field to determine how fish respond to thermal heterogeneity and to identify specific strategies for improving the availability and quality of refuges through targeted restoration activities.
Supplementary Material
Acknowledgments
Remotely sensed river temperature survey data were provided by R. Faux, Quantum Spatial Inc. and D. Essig, Idaho Department of Environmental Quality. We are grateful to the many local, state, federal, tribal and nongovernmental organizations that funded the collection of these data for water quality monitoring and assessment. We thank D. Miller, L. Crozier, and T. Beechie for helpful discussions, and B. Feist, S. Morley, and two anonymous reviewers for constructive feedback on the manuscript. Funding from the North Pacific Landscape Conservation Cooperative and the NOAA Advanced Studies Program supported this work. The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the U.S. Government. This article has been peer reviewed and approved for publication consistent with USGS Fundamental Science Practices (pubs.usgs.gov/circ/1367). Any use of trade, product or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
References
- Angilletta MJ, Niewiarowski PH, Navas CA. The evolution of thermal physiology in ectotherms. J Therm Biol. 2002;27:249–268. [Google Scholar]
- Arismendi I, Johnson SL, Dunham JB, Haggerty R. Descriptors of natural thermal regimes in streams and their responsiveness to change in the Pacific Northwest of North America. Freshwater Biol. 2013;58:880–894. doi: 10.1111/Fwb.12094. [DOI] [Google Scholar]
- Arismendi I, Johnson SL, Dunham JB, Haggerty R, Hockman-Wert D. The paradox of cooling streams in a warming world: Regional climate trends do not parallel variable local trends in stream temperature in the Pacific continental. United States Geophysical Research Letters. 2012;39:L10401. doi: 10.1029/2012gl051448. [DOI] [Google Scholar]
- Armstrong JB, Schindler DE. Going with the flow: spatial distributions of juvenile coho salmon track an annually shifting mosaic of water temperature. Ecosystems. 2013;16:1429–1441. doi: 10.1007/s10021-013-9693-9. [DOI] [Google Scholar]
- Arrigoni AS, Poole GC, Mertes LAK, O’Daniel SJ, Woessner WW, Thomas SA. Buffered, lagged, or cooled? Disentangling hyporheic influences on temperature cycles in stream channels. Wat Res Research. 2008;44:W09418. doi: 10.1029/2007wr006480. [DOI] [Google Scholar]
- Beechie T, et al. Restoring salmon habitat for a changing climate. River Res Appl. 2012;29:939–960. doi: 10.1002/rra.2590. [DOI] [Google Scholar]
- Breau C, Cunjak RA, Peake SJ. Behaviour during elevated water temperatures: can physiology explain movement of juvenile Atlantic salmon to cool water? J Anim Ecol. 2011;80:844–853. doi: 10.1111/j.1365-2656.2011.01828.x. [DOI] [PubMed] [Google Scholar]
- Breiman L. Random forests. Machine Learning. 2001;45:5–32. [Google Scholar]
- Brett JR. Energetic responses of salmon to temperature - study of some thermal relations in physiology and freshwater ecology of sockeye salmon (Oncorhynchus nerka) Am Zool. 1971;11:99–113. [Google Scholar]
- Brewitt KS, Danner EM. Spatio-temporal temperature variation influences juvenile steelhead (Oncorhynchus mykiss) use of thermal refuges. Ecosphere. 2014;5:art92. doi: 10.1890/es14-00036.1. [DOI] [Google Scholar]
- Chiaramonte LV, Ray RA, Corum RA, Soto T, Hallett SL, Bartholomew JL. Klamath River thermal refuge provides juvenile salmon reduced exposure to the parasite Ceratonova shasta. Trans Am Fish Soc. 2016;145:810–820. doi: 10.1080/00028487.2016.1159612. [DOI] [Google Scholar]
- Crozier LG, Hutchings JA. Plastic and evolutionary responses to climate change in fish. Evolutionary Applications. 2014;7:68–87. doi: 10.1111/eva.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier LG, Zabel RW, Hockersmith EE, Achord S. Interacting effects of density and temperature on body size in multiple populations of Chinook salmon. J Anim Ecol. 2010;79:342–349. doi: 10.1111/j.1365-2656.2009.01641.x. [DOI] [PubMed] [Google Scholar]
- Cutler DR, Edwards TC, Jr, Beard KH, Cutler A, Hess KT, Gibson J, Lawler JJ. Random forests for classification in ecology. Ecology. 2007;88:2783–2792. doi: 10.1890/07-0539.1. [DOI] [PubMed] [Google Scholar]
- Dalton MM, Mote PW, Snover AK. Climate change in the Northwest: implications for our landscapes, waters, and communities. Oregon Climate Change Research Institute; Washington, D.C.: 2013. [Google Scholar]
- Dietrich JP, Van Gaest AL, Strickland SA, Arkoosh MR. The impact of temperature stress and pesticide exposure on mortality and disease susceptibility of endangered Pacific salmon. Chemosphere. 2014;108:353–359. doi: 10.1016/j.chemosphere.2014.01.079. [DOI] [PubMed] [Google Scholar]
- Dugdale SJ, Bergeron NE, St-Hilaire A. Temporal variability of thermal refuges and water temperature patterns in an Atlantic salmon river. Remote Sens Environ. 2013;136:358–373. doi: 10.1016/j.rse.2013.05.018. [DOI] [Google Scholar]
- Dugdale SJ, Bergeron NE, St-Hilaire A. Spatial distribution of thermal refuges analysed in relation to riverscape hydromorphology using airborne thermal infrared imagery. Remote Sens Environ. 2015a;160:43–55. doi: 10.1016/j.rse.2014.12.021. [DOI] [Google Scholar]
- Dugdale SJ, Franssen J, Corey E, Bergeron NE, Lapointe M, Cunjak RA. Main stem movement of Atlantic salmon parr in response to high river temperature. Ecol Freshw Fish Preprint. 2015b doi: 10.1111/eff.12224. [DOI] [Google Scholar]
- Ebersole JL, Liss WJ, Frissell CA. Cold water patches in warm streams: physicochemical characteristics and the influence of shading. J Am Water Resour Assoc. 2003a;39:355–368. [Google Scholar]
- Ebersole JL, Liss WJ, Frissell CA. Thermal heterogeneity, stream channel morphology, and salmonid abundance in northeastern Oregon streams. Can J Fish Aquat Sci. 2003b;60:1266–1280. doi: 10.1139/f03-107. [DOI] [Google Scholar]
- Ebersole JL, Wigington PJ, Leibowitz SG, Comeleo RL, Sickle JV. Predicting the occurrence of cold-water patches at intermittent and ephemeral tributary confluences with warm rivers. Freshwater Science. 2015;34:111–124. doi: 10.1086/678127. [DOI] [Google Scholar]
- Fenkes M, Shiels HA, Fitzpatrick JL, Nudds RL. The potential impacts of migratory difficulty, including warmer waters and altered flow conditions, on the reproductive success of salmonid fishes. Comp Biochem Physiol A Mol Integr Physiol. 2016;193:11–21. doi: 10.1016/j.cbpa.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flitcroft RL, Burnett KM, Reeves GH, Ganio LM. Do network relationships matter? Comparing network and instream habitat variables to explain densities of juvenile coho salmon (Oncorhynchus kisutch) in mid-coastal Oregon, USA Aquatic Conservation: Marine and Freshwater. Ecosystems. 2012;22:288–302. doi: 10.1002/aqc.2228. [DOI] [Google Scholar]
- Fukushima M, Shimazaki H, Rand PS, Kaeriyama M. Reconstructing Sakhalin taimen Parahucho Perryi historical distribution and identifying causes for local extinctions. Trans Am Fish Soc. 2011;140:1–13. doi: 10.1080/00028487.2011.544999. [DOI] [Google Scholar]
- Fullerton AH, et al. Rethinking the longitudinal stream temperature paradigm: region-wide comparison of thermal infrared imagery reveals unexpected complexity of river temperatures. Hydrological Processes. 2015;29:4719–4737. doi: 10.1002/hyp.10506. [DOI] [Google Scholar]
- Hamlet AF. Assessing water resources adaptive capacity to climate change impacts in the Pacific Northwest Region of North America Hydrology and Earth System Sciences. Discussions. 2010;7:4437–4471. doi: 10.5194/hessd-7-4437-2010. [DOI] [Google Scholar]
- Hamlet AF, Elsner MM, Mauger GS, Lee S-Y, Tohver I, Norheim RA. An overview of the Columbia basin climate change scenarios project: approach, methods, and summary of key results. Atmosphere-Ocean. 2013;51:392–415. doi: 10.1080/07055900.2013.819555. [DOI] [Google Scholar]
- Handcock RN, Torgersen CE, Cherkauer KA, Gillespie AR, Tockner K, Faux RN, Tan J. Thermal infrared remote sensing of water temperature in riverine landscapes. In: Carbonneau P, Piégay H, editors. Fluvial Remote Sensing for Science and Management. Vol. 1. John Wiley & Sons; Hoboken, NJ; Chichester, UK.: 2012. pp. 85–113. [DOI] [Google Scholar]
- Harvey BC, Nakamoto RJ. Effects of steelhead density on growth of coho salmon in a small coastal California stream. Trans Am Fish Soc. 1996;125:237–243. [Google Scholar]
- Hasler CT, Mossop B, Patterson DA, Hinch SG, Cooke SJ. Swimming activity of migrating Chinook salmon in a regulated river. Aquatic Biology. 2012;17:47–56. doi: 10.3354/ab00460. [DOI] [Google Scholar]
- Hinch SG, Cooke SJ, Farrell AP, Miller KM, Lapointe M, Patterson DA. Dead fish swimming: a review of research on the early migration and high premature mortality in adult Fraser River sockeye salmon Oncorhynchus nerka. J Fish Biol. 2012;81:576–599. doi: 10.1111/j.1095-8649.2012.03360.x. [DOI] [PubMed] [Google Scholar]
- Isaak DJ, Rieman BE. Stream isotherm shifts from climate change and implications for distributions of ectothermic organisms. Glob Change Biol. 2013;19:742–751. doi: 10.1111/gcb.12073. [DOI] [PubMed] [Google Scholar]
- Isaak DJ, Wollrab S, Horan D, Chandler G. Climate change effects on stream and river temperatures across the northwest U.S. from 1980–2009 and implications for salmonid fishes. Clim Change. 2012;113:499–524. doi: 10.1007/s10584-011-0326-z. [DOI] [Google Scholar]
- Isaak DJ, et al. Slow climate velocities of mountain streams portend their role as refugia for cold-water biodiversity. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1522429113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaak DJ, Young MK, Nagel DE, Horan DL, Groce MC. The cold-water climate shield: delineating refugia for preserving salmonid fishes through the 21st century. Glob Change Biol. 2015;21:2541–2553. doi: 10.1111/gcb.12879. [DOI] [PubMed] [Google Scholar]
- Jefferson AJ. Seasonal versus transient snow and the elevation dependence of climate sensitivity in maritime mountainous regions. Geophysical Research Letters. 2011;38:L16402. doi: 10.1029/2011gl048346. [DOI] [Google Scholar]
- Kaushal SS, et al. Rising stream and river temperatures in the United States. Front Ecol Environ. 2010;8:461–466. doi: 10.1890/090037. [DOI] [Google Scholar]
- Keefer ML, Caudill CC. Estimating thermal exposure of adult summer steelhead and fall Chinook salmon migrating in a warm impounded river. Ecol Freshw Fish Preprint. 2015 doi: 10.1111/eff.12238. [DOI] [Google Scholar]
- Keefer ML, Peery CA, High B. Behavioral thermoregulation and associated mortality trade-offs in migrating adult steelhead (Oncorhynchus mykiss): variability among sympatric populations. Can J Fish Aquat Sci. 2009;66:1734–1747. doi: 10.1139/f09-131. [DOI] [Google Scholar]
- Keppel G, Mokany K, Wardell-Johnson GW, Phillips BL, Welbergen JA, Reside AE. The capacity of refugia for conservation planning under climate change. Front Ecol Environ. 2015;13:106–112. doi: 10.1890/140055. [DOI] [Google Scholar]
- Kingsland SE. Creating a science of nature reserve design: Perspectives from history. Environmental Modeling & Assessment. 2002;7:61–69. [Google Scholar]
- Kurylyk BL, MacQuarrie KTB, Linnansaari T, Cunjak RA, Curry RA. Preserving, augmenting, and creating cold-water thermal refugia in rivers: concepts derived from research on the Miramichi River, New Brunswick (Canada) Ecohydrology. 2015;8:1095–1108. doi: 10.1002/eco.1566. [DOI] [Google Scholar]
- Lindeman AA, Grant JWA, Desjardins CM. Density-dependent territory size and individual growth rate in juvenile Atlantic salmon (Salmo salar) Ecol Freshw Fish. 2015;24:15–22. doi: 10.1111/eff.12120. [DOI] [Google Scholar]
- Lisi PJ, Schindler DE, Bentley KT, Pess GR. Association between geomorphic attributes of watersheds, water temperature, and salmon spawn timing in Alaskan streams. Geomorphology. 2013;185:78–86. doi: 10.1016/j.geomorph.2012.12.013. [DOI] [Google Scholar]
- Luce C, Staab B, Kramer M, Wenger S, Isaak D, McConnell C. Sensitivity of summer stream temperatures to climate variability in the Pacific Northwest. Wat Res Research. 2014;50:3428–3443. doi: 10.1002/2013wr014329. [DOI] [Google Scholar]
- Mantua N, Tohver I, Hamlet A. Climate change impacts on streamflow extremes and summertime stream temperature and their possible consequences for freshwater salmon habitat in Washington State. Clim Change. 2010;102:187–223. doi: 10.1007/s10584-010-9845-2. [DOI] [Google Scholar]
- Marcos-Lopez M, Gale P, Oidtmann BC, Peeler EJ. Assessing the impact of climate change on disease emergence in freshwater fish in the United Kingdom Transboundary and Emerging. Diseases. 2010;57:293–304. doi: 10.1111/j.1865-1682.2010.01150.x. [DOI] [PubMed] [Google Scholar]
- Mayer TD. Controls of summer stream temperature in the Pacific Northwest. J Hydrol. 2012;475:323–335. doi: 10.1016/j.jhydrol.2012.10.012. [DOI] [Google Scholar]
- McCullough DA, et al. Research in thermal biology: burning questions for coldwater stream fishes. Rev Fish Sci. 2009;17:90–115. doi: 10.1080/10641260802590152. [DOI] [Google Scholar]
- Minder JR, Mote PW, Lundquist JD. Surface temperature lapse rates over complex terrain: Lessons from the Cascade Mountains. Journal of Geophysical Research. 2010;115 doi: 10.1029/2009jd013493. [DOI] [Google Scholar]
- Monk WA, Wilbur NM, Curry RA, Gagnon R, Faux RN. Linking landscape variables to cold water refugia in rivers. J Environ Manage. 2013;118:170–176. doi: 10.1016/j.jenvman.2012.12.024. [DOI] [PubMed] [Google Scholar]
- Morelli TL, et al. Managing Climate Change Refugia for Climate Adaptation. PLoS ONE. 2016;11:e0159909. doi: 10.1371/journal.pone.0159909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz NJ, Farrell AP, Heath JW, Neff BD. Adaptive potential of a Pacific salmon challenged by climate change. Nature Climate Change. 2014;5:163–166. doi: 10.1038/nclimate2473. [DOI] [Google Scholar]
- NMFS (National Marine Fisheries Service) Jeopardy and Destruction or Adverse Modification of Critical Habitat: Endangered Species Act Biological Opinion for “The Environmental Protection Agency’s Proposal Approval of Certain Oregon Water Quality Standards Including Temperature and Intragravel Dissolved Oxygen”. West Coast Region, Portland, Oregon: 2015. (NMFS No. WCR-2013-76). https://pcts.nmfs.noaa.gov/pcts-web/dispatcher/trackable/WCR-2013-76?overrideUserGroup=PUBLIC&referer=%2fpcts-web%2fpublicAdvancedQuery.pcts%3fsearchAction%3dSESSION_SEARCH. [Google Scholar]
- Orr HG, et al. Detecting changing river temperatures in England and Wales. Hydrological Processes. 2015;29:752–766. doi: 10.1002/hyp.10181. [DOI] [Google Scholar]
- Palmer J, et al. EPA Region 10 Guidance for Pacific Northwest state and tribal temperature water quality standards. Region 10 Office of Water, Seattle, WA: 2003. (EPA 910-B-03-002). http://www.epa.gov/region10/pdf/water/final_temperature_guidance_2003.pdf. [Google Scholar]
- Penaluna BE, et al. Local variability mediates vulnerability of trout populations to land use and climate change. PLoS ONE. 2015;10:e0135334. doi: 10.1371/journal.pone.0135334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson EE, et al. Modelling dendritic ecological networks in space: an integrated network perspective. Ecol Lett. 2013;16:707–719. doi: 10.1111/ele.12084. [DOI] [PubMed] [Google Scholar]
- Petty JT, Hansbarger JL, Huntsman BM, Mazik PM. Brook trout movement in response to temperature, flow, and thermal refugia within a complex Appalachian riverscape. Trans Am Fish Soc. 2012;141:1060–1073. doi: 10.1080/00028487.2012.681102. [DOI] [Google Scholar]
- Poole GC, et al. The case for regime-based water quality standards. Bioscience. 2004;54:155–161. [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. URL www.R-project.org/ [Google Scholar]
- Richter A, Kolmes SA. Maximum temperature limits for Chinook, coho, and chum salmon, and steelhead trout in the Pacific Northwest. Rev Fish Sci. 2005;13:23–49. doi: 10.1080/10641260590885861. [DOI] [Google Scholar]
- Schlosser IJ. Critical landscape attributes that influence fish population-dynamics in headwater streams. Hydrobiologia. 1995;303:71–81. doi: 10.1007/978-94-017-3360-1_7. [DOI] [Google Scholar]
- Simberloff D, Abele LG. Refuge design and island biogeographic theory: effects of fragmentation. The American Naturalist. 1982;120:41–50. doi: 10.1086/283968. [DOI] [Google Scholar]
- Snyder CD, Hitt NP, Young JA. Accounting for groundwater in stream fish thermal habitat responses to climate change. Ecol Appl. 2015;25:1397–1419. doi: 10.1890/14-1354.1. [DOI] [PubMed] [Google Scholar]
- Steel EA, Beechie TJ, Torgersen CE, Fullerton AH. Envisioning, quantifying, and managing thermal regimes on river networks. Bioscience. 2017;67:506–522. [Google Scholar]
- Steel EA, Sowder C, Peterson EE. Spatial and temporal variation of water temperature regimes on the Snoqualmie River network. J Am Water Resour Assoc. 2016;52:769–787. doi: 10.1111/1752-1688.12423. [DOI] [Google Scholar]
- Steel EA, Tillotson A, Larsen DA, Fullerton AH, Denton KP, Beckman BR. Beyond the mean: The role of variability in predicting ecological effects of stream temperature on salmon. Ecosphere. 2012;3:art104. doi: 10.1890/es12-00255.1. [DOI] [Google Scholar]
- Sutton R, Soto T. Juvenile coho salmon behavioural characteristics in Klamath river summer thermal refugia. River Res Appl. 2012;28:338–346. doi: 10.1002/rra.1459. [DOI] [Google Scholar]
- Sutton RJ, Deas ML, Tanaka SK, Soto T, Corum RA. Salmonid observations at a Klamath River thermal refuge under various hydrological and meteorological conditions. River Res Appl. 2007;23:775–785. doi: 10.1002/Rra.1026. [DOI] [Google Scholar]
- Tague C, Farrell M, Grant G, Lewis S, Rey S. Hydrogeologic controls on summer stream temperatures in the McKenzie River basin, Oregon. Hydrological Processes. 2007;21:3288–3300. [Google Scholar]
- Tague C, Grant G, Farrell M, Choate J, Jefferson A. Deep groundwater mediates streamflow response to climate warming in the Oregon Cascades. Clim Change. 2008;86:189–210. doi: 10.1007/s10584-007-9294-8. [DOI] [Google Scholar]
- Taylor KE, Stouffer RJ, Meehl GA. An Overview of CMIP5 and the Experiment Design Bulletin of the American. Meteorological Society. 2012;93:485–498. doi: 10.1175/bams-d-11-00094.1. [DOI] [Google Scholar]
- Tohver IM, Hamlet AF, Lee S-Y. Impacts of 21st-Century climate change on hydrologic extremes in the Pacific Northwest region of North America JAWRA. Journal of the American Water Resources Association. 2014;50:1461–1476. doi: 10.1111/jawr.12199. [DOI] [Google Scholar]
- Torgersen CE, Ebersole JL, Keenan DM. Primer for identifying cold-water refuges to protect and restore thermal diversity in riverine landscapes. Region 10, US. Environmental Protection Agency; Seattle, WA: 2012. (Agreement No. DW-14-95755001-0). [Google Scholar]
- Torgersen CE, Price DM, Li HW, McIntosh BA. Multiscale thermal refugia and stream habitat associations of Chinook salmon in northeastern. Oregon Ecol Appl. 1999;9:301–319. [Google Scholar]
- Wade AA, et al. Steelhead vulnerability to climate change in the Pacific Northwest. J Appl Ecol. 2013;50:1093–1104. doi: 10.1111/1365-2664.12137. [DOI] [Google Scholar]
- Wawrzyniak V, Piégay H, Allemand P, Vaudor L, Goma R, Grandjean P. Effects of geomorphology and groundwater level on the spatio-temporal variability of riverine cold water patches assessed using thermal infrared (TIR) remote sensing. Remote Sens Environ. 2016;175:337–348. doi: 10.1016/j.rse.2015.12.050. [DOI] [Google Scholar]
- Welty EZ. Linbin: binning and plotting of linearly referenced data. R package version 0.1.0. 2015 http://cran.r-project.org/web/packages/linbin/
- Whitney JE, et al. Physiological basis of climate change impacts on North American inland fishes. Fisheries. 2016;41:332–345. doi: 10.1080/03632415.2016.1186656. [DOI] [Google Scholar]
- Woolnough DA, Downing JA, Newton TJ. Fish movement and habitat use depends on water body size and shape. Ecol Freshw Fish. 2009;18:83–91. doi: 10.1111/j.1600-0633.2008.00326.x. [DOI] [Google Scholar]
- Wu H, Kimball JS, Elsner MM, Mantua N, Adler RF, Stanford J. Projected climate change impacts on the hydrology and temperature of Pacific Northwest rivers. Wat Res Research. 2012;48:W11530. doi: 10.1029/2012wr012082. [DOI] [Google Scholar]
- Zeug SC, Albertson LK, Lenihan H, Hardy J, Cardinale B. Predictors of Chinook salmon extirpation in California’s Central Valley. Fish Manag Ecol. 2011;18:61–71. doi: 10.1111/j.1365-2400.2010.00769.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


