Version Changes
Revised. Amendments from Version 2
Minute changes have been made in the Results and Discussion sections as per referee input in version 2.
Abstract
Background: Ralstonia spp. is a major pathogenic microbe for tomato, which invades the roots of diverse plant hosts and colonizes xylem vessels causing wilt, especially in tropical, subtropical and warm-temperate regions. Ralstonia spp. produces several virulence factors helping it to invade the plant’s natural defense mechanism. Native isolates of Trichoderma spp., Pseudomonas fluorescens and Bacillus subtilis can be used as biocontrol agents to control the bacterial wilt and combined application of these beneficial microbes can give better results.
Methods: Bacterial wilt infection in the field was identified by field experts and the infected plant part was used to isolate Ralstonia spp. in CPG media and was positively identified. Subsequently, the efficacy of the biocontrol agents was tested and documented using agar well diffusion technique and digital microscopy. 2ml of the microbial concentrate (10 9 cells/ml) was mixed in one liter of water and was applied in the plant root at the rate of 100 ml per plant as a treatment method.
Results: It was observed that the isolated Trichoderma spp. AA2 and Pseudomonas fluorescens PFS were most potent in inhibiting the growth of Ralstonia spp. , showing ZOI 20.67 mm and 22.33 mm, respectively. Digital microscopy showed distinct inhibitory effect on the growth and survival of Ralstonia spp . The results from the field data indicated that Trichoderma spp. and Pseudomonas fluorescens alone were able to prevent 92% and 96% of the infection and combination of both were more effective, preventing 97% of infection. Chemical control methods prevented 94% of infection. Bacillus subtilis could only prevent 84 % of the infection.
Conclusions: Antagonistic effect against Ralstonia spp. shown by native isolates of Trichoderma spp. and P. fluorescens manifested the promising potential as biocontrol agents. Combined application gave better results. Results shown by Bacillus subtilis were not significant.
Keywords: Trichoderma, Pseudomonas fluorescence, Bacillus subtilis, Ralstonia solanacearum, biocontrol agent, bio-efficacy, bacterial wilt, tomato
Author endorsement
Dr. Sushil Thapa confirms that the author has an appropriate level of expertise to conduct this research, and confirms that the submission is of an acceptable scientific standard. Dr. Sushil Thapa declares he has no competing interests. Affiliation: Texas A&M AgriLife Research, Amarillo, TX 79106, USA.
Introduction
Bacterial wilt caused by Ralstonia spp. is a major pathogenic microbe for tomatoes ( Solanum lycopersicum L). It invades the roots of diverse plant hosts from the soil and aggressively colonizes the xylem vessels, causing bacterial wilt disease 1– 3. It is a devastating plant disease most commonly found in tropical, subtropical and warm-temperate regions 4, 5. Ralstonia spp. produces several known virulence factors, including extracellular polysaccharide (EPS), and a combination of plant cell wall-degrading enzymes, such as endoglucanase (EG) and polygalacturonase (PG). Mutants lacking EPS and EG shows reduced virulence 6– 8. The major virulence factors for this pathogen are plant cell wall-degrading polygalacturonases (PGs) 9.
Various biocontrol agents are used to control the bacterial wilt caused by Ralstonia spp. Trichoderma harzianum 10, 11, Trichoderma viride 12, Trichoderma asperellum 13, Trichoderma virens 14, Pseudomonas fluorescens 15, 16 and Bacillus subtilis 17 are used as biocontrol agents to control bacterial wilt. Combination treatment methods using two or more of these agents are more effective in managing the disease than treatment using a single biocontrol agent 10, 18, 19. Chemical bactericides and fungicides induce resistance in pathogens during long-term use, which ultimately makes the pathogen tolerant to these chemical applications 20– 23. Hence, there should be a focus on the use of biological methods to control plant disease.
This study focuses on evaluating the efficacy of different native isolates of Trichoderma species, B. subtilis and P. fluorescens against bacterial wilt disease caused by the pathogen Ralstonia spp. in the tomato plant. The study hypothesizes that the native isolates of Trichoderma spp., B. subtilis and P. fluorescens can be used as bioantagonistic agents to control bacterial wilt ( Ralstonia spp.) of tomato. This study tries to establish the hypothesis by the microscopic examinations, agar well diffusion technique and field trials in infected tomato plots, by calculating their efficacy and comparing them with chemical methods of treatment.
Methods
Selection of the study plot
The sample collection and field trials were done at Agro Narayani Farm, Sukranagar, Chitwan District, Nepal (Latitude: 27.582016 and Longitude: 84.272259) where the bacterial wilt infection was recorded in the previous harvest (done 3 months before the test). Also, Ralstonia spp. were observed in the Casamino acid-Peptone-Glucose (CPG) Agar plates from the soil samples. The tests in the field were conducted from 11 March 2017 to 8 July 2017 for 120 days in new transplants. At the time of transplant, compost fertilizer at the rate 2.94 kg/m 2, urea at the rate of 23.59 gm/m 2, potash at the rate of 29.49 gm/m 2, DAP 19.66 gm/m 2, borax at the rate 1.97 gm/m 2 and zinc at the rate 1.97 gm/m 2 were applied. At 45 days and 90 days of transplant NPK 20:20:20 was applied at the rate 9.83/m 2. The spacing of the plant was 50 cm in double row system of 50 × 50 cm. The plot size was 50 m 2 and total of 8 plots were used having 100 plants per plot. Weather data were also collected from online resources as a reference.
Identification of bacterial wilt infection in the field
Physical symptoms, such as wilting of young leaves, discolored tissue at the dissected part of the stem base, and white, slimy ooze when the dissected part of the plant was kept in the glass of water, were used for the identification of infected plants 24.
Isolation and identification of Ralstonia spp.
Bacterial wilt infection in tomato plants ( Solanum lycopersicum L. var. Manisha) grown in Chitwan, Nepal, was positively identified by S. Yendyo of Kishan Call Center (KCC). Six whole plants were brought to the Quality Control laboratory of Agricare Nepal Pvt. Ltd. in a sterile bag for the isolation of the pathogen.
Ralstonia spp. was isolated from dissected sections of the infected tomato plants on Casamino acid-Peptone-Glucose (CPG) Agar (casein hydrolysate 1 g/l, peptone 10 g/l, glucose 5g/l, agar 15g/l) which is preferred media for isolation of Ralstonia spp. The stems of the infected plant were washed three times with autoclaved distilled water and then blot dried. After drying the stem were washed with 80% ethanol solution, then 1% sodium hypochlorite (NaOCl) solution was applied for 2 minutes. Final washing was done with autoclaved distilled water three times. The xylem of 2–3 cm from the stem was dissected and the sap was rubbed in CPG medium and inoculated for 48h at 28°C. Identification was done on the basis of the morphology of the colony on CPG medium, Gram staining and microscopic examination 25, 26.
Isolation of antagonists
A total of 13 strains were used in this study (see Table 1). Six strains of Trichoderma spp., two strains of Pseudomonas fluorescens and one strain of Bacillus subtilis (GenBank Accession No. MG952584) were isolated from soils and root soils collected from different sites in Nepal. ATCC 13525 strain of P. fluorescens from Microbiologics, St. Cloud, MN 56303, France was used as the reference for isolated Pseudomonas species. Trichoderma harzianum, Trichoderma virens and Trichoderma asperellum strains were provided by Tamil Nadu Agricultural University (TNAU), Coimbatore, Andhra Pradesh, India, and were used as reference species for isolated Trichoderma spp for two purposes. First, the reference species were used to identify the isolated species through morphological and microscopic analysis and second, the reference species were used to compare the antagonistic effects exhibited by the native isolates. All the species were collected from the tropical region except Bacillus subtilis which was collected from mid hill regions of Nepal. The reason behind this was that the site from where the samples were collected was unaffected by the wilt disease compared to nearby sites where the bacterial wilt was severely present. The testing of the soil samples from the unaffected site revealed the substantial presence of Bacillus subtilis which may have induced systemic resistance in the plants.
Table 1. Biocontrol agents used to analyze bio-efficacy against Ralstonia spp.
This includes the shortcode given to the isolates for identification in this study.
| Name of strains and source | Source | Code
given |
|---|---|---|
| Trichoderma spp. | Parthenium hysterophorus L. rhizoplane soil | AA2 |
| Trichoderma spp. | Cannabis sativa L. rhizoplane soil | AG3 |
| Trichoderma spp. | Solanum viarum Dunal rhizoplane soil | AKD |
| Trichoderma spp. | Agricare Nepal Pvt. Ltd. top soil | A5 |
| Trichoderma spp. | Brassica juncea L. spp. rhizoplane soil | A9 |
| Trichoderma spp. | Chitwan National Park top soil | A10 |
| Trichoderma harzianum | TNAU, India | ATH |
| Trichoderma virens | TNAU, India | ATV |
| Trichoderma asperellum | TNAU, India | ATA |
| Bacillus subtilis ( MG952584) | Bhaktapur top soil | BS |
| Pseudomonas fluorescens | Parthenium hysterophorus L. rhizoplane soil | PFS |
| Pseudomonas fluorescens | Chenopodium album L. rhizoplane soil | PFB |
| Pseudomonas fluorescens | ATCC 13525 | PFA |
Trichoderma spp. were isolated by serial dilution method using TSM agar plate (K 2HPO 4: 0.9 g/l, MgSO 4: 0.2 g/l, KCl: 0.15 g/l, NH 4Cl: 1.05 g/l, Glucose 3 g/l, Rose Bengal: 0.15 g/l, agar 20 g/l, streptomycin: 100 mg/l, tetracycline: 50 mg/l) 27. 1 gm of each soil samples were suspended in 9 ml of sterile distilled water and vortexed (Accumax India, New Delhi-110058, India) for 5 min. The soil suspension was then serially diluted to 10 -3 and 10 -4. Pour plate technique was used by mixing 1 ml of the diluted soil suspension in 3 TSM agar plates for each sample and incubated at 25°C for 5 days. The strains were purified on TSM agar plates using sub-culture technique.
Pseudomonas fluorescens were isolated by serial dilution method using King’s B agar (Peptone: 20g/l, K 2HPO 4: 1.5 g/l, MgSO 4: 1.5 g/l, glycerol: 10 ml/l, agar: 20g/l) 28. 1 gm of each soil samples were suspended in 9 ml of sterile distilled water and vortexed (Accumax India, New Delhi-110058, India) for 5 min. The soil suspension was then serially diluted to 10 -6. Pour plate technique was used by mixing 1 ml of the diluted soil suspension in 3 King’s B agar plates for each sample and incubated at 27°C for 48 h and the fluorescence was observed in UV light. The fluorescent strains were purified on King’s B agar plates using sub-culture technique.
Bacillus subtilis was isolated by serial dilution method using Nutrient Agar (Peptic digest of animal tissue: 5 g/l, NaCl: 5 g/l, beef extract: 1.5 g/l, yeast extract: 1.5 g/l, agar: 20g/l) 29. 1 gm of each soil samples were suspended in 9 ml of sterile distilled water and vortexed (Accumax India, New Delhi-110058, India) for 5 min. The soil suspension was then serially diluted to 10 -6. Pour plate technique was used by mixing 1 ml of the diluted soil suspension in 3 NA plates for each sample and incubated at 27°C for 48 h. The strains were purified on NA agar plates by using sub-culture technique.
Evaluating the efficacy of antagonists against Ralstonia spp.
All thirteen isolates were screened against Ralstonia spp . by agar well diffusion technique 30. Ralstonia spp . on CPG agar plates were transferred to the nutrient broth and shaken in a rotary shaker (Talboys, Henry Troemner, LLC, USA) at 100 rpm at 27°C for 24 h. Similarly, the TSM, King’ B and NB were prepared for all Trichoderma spp., P. fluorescens and B. subtilis, respectively, and incubated for 7 days, 48 h and 48h, respectively. After incubation of the antagonists, 5 ml of broth suspension were centrifuged at 5000 rpm for 5 min and the supernatant was stored at 4°C for further procedure. Then, Ralstonia spp . suspension of 10 8 cells/ml was prepared as per McFarland 0.5 turbidity method 31 and was swabbed on NA plates. Holes of 5 mm were punched into the agar plate and 40 µl of the supernatant prepared were added separately and the plates were incubated at 27°C for 48 h. Inhibition of Ralstonia spp . growth was assessed by measuring the radius in mm of the zone of inhibition (ZOI) after incubation.
For microscopic visualization of the inhibition, CPG agar plates were prepared to provide the most favorable growth to Ralstonia spp. , and the respective 5 mm mycelial discs of Trichoderma species were added in the center of the plate after cotton swabbing from the CPG broth of Ralstonia spp. For B. subtilis and P. fluorescens, the line was streaked parallel to the streak of Ralstonia spp. in two different CPG agar plates using dual culture technique. After 72 hr of incubation, live microscopic examination on the culture plate was done using a digital microscope (Olympus CX-43, Tokyo, Japan). Images were captured to visualize the interaction of the individual strains of biocontrol agents with Ralstonia spp .
Effect of sucrose on the population of biocontrol agents
There is common practice in Nepal of keeping bio-based products in 5–10% (w/v) sucrose solution for 2–4 h before application to the plant. Hence, to evaluate the effect of 5% (w/v) sucrose solution on the cell number (growth) of the biocontrol agents, 1 ml of concentrate containing 1 × 10 -9 cells ml -1 was kept in 5% sucrose solution, made using autoclaved distilled water. Cell count was taken using a Hemocytometer (Reichert, Buffallo, NY, USA) with trypan blue at 1 h, 2 h, 3 h and 24 h to observe the effect on the microbial population.
Evaluating the effects of biocontrol agents in the field
For the field study, the concentrates containing 10 9 cells/ml of the respective biocontrol agent were used. The densities of the cells were determined using Hemocytometer (Reichert, Buffallo, NY, USA) 32. The TSM broth of all six isolated native Trichoderma species viz. AA2, AG3, AKD, A5, A9 and A10 were mixed in equal proportion to prepare 1 liter of concentrate containing 10 9 cells/ml. Similarly, two native P. fluorescens species viz. PFB and PFS were mixed in an equal proportion to prepare 1 liter of concentrate containing 10 9 cells/ml. Concentrate of native B. subtilis viz. BS containing 10 9 cells/ml was used to analyze the effect of B. subtilis as a possible biocontrol agent.
Before the application in the tomato plots at Agro Narayani Farm, the prepared concentrates of biocontrol agents were taken to the field and were further diluted at the rate of 2ml/l of tap water containing 5% (w/v) sucrose. After 2 h of incubation in 5% sucrose water, the diluted solutions were applied in the root of tomato plants at the rate of 100 ml per plant. The processes of applications were repeated every 7 days for 8 weeks (total of 8 applications) by preparing fresh dilutions in 5% sucrose solutions 2 h prior to applications. Effects after the 8 weeks of continuous application were measured in the field by identifying the number of plants that underwent recovery after treatment. 6 plots were treated with the biocontrol agent and 2 plots were used as controls. Chemical treatment was done in one plot (positive control plot) using the combination of Agricin (9% Streptomycin Sulphate and 1% Tetracycline Hydrochloride) at the rate of 100 ml of 0.1% (w/v) solution per plant from Agricare Nepal Pvt. Ltd., Nepal and Bavistin (50% carbendazim) at the rate of 0.2% (w/v) solution per plant from Crystal Crop Protection Pvt. Ltd., India. For negative control, no treatment methods were selected in one plot.
The treatment plots were designed such that the effects of the individual biocontrol agent and effects of combination treatment can be studied ( Table 2).
Table 2. Design of treatment plot to study the effect of different treatment methods on controlling the bacterial disease.
One plot comprised of 100 tomato plants and 8 plots in total were studied; area of 50 m 2 per plot.
| Plot (100
plants/Plot) |
Treatment |
|---|---|
| 1 | BS ( Bacillus subtilis) |
| 2 | TV ( Trichoderma spp. mix) |
| 3 | G ( Pseudomonas fluorescens) |
| 4 | BS+TV ( Bacillus subtilis and Trichoderma spp. Mix) |
| 5 | TV+G ( Trichoderma spp. Mix and Pseudomonas fluorescens) |
| 6 | TV+G+BS ( Bacillus subtilis, Trichoderma spp. Mix and Pseudomonas fluorescens) |
| 7 | No application |
| 8 | Chemical application (Agricin: 9% Streptomycin Sulphate and 1% Tetracycline
Hydrochloride + Bavistin: 50% carbendazim) |
Statistical analysis
Statistical analysis of the data was done using IBM SPSS Statistics (ver 23) and figures and data were made through Microsoft Excel 2007 and Microsoft Word 2007.
Results
Weather data
Weather data of Chitwan District for temperature, humidity and rainfall were collected from www.worldweatheronline.com from March 2017 to July 2017 (see Table 3).
Table 3. Data on average weather over the study period.
| Month | Maximum
temperature °C |
Minimum
temperature °C |
Average
temperature °C |
Rainfall
(mm) |
Cloud
(%) |
Humidity
(%) |
|---|---|---|---|---|---|---|
| March | 26 | 11 | 21 | 42.3 | 16 | 47 |
| April | 32 | 17 | 27 | 184 | 8 | 43 |
| May | 32 | 19 | 28 | 319.5 | 13 | 54 |
| Jun | 32 | 21 | 28 | 536 | 24 | 71 |
| July | 30 | 21 | 27 | 791.8 | 49 | 84 |
Source: www.worldweatheronline.com
The bacterial wilt outbreak was reported in the late May, 2017, when the temperature and humidity level increased. Higher temperature and moisture favors the growth of Ralstonia spp. 33
Identification of bacterial wilt in the field
From the field examination of the tomato plants, observation revealed that the leaves were flaccid, adventitious roots started to appear on the stem and ooze appeared after dipping the stem in water. Also, field experts from KCC confirmed the presence of bacterial wilt infection, due to their years of experience in plant disease diagnosis.
Isolation and identification of Ralstonia spp.
Infected plant saps from six xylems showed similar bacterial colonies on CPG medium. All the colonies were similar to a virulent type, as the appearance was white or cream-colored, irregularly-round, fluidal, and opaque on CPG medium 34, 35. Gram staining and observation using a microscope showed that the bacteria were gram negative, rod-shaped and non-spore forming, which further confirmed that the bacteria was Ralstonia spp.
Evaluating the efficacy of antagonists against Ralstonia spp.
Strains tested showed antagonistic effect against Ralstonia spp., with inhibition zone radii ranging from 13 to 21.33 mm ( Table 4). P. fluorescens PFS isolated from Parthenium hysterophorus L. rhizoplane soil was most potent compared to other P. fluorescens strains. Trichoderma virens ATV and Trichoderma harzianum ATH provided by TNAU were the least and most potent species. Among six natively isolated Trichoderma sp., AA2 isolated from Parthenium hysterophorus L. rhizoplane soil was most potent and AKD and A9 were least potent. However, the activities of native Trichoderma spp. were satisfactory in the term of inhibition zone shown. Bacillus subtilis BS isolated from Bhaktapur top soil did not show satisfactory inhibition activity. The complete photographs showing zone of inhibitions can be retrieved from data availability section.
Table 4. Inhibition zone made by the isolates used as biocontrol agents against Ralstonia spp. using agar well diffusion technique.
All the data are generated using three replications. Values are means (±SE) zone of inhibition (ZOI) in mm against Ralstonia spp. (n=3, P <0.05). S30 denotes streptomycin sulphate used at the dilution of 30 mcg. 5 mm diameter of punch hole is included in the data. Code is in reference to Table 1.
| Code | ZOI (in mm) |
|---|---|
| AA2 | 20.67 ± 0.88 |
| AG3 | 18.33 ± 0.33 |
| AKD | 17.00 ± 0.58 |
| A5 | 19.67 ± 0.67 |
| A9 | 17.00 ± 0.58 |
| A10 | 17.33 ± 0.66 |
| ATH | 21.33 ± 0.88 |
| ATV | 16.33 ± 0.66 |
| ATA | 18.00 ± 0.58 |
| BS | 13.00 ± 1.53 |
| PFS | 22.33 ± 3.38 |
| PFB | 16.33 ± 0.33 |
| PFA | 21.00 ± 0.58 |
| S30 | 37.67 ± 1.33 |
The effect of biocontrol agents against Ralstonia spp. was analyzed at a microscopic level in dark phase using image analyzer (Olympus CX-43, Tokyo, Japan). Figure 1– Figure 3 show the distinct inhibitory effect on the growth and survival of Ralstonia spp. caused by different biocontrol agents. From the figures, it can be seen that the most of the pathogenic cells ( Ralstonia spp.) were either killed or growth was retarded or limited in or towards the region of growth of antagonists as compared to the region away from the growth of antagonists.
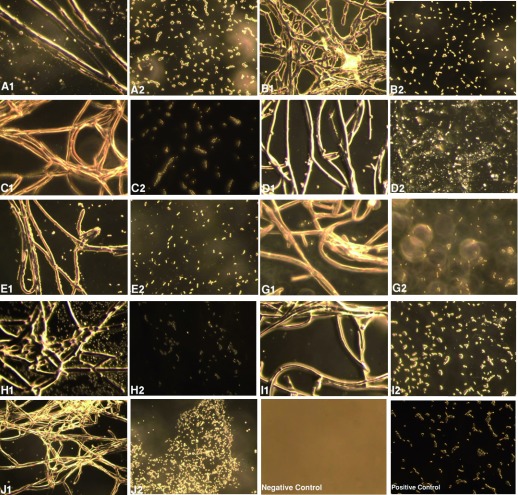
Figure 1. Digital images (400X) of microscopic analysis in dark phase representing the interactions of different Trichoderma species against Ralstonia spp.
A1–J1 shows strains AA2, AG3, AKD, A5, A9, A10, ATA, ATH and ATV, respectively (see Table 1), growing on Ralstonia spp., which was cotton swabbed onto CPG agar plates. A2–J2 represents growth of Ralstonia spp . 4 cm away from the growth of Trichoderma spp., viz., A2, AG3, AKD, A5, A9, A10, ATA, ATH and ATV, respectively, on the plate. Negative control (i.e., blank plate without any swabbing) and positive controls (i.e. Ralstonia spp. without Trichoderma species) are also included.
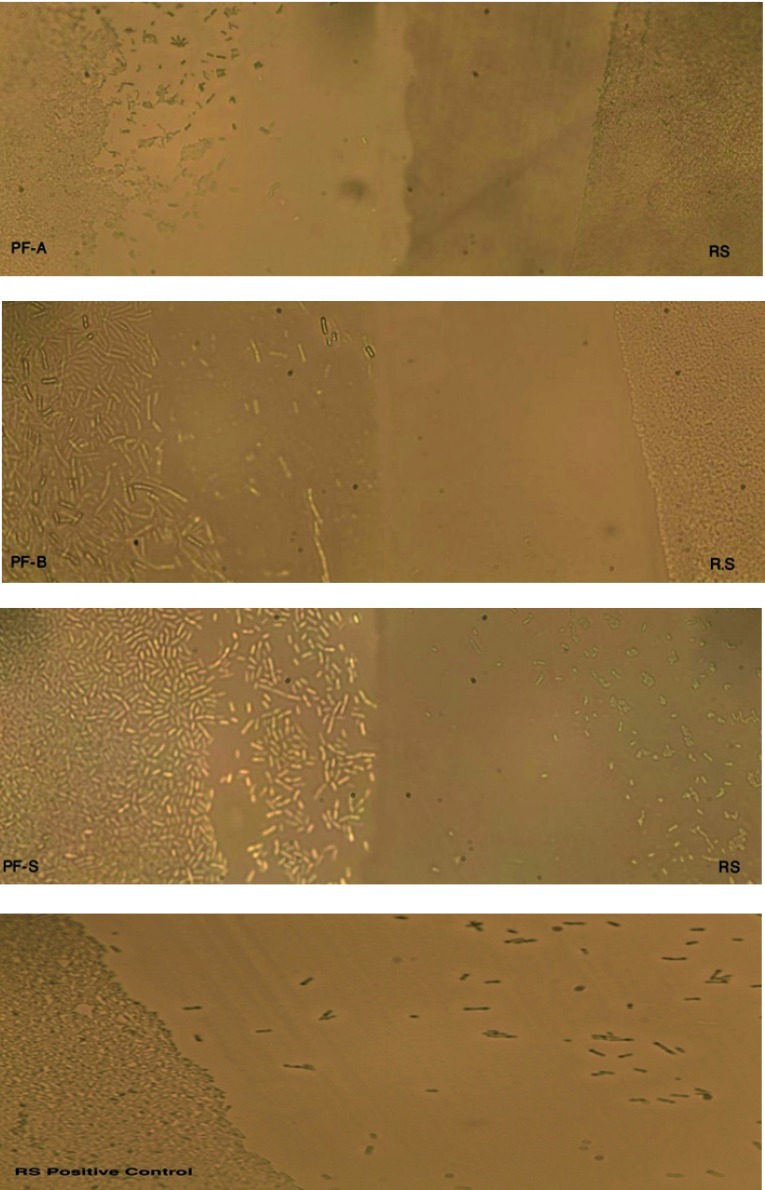
Figure 2. Digital images (400X) of microscopic analysis in bright field representing the interactions of Pseudomonas fluorescens species against Ralstonia spp.
PFA, PFB and PFS represent P. fluorescens (PF) species (see Table 1), and RS represents Ralstonia spp . Both PF and RS were streaked near to each other to see the interaction between the two species.
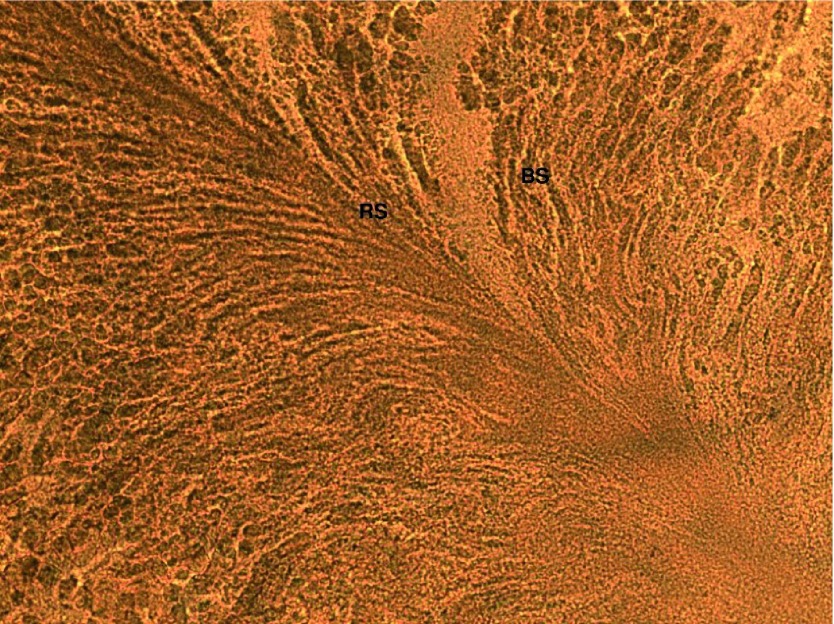
Figure 3. Digital image (400X) of microscopic analysis in bright field representing the interaction of Bacillus subtilis against Ralstonia spp.
BS represents Bacillus subtilis species (see Table 1), and RS represents Ralstonia spp . Both BS and RS were streaked near to each other to see the interaction between two species. BS has completely overgrown the RS streak on the CPG agar plate, which suggests that there has been an interaction between RS and BS and BS is dominant over RS.
The digital images from Figure 1 reveal that the population of Ralstonia spp. is significantly less and most of the cells are dead in the region of growth of samples treated with Trichoderma spp., compared to the region 4 cm far from the growth of Trichoderma spp as the Ralstonia is a rod-shaped bacteria but near the growth region of Trichoderma, most of the bacterial cells clearly seems to be round and distorted in shape with much lower population density than the region far away This indicates the fact that the Ralstonia cells that got inoculated in the plate during the cotton swab were unable to multiply and grow in the zone where Trichoderma was growing.
The images from Figure 2 reveal that PF tended to grow on the side of RS, whereas RS tended to restrict the growth towards the PF species. RS positive control (without PF streak nearby) tended to spread, which confirms the spreading pattern of RS. The microscopic study was to verify a fact that the incompatible species does not grow towards each other and the growth of dominant microbes always surpasses the growth of other recessive microbes. The same phenomenon was observed in the microscopic analysis as Ralstonia spp. formed a clear boundary or showed restricted spreading in the region of the streak as compared to the Positive control but the streak of Pseudomonas fluorescence was easily growing towards the Ralstonia spp.
Effect of sucrose on the population of biocontrol agents
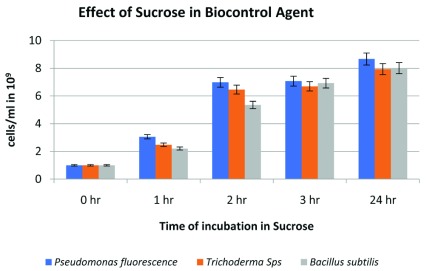
The effect of sucrose (5% w/v) in the concentrate mixture of individual biocontrol agents was analyzed ( Figure 4), which showed that there was a profound increase in the number of cells of biocontrol agents after 2h of incubation compared with the initial population. The cell count between 2h and 3 h of incubation was not significant as compared with 2 h and 24 h of incubation. Thus, 2 h of incubation in sucrose solution can be considered as optimal time, as lengthier time can result in the growth of contaminant in the solution whilst using tap water in the field.
Figure 4. Effect of sucrose on the growth of Pseudomonas fluorescens, Trichoderma spp. and Bacillus subtilis.
An increase in a number of cells of biocontrol agents was seen over time when these agents were kept in 5% (w/v) sucrose solution (n=3). The error bar represents the 5% error in independently performed experiments. The complete data are available in data availability section of this manuscript.
Evaluating the effects of biocontrol agents in the field
Before applications, 2ml of respective biocontrol agents of (10 9 cells/ml) were incubated in 5% (w/v) of sucrose solution and incubated for 2 hr. The prepared dilution was thus applied at the rate of 100 ml per plant in the root region every week. 8 applications were done over 8 weeks. 8 plots (100 plants/plot) were selected, out of which one plot was used as positive control/chemical treatment plot (Agricin+Bavistin) and one plot as negative control/zero treatment plot (no treatment given).
The results are displayed in Table 5 and show that the application inhibited the bacterial wilt infection ( Ralstonia spp.) in tomato plants by and the highest rate of plant recovered was 97% from treatment using antagonists, which was comparable with the plant recovery of 94% using the chemical treatment (Agricin and Bavistin). Only 37% of plants were recovered in the plot where no treatment methods were applied. Field Images ( Figure 5) show a clear visualization of growth of plants and severity of infection in treated and untreated plot. The complete photographs of the field trial can be retrieved from the data availability section of this manuscript.
Table 5. Effect of applying biocontrol agents to tomato plants infected with bacterial wilt.
There was a significant recovery of plants using a mixture of Trichoderma species and Pseudomonas fluorescens, and the recovery rate was higher than that of chemical treatment. Bacillus subtilis did not show significant recovery rate.
| S.N. | Treatment plot (100 plants
per plot) |
No. of plants
recovered |
No. of plants
infected |
% recovery |
|---|---|---|---|---|
| 1 | BS | 84 | 16 | 84 |
| 2 | TV | 92 | 8 | 92 |
| 3 | G | 96 | 4 | 96 |
| 4 | BS+TV | 95 | 5 | 95 |
| 5 | TV+G | 97 | 3 | 97 |
| 6 | TV+G+BS | 97 | 3 | 97 |
| 7 | Positive control (chemical
treatment) |
94 | 6 | 94 |
| 8 | Negative Control (w/o
treatment) |
37 | 63 | 37 |
Figure 5. Differences in the field between treated and untreated plots.
Field images clearly reveal that the treatment with biocontrol agents has helped to eliminate the bacterial wilt disease in the field after 8 weeks of application. ( A) shows the growth and vigor of plants treated with biocontrol agents; ( B) shows growth and severity of infection occurred in the untreated plot.
Discussion
This research covers the results of the effectiveness of native biocontrol agents in both laboratory and field settings. This research also provides the application strategies of biocontrol agents at the field level. Also, from literature reviews 10– 17, it has been shown that these biocontrol agents can be used to control various other bacterial and fungal diseases, such as Fusarium wilt beside bacterial wilt. Hence, application of these biocontrol agents can also help to prevent other diseases in various crops.
The present results, using microscopy, showed that different species of Trichoderma and Pseudomonas fluorescens clearly hinders the growth of Ralstonia spp., which causes bacterial wilt in tomato plants. Bacillus subtilis did not show a significant hindrance. Trichoderma spp. secret different compounds against bacteria and also produce various secondary metabolites that promote plant growth and yield 36– 38. P. fluorescens produces various compounds that suppress the growth of Ralstonia spp. and also induces systemic resistance in the plant 39– 41. B. subtilis is well known to induce systemic resistance in plants by secreting various kinds of lipopeptides and secondary metabolites, and this agent also improves plant growth 42– 44. Analysis of data from research, field trials and scientific journal reviews suggests that the application of B. subtilis may not immediately show results, but a continuous application of this strain in the agricultural field will slowly induce resistance of plants against pathogenic diseases 42. Thus, evidence from scientific research show that Trichoderma spp. and P. fluorescens are effective biocontrol agents against bacterial wilt in compared to Bacillus subtilis.
Pre-application of biocontrol agents can successfully prevent the disease attack 45, induce systemic disease resistance in plants and increase the yield from secondary metabolites secreted by the beneficial bacteria in the biocontrol agent. Thus, we recommend that farmers should continuously apply biocontrol agents in their field so that systemic resistance can get induced in plants and also the plants get protected from invasion of pathogens. Although the cost of production get increased but the farmers can sell their products by considering them as IPM (Integrated Pest Management) product or as an organic product, giving better monetary value for the farmers and health benefits for consumers.
From the results, 2 h incubation of the biocontrol agent in 5% (w/v) sucrose solution was judged as suitable practice being carried out in Nepal for application of biocontrol agents due to two facts. First, it was seen that number of cells of biocontrol agents were increased during the incubation period as observed in Figure 4. Second, the water farmers use for drip irrigation generally is unsterilized and comes from an underground source, which may promote the growth of contaminants in sucrose solution if kept for longer periods.
Trichoderma spp. and Pseudomonas fluorescens provide better results in controlling bacterial wilt in tomato. Bacillus subtilis did not perform well in the immediate control of disease. Data from Table 5 reveals that biocontrol agents can be used as the sole method to control bacterial wilt, and the use of chemical methods can be avoided in the field. Also, combination therapy using both Trichoderma spp. and P. fluorescens seems to be more effective than treatment using each individual biocontrol agent. A 97% control rate was achieved using combination treatment in the field.
For decades, microbiologists have identified pathogen through the use of phenotypic methods 46. We authors agree with the fact that 16S rRNA sequencing should be done to identify to the species level for all the microbes used in this manuscript but due to unavailability of the sequencing service in low-income country like Nepal and several unclear quarantine policies in Nepal, researchers in Nepal can only identify to the genus level of microbes by phenotypic methods. If the species has recently diverged then 16S rRNA sequencing alone will not be adequate to assign a species rather it needs additional analytical procedures like sequencing protein coding genes or the intergenic spacer region of the ribosomal gene complex 46.
Conclusions
In the present study, Trichoderma spp. and P. fluorescens seem to be the best biocontrol agents in controlling bacterial wilt induced by Ralstonia spp. The zone of inhibition shown by the various antagonists reveals that native isolates were successful in inhibiting the growth of Ralstonia spp . The digital microscopy also supports the antagonistic effects of the native isolates.
Also during field application, mixing with 5% (w/v) of sucrose solution and keeping it for 2 h seems to be an effective strategy in better management of bacterial wilt. The application strategies of biocontrol agents with the rate of 100 ml per plant per week successfully recovered the plants from the attack of the pathogen. However, the application rate and amount of biocontrol agents can be varied according to disease severity. Also, the application of the multiple numbers of biocontrol agents can be performed to achieve better results. Hence, native isolates of Trichoderma spp. and Pseudomonas fluorescens can be used as biocontrol agents to control the bacterial wilt and combined application of these beneficial microbes as bioantagonist can give better results in controlling bacterial wilt infection by Ralstonia spp. Results shown by Bacillus subtilis were not significant but the scientific researches shows that it can induce systemic resistance in plant with time
Data availability
The data referenced by this article are under copyright with the following copyright statement: Copyright: © 2018 Yendyo S et al.
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication). http://creativecommons.org/publicdomain/zero/1.0/
OSF: Raw values of zone of inhibition by antagonist against Ralstonia spp. ZOI is shown in mm and the data were used for statistical analysis. http://doi.org/10.17605/OSF.IO/9TQCE 47
OSF: Raw values of the 5% sucrose treatment. The average of these values in cells/ml was taken to create the data in the manuscript. http://doi.org/10.17605/OSF.IO/Q8FVU 48
Figshare: Images of the plates showing the zone of inhibition by different antagonists against Ralstonia spp. Clear zone of inhibition obtained by agar well diffusion technique indicates the bioefficacy of the selected bioantagonists against Ralstonia spp. https://doi.org/10.6084/m9.figshare.5562058.v3 49
Figshare: Raw digital images (400X) of microscopic analysis representing the interactions of different antagonist against Ralstonia spp. Collection of raw images obtained from digital microscopy in both dark field and bright field microscopy at 400 X zoom. https://doi.org/10.6084/m9.figshare.5561968.v2 50
Figshare: Field Images of plot design showing pictures of before the treatment and effects after the treatment. There is significant decrease in the occurrence of disease for the treated plots whereas the bacterial wilt has severely affected in the untreated plot. https://doi.org/10.6084/m9.figshare.5562373.v2 51
Data are available under the terms of the Creative Commons Attribution 4.0 license (CC-BY 4.0).
Acknowledgements
We would first like to thank Ms. Mona Sharma, Public Private Partnership Manager, Winrock International, Nepal and USAID for providing moral and financial support to conduct this research. We like to thank Prof. Dr. Sevugapperumal Nakkeeran, Department of Plant Pathology, Tamil Nadu Agricultural University (TNAU), Coimbatore, India for his supportive ideas in the research. We would also like to thank Ms. Santoshi Sharma, Ms. Shushilata Sapkota and Ms. Sweta Shrestha, interns at Agricare Nepal Pvt. Ltd., Nepal, Mr. Sarkal Jyakhwo, Field Technician at Kishan Call Center, Nepal and Mr. Aashish Khanal, Production Officer at Agricare Nepal Pvt. Ltd., Nepal for their supportive efforts during the project. Lastly, we would like to thank Mr. Deepak Gurung, the tomato farmer in Sukranagar, Chitwan, Nepal for reporting the problem of bacterial wilt and providing his field for the plot design and the study.
Funding Statement
This research was funded as a part of public private partnership activities of Agricare Nepal Pvt. Ltd with Winrock International Nepal for USAID’s KISAN Project, the Presidential Feed the Future Initiative to develop bio-products locally in Nepal. The grant number was AID-367-C-13-00004 with sub-awardee DUNS number 557770037.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 3; referees: 2 approved]
References
- 1. Tans-Kersten J, Huang H, Allen C: Ralstonia solanacearum needs motility for invasive virulence on tomato. J Bacteriol. 2001;183(12):3597–3605. 10.1128/JB.183.12.3597-3605.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pradhanang PM, Momol MT, Olson SM, et al. : Effects of plant essential oils on Ralstonia solanacearum population density and bacterial wilt incidence in tomato. Plant Dis. 2003;87(4):423–427. 10.1094/PDIS.2003.87.4.423 [DOI] [PubMed] [Google Scholar]
- 3. Anith KN, Momol MT, Kloepper JW, et al. : Efficacy of plant growth-promoting rhizobacteria, acibenzolar- S-methyl, and soil amendment for integrated management of bacterial wilt on tomato. Plant Dis. 2004;88(6):669–673. 10.1094/PDIS.2004.88.6.669 [DOI] [PubMed] [Google Scholar]
- 4. Buddenhagen IW, Kelman A: Biological and physiological aspects of bacterial wilt caused by Pseudomonas solanacearum. Annu Rev Phytopathol. 1964;2(1):203–230. 10.1146/annurev.py.02.090164.001223 [DOI] [Google Scholar]
- 5. Hayward AC: Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Annu Rev Phytopathol. 1991;29(1):65–87. 10.1146/annurev.py.29.090191.000433 [DOI] [PubMed] [Google Scholar]
- 6. Denny TP, Baek SR: Genetic Evidence that Extracellular Polysaccharide Is a Virulence Factor of Pseudomonas solanacearum. Mol Plant Microbe Interact. 1991;4(2):198–206. 10.1094/MPMI-4-198 [DOI] [Google Scholar]
- 7. Kao CC, Barlow E, Sequeira L: Extracellular polysaccharide is required for wild-type virulence of Pseudomonas solanacearum. J Bacteriol. 1992;174(3):1068–1071. 10.1128/jb.174.3.1068-1071.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roberts DP, Denny TP, Schell MA: Cloning of the egl gene of Pseudomonas solanacearum and analysis of its role in phytopathogenicity. J Bacteriol. 1988;170(4):1445–1451. 10.1128/jb.170.4.1445-1451.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang Q, Allen C: Polygalacturonases are required for rapid colonization and full virulence of Ralstonia solanacearum on tomato plants. Physiol Mol Plant Pathol. 2000;57(2):77–83. 10.1006/pmpp.2000.0283 [DOI] [Google Scholar]
- 10. Maketon M, Apisitsantikul J, Siriraweekul C: Greenhouse evaluation of Bacillus subtilis AP-01 and Trichoderma harzianum AP-001 in controlling tobacco diseases. Braz J Microbiol. 2008;39(2):296–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barua L, Bora BC: Comparative efficacy of Trichoderma harzianum and Pseudomonas fluorescens against Meloidogyne incognita and Ralstonia solanacearum complex in brinjal. Indian J Nematol. 2008;38(1):86–89. Reference Source [Google Scholar]
- 12. Singh R, Gangwar SP, Singh D, et al. : Medicinal plant Coleus forskohlii Briq.: disease and management. Med Plants. 2011;3(1):1–7. 10.5958/j.0975-4261.3.1.001 [DOI] [Google Scholar]
- 13. Srinivas C: Efficacy of Trichoderma asperellum against Ralstonia solanacearum under greenhouse conditions. Ann Plant Sci. 2013;2(09):342–350. Reference Source [Google Scholar]
- 14. Mathew SK, Manimala R, Surendra Gopal K, et al. : Effect of Microbial Antagonists on the Management of Bacterial Wilt in Tomato. Rec Trends Hortic Biotechnol. 2007;823–828. Reference Source [Google Scholar]
- 15. Ramesh R, Joshi AA, Ghanekar MP: Pseudomonads: major antagonistic endophytic bacteria to suppress bacterial wilt pathogen, Ralstonia solanacearum in the eggplant ( Solanum melongena L.). World J Microbiol Biotechnol. 2009;25(1):47–55. 10.1007/s11274-008-9859-3 [DOI] [Google Scholar]
- 16. Aliye N, Fininsa C, Hiskias Y: Evaluation of rhizosphere bacterial antagonists for their potential to bioprotect potato ( Solanum tuberosum) against bacterial wilt ( Ralstonia solanacearum). Biol Control. 2008;47(3):282–288. 10.1016/j.biocontrol.2008.09.003 [DOI] [Google Scholar]
- 17. Ji X, Lu G, Gai Y, et al. : Biological control against bacterial wilt and colonization of mulberry by an endophytic Bacillus subtilis strain. FEMS Microbiol Ecol. 2008;65(3):565–573. 10.1111/j.1574-6941.2008.00543.x [DOI] [PubMed] [Google Scholar]
- 18. Liu HX, Li SM, Luo YM, et al. : Biological control of Ralstonia wilt, Phytophthora blight, Meloidogyne root-knot on bell pepper by the combination of Bacillus subtilis AR12, Bacillus subtilis SM21 and Chryseobacterium sp. R89. Eur J Plant Pathol. 2014;139(1):107–116. 10.1007/s10658-013-0369-2 [DOI] [Google Scholar]
- 19. Larkin RP, Fravel DR: Efficacy of various fungal and bacterial biocontrol organisms for control of Fusarium wilt of tomato. Plant Dis. 1998;82(9):1022–1028. 10.1094/PDIS.1998.82.9.1022 [DOI] [PubMed] [Google Scholar]
- 20. Brent KJ, Hollomon DW: Fungicide resistance in crop pathogens: how can it be managed?Brussels: GIFAP.1995. Reference Source [Google Scholar]
- 21. Ma Z, Michailides TJ: Advances in understanding molecular mechanisms of fungicide resistance and molecular detection of resistant genotypes in phytopathogenic fungi. Crop Prot. 2005;24(10):853–863. 10.1016/j.cropro.2005.01.011 [DOI] [Google Scholar]
- 22. McManus PS, Stockwell VO, Sundin GW, et al. : Antibiotic use in plant agriculture. Annu Rev Phytopathol. 2002;40(1):443–465. 10.1146/annurev.phyto.40.120301.093927 [DOI] [PubMed] [Google Scholar]
- 23. Cooksey DA: Genetics of bactericide resistance in plant pathogenic bacteria. Annu Rev Phytopathol. 1990;28(1):201–219. 10.1146/annurev.py.28.090190.001221 [DOI] [Google Scholar]
- 24. Vanitha SC, Niranjana SR, Mortensen CN, et al. : Bacterial wilt of tomato in Karnataka and its management by Pseudomonas fluorescens. Biocontrol. 2009;54(5):685–695. 10.1007/s10526-009-9217-x [DOI] [Google Scholar]
- 25. Coutinho TA, Roux J, Riedel KH, et al. : First report of bacterial wilt caused by Ralstonia solanacearum on eucalypts in South Africa. For Pathol. 2000;30(4):205–210. 10.1046/j.1439-0329.2000.00205.x [DOI] [Google Scholar]
- 26. Spraker JE, Jewell K, Roze LV, et al. : A volatile relationship: profiling an inter-kingdom dialogue between two plant pathogens, Ralstonia solanacearum and Aspergillus flavus. J Chem Ecol. 2014;40(5):502–13. 10.1007/s10886-014-0432-2 [DOI] [PubMed] [Google Scholar]
- 27. Vargas Gil S, Pastor S, March GJ: Quantitative isolation of biocontrol agents Trichoderma spp., Gliocladium spp. and actinomycetes from soil with culture media. Microbiol Res. 2009;164(2):196–205. 10.1016/j.micres.2006.11.022 [DOI] [PubMed] [Google Scholar]
- 28. Johnsen K, Nielsen P: Diversity of Pseudomonas strains isolated with King's B and Gould's S1 agar determined by repetitive extragenic palindromic-polymerase chain reaction, 16S rDNA sequencing and Fourier transform infrared spectroscopy characterisation. FEMS Microbiol Lett. 1999;173(1):155–162. 10.1111/j.1574-6968.1999.tb13497.x [DOI] [PubMed] [Google Scholar]
- 29. Carls RA, Hanson RS: Isolation and characterization of tricarboxylic acid cycle mutants of Bacillus subtilis. J Bacteriol. 1971;106(3):848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boukaew S, Chuenchit S, Petcharat V: Evaluation of Streptomyces spp. for biological control of Sclerotium root and stem rot and Ralstonia wilt of chili pepper. BioControl. 2011;56(3):365–374. 10.1007/s10526-010-9336-4 [DOI] [Google Scholar]
- 31. Doughari JH: Antimicrobial activity of Tamarindus indica Linn. Trop J Pharm Res. 2006;5(2):597–603. 10.4314/tjpr.v5i2.14637 [DOI] [Google Scholar]
- 32. Louis KS, Siegel AC: Cell viability analysis using trypan blue: manual and automated methods. Methods Mol Biol. 2011;740:7–12. 10.1007/978-1-61779-108-6_2 [DOI] [PubMed] [Google Scholar]
- 33. Alvarez B, Biosca EG, López MM: On the life of Ralstonia solanacearum, a destructive bacterial plant pathogen. Current research, technology and education topics in applied microbiology and microbial biotechnology. 2010;1:267–279. Reference Source [Google Scholar]
- 34. Williamson L, Nakaho K, Hudelson B, et al. : Ralstonia solanacearum race 3, biovar 2 strains isolated from geranium are pathogenic on potato. Plant Dis. 2002;86(9):987–991. 10.1094/PDIS.2002.86.9.987 [DOI] [PubMed] [Google Scholar]
- 35. Kumar A, Prameela TP, Suseelabhai R: A unique DNA repair and recombination gene ( recN) sequence for identification and intraspecific molecular typing of bacterial wilt pathogen Ralstonia solanacearum and its comparative analysis with ribosomal DNA sequences. J Biosci. 2013;38(2):267–278. 10.1007/s12038-013-9312-0 [DOI] [PubMed] [Google Scholar]
- 36. Tapwal A, Singh U, Singh G, et al. : In vitro antagonism of Trichoderma viride against five phytopathogens. Pest Technol. 2011;5(1):59–62. Reference Source [Google Scholar]
- 37. Mukherjee PK, Horwitz BA, Herrera-Estrella A, et al. : Trichoderma research in the genome era. Annu Rev Phytopathol. 2013;51:105–129. 10.1146/annurev-phyto-082712-102353 [DOI] [PubMed] [Google Scholar]
- 38. Ruano-Rosa D, Cazorla FM, Bonilla N, et al. : Biological control of avocado white root rot with combined applications of Trichoderma spp. and rhizobacteria. Eur J Plant Pathol. 2014;138(4):751–762. 10.1007/s10658-013-0347-8 [DOI] [Google Scholar]
- 39. Li H, Li H, Bai Y, et al. : The use of Pseudomonas fluorescens P13 to control sclerotinia stem rot ( Sclerotinia sclerotiorum) of oilseed rape. J Microbiol. 2011;49(6):884–889. 10.1007/s12275-011-1261-4 [DOI] [PubMed] [Google Scholar]
- 40. Park KH, Lee CY, Son HJ: Mechanism of insoluble phosphate solubilization by Pseudomonas fluorescens RAF15 isolated from ginseng rhizosphere and its plant growth-promoting activities. Lett Appl Microbiol. 2009;49(2):222–228. 10.1111/j.1472-765X.2009.02642.x [DOI] [PubMed] [Google Scholar]
- 41. Alsohim AS, Taylor TB, Barrett GA, et al. : The biosurfactant viscosin produced by Pseudomonas fluorescens SBW25 aids spreading motility and plant growth promotion. Environ Microbiol. 2014;16(7):2267–2281. 10.1111/1462-2920.12469 [DOI] [PubMed] [Google Scholar]
- 42. Ongena M, Jourdan E, Adam A, et al. : Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environ Microbiol. 2007;9(4):1084–1090. 10.1111/j.1462-2920.2006.01202.x [DOI] [PubMed] [Google Scholar]
- 43. Kloepper JW, Ryu CM, Zhang S: Induced Systemic Resistance and Promotion of Plant Growth by Bacillus spp. Phytopathology. 2004;94(11):1259–1266. 10.1094/PHYTO.2004.94.11.1259 [DOI] [PubMed] [Google Scholar]
- 44. Bernal G, Illanes A, Ciampi L: Isolation and partial purification of a metabolite from a mutant strain of Bacillus sp. with antibiotic activity against plant pathogenic agents. Electron J Biotechnol. 2002;5(1):7–8. 10.2225/vol5-issue1-fulltext-4 [DOI] [Google Scholar]
- 45. Ippolito A, Nigro F: Impact of preharvest application of biological control agents on postharvest diseases of fresh fruits and vegetables. Crop Prot. 2000;19(8–10):715–723. 10.1016/S0261-2194(00)00095-8 [DOI] [Google Scholar]
- 46. Tang YW, Ellis NM, Hopkins MK, et al. : Comparison of phenotypic and genotypic techniques for identification of unusual aerobic pathogenic gram-negative bacilli. J Clin Microbiol. 1998;36(12):3674–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pandey BR, Yendyo S, Ramesh GC: Raw values of zone of inhibition by antagonist against Ralstonia spp.2017. Data Source [Google Scholar]
- 48. Pandey BR, Yendyo S, Ramesh GC: Raw values of the 5% sucrose treatment.2017. Data Source [Google Scholar]
- 49. Pandey BR, Yendyo S, Ramesh GC: Images of the plates showing the zone of inhibition by different antagonists against Ralstonia spp. figshare. 2017. Data Source [Google Scholar]
- 50. Pandey BR, Yendyo S, Ramesh GC: Raw digital images (400X) of microscopic analysis representing the interactions of different antagonist against Ralstonia spp. figshare. 2017. Data Source [Google Scholar]
- 51. Pandey BR, Yendyo S, Ramesh GC: Field Images of plot design showing pictures of before the treatment and effects after the treatment. figshare. 2017. Data Source [Google Scholar]