Abstract
Pseudomonas aeruginosa is a complex gram-negative facultative anaerobe replete with a variety of arsenals to activate, modify, and destroy host defense mechanisms. The microbe is a common cause of nosocomial infections and an antibiotic-resistant priority pathogen. In the lung, P. aeruginosa disrupts upper and lower airway homeostasis by damaging the epithelium and evading innate and adaptive immune responses. The biology of these interactions is essential to understand P. aeruginosa pathogenesis. P. aeruginosa interacts directly with host cells via flagella, pili, lipoproteins, lipopolysaccharides, and the type III secretion system localized in the outer membrane. P. aeruginosa quorum-sensing molecules regulate the release of soluble factors that enhance the spread of infection. These characteristics of P. aeruginosa differentially affect lung epithelial, innate, and adaptive immune cells involved in the production of mediators and the recruitment of additional immune cell subsets. Pathogen interactions with individual host cells and in the context of host acute lung infection are discussed to reveal pathways that may be targeted therapeutically.
Keywords: quorum sensing, type III secretion system, immunology, therapeutics, Pseudomonas aeruginosa
Contents
Clinical Presentation and Standard Therapy of Acute Nosocomial Pneumonia
The Bacterium
Adhesion Factors
Membrane Components
Secretion Systems
QS
The Innate Immune Response
Phagocytes
Innate Lymphoid Cells
Bronchial Epithelium
Alveolar Epithelium
The Adaptive Immune Response
T Cells and BALT
B Cells
Pathophysiology of P. aeruginosa Pneumonia
Novel Therapeutic Interventions
Interventions Targeting the Pathogen
Protein Epitope Mimetics
Iron
Pyocyanin
Adhesion Factors
Bacteriocins and QS Inhibition
Bacteriophages
Interventions Targeting the Host
IL-22
Desulfated Heparin
ACE2 Activation
Interventions Targeting the Host and the Pathogen
Indoles
Cationic Molecules
Conclusions
Pseudomonas aeruginosa is a predominant organism within the hospital environment, an increasingly multidrug-resistant microbe, and the most common gram-negative pathogen causing nosocomial pneumonia in the United States (1, 2). Nearly all P. aeruginosa infections are associated with compromised host defenses, which may include patients with severe burns, diabetes, cancer, organ transplants, or additional immunodeficiencies (3). In the lung, P. aeruginosa is known to opportunistically colonize patients with cystic fibrosis (4) and chronic obstructive pulmonary disease (5). The biology and impact of P. aeruginosa chronic lung infection in patients with cystic fibrosis and chronic obstructive pulmonary disease have been extensively reviewed elsewhere (6–9). The aim of this review is to assess the pathogenesis of acute P. aeruginosa lung infections and to provide insight into potential host- and pathogen-associated therapeutic targets.
P. aeruginosa is a leading cause of acute nosocomial infections and pneumonias in particular (10–12). Nosocomial pneumonia has a mortality rate ranging from 13% to 50%, lengthens hospital stays, and adds approximately US$40,000 in excess cost per patient (13, 14). Ventilator-associated pneumonia (VAP) is a significant cause of morbidity and mortality in critically ill patients, and the isolation of P. aeruginosa is associated with worse clinical outcomes (15). Prior exposure to quinolones and carbapenems, commonly used in ICUs, is linked to the development of multidrug-resistant P. aeruginosa (16), making this pathogen of great concern. P. aeruginosa expresses efflux pumps, β-lactamases, impermeable outer membrane proteins, and an adaptable genome that allows the microbe to acquire resistance to many classes of antibiotics (17). The number of multidrug-resistant P. aeruginosa strains (resistant to one or more drugs in three or more antibiotic categories) has steadily increased, and many clinical isolates possess carbapenem resistance or, rarely, colistin resistance (10, 18). Because of the increasing frequency of antimicrobial resistance in P. aeruginosa, the World Health Organization has listed it as a “Priority 1: Critical” pathogen in need of research and development of new therapeutic approaches to treating infections (19). The genetic diversity in antibiotic-resistant P. aeruginosa strains obtained from clinical isolates highlights the ability of the microbe to selectively adapt to environmental challenges (20). Therefore, the biology of the bacteria and the host microenvironment are essential to disease progression.
Clinical Presentation and Standard Therapy of Acute Nosocomial Pneumonia
P. aeruginosa accounts for up to 18% of nosocomial pneumonia cases, making it one of the most frequently isolated pathogens (10–13). The diagnosis of nosocomial pneumonia is associated with the appearance of new or progressive radiographic infiltrate plus fever, purulent sputum, leukocytosis, and a decline in oxygenation (21). Hospital-acquired pneumonia (HAP) is defined as pneumonia occurring 48 hours or more after admission to the hospital, whereas VAP is defined as pneumonia occurring 48 hours after endotracheal intubation (13). Some of the complications associated with pseudomonal HAP and VAP are empyema (in 4–8% of patients), bacteremia (in 4–17% of patients), and shock (in up to 46% of patients) (22, 23). Attributable mortality rates associated with VAP and caused by P. aeruginosa are estimated to range from 13% to 32% (14, 24). Delayed or inappropriate therapy is associated with increased mortality, extended hospital stays, and the development of resistant organisms (13). Therefore, microbial identification and selection of targeted therapies for this pathogen are essential to effective treatment. Initial empiric therapy for HAP and VAP includes a β-lactam (antipseudomonal penicillin or cephalosporin, or a carbapenem) plus a fluoroquinolone (ciprofloxacin, levofloxacin) or aminoglycoside (amikacin, gentamicin, tobramycin) (13). In some cases of multidrug-resistant P. aeruginosa, polymyxins (colistin) may also be considered (25). The prevalence of P. aeruginosa nosocomial infections, their associated morbidity and mortality, and the increased presence of multidrug-resistant P. aeruginosa strains (10, 18) highlight the need for new therapeutic approaches. In the sections that follow, we discuss the biology of P. aeruginosa and the host response to highlight potential molecules and pathways for targeted intervention.
The Bacterium
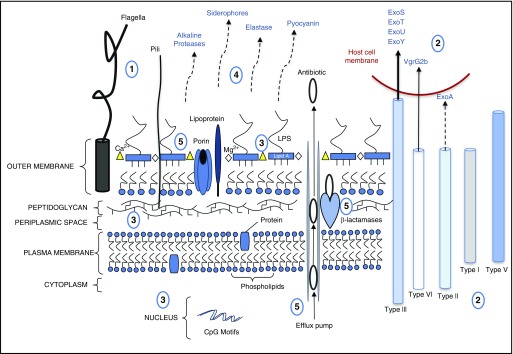
P. aeruginosa is a gram-negative, rod-shaped, facultative anaerobe that adheres to host airway epithelia via its flagellum, pili, and components of the outer plasma membrane (26). P. aeruginosa breach of the epithelium may require weakened tight junctions, as occurs during cell proliferation or death, or in response to a significant focal concentration of the bacterium, which circumvents the immune response and allows for P. aeruginosa exotoxin-S (ExoS)-dependent cytotoxic damage to the epithelium (27, 28). Individuals with a compromised immune system or a damaged epithelium, often present in mechanically ventilated patients, are highly susceptible to P. aeruginosa infections (3, 29). In addition, previous exposure to antibiotics has been identified as an additional risk factor (30), which may be linked to antibiotic-induced changes in the lung microbiome (31) and the innate immune subsets (e.g., macrophages) that reside in the microenvironment (32). The mechanisms that allow P. aeruginosa to home in on damaged tissue or colonize hospitalized patients with different underlying disorders (e.g., extrapulmonary infection, congestive heart failure, renal failure, or surgery) are not fully elucidated (27, 29, 30, 33, 34). Quorum sensing (QS), or the ability of the bacterium to “sense” and respond to its environment, in association with adhesion factors, membrane components, and secretion systems, is fundamental to the initiation, propagation, and maintenance of acute P. aeruginosa infection (35) (Figure 1).
Figure 1.
Pseudomonas aeruginosa structure and biology with respect to host interactions. (1) P. aeruginosa adheres to the host via flagella (e.g., binds to mucin, asialo ganglio-N-tetraosylceramide 1 [GM1], heparin sulfate proteoglycans, Toll-like receptor [TLR]-5, and TLR-2) and pili (e.g., binds to asialo GM1, asialo GM2, and N-glycans). (2) The microbe uses five secretion systems (type I, II, III, V, and VI). Type II secretion system releases exotoxin (Exo)A. Direct cell contact enables T3SS to inject toxins into host cells. T6SS-mediated injection of the effector molecule VgrG2b permits microtubule-dependent internalization of the pathogen. (3) Membrane proteins (lipoprotein:TLR-2, peptidoglycan:nucleotide-binding oligomerization domain [NOD]1 or NOD2) and molecules (LPS:TLR-4) as well as nucleic DNA motifs (CpG:TLR-9) also interact with host receptors. (4) Quorum-sensing (QS) molecules regulate the release of soluble factors (proteases, siderophores, elastase, and pyocyanin) in propagating infection. Pyocyanin also interacts with the host receptor, aryl hydrocarbon receptor. (5) P. aeruginosa efflux pumps, β-lactamases, and porins that regulate slow diffusion of solutes mediate antibiotic resistance.
Adhesion Factors
P. aeruginosa flagellum and pili play roles in the motility and adherence of the bacterium to host tissue and are therefore therapeutic targets of interest (36, 37). P. aeruginosa has a single, polar flagellum (38) that is essential for motility and establishing acute infections (39). The flagellar cap protein, FliD, is responsible for adhesion to mucins in the upper airways (40). On the apical surface of respiratory epithelial cells, both flagella and pili bind to glycosphingolipids called asialo ganglio-N-tetraosylceramides (41, 42), and on the basolateral surface, flagella and pili respectively bind heparan sulfate proteoglycans and N-glycans (26). Induced cell signals from interactions between glycosphingolipids and flagella or pili on the apical surface of respiratory epithelial cells initiate the transcription and mobilization of Toll-like receptor (TLR)-2 and TLR-5, where the latter is another well-characterized flagellar receptor (41). Damaged or immature epithelial cells express higher quantities of glycosphingolipids and heparan sulfate proteoglycans, which may explain P. aeruginosa’s predisposition for these cells (33). Pili can also operate as receptors for certain bacteriophages, and these bacterial viruses are currently being examined therapeutically in the treatment of P. aeruginosa infections (43).
Membrane Components
The P. aeruginosa outer membrane exhibits very low permeability because of the expression of certain porins and lipoproteins (44). These outer membrane proteins provide maintenance of cell structure, passive and active transport of extracellular molecules, adhesion to other cells, activation of TLR-2, and antibiotic resistance via various mechanisms (45–47). For example, the outer membrane porin F (OprF) allows nonspecific diffusion of solutes. However, in P. aeruginosa, OprF has much lower permeability than other gram-negative bacteria (48). This reduced permeability decreases the free diffusion of small hydrophilic antibiotics (e.g., β-lactams and tetracyclines) across the cell membrane and offers β-lactamases and efflux pumps greater efficiency in removing antibiotics that may gain access to the bacterium (44, 48). Outer membrane protein H (OprH) overexpression confers antibiotic resistance to cationic peptides and antibiotics such as aminoglycosides and polymyxin B by displacing Mg2+ and Ca2+ from the outer membrane and making tight cross-linking interactions with LPS, which consequently reduces outer membrane permeability (49). Increased production of additional proteins by the microbe, which may operate in either the diffusion or efflux of antibiotics (e.g., OprM, OprJ, and OprN), is also linked to multidrug resistance (45), whereas OprI is an identified susceptible target and potential receptor for the internalization of host cationic antimicrobial peptides/proteins (e.g., LL-37 and defensins) (50, 51). Therapeutic opportunities may therefore exist in targeting outer membrane proteins.
Also within the gram-negative outer membrane are molecules of LPS (Figure 1). This virulence factor contains a lipid A moiety that is recognized by the host cell TLR-4 complex consisting of TLR-4, the coreceptor MD-2, and either membrane or soluble CD14 (52). In addition to the lipid A moiety, LPS consists of inner and outer core oligosaccharides and the O antigen (53). The O antigen can also serve as a receptor for bacteriophages (54). However, the heterogeneity of the lipid A and O antigen components in P. aeruginosa limits the opportunities to target LPS (52). Recent advances in understanding LPS biogenesis and the transport proteins involved in transporting the molecule from the inner membrane to the outer membrane have identified targets in modifying LPS virulence that warrant further study (53).
Peptidoglycan, another structural component of the membrane, is located just below the outer membrane (Figure 1). The muropeptides contained in peptidoglycan from either gram-negative or gram-positive bacteria are known to activate the intracellular innate immune receptor nucleotide-binding oligomerization domain (NOD)-2 (55). NOD-1, however, primarily responds to a unique muropeptide found in gram-negative bacteria (56). Because TLR-4 and NOD-1 are specific to gram-negative bacteria and induce synergistic cell signals, attempts to block or antagonize these pathways in severe infections have been investigated, albeit without success (57, 58). Routes of administration, animal models versus human clinical syndromes, patient comorbidities, and type, location, and duration of infection may contribute to the difficulties in developing effective interventions that target TLRs and NODs.
Secretion Systems
P. aeruginosa has secretion systems that release virulence factors directly into targeted cells or into the extracellular space. Gram-negative bacteria use at least seven secretion systems to transport bacterial proteins (59). P. aeruginosa secretes proteins via type I, type II, type III, type V, and type VI, but the major virulence factors of P. aeruginosa are secreted via either the type II secretion system (T2SS) or type III secretion system (T3SS) (60). General secretory pathway molecules have been identified in the transmembrane assembly of P. aeruginosa T2SS (61) that could be potential targets in antagonizing T2SS function. ExoA, elastases, lipases, and proteases are released into the extracellular space via the T2SS (62). Additional exotoxins, ExoS, ExoT, ExoU, and ExoY, are injected directly into host cells by the T3SS needle-like apparatus, which consists of a network of proteins (63). One product of the T3SS needle complex, PcrV, has been assessed as a therapeutic target (64, 65). Other molecules involved in the needle complex, translocation apparatus, or associated regulatory proteins and chaperones may also represent areas for therapeutic interventions.
QS
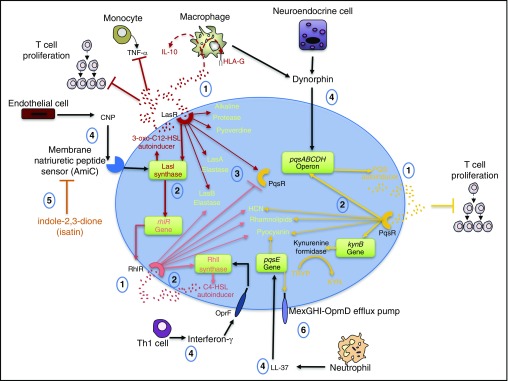
QS is a form of bacterial cell-to-cell communication that regulates gene expression. P. aeruginosa has three well-described QS pathways termed Las, Rhl, and Pqs that respectively generate the autoinducers 3-oxo-C12-homoserine lactone (3-oxo-C12-HSL), N-butyryl homoserine lactone (C4-HSL), and 2-heptyl-3-hydroxy-4-quinolone (PQS) (66). Each autoinducer freely diffuses into cells, and at high bacterial cell densities, these molecules reach a threshold concentration and bind to their respective receptor, which also serves as an inducible transcription factor for the induction of genes involved in the production of autoinducers, QS receptors, and virulence genes (67, 68). The Las pathway activates the transcription of the Las synthase, LasI, for the induction of 3-oxo-C12-HSL, the Rhl receptor (RhlR), and the RhlR cognate synthase, RhlI (68). In addition, the Las receptor (LasR) activates the transcription of the Pqs receptor (PqsR), but the transcription is negatively regulated by RhlR (69). The Pqs pathway is also dependent on anthranilate generated from chorismate (a nonmammal precursor of aromatic amino acids) by P. aeruginosa anthranilate synthases or via the kynurenine pathway, where tryptophan is catabolized (70, 71). PQS is produced by proteins that are encoded by the pqsABCDH genes, where pqsE is not involved but is linked to the increased production of pyocyanin and the expression of the efflux pump, MexGHI-OpmD (72). Additional virulence factors are generated by each pathway (35, 73, 74) and are highlighted in Figure 2.
Figure 2.
Quorum sensing (QS). Pseudomonas aeruginosa QS is a bacterial intercellular communication system that also interacts with the host. (1) The autoinducers 3-oxo-C12-homoserine lactone (3-oxo-C12-HSL), N-butyryl homoserine lactone (C4-HSL), and 2-heptyl-3-hydroxy-4-quinolone (PQS) activate their receptors (LasR, RhIR, and PqsR, respectively) and modulate the immune response. 3-oxo-C12-HSL inhibits tumor necrosis factor (TNF)-α release and induces IL-10 and HLA-G expression in myeloid cells. Both 3-oxo-C12-HSL and PQS can inhibit T-cell proliferation. (2) The receptors’ binding their ligands stimulates the transcription of autoinducer enzymes and genes (e.g., LasI synthase, RhlI synthase, and PqsABCDH operon) and the transcription of virulence factors: elastases, proteases, siderophores (pyoverdine), reactive oxygen species (hydrogen cyanide [HCN]), and pyocyanin. PqsR activation also generates kynurenine formidase involved in the catabolism of tryptophan (TRYP) into kynurenine (KYN). (3) LasR activates while RhlR suppresses PqsR transcription. (4) Environmental stimuli such as IFN-γ, C natriuretic peptide (CNP), dynorphin, and the antimicrobial peptide LL-37 can induce the activation of the QS system at various points. CNPs secreted from endothelial cells are detected by the membrane natriuretic peptide sensor AmiC, which activates the transcription of lasI. Likewise, when IFN-γ binds to the outer membrane porin F (OprF), rhlI transcription is stimulated. Dynorphin activates the pqsABCDH operon. (5) The indole isatin antagonizes AmiC activation. (6) LL-37 activates pqsE, which generates the multidrug efflux pump MexGHI-OpmD. Th1 = T-helper cell type 1.
In addition, activation of QS results in the production of virulence factors that not only damage host cells but also exhibit crosstalk with host cells through direct and indirect mechanisms (60) (Figure 2). For example, the Las pathway generates the siderophore pyoverdine, which sequesters iron away from host cells (35), and this pathway also generates the autoinducer 3-oxo-C12-HSL, which induces myeloid cell production of IL-10, the expression of the tolerogenic nonclassical class I HLA-G, and an inhibition of tumor necrosis factor (TNF)-α release (75, 76). Both 3-oxo-C12-HSL and the PQS autoinducer inhibit in vitro mitogen-induced human T-cell proliferation and IL-2 release (76). The Rhl and Pqs pathways produce hydrogen cyanide and pyocyanin that generate reactive oxygen species (ROS) (35, 73, 77). Pyocyanin also induces the activation of a characterized host receptor called the aryl hydrocarbon receptor (AHR) (78). This receptor is also activated by kynurenine generated in the Pqs system (79). Adding to the complexity, AHR is an identified receptor for various xenobiotic chemicals, a transcriptional activator of cytochrome P450 enzymes, and an essential factor in the development and function of hematopoietic cells (80).
Moreover, the QS pathways are activated by host factors (Figure 2). Although natriuretic peptides are predominantly characterized as cardiac hormones known to regulate blood pressure (through control of sodium and water balance, for example) (81), endothelial cell-produced C-type natriuretic peptide also induces LasI transcription and the subsequent production of soluble 3-oxo-C12-HSL (81, 82). The human cathelicidin, LL-37, is a host defense peptide secreted by a variety of cells (e.g., macrophages, natural killer cells, and epithelial cells) but is best characterized in neutrophils (83). Treatment of P. aeruginosa with LL-37 induces the production of pyocyanin, which may occur through the activation of pqsE (84). Dynorphin, a κ-opioid peptide produced by pulmonary neuroendocrine cells and alveolar macrophages, has been implicated in the activation of pqsABCDE (85, 86). Last, IFN-γ has been shown to bind to the outer membrane protein OprF, subsequently activating the Rhl system and the production of C4-HSL and pyocyanin (87). The above QS studies reflect a constant interplay between the microbe and the host and highlight the need to understand the biology of both the microbe as well as the host response in designing novel therapies.
The Innate Immune Response
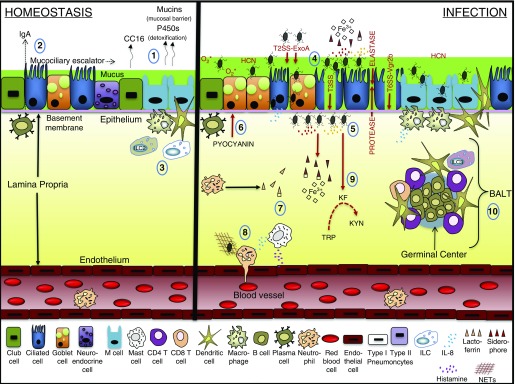
In the development of a pulmonary bacterial infection, the microbe must first overcome innate host defense responses. Goblet, ciliated, basal, club, and neuroendocrine cells line the epithelium in the upper airway mucosa and contribute to an extensive mucociliary escalator to defend against particulate matter and organisms (88). Mucins protect the epithelium in association with plasma cell–secreted immunoglobulins, which opsonize foreign matter (89, 90). P450 enzymes detoxify xenobiotics throughout the lung (91). In the alveolar lumen, macrophages are charged with regularly clearing debris, surfactant, and microorganisms in maintaining lung compliance and protecting the epithelium composed of type I and II pneumocytes (92, 93) (Figures 3 and 4).
Figure 3.
Upper airway homeostasis and infection. (1) During homeostasis, the respiratory bronchial epithelium (club cell, ciliary, goblet, neuroendocrine, microfold [M] cells) protects against environmental insult by maintaining tight junctions between cells, producing mucin and p450 detoxifying enzymes and generating the antiinflammatory molecule club secretory protein (CC16). (2) Plasma cells generate antibody to opsonize foreign pathogens, and the mucociliary escalator acts as an additional host defense mechanism. (3) The vascular endothelium is separated from the epithelium by the lamina propria, where dendritic cells, macrophages, and innate lymphoid cells (ILCs) that are mostly ILC2 localize. (4) During infection, Pseudomonas aeruginosa adheres to the epithelium and injects toxins through the type III secretion system (T3SS) and the effector molecule involved in T6SS-induced internalization, VgrG2b. (5) Activation of quorum sensing (QS) encourages the accumulation of P. aeruginosa and the production of QS molecules that in turn generate exotoxin (Exo)A, hydrogen cyanide (HCN), reactive oxygen species, proteases, and elastase in the destruction of the epithelium. (6). QS also induces the production of pyocyanin, which is characterized in goblet cell hyperplasia and enhanced mucin production. (7) Released siderophores compete with cells in the microenvironment for available iron. (8) Mast cell production of histamine and IL-8 production by various cells induce the recruitment of neutrophils. (9) The production of kynurenine formidase (KF) induces tryptophan (TRP) catabolism into kynurenine (KYN). (10) Infection triggers cell signals for the formation of the inducible bronchus-associated lymphoid tissue (BALT) that coordinates with the lymphatic system in mediating secondary responses. NET = neutrophil extracellular trap.
Figure 4.
Lower airway homeostasis and infection. (1) The alveolar epithelium consists of mainly type I pneumocytes that mediate gas exchange and type II pneumocytes that secrete surfactant. (2) Intact elastin fibers provide structural support and aid in expansion of alveoli during respiration. (3) Macrophages persistently survey the lumen for debris and antigen that may or may not be coated with opsonins. (4) Additional innate immune cells (dendritic cells and mast cells) stand vigil over their microenvironment. (5) During infection, Pseudomonas aeruginosa adheres to the epithelium and injects toxins through the type III secretion system (T3SS). (6) In some instances, P. aeruginosa will proliferate within type I or II pneumocytes. (7) Activation of quorum sensing (QS) encourages the accumulation of P. aeruginosa and the production of QS molecules that in turn generate exotoxin (Exo)A, hydrogen cyanide (HCN), reactive oxygen species, proteases, and elastase in the destruction of the epithelium, surfactant, opsonins, and elastin. (8) Released siderophores compete with cells in the microenvironment for available iron. (9) The production of kynurenine formidase (KF) induces tryptophan (TRP) catabolism into kynurenine (KYN). (10) Innate immune cells interact with the pathogen and begin to recruit additional immune subsets.
Phagocytes
During infection, macrophages and neutrophils preferentially phagocytose motile bacteria irrespective of opsonization (94). Nonopsonic phagocytosis mechanisms are not well defined, might be strain specific, and may require P. aeruginosa flagella, macrophage complement receptor 3 (CR3), or CD14 (95, 96). Antibodies (IgA, IgG) (97), complement (C2, C3, C3b, C4) (98–100), and surfactant protein-A (101) opsonize P. aeruginosa and enhance phagocytic uptake via binding interactions with Fc or complement receptors. To evade these mechanisms, P. aeruginosa uses 3-oxo-C12-HSL to modulate the activation state of myeloid cells (75, 76) (Figure 2). The virulence factors elastase and alkaline protease degrade opsonins (100, 102–104). ExoA and pyocyanin induce apoptosis (62, 105), and pyocyanin also impairs macrophage ingestion of apoptotic cells (106). In a murine model of P. aeruginosa pneumonia, macrophages and neutrophils are also susceptible to T3SS ExoS cytotoxicity (28). P. aeruginosa–induced release of IL-8 by macrophages (107), mast cells (108), and epithelial cells (109) elicits the recruitment of neutrophils from the peripheral blood (110). To replace a depleted pool of macrophages in the lung is more complex, given that the vast majority of tissue-resident macrophages originate from the yolk sac and not hematopoietic stem cell precursors (111). In a murine Escherichia coli primary pulmonary infection model followed by secondary P. aeruginosa pneumonia, populations of dendritic cells (DCs) and macrophages were reconstituted in an altered phenotype involving diminished antigen presentation and reduced production of proinflammatory cytokines (IL-6, TNF-α, IL-12) (112). CD11b+ DC populations characterized in this study exhibited increased B-lymphocyte–induced maturation protein (Blimp)-1, which is an identified transcription factor regulated by AHR (112, 113). In neutrophils, the P. aeruginosa–produced AHR ligand, pyocyanin, induces nicotinamide adenine dinucleotide phosphate(H) (NADPH) oxidase and the uncontrolled release of neutrophil extracellular traps (NETs) (114). Neutrophil bacterial uptake also involves the activation of NADPH oxidase, the release of antibacterial molecules, and ROS involved in the killing of the pathogen (115). These activities may be countered by an additional P. aeruginosa–derived AHR ligand, kynurenine, which scavenges neutrophil ROS (116). In addition, T3SS blocks neutrophil ROS production via ADP-ribosyltransferase activities of ExoS and ExoT (117). These competing processes may allow the microbe to evade host phagocytes and interact with additional immune subsets and the epithelium.
Innate Lymphoid Cells
Lung innate lymphoid cells (ILCs) are composed of three subsets (ILC1, ILC2, ILC3), which are derived from common lymphoid progenitors. The ILC2s have been characterized in murine and human lung tissue and may be recruited to the airway (118). This subset is maintained by various cytokines (e.g., IL-33 and IL-25) and is implicated in the maintenance of lung homeostasis (118, 119). In a murine model, P. aeruginosa infection altered mediator production and induced the formation of the ILC3 variant (120). This subgroup is formed not only in response to IL-1β and IL-23 but also in response to AHR cell signals (119), suggesting that the AHR ligands, particularly pyocyanin and kynurenine from QS (Figure 2), or the production of kynurenine via indoleamine-2,3-dioxygenase (IDO) released from CpG DNA-activated dendritic cells (121), may influence the proliferation or recruitment of ILC3s (122). These cells are also characterized by the production of IL-17 and IL-22 (119), which are essential cytokines in the formation of bronchus-associated lymphoid tissues (BALT) during the adaptive immune response (123).
Bronchial Epithelium
P. aeruginosa flagella and pili interact with mucin and cell surface receptors on the apical surface of the respiratory bronchial epithelium (41, 42) (Figures 1 and 3). Epithelial cell invasion by the microbe may be induced by T6SS-mediated injection of the effector molecule VgrG2b, which targets the host cell microtubule network and permits microtubule-dependent internalization of the pathogen (124, 125). This mechanism may be linked to the activation of epithelial cell signals, specifically phosphatidylinositol 3-kinase (PI3K) and protein kinase B/Akt (Akt), which are required for internalization of the bacterium (126). Release of ExoA via T2SS (62) and injection of additional toxins via T3SS (63) into epithelial cells damage the epithelium and provide pathways to the basolateral surfaces. There the bacteria preferentially adhere and localize via binding interactions between the epithelium and bacterial flagella (26, 127) (Figure 3). QS activates cell signals involved in the production of virulence factors (35) (Figure 2). Proteases and elastases degrade mucin (128), IgA (102), and the epithelial tight junctions (3). Pyocyanin slows ciliary beating (129) and induces goblet cell hyperplasia and mucin hypersecretion (130), possibly as a result of increased club cell differentiation into goblet cells (130, 131). Furthermore, the differentiation of club cells may also involve pyocyanin or kynurenine (79) binding to AHR in club cells, because AHR is highly expressed in these cells and known to bind additional AHR ligands in human club cells in vitro (132). Distinct xenobiotic AHR ligands (curcumin, indole-3-carbinol, 2,3,7,8-tetrachlorodibenzo-p-dioxin) are also characterized in modulating lung inflammation and fibrosis (133), suggesting that microbe AHR ligands are integral to the host response.
Alveolar Epithelium
In the alveolar epithelium, type I pneumocytes cover approximately 95% of the gas exchange surface area, and the type II pneumocytes cover the remaining area and secrete surfactant in maintaining lung compliance (134). P. aeruginosa type IV pilus accounts for about 90% of the adherence capability of the microbe to the adenocarcinomic human alveolar basal epithelial cell line, A549 (135). Invasion of rat and murine type I–like pneumocytes in vitro and in vivo is mediated by P. aeruginosa coopting host cell caveolin-2–dependent lipid raft endocytosis (136, 137), suggesting that this host–microbe interaction could be a therapeutic target. In cell lines and murine models, type I and type II pneumocytes endocytose P. aeruginosa, protect the bacteria from host defenses by shielding them from phagocytes, and allow bacterial proliferation (138, 139). In a murine pneumonia model, type I pneumocytes accounted for nearly 40% of all cells injected with ExoS 18 to 24 hours after P. aeruginosa infection (28). Damaged type I pneumocytes are replaced by type II pneumocytes, and this increases the production of surfactant and decreases gas exchange in the alveoli (134). In A549 cells, pyocyanin accepts electrons from nicotinamide adenine dinucleotide(H) (NADH), NADPH, or reduced glutathione and transfers the electrons to O2, generating superoxide (O2*) and hydrogen peroxide (H2O2) (77). These ROSs also induce the release of histamine from murine primary mast cells (140), leading to increased vascular permeability and therefore encouraging immune cell influx from circulation (Figure 4).
The Adaptive Immune Response
The adaptive immune response is a delayed response that requires directives from the innate immune system for the generation of pathogen-specific lymphocytes. P. aeruginosa antigens in the lung are taken up, processed, and presented to T-cell subsets mainly by DCs (141, 142). Additional professional antigen-presenting cells, such as B cells and macrophages, can also perform this function (143). Atypical epithelial cells called microfold (M) cells and ILC3s may also present antigen and interact with T-cell subsets (143, 144). In mice, M cells are localized above nasopharynx-associated lymphoid tissue and BALT (145) and may translocate pathogens to macrophages and dendritic cells directly beneath the epithelium (145, 146). The activation of T cells occurs mostly in lymph nodes but can also occur in the BALT, where distinct subsets (T-helper cell type 22 [Th22], Th17, T regulatory) are generated (123). Animal studies examining the effect of Th1 cytokines, such as IFN-γ and IL-18, in response to P. aeruginosa pulmonary infection, demonstrate that a Th1 response impairs bacterial clearance in the lung (147, 148). This contrasts with a murine model involving P. aeruginosa subcutaneous footpad injections, where IFN-γ is critical to the local containment of bacteria and the prevention of systemic dissemination (149). Possibly, this is due to the ability of P. aeruginosa to “sense” IFN-γ through the QS system in the lung microenvironment, thus leading to increased virulence (Figure 2). This may explain the proliferation of additional T-cell subsets and B cells that can both be influenced by AHR ligands.
T Cells and BALT
The most robust adaptive immune response occurs within the regional mediastinal lymph nodes, where migratory dendritic cells interact with T cells to generate and induce the proliferation of antigen-specific T cells (142, 150). Dendritic cells pulsed with the heat-killed bacterium in vitro are able to protect mice against an in vivo lethal challenge in CD8−/− but not CD4−/− mice, indicating a requirement for CD4+ cells (151). Subsets of CD4 cells that are generated after P. aeruginosa challenge include Th17 and Th22 cells (152) that provide cytokines (IL-17, IL-22) in promoting the organization of B cells and T cells into BALT (123). IL-17 also contributes to lymphangiogenesis within BALT (153), forming around major bronchi and pulmonary blood vessels, thus allowing for enhanced recruitment of innate and adaptive immune cells into the lung (154). IL-17 and IL-22 are also important to host defense, as deletion or blockade of IL-17 or IL-22 in murine models of P. aeruginosa and other gram-negative pulmonary infections results in increased lung injury and mortality (155–158). Cell signals that yield IL-17 and IL-22 in T-cell subsets are similar, particularly in that some murine Th17 cells can produce both IL-17 and IL-22 (159). Similar to the ILC3s (119), both Th17 and Th22 cells are regulated by the cytokines IL-1β and IL-23 (160, 161) and are influenced by the transcription factor AHR.
Moreover, the activation of T-cell subsets by AHR is critical to the development and function of immune responses. The microenvironment and type of AHR ligand determine the formation of Th17, Th22, the immunosuppressive T regulatory lymphocytes (80), and ILC3s (120, 162). P. aeruginosa pigment molecules, pyocyanin and 1-hydroxyphenazine, in association with the tryptophan catabolic product kynurenine, produced by both the bacterium and host, appear sufficient to activate AHR during infection (78, 79, 163). In a rat catheter-related lung infection model examining P. aeruginosa QS mutants, the production of suppressive cytokines (IL-10, TGF-β) and the presence of T regulatory lymphocytes were higher in the animals infected with the wild-type bacteria than in animals challenged with the mutants (164), suggesting that QS molecules also affect the induction of certain T-cell subsets.
B Cells
In a murine model of P. aeruginosa lung infection, IL-17–producing γδ T cells were found to be essential for B-cell production of antibodies detected in serum and bronchial lavage fluid (165). An additional model demonstrated that B cells also produced IL-17, but in mice lacking B cells (μMT mice), pathogenesis was not affected (166), which highlights the lack of target specificity of the antibodies generated. Of interest, B-cell receptor cross-linking significantly induces the expression of AHR, and AHR activation affects the processes of class-switch recombination and plasma cell differentiation, which induce the formation of distinct effector B cells and antibody-secreting cells, respectively (113). The production of AHR ligands by the host and microbe may therefore affect the humoral response to P. aeruginosa infection. These adaptive immune responses involving lymphocyte subsets, coupled with innate host defense mechanisms, provide insight into the pathophysiology of clinically evident P. aeruginosa pneumonia.
Pathophysiology of P. aeruginosa Pneumonia
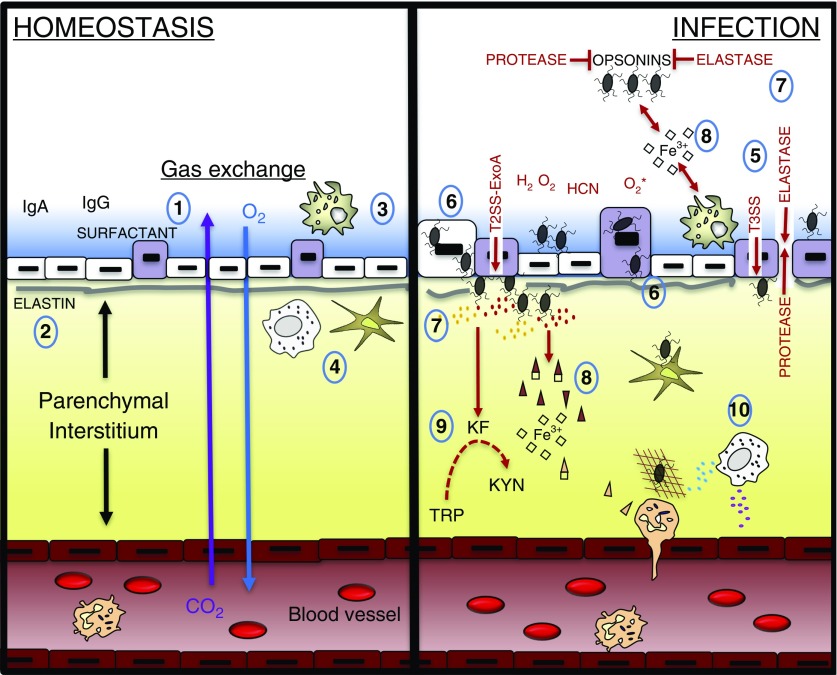
Barriers exist in the respiratory tract to prevent the establishment of infection, such as the presence of mucus, opsonins, innate immune cells, and additional factors (Figures 3 and 4). These barriers may be altered by physical injury (29), a compromised immune system (3), or previous exposure to antibiotics (30), which render the host susceptible to P. aeruginosa lung infections. Activation of P. aeruginosa QS alters innate and adaptive responses (Figure 2) and, along with the associated cytotoxic effects of the virulence factors, allows for the establishment of a severe lower respiratory tract infection, as discussed in detail above (Figures 5 and 6). The corresponding inflammatory response includes P. aeruginosa–mediated release of IL-8 by various cell types and the recruitment of neutrophils to the lung (110). NET proteins can induce endothelial and epithelial cell death, alter the extracellular matrix, and expose autoantigens, which may contribute to mucus viscosity, autoantibody production, and impaired lung function in certain P. aeruginosa infections (167, 168). Red hepatization, tissue necrosis, thrombosis of the blood vessels, and obstruction of small airways by lymphocytic infiltrates are linked to the oxidative effects of QS-mediated release of pyocyanin in the murine lung (169). These various interactions combined with sequestration of iron by the pathogen converge to create a hypoxic environment where host lung function declines (170, 171).
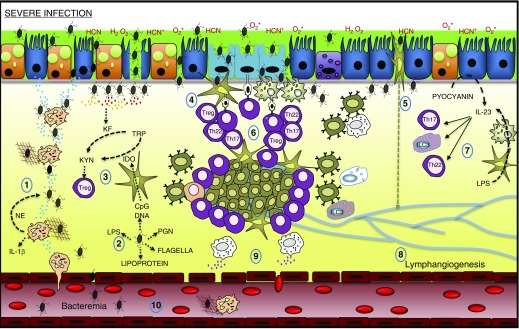
Figure 5.
Upper airway severe infection. (1) IL-8 produced by epithelial cells and additional cell types encourages the recruitment of neutrophils that trap Pseudomonas aeruginosa with neutrophil extracellular traps and degrade P. aeruginosa cell walls with neutrophil elastase (NE). Active IL-1β can also be generated by NE. (2) Release of components of the bacterial wall induces the activation of immune and nonimmune cells. (3) CpG DNA induces the production of indoleamine-2,3-dioxygenase (IDO) that synergistically with P. aeruginosa kynurenine formidase (KF) induces the catabolism of tryptophan (TRP) to kynurenine (KYN), which encourages the production of T regulatory lymphocytes (Tregs). (4) Antigen may be directly presented by microfold (M) cells or transported through M cells to dendritic cells and macrophages under the epithelium. (5) Dendritic cells transport antigen retrieved from the epithelium directly to draining lymph nodes. (6) P. aeruginosa infections generally induce the production of T-cell subsets (T-helper cell type 22 [Th22], Th17, and Tregs). Th22 and Th17 provide cytokines (IL-22 and IL-17, respectively) in promoting the organization of B cells and T cells into bronchus-associated lymphoid tissue. (7) Th22, Th17, and innate lymphoid cell (ILC) 3 are known to be induced by IL-23, which can be generated by P. aeruginosa–activated epithelial cells, dendritic cells, and macrophages. (8) Excessive inflammation induces the formation of new lymphatic vessels. (9) Mast cells regulate vascular dilation via histamine production. (10) Breaks in the vascular endothelium may, in some cases, lead to infection spread or bacteremia. HCN = hydrogen cyanide; PGN = peptidoglycan.
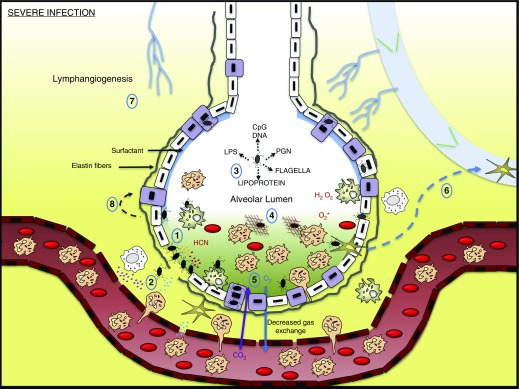
Figure 6.
Lower airway severe infection. (1) Pseudomonas aeruginosa production of quorum-sensing molecules degrades the epithelium and the supporting elastin fibers. (2) Production of histamine by mast cells and IL-8 by various cells in the microenvironment encourages the recruitment of immune cells into the alveolus. (3) Components of P. aeruginosa activate immune cells and induce the production of mediators. (4) Leakage of fluid from the interstitium and mediators produced from immune and nonimmune cells fill the lumen space. This edema, in association with reactive oxygen species and various P. aeruginosa toxins, induces hypoxia. (5) Gas exchange is reduced. (6) Dendritic cells migrate to draining lymph nodes. (7) New lymphatic vessels are formed. (8) Damaged type I pneumocytes are replaced by type II pneumocytes. HCN = hydrogen cyanide; PGN = peptidoglycan.
Moreover, ineffective clearance of the pathogen occurs in response to P. aeruginosa–mediated phagocyte apoptosis (62, 105). This decreased clearance allows the bacteria to further degrade tight junctions (172), which induces the expression of adhesion molecules (173) that permit fluid and immune cell influx into the lung parenchyma and subsequently impairs gas exchange. Preferential damage to club cells in the bronchioles and type I pneumocytes in the alveoli increases the proliferation of mucin-producing goblet cells and surfactant-generating type II pneumocytes, respectively, thus altering the mix of cytochrome P450 enzymes that are produced by these cells to mediate the clearance and detoxification of AHR ligands (91). The AHR ligand, pyocyanin, also enhances the production of free radicals (77) that are also produced by neutrophil respiratory burst activity (115). Increased production of ROS, damage-associated molecular patterns, and pathogen-associated molecular patterns sustain cellular inflammasome activation and induce the production of mediators that enhance the alveolar filling process, dramatically decreasing gas exchange within the alveoli (174).
Last, bacterial virulence factors also have a role in promoting bacteremia. P. aeruginosa elastase degrades surfactant proteins A and D, which are known to opsonize bacteria and affect the phenotype and function of macrophages (93, 175). P. aeruginosa proteases that degrade tight junctions also degrade IL-22 (176), which protects the integrity of the epithelium (156). The combined effect is increased destruction of the epithelial barrier, which may allow the bacteria to interact with the endothelium, where proteases and toxins released from T2SS and T3SS further disrupt endothelial tight junctions and destroy endothelial cells (172). Migration of P. aeruginosa into the bloodstream produces bacteremia (29). In a murine in vivo model of severe infection, TLR-4–activated platelet-bound neutrophils within the vasculature significantly and predominantly induced NET activity, mediating intravascular bacterial trapping while at the same time causing tissue damage (177). In summary, the delicate balance between bacterial factors and host responses determines the severity of infection and thus host outcomes. Therefore, modulating bacterial factors, the host response to infection, or both offer opportunities for novel therapeutic interventions.
Novel Therapeutic Interventions
The acquisition of resistance genes (e.g., genes for β-lactamases and enzymes inactivating aminoglycosides), overexpression of efflux pumps, or decreased expression of porins may affect treatment success of P. aeruginosa infections (178). Ceftolozane/tazobactam and ceftazidime/avibactam are new combinations of β-lactam/β-lactamase inhibitors that have been approved for use against certain infections caused by multidrug-resistant strains of P. aeruginosa (179). Vaccines using live-attenuated or irradiated P. aeruginosa (180–182), the LPS O-antigen (52), a 3-oxo-C12-HSL-carrier protein conjugate (183), and various recombinant proteins (PcrV [184, 185], flagellin B [186], OprL [187], OprF [186], OprI [184, 186], OprF/I fusion [188], pili [189, 190], T6SS hemolysin coregulated protein 1 [184]) are being investigated to prevent P. aeruginosa infections. Because P. aeruginosa is a World Health Organization “Priority 1: Critical” pathogen in need of new approaches to treatment (19), preclinical models of biologics are being assessed (Table 1). Expanding on these factors that are unique to the pathogen as well as those linked to the host response may aid in devising new therapeutic interventions to combat P. aeruginosa infections.
Table 1.
Biologics Targeting Pseudomonas aeruginosa Pneumonia in Animal Models
| Target | Biologic | Model | Outcomes |
|---|---|---|---|
| LPS transport protein LptD | Intratracheal POL7001 protein epitope mimetic molecule (192) | Mouse | ↓ cfu/lung |
| Acute pneumonia | ↓ Leukocyte recruitment | ||
| ↓ Cytokine/chemokine profiles | |||
| Bacteriophage-induced Pseudomonas aeruginosa lysis | Intranasal PAK-P1 bacteriophage (245) | Mouse | ↓ IL-6 and TNF-α |
| Acute pneumonia | ↑ Survival rate | ||
| ↓ Bacterial load | |||
| Bacteriophage-induced P. aeruginosa lysis | Intranasal YH6 or YH30-phage (246, 247) | Mouse or mink | ↓ cfu/lung, spleen, blood |
| Hemorrhagic pneumonia | ↑ Survival rate | ||
| PcrV, nonspecific P. aeruginosa neutralization, FcγRs on target cells | Intravenous anti-PcrV antibodies and immunoglobulins (248) | Mouse | ↓ cfu/lung |
| Lethal P. aeruginosa infection | ↑ Survival rate | ||
| ↓ Cytokines | |||
| Nonspecific P. aeruginosa neutralization, FcγRs on target cells | Intravenous immunoglobulins (249) | Immunocompromised mice | ↑ Serum TGF-β |
| Acute pneumonia | ↑ T regulatory cells | ||
| ↓ Lung injury | |||
| Lymphocytes | Recombinant human IL-7 immunotherapy via subcutaneous injection (250) | Murine secondary pneumonia (intratracheal P. aeruginosa infection subsequent to cecal ligation puncture) | ↑ Lung ILCs and CD8 T cells expressing IL-17, IFN-γ, TNF-α |
| ↑ Survival rate | |||
| ↑ Spleen T cells expressing IFN-γ, TNF-α, IL-17, IL-22 | |||
| Pyocin S2, pyocin AP41 (DNase activity), pyocin S5 (pore-forming toxin), pyocin L1 (cytotoxic mechanism is unknown) | Intranasal or peritoneal recombinant protein toxins (molecules produced by P. aeruginosa to inhibit the growth of closely related strains) “pyocin cocktail” (251) | Murine acute lung injury | ↓ Bacterial counts |
| ↑ Survival rate | |||
| Pyocin SD2 (tRNase activity) | Intranasal recombinant P. aeruginosa SD2 1 h postinfection (209) | Murine | ↓ Bacterial counts |
| Acute lung injury | ↑ Survival rate | ||
| 3-oxo-C12-HSL | Intratracheal lactonase (252) | Rat | ↑ Survival rate |
| Acute lung injury | ↓ Lung injury | ||
| All TLR-4/MD2–expressing cells, primarily immune cells | Intraperitoneal TLR-4/MD2 agonistic monoclonal antibody UT12 (253) | Murine chronic lung infection (bronchi-implanted sterile plastic P. aeruginosa–coated tubes) | ↓ cfu counts/lung |
| ↑ Neutrophil recruitment and bactericidal activity | |||
| P. aeruginosa outer membrane | Aerosolized recombinant human lysozyme (254) | Hamster | ↓ cfu/lung |
| Acute pneumonia | ↓ BAL neutrophils | ||
| ↓ Lung injury | |||
| P. aeruginosa flagella | Intravenous anti–type a and type b flagellar protein antibodies (36) | Rat | ↑ PaO2 |
| Acute pneumonia | ↓ Respiratory rate | ||
| ↓ Lung injury | |||
| P. aeruginosa quorum sensing | Subcutaneous injections of a halogenated furanone (C-30) (255) | Murine | ↓ cfu counts/lung |
| Acute lung injury | ↓ QS as identified by LasB-GFP reporter in infected lungs |
Definition of abbreviations: cfu = colony-forming unit; HSL = homoserine lactone; ILC = innate lymphoid cell; LasB-GFP = las gene that encodes elastase labeled with green fluorescent protein; QS = quorum sensing; TGF = transforming growth factor; TNF = tumor necrosis factor; TLR = Toll-like receptor.
Interventions Targeting the Pathogen
Protein Epitope Mimetics
Small synthetic molecules called protein epitope mimetics resemble functional epitopes of physiologically important proteins. A protein epitope mimetic of LptD, a porin protein critical to LPS transport to the outer membrane, has demonstrated antimicrobial activity in vitro (191). Intratracheal administration of this mimetic significantly reduced lung bacterial counts and pulmonary inflammation in an in vivo P. aeruginosa pneumonia mouse model (192) (Table 1). LptE forms a complex with LptD and is being similarly assessed as a target for peptidomimetic antibiotics (191, 193). In addition to targeting LPS transport, examination of antibiotic resistance OM proteins (e.g., OprF, -H, -J, -L, -M, and -N) or structural proteins of secretion systems (T2SS, T3SS, and T6SS) as potential targets of protein mimetics or binding molecules such as aptamers may provide a needed mechanism in treating resistant P. aeruginosa infections.
Iron
P. aeruginosa siderophores, pyochelin and pyoverdine, sequester iron from the surrounding environment and are respectively internalized by their outer membrane receptors, FpvA and FptA (194). Tagging pyochelin or pyoverdine with an antibiotic or a redox-inactive metal ion such as gallium, which interferes with P. aeruginosa iron metabolism, has been proposed as a potential therapeutic intervention (194). In the absence of siderophores, some P. aeruginosa strains may use another iron chelating system involving nicotianamine (171), which could be similarly explored.
Pyocyanin
Strategies to neutralize pyocyanin activity or block pyocyanin production in the host with antioxidants, antibodies, or acyl-homoserine lactone analogs may prevent severe P. aeruginosa infections (195, 196) (Table 1). Selective estrogen receptor modulators have unique effects on pyocyanin production. One member of this class of drugs, raloxifene, binds and inhibits the activity of a P. aeruginosa biosynthesis protein, PhzB2, involved in the conversion of chorismate to pyocyanin (197). In additional in vitro analyses, raloxifene and similar compounds (toremifene, tamoxifen) in combination with polymyxin B were shown to exhibit synergistic activity against polymyxin-resistant P. aeruginosa proliferation and survival (198), suggesting that modifying cell signals leading to pyocyanin production may be beneficial in severe infections.
Adhesion Factors
Because flagella and pili are the primary means by which P. aeruginosa adheres to the host (36, 37), these molecules are being explored as therapeutic targets. Human antiflagellar antibodies administered intravenously have shown efficacy in a rat P. aeruginosa pneumonia model (36) (Table 1). Studies with chicken antiflagellar antibodies have shown a potential prophylactic effect against P. aeruginosa colonization in patients with cystic fibrosis who gargled the antibodies (199, 200). In a murine burn wound sepsis model involving P. aeruginosa, mouse antiflagellar antibodies given intraperitoneally significantly improved morbidity and mortality (201), highlighting the therapeutic potential of antiflagellar antibodies. With respect to pili, extensive study of P. aeruginosa type IV pili biogenesis is anticipated to identify small molecule inhibitors that may block the dynamic assembly and disassembly of pili or the adhesion of pili (202, 203). However, P. aeruginosa pili and flagella are also susceptible to genetic changes in expression in certain microenvironments and species, and this may create difficulties in targeting these adhesive factors (204–206).
Bacteriocins and QS Inhibition
Bacteriocins are bacterial toxins that an individual bacterium uses to kill neighboring bacteria (207). Bacteria have also devised mechanisms to inhibit QS of neighboring bacteria. This phenomenon is termed quorum quenching and occurs by bacterial production of bioactive molecules that affect a neighboring cell’s autoinducer gene activation, structure, or receptor binding interactions (208). Attempts to identify and engineer P. aeruginosa–specific bacteriocins called pyocins, native quorum quenchers, and synthetic or plant-derived QS inhibitors as antimicrobial agents against P. aeruginosa are in process. For example, the pyocin SD2 binds the common polysaccharide antigen of P. aeruginosa LPS and interacts with an outer membrane receptor, which facilitates the killing of the microbe (209). In diabetic foot ulcer isolates, a quorum quencher lactonase (SsoPox-W263I), which cleaves acyl side chains from 3-oxo-C12-HSL (210), inhibits the virulence of P. aeruginosa by decreasing the secretion of proteases and pyocyanin (211). The PA2385 gene of P. aeruginosa encodes a quorum quencher acylase that removes the fatty acid side chain from 3-oxo-C12-HSL and effectively reduces the production of PQS, elastase, and pyocyanin in vitro (212). A synthetic C4-HSL inhibitor, N-(2-pyrimidyl) butanamide, called C11, decreases expression levels of both las and rhl virulence genes (213). Targeting pqsA via substrates that bind the gene reduces autoinducer production for this pathway (214). The compounds brominated furanone C-30 and 5-fluorouracil as well as plant-derived zingerone from ginger root and garlic extract also inhibit P. aeruginosa QS systems in vitro (215–218). Additional in vivo studies are highlighted in Table 1.
Bacteriophages
Bacteriophages are ubiquitously expressed in the biosphere and provide a reservoir of genetic diversity to the bacterial hosts they infect (219). These bacterial viruses, consisting of a single-stranded or double-stranded DNA or RNA, either integrate their genome into the host chromosome or inject and replicate their DNA in the host, resulting in lytic death and the release of progeny phages (219, 220). Lytic phages (Myoviridae, Podoviridae, Siphoviridae) are being assessed for the treatment of P. aeruginosa infections (220). Some of these bacteriophages also bind to additional intervention targets. For example, the Myoviridae phage ϕCTX uses LPS as a receptor, and the Podoviridae 116 phage as well as Siphoviridae D3112 and B3 phages bind type IV pili (219). A recently isolated Myoviridae, OMKO1, depends on the porin OprM for entry, and, in the absence of OprM, P. aeruginosa becomes resistant to the phage but susceptible to drugs such as tetracycline and erythromycin, because these antibiotics are commonly removed from the bacterium by OprM-specific multidrug efflux systems (MexAb-OprM, MexXY-OprM) (221). This research suggests that microbial defenses involved in the generation of phage resistance may also increase the microbe’s susceptibility to antibiotics, which may suggest that these approaches could be combined therapeutically (221). Bacteriophages, used individually or as a mixture of various phages, have also shown improved survival in some animal models of P. aeruginosa infection (220) (Table 1). Despite regulatory hurdles involved in the manufacturing and handling of bacteriophages for personalized patient use as well as safety concerns regarding the host immune response and changes to mucosal microbiota (222–224), bacteriophages continue to be evaluated as noted in a clinical trial involving patients with severe burns or diabetic foot ulcers (225).
Interventions Targeting the Host
IL-22
In animal models of ventilator-induced lung injury or allergic inflammation, inhalation or intranasal doses of IL-22 ameliorated disease progression (226). Because IL-22 supports epithelial integrity (156, 227, 228), and the activity of the cytokine may be weakened by P. aeruginosa protease degradation (176), inhalation therapy with IL-22 may be an approach to protect host airways against P. aeruginosa damage.
Desulfated Heparin
During a bacterial infection, heparin may inhibit the activation of platelets and disrupts neutrophil NET activity (229, 230). Heparin also inhibits mast cell degranulation, which may be important in mast cell protection of the host epithelium (231). However, the clinical use of heparin in the treatment of patients with severe infections has not shown significant benefits (229). Partially desulfated heparin (2-O, 3-O-desulfated heparin: ODSH), a heparin derivative with significant antiinflammatory properties but minimal anticoagulative effects, reduced P. aeruginosa–induced neutrophilic lung injury and also increased P. aeruginosa clearance when delivered subcutaneously to mice. This may be due to the ability of ODSH to inhibit neutrophil elastase-induced macrophage release of the proinflammatory molecule high-mobility group box 1, which is known to bind to TLR-2, TLR-4, and the receptor for advanced glycation end products (RAGE) (232, 233). Understanding the functions of ODSH with respect to mast cells, platelets, or additional cells in the lung may increase insight into the use of ODSH as a therapeutic for P. aeruginosa infections.
ACE2 Activation
Mast cells produce heparin and renin (234). The latter cleaves angiotensinogen into angiotensin I, which is also cleaved by angiotensin-converting enzyme (ACE) into angiotensin II, which then binds type 1 and type 2 receptors involved in vasoconstriction and blood pressure regulation (235). The ACE homolog, ACE2, further cleaves angiotensin II into angiotensin 1 to 7, inactivating angiotensin II, thus functioning as an endogenous inhibitor of the ACE pathway (235). In a murine ACE2 knockout model of lung injury, knockout mice exhibited increased vascular permeability, lung edema, and neutrophil influx in association with decreased lung function, which is a phenotype that was rescued with the administration of recombinant ACE2 or with a pharmacological inhibitor of the angiotensin II type 1 receptor. Additional research in this study also revealed that ACE knockout mice showed reduced disease severity, whereas ACE2 knockout mice increased disease severity in an endotoxin-induced model of lung injury (236), highlighting the divergent function of these two enzymes. These responses may be explained by ACE2 catabolism of angiotensin II into seven peptides (angiotensin 1–7) that exhibit additional mechanisms that are not yet elucidated (235). These studies emphasize the potential of angiotensin II type 1 receptor inhibitors, ACE inhibitors, ACE2, and possibly angiotensins 1 to 7 in treating lung infections.
Interventions Targeting the Host and the Pathogen
Indoles
C-type natriuretic peptide is produced by lung epithelial, club, and endothelial cells and activates QS via the bacterial sensor protein, AmiC (237). The molecule AmiC has structural and pharmacological profiles similar to those of the human C-type natriuretic peptide receptor and is similarly inhibited by an interspecies indole called indole-2,3-dione or isatin, thus inhibiting QS-mediated virulence (237). The molecules 7-fluoroindole and curcumin are also able to inhibit P. aeruginosa QS signals in vitro and block pyocyanin production (238, 239). Indoles and curcumin are AHR ligands where dietary indole-3-carbinol and intraperitoneally administered curcumin have been shown to suppress LPS-induced lung injury in murine models (240, 241). If AHR ligands, particularly the indoles, also affect the C-type natriuretic peptide receptor AmiC is not clear. Nonetheless, nontoxic AHR ligands may be beneficial in targeting P. aeruginosa virulence factors and modulating the immune response to P. aeruginosa.
Cationic Molecules
Lactoferrin and LL-37 are both cationic molecules released from neutrophils that can then neutralize LPS but also function in the activation of TLR-4 (83, 242). Although the characterized functions of these molecules confound possible therapeutic approaches, they are pivotal to understanding pathogenesis. For example, lactoferrin also sequesters iron as a mechanism of host defense, where P. aeruginosa pyoverdine has been found to acquire the metal from lactoferrin (243). The peptide fragment of lactoferrin, lactoferricin, also exhibits P. aeruginosa antimicrobial functions but lacks the ability to sequester iron, thereby suggesting an alternative antibacterial mechanism (244). LL-37 is an activator of the QS system (35) (Figure 2), suggesting that blocking LL-37 activity could be therapeutic. Continued exploration of lactoferrin, lactoferricin, and LL-37 may yield valuable insight into P. aeruginosa infections and treatment.
Conclusions
Current therapies are increasingly insufficient in the treatment of P. aeruginosa infections. The ability of the microbe to readily adapt to the host microenvironment, by modulating the expression of cell surface molecules and virulence factors, directly affects the efficiency of the host’s innate and adaptive immune responses. This constant interplay between the microbe and host requires new and innovative approaches to effectively eradicate the pathogen from the most vulnerable populations. With increased resistance to antibiotics, effective treatments in the future may include combined therapies involving a QS inhibitor, pyocins, bacteriophages, an immunomodulatory agent, or neutralizing antibody or aptamer with conventional antibiotic therapy to increase the clinical cure rate and improve survival.
Acknowledgments
Acknowledgment
The authors thank informationist Judith Welsh, National Institutes of Health Library, for developing database search strategies and performing literature searches in support of this literature review. They also thank Anthony F. Suffredini, M.D., for critical review of the manuscript.
Footnotes
Supported by NIH, Clinical Center, Intramural Research Funds.
Author Contributions: All authors contributed to the work presented in this paper. P.T.-P. and C.S.C. jointly conceived the study. Article format and design was set forth by C.S.C. P.T.-P., T.B., and C.S.C. wrote and edited the manuscript. T.B. and C.S.C. illustrated the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201705-1043SO on October 31, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kizny Gordon AE, Mathers AJ, Cheong EYL, Gottlieb T, Kotay S, Walker AS, et al. The hospital water environment as a reservoir for carbapenem-resistant organisms causing hospital-acquired infections: a systematic review of the literature. Clin Infect Dis. 2017;64:1435–1444. doi: 10.1093/cid/cix132. [DOI] [PubMed] [Google Scholar]
- 2.Nathwani D, Raman G, Sulham K, Gavaghan M, Menon V. Clinical and economic consequences of hospital-acquired resistant and multidrug-resistant Pseudomonas aeruginosa infections: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2014;3:32. doi: 10.1186/2047-2994-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gellatly SL, Hancock RE. Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis. 2013;67:159–173. doi: 10.1111/2049-632X.12033. [DOI] [PubMed] [Google Scholar]
- 4.Mayer-Hamblett N, Rosenfeld M, Gibson RL, Ramsey BW, Kulasekara HD, Retsch-Bogart GZ, et al. Pseudomonas aeruginosa in vitro phenotypes distinguish cystic fibrosis infection stages and outcomes. Am J Respir Crit Care Med. 2014;190:289–297. doi: 10.1164/rccm.201404-0681OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy TF, Brauer AL, Eschberger K, Lobbins P, Grove L, Cai X, et al. Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:853–860. doi: 10.1164/rccm.200709-1413OC. [DOI] [PubMed] [Google Scholar]
- 6.Murphy TF. Pseudomonas aeruginosa in adults with chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2009;15:138–142. doi: 10.1097/MCP.0b013e328321861a. [DOI] [PubMed] [Google Scholar]
- 7.Bhagirath AY, Li Y, Somayajula D, Dadashi M, Badr S, Duan K. Cystic fibrosis lung environment and Pseudomonas aeruginosa infection. BMC Pulm Med. 2016;16:174. doi: 10.1186/s12890-016-0339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Folkesson A, Jelsbak L, Yang L, Johansen HK, Ciofu O, Høiby N, et al. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: an evolutionary perspective. Nat Rev Microbiol. 2012;10:841–851. doi: 10.1038/nrmicro2907. [DOI] [PubMed] [Google Scholar]
- 9.Hassett DJ, Borchers MT, Panos RJ. Chronic obstructive pulmonary disease (COPD): evaluation from clinical, immunological and bacterial pathogenesis perspectives. J Microbiol. 2014;52:211–226. doi: 10.1007/s12275-014-4068-2. [DOI] [PubMed] [Google Scholar]
- 10.Gaynes R, Edwards JR National Nosocomial Infections Surveillance System. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis. 2005;41:848–854. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 11.Weiner LM, Webb AK, Limbago B, Dudeck MA, Patel J, Kallen AJ, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011-2014. Infect Control Hosp Epidemiol. 2016;37:1288–1301. doi: 10.1017/ice.2016.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, et al. Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melsen WG, Rovers MM, Groenwold RH, Bergmans DC, Camus C, Bauer TT, et al. Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis. 2013;13:665–671. doi: 10.1016/S1473-3099(13)70081-1. [DOI] [PubMed] [Google Scholar]
- 15.Parker CM, Kutsogiannis J, Muscedere J, Cook D, Dodek P, Day AG, et al. Canadian Critical Care Trials Group. Ventilator-associated pneumonia caused by multidrug-resistant organisms or Pseudomonas aeruginosa: prevalence, incidence, risk factors, and outcomes. J Crit Care. 2008;23:18–26. doi: 10.1016/j.jcrc.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Montero M, Sala M, Riu M, Belvis F, Salvado M, Grau S, et al. Risk factors for multidrug-resistant Pseudomonas aeruginosa acquisition: impact of antibiotic use in a double case-control study. Eur J Clin Microbiol Infect Dis. 2010;29:335–339. doi: 10.1007/s10096-009-0850-1. [DOI] [PubMed] [Google Scholar]
- 17.Livermore DM. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin Infect Dis. 2002;34:634–640. doi: 10.1086/338782. [DOI] [PubMed] [Google Scholar]
- 18.Zilberberg MD, Shorr AF. Prevalence of multidrug-resistant Pseudomonas aeruginosa and carbapenem-resistant Enterobacteriaceae among specimens from hospitalized patients with pneumonia and bloodstream infections in the United States from 2000 to 2009. J Hosp Med. 2013;8:559–563. doi: 10.1002/jhm.2080. [DOI] [PubMed] [Google Scholar]
- 19.Tacconelli E, Magrini N. World Health Organization. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. 2017 [accepted 2018 Feb 12]. Available from: http://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/ [Google Scholar]
- 20.Aguilar-Rodea P, Zúñiga G, Rodríguez-Espino BA, Olivares Cervantes AL, Gamiño Arroyo AE, Moreno-Espinosa S, et al. Identification of extensive drug resistant Pseudomonas aeruginosa strains: new clone ST1725 and high-risk clone ST233. PLoS One. 2017;12:e0172882. doi: 10.1371/journal.pone.0172882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 22.Crouch Brewer S, Wunderink RG, Jones CB, Leeper KV., Jr Ventilator-associated pneumonia due to Pseudomonas aeruginosa. Chest. 1996;109:1019–1029. doi: 10.1378/chest.109.4.1019. [DOI] [PubMed] [Google Scholar]
- 23.Taylor GD, Buchanan-Chell M, Kirkland T, McKenzie M, Wiens R. Bacteremic nosocomial pneumonia: a 7-year experience in one institution. Chest. 1995;108:786–788. doi: 10.1378/chest.108.3.786. [DOI] [PubMed] [Google Scholar]
- 24.Fujitani S, Sun HY, Yu VL, Weingarten JA. Pneumonia due to Pseudomonas aeruginosa: part I: epidemiology, clinical diagnosis, and source. Chest. 2011;139:909–919. doi: 10.1378/chest.10-0166. [DOI] [PubMed] [Google Scholar]
- 25.Martis N, Leroy S, Blanc V. Colistin in multi-drug resistant Pseudomonas aeruginosa blood-stream infections: a narrative review for the clinician. J Infect. 2014;69:1–12. doi: 10.1016/j.jinf.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Bucior I, Pielage JF, Engel JN. Pseudomonas aeruginosa pili and flagella mediate distinct binding and signaling events at the apical and basolateral surface of airway epithelium. PLoS Pathog. 2012;8:e1002616. doi: 10.1371/journal.ppat.1002616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Golovkine G, Faudry E, Bouillot S, Elsen S, Attrée I, Huber P. Pseudomonas aeruginosa transmigrates at epithelial cell-cell junctions, exploiting sites of cell division and senescent cell extrusion. PLoS Pathog. 2016;12:e1005377. doi: 10.1371/journal.ppat.1005377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rangel SM, Diaz MH, Knoten CA, Zhang A, Hauser AR. The role of ExoS in dissemination of Pseudomonas aeruginosa during pneumonia. PLoS Pathog. 2015;11:e1004945. doi: 10.1371/journal.ppat.1004945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadikot RT, Blackwell TS, Christman JW, Prince AS. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med. 2005;171:1209–1223. doi: 10.1164/rccm.200408-1044SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seligman R, Ramos-Lima LF, Oliveira VdoA, Sanvicente C, Sartori J, Pacheco EF. Risk factors for infection with multidrug-resistant bacteria in non-ventilated patients with hospital-acquired pneumonia. J Bras Pneumol. 2013;39:339–348. doi: 10.1590/S1806-37132013000300011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Segal LN, Blaser MJ. A brave new world: the lung microbiota in an era of change. Ann Am Thorac Soc. 2014;11:S21–S27. doi: 10.1513/AnnalsATS.201306-189MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clarke TB. Early innate immunity to bacterial infection in the lung is regulated systemically by the commensal microbiota via nod-like receptor ligands. Infect Immun. 2014;82:4596–4606. doi: 10.1128/IAI.02212-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bucior I, Mostov K, Engel JN. Pseudomonas aeruginosa-mediated damage requires distinct receptors at the apical and basolateral surfaces of the polarized epithelium. Infect Immun. 2010;78:939–953. doi: 10.1128/IAI.01215-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berube BJ, Rangel SM, Hauser AR. Pseudomonas aeruginosa: breaking down barriers. Curr Genet. 2016;62:109–113. doi: 10.1007/s00294-015-0522-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee J, Zhang L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell. 2015;6:26–41. doi: 10.1007/s13238-014-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landsperger WJ, Kelly-Wintenberg KD, Montie TC, Knight LS, Hansen MB, Huntenburg CC, et al. Inhibition of bacterial motility with human antiflagellar monoclonal antibodies attenuates Pseudomonas aeruginosa-induced pneumonia in the immunocompetent rat. Infect Immun. 1994;62:4825–4830. doi: 10.1128/iai.62.11.4825-4830.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chua SL, Yam JK, Hao P, Adav SS, Salido MM, Liu Y, et al. Selective labelling and eradication of antibiotic-tolerant bacterial populations in Pseudomonas aeruginosa biofilms. Nat Commun. 2016;7:10750. doi: 10.1038/ncomms10750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sampedro I, Parales RE, Krell T, Hill JE. Pseudomonas chemotaxis. FEMS Microbiol Rev. 2015;39:17–46. doi: 10.1111/1574-6976.12081. [DOI] [PubMed] [Google Scholar]
- 39.Balloy V, Verma A, Kuravi S, Si-Tahar M, Chignard M, Ramphal R. The role of flagellin versus motility in acute lung disease caused by Pseudomonas aeruginosa. J Infect Dis. 2007;196:289–296. doi: 10.1086/518610. [DOI] [PubMed] [Google Scholar]
- 40.Arora SK, Ritchings BW, Almira EC, Lory S, Ramphal R. The Pseudomonas aeruginosa flagellar cap protein, FliD, is responsible for mucin adhesion. Infect Immun. 1998;66:1000–1007. doi: 10.1128/iai.66.3.1000-1007.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adamo R, Sokol S, Soong G, Gomez MI, Prince A. Pseudomonas aeruginosa flagella activate airway epithelial cells through asialoGM1 and toll-like receptor 2 as well as toll-like receptor 5. Am J Respir Cell Mol Biol. 2004;30:627–634. doi: 10.1165/rcmb.2003-0260OC. [DOI] [PubMed] [Google Scholar]
- 42.Proft T, Baker EN. Pili in Gram-negative and Gram-positive bacteria: structure, assembly and their role in disease. Cell Mol Life Sci. 2009;66:613–635. doi: 10.1007/s00018-008-8477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim S, Rahman M, Seol SY, Yoon SS, Kim J. Pseudomonas aeruginosa bacteriophage PA1Ø requires type IV pili for infection and shows broad bactericidal and biofilm removal activities. Appl Environ Microbiol. 2012;78:6380–6385. doi: 10.1128/AEM.00648-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hancock RE, Brinkman FS. Function of pseudomonas porins in uptake and efflux. Annu Rev Microbiol. 2002;56:17–38. doi: 10.1146/annurev.micro.56.012302.160310. [DOI] [PubMed] [Google Scholar]
- 45.Masuda N, Sakagawa E, Ohya S. Outer membrane proteins responsible for multiple drug resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:645–649. doi: 10.1128/AAC.39.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nikaido H. Preventing drug access to targets: cell surface permeability barriers and active efflux in bacteria. Semin Cell Dev Biol. 2001;12:215–223. doi: 10.1006/scdb.2000.0247. [DOI] [PubMed] [Google Scholar]
- 47.Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, et al. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol. 2002;169:10–14. doi: 10.4049/jimmunol.169.1.10. [DOI] [PubMed] [Google Scholar]
- 48.Sugawara E, Nagano K, Nikaido H. Alternative folding pathways of the major porin OprF of Pseudomonas aeruginosa. FEBS J. 2012;279:910–918. doi: 10.1111/j.1742-4658.2012.08481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kucharska I, Liang B, Ursini N, Tamm LK. Molecular interactions of lipopolysaccharide with an outer membrane protein from Pseudomonas aeruginosa probed by solution NMR. Biochemistry. 2016;55:5061–5072. doi: 10.1021/acs.biochem.6b00630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin YM, Wu SJ, Chang TW, Wang CF, Suen CS, Hwang MJ, et al. Outer membrane protein I of Pseudomonas aeruginosa is a target of cationic antimicrobial peptide/protein. J Biol Chem. 2010;285:8985–8994. doi: 10.1074/jbc.M109.078725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang TW, Wang CF, Huang HJ, Wang I, Hsu ST, Liao YD. Key residues of outer membrane protein OprI involved in hexamer formation and bacterial susceptibility to cationic antimicrobial peptides. Antimicrob Agents Chemother. 2015;59:6210–6222. doi: 10.1128/AAC.01406-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pier GB. Pseudomonas aeruginosa lipopolysaccharide: a major virulence factor, initiator of inflammation and target for effective immunity. Int J Med Microbiol. 2007;297:277–295. doi: 10.1016/j.ijmm.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okuda S, Sherman DJ, Silhavy TJ, Ruiz N, Kahne D. Lipopolysaccharide transport and assembly at the outer membrane: the PEZ model. Nat Rev Microbiol. 2016;14:337–345. doi: 10.1038/nrmicro.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chaturongakul S, Ounjai P. Phage-host interplay: examples from tailed phages and Gram-negative bacterial pathogens. Front Microbiol. 2014;5:442. doi: 10.3389/fmicb.2014.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Girardin SE, Travassos LH, Hervé M, Blanot D, Boneca IG, Philpott DJ, et al. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J Biol Chem. 2003;278:41702–41708. doi: 10.1074/jbc.M307198200. [DOI] [PubMed] [Google Scholar]
- 56.Girardin SE, Boneca IG, Carneiro LAM, Antignac A, Jéhanno M, Viala J, et al. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science. 2003;300:1584–1587. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- 57.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 58.Opal SM, Laterre PF, Francois B, LaRosa SP, Angus DC, Mira JP, et al. ACCESS Study Group. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA. 2013;309:1154–1162. doi: 10.1001/jama.2013.2194. [DOI] [PubMed] [Google Scholar]
- 59.Green ER, Mecsas J. Bacterial secretion systems: an overview. Microbiol Spectr. 2016;4:1–32. doi: 10.1128/microbiolspec.VMBF-0012-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bleves S, Viarre V, Salacha R, Michel GP, Filloux A, Voulhoux R. Protein secretion systems in Pseudomonas aeruginosa: a wealth of pathogenic weapons. Int J Med Microbiol. 2010;300:534–543. doi: 10.1016/j.ijmm.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 61.Korotkov KV, Sandkvist M, Hol WG. The type II secretion system: biogenesis, molecular architecture and mechanism. Nat Rev Microbiol. 2012;10:336–351. doi: 10.1038/nrmicro2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Michalska M, Wolf P. Pseudomonas exotoxin A: optimized by evolution for effective killing. Front Microbiol. 2015;6:963. doi: 10.3389/fmicb.2015.00963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hauser AR. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat Rev Microbiol. 2009;7:654–665. doi: 10.1038/nrmicro2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.François B, Luyt CE, Dugard A, Wolff M, Diehl JL, Jaber S, et al. Safety and pharmacokinetics of an anti-PcrV PEGylated monoclonal antibody fragment in mechanically ventilated patients colonized with Pseudomonas aeruginosa: a randomized,double-blind, placebo-controlled trial. Crit Care Med. 2012;40:2320–2326. doi: 10.1097/CCM.0b013e31825334f6. [DOI] [PubMed] [Google Scholar]
- 65.Thanabalasuriar A, Surewaard BG, Willson ME, Neupane AS, Stover CK, Warrener P, et al. Bispecific antibody targets multiple Pseudomonas aeruginosa evasion mechanisms in the lung vasculature. J Clin Invest. 2017;127:2249–2261. doi: 10.1172/JCI89652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Papenfort K, Bassler BL. Quorum sensing signal-response systems in Gram-negative bacteria. Nat Rev Microbiol. 2016;14:576–588. doi: 10.1038/nrmicro.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pearson JP, Van Delden C, Iglewski BH. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J Bacteriol. 1999;181:1203–1210. doi: 10.1128/jb.181.4.1203-1210.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsai CS, Winans SC. LuxR-type quorum-sensing regulators that are detached from common scents. Mol Microbiol. 2010;77:1072–1082. doi: 10.1111/j.1365-2958.2010.07279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wade DS, Calfee MW, Rocha ER, Ling EA, Engstrom E, Coleman JP, et al. Regulation of Pseudomonas quinolone signal synthesis in Pseudomonas aeruginosa. J Bacteriol. 2005;187:4372–4380. doi: 10.1128/JB.187.13.4372-4380.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Palmer GC, Jorth PA, Whiteley M. The role of two Pseudomonas aeruginosa anthranilate synthases in tryptophan and quorum signal production. Microbiology. 2013;159:959–969. doi: 10.1099/mic.0.063065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Knoten CA, Wells G, Coleman JP, Pesci EC. A conserved suppressor mutation in a tryptophan auxotroph results in dysregulation of Pseudomonas quinolone signal synthesis. J Bacteriol. 2014;196:2413–2422. doi: 10.1128/JB.01635-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rampioni G, Falcone M, Heeb S, Frangipani E, Fletcher MP, Dubern JF, et al. Unravelling the genome-wide contributions of specific 2-alkyl-4-quinolones and PqsE to quorum sensing in Pseudomonas aeruginosa. PLoS Pathog. 2016;12:e1006029. doi: 10.1371/journal.ppat.1006029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gilbert KB, Kim TH, Gupta R, Greenberg EP, Schuster M. Global position analysis of the Pseudomonas aeruginosa quorum-sensing transcription factor LasR. Mol Microbiol. 2009;73:1072–1085. doi: 10.1111/j.1365-2958.2009.06832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moradali MF, Ghods S, Rehm BH. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol. 2017;7:39. doi: 10.3389/fcimb.2017.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bortolotti D, LeMaoult J, Trapella C, Di Luca D, Carosella ED, Rizzo R. Pseudomonas aeruginosa quorum sensing molecule N-(3-oxododecanoyl)-L-homoserine-lactone induces HLA-G expression in human immune cells. Infect Immun. 2015;83:3918–3925. doi: 10.1128/IAI.00803-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hooi DS, Bycroft BW, Chhabra SR, Williams P, Pritchard DI. Differential immune modulatory activity of Pseudomonas aeruginosa quorum-sensing signal molecules. Infect Immun. 2004;72:6463–6470. doi: 10.1128/IAI.72.11.6463-6470.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O’Malley YQ, Reszka KJ, Spitz DR, Denning GM, Britigan BE. Pseudomonas aeruginosa pyocyanin directly oxidizes glutathione and decreases its levels in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L94–L103. doi: 10.1152/ajplung.00025.2004. [DOI] [PubMed] [Google Scholar]
- 78.Moura-Alves P, Faé K, Houthuys E, Dorhoi A, Kreuchwig A, Furkert J, et al. AhR sensing of bacterial pigments regulates antibacterial defence. Nature. 2014;512:387–392. doi: 10.1038/nature13684. [DOI] [PubMed] [Google Scholar]
- 79.Bortolotti P, Hennart B, Thieffry C, Jausions G, Faure E, Grandjean T, et al. Tryptophan catabolism in Pseudomonas aeruginosa and potential for inter-kingdom relationship. BMC Microbiol. 2016;16:137. doi: 10.1186/s12866-016-0756-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Esser C, Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol Rev. 2015;67:259–279. doi: 10.1124/pr.114.009001. [DOI] [PubMed] [Google Scholar]
- 81.Santhekadur PK, Kumar DP, Seneshaw M, Mirshahi F, Sanyal AJ. The multifaceted role of natriuretic peptides in metabolic syndrome. Biomed Pharmacother. 2017;92:826–835. doi: 10.1016/j.biopha.2017.05.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Blier AS, Veron W, Bazire A, Gerault E, Taupin L, Vieillard J, et al. C-type natriuretic peptide modulates quorum sensing molecule and toxin production in Pseudomonas aeruginosa. Microbiology. 2011;157:1929–1944. doi: 10.1099/mic.0.046755-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kahlenberg JM, Kaplan MJ. Little peptide, big effects: the role of LL-37 in inflammation and autoimmune disease. J Immunol. 2013;191:4895–4901. doi: 10.4049/jimmunol.1302005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Strempel N, Neidig A, Nusser M, Geffers R, Vieillard J, Lesouhaitier O, et al. Human host defense peptide LL-37 stimulates virulence factor production and adaptive resistance in Pseudomonas aeruginosa. PLoS One. 2013;8:e82240. doi: 10.1371/journal.pone.0082240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zaborina O, Lepine F, Xiao G, Valuckaite V, Chen Y, Li T, et al. Dynorphin activates quorum sensing quinolone signaling in Pseudomonas aeruginosa. PLoS Pathog. 2007;3:e35. doi: 10.1371/journal.ppat.0030035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mousa SA, Krajnik M, Sobanski P, Kowalewski J, Bloch-Boguslawska E, Zylicz Z, et al. Dynorphin expression, processing and receptors in the alveolar macrophages, cancer cells and bronchial epithelium of lung cancer patients. Histol Histopathol. 2010;25:755–764. doi: 10.14670/HH-25.755. [DOI] [PubMed] [Google Scholar]
- 87.Wu L, Estrada O, Zaborina O, Bains M, Shen L, Kohler JE, et al. Recognition of host immune activation by Pseudomonas aeruginosa. Science. 2005;309:774–777. doi: 10.1126/science.1112422. [DOI] [PubMed] [Google Scholar]
- 88.Tomashefski JF, Farver CF. Anatomy and histology of the lung. In: Tomashefski JF, Cagle PT, Farver CF, Fraire AE, editors. Dail and Hammar’s pulmonary pathology. Vol. I: Nonneoplastic lung disease. New York: Springer New York; 2008. pp. 20–48. [Google Scholar]
- 89.El Kaissouni J, Bene MC, Thionnois S, Monin P, Vidailhet M, Faure GC. Maturation of B cells in the lamina propria of human gut and bronchi in the first months of human life. Dev Immunol. 1998;5:153–159. doi: 10.1155/1998/42138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Williams OW, Sharafkhaneh A, Kim V, Dickey BF, Evans CM. Airway mucus: from production to secretion. Am J Respir Cell Mol Biol. 2006;34:527–536. doi: 10.1165/rcmb.2005-0436SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Castell JV, Donato MT, Gómez-Lechón MJ. Metabolism and bioactivation of toxicants in the lung: the in vitro cellular approach. Exp Toxicol Pathol. 2005;57:189–204. doi: 10.1016/j.etp.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 92.Aggarwal NR, King LS, D’Alessio FR. Diverse macrophage populations mediate acute lung inflammation and resolution. Am J Physiol Lung Cell Mol Physiol. 2014;306:L709–L725. doi: 10.1152/ajplung.00341.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yamashita CM, Veldhuizen RAW, Gill SE. Alveolar macrophages and pulmonary surfactant—more than just friendly neighbours. OA Biology. 2013;1 [Google Scholar]
- 94.Lovewell RR, Patankar YR, Berwin B. Mechanisms of phagocytosis and host clearance of Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol. 2014;306:L591–L603. doi: 10.1152/ajplung.00335.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mahenthiralingam E, Speert DP. Nonopsonic phagocytosis of Pseudomonas aeruginosa by macrophages and polymorphonuclear leukocytes requires the presence of the bacterial flagellum. Infect Immun. 1995;63:4519–4523. doi: 10.1128/iai.63.11.4519-4523.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Heale JP, Pollard AJ, Stokes RW, Simpson D, Tsang A, Massing B, et al. Two distinct receptors mediate nonopsonic phagocytosis of different strains of Pseudomonas aeruginosa. J Infect Dis. 2001;183:1214–1220. doi: 10.1086/319685. [DOI] [PubMed] [Google Scholar]
- 97.Reynolds HY, Kazmierowski JA, Newball HH. Specificity of opsonic antibodies to enhance phagocytosis of Pseudomonas aeruginosa by human alveolar macrophages. J Clin Invest. 1975;56:376–385. doi: 10.1172/JCI108102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mueller-Ortiz SL, Drouin SM, Wetsel RA. The alternative activation pathway and complement component C3 are critical for a protective immune response against Pseudomonas aeruginosa in a murine model of pneumonia. Infect Immun. 2004;72:2899–2906. doi: 10.1128/IAI.72.5.2899-2906.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peterson PK, Kim Y, Schmeling D, Lindemann M, Verhoef J, Quie PG. Complement-mediated phagocytosis of Pseudomonas aeruginosa. J Lab Clin Med. 1978;92:883–894. [PubMed] [Google Scholar]
- 100.Laarman AJ, Bardoel BW, Ruyken M, Fernie J, Milder FJ, van Strijp JA, et al. Pseudomonas aeruginosa alkaline protease blocks complement activation via the classical and lectin pathways. J Immunol. 2012;188:386–393. doi: 10.4049/jimmunol.1102162. [DOI] [PubMed] [Google Scholar]
- 101.Mariencheck WI, Savov J, Dong Q, Tino MJ, Wright JR. Surfactant protein A enhances alveolar macrophage phagocytosis of a live, mucoid strain of P. aeruginosa. Am J Physiol. 1999;277:L777–L786. doi: 10.1152/ajplung.1999.277.4.L777. [DOI] [PubMed] [Google Scholar]
- 102.Heck LW, Alarcon PG, Kulhavy RM, Morihara K, Russell MW, Mestecky JF. Degradation of IgA proteins by Pseudomonas aeruginosa elastase. J Immunol. 1990;144:2253–2257. [PubMed] [Google Scholar]
- 103.Schultz DR, Miller KD. Elastase of Pseudomonas aeruginosa: inactivation of complement components and complement-derived chemotactic and phagocytic factors. Infect Immun. 1974;10:128–135. doi: 10.1128/iai.10.1.128-135.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kuang Z, Hao Y, Walling BE, Jeffries JL, Ohman DE, Lau GW. Pseudomonas aeruginosa elastase provides an escape from phagocytosis by degrading the pulmonary surfactant protein-A. PLoS One. 2011;6:e27091. doi: 10.1371/journal.pone.0027091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Allen L, Dockrell DH, Pattery T, Lee DG, Cornelis P, Hellewell PG, et al. Pyocyanin production by Pseudomonas aeruginosa induces neutrophil apoptosis and impairs neutrophil-mediated host defenses in vivo. J Immunol. 2005;174:3643–3649. doi: 10.4049/jimmunol.174.6.3643. [DOI] [PubMed] [Google Scholar]
- 106.Bianchi SM, Prince LR, McPhillips K, Allen L, Marriott HM, Taylor GW, et al. Impairment of apoptotic cell engulfment by pyocyanin, a toxic metabolite of Pseudomonas aeruginosa. Am J Respir Crit Care Med. 2008;177:35–43. doi: 10.1164/rccm.200612-1804OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Losa García JE, Rodríguez FM, Martín de Cabo MR, García Salgado MJ, Losada JP, Villarón LG, et al. Evaluation of inflammatory cytokine secretion by human alveolar macrophages. Mediators Inflamm. 1999;8:43–51. doi: 10.1080/09629359990711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sun G, Liu F, Lin TJ. Identification of Pseudomonas aeruginosa-induced genes in human mast cells using suppression subtractive hybridization: up-regulation of IL-8 and CCL4 production. Clin Exp Immunol. 2005;142:199–205. doi: 10.1111/j.1365-2249.2005.02909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Look DC, Stoll LL, Romig SA, Humlicek A, Britigan BE, Denning GM. Pyocyanin and its precursor phenazine-1-carboxylic acid increase IL-8 and intercellular adhesion molecule-1 expression in human airway epithelial cells by oxidant-dependent mechanisms. J Immunol. 2005;175:4017–4023. doi: 10.4049/jimmunol.175.6.4017. [DOI] [PubMed] [Google Scholar]
- 110.Russo RC, Garcia CC, Teixeira MM, Amaral FA. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert Rev Clin Immunol. 2014;10:593–619. doi: 10.1586/1744666X.2014.894886. [DOI] [PubMed] [Google Scholar]
- 111.Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Roquilly A, McWilliam HEG, Jacqueline C, Tian Z, Cinotti R, Rimbert M, et al. Local modulation of antigen-presenting cell development after resolution of pneumonia induces long-term susceptibility to secondary infections. Immunity. 2017;47:135–147.e5. doi: 10.1016/j.immuni.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 113.Vaidyanathan B, Chaudhry A, Yewdell WT, Angeletti D, Yen WF, Wheatley AK, et al. The aryl hydrocarbon receptor controls cell-fate decisions in B cells. J Exp Med. 2017;214:197–208. doi: 10.1084/jem.20160789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rada B, Jendrysik MA, Pang L, Hayes CP, Yoo DG, Park JJ, et al. Pyocyanin-enhanced neutrophil extracellular trap formation requires the NADPH oxidase. PLoS One. 2013;8:e54205. doi: 10.1371/journal.pone.0054205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.El-Benna J, Hurtado-Nedelec M, Marzaioli V, Marie JC, Gougerot-Pocidalo MA, Dang PM. Priming of the neutrophil respiratory burst: role in host defense and inflammation. Immunol Rev. 2016;273:180–193. doi: 10.1111/imr.12447. [DOI] [PubMed] [Google Scholar]
- 116.Genestet C, Le Gouellec A, Chaker H, Polack B, Guery B, Toussaint B, et al. Scavenging of reactive oxygen species by tryptophan metabolites helps Pseudomonas aeruginosa escape neutrophil killing. Free Radic Biol Med. 2014;73:400–410. doi: 10.1016/j.freeradbiomed.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 117.Vareechon C, Zmina SE, Karmakar M, Pearlman E, Rietsch A. Pseudomonas aeruginosa effector ExoS inhibits ROS production in human neutrophils. Cell Host Microbe. 2017;21:611–618.e5. doi: 10.1016/j.chom.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lai DM, Shu Q, Fan J. The origin and role of innate lymphoid cells in the lung. Mil Med Res. 2016;3:25. doi: 10.1186/s40779-016-0093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Muir R, Osbourn M, Dubois AV, Doran E, Small DM, Monahan A, et al. Innate lymphoid cells are the predominant source of IL-17A during the early pathogenesis of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2016;193:407–416. doi: 10.1164/rccm.201410-1782OC. [DOI] [PubMed] [Google Scholar]
- 121.Baban B, Chandler PR, Sharma MD, Pihkala J, Koni PA, Munn DH, et al. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol. 2009;183:2475–2483. doi: 10.4049/jimmunol.0900986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gasteiger G, Fan X, Dikiy S, Lee SY, Rudensky AY. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science. 2015;350:981–985. doi: 10.1126/science.aac9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hwang JY, Randall TD, Silva-Sanchez A. Inducible bronchus-associated lymphoid tissue: taming inflammation in the lung. Front Immunol. 2016;7:258. doi: 10.3389/fimmu.2016.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sana TG, Berni B, Bleves S. The T6SSs of Pseudomonas aeruginosa strain PAO1 and their effectors: beyond bacterial-cell targeting. Front Cell Infect Microbiol. 2016;6:61. doi: 10.3389/fcimb.2016.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sana TG, Baumann C, Merdes A, Soscia C, Rattei T, Hachani A, et al. Internalization of Pseudomonas aeruginosa strain PAO1 into epithelial cells is promoted by interaction of a T6SS effector with the microtubule network. MBio. 2015;6:e00712. doi: 10.1128/mBio.00712-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kierbel A, Gassama-Diagne A, Mostov K, Engel JN. The phosphoinositol-3-kinase-protein kinase B/Akt pathway is critical for Pseudomonas aeruginosa strain PAK internalization. Mol Biol Cell. 2005;16:2577–2585. doi: 10.1091/mbc.E04-08-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tsang KW, Rutman A, Tanaka E, Lund V, Dewar A, Cole PJ, et al. Interaction of Pseudomonas aeruginosa with human respiratory mucosa in vitro. Eur Respir J. 1994;7:1746–1753. doi: 10.1183/09031936.94.07101746. [DOI] [PubMed] [Google Scholar]
- 128.Houdret N, Ramphal R, Scharfman A, Perini JM, Filliat M, Lamblin G, et al. Evidence for the in vivo degradation of human respiratory mucins during Pseudomonas aeruginosa infection. Biochim Biophys Acta. 1989;992:96–105. doi: 10.1016/0304-4165(89)90055-x. [DOI] [PubMed] [Google Scholar]
- 129.Wilson R, Pitt T, Taylor G, Watson D, MacDermot J, Sykes D, et al. Pyocyanin and 1-hydroxyphenazine produced by Pseudomonas aeruginosa inhibit the beating of human respiratory cilia in vitro. J Clin Invest. 1987;79:221–229. doi: 10.1172/JCI112787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hao Y, Kuang Z, Walling BE, Bhatia S, Sivaguru M, Chen Y, et al. Pseudomonas aeruginosa pyocyanin causes airway goblet cell hyperplasia and metaplasia and mucus hypersecretion by inactivating the transcriptional factor FoxA2. Cell Microbiol. 2012;14:401–415. doi: 10.1111/j.1462-5822.2011.01727.x. [DOI] [PubMed] [Google Scholar]
- 131.Harrod KS, Jaramillo RJ. Pseudomonas aeruginosa and tumor necrosis factor-alpha attenuate Clara cell secretory protein promoter function. Am J Respir Cell Mol Biol. 2002;26:216–223. doi: 10.1165/ajrcmb.26.2.4718. [DOI] [PubMed] [Google Scholar]
- 132.Chang H, Chang LW, Cheng YH, Tsai WT, Tsai MX, Lin P. Preferential induction of CYP1A1 and CYP1B1 in CCSP-positive cells. Toxicol Sci. 2006;89:205–213. doi: 10.1093/toxsci/kfj025. [DOI] [PubMed] [Google Scholar]
- 133.Beamer CA, Shepherd DM. Role of the aryl hydrocarbon receptor (AhR) in lung inflammation. Semin Immunopathol. 2013;35:693–704. doi: 10.1007/s00281-013-0391-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McElroy MC, Kasper M. The use of alveolar epithelial type I cell-selective markers to investigate lung injury and repair. Eur Respir J. 2004;24:664–673. doi: 10.1183/09031936.04.00096003. [DOI] [PubMed] [Google Scholar]
- 135.Hahn HP. The type-4 pilus is the major virulence-associated adhesin of Pseudomonas aeruginosa--a review. Gene. 1997;192:99–108. doi: 10.1016/s0378-1119(97)00116-9. [DOI] [PubMed] [Google Scholar]
- 136.Zaas DW, Duncan MJ, Li G, Wright JR, Abraham SN. Pseudomonas invasion of type I pneumocytes is dependent on the expression and phosphorylation of caveolin-2. J Biol Chem. 2005;280:4864–4872. doi: 10.1074/jbc.M411702200. [DOI] [PubMed] [Google Scholar]
- 137.Zaas DW, Swan ZD, Brown BJ, Li G, Randell SH, Degan S, et al. Counteracting signaling activities in lipid rafts associated with the invasion of lung epithelial cells by Pseudomonas aeruginosa. J Biol Chem. 2009;284:9955–9964. doi: 10.1074/jbc.M808629200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chi E, Mehl T, Nunn D, Lory S. Interaction of Pseudomonas aeruginosa with A549 pneumocyte cells. Infect Immun. 1991;59:822–828. doi: 10.1128/iai.59.3.822-828.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schmiedl A, Kerber-Momot T, Munder A, Pabst R, Tschernig T. Bacterial distribution in lung parenchyma early after pulmonary infection with Pseudomonas aeruginosa. Cell Tissue Res. 2010;342:67–73. doi: 10.1007/s00441-010-1036-y. [DOI] [PubMed] [Google Scholar]
- 140.Son A, Nakamura H, Kondo N, Matsuo Y, Liu W, Oka S, et al. Redox regulation of mast cell histamine release in thioredoxin-1 (TRX) transgenic mice. Cell Res. 2006;16:230–239. doi: 10.1038/sj.cr.7310031. [DOI] [PubMed] [Google Scholar]
- 141.Thornton EE, Looney MR, Bose O, Sen D, Sheppard D, Locksley R, et al. Spatiotemporally separated antigen uptake by alveolar dendritic cells and airway presentation to T cells in the lung. J Exp Med. 2012;209:1183–1199. doi: 10.1084/jem.20112667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Caucheteux SM, Torabi-Parizi P, Paul WE. Analysis of naïve lung CD4 T cells provides evidence of functional lung to lymph node migration. Proc Natl Acad Sci USA. 2013;110:1821–1826. doi: 10.1073/pnas.1221306110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kambayashi T, Laufer TM. Atypical MHC class II-expressing antigen-presenting cells: can anything replace a dendritic cell? Nat Rev Immunol. 2014;14:719–730. doi: 10.1038/nri3754. [DOI] [PubMed] [Google Scholar]
- 144.Miller H, Zhang J, Kuolee R, Patel GB, Chen W. Intestinal M cells: the fallible sentinels? World J Gastroenterol. 2007;13:1477–1486. doi: 10.3748/wjg.v13.i10.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mutoh M, Kimura S, Takahashi-Iwanaga H, Hisamoto M, Iwanaga T, Iida J. RANKL regulates differentiation of microfold cells in mouse nasopharynx-associated lymphoid tissue (NALT) Cell Tissue Res. 2016;364:175–184. doi: 10.1007/s00441-015-2309-2. [DOI] [PubMed] [Google Scholar]
- 146.Nair VR, Franco LH, Zacharia VM, Khan HS, Stamm CE, You W, et al. Microfold cells actively translocate mycobacterium tuberculosis to initiate infection. Cell Reports. 2016;16:1253–1258. doi: 10.1016/j.celrep.2016.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schultz MJ, Rijneveld AW, Speelman P, van Deventer SJ, van der Poll T. Endogenous interferon-gamma impairs bacterial clearance from lungs during Pseudomonas aeruginosa pneumonia. Eur Cytokine Netw. 2001;12:39–44. [PubMed] [Google Scholar]
- 148.Schultz MJ, Knapp S, Florquin S, Pater J, Takeda K, Akira S, et al. Interleukin-18 impairs the pulmonary host response to Pseudomonas aeruginosa. Infect Immun. 2003;71:1630–1634. doi: 10.1128/IAI.71.4.1630-1634.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kastenmüller W, Torabi-Parizi P, Subramanian N, Lämmermann T, Germain RN. A spatially-organized multicellular innate immune response in lymph nodes limits systemic pathogen spread. Cell. 2012;150:1235–1248. doi: 10.1016/j.cell.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Worbs T, Hammerschmidt SI, Förster R. Dendritic cell migration in health and disease. Nat Rev Immunol. 2017;17:30–48. doi: 10.1038/nri.2016.116. [DOI] [PubMed] [Google Scholar]
- 151.Worgall S, Kikuchi T, Singh R, Martushova K, Lande L, Crystal RG. Protection against pulmonary infection with Pseudomonas aeruginosa following immunization with P. aeruginosa-pulsed dendritic cells. Infect Immun. 2001;69:4521–4527. doi: 10.1128/IAI.69.7.4521-4527.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bayes HK, Bicknell S, MacGregor G, Evans TJ. T helper cell subsets specific for Pseudomonas aeruginosa in healthy individuals and patients with cystic fibrosis. PLoS One. 2014;9:e90263. doi: 10.1371/journal.pone.0090263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chauhan SK, Jin Y, Goyal S, Lee HS, Fuchsluger TA, Lee HK, et al. A novel pro-lymphangiogenic function for Th17/IL-17. Blood. 2011;118:4630–4634. doi: 10.1182/blood-2011-01-332049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Baluk P, Adams A, Phillips K, Feng J, Hong YK, Brown MB, et al. Preferential lymphatic growth in bronchus-associated lymphoid tissue in sustained lung inflammation. Am J Pathol. 2014;184:1577–1592. doi: 10.1016/j.ajpath.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liu J, Feng Y, Yang K, Li Q, Ye L, Han L, et al. Early production of IL-17 protects against acute pulmonary Pseudomonas aeruginosa infection in mice. FEMS Immunol Med Microbiol. 2011;61:179–188. doi: 10.1111/j.1574-695X.2010.00764.x. [DOI] [PubMed] [Google Scholar]
- 156.Manni ML, Robinson KM, Alcorn JF. A tale of two cytokines: IL-17 and IL-22 in asthma and infection. Expert Rev Respir Med. 2014;8:25–42. doi: 10.1586/17476348.2014.854167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wu W, Huang J, Duan B, Traficante DC, Hong H, Risech M, et al. Th17-stimulating protein vaccines confer protection against Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med. 2012;186:420–427. doi: 10.1164/rccm.201202-0182OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 161.Dudakov JA, Hanash AM, van den Brink MR. Interleukin-22: immunobiology and pathology. Annu Rev Immunol. 2015;33:747–785. doi: 10.1146/annurev-immunol-032414-112123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sonnenberg GF, Artis D. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat Med. 2015;21:698–708. doi: 10.1038/nm.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Noakes R. The aryl hydrocarbon receptor: a review of its role in the physiology and pathology of the integument and its relationship to the tryptophan metabolism. Int J Tryptophan Res. 2015;8:7–18. doi: 10.4137/IJTR.S19985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Feng L, Xiang Q, Ai Q, Wang Z, Zhang Y, Lu Q. Effects of quorum sensing systems on regulatory T cells in catheter-related Pseudomonas aeruginosa biofilm infection rat models. Mediators Inflamm. 2016;2016:4012912. doi: 10.1155/2016/4012912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pan T, Tan R, Li M, Liu Z, Wang X, Tian L, et al. IL17-producing γδ T cells may enhance humoral immunity during pulmonary Pseudomonas aeruginosa infection in mice. Front Cell Infect Microbiol. 2016;6:170. doi: 10.3389/fcimb.2016.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bayes HK, Ritchie ND, Evans TJ. Interleukin-17 is required for control of chronic lung infection caused by Pseudomonas aeruginosa. Infect Immun. 2016;84:3507–3516. doi: 10.1128/IAI.00717-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sørensen OE, Borregaard N. Neutrophil extracellular traps - the dark side of neutrophils. J Clin Invest. 2016;126:1612–1620. doi: 10.1172/JCI84538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Porto BN, Stein RT. Neutrophil extracellular traps in pulmonary diseases: too much of a good thing? Front Immunol. 2016;7:311. doi: 10.3389/fimmu.2016.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lau GW, Ran H, Kong F, Hassett DJ, Mavrodi D. Pseudomonas aeruginosa pyocyanin is critical for lung infection in mice. Infect Immun. 2004;72:4275–4278. doi: 10.1128/IAI.72.7.4275-4278.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Worlitzsch D, Tarran R, Ulrich M, Schwab U, Cekici A, Meyer KC, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest. 2002;109:317–325. doi: 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gi M, Lee KM, Kim SC, Yoon JH, Yoon SS, Choi JY. A novel siderophore system is essential for the growth of Pseudomonas aeruginosa in airway mucus. Sci Rep. 2015;5:14644. doi: 10.1038/srep14644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Golovkine G, Faudry E, Bouillot S, Voulhoux R, Attrée I, Huber P. VE-cadherin cleavage by LasB protease from Pseudomonas aeruginosa facilitates type III secretion system toxicity in endothelial cells. PLoS Pathog. 2014;10:e1003939. doi: 10.1371/journal.ppat.1003939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Moldoveanu B, Otmishi P, Jani P, Walker J, Sarmiento X, Guardiola J, et al. Inflammatory mechanisms in the lung. J Inflamm Res. 2009;2:1–11. [PMC free article] [PubMed] [Google Scholar]
- 174.dos Santos G, Kutuzov MA, Ridge KM. The inflammasome in lung diseases. Am J Physiol Lung Cell Mol Physiol. 2012;303:L627–L633. doi: 10.1152/ajplung.00225.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mariencheck WI, Alcorn JF, Palmer SM, Wright JR. Pseudomonas aeruginosa elastase degrades surfactant proteins A and D. Am J Respir Cell Mol Biol. 2003;28:528–537. doi: 10.1165/rcmb.2002-0141OC. [DOI] [PubMed] [Google Scholar]
- 176.Guillon A, Brea D, Morello E, Tang A, Jouan Y, Ramphal R, et al. Pseudomonas aeruginosa proteolytically alters the interleukin 22-dependent lung mucosal defense. Virulence. 2017;8:810–820. doi: 10.1080/21505594.2016.1253658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 178.Mesaros N, Nordmann P, Plésiat P, Roussel-Delvallez M, Van Eldere J, Glupczynski Y, et al. Pseudomonas aeruginosa: resistance and therapeutic options at the turn of the new millennium. Clin Microbiol Infect. 2007;13:560–578. doi: 10.1111/j.1469-0691.2007.01681.x. [DOI] [PubMed] [Google Scholar]
- 179.Gentile I, Maraolo AE, Borgia G. What is the role of the new β-lactam/β-lactamase inhibitors ceftolozane/tazobactam and ceftazidime/avibactam? Expert Rev Anti Infect Ther. 2016;14:875–878. doi: 10.1080/14787210.2016.1233060. [DOI] [PubMed] [Google Scholar]
- 180.Priebe GP, Meluleni GJ, Coleman FT, Goldberg JB, Pier GB. Protection against fatal Pseudomonas aeruginosa pneumonia in mice after nasal immunization with a live, attenuated aroA deletion mutant. Infect Immun. 2003;71:1453–1461. doi: 10.1128/IAI.71.3.1453-1461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kamei A, Wu W, Traficante DC, Koh AY, Van Rooijen N, Pier GB, et al. Collaboration between macrophages and vaccine-induced CD4+ T cells confers protection against lethal Pseudomonas aeruginosa pneumonia during neutropenia. J Infect Dis. 2013;207:39–49. doi: 10.1093/infdis/jis657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li Y, Wang Z, Liu X, Tang J, Peng B, Wei Y. X-ray irradiated vaccine confers protection against pneumonia caused by Pseudomonas aeruginosa. Sci Rep. 2016;6:18823. doi: 10.1038/srep18823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Miyairi S, Tateda K, Fuse ET, Ueda C, Saito H, Takabatake T, et al. Immunization with 3-oxododecanoyl-L-homoserine lactone-protein conjugate protects mice from lethal Pseudomonas aeruginosa lung infection. J Med Microbiol. 2006;55:1381–1387. doi: 10.1099/jmm.0.46658-0. [DOI] [PubMed] [Google Scholar]
- 184.Yang F, Gu J, Yang L, Gao C, Jing H, Wang Y, et al. Protective efficacy of the trivalent Pseudomonas aeruginosa vaccine candidate PcrV-OprI-Hcp1 in murine pneumonia and burn models. Sci Rep. 2017;7:3957. doi: 10.1038/s41598-017-04029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hamaoka S, Naito Y, Katoh H, Shimizu M, Kinoshita M, Akiyama K, et al. Efficacy comparison of adjuvants in PcrV vaccine against Pseudomonas aeruginosa pneumonia. Microbiol Immunol. 2017;61:64–74. doi: 10.1111/1348-0421.12467. [DOI] [PubMed] [Google Scholar]
- 186.Hassan R, El-Naggar W, Abd El-Aziz AM, Shaaban M, Kenawy HI, Ali YM. Immunization with outer membrane proteins (OprF and OprI) and flagellin B protects mice from pulmonary infection with mucoid and nonmucoid Pseudomonas aeruginosa. J Microbiol Immunol Infect. doi: 10.1016/j.jmii.2016.08.014. [online ahead of print] 20 Feb 2017; DOI: 10.1016/j.jmii.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 187.Gao C, Yang F, Wang Y, Liao Y, Zhang J, Zeng H, et al. Vaccination with a recombinant OprL fragment induces a Th17 response and confers serotype-independent protection against Pseudomonas aeruginosa infection in mice. Clin Immunol. doi: 10.1016/j.clim.2017.09.022. [online ahead of print] 29 Sept 2017; DOI: 10.1016/j.clim.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 188.Rello J, Krenn CG, Locker G, Pilger E, Madl C, Balica L, et al. A randomized placebo-controlled phase II study of a Pseudomonas vaccine in ventilated ICU patients. Crit Care. 2017;21:22. doi: 10.1186/s13054-017-1601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ohama M, Hiramatsu K, Miyajima Y, Kishi K, Nasu M, Kadota J. Intratracheal immunization with pili protein protects against mortality associated with Pseudomonas aeruginosa pneumonia in mice. FEMS Immunol Med Microbiol. 2006;47:107–115. doi: 10.1111/j.1574-695X.2006.00069.x. [DOI] [PubMed] [Google Scholar]
- 190.Banadkoki AZ, Keshavarzmehr M, Afshar Z, Aleyasin N, Fatemi MJ, Behrouz B, et al. Protective effect of pilin protein with alum+naloxone adjuvant against acute pulmonary Pseudomonas aeruginosa infection. Biologicals. 2016;44:367–373. doi: 10.1016/j.biologicals.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 191.Srinivas N, Jetter P, Ueberbacher BJ, Werneburg M, Zerbe K, Steinmann J, et al. Peptidomimetic antibiotics target outer-membrane biogenesis in Pseudomonas aeruginosa. Science. 2010;327:1010–1013. doi: 10.1126/science.1182749. [DOI] [PubMed] [Google Scholar]
- 192.Cigana C, Bernardini F, Facchini M, Alcalá-Franco B, Riva C, De Fino I, et al. Efficacy of the novel antibiotic POL7001 in preclinical models of Pseudomonas aeruginosa pneumonia. Antimicrob Agents Chemother. 2016;60:4991–5000. doi: 10.1128/AAC.00390-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Moehle K, Kocherla H, Bacsa B, Jurt S, Zerbe K, Robinson JA, et al. Solution structure and dynamics of LptE from Pseudomonas aeruginosa. Biochemistry. 2016;55:2936–2943. doi: 10.1021/acs.biochem.6b00313. [DOI] [PubMed] [Google Scholar]
- 194.Wilson BR, Bogdan AR, Miyazawa M, Hashimoto K, Tsuji Y. Siderophores in iron metabolism: from mechanism to therapy potential. Trends Mol Med. 2016;22:1077–1090. doi: 10.1016/j.molmed.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Miller LC, O’Loughlin CT, Zhang Z, Siryaporn A, Silpe JE, Bassler BL, et al. Development of potent inhibitors of pyocyanin production in Pseudomonas aeruginosa. J Med Chem. 2015;58:1298–1306. doi: 10.1021/jm5015082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.O’Loughlin CT, Miller LC, Siryaporn A, Drescher K, Semmelhack MF, Bassler BL. A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc Natl Acad Sci USA. 2013;110:17981–17986. doi: 10.1073/pnas.1316981110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ho Sui SJ, Lo R, Fernandes AR, Caulfield MD, Lerman JA, Xie L, et al. Raloxifene attenuates Pseudomonas aeruginosa pyocyanin production and virulence. Int J Antimicrob Agents. 2012;40:246–251. doi: 10.1016/j.ijantimicag.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hussein MH, Schneider EK, Elliott AG, Han M, Reyes-Ortega F, Morris F, et al. From breast cancer to antimicrobial: combating extremely resistant gram-negative “superbugs” using novel combinations of polymyxin B with selective estrogen receptor modulators. Microb Drug Resist. 2017;23:640–650. doi: 10.1089/mdr.2016.0196. [DOI] [PubMed] [Google Scholar]
- 199.Kollberg H, Carlander D, Olesen H, Wejåker PE, Johannesson M, Larsson A. Oral administration of specific yolk antibodies (IgY) may prevent Pseudomonas aeruginosa infections in patients with cystic fibrosis: a phase I feasibility study. Pediatr Pulmonol. 2003;35:433–440. doi: 10.1002/ppul.10290. [DOI] [PubMed] [Google Scholar]
- 200.Nilsson E, Amini A, Wretlind B, Larsson A. Pseudomonas aeruginosa infections are prevented in cystic fibrosis patients by avian antibodies binding Pseudomonas aeruginosa flagellin. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;856:75–80. doi: 10.1016/j.jchromb.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 201.Barnea Y, Carmeli Y, Neville LF, Kahel-Reifer H, Eren R, Dagan S, et al. Therapy with anti-flagellin A monoclonal antibody limits Pseudomonas aeruginosa invasiveness in a mouse burn wound sepsis model. Burns. 2009;35:390–396. doi: 10.1016/j.burns.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 202.Leighton TL, Dayalani N, Sampaleanu LM, Howell PL, Burrows LL. Novel role for PilNO in type IV pilus retraction revealed by alignment subcomplex mutations. J Bacteriol. 2015;197:2229–2238. doi: 10.1128/JB.00220-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Leighton TL, Yong DH, Howell PL, Burrows LL. Type IV pilus alignment subcomplex proteins PilN and PilO form homo- and heterodimers in vivo. J Biol Chem. 2016;291:19923–19938. doi: 10.1074/jbc.M116.738377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kus JV, Tullis E, Cvitkovitch DG, Burrows LL. Significant differences in type IV pilin allele distribution among Pseudomonas aeruginosa isolates from cystic fibrosis (CF) versus non-CF patients. Microbiology. 2004;150:1315–1326. doi: 10.1099/mic.0.26822-0. [DOI] [PubMed] [Google Scholar]
- 205.Kiewitz C, Tümmler B. Sequence diversity of Pseudomonas aeruginosa: impact on population structure and genome evolution. J Bacteriol. 2000;182:3125–3135. doi: 10.1128/jb.182.11.3125-3135.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tart AH, Wolfgang MC, Wozniak DJ. The alternative sigma factor AlgT represses Pseudomonas aeruginosa flagellum biosynthesis by inhibiting expression of fleQ. J Bacteriol. 2005;187:7955–7962. doi: 10.1128/JB.187.23.7955-7962.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bakkal S, Robinson SM, Ordonez CL, Waltz DA, Riley MA. Role of bacteriocins in mediating interactions of bacterial isolates taken from cystic fibrosis patients. Microbiology. 2010;156:2058–2067. doi: 10.1099/mic.0.036848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kalia VC, Wood TK, Kumar P. Evolution of resistance to quorum-sensing inhibitors. Microb Ecol. 2014;68:13–23. doi: 10.1007/s00248-013-0316-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.McCaughey LC, Josts I, Grinter R, White P, Byron O, Tucker NP, et al. Discovery, characterization and in vivo activity of pyocin SD2, a protein antibiotic from Pseudomonas aeruginosa. Biochem J. 2016;473:2345–2358. doi: 10.1042/BCJ20160470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Czajkowski R, Jafra S. Quenching of acyl-homoserine lactone-dependent quorum sensing by enzymatic disruption of signal molecules. Acta Biochim Pol. 2009;56:1–16. [PubMed] [Google Scholar]
- 211.Guendouze A, Plener L, Bzdrenga J, Jacquet P, Rémy B, Elias M, et al. Effect of quorum quenching lactonase in clinical isolates of Pseudomonas aeruginosa and comparison with quorum sensing inhibitors. Front Microbiol. 2017;8:227. doi: 10.3389/fmicb.2017.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sio CF, Otten LG, Cool RH, Diggle SP, Braun PG, Bos R, et al. Quorum quenching by an N-acyl-homoserine lactone acylase from Pseudomonas aeruginosa PAO1. Infect Immun. 2006;74:1673–1682. doi: 10.1128/IAI.74.3.1673-1682.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Furiga A, Lajoie B, El Hage S, Baziard G, Roques C. Impairment of Pseudomonas aeruginosa biofilm resistance to antibiotics by combining the drugs with a new quorum-sensing inhibitor. Antimicrob Agents Chemother. 2015;60:1676–1686. doi: 10.1128/AAC.02533-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ji C, Sharma I, Pratihar D, Hudson LL, Maura D, Guney T, et al. Designed small-molecule inhibitors of the anthranilyl-CoA synthetase PqsA block quinolone biosynthesis in Pseudomonas aeruginosa. ACS Chem Biol. 2016;11:3061–3067. doi: 10.1021/acschembio.6b00575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ueda A, Attila C, Whiteley M, Wood TK. Uracil influences quorum sensing and biofilm formation in Pseudomonas aeruginosa and fluorouracil is an antagonist. Microb Biotechnol. 2009;2:62–74. doi: 10.1111/j.1751-7915.2008.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.García-Contreras R, Martínez-Vázquez M, Velázquez Guadarrama N, Villegas Pañeda AG, Hashimoto T, Maeda T, et al. Resistance to the quorum-quenching compounds brominated furanone C-30 and 5-fluorouracil in Pseudomonas aeruginosa clinical isolates. Pathog Dis. 2013;68:8–11. doi: 10.1111/2049-632X.12039. [DOI] [PubMed] [Google Scholar]
- 217.Kumar L, Chhibber S, Kumar R, Kumar M, Harjai K. Zingerone silences quorum sensing and attenuates virulence of Pseudomonas aeruginosa. Fitoterapia. 2015;102:84–95. doi: 10.1016/j.fitote.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 218.Rasmussen TB, Bjarnsholt T, Skindersoe ME, Hentzer M, Kristoffersen P, Köte M, et al. Screening for quorum-sensing inhibitors (QSI) by use of a novel genetic system, the QSI selector. J Bacteriol. 2005;187:1799–1814. doi: 10.1128/JB.187.5.1799-1814.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ceyssens PJ, Lavigne R. Bacteriophages of Pseudomonas. Future Microbiol. 2010;5:1041–1055. doi: 10.2217/fmb.10.66. [DOI] [PubMed] [Google Scholar]
- 220.Pires DP, Vilas Boas D, Sillankorva S, Azeredo J. Phage therapy: a step forward in the treatment of Pseudomonas aeruginosa infections. J Virol. 2015;89:7449–7456. doi: 10.1128/JVI.00385-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chan BK, Sistrom M, Wertz JE, Kortright KE, Narayan D, Turner PE. Phage selection restores antibiotic sensitivity in MDR Pseudomonas aeruginosa. Sci Rep. 2016;6:26717. doi: 10.1038/srep26717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Fauconnier A. Regulating phage therapy: the biological master file concept could help to overcome regulatory challenge of personalized medicines. EMBO Rep. 2017;18:198–200. doi: 10.15252/embr.201643250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cooper CJ, Khan Mirzaei M, Nilsson AS. Adapting drug approval pathways for bacteriophage-based therapeutics. Front Microbiol. 2016;7:1209. doi: 10.3389/fmicb.2016.01209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Tetz G, Tetz V. Bacteriophage infections of microbiota can lead to leaky gut in an experimental rodent model. Gut Pathog. 2016;8:33. doi: 10.1186/s13099-016-0109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kingwell K. Bacteriophage therapies re-enter clinical trials. Nat Rev Drug Discov. 2015;14:515–516. doi: 10.1038/nrd4695. [DOI] [PubMed] [Google Scholar]
- 226.Mühl H, Scheiermann P, Bachmann M, Härdle L, Heinrichs A, Pfeilschifter J. IL-22 in tissue-protective therapy. Br J Pharmacol. 2013;169:761–771. doi: 10.1111/bph.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Besnard AG, Sabat R, Dumoutier L, Renauld JC, Willart M, Lambrecht B, et al. Dual role of IL-22 in allergic airway inflammation and its cross-talk with IL-17A. Am J Respir Crit Care Med. 2011;183:1153–1163. doi: 10.1164/rccm.201008-1383OC. [DOI] [PubMed] [Google Scholar]
- 228.Hoegl S, Bachmann M, Scheiermann P, Goren I, Hofstetter C, Pfeilschifter J, et al. Protective properties of inhaled IL-22 in a model of ventilator-induced lung injury. Am J Respir Cell Mol Biol. 2011;44:369–376. doi: 10.1165/rcmb.2009-0440OC. [DOI] [PubMed] [Google Scholar]
- 229.Allen KS, Sawheny E, Kinasewitz GT. Anticoagulant modulation of inflammation in severe sepsis. World J Crit Care Med. 2015;4:105–115. doi: 10.5492/wjccm.v4.i2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Junkins RD, Carrigan SO, Wu Z, Stadnyk AW, Cowley E, Issekutz T, et al. Mast cells protect against Pseudomonas aeruginosa-induced lung injury. Am J Pathol. 2014;184:2310–2321. doi: 10.1016/j.ajpath.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 232.Zheng S, Kummarapurugu AB, Afosah DK, Sankaranarayanan NV, Boothello RS, Desai UR, et al. 2-O, 3-O desulfated heparin blocks high mobility group box 1 release by inhibition of p300 acetyltransferase activity. Am J Respir Cell Mol Biol. 2017;56:90–98. doi: 10.1165/rcmb.2016-0069OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Griffin KL, Fischer BM, Kummarapurugu AB, Zheng S, Kennedy TP, Rao NV, et al. 2-O, 3-O-desulfated heparin inhibits neutrophil elastase-induced HMGB-1 secretion and airway inflammation. Am J Respir Cell Mol Biol. 2014;50:684–689. doi: 10.1165/rcmb.2013-0338RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Krystel-Whittemore M, Dileepan KN, Wood JG. Mast cell: a multi-functional master cell. Front Immunol. 2016;6:620. doi: 10.3389/fimmu.2015.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Jiang F, Yang J, Zhang Y, Dong M, Wang S, Zhang Q, et al. Angiotensin-converting enzyme 2 and angiotensin 1-7: novel therapeutic targets. Nat Rev Cardiol. 2014;11:413–426. doi: 10.1038/nrcardio.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Rosay T, Bazire A, Diaz S, Clamens T, Blier AS, Mijouin L, et al. Pseudomonas aeruginosa expresses a functional human natriuretic peptide receptor ortholog: involvement in biofilm formation. MBio. 2015;6 doi: 10.1128/mBio.01033-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Lee JH, Kim YG, Cho MH, Kim JA, Lee J. 7-fluoroindole as an antivirulence compound against Pseudomonas aeruginosa. FEMS Microbiol Lett. 2012;329:36–44. doi: 10.1111/j.1574-6968.2012.02500.x. [DOI] [PubMed] [Google Scholar]
- 239.Sethupathy S, Prasath KG, Ananthi S, Mahalingam S, Balan SY, Pandian SK. Proteomic analysis reveals modulation of iron homeostasis and oxidative stress response in Pseudomonas aeruginosa PAO1 by curcumin inhibiting quorum sensing regulated virulence factors and biofilm production. J Proteomics. 2016;145:112–126. doi: 10.1016/j.jprot.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 240.Kim J, Jeong SW, Quan H, Jeong CW, Choi JI, Bae HB. Effect of curcumin (Curcuma longa extract) on LPS-induced acute lung injury is mediated by the activation of AMPK. J Anesth. 2016;30:100–108. doi: 10.1007/s00540-015-2073-1. [DOI] [PubMed] [Google Scholar]
- 241.Jiang J, Kang TB, Shim W, Oh NH, Kim TJ, Lee KH. Indole-3-carbinol inhibits LPS-induced inflammatory response by blocking TRIF-dependent signaling pathway in macrophages. Food Chem Toxicol. 2013;57:256–261. doi: 10.1016/j.fct.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 242.Curran CS, Demick KP, Mansfield JM. Lactoferrin activates macrophages via TLR4-dependent and -independent signaling pathways. Cell Immunol. 2006;242:23–30. doi: 10.1016/j.cellimm.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 243.Xiao R, Kisaalita WS. Iron acquisition from transferrin and lactoferrin by Pseudomonas aeruginosa pyoverdin. Microbiology. 1997;143:2509–2515. doi: 10.1099/00221287-143-7-2509. [DOI] [PubMed] [Google Scholar]
- 244.Sánchez-Gómez S, Ferrer-Espada R, Stewart PS, Pitts B, Lohner K, Martínez de Tejada G. Antimicrobial activity of synthetic cationic peptides and lipopeptides derived from human lactoferricin against Pseudomonas aeruginosa planktonic cultures and biofilms. BMC Microbiol. 2015;15:137. doi: 10.1186/s12866-015-0473-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Debarbieux L, Leduc D, Maura D, Morello E, Criscuolo A, Grossi O, et al. Bacteriophages can treat and prevent Pseudomonas aeruginosa lung infections. J Infect Dis. 2010;201:1096–1104. doi: 10.1086/651135. [DOI] [PubMed] [Google Scholar]
- 246.Yang M, Du C, Gong P, Xia F, Sun C, Feng X, et al. Therapeutic effect of the YH6 phage in a murine hemorrhagic pneumonia model. Res Microbiol. 2015;166:633–643. doi: 10.1016/j.resmic.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 247.Gu J, Li X, Yang M, Du C, Cui Z, Gong P, et al. Therapeutic effect of Pseudomonas aeruginosa phage YH30 on mink hemorrhagic pneumonia. Vet Microbiol. 2016;190:5–11. doi: 10.1016/j.vetmic.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 248.Katoh H, Yasumoto H, Shimizu M, Hamaoka S, Kinoshita M, Akiyama K, et al. IV immunoglobulin for acute lung injury and bacteremia in Pseudomonas aeruginosa pneumonia. Crit Care Med. 2016;44:e12–e24. doi: 10.1097/CCM.0000000000001271. [DOI] [PubMed] [Google Scholar]
- 249.Li J, Chen T, Yuan C, Zhao G, Xu M, Li X, et al. Effect of intravenous immunoglobulin on the function of Treg cells derived from immunosuppressed mice with Pseudomonas aeruginosa pneumonia. PLoS One. 2017;12:e0176843. doi: 10.1371/journal.pone.0176843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Shindo Y, Fuchs AG, Davis CG, Eitas T, Unsinger J, Burnham CD, et al. Interleukin 7 immunotherapy improves host immunity and survival in a two-hit model of Pseudomonas aeruginosa pneumonia. J Leukoc Biol. 2017;101:543–554. doi: 10.1189/jlb.4A1215-581R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.McCaughey LC, Ritchie ND, Douce GR, Evans TJ, Walker D. Efficacy of species-specific protein antibiotics in a murine model of acute Pseudomonas aeruginosa lung infection. Sci Rep. 2016;6:30201. doi: 10.1038/srep30201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Hraiech S, Hiblot J, Lafleur J, Lepidi H, Papazian L, Rolain JM, et al. Inhaled lactonase reduces Pseudomonas aeruginosa quorum sensing and mortality in rat pneumonia. PLoS One. 2014;9:e107125. doi: 10.1371/journal.pone.0107125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Nakamura S, Iwanaga N, Seki M, Fukudome K, Oshima K, Miyazaki T, et al. Toll-like receptor 4 agonistic antibody promotes host defense against chronic Pseudomonas aeruginosa lung infection in mice. Infect Immun. 2016;84:1986–1993. doi: 10.1128/IAI.01384-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Bhavsar T, Liu M, Hardej D, Liu X, Cantor J. Aerosolized recombinant human lysozyme ameliorates Pseudomonas aeruginosa-induced pneumonia in hamsters. Exp Lung Res. 2010;36:94–100. doi: 10.3109/01902140903154608. [DOI] [PubMed] [Google Scholar]
- 255.Hentzer M, Wu H, Andersen JB, Riedel K, Rasmussen TB, Bagge N, et al. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 2003;22:3803–3815. doi: 10.1093/emboj/cdg366. [DOI] [PMC free article] [PubMed] [Google Scholar]