Abstract
Rationale: Pregnancies complicated by antenatal stress, including preeclampsia (PE) and chorioamnionitis (CA), increase the risk for bronchopulmonary dysplasia (BPD) in preterm infants, but biologic mechanisms linking prenatal factors with BPD are uncertain. Levels of sFlt-1 (soluble fms-like tyrosine kinase 1), an endogenous antagonist to VEGF (vascular endothelial growth factor), are increased in amniotic fluid and maternal blood in PE and associated with CA.
Objectives: Because impaired VEGF signaling has been implicated in the pathogenesis of BPD, we hypothesized that fetal exposure to sFlt-1 decreases lung growth and causes abnormal lung structure and pulmonary hypertension during infancy.
Methods: To test this hypothesis, we studied the effects of anti–sFlt-1 monoclonal antibody (mAb) treatment on lung growth in two established antenatal models of BPD that mimic PE and CA induced by intraamniotic (i.a.) injections of sFlt-1 or endotoxin, respectively. In experimental PE, mAb was administered by three different approaches, including antenatal treatment by either i.a. instillation or maternal uterine artery infusion, or by postnatal intraperitoneal injections.
Results: With each strategy, mAb therapy improved infant lung structure as assessed by radial alveolar count, vessel density, right ventricular hypertrophy, and lung function. As found in the PE model, the adverse lung effects of i.a. endotoxin were also reduced by antenatal or postnatal mAb therapy.
Conclusions: We conclude that treatment with anti–sFlt-1 mAb preserves lung structure and function and prevents right ventricular hypertrophy in two rat models of BPD of antenatal stress and speculate that early mAb therapy may provide a novel strategy for the prevention of BPD.
Keywords: preeclampsia, chorioamnionitis, pulmonary hypertension, bronchopulmonary dysplasia, vascular endothelial growth factor
Bronchopulmonary dysplasia (BPD), the chronic lung disease of infancy that follows preterm birth, is characterized by high mortality, frequent respiratory hospitalizations, exercise intolerance, pulmonary hypertension (PH), and altered lung function that continue into adulthood (1–4). Advances in perinatal medicine, including the use of maternal steroids, surfactant therapy, and better respiratory and nutritional support, have improved survival, yet the incidence of BPD remains unchanged (5). Epidemiologic studies have shown that maternal factors, especially preeclampsia (PE) and chorioamnionitis (CA), are associated with an increased risk for preterm birth as well as high susceptibility for the development of BPD (6–16). As highlighted in recent NIH workshops (6, 7), mechanisms by which complications of pregnancy increase the risk for BPD remain elusive, but early identification of at-risk preterm newborns may lead to novel preventive strategies to reduce the development of BPD.
Past studies of BPD pathogenesis suggest that early disruption of lung vascular development may contribute to sustained abnormalities of alveolar growth and PH (17–19). Decreased angiogenic signaling through direct inhibition of VEGF (vascular endothelial growth factor) activity at birth is sufficient to impair angiogenesis and distal airspace growth and cause PH in neonatal and infant rats (17, 18). Clinically, lung VEGF expression is reduced in infants dying with BPD (20), and echocardiogram signs of PH at postnatal Day 7 are strongly associated with the subsequent development of severe BPD and late PH (21, 22). These findings support the “vascular hypothesis” of BPD, which suggests that early endothelial cell injury impairs lung vascular and alveolar growth and that reduced production of “angiocrine factors” from the developing endothelium further disrupts lung epithelial and mesenchymal development (18).
In pregnancies complicated by preeclampsia, marked elevations of soluble VEGF receptor-1 (sFlt-1[soluble fms-like tyrosine kinase 1]), an endogenous VEGF inhibitor, in maternal blood and amniotic fluid precede the development of signs of PE (23–30). Placental pathology suggestive of vascular underperfusion are strongly associated with low levels of proangiogenic factors (such as VEGF) and elevated levels of sFlt-1 in maternal and cord blood (15, 31, 32), and are linked with intrauterine growth restriction (IUGR) and high risk for subsequent development of BPD with PH (15, 31). Decreased VEGF and increased sFlt-1 in tracheal fluid samples from low gestational age preterm infants are strongly associated with BPD risk (33, 34), suggesting that an early imbalance of pro- and antiangiogenic factors may contribute to the pathogenesis of BPD due to early disruption of lung vascular growth.
Pregnancies complicated by chorioamnionitis (CA) have been linked with high risk for premature birth and increased susceptibility for BPD (8–11, 35, 36). Previous models of CA have shown that intrauterine exposure to endotoxin (ETX) has complex effects but generally stimulates lung inflammation, alters lung vascular structure in fetal sheep (37), and can cause severe PH with sustained abnormalities of lung structure in infant rats (38–40). In addition to increased levels of inflammatory mediators, infants born in pregnancies complicated by CA have high sFlt-1 protein levels, which would likely decrease VEGF signaling and disrupt angiogenesis (39). Whether early restoration of lung VEGF signaling by reducing perinatal sFlt-1 exposure could enhance lung structure and function in experimental CA is unknown.
Therefore, we hypothesized that fetal exposure to high levels of sFlt-1, an endogenous inhibitor of VEGF, contributes to impaired lung vascular growth, reduced alveolarization, and high risk for PH in experimental models of PE and CA. To address this hypothesis, we studied the effects of treatment with a selective anti–sFlt-1 monoclonal antibody (mAb) on perinatal survival, lung structure and function, and PH in experimental models of BPD that mimic PE and CA. We first studied the antenatal and postnatal effects of mAb administration on lung structure and right ventricular hypertrophy (RVH) in an experimental model of PE caused by intraamniotic (i.a.) injection of sFlt-1. We then examined the effects of antenatal and postnatal anti–sFlt-1 mAb treatment in a second model of experimental BPD induced by i.a. injections of ETX to mimic CA. We found that antenatal and postnatal treatment with anti–sFlt-1 mAb preserved lung growth and improved RVH in infant rats in both models. These data support the concept that early interventions that lower sFlt-1 levels, such as with anti–sFlt-1 mAb therapy, may provide a novel strategy and therapeutic window for the prevention of BPD in preterm infants who are identified as being at risk because of exposure to antenatal stress.
Methods
Animals
All procedures and protocols were approved by the Institutional Animal Care and Use Committee at the University of Colorado Health Sciences Center. Charles River Laboratories provided pregnant Sprague-Dawley rats, which were maintained in room air at Denver’s altitude (1,600 m; 630 mm Hg barometric pressure; 122 mm Hg inspired oxygen tension) for at least 1 week before giving birth. Animals received ad libitum feeds and 12-hour alternating day–night cycles. Rats were killed with an intraperitoneal injection of pentobarbital sodium.
Study Design and Methods
Drug treatments
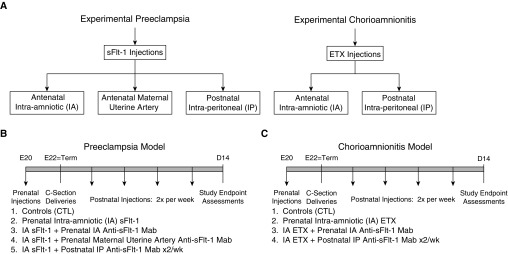
Time-dated pregnant rats received i.a. injections at Day 20 of gestation (term, 22 d) by standard methods, as previously described (41) (Figure 1). By random assignment, pregnant rats were administered i.a. injections of normal saline (NS; 50 μl per amniotic sac) for the control (CTL) groups, sFlt-1 (1 μg of recombinant human sFlt-1-Fc diluted to 50 μl with NS per sac) for the PE model, and ETX (10 μg of Escherichia coli 055:B55 ETX diluted to 50 μl with NS per sac) for the CA model.
Figure 1.
Study design. This figure illustrates the study protocols involving antenatal models of bronchopulmonary dysplasia caused by intraamniotic injections of sFlt-1 (soluble fms-like tyrosine kinase-1; experimental preeclampsia) and endotoxin (ETX) (experimental chorioamnionitis). (A) Strategies include the use of anti–sFlt-1 monoclonal antibody (mAb) for antenatal and postnatal interventions, as shown. (B and C) Study endpoints as assessed on postnatal Day 14 include lung histology with morphometric analysis for radial alveolar counts and lung vessel density, right ventricular hypertrophy, and lung mechanics.
The effects of the anti–sFlt-1 mAb (Shire Pharmaceuticals) were studied in each of the experimental groups (CTL, sFlt-1–treated, and ETX-treated) by random assignment. For the PE model, three different mAb treatment strategies were studied. To examine the effects of antenatal mAb treatment, pregnant rats that received i.a. sFlt-1 or saline were randomized to treatment with mAb or saline (CTL) that was administered either by injection into the amniotic sac (1.5 μg per sac) or by direct bolus infusion into the maternal uterine artery (1 or 10 mg/kg). To determine the effects of postnatal mAb treatment, mAb (1 or 10 mg/kg) or saline was administered to the rat pups by intraperitoneal injection twice weekly for 2 weeks (on postnatal Days 1, 4, 8, and 12) (Figure 1). These doses were established from preliminary studies in our laboratory that examined the efficacy of study doses used in these experiments.
In control groups (CTL), five litters of fetal rats received i.a. NS injections. For the PE model, six litters received sFlt-1 with or without mAb treatment, as described above. The number of treated litters included between one and four litters for each dose and for each intervention. For studies of postnatal treatment after i.a. sFlt-1, each neonatal rat received intraperitoneal doses of a control IgG carrier for two litters, low-dose mAb for two litters, or higher-dose anti–sFlt-1 for two litters (Figure 1B).
For the CA model, a similar protocol was followed as described for the PE model. Seven litters received ETX alone, three litters were treated with antenatal mAb by injection into the amniotic sac (1.5 μg per sac), and neonatal rats were treated with intraperitoneal doses of control IgG (1 mg/kg), two different doses of mAb (1 or 10 mg/kg), or saline (Figure 1C).
Surgery for drug administration
After pretreatment with buprenorphine (0.01–0.05 μg by intramuscular injection), laparotomy was performed on each dam under general anesthesia with 1 to 2% isoflurane inhalation via face mask (Matrx model VIP3000; Midmark) (see online supplement for details).
Cesarean section delivery
Two days after i.a. injections, cesarean section was performed on pregnant rats under general anesthesia (see online supplement for details). At the time of i.a. injection, pups were directly visualized through the uterine wall to ensure viability and to allow for survival assessments from the time of the i.a. injection throughout the early hours during the transition and the entire postnatal study period.
Tissue collection for studies
Rat lungs were harvested at birth and 14 days of age for histological and physiological assessments (see online supplement for details).
Study Measurements
Tissue for histological analysis
Animals were killed with intraperitoneal pentobarbital sodium. A catheter was placed in the trachea, the lungs were inflated with 4% paraformaldehyde, and constant pressure was maintained at 20 cm H2O for 60 minutes. A 2-mm-thick transverse section was taken from the midplane of the left lobe of the fixed lungs per animal to process and embed in paraffin wax.
Immunohistochemistry
Vessel density was quantified after performing immunostaining with von Willebrand factor, an endothelial cell–specific marker.
Morphometric analysis
Alveolarization was assessed by the radial alveolar count (RAC) method described by Emery and Mithal (42, 43) (see online supplement for details).
Assessment of RVH
The right ventricle (RV) and left ventricle plus septum (LV + S) were dissected and weighed. The ratios of RV to LV + S weights [RV/(LV + S)] were used to evaluate RVH.
Western blot analysis
Frozen lung samples were prepared for Western blot analysis by standard methods for assays of lung VEGF, VEGFR2, phsopho-VEGFR2, eNOS (endothelial nitric oxide synthase), and PlGF (placental growth factor) protein content (see online supplement).
Lung function
Lung function was determined in 14-day-old pups with the flexiVent system (SCIREQ).
Statistical Analysis
Statistical analysis was performed with the InStat 3.0 software package (Prism 7.0 Software Package; GraphPad) (see online supplement for details).
Results
Preeclampsia Model
Antenatal treatment: i.a. mAb treatment
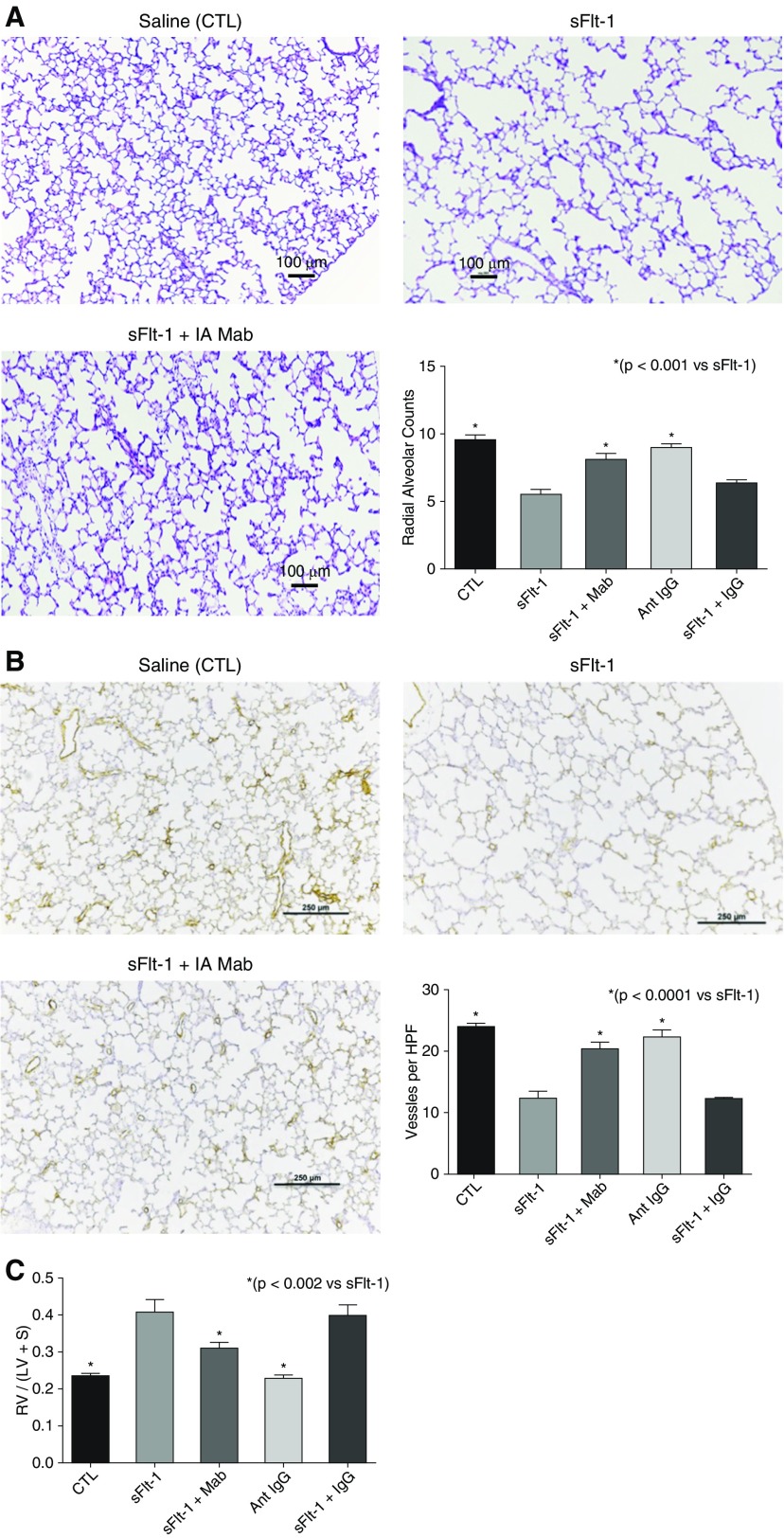
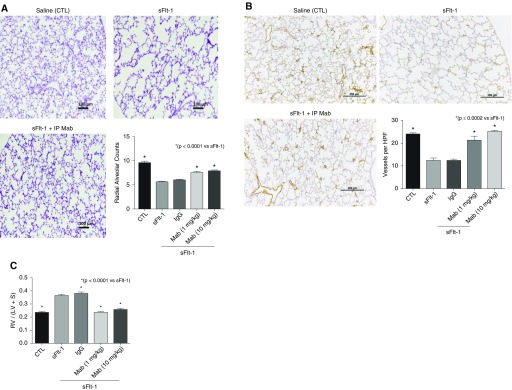
Birth weights were not different from control pups after exposure to sFlt-1, and survival of rats throughout the postnatal study period did not differ between CTL and sFlt-1 groups. Lung histology and morphometric analysis showed that i.a. sFlt-1 reduced RAC by 58% (P < 0.001), which was increased by antenatal i.a. mAb treatment (Figure 2A). Intraamniotic sFlt-1 treatment also reduced pulmonary vessel density in the setting of antenatal i.a. mAb treatment in 14-day-old rats, showing qualitative improvement after treatment (Figure 2C). Pulmonary vessel density was reduced by 49% from control values (P < 0.001), which was increased by i.a. mAb therapy (P < 0.001; Figure 2B). In addition, RVH was increased by 64% above CTL values (P < 0.001), which was improved with i.a. anti–sFlt-1 mAb therapy (P < 0.002 vs. antenatal sFlt-1 alone; Figure 2C).
Figure 2.
Effects of prenatal intraamniotic (i.a.) monoclonal antibody (mAb) treatment on lung structure and right ventricular hypertrophy (RVH) in experimental preeclampsia. (A) In comparison with saline control rats (CTL), i.a. exposure to sFlt-1 (soluble fms-like tyrosine kinase 1) decreases septation and causes lung simplification. Intraamniotic treatment with mAb enhances lung structure, as quantified by radial alveolar counts. Micrographs are representative and were obtained at the same magnification (scale bars, 100 μm). (B) Lung histology and vessel counts after immunostaining of endothelial cells with von Willebrand factor show that sFlt-1–exposed rats have decreased vascular density when compared with control rats and that i.a. mAb treatment restores vessel density at 14 days (scale bars, 250 μm). (C) In addition, antenatal treatment with mAb improved RVH after i.a. sFlt-1 exposure (preeclampsia). As shown, i.a. injection with nonspecific IgG did not affect radial alveolar counts (A), vessel density (B), or RVH (C) in saline control rats and did not alter the adverse effects of i.a. sFlt-1. Error bars show SEM. Numbers of pups studied in each group are: CTL, n = 22; sFlt-1, n = 11; sFlt-1 + mAb, n = 11; Ant IgG, n = 13; sFlt-1 + IgG, n = 12. HPF = high-power field; LV = left ventricle; RV = right ventricle; S = septum.
Antenatal treatment: maternal uterine artery mAb injection
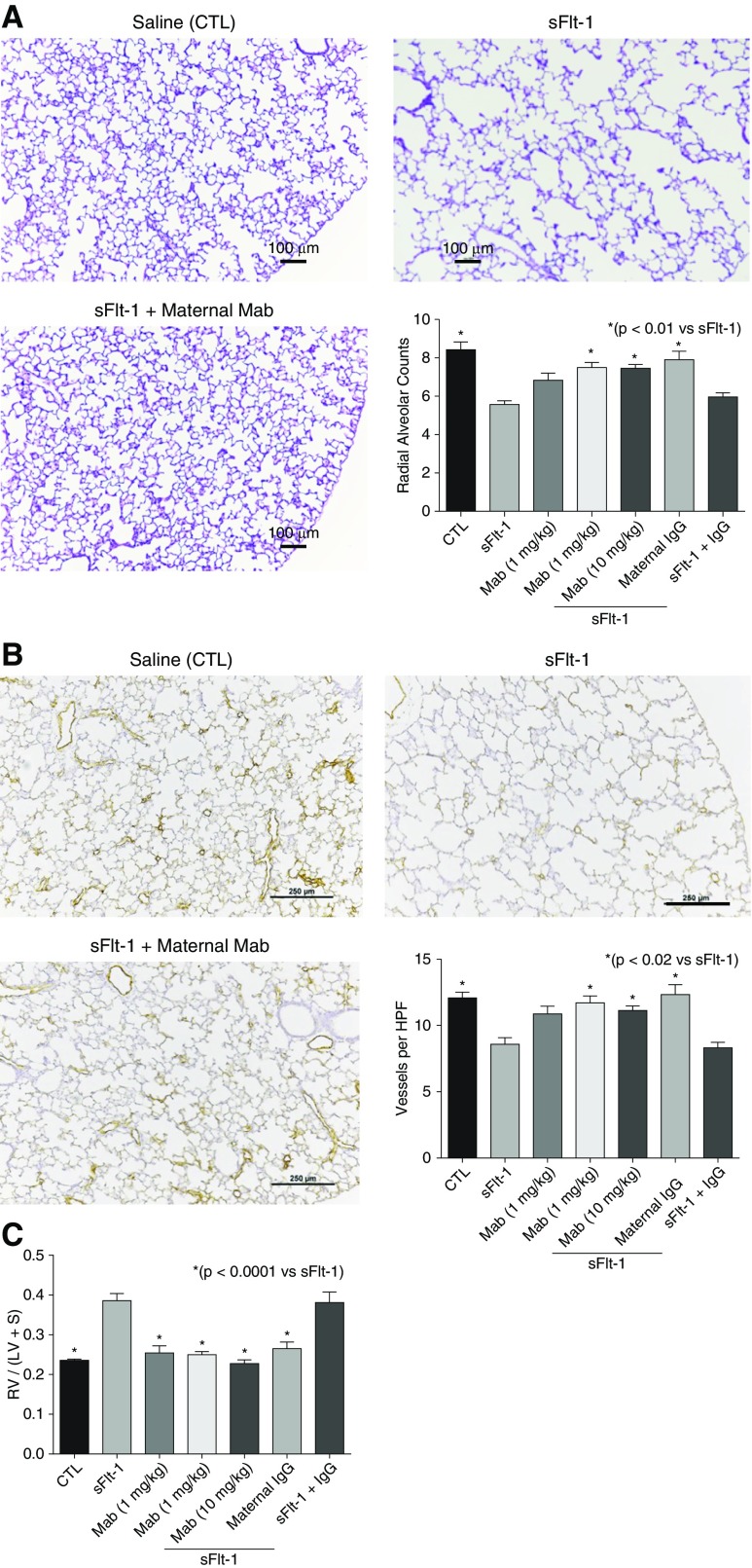
In studies of the PE model, antenatal anti–sFlt-1 mAb treatment via the maternal uterine artery showed a 95% survival rate at birth for all groups. Birth weights were not different in any of the treatment groups. As observed with the i.a. mAb injection studies, lung histology showed that antenatal treatment via uterine artery injection improved lung structure at 14 days after i.a. sFlt-1 exposure (Figure 3A). By morphometric analysis, i.a. sFlt-1 decreased RAC by 34% in comparison with control lungs (P < 0.001), and maternal uterine artery injection of mAb returned RAC to control values (P < 0.01; Figure 3A). Similarly, lung vessel density at Day 14 was preserved after maternal mAb treatment after i.a. sFlt-1 exposure (Figure 3B). Although i.a. sFlt-1 decreased vessel density to 71% of control levels, maternal mAb treatment preserved vessel density (P < 0.02 vs. sFlt-1). In addition, uterine artery anti- mAb treatment improved RVH at Day 1 (P < 0.0001 vs. sFlt-1; Figure 3C).
Figure 3.
Cardiopulmonary effects of prenatal maternal uterine artery anti–sFlt-1 (soluble fms-like tyrosine kinase 1) monoclonal antibody (mAb) injection in experimental preeclampsia. (A) As shown, distal lung growth is enhanced by maternal mAb infusion after intraamniotic (i.a.) sFlt-1 exposure. In comparison with control (CTL), radial alveolar counts are decreased after i.a. sFlt-1 exposure and are restored after maternal uterine artery mAb infusion. Scale bars, 100 μm. (B) Similarly, lung vessel density is reduced after i.a. sFlt-1 exposure but is enhanced after prenatal maternal uterine artery mAb treatment. Scale bars, 250 μm. (C) In addition, uterine artery infusion of mAb improved the development of right ventricular hypertrophy after sFlt-1 exposure in utero. As shown, uterine artery injection with nonspecific IgG did not affect radial alveolar counts (A), vessel density (B), or right ventricular hypertrophy (C) in saline control rats and did not alter the adverse effects of i.a. sFlt-1. Error bars show SEM. Numbers of pups studied in each group are: CTL, n = 32; sFlt-1, n = 23; mAb alone, n = 10; sFlt-1 + mAb (1 mg/kg), n = 19; sFlt-1 + mAb (10 mg/kg), maternal IgG, n = 15; sFlt-1 + IgG, n = 12. HPF = high-power field; LV = left ventricle; RV = right ventricle; S = septum.
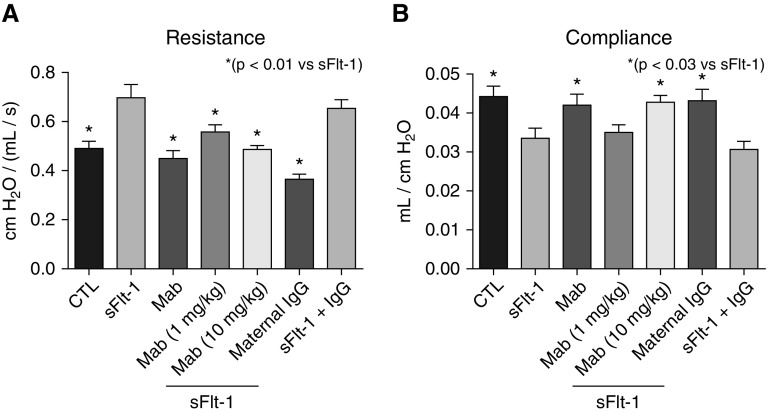
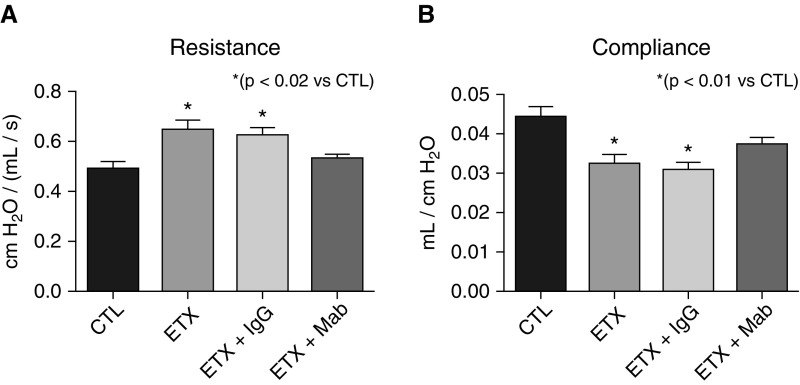
The impact of antenatal sFlt-1 exposure and treatment with mAb was further evaluated by changes in lung function (Figure 4). At 14 days, lung resistance was increased by 42% above values measured in CTL rats after i.a. sFlt-1 exposure (P < 0.007). Lung resistance after antenatal maternal uterine artery mAb treatment was equivalent to control values (P < 0.003 vs. sFlt-1; Figure 4A). Antenatal i.a. sFlt-1 exposure decreased lung compliance by 24% from CTL (P < 0.02), which was also improved with early mAb treatment, but only at the higher dose (P < 0.03; Figure 4B).
Figure 4.
Effects of intraamniotic (i.a.) sFlt-1 (soluble fms-like tyrosine kinase 1) exposure and uterine artery monoclonal antibody (mAb) treatment on lung function as assessed on 14-day-old rats. (A) Intraamniotic exposure to sFlt-1 increased infant lung resistance, which was improved with maternal mAb therapy. (B) Similarly, i.a. exposure to sFlt-1 decreased lung compliance, which was increased in rats treated by maternal mAb infusion before birth. As shown, treatment with nonspecific IgG did not affect lung resistance or compliance in control (CTL) or sFlt-1–exposed pups. Error bars show SEM. Numbers of pups studied in each group are: CTL, n = 32; sFlt-1, n = 23; mAb alone, n = 10; sFlt-1 + mAb (1 mg/kg), n = 19; sFlt-1 + mAb (10 mg/kg), maternal IgG, n = 15; sFlt-1 + IgG, n = 12.
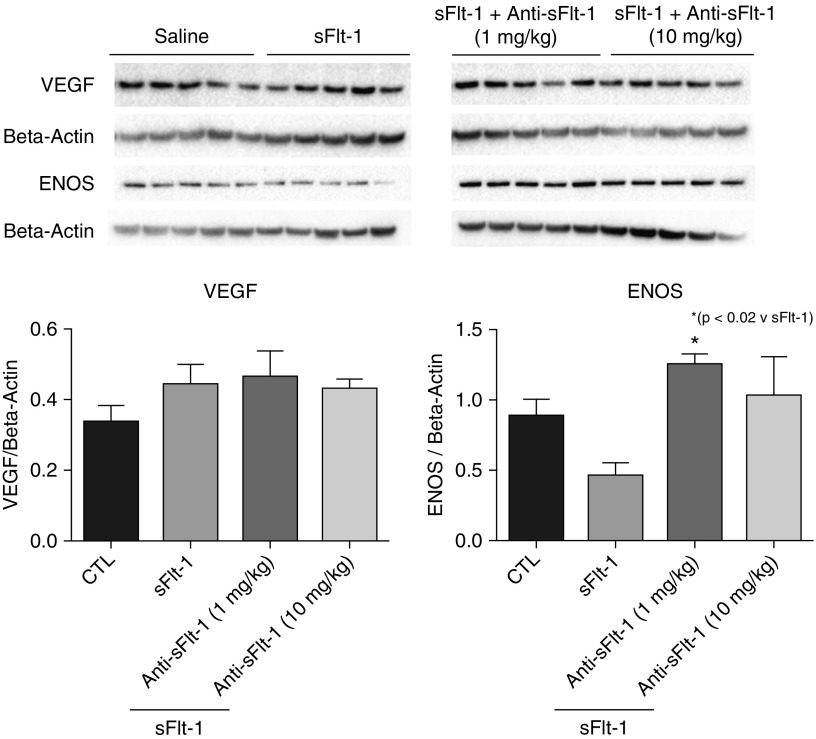
To determine whether antenatal mAb treatment caused early changes in lung VEGF signaling at birth, we assayed lung tissue homogenates for VEGF, VEGFR2, phosphorylated VEGFR2, eNOS, and PlGF protein expression Western blot analysis. We found that lung eNOS protein expression was decreased after i.a. sFlt-1 exposure and that these levels were increased after antenatal mAb treatment to control levels (P < 0.05 vs. sFlt-1; Figure 5). Total lung VEGF, VEGFR2, phosphorylated VEGFR2, or PlGF expression were not different between the study groups (data not shown).
Figure 5.
Effects of intraamniotic (i.a.) sFlt-1 (soluble fms-like tyrosine kinase 1) exposure on lung VEGF (vascular endothelial growth factor) and eNOS (endothelial nitric oxide synthase) protein expression at birth. In comparison with control rats (CTL), lung VEGF protein content was not different after i.a. sFlt-1 exposure with or without maternal monoclonal antibody (mAb) treatment. However, lung eNOS protein content was increased by maternal uterine artery mAb infusion. Error bars show SEM.
Postnatal treatment: intraperitoneal mAb injection
After antenatal exposure to i.a. sFlt-1, fetal rat pups were delivered and randomized to postnatal saline or mAb treatment, as described above (Figure 1). As observed with antenatal mAb treatment, postnatal mAb therapy restored lung structure in this model (Figure 6). Late mAb treatment increased lung growth after antenatal sFlt-1 exposure, as assessed by RAC and vessel density (Figures 6A and 6B). The development of RVH after antenatal sFlt-1 exposure was blunted by postnatal mAb therapy (Figure 6C).
Figure 6.
Effects of postnatal intraperitoneal (i.p.) monoclonal antibody (mAb) therapy after intraamniotic (i.a.) sFlt-1 (soluble fms-like tyrosine kinase 1) exposure. (A–C) In comparison with lungs from untreated rats that were exposed to i.a. sFlt-1, postnatal mAb therapy improved lung histology and increased radial alveolar counts (A), increased vessel density (B), and improved the development of right ventricular hypertrophy (C). Postnatal treatment with nonspecific IgG had no effect on lung structure or right ventricular hypertrophy. Scale bars: (A) 100 μm; (B) 250 μm. Error bars show SEM. Numbers of pups studied in each group are: control (CTL), n = 20; sFlt-1, n = 12; sFlt-1 + IgG, n = 11; sFlt-1 + mAb (1 mg/kg), n = 12; sFlt-1 + mAb (10 mg/kg), n = 12. HPF = high-power field; LV = left ventricle; RV = right ventricle; S = septum.
Chorioamnionitis Model
Increased BAL fluid and serum sFlt-1 levels after i.a. ETX exposure
To determine whether antenatal stress induced by exposure to i.a. ETX upregulates sFlt-1 in the newborn rat pup, we measured sFlt-1 levels by ELISA from tracheal fluid and blood levels shortly after cesarean section delivery. In comparison with saline-treated control rats, we found that serum sFlt-1 protein levels were markedly increased in samples from newborn rat pups at birth after either i.a. sFlt-1 or i.a. ETX exposure, showing a 2.5- and 4.6-fold increase in levels, respectively (Figure 7A). Interestingly, circulating levels in rat pups after i.a. ETX exposure were even greater than those measured from samples after i.a. exposure to sFlt-1. We also found that i.a. ETX exposure increased tracheal fluid sFlt-1 levels by nearly 3.5-fold (Figure 7B).
Figure 7.
Effects of prenatal intraamniotic (i.a.) sFlt-1 (soluble fms-like tyrosine kinase 1) and i.a. endotoxin (ETX) on sFlt-1 content in newborn rats at birth. (A) Antenatal exposure to sFlt-1 or ETX markedly increased serum sFlt-1 levels in newborn rat pups by 2.5- and 4.6-fold, respectively. (B) In addition, i.a. ETX increased sFLT-1 levels in BAL fluid (BALF) after birth. CTL = control.
Antenatal treatment: i.a. mAb injection
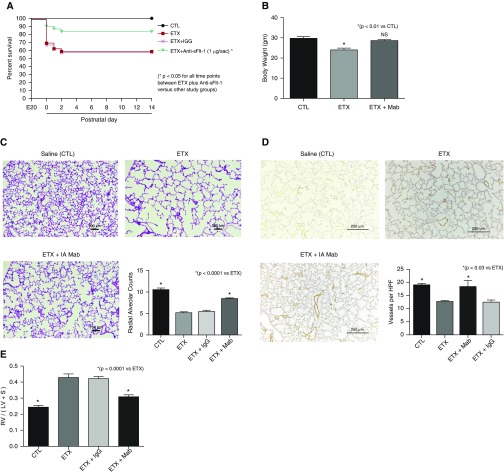
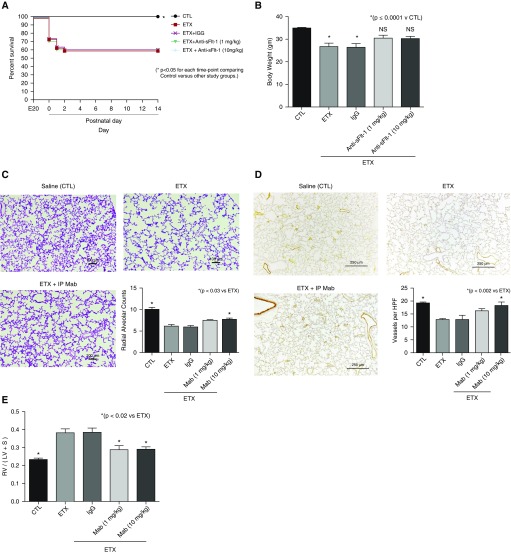
Antenatal ETX exposure reduced survival of rat pups to 70% at birth, in contrast with 100% survival of saline-control pups (Figure 8A). Over the first days of life, i.a. ETX had a survival rate of 58%, which was not altered by treatment with a nonspecific IgG. In contrast, antenatal treatment with mAb after i.a. ETX exposure improved neonatal survival to 90% (P < 0.01) and increased survival at 2 weeks to 76%. Pups treated with i.a. ETX had a 19% decrease in body weight at 2 weeks when compared with control pups (P < 0.01), which was prevented after mAb therapy (Figure 8B). Lung histology and morphometric analysis demonstrate that i.a. mAb treatment enhanced distal airspace and vascular growth when compared with i.a. ETX–exposed pups (Figures 8C and 8D). Exposure to i.a. ETX increased RVH by 76% compared with control rats, with partial decrease after i.a. anti–sFlt-1 mAb dosing to 26% higher than control level (P < 0.0001 vs. ETX; Figure 8E). Finally, we found that i.a. ETX exposure caused elevated lung resistance and reduced compliance by 32 and 27%, respectively (Figures 9A and 9B). Antenatal i.a. anti–sFlt-1 mAb treatment improved lung resistance and compliance toward control values (Figure 9).
Figure 8.
Effects of prenatal intraamniotic (i.a.) monoclonal antibody (mAb) treatment on lung structure and right ventricular hypertrophy (RVH) after i.a. endotoxin (ETX) exposure. (A) In comparison with control rats (CTL), i.a. exposure to ETX reduced survival in rat pups after birth, and neonatal survival was improved by antenatal mAb treatment. Numbers of pups studied for the survival aim included 30 fetuses for the groups receiving either saline, ETX alone, or ETX plus mAb, and 20 fetuses for the ETX plus IgG groups. *P < 0.05 at each time point for comparisons between ETX plus mAb versus each of the other study groups. (B–E) Antenatal mAb therapy improved body weight (B), distal lung growth (C), and vessel density (D), and improved RVH (E). As shown, i.a. treatment with nonspecific IgG did not affect lung structure or RVH. Scale bars: (C) 100 μm; (D) 250 μm. Error bars show SEM. Numbers of each group for panels B–E are: CTL, n = 11; ETX, n = 10; ETX + mAb, n = 10; ETX + IgG, n = 10. HPF = high-power field; LV = left ventricle; NS = not significant; RV = right ventricle; S = septum; sFlt-1 = soluble fms-like tyrosine kinase 1.
Figure 9.
Effects of antenatal monoclonal antibody (mAb) treatment on lung function after intraamniotic (i.a.) endotoxin (ETX) exposure as assessed in 14-day-old rats by flexiVent measurements. As shown, i.a. ETX increased lung resistance (A) and decreased lung compliance (B), which were improved with antenatal mAb injections but not nonspecific IgG treatment. Error bars show SEM. Numbers of pups studied in each group are: control (CTL), n = 11; ETX, n = 10; ETX + mAb, n = 10; ETX + IgG, n = 10.
Postnatal treatment: intraperitoneal mAb injections
In the chorioamnionitis model, survival was not different between ETX and ETX plus postnatal mAb treatment at 1 mg/kg or 10 mg/kg (Figure 10A). Body weight at 2 weeks was reduced after i.a. ETX and i.a. ETX plus nonspecific IgG exposure as compared with control rats. Postnatal mAb treatment improved somatic growth and was not different from CTL rats (Figure 10B). Lung histology showed increased septation and alveolar growth with postnatal mAb therapy after i.a. ETX exposure (Figure 10C). Compared with control, i.a. ETX exposure decreased RAC by 61% (P < 0.0001), with a subsequent increase in RAC to 77% of control values after mAb treatment (P < 0.03 vs. ETX). Similarly, postnatal mAb treatment at the higher dose improved lung vessel density at Day 14 (Figure 10D). Finally, postnatal mAb therapy reduced RVH by 63% when compared with infant rats exposed to i.a. ETX without therapy (P < 0.0001; Figure 10E).
Figure 10.
Effects of postnatal monoclonal antibody (mAb) treatment on lung structure, vessel density, and right ventricular hypertrophy (RVH) after intraamniotic (i.a.) endotoxin (ETX) exposure. (A) Survival was not different between study groups. Thirty fetuses were included in each study group. *P < 0.05 at each time point for comparisons between ETX plus mAb versus each of the other study groups (*P < 0.05 for each time point comparing control vs. other study groups). (B–E) Compared with saline control rats (CTL), postnatal mAb treatment improved body weight at Day 14 (B), increased lung alveolarization (C), improved vessel density (D), and reduced RVH after i.a. ETX exposure (E). Postnatal injections with nonspecific IgG had no effect on lung structure or RVH. Scale bars: (C) 100 μm; (D) 250 μm. Error bars show SEM. Numbers of pups studied in each group for data in B–E are: CTL, n = 11; ETX, n = 10; ETX + IgG, n = 10; ETX + mAb (1 mg/kg), n = 9; ETX + mAb (10 mg/kg), n = 10. HPF = high-power field; i.p. = intraperitoneal; LV = left ventricle; NS = not significant; RV = right ventricle; S = septum; sFlt-1 = soluble fms-like tyrosine kinase 1.
Discussion
Strong epidemiologic evidence has shown that antenatal factors are linked with the pathogenesis of BPD after preterm birth, yet little is understood about underlying biologic mechanisms that modulate respiratory outcomes of prematurity (6, 7). We report that antenatal exposure to i.a. sFlt-1 or ETX, which were used to model fetal exposures in the settings of PE and CA, respectively, caused sustained abnormalities of lung growth and RVH in infant rats. Antenatal treatment with a selective anti–sFlt-1 mAb preserved lung structure and function and improved RVH during infancy after i.a. exposure to sFlt-1, whether mAb was administered by direct instillation in the amniotic sac or infused into the uterine artery. Postnatal mAb treatment also improved lung growth and RVH after sFlt-1 exposure. To determine whether sFlt-1 may also contribute to the pathobiology of infant lung disease in an experimental model of CA, we first measured sFlt-1 levels in BAL fluid and blood from neonatal rat pups exposed to antenatal ETX and found that sFlt-1 protein was markedly increased shortly after birth. As observed in the sFlt-1 model, antenatal or postnatal treatment with sFlt-1 mAb improved neonatal survival, increased somatic growth, and restored lung growth in this model of CA. Overall, these findings suggest that the late adverse effects of antenatal factors, such as those associated with PE and CA, may be mediated through increased expression of sFlt-1, and that early intervention with anti–sFlt-1 mAb may provide a novel preventive strategy to improve late respiratory outcomes of preterm infants.
Past studies exploring the pathogenesis of BPD have traditionally examined the impact of postnatal injuries due to such adverse stimuli as prolonged hyperoxia, adverse ventilator strategies, and others, with few studies using animal models to explore prenatal mechanisms (44, 45). Growing clinical data, however, support the hypothesis that BPD likely has its origins during fetal life and that antenatal events are potent determinants of the persistent high rate of BPD (6, 7, 46). Two recent prospective cohort studies show that antenatal factors identified on the first day of life strongly predict subsequent risk for BPD and respiratory morbidities during early childhood, and that adding postnatal factors and even the BPD diagnosis to statistical models did not further enhance the ability of antenatal determinants to predict late lung disease (16, 47). Several studies have shown that preterm infants with IUGR or low birth weight z scores are at high risk for late respiratory morbidities and abnormal lung function at school age (48–51). Maternal smoking, hypertensive disorders of pregnancy, and PE have also been strongly associated with high risk for BPD after preterm birth (16, 47). In a cohort study of preterm infants, the risk for BPD was increased in the setting of PE, even after adjusting the analytic model for IUGR (52).
Recent clinical studies suggest that placental dysfunction, as reflected by vascular lesions that reflect maternal vascular “underperfusion,” are predictive for BPD and PH (15, 31, 32). Cord blood levels of angiogenic factors are decreased in association with placental findings of maternal vascular underperfusion (31). Other cord blood angiogenic biomarkers, such as decreased endothelial colony-forming cells, are associated with severe BPD in preterm infants (53, 54). Disruption of placentation and abnormal perfusion likely represent failed placental angiogenesis (55) and further suggest that abnormal angiogenesis vascular signaling may contribute to postnatal diseases in the newborn.
Inhibition of angiogenesis at birth impairs alveolarization and causes PH in infant rats (17, 18). This work led to the “vascular hypothesis” of BPD, in which disruption of angiogenesis alters angiocrine signaling and endothelial–epithelial communication, which decreases airspace growth (19). Recent studies suggest that the developing endothelial cell plays a key role in the regulation and coordination of epithelial growth and distal airspace structure through the production of critical “angiocrines,” such as nitric oxide, hepatocyte growth factor (56), vitamin A (57), insulin growth factor-1, and others. As a decoy receptor, sFlt-1 inhibits VEGF activity by directly binding free VEGF protein, which decreases downstream activation of membrane-associated VEGF receptors (58, 59). Past studies have shown that disruption of VEGF activity affects several signaling pathways, including activation of the VEGF–NO pathway (60). In this study, lung eNOS protein content, which is strongly dependent on VEGF activity, was markedy reduced after i.a. exposure to sFlt-1 and increased by mAb treatment. The effect of mAb treatment on preservation of lung growth is likely mediated by restoration of VEGF signaling during this critical developmental window.
Preserving normal vascular growth and endothelial function in the developing lung after preterm birth may not only reduce the risk for PH but also further enhance airspace growth and improve respiratory health. This concept is further supported by prospective clinical studies that have shown that early echocardiogram findings of pulmonary vascular disease at Day 7 after preterm birth are strongly associated with the development and severity of BPD and PH at 36 weeks’ corrected age (21, 22). Importantly, early signs of PH in preterm infants were also associated with a subsequently worse respiratory course during the initial hospitalization as well as late respiratory outcomes during infancy (21). Overall, these findings suggest that early interventions that enhance endothelial survival and function may provide novel approaches toward the prevention of BPD.
Potential limitations of these findings include the use of exogenous sFlt-1 and ETX to provide experimental models to study the fetal impact of PE and CA, respectively. The rationale for developing models involving i.a. exposure to these stimuli are based on extensive data from clinical studies (23–30, 35–39). However, PE and CA are complex diseases that involve multiple interactive pathways beyond VEGF signaling, and further studies are needed to more fully characterize the roles of alternate mechanisms. Future studies are warranted to determine whether the impact of mAb on infant lung structure was primarily related to its protective effects on placental structure and function or directly related to its impact on the fetus or newborn rat pup. Extensive studies are also needed to examine the effects of mAb on the immature retina, brain, kidney, and other organs during development and in response to injury. Finally, the use of antenatal models to explore BPD mechanisms provides new insights into potential disease pathobiology and interventions; however, human BPD remains a disease of multifactorial etiologies.
We conclude that antenatal or postnatal anti–sFlt-1 mAb treatment preserves lung structure in infant rat models of BPD due to prenatal stress. Establishing mechanistic links between antenatal factors with disease pathogenesis and how these factors impact respiratory outcomes is essential for further advances that may lead to primary prevention of the late respiratory sequelae of prematurity.
Footnotes
Supported in part by NHLBI grant HL68702 (S.H.A.) and a grant from Shire Pharmaceuticals.
Author Contributions: Conception and design: all authors; analysis and interpretation: all authors; drafting the manuscript: B.W., A.P., G.S., and S.H.A.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201707-1371OC on December 21, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Northway WH, Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease: bronchopulmonary dysplasia. N Engl J Med. 1967;276:357–368. doi: 10.1056/NEJM196702162760701. [DOI] [PubMed] [Google Scholar]
- 2.Kinsella JP, Greenough A, Abman SH. Bronchopulmonary dysplasia. Lancet. 2006;367:1421–1431. doi: 10.1016/S0140-6736(06)68615-7. [DOI] [PubMed] [Google Scholar]
- 3.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357:1946–1955. doi: 10.1056/NEJMra067279. [DOI] [PubMed] [Google Scholar]
- 4.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 5.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314:1039–1051. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McEvoy CT, Jain L, Schmidt B, Abman S, Bancalari E, Aschner JL. Bronchopulmonary dysplasia: NHLBI workshop on the primary prevention of chronic lung disease. Ann Am Thorac Soc. 2014;11:S146–S153. doi: 10.1513/AnnalsATS.201312-424LD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manuck TA, Levy PT, Gyamfi-Bannerman C, Jobe AH, Blaisdell CJ. Prenatal and perinatal determinants of lung health and disease in early life. JAMA Pediatr. 2016;170:e154577. doi: 10.1001/jamapediatrics.2015.4577. [DOI] [PubMed] [Google Scholar]
- 8.Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. 2015;213:S29–S52. doi: 10.1016/j.ajog.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics. 1996;97:210–215. [PubMed] [Google Scholar]
- 10.Van Marter LJ, Dammann O, Allred EN, Leviton A, Pagano M, Moore M, et al. Developmental Epidemiology Network Investigators. Chorioamnionitis, mechanical ventilation, and postnatal sepsis as modulators of chronic lung disease in preterm infants. J Pediatr. 2002;140:171–176. doi: 10.1067/mpd.2002.121381. [DOI] [PubMed] [Google Scholar]
- 11.Hansen AR, Barnés CM, Folkman J, McElrath TF. Maternal preeclampsia predicts the development of bronchopulmonary dysplasia. J Pediatr. 2010;156:532–536. doi: 10.1016/j.jpeds.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Bose C, Van Marter LJ, Laughon M, O’Shea TM, Allred EN, Karna P, et al. Extremely Low Gestational Age Newborn Study Investigators. Fetal growth restriction and chronic lung disease among infants born before the 28th week of gestation. Pediatrics. 2009;124:e450–e458. doi: 10.1542/peds.2008-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lal MK, Manktelow BN, Draper ES, Field DJ. Chronic lung disease of prematurity and intrauterine growth retardation: a population-based study. Pediatrics. 2003;111:483–487. doi: 10.1542/peds.111.3.483. [DOI] [PubMed] [Google Scholar]
- 14.Reiss I, Landmann E, Heckmann M, Misselwitz B, Gortner L. Increased risk of bronchopulmonary dysplasia and increased mortality in very preterm infants being small for gestational age. Arch Gynecol Obstet. 2003;269:40–44. doi: 10.1007/s00404-003-0486-9. [DOI] [PubMed] [Google Scholar]
- 15.Mestan KK, Check J, Minturn L, Yallapragada S, Farrow KN, Liu X, et al. Placental pathologic changes of maternal vascular underperfusion in bronchopulmonary dysplasia and pulmonary hypertension. Placenta. 2014;35:570–574. doi: 10.1016/j.placenta.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrow LA, Wagner BD, Ingram DA, Poindexter BB, Schibler K, Cotten CM, et al. Antenatal determinants of bronchopulmonary dysplasia and late respiratory disease in preterm infants. Am J Respir Crit Care Med. 2017;196:364–374. doi: 10.1164/rccm.201612-2414OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, et al. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol. 2000;279:L600–L607. doi: 10.1152/ajplung.2000.279.3.L600. [DOI] [PubMed] [Google Scholar]
- 18.Le Cras TD, Markham NE, Tuder RM, Voelkel NF, Abman SH. Treatment of newborn rats with a VEGF receptor inhibitor causes pulmonary hypertension and abnormal lung structure. Am J Physiol Lung Cell Mol Physiol. 2002;283:L555–L562. doi: 10.1152/ajplung.00408.2001. [DOI] [PubMed] [Google Scholar]
- 19.Abman SH. Bronchopulmonary dysplasia: “a vascular hypothesis”. Am J Respir Crit Care Med. 2001;164:1755–1756. doi: 10.1164/ajrccm.164.10.2109111c. [DOI] [PubMed] [Google Scholar]
- 20.Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;164:1971–1980. doi: 10.1164/ajrccm.164.10.2101140. [DOI] [PubMed] [Google Scholar]
- 21.Mourani PM, Sontag MK, Younoszai A, Miller JI, Kinsella JP, Baker CD, et al. Early pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2015;191:87–95. doi: 10.1164/rccm.201409-1594OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirza H, Ziegler J, Ford S, Padbury J, Tucker R, Laptook A. Pulmonary hypertension in preterm infants: prevalence and association with bronchopulmonary dysplasia. J Pediatr. 2014;165:909–14.e1. doi: 10.1016/j.jpeds.2014.07.040. [DOI] [PubMed] [Google Scholar]
- 23.Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Pre-eclampsia. Lancet. 2016;387:999–1011. doi: 10.1016/S0140-6736(15)00070-7. [DOI] [PubMed] [Google Scholar]
- 24.Sircar M, Thadhani R, Karumanchi SA. Pathogenesis of preeclampsia. Curr Opin Nephrol Hypertens. 2015;24:131–138. doi: 10.1097/MNH.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 25.Schrey-Petersen S, Stepan H. Anti-angiogenesis and preeclampsia in 2016. Curr Hypertens Rep. 2017;19:6. doi: 10.1007/s11906-017-0706-5. [DOI] [PubMed] [Google Scholar]
- 26.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 28.Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennström M, et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374:13–22. doi: 10.1056/NEJMoa1414838. [DOI] [PubMed] [Google Scholar]
- 29.Allen RE, Rogozinska E, Cleverly K, Aquilina J, Thangaratinam S. Abnormal blood biomarkers in early pregnancy are associated with preeclampsia: a meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2014;182:194–201. doi: 10.1016/j.ejogrb.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 30.Wu FTH, Stefanini MO, Mac Gabhann F, Kontos CD, Annex BH, Popel AS. A systems biology perspective on sVEGFR1: its biological function, pathogenic role and therapeutic use. J Cell Mol Med. 2010;14:528–552. doi: 10.1111/j.1582-4934.2009.00941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mestan KK, Gotteiner N, Prota N, Grobman W, Su EJ, Ernst LM. Cord blood biomarkers of placental maternal vascular underperfusion predict bronchopulmonary dysplasia-associated pulmonary hypertension. J Pediatr. 2017;185:33–41. doi: 10.1016/j.jpeds.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korzeniewski SJ, Romero R, Chaiworapongsa T, Chaemsaithong P, Kim CJ, Kim YM, et al. Maternal plasma angiogenic index-1 (placental growth factor/soluble VEGF receptor-1) is a biomarker for the burden of placental lesions consistent with uteroplacental underperfusion: a longitudinal case-cohort study. Am J Obstet Gynecol. 2016;214:619.e1–619.e17. doi: 10.1016/j.ajog.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lassus P, Turanlahti M, Heikkilä P, Andersson LC, Nupponen I, Sarnesto A, et al. Pulmonary vascular endothelial growth factor and Flt-1 in fetuses, in acute and chronic lung disease, and in persistent pulmonary hypertension of the newborn. Am J Respir Crit Care Med. 2001;164:1981–1987. doi: 10.1164/ajrccm.164.10.2012036. [DOI] [PubMed] [Google Scholar]
- 34.Hasan J, Beharry KD, Valencia AM, Strauss A, Modanlou HD. Soluble vascular endothelial growth factor receptor 1 in tracheal aspirate fluid of preterm neonates at birth may be predictive of bronchopulmonary dysplasia/chronic lung disease. Pediatrics. 2009;123:1541–1547. doi: 10.1542/peds.2008-1670. [DOI] [PubMed] [Google Scholar]
- 35.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 36.Tita ATN, Andrews WW. Diagnosis and management of clinical chorioamnionitis. Clin Perinatol. 2010;37:339–354. doi: 10.1016/j.clp.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kallapur SG, Bachurski CJ, Le Cras TD, Joshi SN, Ikegami M, Jobe AH. Vascular changes after intra-amniotic endotoxin in preterm lamb lungs. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1178–L1185. doi: 10.1152/ajplung.00049.2004. [DOI] [PubMed] [Google Scholar]
- 38.Mandell E, Powers KN, Harral JW, Seedorf GJ, Hunter KS, Abman SH, et al. Intrauterine endotoxin-induced impairs pulmonary vascular function and right ventricular performance in infant rats and improvement with early vitamin D therapy. Am J Physiol Lung Cell Mol Physiol. 2015;309:L1438–L1446. doi: 10.1152/ajplung.00302.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Been JV, Zimmermann LJI, Debeer A, Kloosterboer N, van Iwaarden JF. Bronchoalveolar lavage fluid from preterm infants with chorioamnionitis inhibits alveolar epithelial repair. Respir Res. 2009;10:116. doi: 10.1186/1465-9921-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mandell E, Seedorf G, Gien J, Abman SH. Vitamin D treatment improves survival and infant lung structure after intra-amniotic endotoxin exposure in rats: potential role for the prevention of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2014;306:L420–L428. doi: 10.1152/ajplung.00344.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang JR, Karumanchi SA, Seedorf G, Markham N, Abman SH. Excess soluble vascular endothelial growth factor receptor-1 in amniotic fluid impairs lung growth in rats: linking preeclampsia with bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2012;302:L36–L46. doi: 10.1152/ajplung.00294.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cooney TP, Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal 1--postnatal lung growth. Thorax. 1982;37:572–579. doi: 10.1136/thx.37.8.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Emery JL, Mithal A. The number of alveoli in the terminal respiratory unit of man during late intrauterine life and childhood. Arch Dis Child. 1960;35:544–547. doi: 10.1136/adc.35.184.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hilgendorff A, Reiss I, Ehrhardt H, Eickelberg O, Alvira CM. Chronic lung disease in the preterm infant. Lessons learned from animal models. Am J Respir Cell Mol Biol. 2014;50:233–245. doi: 10.1165/rcmb.2013-0014TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ambalavanan N, Morty RE. Searching for better animal models of BPD: a perspective. Am J Physiol Lung Cell Mol Physiol. 2016;311:L924–L927. doi: 10.1152/ajplung.00355.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mandell EW, Abman SH. Fetal vascular origins of bronchopulmonary dysplasia. J Pediatr. 2017;185:7–10.e1. doi: 10.1016/j.jpeds.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 47.Keller RL, Feng R, DeMauro SB, Ferkol T, Hardie W, Rogers EE, et al. Bronchopulmonary dysplasia and perinatal characteristics predict 1-year respiratory outcomes in newborns born at extremely low gestational age: a prospective cohort study. J Pediatr. 2017;187:89–97.e3. doi: 10.1016/j.jpeds.2017.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeitlin J, El Ayoubi M, Jarreau PH, Draper ES, Blondel B, Künzel W, et al. MOSAIC Research Group. Impact of fetal growth restriction on mortality and morbidity in a very preterm birth cohort. J Pediatr. 2010;157:733–9.e1. doi: 10.1016/j.jpeds.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Eriksson L, Haglund B, Odlind V, Altman M, Ewald U, Kieler H. Perinatal conditions related to growth restriction and inflammation are associated with an increased risk of bronchopulmonary dysplasia. Acta Paediatr. 2015;104:259–263. doi: 10.1111/apa.12888. [DOI] [PubMed] [Google Scholar]
- 50.Ronkainen E, Dunder T, Kaukola T, Marttila R, Hallman M. Intrauterine growth restriction predicts lower lung function at school age in children born very preterm. Arch Dis Child Fetal Neonatal Ed. 2016;101:F412–F417. doi: 10.1136/archdischild-2015-308922. [DOI] [PubMed] [Google Scholar]
- 51.Greenough A, Yuksel B, Cheeseman P. Effect of in utero growth retardation on lung function at follow-up of prematurely born infants. Eur Respir J. 2004;24:731–733. doi: 10.1183/09031936.04.00060304. [DOI] [PubMed] [Google Scholar]
- 52.Ozkan H, Cetinkaya M, Koksal N. Increased incidence of bronchopulmonary dysplasia in preterm infants exposed to preeclampsia. J Matern Fetal Neonatal Med. 2012;25:2681–2685. doi: 10.3109/14767058.2012.708371. [DOI] [PubMed] [Google Scholar]
- 53.Borghesi A, Massa M, Campanelli R, Bollani L, Tzialla C, Figar TA, et al. Circulating endothelial progenitor cells in preterm infants with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2009;180:540–546. doi: 10.1164/rccm.200812-1949OC. [DOI] [PubMed] [Google Scholar]
- 54.Baker CD, Balasubramaniam V, Mourani PM, Sontag MK, Black CP, Ryan SL, et al. Cord blood angiogenic progenitor cells are decreased in bronchopulmonary dysplasia. Eur Respir J. 2012;40:1516–1522. doi: 10.1183/09031936.00017312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Su EJ, Xin H, Yin P, Dyson M, Coon J, Farrow KN, et al. Impaired fetoplacental angiogenesis in growth-restricted fetuses with abnormal umbilical artery Doppler velocimetry is mediated by aryl hydrocarbon receptor nuclear translocator (ARNT) J Clin Endocrinol Metab. 2015;100:E30–E40. doi: 10.1210/jc.2014-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seedorf G, Metoxen AJ, Rock R, Markham N, Ryan S, Vu T, et al. Hepatocyte growth factor as a downstream mediator of vascular endothelial growth factor-dependent preservation of growth in the developing lung. Am J Physiol Lung Cell Mol Physiol. 2016;310:L1098–L1110. doi: 10.1152/ajplung.00423.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yun EJ, Lorizio W, Seedorf G, Abman SH, Vu TH. VEGF and endothelium-derived retinoic acid regulate lung vascular and alveolar development. Am J Physiol Lung Cell Mol Physiol. 2016;310:L287–L298. doi: 10.1152/ajplung.00229.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kendall RL, Wang G, Thomas KA. Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its heterodimerization with KDR. Biochem Biophys Res Commun. 1996;226:324–328. doi: 10.1006/bbrc.1996.1355. [DOI] [PubMed] [Google Scholar]
- 59.Shibuya M. Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct Funct. 2001;26:25–35. doi: 10.1247/csf.26.25. [DOI] [PubMed] [Google Scholar]
- 60.Gale NW, Yancopoulos GD. Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev. 1999;13:1055–1066. doi: 10.1101/gad.13.9.1055. [DOI] [PubMed] [Google Scholar]