Abstract
Rationale: In addition to their well-known function as antibody-producing cells, B lymphocytes can markedly influence the course of infectious or noninfectious diseases via antibody-independent mechanisms. In tuberculosis (TB), B cells accumulate in lungs, yet their functional contribution to the host response remains poorly understood.
Objectives: To document the role of B cells in TB in an unbiased manner.
Methods: We generated the transcriptome of B cells isolated from Mycobacterium tuberculosis (Mtb)-infected mice and validated the identified key pathways using in vitro and in vivo assays. The obtained data were substantiated using B cells from pleural effusion of patients with TB.
Measurements and Main Results: B cells isolated from Mtb-infected mice displayed a STAT1 (signal transducer and activator of transcription 1)-centered signature, suggesting a role for IFNs in B-cell response to infection. B cells stimulated in vitro with Mtb produced type I IFN, via a mechanism involving the innate sensor STING (stimulator of interferon genes), and antagonized by MyD88 (myeloid differentiation primary response 88) signaling. In vivo, B cells expressed type I IFN in the lungs of Mtb-infected mice and, of clinical relevance, in pleural fluid from patients with TB. Type I IFN expression by B cells induced an altered polarization of macrophages toward a regulatory/antiinflammatory profile in vitro. In vivo, increased provision of type I IFN by B cells in a murine model of B cell–restricted Myd88 deficiency correlated with an enhanced accumulation of regulatory/antiinflammatory macrophages in Mtb-infected lungs.
Conclusions: Type I IFN produced by Mtb-stimulated B cells favors macrophage polarization toward a regulatory/antiinflammatory phenotype during Mtb infection.
Keywords: B lymphocytes, macrophages, tuberculosis, IFN
At a Glance Commentary
Scientific Knowledge on the Subject
The role played by T cells in tuberculosis (TB) has been thoroughly investigated. In marked contrast, the contribution of B cells in immunity to TB, which has mostly been explored for their ability to produce antibodies, remains poorly understood, despite their massive accumulation in lung lesions of both patients with TB and experimentally infected animals.
What This Study Adds to the Field
Here we show that B cells can be directly stimulated by Mycobacterium tuberculosis in an innate manner to produce type I IFN to subsequently modulate the polarization of macrophages toward a regulatory/antiinflammatory profile in vitro and in infected lungs. This pathway was observed in a murine model of TB and in B cells isolated from patients with TB. Our observations reveal B cells as novel regulators of immunity to TB through type I IFN–mediated polarization of myeloid cells.
Infection with Mycobacterium tuberculosis (Mtb) leads to the formation of lung lesions, the granulomas, which contain macrophages and other cell types and are surrounded by various lymphocyte populations, including B lymphocytes (1–4). The presence of B cells at the site of infection suggests that they may contribute to host–pathogen interaction locally. Several studies attempted to delineate the antibody-mediated roles of B cells and the impact of their total deficiency in tuberculosis (TB) (5–10). Studies performed with B cell–deficient mice yielded conflicting results, with some studies concluding that B cells played no apparent function in TB and others concluding that B cells contributed to protection against Mtb (2, 6, 8, 11, 12). In humans, the depletion of B cells in patients treated with rituximab did not increase the risk of TB reactivation (13, 14), and in macaques rituximab administration to Mtb-infected animals had limited effects at the individual granuloma level (15). These studies suggest a moderate role for B cells in immunity to Mtb. However, they used approaches that might not be suitable to reveal more complex functions of B cells, in particular those mediated through the production of cytokines, whose relevance during infection by intracellular bacterial pathogens has received increasing experimental evidence (16–18). Indeed, B cells can play either favorable or detrimental roles during infection, depending on the cytokines they produce, and the depletion of the whole B-cell compartment may not be suitable to reveal such potentially antagonistic B-cell activities. The aim of our study was to investigate the eventual antibody-independent functions of B cells in an unbiased manner. For this, we analyzed the transcriptome of B cells isolated from the lungs and spleen of Mtb-infected mice. This revealed a STAT1 (signal transducer and activator of transcription 1)-centered signature, which pointed to the ability of B cells to both produce and respond to type I IFN. We identified STING (stimulator of interferon genes) and Mincle as positive regulators, and myeloid differentiation primary response gene 88 (MyD88) as a negative regulator of type I IFN production by Mtb-stimulated B cells. Type I IFN production by B cells drove macrophages toward an antiinflammatory phenotype in vitro. Mice with a B cell–specific Myd88 deficiency harbored B cells that overexpressed type I IFN and displayed an abnormal accumulation of antiinflammatory myeloid cells in infected lungs compared with control mice. This was associated with reduced signs of inflammation and increased Mtb burden in lungs. Importantly, B cells purified from the pleural fluid of patients with TB displayed a massive type I IFN expression, and supernatants of Mtb-stimulated human B cells also polarized human macrophages toward an antiinflammatory profile in vitro. Altogether, our data reveal that type I IFN expression in B cells impacts macrophage polarization toward an antiinflammatory/regulatory phenotype during TB and unravel a previously unanticipated role for B cells in this disease.
Methods
Patients with TB
Human studies were performed in accordance with the Declaration of Helsinki (2013) of the World Medical Association and have been approved by the Ethics Committees of Hospital F.J Muñiz, Academia Nacional de Medicina, and Instituto Vaccarezza from Buenos Aires, Argentina. Patients with TB with or without moderate and large pleural effusions were identified at the Servicio de Tisioneumonología. Written informed consent was obtained before sample collection.
Mice
All mice were on the C57BL/6 background. All of the procedures, including animal studies, were conducted in strict accordance with French laws and regulations in compliance with the European Community council directive 68/609/EEC guidelines and their implementation in France. All protocols were approved by the Comité d’Ethique Midi-Pyrénées (MP/11/13/02/11 and MP/07/80/11/12).
Full methods are provided in online supplement.
Results
B Cells Purified from the Lungs and Spleen of Mtb-infected Mice Display a STAT1-centered Gene Expression Signature
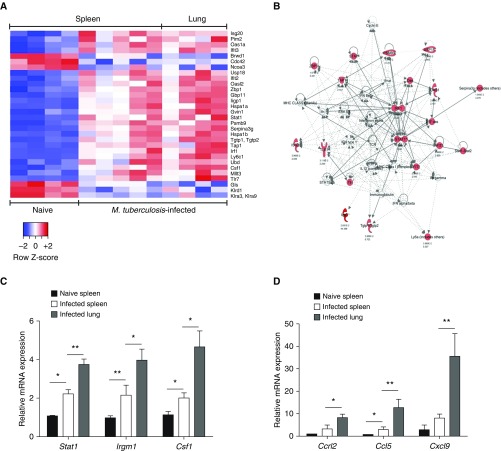
To address whether B cells might perform antibody-independent functions in TB, as observed in other bacterial infections (17–19), we performed a genome-wide transcriptome analysis of B cells isolated from the lungs and spleen of Mtb-infected mice, in comparison with splenic B cells from naive mice. Strikingly, B cells from infected mice differentially expressed a limited number (30) of genes (Figure 1A; see Table E1 in the online supplement) compared with naive controls. Ingenuity Pathway Analysis indicated that the differentially expressed genes formed a network centered on STAT1, a master transcription factor of the IFN response (Figure 1B). The higher expression of the STAT1 signature genes Stat1 (signal transducer and activator of transcription 1), Irgm1 (immunity-related GTPase family M member 1), Csf1 (colony-stimulating factor 1), Ccrl2 (C-C motif chemokine receptor–like 2), Ccl5 (C-C motif chemokine ligand 5), and Cxcl9 (C-X-C motif chemokine ligand 9) in B cells from the lungs of infected mice was confirmed by quantitative reverse transcriptase–polymerase chain reaction (Figures 1C and 1D).
Figure 1.
B cells from Mycobacterium tuberculosis (Mtb)-infected mice display a STAT1 signature. (A) Heat map of the differentially expressed genes (selected on the basis of an adjusted P value [Benjamini-Hochberg procedure] < 0.05 and a fold change > 2 or <0.5) both between B cells from the spleen of naive C57BL/6 mice and B cells from the spleen of Mtb-infected mice on the one side, as well as between B cells from the spleen of naive C57BL/6 mice and B cells from the lung of infected mice after 21 days of infection on the other side (we had to pool the B cells from three independent mice to obtain the necessary amount of mRNA to perform microarrays, and four to five independent microarrays were performed for each of the three conditions indicated above). (B) Main network deduced from the Ingenuity Pathway Analysis involved in B cells from Mtb-infected lungs and spleens, as compared with naive spleens. Solid lines and dotted lines indicate direct and indirect interactions, respectively. Differentially expressed genes present in the pathways are represented in red. Light red, 2 < fold change < 10; dark red, fold change > 10. (C) Quantitative reverse transcriptase–polymerase chain reaction analysis of mRNA expression of the Stat1, Irgm1, and Csf1 genes found to be up-regulated in the transcriptome of B cells purified from the spleen of naive mice or from spleen and lung of Mtb-infected C57BL/6 mice. (For each sample, B cells were pooled from three independent mice. Four to five independent infection experiments were performed.) (D) As in C, except that the Ccrl2, Ccl5, and Cxcl9 genes were analyzed. Data represent mean ± SEM and were analyzed by the two-tailed Mann-Whitney test. *P ≤ 0.05; **P ≤ 0.01.
Type I IFNs Are Chief Cytokines Induced in Lung B Cells on Mtb Infection and Reflect an Innate B-Cell Response
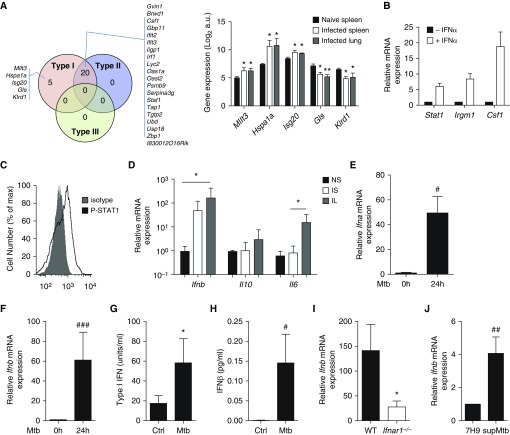
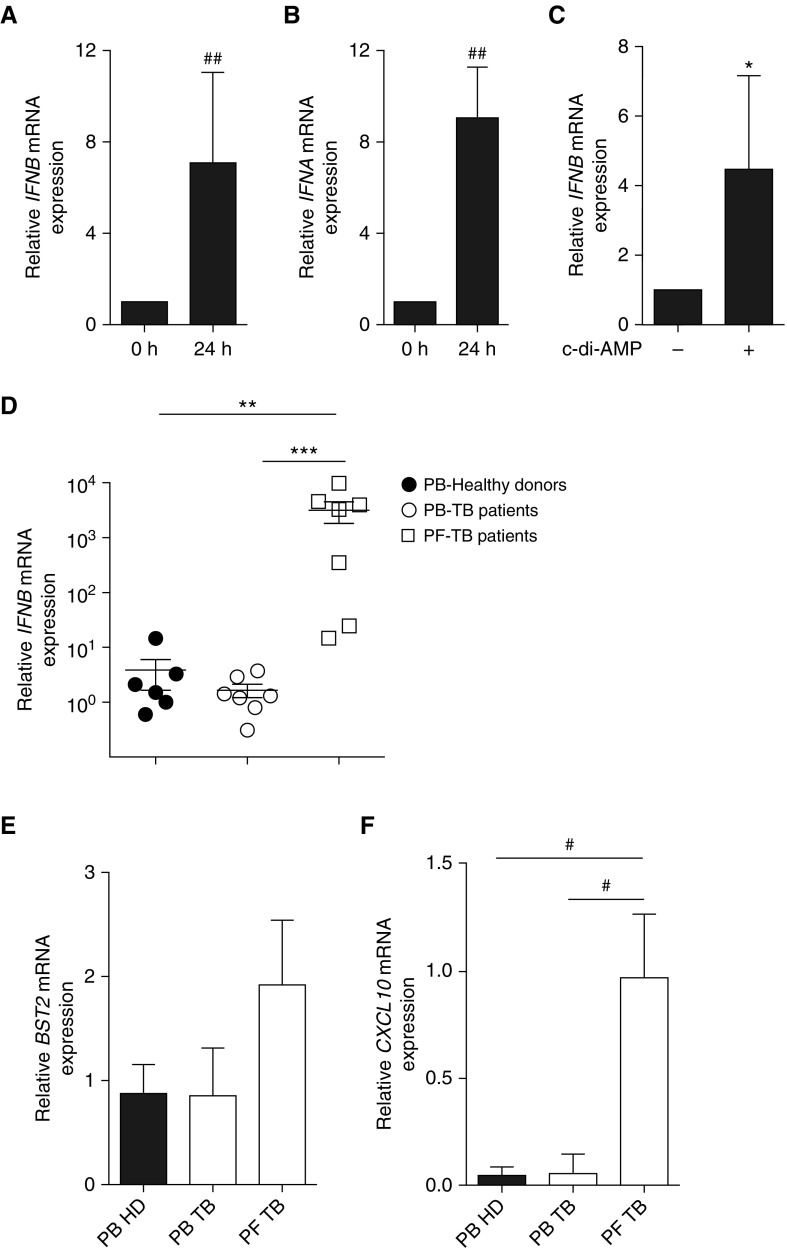
Interrogation of the Interferome database (20) indicated that 20 out of the 30 genes of the B-cell signature were regulated by both type I and type II, but not by type III IFN, and that 5 of them, namely Mllt3 (mixed-lineage leukemia; translocated to, 3), Hspa1a (heat shock protein family A, member A1), Isg20 (interferon-stimulated gene 20), Gls (glutaminase), and Klrd1 (killer cell lectin-like receptor D1), were specifically regulated by type I IFN (Figure 2A), suggesting that the STAT1 signature reflected an effect of type I IFN on B cells. Consistent with this possibility, naive B cells stimulated with type I IFN in vitro displayed a similar gene signature (Figure 2B), and B cells recovered from the lungs of Mtb-infected mice at 3 weeks after infection showed increased STAT1 phosphorylation after stimulation with type I IFN ex vivo (Figure 2C). Taken together, these data suggest that B cells were exposed to type I IFN in infected mice. Because type I IFN could act in an autocrine manner (21), we next investigated whether B cells expressed type I IFN during infection. B cells isolated from the lungs and spleen of infected mice indeed displayed a massive up-regulation of the Ifnb transcripts, compared with B cells from naive mice (Figure 2D). In comparison, the levels of Il-6 and Il-10 mRNA, which have previously been identified as important mediators of the antibody-independent functions of B cells in other diseases (16, 19), showed only a modest increase (although significant in for Il-6) (Figure 2D). Thus, type I IFNs are the chief cytokines induced in lung B cells on Mtb infection. This possibly involved a direct interaction between Mtb and B cells, because Mtb elicited type I IFN expression in naive spleen B cells in vitro within 24 hours (Figures 2E and 2F). Similar results were obtained after 4 hours of stimulation (Figure E1), underlining a rapid innate response. B cell–derived type I IFN protein was detected in the B-cell culture supernatants using a type I IFN–specific reporter cell line (Figure 2G) and ELISA (Figure 2H). Type I IFN amplified its induction in an autocrine manner, because its expression was markedly reduced in B cells lacking the type I IFN receptor subunit IFNAR1 (Figure 2I). B-cell infection per se was not necessary, as filtered supernatants from Mtb cultures also induced type I IFN expression in B cells, implicating secreted Mtb components in this process (Figure 2J). We conclude that B cells produce and respond to type I IFN during Mtb infection.
Figure 2.
B cells from Mycobacterium tuberculosis (Mtb)-infected mice produce and respond to type I IFN. (A) The Venn diagram shows the differentially expressed genes known to be regulated by type I, type II, and/or type III IFN according to Interferome analysis. The histogram on the right indicates the relative expression of the five genes regulated only by type I IFN in B cell samples from naive spleen or Mtb-infected spleen or lungs (microarray data). (B) B cells purified from the spleen of naive C57BL/6 mice were stimulated for 24 hours with IFNα or not, and the mRNA expression of IFN-stimulated genes was analyzed by quantitative reverse transcriptase–polymerase chain reaction (n = 3). (C) Overlay of flow cytometry histograms showing phospho-STAT1 staining in lung B cells from Mtb-infected mice stimulated for 15 minutes with IFNα (n = 3; a representative experiment out of two independent experiments is shown). (D) Expression of Ifnb, Il6, and Il10 in B cells purified from the spleen of naive (NS) or Mtb-infected (IS) C57BL/6 mice and from the lungs of Mtb-infected (IL) C57BL/6 mice after 21 days of infection. (For each sample, B cells were pooled from three independent mice. Four to five independent infection experiments were performed.) (E) Ifna mRNA induction in B cells purified from naive C57BL/6 spleens on in vitro 24-hour stimulation or not with Mtb (multiplicity of infection [MOI] = 0.3); n = 12). (F) As in E, except that Ifnb mRNA induction was measured (n = 12). (G) Activity of type I IFN measured using a reporter assay in the supernatant of naive splenic B cells stimulated or not for 6 days with Mtb (MOI = 0.3; n = 8 independent preparations of B cells per group). (H) Concentrations of IFNβ measured by ELISA in the supernatants of naive splenic B cells stimulated for 6 days or not with Mtb (MOI = 0.3; n = 13 independent preparations of B cells per group). (I) Ifnb mRNA expression in B cells purified from the spleen of WT or Ifnar1−/− mice on in vitro stimulation for 24 hours with Mtb (MOI = 0.3); fold change is relative to respective expression before stimulation (n = 5). (J) Ifnb mRNA expression in B cells purified from the spleen of C57BL/6 mice on in vitro stimulation (supMtb) for 24 hours or not (7H9 medium) with Mtb-culture supernatant (n = 7). Data represent mean ± SEM and were analyzed using the nonparametric two-tailed Mann-Whitney test or the Wilcoxon test (A, B, D, G, and I), *P ≤ 0.05; **P ≤ 0.01. For E, F, H, and J, we used the parametric two-tailed Student paired t-test (#P ≤ 0.05; ##P ≤ 0.01; ###P ≤ 0.001). a.u. = arbitrary units; Ctrl = control; WT = wild type.
Type I IFN Expression in Mtb-stimulated B Cells Involves Innate Receptors
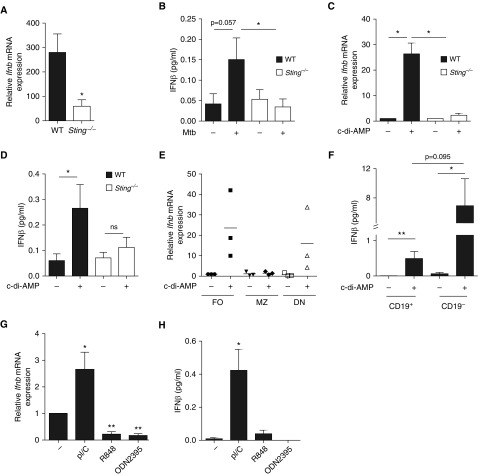
We next sought to identify the molecular mechanisms controlling type I IFN expression in B cells exposed to Mtb and tested the involvement of distinct innate sensors. The cytosolic dinucleotide sensor STING (22, 23) contributed to type I IFN expression in B cells stimulated with Mtb or cyclic-di-AMP (c-di-AMP), a secreted mycobacterial STING ligand (Figures 3A–3D) (22, 24). In addition, the C-type lectin Mincle (25) also contributed to type I IFN expression in B cells stimulated with Mtb, albeit to a lower extent than STING (Figure E2). We thus used c-di-AMP to further address which B-cell subset(s) contributed to this response. CD21lowCD23hi follicular and CD21−CD23− B cells contributed most to type I IFN production (Figure 3E). This particular mode of activation could operate in lung B cells, because B cells from Mtb-infected lungs also expressed type I IFN on c-di-AMP stimulation, although in smaller amounts than non-B cells taken for comparison (Figure 3F). Testing the role of other innate receptors, we found that type I IFN expression in B cells could also be triggered by a TLR3 (Toll-like receptor 3) ligand, suggesting a role for the adaptor TIR-domain–containing adapter-inducing IFN-β (TRIF), but not by ligands of TLR7/8 or 9, which signal via MyD88 (Figures 3G and 3H).
Figure 3.
STING and its ligand trigger type I IFN expression in B cells. (A) Ifnb mRNA expression in B cells purified from the spleen of wild type (WT, solid bars) or Sting−/− (open bars) on stimulation for 24 hours with Mycobacterium tuberculosis (Mtb) (multiplicity of infection = 0.3); fold change represents expression after 24 hours of stimulation relative to respective expression before stimulation (n = 5–10 mice per group). (B) Concentration of IFNβ in the supernatants of naive splenic B cells from WT or Sting−/− mice stimulated for 6 days or not with Mtb (n = 4 per group). (C) Ifnb mRNA expression in B cells purified from the spleen of naive WT (solid bars) or Sting−/− (open bars) mice stimulated (+) or not (−) with c-di-AMP during 24 hours (n = 4 per group). (D) Concentration of IFNβ in the supernatants of naive splenic B cells from WT or Sting−/− mice stimulated for 3 days or not with c-di-AMP (n = 5 per group). (E) Ifnb mRNA expression in CD21lowCD23hi follicular (FO), CD21hiCD23low marginal zone (MZ), and CD21−CD23− double-negative (DN) B cells sorted from the spleen of naive C57BL/6 mice on 24-hour stimulation (+) or not (−) with c-di-AMP; fold change is relative to unstimulated follicular B cells (n = 3). Each symbol represents B cells purified from an individual mouse. (F) Concentration of IFNβ in the supernatants of CD19+ and CD19− lung cells purified from Mtb-infected C57BL/6 mice on 24-hour ex vivo stimulation (+) or not (−) with c-di-AMP (for each sample, B cells were pooled from three independent mice, and we performed four to five independent infection experiments). (G) Ifnb mRNA expression in splenic B cells from WT mice stimulated or not with the indicated TLR ligands during 24 hours (n = 4–7). (H) Concentration of IFNβ in the supernatants of splenic B cells from WT mice stimulated or not with the indicated TLR ligands during 3 days (n = 4 per group). Data represent mean ± SEM and were analyzed using the two-tailed (A, C, E, F, G, and H) or one-tailed (B and D) Mann-Whitney test. *P ≤ 0.05; **P ≤ 0.01. One-tailed Mann-Whitney was used in B and D, because protein expression is expected to positively correlate with mRNA expression. ns = not significant; TLR = Toll-like receptor.
Type I IFN Production by B Cells on Mtb Stimulation Is Antagonized by MyD88 Signaling
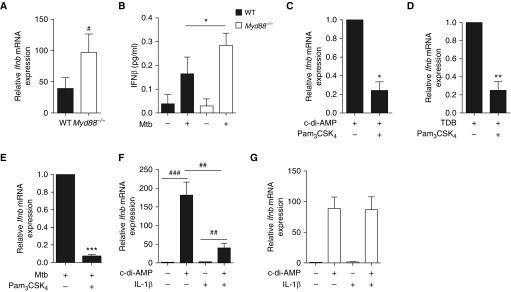
We next tested the effect of MyD88 signaling on type I IFN expression in Mtb-stimulated B cells. Myd88 (myeloid differentiation primary response 88) deficiency resulted in an increased type I IFN expression at both transcriptional (Figure 4A) and protein (Figure 4B) levels. Accordingly, stimulation of B cells with the TLR2 agonist Pam3CSK4, which signals via MyD88, downregulated type I IFN expression induced by the agonists of STING (Figure 4C) or Mincle (Figure 4D), or Mtb (Figure 4E). As expected, IL-1β also inhibited type I IFN expression in B cells in a MyD88-dependent manner (Figures 4F and 4G). In sum, we found that the amount of type I IFN produced by B cells is regulated by the balance between distinct innate signaling pathways. These results illustrate further the possible antagonism between TLR-MyD88 and IFN signaling (26–28).
Figure 4.
MyD88 signaling negatively regulates type I IFN expression in B cells. (A) Ifnb mRNA expression in B cells purified from the spleen of Myd88−/− (open bars) and wild type (WT, solid bars) control mice on stimulation for 24 hours with Mycobacterium tuberculosis (Mtb) (multiplicity of infection = 0.3); fold change represents expression after stimulation relative to respective expression before stimulation (set to 1) (n = 10 mice per group). (B) Concentration of IFNβ in the supernatants of naive splenic B cells from WT or Myd88−/− mice stimulated for 6 days or not with Mtb (n = 3). (C–E) Ifnb mRNA expression in B cells purified from the spleen of WT mice (n = 4–8 mice per group) on 24-hour in vitro stimulation with either c-di-AMP (C), TDB (D) or Mtb (E) in the presence (+) or absence (−) of the TLR2 agonist Pam3CSK4. (F) B cells from WT mice were stimulated with c-di-AMP and/or IL1β, then IFNβ expression was analyzed at the mRNA level (n = 6). (G) As in F, except that Myd88−/− B cells were used (n = 4). Data represent mean ± SEM and were analyzed by using the two-tailed Mann-Whitney test or the two-tailed Wilcoxon test (B–E) (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001). The two-tailed Student paired t test was used for A and F (#P ≤ 0.05; ##P ≤ 0.01; ###P ≤ 0.001). TDB = trehalose-6,6-dibehenate.
Pleural Fluid B Cells Express Type I IFN in Patients with TB
An excessive type I IFN signature distinguished patients with TB from latently Mtb-infected individuals (29, 30). To address whether B cells could contribute to the type I IFN response in TB, we next assessed whether human B cells produced and responded to type I IFN on Mtb stimulation in vitro and whether this pathway was operative in clinical TB. Human B cells purified from the blood of healthy donors up-regulated type I IFN (both α and β) expression on stimulation with Mtb (Figures 5A and 5B) or c-di-AMP (Figure 5C) in vitro. Remarkably, B cells from the pleural fluid (PF) of patients with TB displayed a markedly increased abundance of type I IFN transcripts, compared with B cells purified from the blood of healthy donors or patients with TB, indicating that this response was particularly present in infected lungs during disease (Figure 5D). PF B cells also responded to type I IFN in vivo, because they displayed an increased expression of BST2 (bone marrow stromal cell antigen 2) and CXCL10 (Figures 5E and 5F), two genes belonging to the type I IFN signature in patients with active TB (29). These results show that B cells locally express and respond to type I IFN in the infected lungs during clinical TB.
Figure 5.
Expression of IFNβ in blood B cells from healthy donors after Mycobacterium tuberculosis (Mtb) stimulation and in pleural B cells of patients with tuberculosis (TB). (A) Induction of IFNB mRNA expression in peripheral blood (PB) B cells of healthy donors stimulated or not for 24 hours with Mtb (multiplicity of infection = 0.3; n = 8). (B) IFNA mRNA expression in B cells purified from peripheral blood mononuclear cells of healthy donor as in A (n = 8). (C) IFNB mRNA expression in B cells purified from the blood of healthy donors stimulated (+) or not (−) with c-di-AMP for 24 hours (n = 4 per group). (D–F) mRNA expression of IFNB (D), BST2 (E), and CXCL10 (F) in B cells from peripheral blood of healthy donors, patients with TB, and in B cells from pleural fluid (PF) of patients with TB. Each symbol represents an independent donor (n = 6–7 individuals per group). The expression level was arbitrarily set to 1 for one sample from the peripheral blood of healthy donors group, and the values for the other samples were calculated relative to this reference. Data represent mean ± SEM and were analyzed using the two-tailed Mann-Whitney test or the two-tailed Wilcoxon test (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001) except for panels A, B, and F, where a two-tailed Student paired t-test was used (#P ≤ 0.05; ##P ≤ 0.01). HD = healthy donor.
Mtb-stimulated B Cells Drive Macrophage Polarization toward an Antiinflammatory/Regulatory Profile in Both Mice and Humans
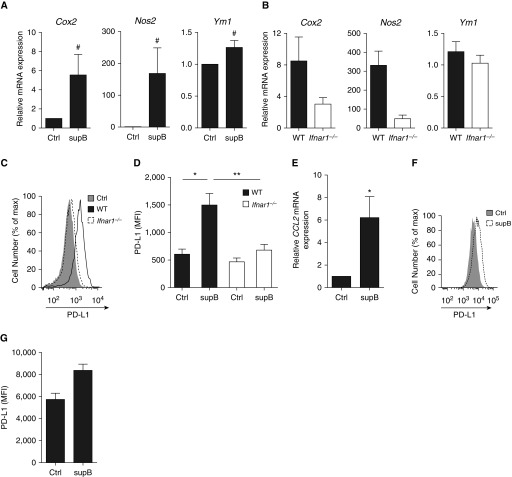
Considering that B cells can directly influence the activity of cells located in their microenvironment through cytokine production (16, 19), are in close contact with macrophages in TB lesions (31), and have already been shown to modulate macrophage activity (18, 32, 33), we next assessed whether type I IFN produced by Mtb-stimulated B cells could affect macrophage polarization. Macrophages treated with the supernatant from Mtb-stimulated B cells exhibited an enhanced expression of Cox2 (cyclooxygenase 2), Nos2 (nitric oxide synthase 2), and Ym1 (Figure 6A), which depended on type I IFN (Figure 6B). A profound IFNAR1-dependent induction of IFN-stimulated genes, including Ccl2 and Tnfsf10 (tumor necrosis factor superfamily member 10), was also triggered in treated macrophages (Figure E3). In addition, these macrophages displayed an enhanced expression of the regulatory/antiinflammatory molecules PD-L1 (programmed death-ligand 1) and IL-10 (Figure 6C and 6D, Figure E4) as well as a decreased production of IL-1β (Figure E4B). A similar IFNAR-1–dependent expression profile was triggered in macrophages treated with the supernatant of c-di-AMP–stimulated B cells, confirming the involvement of type I IFN triggered by STING in this altered macrophage polarization (Figure E5). Similarly, supernatant of Mtb-activated human B cells induced human macrophages to express IFN-stimulated genes, such as CCL2 (Figure 6E) and PD-L1 (Figures 6F and 6G). These results demonstrate that type I IFN produced by Mtb-activated B cells can directly drive macrophages toward an antiinflammatory/regulatory phenotype and suggest that B cells can directly influence adjacent macrophages via the local production of type I IFN in patients with active TB.
Figure 6.
IFNβ production by B cells polarizes macrophages in vitro toward an antiinflammatory phenotype. (A) Cox2, Nos2, and Ym1 mRNA expression in wild type (WT) macrophages first conditioned or not with supernatant of Mycobacterium tuberculosis (Mtb)-stimulated B cells and then infected with Mtb (multiplicity of infection [MOI] = 0.5; n = 6–7). (B) Same as in A, but in WT or Ifnar1−/− macrophages (MOI = 0.5; n = 4). (C) Overlay of flow cytometry histograms and (D) mean fluorescence intensity (MFI) of PD-L1 surface expression on macrophages from the bone marrow of naive WT or Ifnar1−/− mice and incubated (supB) or not (Ctrl) for 24 hours with supernatants of Mtb-stimulated B cells (n = 4 independent preparations of B cells per group). (E) CCL2 mRNA expression in human monocyte-derived macrophages incubated with supernatants of human B cells stimulated or not with Mtb (n = 4). (F) Overlay of flow cytometry histograms and cumulative geometric MFI representing PD-L1 surface expression on macrophages incubated with supernatants of B cells stimulated or not with Mtb (n = 4). Results were analyzed using the two-tailed Mann-Whitney test or the two-tailed Wilcoxon test (*P ≤ 0.05; **P ≤ 0.01) except for A, where a two-tailed Student paired t-test was used (#P ≤ 0.05).
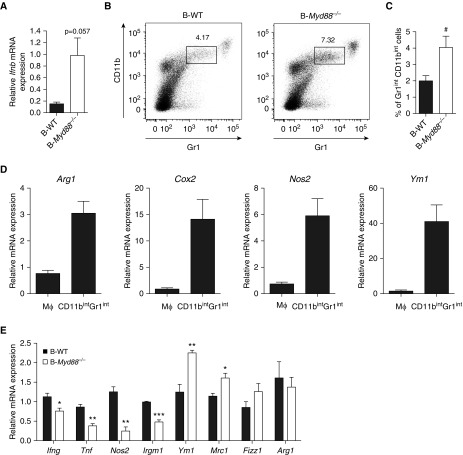
Excessive production of type I IFN by B cells is associated with altered macrophage polarization in the lungs of Mtb-infected mice. Because increased levels of type I IFN were associated with clinical TB (29), and because B cells from the PF of patients with TB expressed high levels of type I IFN (Figure 5D), we investigated the consequence of type I IFN overexpression in B cells in the mouse. To generate a model in which only B cells overexpress type I IFN, we took advantage of the fact that type I IFN production by B cells was inhibited by MyD88 signaling. Therefore, we generated mixed bone marrow chimera (34, 35), in which only B cells lacked MyD88 (B-Myd88−/−), as well as their corresponding controls with wild-type B cells (B-WT, B-CTRL). As expected, B cells from the lungs of Mtb-infected B-Myd88−/− mice expressed more type I IFN transcripts than their controls (Figure 7A). Remarkably, B-Myd88−/− mice harbored in lungs an increased proportion of CD11bintGr1int cells (Figures 7B and 7C), resembling a population of Mtb-permissive monocytes/macrophages known to develop in a type I IFN–dependent manner (36). Further characterization of CD11bintGr1int cells from infected mice revealed that they expressed high levels of Arg1 (arginase 1), Cox2, iNOS (inducible nitric oxide synthase), and Ym1 compared with “classical” macrophages (Figure 7D). These data suggest that B-Myd88−/− mice display an increased accumulation of antiinflammatory monocytes/macrophages compared with control mice. In keeping with this, they also showed in total lung an increased expression of genes characteristic of antiinflammatory and tissue repair–driving M2 macrophages, such as Ym1 and Mrc1 (mannose receptor C-type 1) (37, 38) (Figure 7E), with other genes associated with M2 macrophages showing a similar trend to increased expression (Fizz1 [found in inflammatory zone 1]) or unaffected (Arg1) (Figure 7E), compared with controls. In contrast, the proinflammatory genes Ifng, Tnfa (tumor necrosis factor α), Nos2, and Irgm1 were expressed at lower levels in infected chimeric animals than in controls (Figure 7E). These data are therefore consistent with our initial hypothesis that B-Myd88−/− mice would, as a result of excessive type I IFN production by B cells, show an altered macrophage polarization toward an antiinflammatory/regulatory profile. This B cell–mediated effect specifically affected macrophages, because similar frequencies of infiltrating T (both CD4+ and CD8+) and B cells were observed in B-Myd88−/− mice compared with their B-WT counterparts (Figure E6A). Of note, the altered macrophage phenotype in B-Myd88−/− was associated with slightly increased Mtb loads (Figure E6B) and reduced signs of inflammation in lungs (Figure E6C). Collectively, our data reveal that innate production of type I IFN by B cells correlates with an altered polarization of lung macrophages during Mtb infection.
Figure 7.
Excessive production of type I IFN by B cells is associated with altered macrophage polarization and reduced inflammation in the lungs of Mycobacterium tuberculosis (Mtb)-infected mice. Mixed bone marrow chimeras were generated, in which B cells were competent (B-WT, i.e., 80% μMT + 20% wild type [WT]→ WT, solid bars) or deficient (B-Myd88−/−, i.e., 80% μMT + 20% Myd88−/− →WT, open bars) for Myd88. B-CTRL mice (80% WT + 20% MyD88−/−→WT) lacking Myd88 expression on 20% total hematopoietic cells were also used as control. These mice were infected with 1,000 cfu Mtb, H37Rv strain and their lungs analyzed 6 weeks later. (A) Ifnb mRNA expression in B cells purified from the lungs of Mtb-infected B-WT and B-Myd88−/− mice (for each sample, B cells were pooled from three independent mice, and we performed firve independent infection experiments). The expression level was arbitrarily set to 1 for one sample from the B-Myd88−/− group, and the values for the other samples were calculated relative to this reference. (B) Representative dot plot of CD11b versus Gr-1 staining in the lungs of B-WT or and B-Myd88−/− mice infected for 42 days with Mtb. The gates indicate the percentage of CD11bintGr-1int cells among total lung cells. One representative experiment out of two independent experiments is shown. (C) Percentage of Gr1intCD11bint cells in the lung of B-WT or B-Myd88−/− mice (n = 7). (D) CD11b+Gr1− (Mϕ) and CD11bintGr1int cells were sorted by fluorescence-activated cell sorter from the lungs of C57BL/6 mice infected with Mtb for 42 days and then analyzed for the expression of the indicated genes (n = 5). (E) mRNA expression of proinflammatory cytokines and antiinflammatory genes in the lung of B-WT and B-Myd88−/− mice (n = 3). Data represent mean ± SEM, are representative of two independent experiments, and were analyzed using the two-tailed Mann-Whitney test. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001 except for C, where a two-tailed Student paired t-test was used (#P ≤ 0.05). cfu = colony-forming units; CTRL = control.
Discussion
Our study reveals that innate signaling in B cells leads, through type I IFN production, to the modulation of macrophage polarization in TB. In particular, our data demonstrate that on Mtb stimulation, B cells of murine or human origin produce type I IFN. Although B cells produce only low amounts of these cytokines, such low amounts have already been reported for type I IFN in other settings and thus appear to be a normal feature of the type I IFN response (39). Here, we found that type I IFN production by Mtb-stimulated B cells was regulated by the integration of several pathways, whose dysregulation led to important functional consequences in TB. At the molecular level, we identified several ligands, together with their corresponding host sensors, namely STING and, to a lesser extent, Mincle, as contributors to type I IFN expression in B cells during Mtb stimulation. We also show that intrinsic MyD88 signaling, associated with either TLR or IL-1 receptor triggering, is a potent inhibitor of type I IFN production by B cells, which further illustrates the antagonism between MyD88 signaling and IFN production (28). Whether additional pathways such as the TRIF-dependent one could be involved in type I IFN production by B cells remains to be explored, yet seems likely given the capacity of TLR3 agonist to trigger such a response.
From a clinical viewpoint, we report that type I IFN expression in B cells is dramatically increased in the PF of patients with TB compared with peripheral blood B cells of patients with TB or healthy donors. This is in contrast with the reported type I IFN signature observed in blood myeloid cells of patients with TB, compared with latently infected individuals or healthy subjects (29, 30, 40), but similar to the situation reported in blood T cells of patients with TB (31). We can think of three possible explanations for the local expression of type I IFN in TB. First, it is possible that lymphocytes are in nonresponsive state specifically in blood in TB. This would be consistent with the fact that peripheral B cells from patients with TB have been reported to be hyporesponsive to stimulation (41). Alternatively, this might reflect a preferential trapping of modulated lymphocytes in the lungs and possibly secondary lymphoid organs, whereas modulated myeloid cells might be more prone to circulate from the affected tissue to the blood. Finally, this might indicate a different threshold of activation for B cells and myeloid cells by Mtb, so that B cells only respond to Mtb-derived molecules when these are present at a high concentration such as in the infected lung, but not in the blood, whereas myeloid cells might be able to respond to the lower microbial compound concentrations in blood. Thus, our data suggest that type I IFN production by B cells might be important locally, at the site of TB infection and inflammation. B cells are known to be in close proximity to other immune cells, including monocytes and macrophages, in the lungs of patients with TB or infected animals (3, 31). Our in vitro data unambiguously demonstrate that Mtb-stimulated B cells drive macrophage polarization toward an antiinflammatory profile in a type I IFN–dependent manner. B cells were previously reported to modulate macrophage polarization in various models of infection, autoimmunity, and cancer (18, 32, 42). In particular, B cells polarized macrophages toward an alternatively activated M2 state through IL-10 production in a murine melanoma model (42). By contrast, a similar action of B cells on macrophages was reported to be independent of IL-10 in the context of TB (33). Our data provide direct evidence that type I IFN produced by Mtb-stimulated B cells contributes to macrophage polarization in vitro, which might explain this apparent discrepancy. This is in agreement with our in vivo data showing that overproduction of type I IFN by B cells in a mouse model of B cell–restricted Myd88 deficiency correlates with the accumulation of antiinflammatory/regulatory macrophages during Mtb infection in infected lungs, which is locally associated with increased bacterial burden and reduced pathology.
Overexpression of type I IFN is generally detrimental in TB (43, 44). In particular, hypervirulent strains of Mtb induce increased production of type I IFN (45, 46), and patients with active TB disease show a type I IFN–inducible gene expression profile in their blood cells (29, 47). Among the proposed mechanisms for the detrimental action of type I IFN in TB is the induction of IL-10 secretion by myeloid cells and macrophages, which leads to a reduced expression of protective IL-12 and tumor necrosis factor-α (28, 43), as well as an increased induction of myeloid-derived suppressor cells (36, 48) known to be permissive for Mtb replication (48). Here we show that type I IFN overproduction by B cells in our mouse model of B cell–restricted Myd88 deficiency is associated with an accumulation of CD11bintGr1int cells. Myeloid cells sharing similar phenotypic characteristics were previously reported as Mtb-permissive macrophages, whose accumulation depended on type I IFN (37). Because MyD88 signaling controls the production of IL-6 and IL-10 by B cells (49), we cannot exclude that these cytokines play a part in the observed phenotype. However, beyond the fact that the major cellular changes observed in B-Myd88−/− mice are known to be inducible by type I IFN, we do not favor this possibility, because Il6 and Il10 mRNA were only induced at modest levels in B cells isolated from the lungs of wild-type mice, and it is most likely that their expression was even lower in Myd88-deficient B cells.
Overall, hyperproduction of type I IFN production by B cells correlates with increased Mtb burden in lungs, which suggests that this B-cell activity negatively affects the control of bacterial replication and is in agreement with the belief that type I IFNs are detrimental in TB. How low concentrations of type I IFN can induce significant biological responses is a complex question, whose answer likely rests on parameters such as the diversity of type I IFNs, their differential affinity for the IFNAR receptor, the properties of type I IFN signaling pathways, and the timing and duration of expression (50). In conclusion, our study reveals type I IFN production as a novel antibody-independent function of B cells in immunity to TB.
Acknowledgments
Acknowledgment
The authors thank G. Lugo-Villarino for helpful comments, F. Moreau and L. Lepourry for help with animal experiments, F. Capilla for technical assistance in histology, and L. Trouilh and S. Lamarre for help with microarray experiments and statistical analysis. They also thank A. Debin for the kind gift of reagents and G. Czapliki for help with statistical analysis. They also thank A. Peixoto and the members of the TRI core facility for cell sorting and flow cytometry analysis and the members of the ANEXPLO GenoToul core facility for animal experiments.
Footnotes
Supported by Centre National de la Recherche Scientifique, University of Toulouse–Université Paul Sabatier, Institut Pasteur, Agence Nationale de la Recherche grants B-TB ANR-12-BSV3-0002 and ANR-11-EQUIPEX-0003 (O.N.), the European Union grants NEWTBVAC 241745 and TBVAC2020 643381 (O.N. and S.H.E.K.), grant ERA-INFECT ABIR (S.F. and S.H.E.K), the Bettencourt-Schueller Foundation, the Fondation pour la Recherche Médicale grant DEQ20160334902 (O.N.), and Deutsche Forschungsgemeinschaft grants SFB 796 and TP B6 (R.L.) and SFB TRR10 (S.F.). A.O’G. is funded by the Francis Crick Institute, which receives its core funding from Cancer Research UK (FC001126), the UK Medical Research Council (FC001126), and the Wellcome Trust (FC001126); by the UK Medical Research Council (MR/U117565642/1); and by the European Research Council (294682-TB-PATH). A.B. held a fellowship from the Fondation pour la Recherche Médicale.
Author Contributions: A.B. performed most of the experiments and data analysis, designed the study, and wrote the manuscript. I.S., P.S., A.C., L.T., B.G., and T.A.-S. contributed to some experiments. I.M., L.J., P.B., and V.A.-L. performed the microarray data analysis. P.S., R.L., J.R., M.D.C.S., A.G.L., and A.O’G. provided key biological material. S.H.E.K. helped with the writing of the manuscript. S.F., O.N., and D.H. designed the study, performed some experiments, and wrote the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201707-1475OC on November 21, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kahnert A, Höpken UE, Stein M, Bandermann S, Lipp M, Kaufmann SH. Mycobacterium tuberculosis triggers formation of lymphoid structure in murine lungs. J Infect Dis. 2007;195:46–54. doi: 10.1086/508894. [DOI] [PubMed] [Google Scholar]
- 2.Maglione PJ, Xu J, Chan J. B cells moderate inflammatory progression and enhance bacterial containment upon pulmonary challenge with Mycobacterium tuberculosis. J Immunol. 2007;178:7222–7234. doi: 10.4049/jimmunol.178.11.7222. [DOI] [PubMed] [Google Scholar]
- 3.Tsai MC, Chakravarty S, Zhu G, Xu J, Tanaka K, Koch C, et al. Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cell Microbiol. 2006;8:218–232. doi: 10.1111/j.1462-5822.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 4.Ulrichs T, Kosmiadi GA, Trusov V, Jörg S, Pradl L, Titukhina M, et al. Human tuberculous granulomas induce peripheral lymphoid follicle-like structures to orchestrate local host defence in the lung. J Pathol. 2004;204:217–228. doi: 10.1002/path.1628. [DOI] [PubMed] [Google Scholar]
- 5.Chan J, Mehta S, Bharrhan S, Chen Y, Achkar JM, Casadevall A, et al. The role of B cells and humoral immunity in Mycobacterium tuberculosis infection. Semin Immunol. 2014;26:588–600. doi: 10.1016/j.smim.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kozakiewicz L, Chen Y, Xu J, Wang Y, Dunussi-Joannopoulos K, Ou Q, et al. B cells regulate neutrophilia during Mycobacterium tuberculosis infection and BCG vaccination by modulating the interleukin-17 response. PLoS Pathog. 2013;9:e1003472. doi: 10.1371/journal.ppat.1003472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kozakiewicz L, Phuah J, Flynn J, Chan J. The role of B cells and humoral immunity in Mycobacterium tuberculosis infection. Adv Exp Med Biol. 2013;783:225–250. doi: 10.1007/978-1-4614-6111-1_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson CM, Cooper AM, Frank AA, Bonorino CB, Wysoki LJ, Orme IM. Mycobacterium tuberculosis aerogenic rechallenge infections in B cell-deficient mice. Tuber Lung Dis. 1997;78:257–261. doi: 10.1016/s0962-8479(97)90006-x. [DOI] [PubMed] [Google Scholar]
- 9.Lu LL, Chung AW, Rosebrock TR, Ghebremichael M, Yu WH, Grace PS, et al. A functional role for antibodies in tuberculosis. Cell. 2016;167:433–443.e14. doi: 10.1016/j.cell.2016.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casadevall A. Antibodies to Mycobacterium tuberculosis. N Engl J Med. 2017;376:283–285. doi: 10.1056/NEJMcibr1613268. [DOI] [PubMed] [Google Scholar]
- 11.Kondratieva TK, Rubakova EI, Linge IA, Evstifeev VV, Majorov KB, Apt AS. B cells delay neutrophil migration toward the site of stimulus: tardiness critical for effective bacillus Calmette-Guérin vaccination against tuberculosis infection in mice. J Immunol. 2010;184:1227–1234. doi: 10.4049/jimmunol.0902011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner J, Frank AA, Brooks JV, Gonzalez-Juarrero M, Orme IM. The progression of chronic tuberculosis in the mouse does not require the participation of B lymphocytes or interleukin-4. Exp Gerontol. 2001;36:537–545. doi: 10.1016/s0531-5565(00)00257-6. [DOI] [PubMed] [Google Scholar]
- 13.Atzeni F, Batticciotto A, Masala IF, Talotta R, Benucci M, Sarzi-Puttini P. Infections and biological therapy in patients with rheumatic diseases. Isr Med Assoc J. 2016;18:164–167. [PubMed] [Google Scholar]
- 14.Liao TL, Lin CH, Chen YM, Chang CL, Chen HH, Chen DY. Different risk of tuberculosis and efficacy of isoniazid prophylaxis in rheumatoid arthritis patients with biologic therapy: a nationwide retrospective cohort study in Taiwan. PLoS One. 2016;11:e0153217. doi: 10.1371/journal.pone.0153217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phuah J, Wong EA, Gideon HP, Maiello P, Coleman MT, Hendricks MR, et al. Effects of B cell depletion on early Mycobacterium tuberculosis infection in cynomolgus macaques. Infect Immun. 2016;84:1301–1311. doi: 10.1128/IAI.00083-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fillatreau S. Novel regulatory functions for Toll-like receptor-activated B cells during intracellular bacterial infection. Immunol Rev. 2011;240:52–71. doi: 10.1111/j.1600-065X.2010.00991.x. [DOI] [PubMed] [Google Scholar]
- 17.Neves P, Lampropoulou V, Calderon-Gomez E, Roch T, Stervbo U, Shen P, et al. Signaling via the MyD88 adaptor protein in B cells suppresses protective immunity during Salmonella typhimurium infection. Immunity. 2010;33:777–790. doi: 10.1016/j.immuni.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Shen P, Roch T, Lampropoulou V, O’Connor RA, Stervbo U, Hilgenberg E, et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature. 2014;507:366–370. doi: 10.1038/nature12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fillatreau S. Cytokine-producing B cells as regulators of pathogenic and protective immune responses. Ann Rheum Dis. 2013;72:ii80–ii84. doi: 10.1136/annrheumdis-2012-202253. [DOI] [PubMed] [Google Scholar]
- 20.Rusinova I, Forster S, Yu S, Kannan A, Masse M, Cumming H, et al. Interferome v2.0: an updated database of annotated interferon-regulated genes. Nucleic Acids Res. 2013;41:D1040–D1046. doi: 10.1093/nar/gks1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai P, Wang W, Cao H, Avogadri F, Dai L, Drexler I, et al. Modified vaccinia virus Ankara triggers type I IFN production in murine conventional dendritic cells via a cGAS/STING-mediated cytosolic DNA-sensing pathway. PLoS Pathog. 2014;10:e1003989. doi: 10.1371/journal.ppat.1003989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dey B, Dey RJ, Cheung LS, Pokkali S, Guo H, Lee JH, et al. A bacterial cyclic dinucleotide activates the cytosolic surveillance pathway and mediates innate resistance to tuberculosis. Nat Med. 2015;21:401–406. doi: 10.1038/nm.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin L, Hill KK, Filak H, Mogan J, Knowles H, Zhang B, et al. MPYS is required for IFN response factor 3 activation and type I IFN production in the response of cultured phagocytes to bacterial second messengers cyclic-di-AMP and cyclic-di-GMP. J Immunol. 2011;187:2595–2601. doi: 10.4049/jimmunol.1100088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang J, Bai Y, Zhang Y, Gabrielle VD, Jin L, Bai G. Deletion of the cyclic di-AMP phosphodiesterase gene (cnpB) in Mycobacterium tuberculosis leads to reduced virulence in a mouse model of infection. Mol Microbiol. 2014;93:65–79. doi: 10.1111/mmi.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoenen H, Bodendorfer B, Hitchens K, Manzanero S, Werninghaus K, Nimmerjahn F, et al. Cutting edge: Mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose-dibehenate. J Immunol. 2010;184:2756–2760. doi: 10.4049/jimmunol.0904013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benson SA, Ernst JD. TLR2-dependent inhibition of macrophage responses to IFN-gamma is mediated by distinct, gene-specific mechanisms. PLoS One. 2009;4:e6329. doi: 10.1371/journal.pone.0006329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fortune SM, Solache A, Jaeger A, Hill PJ, Belisle JT, Bloom BR, et al. Mycobacterium tuberculosis inhibits macrophage responses to IFN-gamma through myeloid differentiation factor 88-dependent and -independent mechanisms. J Immunol. 2004;172:6272–6280. doi: 10.4049/jimmunol.172.10.6272. [DOI] [PubMed] [Google Scholar]
- 28.Mayer-Barber KD, Andrade BB, Oland SD, Amaral EP, Barber DL, Gonzales J, et al. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature. 2014;511:99–103. doi: 10.1038/nature13489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–977. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cliff JM, Kaufmann SH, McShane H, van Helden P, O’Garra A. The human immune response to tuberculosis and its treatment: a view from the blood. Immunol Rev. 2015;264:88–102. doi: 10.1111/imr.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chakravarty SD, Zhu G, Tsai MC, Mohan VP, Marino S, Kirschner DE, et al. Tumor necrosis factor blockade in chronic murine tuberculosis enhances granulomatous inflammation and disorganizes granulomas in the lungs. Infect Immun. 2008;76:916–926. doi: 10.1128/IAI.01011-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li R, Rezk A, Miyazaki Y, Hilgenberg E, Touil H, Shen P, et al. Canadian B cells in MS Team. Proinflammatory GM-CSF-producing B cells in multiple sclerosis and B cell depletion therapy. Sci Transl Med. 2015;7:310ra166. doi: 10.1126/scitranslmed.aab4176. [DOI] [PubMed] [Google Scholar]
- 33.Torrado E, Fountain JJ, Robinson RT, Martino CA, Pearl JE, Rangel-Moreno J, et al. Differential and site specific impact of B cells in the protective immune response to Mycobacterium tuberculosis in the mouse. PLoS One. 2013;8:e61681. doi: 10.1371/journal.pone.0061681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fillatreau S, Gray D. T cell accumulation in B cell follicles is regulated by dendritic cells and is independent of B cell activation. J Exp Med. 2003;197:195–206. doi: 10.1084/jem.20021750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 36.Antonelli LR, Gigliotti Rothfuchs A, Gonçalves R, Roffê E, Cheever AW, Bafica A, et al. Intranasal Poly-IC treatment exacerbates tuberculosis in mice through the pulmonary recruitment of a pathogen-permissive monocyte/macrophage population. J Clin Invest. 2010;120:1674–1682. doi: 10.1172/JCI40817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodero MP, Decalf J, Bondet V, Hunt D, Rice GI, Werneke S, et al. Detection of interferon alpha protein reveals differential levels and cellular sources in disease. J Exp Med. 2017;214:1547–1555. doi: 10.1084/jem.20161451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dorhoi A, Yeremeev V, Nouailles G, Weiner J, III, Jörg S, Heinemann E, et al. Type I IFN signaling triggers immunopathology in tuberculosis-susceptible mice by modulating lung phagocyte dynamics. Eur J Immunol. 2014;44:2380–2393. doi: 10.1002/eji.201344219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joosten SA, van Meijgaarden KE, Del Nonno F, Baiocchini A, Petrone L, Vanini V, et al. Patients with tuberculosis have a dysfunctional circulating B-cell compartment, which normalizes following successful treatment. PLoS Pathog. 2016;12:e1005687. doi: 10.1371/journal.ppat.1005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong S-C, Puaux A-L, Chittezhath M, Shalova I, Kajiji TS, Wang X, et al. Macrophage polarization to a unique phenotype driven by B cells. Eur J Immunol. 2010;40:2296–2307. doi: 10.1002/eji.200940288. [DOI] [PubMed] [Google Scholar]
- 43.McNab FW, Ewbank J, Howes A, Moreira-Teixeira L, Martirosyan A, Ghilardi N, et al. Type I IFN induces IL-10 production in an IL-27-independent manner and blocks responsiveness to IFN-γ for production of IL-12 and bacterial killing in Mycobacterium tuberculosis-infected macrophages. J Immunol. 2014;193:3600–3612. doi: 10.4049/jimmunol.1401088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP. The immune response in tuberculosis. Annu Rev Immunol. 2013;31:475–527. doi: 10.1146/annurev-immunol-032712-095939. [DOI] [PubMed] [Google Scholar]
- 45.Manca C, Tsenova L, Freeman S, Barczak AK, Tovey M, Murray PJ, et al. Hypervirulent M. tuberculosis W/Beijing strains upregulate type I IFNs and increase expression of negative regulators of the Jak-Stat pathway. J Interferon Cytokine Res. 2005;25:694–701. doi: 10.1089/jir.2005.25.694. [DOI] [PubMed] [Google Scholar]
- 46.Ordway D, Henao-Tamayo M, Harton M, Palanisamy G, Troudt J, Shanley C, et al. The hypervirulent Mycobacterium tuberculosis strain HN878 induces a potent TH1 response followed by rapid down-regulation. J Immunol. 2007;179:522–531. doi: 10.4049/jimmunol.179.1.522. [DOI] [PubMed] [Google Scholar]
- 47.Maertzdorf J, Weiner J, III, Mollenkopf HJ, Bauer T, Prasse A, Müller-Quernheim J, et al. TBornotTB Network. Common patterns and disease-related signatures in tuberculosis and sarcoidosis. Proc Natl Acad Sci USA. 2012;109:7853–7858. doi: 10.1073/pnas.1121072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knaul JK, Jörg S, Oberbeck-Mueller D, Heinemann E, Scheuermann L, Brinkmann V, et al. Lung-residing myeloid-derived suppressors display dual functionality in murine pulmonary tuberculosis. Am J Respir Crit Care Med. 2014;190:1053–1066. doi: 10.1164/rccm.201405-0828OC. [DOI] [PubMed] [Google Scholar]
- 49.Lampropoulou V, Hoehlig K, Roch T, Neves P, Calderón Gómez E, Sweenie CH, et al. TLR-activated B cells suppress T cell-mediated autoimmunity. J Immunol. 2008;180:4763–4773. doi: 10.4049/jimmunol.180.7.4763. [DOI] [PubMed] [Google Scholar]
- 50.Tomasello E, Pollet E, Vu Manh T-P, Uzé G, Dalod M. Harnessing mechanistic knowledge on beneficial versus deleterious IFN-I effects to design innovative immunotherapies targeting cytokine activity to specific cell types. Front Immunol. 2014;5:526. doi: 10.3389/fimmu.2014.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]