Abstract
Rationale: A 10-year gap in the median age of survival for patients with cystic fibrosis (CF) was reported between patients living in Canada compared with patients living in the United States.
Objectives: Because both malnutrition and poor lung function are associated with an increased risk of mortality in CF, we investigated the temporal and longitudinal trends in lung function and nutrition between Canada and the United States.
Methods: This cohort study used Canadian CF Registry and U.S. CF Foundation Patient Registry data from 1990 to 2013. A unified dataset was created to harmonize the variables collected within the two registries for the purpose of comparing outcomes between the two countries.
Measurements and Main Results: We conducted three analyses: survival differences by birth cohort; population trends for FEV1 and body mass index (BMI) over time; and individual patient FEV1 and BMI trajectories. The study included a total of 37,772 patients in the United States and 5,149 patients in Canada. Patients with CF experienced significant improvements in nutritional status and lung function in both Canada and the United States during the study. In addition, the survival gap between the two countries is narrowing within younger birth cohorts. The improvements for the patients within the United States were most prominent in the BMI trajectories, where patients born after 1990 in the United States have higher BMI that has persisted over time.
Conclusions: The reasons for the observed improvements, and catch-up in the United States, are likely multifactorial and include the introduction of high-fat, high-calorie diets; introduction of newborn screening; and/or improved access to care for CF children in the United States.
Keywords: cystic fibrosis, survival, lung function, nutrition, international comparison
At a Glance Commentary
Scientific Knowledge on the Subject
A survival gap between two sets of patients with cystic fibrosis (CF) has recently been identified, with those patients living in Canada living 10 years longer. Identifying lung function and nutritional status may explain this difference and may be key to closing the gap.
What This Study Adds to the Field
We have demonstrated that the survival gap between patients in both the United States and Canada is closing within the youngest birth cohorts. Although patients with CF in both countries have improved nutritional status and lung function, nutritional status and lung function of children with CF in the United States have improved faster. This improvement has led to the finding that patients born after 1990 in the United States have a clear body mass index advantage compared to Canada; this improvement has persisted over time.
Based on the cystic fibrosis (CF) national registry data from 2009 to 2013, a 10-year gap in the median age of survival for patients with CF was reported between Canada and the United States (1). Although survival is a key outcome for monitoring the health of individuals living with CF, several intermediate clinical outcomes may provide insight into the health of the CF population to help explain the observed differences in survival between the two countries.
It is well established that both malnutrition and poor lung function are associated with an increased risk of mortality in CF; consequently, treatments and interventions aim to maintain good nutrition and preserve lung function (2, 3). Nutritional status is most commonly monitored by tracking changes in body mass index (BMI) in adults, or BMI-for-age percentile (BMI percentile) in children (4). Lung function is monitored by tracking changes in FEV1 measured by spirometry. In particular, earlier implementation of aggressive nutritional support with a high-fat, high-calorie diet in Canada is thought to explain part of the observed difference in the survival rate. A comparative analysis of survival between CF clinics in Toronto (Canada) and Boston (United States) between 1972 and 1981 found a 9-year survival advantage in patients followed in Toronto, where the nutritional intervention was implemented in the early 1970s (5). The high-fat, high-calorie diet was subsequently implemented around the world and is now considered the standard of care for patients with CF.
The national annual CF registry reports in both Canada and the United States have demonstrated improvements in survival in more recent birth cohorts relative to earlier epochs, as well as cross-sectional improvements in the median lung function and nutritional status of patients with CF. However, the statistics within the reports cannot be directly compared between countries because of differences in reporting and key patient demographics (6).
The objectives of this study were to use the unified Canadian and U.S. CF dataset to compare 1) survival differences by birth cohorts, 2) population-level changes in lung function and nutritional status from 1990 until 2013, and 3) the rate of decline of lung function and nutritional status within patients between the two countries.
Methods
Study Design
This population-based cohort study used prospectively collected Canadian CF Registry (CCFR) and U.S. CF Foundation Patient Registry (USCFFPR) data from 1990 to 2013 inclusive. Analyses of these data for this study were approved by the Research Ethics Board at St. Michael’s Hospital, Toronto, Ontario (Research Ethics Board #14-148), and at Seattle Children’s Hospital (Institutional Research Board #15294).
Study Population
A detailed description of both registries as well as information on how individuals are accrued and monitored within each registry have previously been published (1). Briefly, the CCFR and the USCFFPR contain detailed demographic and clinical information on patients who have a confirmed diagnosis of CF and are receiving clinical care at accredited CF centers (see the online supplement).
Outcomes
Weight, height, and lung function measurements from the first stable measurement of each year were used for analysis. A stable measurement was defined as one that is taken from a routine outpatient clinic visit when the patient was not being treated for a pulmonary exacerbation. FEV1 was expressed as a percentage of the predicted values using Global Lung Function Initiative reference equations (7). BMI percentile was calculated for children between 6 and 18 years of age by using the Centers for Disease Control and Prevention growth charts (8). For individuals 19 years of age and older, subjects were classified into BMI categories based on World Health Organization guidelines (9) (see the online supplement). To have a continuous measure of nutritional status, we also calculated BMI percentiles for adults, assuming the expected BMI distribution of a 19-year-old.
Statistical Analyses
To evaluate the differences between demographic and clinical factors between the two countries, a standardized difference (Std. Diff.) greater than 10% was used to determine statistical significance (10).
Survival Differences by Birth Cohort
Individuals were categorized by year of birth into three birth cohorts: (1) before 1980, representing a period during which the high-fat diet was implemented in Canada but not the United States; (2) 1980–1989, representing a period during which both countries had the high-fat diet implemented; and (3) 1990 onward, representing a contemporary cohort (see the online supplement).
Population Trends over Time
To examine how FEV1% predicted and BMI (or BMI percentile) have changed on a population level over time between the two countries, the annual median value was calculated for each country from 1990 to 2013. A simple linear regression model was fit to determine the trend in median FEV1 (or BMI) over the time period (see the online supplement). Analyses were stratified by three age groups: 6–18 years, 19–40 years, and 41–65 years. The age categories were chosen to reflect the pediatric population, the adult population, and older adults who represent “survivors.” (11)
Individual Patient-Level Trajectories
Individual trajectories of FEV1% predicted (and BMI percentile) with age were modeled using linear mixed-effects models as previously described (12), which included an interaction term between country and age. The best model was selected based on the Bayesian information criterion. All analyses were stratified by birth cohort, as defined previously.
The longitudinal analyses were adjusted for covariates that might explain the observed differences between the countries. For all analyses, a strict P value was used for statistical significance (P < 0.0001) to avoid misinterpretation due to the large sample size (13). R version 3.3.0 was used to analyze the data.
Results
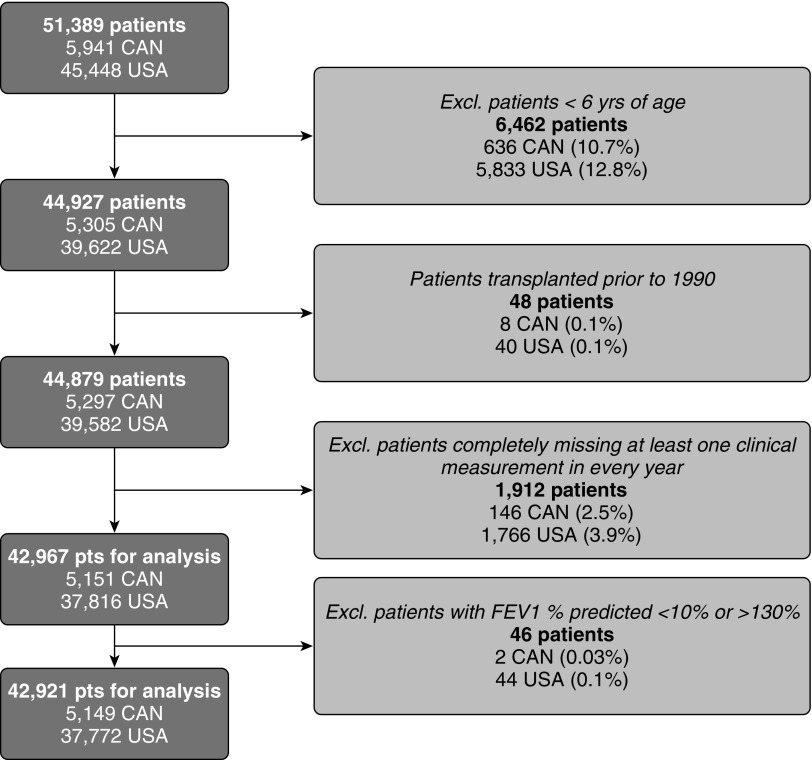
A total of 45,448 and 5,941 subjects are captured in the U.S. and Canadian cohorts, respectively, of which 37,772 and 5,149 were included in these analyses (Figure 1). The demographic characteristics of the patients with CF included in the study are presented in Table 1. The countries were similar with respect to the majority of the demographic characteristics, except a higher proportion of subjects were asymptomatic at the time of diagnosis in the United States compared with Canada (9.8% vs. 4.5%; Std. Diff., 17.4), which corresponds to a higher prevalence of newborn-screened subjects in the United States (5.4% vs. 0.3%; Std. Diff., 30.7). U.S. patients also had a higher prevalence of neonatal bowel obstruction (17.9% vs. 13.6%; Std. Diff., 11.1). More patients in the United States had a missing genotype (14% vs. 7%; Std. Diff., 24.3). Because genotype and pancreatic status are highly correlated, pancreatic status was used as a marker for disease severity in the analyses. There were more patients in the pre-1980 birth cohort in Canada (38.5% vs. 32.6%; Std. Diff., 12.3), likely reflecting the greater median age of survival observed in Canada. In the United States for the most recent birth cohort (born after 1990), there was a slightly greater proportion of patients (41.3% vs. 36.3%; Std. Diff., 10.5).
Figure 1.
Description of the study population. CAN = Canada; Excl. = excluded; pts = patients.
Table 1.
Demographic Characteristics of Patients with CF in Canada and United States Followed between 1990 and 2013
| Variable | Canada | United States | Std. Diff.* |
|---|---|---|---|
| N | 5,149 | 37,772 | |
| Sex | |||
| Female | 2,421 (47.0) | 18,108 (47.9) | 1.8 |
| Male | 2,728 (53.0) | 19,664 (52.1) | |
| Age at diagnosis | |||
| Median (range) | 0.7 (0–73.8) | 0.6 (0–81.7) | 0.9 |
| <2 yr | 3,271 (63.5) | 24,151 (63.9) | 0.9 |
| ≥2 yr | 1,878 (36.5) | 13,621 (36.1) | |
| Race | |||
| White | 4,836 (93.9) | 35,456 (93.9) | |
| Other | 199 (3.9) | 2,296 (6.1) | 9.8 |
| Unknown or N/A | 114 (2.2) | 20 (0.1) | |
| Genotype | |||
| Homozygous for F508del | 2,364 (45.9) | 14,943 (39.6) | 6.2 |
| Heterozygous for F508del | 1,870 (36.3) | 12,616 (33.4) | 0.0 |
| Other | 554 (10.8) | 4,747 (12.6) | 9.3 |
| Missing | 361 (7.0) | 5,466 (14.5) | 24.3 |
| Pancreatic status | |||
| Sufficient | 760 (14.8) | 4,342 (11.5) | 9.7 |
| Insufficient | 4,389 (85.2) | 33,430 (88.5) | |
| Neonatal bowel obstruction | |||
| No | 4,354 (84.6) | 31,013 (82.1) | 11.1 |
| Yes | 700 (13.6) | 6,759 (17.9) | |
| Missing | 95 (1.8) | 0 (0) | |
| Condition at diagnosis | |||
| Asymptomatic | 232 (4.5) | 3,701 (9.8) | 17.4 |
| Symptomatic | 4,050 (78.7) | 33,102 (87.6) | |
| Missing | 867 (16.8) | 969 (2.6) | |
| Newborn screening | |||
| No | 5,089 (98.8) | 35,749 (94.6) | 30.7 |
| Yes | 16 (0.3) | 2,023 (5.4) | |
| Missing | 44 (0.9) | 0 (0) | |
| CF-related diabetes | |||
| Never | 3,777 (73.4) | 26,836 (71.0) | 5.2 |
| Ever | 1,372 (26.6) | 10,936 (29.0) | |
| Birth cohort | |||
| <1980 | 1,981 (38.5) | 12,309 (32.6) | 12.3 |
| 1980–1989 | 1,297 (25.2) | 9,814 (26.0) | 1.8 |
| ≥1990 | 1,871 (36.3) | 15,649 (41.4) | 10.5 |
Definition of abbreviations: CF = cystic fibrosis; N/A = not available; Std. Diff. = standardized difference.
Data are n (%) unless otherwise indicated.
P values are heavily influenced by sample size, and thus the large sample sizes herein will render very small differences statistically significant. As such, the standardized difference was used to determine statistical significance. The standardized difference is the mean difference as a percentage of the average SD. A standardized difference greater than 10 is generally used to determine those variables that remain sufficiently different between the two countries.
Survival Differences by Birth Cohort
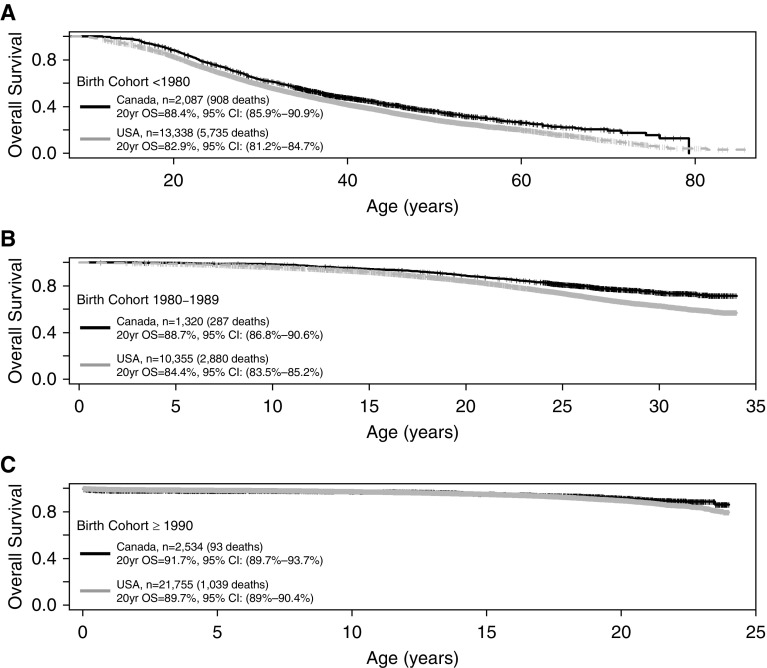
There have been temporal improvements in survival in consecutive birth cohorts (Figure 2), with the gap in the proportion of patients surviving to age 20 between Canada and the United States decreasing from 5.5% in those born before 1980 (88.4% [95% confidence interval (CI), 85.9–90.9%] in Canada vs. 82.9% [95% CI, 81.2–84.7%] in the United States), to 4.3% in the 1980–1990 cohort (88.7% [95% CI, 86.8–90.6%] in Canada vs. 84.4% [95% CI, 83.5–85.2%] in the United States), and 2.0% in the youngest cohort born after 1990 (91.7% [95% CI, 89.7–93.7%] in Canada vs. 89.7% [95% CI, 89–90.4%) in the United States). An interaction between birth cohort and country from 1990 to 2013 was statistically significant (P = 0.00024), indicating that the difference in survival between the two countries varies depending on the birth cohort.
Figure 2.
Probability of survival by birth cohort and country. The probability of surviving to age 20 narrows between Canada and the United States from a difference of 5.5% in the oldest cohort (born before 1980) (A) to 4.3% in the middle cohort (born 1980–1989) (B) and 2.0% in the youngest cohort (born after 1990) (C). CI = confidence interval; OS = overall survival.
Population Trends over Time
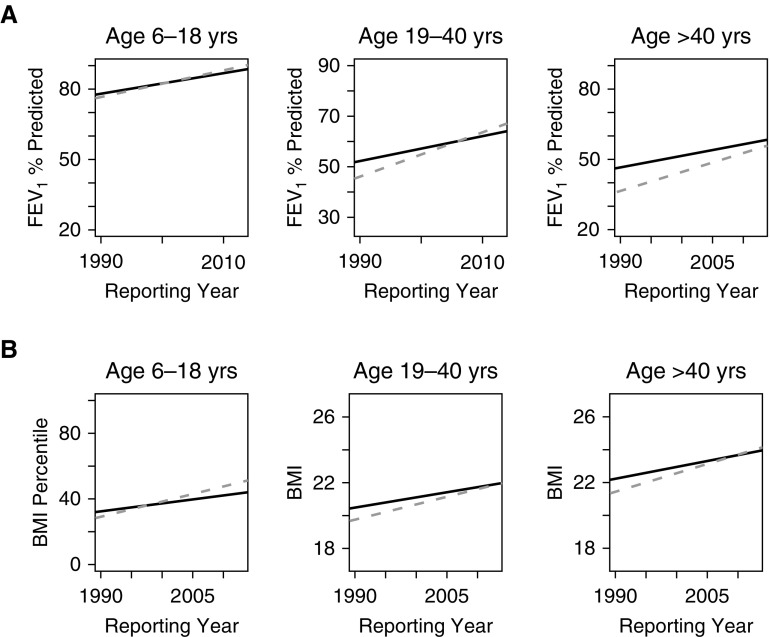
At the population level, Canadian patients had higher median FEV1% predicted in 1990 in all three age groups, with the gap narrowing between the countries in younger groups (Figure 3A; see Table E1A in the online supplement). In addition, there have been temporal improvements in median lung function in both countries for all three age groups during the 23-year study period. However, in all three age groups, the rate of improvement was greater in the United States compared with Canada. In the pediatric group, the median FEV1% predicted in the United States surpassed that in Canada by 2002, and in the young adult group (19–40 yr), the U.S. patients surpassed those in Canada by 2006. In those more than 40 years of age, the U.S. median FEV1% predicted remained below Canada for the entire study period although the rate of change in the United States was faster compared with Canada.
Figure 3.
Median (A) FEV1 and (B) BMI percentile/BMI (kg/m2) from 1990 to 2013 by country, stratified by age group. Canada (solid line) had significantly higher FEV1 and BMI for all three age groups at the start of the study (1990), whereas the rate of improvement with time was greater in the United States (dashed line) compared with Canada (Table E1). BMI = body mass index.
We repeated the analysis excluding individuals who died within the study period to evaluate the impact of survivorship bias (Figure E1); the rate of improvement was consistent with our primary analyses in that there was a faster rate of improvement in the United States for both the 6–18 year group and the 19–40 year group. However, the rate of improvement was attenuated in the older cohort (age 40+) with no difference in the rate of improvement between the two countries. Because more patients in the United States died, surviving patients who inherently would have higher lung function cause the median to be higher, suggesting an impact of survivorship bias.
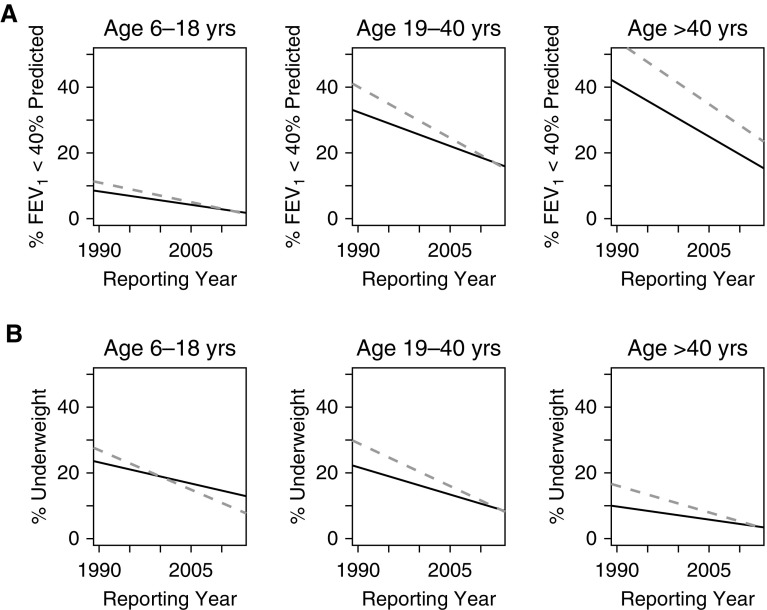
We also examined the proportion of patients with severe lung disease (FEV1% predicted < 40%). Canada had a lower proportion of patients with severe lung disease in 1990 for all three age groups (Figure 4A). However, in children (6–18 yr) and young adults (19–40 yr), the percentage of patients with severe lung disease decreased at a faster rate in the United States compared with Canada. In the older age group, Canada had fewer patients with low lung function over the entire study, whereas the rate of change was similar between the two countries.
Figure 4.
Proportion of patients (A) with severe lung disease (FEV1 < 40% predicted) and (B) who are underweight (BMI percentile <12.5% in children; BMI <18.5 in adults) from 1990 to 2013 by country, stratified by age group. Canada (solid line) had a significantly lower proportion of patients with severe lung disease or who are underweight for all three age groups at the start of the study (1990), whereas the rate of improvement with time was greater in the United States (dashed line) than in Canada. BMI = body mass index.
Similar results were seen for BMI percentile and BMI (Figure 3B; Table E1B). Canadian patients had better nutritional status in 1990 for all three age groups, whereas the rate of improvement in BMI was faster in the United States compared with Canada for all three age groups. In the pediatric age group, median BMI percentile surpassed Canada by 1997, whereas the young adult group caught up by 2013. The oldest group surpassed Canada in terms of BMI in 2009. Of note, the proportion of overweight individuals in 2013 was greater in the United States compared with Canada (19.0% vs. 16.8%; Std. Diff., 5.8). The sensitivity analysis restricted to patients who were alive for the entire study period showed similar results to those observed for lung function. The United States continued to show a faster rate of improvement in nutritional status in the children and young adults, but that rate of improvement was similar to Canada for the oldest cohort, suggesting part of the improvement seen in the United States was due to survivorship bias (Figure E1). Another sensitivity analysis restricted to those patients who are homozygous for F508del had very similar trends over time for lung function and BMI (Figure E2). We also examined the proportion of patients who were malnourished based on BMI/BMI percentile (Figure 4B). Canada had a lower proportion of patients who are underweight in 1990 for all three age groups; however, the proportion of patients who are underweight decreased at a faster rate in the United States compared with Canada in children (6–18 yr), in young adults (19–40%), and in older adults (more than 40 yr).
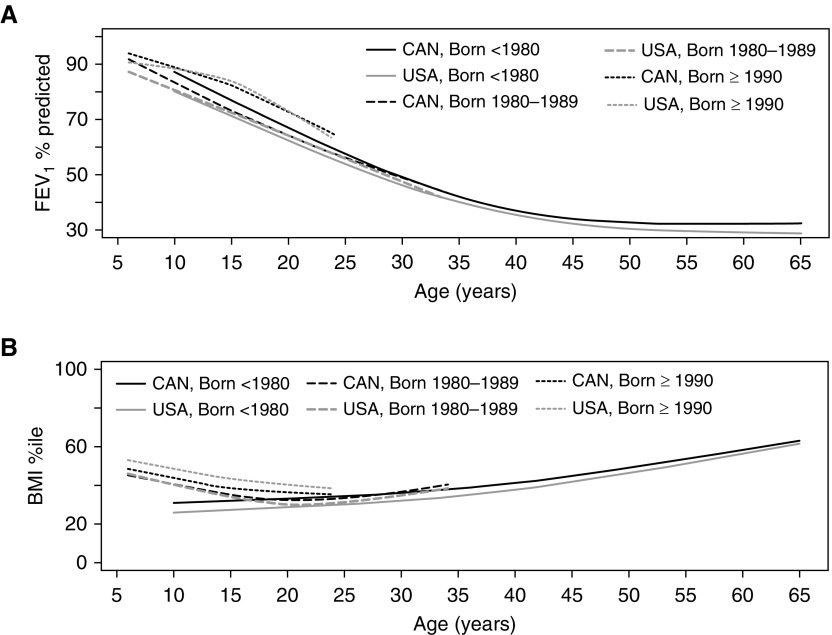
Individual Patient-Level Trajectories of Lung Function and Nutritional Status
In the youngest cohort (born in or after 1990), there were clear and substantial improvements in the rate of lung function decline compared with older cohorts in both countries (Figure 5A); nonetheless there were significant differences between the two countries in both lung function at the first measurement, with Canada having higher lung function, and the rate of lung function decline, with a more pronounced adolescent decline observed in the United States (Figure 5A; Table E2). In the middle cohort (born between 1980 and 1990), Canadian patients had higher initial lung function measurements, with the rate of decline differing between the countries, particularly in childhood where Canadians had a faster rate of decline. Finally, the most striking differences between the two countries can be observed in the oldest cohort (born before 1980), where lung function in Canadian patients was on average 8% higher than in the United States at approximately 10 years of age, and the difference in the rate of decline is most pronounced in adolescents. The observed differences in the pattern of lung function decline within each of the cohorts were not attenuated after adjusting for known confounders (Table E2), namely newborn screening (for only the youngest cohort), pancreatic status, age at diagnosis, cystic fibrosis–related diabetes, B. cepacia, P. aeruginosa, MRSA, and being underweight (BMI). In an analysis restricted to those patients who are homozygous for F508del, the lung function in older ages for the United States passed that in Canada, but this could be an artifact of the lack of genotyping available for this cohort (Figure E3).
Figure 5.
Rate of decline of (A) FEV1% predicted and (B) BMI percentiles by age and country, stratified by birth cohort. Canadian patients consistently started with higher lung function at earlier ages, whereas the U.S. patients experienced improvements in the rate of decline relative to Canada in younger cohorts. The absolute differences in BMI between Canada and the United States were unique to each birth cohort, with Canadian patients having a BMI advantage in the oldest cohort (born before 1980), whereas U.S. patients now have a BMI advantage in the youngest cohort (born after 1990). BMI = body mass index; CAN = Canada.
The adjusted differences between the two countries are summarized in Table E3 for two hypothetical patients with CF from the 1980–1989 birth cohort. Case 1 represents a male patient with mild disease, whereas Case 2 represents a female patient with more severe disease. Similar to the unadjusted model, the differences observed between the countries for the middle cohort (born 1980–1989) for these two hypothetical patients are greatest in childhood, when a patient in Canada has better lung function, and later in adulthood, when a U.S. patient has better lung function. The crossover in lung function is observed around the age of 20 years.
The improvements in the United States were most prominent in the BMI percentile trajectories. Canadian patients born before 1980 start with a clear BMI advantage that persisted across the life span, whereas the rate of change over time in BMI was similar between the two countries. The middle cohort (1980–1989), when both countries were exposed to aggressive nutritional interventions, experienced similar outcomes in terms of BMI. Finally, in the youngest cohort (born in or after 1990), children in the United States started with a clear BMI advantage, which persisted over time, with equivalent rates of decline through to early adulthood between the countries. In the adjusted multivariable model (Table E4), the overall interpretation of the differences and rate of decline was similar, although attenuated, suggesting that BMI is more sensitive to other patient characteristics such as the presence of infection, cystic fibrosis–related diabetes, and so on.
Discussion
This study confirms that patients with CF in both Canada and the United States have experienced significant improvements in nutritional status and lung function. Notably, patients in the United States have experienced faster rates of improvement during this time for both outcomes. Although the youngest American patients (born after 1990) have surpassed Canadian patients in both lung function and nutritional status, this has yet to lead to better survival at age 20. Our data have demonstrated that the survival gap between the patients from both countries appears to be decreasing in younger birth cohorts. However, the survival gap persists in the oldest patients, which is likely a significant driver of the recently noted 10-year survival advantage for Canadians living with CF (1).
The faster rate of improvement in both lung function and nutritional status in the United States may reflect the effects of nutritional interventions that were implemented approximately 10 years later in the United States compared with Canada. This is most evident in the individual trajectory results: nutritional status is similar in the middle cohort when both countries were exposed to high-calorie, high-fat diets, whereas in the oldest cohort, Canadians born in the 1970s would have received aggressive nutritional supplementation from birth, resulting in persistently improved outcomes later in life. Another possible contributing factor to a faster rate of improvement in the United States compared with Canada, particularly in the youngest patients, involves earlier implementation of newborn screening in the United States as these patients would have had opportunities for treatment at an earlier age. Newborn screening was not fully implemented in all U.S. states until 2010 (14), although some states (notably Colorado and Wisconsin) had employed state-level newborn screening for decades. Newborn screening in Canada was initiated in 2007 in specific provinces but was not implemented in all provinces within the study period. This makes it unlikely that differences seen in this analysis can be explained by the use of newborn screening. The study period also has encompassed a major quality improvement initiative by the U.S. CF Foundation within the clinical care setting in CF. This effort, including benchmarking of high-performing clinical sites (15) together with the integration of metrics in the CFF registry (16), has focused on nutrition and lung function outcomes and may be a driving factor in the rapid rate of improvement seen in the United States (17). Additional factors, such as differential use of medications between the countries, may impact lung function; however, medication usage was not captured within the registry for the entire study period.
Lung function is one of the best predictors of survival, and our findings highlight improved health outcomes in U.S. patients with CF: therefore we might expect that the gap in survival between Canada and the United States to decrease. The differences in median lung function between the countries are greatest in the subjects who are 40 years of age and older, and this is the only group that showed a persistent difference for the entire study period. These findings suggest that the overall 10-year survival gap that was observed between the countries (1) is primarily driven by survival differences in older individuals, and possibly by the treatment options, such as transplantation. Furthermore, our data showed that there are fewer patients with severe lung disease in Canada, particularly in older patients, suggesting the survival advantage seen in Canada is driven, at least in part, by having fewer patients with severe lung disease.
The fact that the United States has now surpassed Canada in lung function and nutritional status in patients younger than 40 years cannot be fully explained by changes in the 1990s for either nutritional support of children or newborn screening. One potential explanation is that the Medicaid Children’s Health Insurance Program (CHIP) was signed into law in the United States in 1997. This law provides federal matching funds to U.S. states to provide health coverage to children in families where household income is too high to qualify for Medicaid, but who cannot afford private coverage. The introduction of this program improved access and quality of health care for children and adolescents in the United States (18, 19). No similar program exists for young adults in the United States. Further work regarding the impact of insurance status on clinical outcomes in CF is warranted to devise nationwide approaches to care for the growing population of adults living with CF in the United States, particularly if they suffer from severe lung disease (20).
International comparisons provide opportunities to assess how healthcare systems function. Such comparisons can lead to new hypotheses regarding differences in health outcomes that could influence healthcare policy. Goss and colleagues compared lung function and BMI using U.S. CF registry and U.K. cross-sectional data from 2010 and showed that U.S. children and young adults had higher lung function compared with those in the United Kingdom. There was no difference seen in older adults (21). Differences between the use of CF-specific therapies in young children with CF could potentially explain these differences (markedly higher rates of use of hypertonic saline and rhDNase, both with demonstrated efficacy in CF [22, 23]). Similarly, Martin and colleagues compared data on lung function and nutritional status in children, captured in the 2003 U.S. and Australian CF registries, and they found that Australian children had better nutritional outcomes, whereas there were no differences observed in lung function. The better outcomes in Australia were attributed to the earlier implementation of newborn screening. Our Canadian–U.S. comparison complements these findings, providing insight into the trends over time at both the population and individual levels. Further work is necessary to understand why differences in survival persist despite improved clinical outcomes in later birth cohorts.
The strengths of the study include the longitudinal nature of the data over 23 years, which allowed investigation of trends at both the population and individual patient levels. The unified dataset for the two countries was created in a systematic way with clear definitions for all variables to ensure that similar outcomes are compared (1). Both registries have a high participation rate and capture the majority of the patients with CF nationally. Nonetheless this analysis has a number of key limitations. First, given differences in the use of newborn screening between the countries, differences could be attributed to having milder genotype and phenotypes of CF in the United States compared with Canada (24). To address this concern, all analyses were adjusted for both newborn screening and age of diagnosis. These adjusted analyses reaffirmed the unadjusted comparisons. Another important limitation is that we have already demonstrated improved survival in Canada compared with the United States; this could lead to comparisons of the healthier U.S. survivors when comparing them to Canada. However, survivor bias does not completely explain the improvements that were seen in the United States compared with Canada, except in the oldest cohort. Because the proportion of patients with CF over age 40 who had severe pulmonary impairment and were malnourished was lower in Canada compared with the United States, healthier U.S. survivors may at least partially explain the observed results. We performed several sensitivity analyses to account for this potential bias. Although the consistency of these findings suggests the observations are robust, each of the sensitivity analyses has its own strengths and limitations. For instance, the restricted analysis of patients alive during the study period showed similar results to the main analysis, suggesting that survivor bias is not the reason for the differential improvements between the two countries. However, future work using join modeling techniques that model both longitudinal outcomes and time to death is necessary to investigate this issue more rigorously (25). Similarly, the analyses restricted to patients who are homozygous for the F508del mutation may be biased in the oldest group, because many patients in this cohort were not genotyped and the sample size is limited. We limited the study population to patients older than 6 years of age such that the study population would be the same for both lung function and nutrition outcomes. The observed catch-up in nutrition in the youngest cohort may be better studied in children less than 6 years of age. Finally, prior published comparisons of medication use in the two countries suggest that patients in the United States are treated more than in Canada (1); however, we were unable to investigate whether the observed differences are associated with patterns of medication use, because treatment details were only available in the Canadian registry since 2011.
In conclusion, we have demonstrated both a narrowing of the survival gap between Canadian and U.S. patients in more recent birth cohorts, and rapid improvement, or catch-up, in both nutritional status and lung function for patients in the United States. The reasons for these improvements are likely multifactorial and include the delayed introduction of high-fat, high-calorie diets in the United States; earlier introduction of newborn screening in the United States; sustained quality improvement initiatives within the United States; and/or improved access to care for CF children in the United States with the advent of the Medicaid CHIP program in 1997. Further work is needed to determine whether the observed improvements can be explained by access to CF therapeutics and treatment options such as lung transplantation.
Footnotes
Supported by the Cystic Fibrosis Foundation (C.H.G. and A.L.S.), the NIH (R01HL113382, R01AI101307, U M1HL119073, P30DK089507, and UL1TR000423) (C.H.G.), and the Food and Drug Administration (R01FD003704) (C.H.G.).
Author Contributions: C.H.G. and A.L.S. managed the study’s inception, the study design, supervising the conduction of the study, discussing the data and writing, and revising the manuscript. C.H.G. and A.L.S. were co–principal investigators on this study. J.S., S.S., B.M., K.P., J.O., A.F., A.E., and B.S.Q. contributed to study conception and study design, data management, and statistical analyses. All authors participated in data analysis and interpretation, drafting and/or revising the manuscript for intellectual content, and editing the manuscript for final approval.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201707-1541OC on November 3, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Stephenson AL, Sykes J, Stanojevic S, Quon BS, Marshall BC, Petren K, et al. Survival comparison of patients with cystic fibrosis in Canada and the United States: a population-based cohort study. Ann Intern Med. 2017;166:537–546. doi: 10.7326/M16-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stephenson AL, Tom M, Berthiaume Y, Singer LG, Aaron SD, Whitmore GA, et al. A contemporary survival analysis of individuals with cystic fibrosis: a cohort study. Eur Respir J. 2015;45:670–679. doi: 10.1183/09031936.00119714. [DOI] [PubMed] [Google Scholar]
- 3.Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, Marshall BC. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol. 2001;153:345–352. doi: 10.1093/aje/153.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuczmarski R, Ogden CL, Guo S, Grummer-Strawn L, Flegal KM, Mei Z, et al. CDC growth charts. Hyattsville, MD: National Center for Health Statistics; 2000. [Google Scholar]
- 5.Corey M, McLaughlin FJ, Williams M, Levison H. A comparison of survival, growth, and pulmonary function in patients with cystic fibrosis in Boston and Toronto. J Clin Epidemiol. 1988;41:583–591. doi: 10.1016/0895-4356(88)90063-7. [DOI] [PubMed] [Google Scholar]
- 6.Sykes J, Stanojevic S, Goss CH, Quon BS, Marshall BC, Petren K, et al. A standardized approach to estimating survival statistics for population-based cystic fibrosis registry cohorts. J Clin Epidemiol. 2016;70:206–213. doi: 10.1016/j.jclinepi.2015.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000:1–27. [PubMed] [Google Scholar]
- 9.World Health Organization. BMI classification. 2013 [accessed 2012 Sept 15]. Available from: http://apps.who.int/bmi/index.jsp?introPage=intro_3.html.
- 10.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38:1228–1234. [Google Scholar]
- 11.Hodson ME, Simmonds NJ, Warwick WJ, Tullis E, Castellani C, Assael B, et al. An international/multicentre report on patients with cystic fibrosis (CF) over the age of 40 years. J Cyst Fibros. 2008;7:537–542. doi: 10.1016/j.jcf.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Taylor-Robinson D, Whitehead M, Diderichsen F, Olesen HV, Pressler T, Smyth RL, et al. Understanding the natural progression in %FEV1 decline in patients with cystic fibrosis: a longitudinal study. Thorax. 2012;67:860–866. doi: 10.1136/thoraxjnl-2011-200953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stang A, Poole C, Kuss O. The ongoing tyranny of statistical significance testing in biomedical research. Eur J Epidemiol. 2010;25:225–230. doi: 10.1007/s10654-010-9440-x. [DOI] [PubMed] [Google Scholar]
- 14.Farrell PM, White TB, Derichs N, Castellani C, Rosenstein BJ. Cystic fibrosis diagnostic challenges over 4 decades: historical perspectives and lessons learned. J Pediatr. 2017;181S:S16–S26. doi: 10.1016/j.jpeds.2016.09.067. [DOI] [PubMed] [Google Scholar]
- 15.Boyle MP, Sabadosa KA, Quinton HB, Marshall BC, Schechter MS. Key findings of the US Cystic Fibrosis Foundation’s clinical practice benchmarking project. BMJ Qual Saf. 2014;23:i15–i22. doi: 10.1136/bmjqs-2013-002369. [DOI] [PubMed] [Google Scholar]
- 16.Schechter MS, Fink AK, Homa K, Goss CH. The Cystic Fibrosis Foundation Patient Registry as a tool for use in quality improvement. BMJ Qual Saf. 2014;23:i9–i14. doi: 10.1136/bmjqs-2013-002378. [DOI] [PubMed] [Google Scholar]
- 17.Marshall BC, Nelson EC. Accelerating implementation of biomedical research advances: critical elements of a successful 10 year Cystic Fibrosis Foundation healthcare delivery improvement initiative. BMJ Qual Saf. 2014;23:i95–i103. doi: 10.1136/bmjqs-2013-002790. [DOI] [PubMed] [Google Scholar]
- 18.Szilagyi PG, Dick AW, Klein JD, Shone LP, Zwanziger J, McInerny T. Improved access and quality of care after enrollment in the New York State Children’s Health Insurance Program (SCHIP) Pediatrics. 2004;113:e395–e404. doi: 10.1542/peds.113.5.e395. [DOI] [PubMed] [Google Scholar]
- 19.Szilagyi PG, Dick AW, Klein JD, Shone LP, Zwanziger J, Bajorska A, et al. Improved asthma care after enrollment in the State Children’s Health Insurance Program in New York. Pediatrics. 2006;117:486–496. doi: 10.1542/peds.2005-0340. [DOI] [PubMed] [Google Scholar]
- 20.Knapp EA, Fink AK, Goss CH, Sewall A, Ostrenga J, Dowd C, et al. The Cystic Fibrosis Foundation Patient Registry. Design and methods of a National Observational Disease Registry. Ann Am Thorac Soc. 2016;13:1173–1179. doi: 10.1513/AnnalsATS.201511-781OC. [DOI] [PubMed] [Google Scholar]
- 21.Goss CH, MacNeill SJ, Quinton HB, Marshall BC, Elbert A, Knapp EA, et al. Children and young adults with CF in the USA have better lung function compared with the UK. Thorax. 2015;70:229–236. doi: 10.1136/thoraxjnl-2014-205718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, et al. National Hypertonic Saline in Cystic Fibrosis (NHSCF) Study Group. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354:229–240. doi: 10.1056/NEJMoa043900. [DOI] [PubMed] [Google Scholar]
- 23.Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW, et al. The Pulmozyme Study Group. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. N Engl J Med. 1994;331:637–642. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]
- 24.Ren CL, Fink AK, Petren K, Borowitz DS, McColley SA, Sanders DB, et al. Outcomes of infants with indeterminate diagnosis detected by cystic fibrosis newborn screening. Pediatrics. 2015;135:e1386–e1392. doi: 10.1542/peds.2014-3698. [DOI] [PubMed] [Google Scholar]
- 25.Barrett J, Diggle P, Henderson R, Taylor-Robinson D. Joint modelling of repeated measurements and time-to-event outcomes: flexible model specification and exact likelihood inference. J R Stat Soc Series B Stat Methodol. 2015;77:131–148. doi: 10.1111/rssb.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]