Abstract
Mitochondria are the “power house” of a cell continuously generating ATP to ensure its proper functioning. The constant production of ATP via oxidative phosphorylation demands a large electrochemical force that drives protons across the highly selective and low-permeable mitochondrial inner membrane. Besides the conventional role of generating ATP, mitochondria also play an active role in calcium signaling, generation of reactive oxygen species (ROS), stress responses, and regulation of cell-death pathways. Deficiencies in these functions result in several pathological disorders like aging, cancer, diabetes, neurodegenerative and cardiovascular diseases. A plethora of ion channels and transporters are present in the mitochondrial inner and outer membranes which work in concert to preserve the ionic equilibrium of a cell for the maintenance of cell integrity, in physiological as well as pathophysiological conditions. For, e.g., mitochondrial cation channels KATP and BKCa play a significant role in cardioprotection from ischemia–reperfusion injury. In addition to the cation channels, mitochondrial anion channels are equally essential, as they aid in maintaining electro-neutrality by regulating the cell volume and pH. This chapter focusses on the information on molecular identity, structure, function, and physiological relevance of mitochondrial chloride channels such as voltage dependent anion channels (VDACs), uncharacterized mitochondrial inner membrane anion channels (IMACs), chloride intracellular channels (CLIC) and the aspects of forthcoming chloride channels.
Keywords: Anion channels, Chloride intracellular channels (CLICs), Inner membrane anion channel (IMAC), Mitochondria, Mitochondrial permeability transition pore (mPTP), Uncoupling protein (UCP), Voltage dependent anion channel (VDAC)
1 Introduction
Typical ionic concentration and composition of the cytosol dramatically differ from the extracellular environments. All the cells from prokaryotes to eukaryotes maintain a cytosolic pH of ~7.2, with concentration of potassium ions (K+) many fold higher than sodium (Na+) and calcium (Ca2+) ions (Bose et al. 2015; Prindle et al. 2015). In addition to cations, several organic and inorganic anions such as halides exist in cytosol to counter cations and also play an active functional role in cells. Intracellular ions exist in either free or remain bound to cytoplasmic proteins; however, they are classically divided by intracellular membranes, and are enclosed in intracellular organelles. The distribution of intracellular ions in different cellular compartments as well as cytosol is chiefly governed by Gibbs–Donnan effect (Sollner 1955), and is responsible for maintaining physiological functions of the cell.
Cell membranes possess a wide array of ion transporters and channel proteins in order to maintain physiological ionic concentrations (Jentsch et al. 2004). The ionic gradients and membrane potentials across cellular membrane (plasma and intracellular organelle) are key drivers of several physiological processes such as neuro-transmission, cardiac conduction, and muscle contraction and relaxation (Jentsch et al. 2004). Intracellular ion transport is important for cell cycle-regulation, energy production, cellular signaling, and apoptosis (Leanza et al. 2013). Ion transport across the plasma membrane has been discussed in several reviews (Feske et al. 2015; Bates 2015; Singh et al. 2012a; Toro et al. 2014), and therefore in this chapter, emphasis is on the ion channels across mitochondrial membranes.
Several models of energy production in the mitochondrion indicate that the presence of an ion channel on mitochondrial membrane would lower efficiency and effectiveness of the process (O’Rourke 2007). In addition, theoretical opening of any ion channels, for example, a mitoBKCa will readily depolarize mitochondrial membrane within few nanoseconds, hence supporting the earlier hypothesis that mitochondria should not possess ion channels (Singh et al. 2012a; O’Rourke 2007). At the same time, it is well-recognized that membrane potential of inner mitochondrial membrane (IMM) is ~ −180 mV (mV) (Colombini 2012; Colombini and Mannella 1818; O’Rourke et al. 2005). However, when compared to the electric potential of plasma membrane (~ −70 mV), mitochondrial membrane seems to be a better capacitor in storing charge. To further signify the effectiveness of mitochondrial membrane potential (ψmito), we can precisely calculate the charge stored by these cellular capacitors. It is known that the IMM is ~7.0 nm thick (Perkins et al. 1997) thus the voltage gradient across the IMM is 0.180 V per 7.0 ×10−7 cm. This is equivalent to 257,142.90 V per cm which is significantly higher than high-voltage transmission electricity supply lines employing voltage gradient of about 200,000 V per km (and higher to the plasma membrane voltage gradient of 200,000 V per cm as well) (Lodish et al. 2000). Majority of the membrane potential across the mitochondrial inner membrane is generated by proton transport which is directly coupled with ATP production.
Increasing evidence indicate that both outer mitochondrial membrane (OMM) and IMM possess ion channels (Szabo and Zoratti 2014). As stated earlier, until 1980s, scientific community was highly skeptical and divided over existence of typical ion channels in mitochondria (Colombini and Mannella 1818; O’Rourke 2000; Colombini 1979). However, isolation of voltage dependent anion channel (VDAC) from paramecium, and reconstitution in lipid bilayers in 1976 set the ground for mitochondrial ion channels (Schein et al. 1976). This was immediately followed by characterization of permeability transition pore (PTP) (Hunter et al. 1976), and in the last 40 years several ion channels of OMM and IMM were established (Szabo and Zoratti 2014). More recently, mitochondrial ion channels are shown to be the key modulators involved in protection from ischemia–reperfusion (IR) injury, apoptosis, and several other pathophysiological disorders (O’Rourke 2007; O’Rourke et al. 2005; Szabo and Zoratti 2014; O’Rourke 2000). Even though anion channels (VDAC) were the first one to be described in mitochondria (Colombini 1979), majority of the physiological functions are attributed to the cation channels (Szabo and Zoratti 2014). This is chiefly due to non-availability of specific pharmacological agents for anion channels and lack of molecular identity of mitochondrial anion channels residing in IMMs.
Much like plasma membrane anion channels, mitochondrial anion channels are proteinaceous pores which allow passive diffusion of negatively charged ions down their electrochemical gradient. Anion channels may conduct other anions (F−, Br−, I−, or NO−3) better than Cl− but they are often referred as Cl− channels, as Cl− is the most abundant anion, predominantly permeating under physiological conditions (Ashley 2003). Apart from VDAC which resides in the OMM (Colombini and Mannella 1818; Colombini 1979), chloride intracellular channel 4 (CLIC4), and CLIC5 (Ponnalagu et al. 2016a), molecular identity of other inner mitochondrial anion channels is not known. Also classification of anion channels is still ambiguous (Roelfsema et al. 2012). Previous attempts to classify them on the basis of their localization (plasma membrane vs. intracellular membranes), single-channel conductance, and/or regulatory mechanism could not comprehend the diversity of anion channels (Verkman and Galietta 2009). We believe that mitochondrial anion channels can be systematically classified on the basis of their localization, outer membrane anion channels and inner membrane anion channels (IMACs), as well as on the basis of their selectivity. As anion channels are known to be permeable for other ions, we propose to classify them as anion-selective anion channels [ASACs, non-selective for anions], cation-selective anion channels (CSACs, prefer cation over anions), and non-selective anion channels [NSACs, not selective for cations or anions]. These channels will be discussed in detail in following sections.
In this chapter, we have presented an overview of mitochondrial anion channels, investigated their mitochondrial localization as well as distribution, and also show how some of the known anion channel inhibitors affect mitochondrial reactive oxygen species (ROS) generation.
2 Anion Channels of Outer Mitochondrial Membrane
2.1 Voltage Dependent Anion Channels
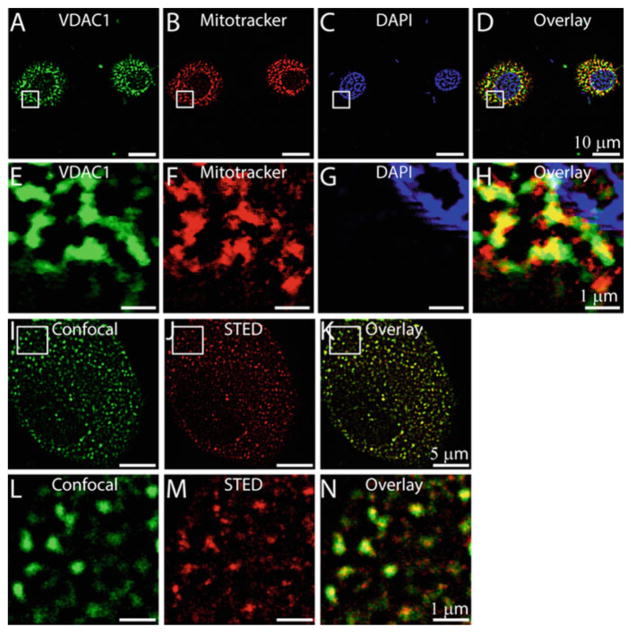
VDAC, an intrinsic membrane channel, is the first channel to be reconstituted and studied at the single-channel level (Colombini and Mannella 1818; Schein et al. 1976). It is a highly conserved protein, homologous to bacterial porins and is localized in the OMM (Colombini 2012; Mertins et al. 2014; Neumann et al. 2010) as also observed in the mitochondria of rat cardiac myogenic cell line, H9C2 (Fig. 1). Geographical distribution of VDAC1 by stimulated emission depletion (STED) nanoscopy also indicates their unique clusters at nano-resolution in H9C2 cells (Fig. 1) as observed earlier in U2OS cells transfected with VDAC (Neumann et al. 2010). It is the principle mode of transport of ions and metabolites across the OMM, and also a probable contact point between the inner and outer membrane (Colombini 1979). VDAC had a serendipitous discovery; it occurred as a result of failed attempts in characterizing voltage-gated calcium sensitive channels from Paramecium aurelia (Colombini and Mannella 1818). It is widely distributed in many species across the taxon, yeast have two (POR1 and POR2), and vertebrates have three isoforms (VDAC1, VDAC2, and VDAC3) (Szabo and Zoratti 2014). Humans, like all other vertebrates canonically encode three different isoforms. It is a well-characterized mitochondrial anion channel in terms of structure and function. Amongst the various isoforms, VDAC1 was the first one to be discovered. VDAC1 and VDAC2 have very strong poreforming characteristics, while VDAC3 forms smaller conductance channels (Checchetto et al. 2014) and consequently modulates the physiological functions of other proteins.
Fig. 1.
Localization of VDAC1 in H9C2 cells. H9C2 cells loaded with mitotracker (b) were labeled with anti-VDAC1 antibody (a), and stained with a nuclear marker, DAPI (c). (d) is merge image of a, b, and c showing colocalization of VDAC1 to the mitochondria of H9C2 cells. e, f, g, and h are enlarged images of the squared regions a, b, c, and d, respectively. STED microscopy of H9C2 cells showing expression of VDAC1 (j, k). Confocal image of H9C2 cells labeled with anti-VDAC1 antibody (i) Corresponding STED image (j), overlay of i and j highlights increased resolution of VDAC1 localization to mitochondria with STED microscopy (k). l, m, and n are enlarged images of the squared region in i, j, and k, respectively
2.1.1 Structural Insights
VDAC, a small, >30 kDa protein consisting of 280 amino acid residues, shows high sequence homology (>80 %) within the same species. Using X-ray crystallography and nuclear magnetic resonance (NMR), three independent studies (Hiller et al. 2008; Ujwal et al. 2008; Bayrhuber et al. 2008) have revealed that VDAC forms β-barrel structures similar to bacterial porins, comprising of 19 unique β-strands connected with 18 loop-like connections, and a short N-terminal α-helix between residues 7 and 17. All the β-barrel strands are oriented in the anti-parallel fashion except strands 1 and 19 which are parallel to each other and close the β-barrel structure (Ujwal et al. 2008). The height and width of the β-barrel were reported to be 35 Å and 40 Å, respectively (Ujwal et al. 2008), and the path for solute passage measures ~15 Å by 1 Å. The N-terminal β-barrel strands (1–4) contribute to conformational stability of VDAC whereas Glu73 is a key residue determining the structural stability of the protein. This residue is conserved in all the VDAC isoforms present in humans. The N-terminal helix folds up inside the β-barrel wall to reach the midpoint of the hydrophobic portion of the membrane. The positively charged residues of the helix face the interior of the β-barrel, while the negatively charged residues interact with the most conserved residues in the β-barrel wall of VDAC proteins (Bayrhuber et al. 2008). As a result the N-terminus is a site for many molecular interactions. The preceding N-terminal region is lightly attached to the β-barrel wall and orients towards the pore outlet (Bayrhuber et al. 2008). Each loop contributes a specific role in channel-gating, ion-selectivity, pore size, and structural stability of VDAC. The long loops fold into the pore lumen and determine the ion-selectivity and the gating of the channel. The short loops provide structural support by linking with the adjacent β-strands. The stability of β-strands is also important for the voltage-gating, and protein–protein interactions of VDAC. Similar to other porins, the C-terminal region extends to the intermembrane space (IMS) (Bayrhuber et al. 2008; Schulz 2002). Various studies indicate the ability of VDAC to form oligomers (Malia and Wagner 2007). Using crystallographic symmetry operators, a model for dimeric form of VDAC was constructed which indicated that the dimeric interface residues reside in β-strands, β1 (Ile-27, Leu-29), β2 (Glu-50, Thr-51), β18 (Leu-257, Leu-259), and β19 (Leu-277) (Bayrhuber et al. 2008).
2.1.2 Electrophysiological Properties
The channel properties of VDAC have been studied mainly by using planar bilayer systems. VDAC forms a large voltage dependent pore with maximum conductance of 4–5 nS in symmetrical 1 M KCl (Szabo and Zoratti 2014; Colombini 2004; Bera and Ghosh 2001). At lower holding potentials of 20–30 mV, VDAC exists in fully open state and exhibits weak anion-selectivity, whereas at higher holding potentials, it is cation selective, and the conductance observed is approximately half of the original conductance shown by the “closed” state of the channel (Szabo and Zoratti 2014). Exposed charge residues of the pore are the key determinants for anion selectivity. Amino-terminal sequence determines the voltage-induced gating of VDAC (Mannella 1997; Shoshan-Barmatz et al. 1797). Although channel activity is observed in bilayers, a consistent conductance is not yet recorded through patch clamping studies (Kinnally and Antonsson 2007). VDAC possesses a binding site for Ca2+ (Gincel et al. 2001) and also transports calcium (Ca2+), ATP, and superoxides. The open state of the channel poorly conducts Ca2+ (PCl−/PCa2+ ~25), while partially closed state still prefers Cl− over Ca2+, with a PCl−/PCa2+ as 1–4.5 (Szabo and Zoratti 2014; Tan and Colombini 1768). Mitochondrial VDAC-like conductance was also observed in excised patches of plasma membrane (Bahamonde et al. 2003) of neuroblastoma cells. Thus, suggesting VDAC as a molecular correlate of the plasma membrane maxi Cl− channels (Bahamonde et al. 2003). In another study, electrophysiological and immunocytochemical evidences showed the activation of VDAC in plasma membrane of neurons during apoptosis (Elinder et al. 2005). These observations are supported by the identification of a VDAC isoform (pl-VDAC) containing a leader sequence for its trafficking to the plasma membrane (Buettner et al. 2000; De Pinto et al. 2010) and also the presence of VDAC in caveolae (Bahamonde et al. 2003).
2.1.3 Regulation of VDAC
VDAC is regulated by many cellular and extracellular factors. It gets phosphorylated by Ser-Thr kinases GSK3β (Das et al. 2008; Sheldon et al. 2011), tyrosine kinase (Salvi et al. 2005), protein kinase A (PKA) (Banerjee and Ghosh 2006), and nima-related kinases (NEK) (Chen et al. 2009; Chen et al. 2010). Phosphorylation regulates the apoptotic activity of VDAC via modulating its interaction with cytoskeletal components (Kerner et al. 1818). However, phosphorylation does not affect the current magnitude and the open probability in the positive clamping potentials, but lowers both in the negative clamping potentials (Bera and Ghosh 2001). Further, it has been shown that phosphorylation of VDAC by JNK3 leads to channel closure and thereby causes cell death (Gupta and Ghosh 2015). VDAC has been shown to interact with multiple factors ranging from pyridine dinucleotides (Lee et al. 1996; Zizi et al. 1994), lipids including cholesterol (Betaneli et al. 2012; Rostovtseva and Bezrukov 2008), Bcl-xL (Malia and Wagner 2007; Arbel et al. 2012), creatine kinases (Beutner et al. 1996; Brdiczka et al. 1994), Ca2+, etc. Interaction of VDAC with tubulin (Sheldon et al. 2011) results in its increased sensitivity to voltages causing a channel closure at potentials as low as 10 mV. It also has been known to be inhibited by gelsolin, a Ca2+-dependent actin-binding regulatory protein, thereby exerting anti-apoptotic effects (Kusano et al. 2000).
2.1.4 Functions
VDAC has been extensively studied with respect to its role in apoptosis. It is known to interact with Bax and Bak to form a large pore and stimulate efflux of cytochrome c (Shimizu et al. 2000; Shimizu et al. 1999; Banerjee and Ghosh 2004) which is a hallmark of apoptosis. Although, VDAC2 is shown to be pro-apoptotic, facilitating sensitivity to t-Bid (Yamagata et al. 2009), its deletion in lymphocytes was fatal (Cheng et al. 2003) but was rescued by deletion of Bak, reasserting VDAC2’s role in apoptosis. There are contradictory reports arguing VDACs (VDAC1 and VDAC3) role in apoptosis; mouse embryonic fibroblasts (MEFs) lacking either one of these isoforms showed no response to their apoptotic response to Bax overexpression and also showed no role of VDAC in cytochrome c release (Baines et al. 2007). Bcl-2 family members like BclxL inhibit VDAC’s activity and exhibit their anti-apoptotic activity (Arbel et al. 2012) via interaction with their N-terminal segment (Malia and Wagner 2007; Abu-Hamad et al. 2009). Overexpression of plant VDACs in jurkat cells (T cell line) induced apoptosis that can be blocked by Bcl-2 and the VDAC inhibitors (Godbole et al. 1642). VDACs have also been widely studied in cancers due to their association with hexokinase 1 (HK1) and hexokinase 2 (HK2) which are over-expressed in glycolytic cancers (Wolf et al. 2011). HKs via binding to VDAC1 provide a metabolic benefit to cells, and suppress apoptosis, causing a cell proliferative advantage and increased resistance to chemotherapy (Shoshan-Barmatz et al. 1848). However, the association of VDAC and HK is also regulated by cyclophilin D (CypD) (Machida et al. 2006) and its acetylation status which is in turn modulated by deacetylases like sirtuin-3 (Shulga et al. 2010; Verma et al. 2013). VDAC is prone to oxidative damage when its tyrosine residues get converted to 3-nitrotyrosine by reactive nitrogen species (RNS) including NO and peroxynitrite, produced upon oxidative stress during aging. Therefore, increased VDAC nitration leads to VDAC-dependent, rapid, and massive cytochrome c release. These functions signify the very important physiological role of VDAC in cell life and death. VDAC has been arguably considered to be a component of mitochondrial permeability transition pore (mPTP) (Zoratti and Szabo 1995), as the electrophysiological property of VDAC resembled to that of mPTP (Szabo et al. 1993). It was also predicted to be in the dimeric state in mPTP complex (Szabo and Zoratti 1993). Molkentin and his group further showed that mitochondria from the VDAC null mutant mice were susceptible to Ca2+ and oxidative stress induced mPTP formation instigating a debatable role of VDACs in mPTP formation (Baines et al. 2007). A recent study showed the SGP7 (mitochondrial AAA protease) which is an essential component of mPTP complex interacts with VDAC (Shanmughapriya et al. 2015). These studies further suggest that VDACs role in forming mPTP complex is dispensable and probably regulates mPTP formation via interacting with modulators like Bax, Bak, and SGP7.
3 Anion Channels of Inner Mitochondrial Membranes
As stated above, it was widely believed that the inner membrane of the mitochondria is unlikely to contain ion channels, as any movement of ions across it would degenerate the proton motive force driving ATP synthesis (O’Rourke 2007). Although, later, multiple conductances were recorded in IMM, no specifics on molecular identity of these channels are known (Szabo and Zoratti 2014). The realization for the existence of ion channel in inner membrane came from early studies on mitochondrial swelling, which were induced by movement of cations and anions into the matrix compartments (O’Rourke 2007).
3.1 Inner Membrane Anion Channel: Historical Perspective and Ion Channel Properties
Around the 1960s, Azzi et al. promoted the idea of Mg2+ governing the permeability of the mitochondrial membrane to univalent cations, which switches to anion, upon increasing pH (Azzi and Azzone 1965; Azzi and Azzone 1967). However, mechanism for this ion extrusion was uncertain. In 1969, Brierley observed that the permeability of the inner membrane to anions is very low at acidic pH in heart mitochondria, which further increased under alkaline conditions (Brierley 1969; Garlid and Beavis 1986). These findings supported the concept of Azzi et al., about the existence of an Mg2+ and pH dependent anion transport pathway in IMM (Garlid and Beavis 1986). Furthermore, the transport of several anions like Cl−, Br−, I−, SCN−, NO3−, PO4−, HCO3−, and SO42− across the mitochondrial membrane was demonstrated (Garlid and Beavis 1986; Stockdale et al. 1970), thus indicating the presence of an IMAC in the mitochondria (Garlid and Beavis 1986).
3.1.1 Electrophysiological Properties of IMAC
Initial patch clamp studies performed on IMM revealed a slightly anion-selective channel with a mean conductance of ~107 pS under symmetrical (cis:trans) 150 mM KCl condition (Sorgato et al. 1987). It was named as mitochondrial centum picosiemens (mCS) channel. This was followed by observations of multiple anion channel conductances, on mitoplasts or mitochondrial membrane vesicles (Ballarin and Sorgato 1995; Borecky et al. 1997; De Marchi et al. 1777; Hayman et al. 1993; Kinnally et al. 1992; Klitsch and Siemen 1991) isolated from different tissues (brown adipose tissue, heart, and liver). Patch clamp studies on mitoplasts isolated from brown fat adipose tissue showed a ~108 pS conductance in symmetrical 150 mM KCl solution (Borecky et al. 1997; Klitsch and Siemen 1991). This channel was a candidate for IMAC as the channel currents were inhibited by purine nucleotides, Mg2+ as well as variations in pH (Borecky et al. 1997). In both liver and cardiac tissues, Sorgato et al. found a ~100 pS voltage-sensitive anion channel (Sorgato et al. 1989). In yet another study, two distinct anion channel currents were observed in sheep mitoplasts with one showing conductance of 100 pS (150 mM KCl) and the other of 50 pS (Hayman et al. 1993). Remarkably, both species of channel activity lasted for 10–15 min and then exhibited irreversible inactivation which could be a result of desensitization of these channels (Sun et al. 2002). PCl−/PK+ values for both the channels were also different. Small conductance channel was shown to be non-selective between anions. Although the channel conductances were similar to that observed by Sorgato et al.(Sorgato et al. 1987), neither of these channels were sensitive to ATP, Mg2+, or pH changes, a property exhibited by IMAC.
Interestingly, three classes of IMM channel activities were recorded in rat cardiac mitochondria, using patch clamp studies (Kinnally et al. 1992; Kinnally et al. 1991). The “107 pS activity” was slightly anion-selective and voltage-dependent (opens with positive potentials towards matrix); a Ca2+ or voltage-dependent “multiple conductance channel” (MCC) activity exhibited a single-channel conductance ranging from ~40 to over 1,000 pS, and a “low-conductance channel” (LCC) showing conductance of ~15 pS that was receptive to pH and Mg2+ changes. Thus, MCC was considered responsible for the Ca2+-induced permeability transition observed in mitochondrial suspensions. LCC was suggested to be similar to IMAC currents with anion selectivity in the order of SCN−>NO3−>Cl−>Pi. All of the IMM channels reported so far are highly regulated and have a very low open probability under physiological conditions.
IMAC currents were also observed even in yeast (both wild type and VDAC less strain), but the properties of these channels differed (Ballarin and Sorgato 1996). Here, two kinds of channel activity were recorded: a small conductance channel of ~45 pS, which decreased at negative holding potentials, and large conductance channel, whose conductance increased at positive holding potentials (~400 pS at −40 mV; ~800 pS at 40 mV). Unlike IMAC, these channels were unaffected by matrix Ca2+ and Mg2+. However, both showed ATP-dependence, indicated by the complete inhibition of the activity of the small channel, and the loss of voltage-dependency for large conductance channel in the presence of nucleotides. Another study revealed the existence of phosphate carrier (PIC) protein, as mitochondria purified from Saccharomyces cerevisiae upon reconstitution in liposomes showed anion-selective single-channel currents in patch clamp experiments (Herick et al. 1997). It exhibited a frequent conductance of ~40 ±10 pS, and was inhibited by higher concentration of phosphate. Although PIC had similar conductance to that of small conducting channel earlier isolated from yeast, its activity was inhibited by Mg2+ and Ca2+ unlike the later one. This further indicated the existence of multiple IMM anion channel conductances in yeast.
In addition, pH-regulated plant inner mitochondrial anion channel (PIMAC) mediated flux of Cl−, NO3−, succinate, malate, and oxaloacetate was shown in potato tuber mitochondria (Beavis and Vercesi 1992), as well as in durum wheat seedlings and Jerusalem artichoke tubers (Laus et al. 2008). Like yeast IMACs, they exhibit different properties than the mammalian IMAC, because of the lack of inhibition by recognized inhibitors of mammalian IMACs. Maize IMAC shows temperature independent open probability in contrast to that of mammalian IMAC, but this level of activity varied with the cold tolerance levels of the maize population. Although these studies point out the presence of IMAC in mitochondria, more data is required to establish the molecular identity of these channels.
3.1.2 IMAC Modulators
Light scattering on swelling mitoplasts was used to describe IMAC in initial studies (Garlid and Beavis 1986; Beavis and Vercesi 1992; Halle-Smith et al. 1988; Powers and Beavis 1991; Zernig et al. 1990). IMAC has been shown to be modulated by palmitoyl-CoA (IC50 ~ 2.5 μM) (Halle-Smith et al. 1988), Mg2+ (Garlid and Beavis 1986; Beavis and Vercesi 1992), (IC50 ~ 38 μM), pH (pIC50 ~ 7.7) (Beavis and Vercesi 1992; Beavis 1992), N,N′-dicyclohexylcarbodiimide, mercurial (chloromercuribenzene sulfonate and mersalyl), and amphiphilic amines such as propranolol and triorganotins (Powers and Beavis 1991). Tributyltin (TBT), a potent triorganotin, inhibits malonate transport via IMAC at 0.9 nmol/mg and 95 % efficiency (Powers and Beavis 1991). IMAC like other chloride channels is reversibly inhibited by stilbene derivatives such as 4,4′-Diisothiocyanato-2,2′-stilbenedisulfonic acid disodium salt (DIDS) (Beavis and Davatol-Hag 1996). Other inhibitors include amiodarone, amitriptyline, dibucaine, Ro 5–4864, and thiol cross linkers, like N-ethylmaleamide. Even inhibitors of adenine nucleotide translocase (ANT) block the anion transport by IMAC (Powers et al. 1994). The plant IMACs unlike that of mammalian are not inhibited by matrix Mg2+, mercurials, or N,N′-dicyclohexylcarbodiimide but by other well-known IMAC inhibitors such as propranolol (IC50 = 14 μM), TBT (IC50 = 4 nmol/mg), and the ANT inhibitors like erythrosin B and Cibacron Blue 3GA. Ca2+ also modulated the activity of IMAC in mitochondrial swelling experiments (Zernig et al. 1990). In addition, benzodiazepines that inhibit mitochondrial benzodiazepine receptor (mBzR) present in the OMM also blocked IMAC activity but the mechanism of inhibition is unclear. Even though the functions of these channels are poorly understood, their tight regulation suggests their import role in maintaining physiology of the cell by possibly regulating mitochondrial function.
3.1.3 Functions
IMACs are mainly implicated in volume homeostasis. IMAC has been proposed to play a role in postischemic electrical and contractile dysfunction in the heart (O’Rourke 2007; Akar et al. 2005; Aon et al. 1762). In adult cardiomyocytes, it was observed that inhibitors of IMAC prevented reverse oscillatory mitochondrial depolarizations induced by substrate deprivation (O’Rourke 2000) and oxidative stress (Aon et al. 2003). In spite of multiple conductance observed in the IMM, it is challenging to assign specific physiological roles due to its ambiguous molecular identity.
3.2 Chloride Intracellular Channel Proteins
These are unique class of intracellular channel proteins consisting of single trans-membrane domain, and exist in dimorphic state of both soluble and integral membrane form (Ashley 2003; Singh 2010). There are seven paralogs of CLIC identified in mammals to date, namely CLIC1–CLIC6 (Singh 2010) which includes CLIC5A and CLIC5B (isoforms of CLIC5). They are also conserved among prokaryotes and widely distributed across other eukaryotic species, including three isoforms, identified in invertebrates (DmCLIC in Drosophila melanogaster, EXC4 and EXL1 in Caenorhabditis elegans) (Berry et al. 2003; Littler et al. 2008) and four in plants (AtDHAR1-4 in Arabidopsis thaliana) (Singh 2010; Littler et al. 2010; Elter et al. 2007). CLICs, as the name specifies, are localized to intracellular organelles (Singh 2010) specifically nuclear membrane (Valenzuela et al. 1997; Ulmasov et al. 2007), secretory vesicles of hippocampal neurons (Chuang et al. 1999), caveolae (Edwards and Kahl 2010), trans-Golgi network (Edwards and Kahl 2010), endoplasmic reticulum, (Duncan et al. 1997; Ponnalagu et al. 2016b) and mitochondria (Ponnalagu et al. 2016a; Edwards and Kahl 2010; Ponnalagu et al. 2016b; Fernandez-Salas et al. 1999).
3.2.1 Structural Insights
CLIC proteins show high sequence and structural similarity to members of omega glutathione-S-transferase (GST) family of proteins (Singh 2010). They contain ~280 amino acid residues, with a conserved C-terminal domain. CLIC5B and CLIC6 contain an additional hydrophobic N-terminal domain (Singh 2010; Littler et al. 2010). Until now, crystal structures of only the soluble forms of CLIC are obtained. CLIC4 and CLIC1 show high structural similarity due to 67 % sequence homology. Mammalian CLICs also share structural similarity to invertebrates. All CLICs contain 10 α-helices (h1, h2, h3, h4a, h4b, h5, h6, h7, h8, and h9) and four β-strands (s1, s2, s3, and s4) except in EXC4, where helix h4 is unbroken but has an additional h10 helix (Littler et al. 2008). Soluble structures of EXC4 and DmCLIC indicate that the putative transmembrane domain is formed by an α-helix (h1) and a β-strand (s2) (Littler et al. 2008). In mammalian CLICs the putative transmembrane domain (C24-46) comprises the pore, and plays a role in ion transport and targeting the protein to the membrane (Singh 2010; Singh and Ashley 2006; Singh and Ashley 2007). For CLIC1 it is shown that modification of Cys24 inhibits the channel activity (Singh and Ashley 2006). Cys24 and its equivalent conserved residues in other CLIC proteins play a key role in the redox-regulation of CLIC1, CLIC4, and CLIC5 (Singh 2010). Also, it is observed that CLIC proteins insert into planar bilayers with the N-terminus located in the luminal side and the C-terminus towards the cytosolic side (Singh and Ashley 2006; Singh and Ashley 2007). Auto insertion of CLIC1 to the bilayers increased with the formation of disulphide bond between the residues Cys24 and Cys59 upon oxidation, indicating the role of Cys24 in integration of soluble form of CLIC to membranes. However, the residue Cys59 is not conserved in all other CLICs, and also it is not very clear how these residues form disulphide bonds in membrane form as they are oriented at opposite directions. Therefore, the role of disulphide bonds in insertion of CLICs is still questionable. As CLICs have single transmembrane domain, it is predicted that at least four molecules of them will be necessary to form a functional ion channel in the membrane (Singh 2010). Conformation flexibility of putative transmembrane increased in an acidic pH of ~5.5, thereby increasing the auto insertion into the membrane and exhibiting enhanced single-channel conductance (Stoychev et al. 2009).
3.2.2 Mitochondrial CLIC Proteins
Even though, CLICs were named as intracellular ion channels, their cellular localization is not systematically explored chiefly due to lack of specific antibodies. However, information on localization of some of the CLIC proteins is available (Singh 2010). Out of six CLIC proteins, CLIC4 was characterized as a putative mitochondrial ion channel protein (Szabo and Zoratti 2014), and was demonstrated to reside in mitochondria of keratinocytes (Fernandez-Salas et al. 2002), where it is involved in Ca2+-induced differentiation of keratinocytes (Suh et al. 2007). Hence, CLIC4 is also known as mitochondrial CLIC4 (mtCLIC4). Furthermore, R (+)-Methylindazone, R (+)-[(6, 7-Dichloro-2-cyclopentyl-2, 3-dihydro-2-methyl-1-oxo-1H-inden-5-yl)-oxy] acetic acid (IAA-94)-sensitive CLIC-like currents were also observed in cardiac mitochondria (Misak et al. 2013) suggesting their presence in cardiac mitochondria. We have recently showed that CLIC4 and CLIC5 are cardiac mitochondrial proteins, where CLIC4 is localized to the OMM and CLIC5 to the IMM of rat cardiac mitochondria (Ponnalagu et al. 2016a; Ponnalagu et al. 2016b). CLIC1 and CLIC2 are chiefly localized to the endoplasmic reticulum of adult cardiomyocytes (Ponnalagu et al. 2016a; Ponnalagu et al. 2016b).
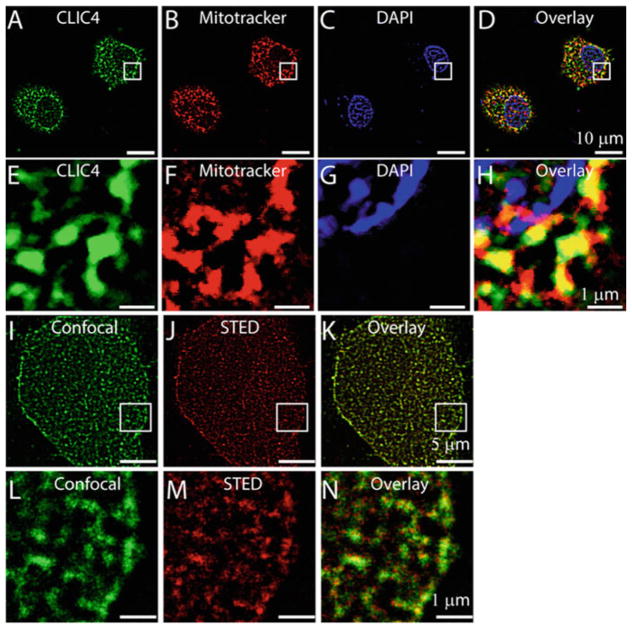
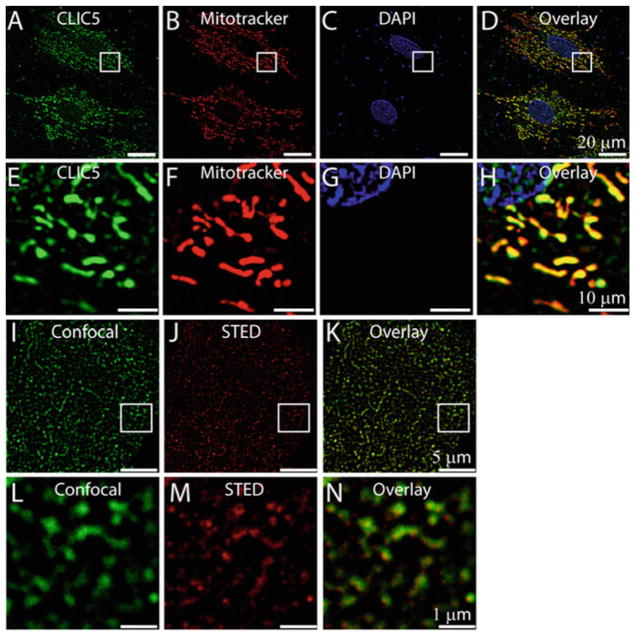
In agreement to these studies here we further show mitochondrial localization of CLIC4 in H9C2 cardiac cells (Fig. 2a–h). We also investigated localization of CLIC5 and found it to be localized to the mitochondria of H9C2 cells (Fig. 3a–h). Interestingly, bioinformatic analysis of CLICs revealed the absence of any mitochondrial targeting sequence (MTS) (Ponnalagu et al. 2016a), further indicating a unique unconventional mechanism for their mitochondrial localization in H9C2 cells. To understand geographical distribution of CLIC4 and CLIC5 in cardiac cells, we acquired super resolution images by using a custom-built STED microscope (Rodríguez et al. 2012). Figures 2i–n and 3i–n revealed a unique cluster distribution of CLIC4 (80 ±10 nm, n =500 clusters) and CLIC5 (98 ±12 nm n =500 clusters) in H9C2 cells. These results indicate that even though CLIC4 and CLIC5 localize to mitochondria of H9C2 cells, they follow a distinct distribution either due to differential localization within the mitochondria as observed in adult cardiac mitochondria (Ponnalagu et al. 2016a; Singh et al. 2012b) or due to unique functional roles.
Fig. 2.
Localization of CLIC4 in H9C2 cells. H9C2 cells loaded with mitotracker (b) were labeled with anti-CLIC4 antibody (a), and stained with DAPI (c). d is merge image of a, b, and c showing colocalization of CLIC4 to the mitochondria of H9C2 cells. e, f, g, and h are enlarged images of the squared regions in a, b, c, and d, respectively. STED microscopy of H9C2 cells showing expression of CLIC4 in the mitochondria (j, k). Confocal image of H9C2 cells labeled with anti-CLIC4 antibody (i). Corresponding STED image (j), overlay of i and j highlights increased resolution of CLIC4 localization to mitochondria with STED microscopy (k). l, m, and n are enlarged images of the squared region in i, j, and k, respectively
Fig. 3.
Localization of CLIC5 in H9C2 cells. Mitotracker-labeled (b) H9C2 cells were incubated with anti-CLIC5 antibody (a), and stained with DAPI (c). d is merge image of a, b, and c showing colocalization of CLIC5 to the mitochondria of H9C2 cells. e, f, g, and h are enlarged images of the squared regions in a, b, c, and d, respectively. STED microscopy of H9C2 cells showing expression of CLIC5 in the mitochondria (j, k). Confocal image of H9C2 cells labeled with anti-CLIC5 antibody (i) Corresponding STED image (j), overlay of i and j highlights increased resolution of CLIC5 localization to mitochondria with STED microscopy (k). l, m, and n are enlarged images of the squared region in i, j, and k, respectively
3.2.3 Electrophysiological Properties
Channel activity of CLIC proteins have been carried out in artificial bilayers (Singh and Ashley 2006; Tulk et al. 2002; Warton et al. 2002) using either recombinant proteins or ectopic expression in mammalian cells (Valenzuela et al. 1997; Tonini et al. 2000). CHO-K1 cells transfected with CLIC1 showed a single-channel conductance of 8 pS in cell and nuclear patch clamping studies (Valenzuela et al. 1997). Conductance of all CLICs varied depending on the lipid composition, recording conditions, and redox environment of the bilayers (Singh 2010; Singh and Ashley 2006). All the CLICs form NSAC as they do not differentiate between cations and anions; however, they turn into anion-selective in the presence of large cations like Tris (Singh 2010; Singh and Ashley 2006). Arg29 and Lys37 in the putative transmembrane domain have been shown to play a role in ion channel function. Lys37 altered the single-channel conductance whereas Arg29 affected the open probability of a single-channel in response to variation in membrane potential (Averaimo et al. 2013). Ionic conductance of CLIC1 and CLIC5 is regulated by actin (Singh et al. 2007), but not for CLIC4. Single-channel biophysics of CLICs in their native environment is not yet reported.
3.2.4 Functions
CLICs are multifunctional proteins. They involve in membrane trafficking, cyto-skeletal function (Berryman et al. 2004), apoptosis (Fernandez-Salas et al. 2002; Suh et al. 2004), cell cycle control (Valenzuela et al. 2000), tubulogenesis (Berry et al. 2003), VEGF-mediated angiogenesis of endothelial cells (Tung et al. 2009), mitosis, and differentiation (Suh et al. 2007). CLIC2 was reported to modulate the function of ryanodine receptors (RyR1 and RyR2) (Board et al. 2004; Takano et al. 2012). In humans, the transcriptional analysis showed the downregulation of CLIC2 in dilated cardiomyopathy patients (Diaz et al. 2010) indicating its possible role in cardiac physiology. CLIC4 also showed a role in regulating the endothelial function, where CLIC4 deficient animals were shown to be resistant to pulmonary hypertension (Wojciak-Stothard et al. 2014). Diaz et al. showed blocking chloride channels using IAA-94, prevented the cardio protective effects of ischemic pre-conditioning (IPC) as there was increased myocardial infarction (MI) due to ischemic/reperfusion (IR) injury in both the heart as well as isolated adult cardiomyocytes (Diaz et al. 1999). It was also shown that other chloride channel blockers like DIDS, 5-Nitro-2-(3-phenylpropylamino) benzoic acid (NPPB), and 4-acetamido-4′-isothiocyanato-stilbene-2,2′-disulfonic acid (SITS), protected hearts from MI further suggesting a probable cardio protective role of IAA-94 specific targets, CLICs (Zheng et al. 2013; Wang et al. 2015).
Overexpression of mtCLIC4 in keratinocytes induced its mitochondrial localization leading to reduced mitochondrial membrane potential and thereby triggering apoptosis (Fernandez-Salas et al. 2002). It was also demonstrated that RNAi suppression of CLIC4 enhanced H2O2-induced glioma cell apoptosis (Xu et al. 2013). Recent study also showed that abrogation of CLIC5 increased the ROS generation of cardiac mitochondria in mice (Ponnalagu et al. 2016a). Thus, these studies provide evidence of CLIC in modulating mitochondrial function which is known to play a role in regulation of apoptosis, cardioprotection from IR injury, and their direct involvement in maintaining cellular physiology.
4 Other Chloride Transporters or Modulators in Mitochondria
Apart from the above mentioned anion channel conductances observed in both IMM and OMM, there are still other upcoming anion channels or modulators maintaining mitochondrial ion flux which needs to be further studied. We have discussed some of them in the following section.
4.1 Mitochondrial Permeability Transition Pore
mPTP is a non-selective channel present in the mitochondrial inner membrane which allows a solute of up to 1.5 kDa to pass through it resulting in dissipation of electrochemical proton gradient, inhibition of ATP synthesis further leading to cell death (Szabo and Zoratti 2014; Bernardi et al. 2006). The conductance of this channel varies from 0.9 to 1.3 pS, with mild anion-selectivity and can also switch to cation-selective channel (Szabo and Zoratti 2014), therefore they are considered as non-selective channels present in the mitochondrial membrane. Opening of the channel is reliant on mitochondrial Ca2+ concentrations and matrix alkalization. Earlier it was shown that PTP is inhibited by submicromolar concentration of an immunosuppressant drug, cyclosporine A (CsA) (Fournier et al. 1987; Crompton et al. 1988; Broekemeier et al. 1989; Davidson and Halestrap 1990) thus indicating the presence of CypD as the molecular component of the channel. Multiple studies have predicted that mPTP comprises of VDAC (Szabo et al. 1993; Szabo and Zoratti 1993), ANT (Kokoszka et al. 2004), CypD, and hexokinase. As discussed earlier in Sect. 2.1.4, interaction of SGP7 and VDAC was found to be essential for formation of mPTP (Shanmughapriya et al. 2015). A latest study has also suggested the likelihood of F1FO ATPase itself as forming a PTP (Halestrap 2014; Bonora et al. 2013). Further, studies on identifying the molecular correlate of mPTP are still underway, and usage of a multidisciplinary approach will certainly provide much desired information (Singh et al. 2012a; Singh 2010; Rodríguez et al. 2012; Singh et al. 2012b; Singh et al. 2009). Activity of the channel is modulated by CsA, matrix acidification, adenine nucleotides, and divalent cations apart from Ca2+(Szabo and Zoratti 2014). In spite of its unclear molecular identity, multiple studies signify its key role in IR injury (Akar et al. 2005; Halestrap et al. 2004), neurodegeneration (Schinzel et al. 2005) in pathological disorders, apoptosis, and progression of cancer (Szabo and Zoratti 2014).
4.2 Maxi-Chloride Channel
This section summarizes the evidence of the presence of Maxi Cl− channel in mitochondria (De Marchi et al. 1777). Patch clamp analysis of liver mitoplast revealed a channel activity similar to monomeric state of PTP. The occurrence of this activity is not found to be consistent in liver mitochondria but was observed more frequently in human colon tumor 116 (HCT116) cells. The conductance of the channel recorded in HCT116 cells was ~400 pS. This channel was considered to be anion-selective in fourfold KCl gradient and at reversal potentials in the range of −26 to −31 mV. The ratio of permeability coefficients reported was PCl−/PK+ ≈7–18. As the channel activity represented the monomeric form of PTP, VDAC was considered as a probable candidate for this channel activity. The channel activity did not differ upon VDAC deletion, thus, ruling out the possibility of VDAC as its molecular component. The channel occasionally switched to cation-selective state. Interestingly, the activity was inhibited by only DIDS and SITS but not by IAA-94, NPPB, or niflumic acid. Properties of this channel were similar to swelling-activated and voltage-inactivated maxi-chloride channels reported earlier (Sabirov et al. 2006). Although molecular structure of the channels is unknown but is considered to have a strong link with PTP.
4.3 Uncoupling Proteins
UCP is a ~33 kDa protein, primarily acting as an H+ carrier in the inner membrane of mitochondria. There are five isoforms of UCP identified in mammals. Anion channel transport was associated with them as it was also demonstrated to play a role in mitochondrial swelling (Nicholls and Lindberg 1973), and Cl− transport was confirmed in vesicles reconstituted with UCP (Huang and Klingenberg 1996; Jezek et al. 1990). There are six transmembrane domains and in case of UCP1 and UCP2, it is shown that transmembrane 2 (TM2) contributes to anion conductance (Yamaguchi et al. 2004). Also, arginine residues of TM2 were shown to be crucial for anion transport (Hoang et al. 2015). The channel exhibited an inward-rectification with a single-channel conductance of 75 ±6 pS at positive holding potentials, and 84 ±8 pS at negative holding potentials. Divalent cations like Mg2+ and Ca2+ aided in the occurrence of these channels. The permeability to ions decreased in the order of Cl−>Br−>F−>SCN−>I−>NO3−>gluconate. Like all other uncharacterized mitochondrial anion channels, the channel activity was sensitive to nucleotides (Huang and Klingenberg 1996). Although, UCP is required to dissipate the proton gradient to provide energy for oxidative phosphorylation, these studies speculate the potential of UCP behaving as an anion channel.
4.4 Vacuolating Cytotoxin A
VacA, the vacuolating cytotoxin A, as the name implies forms large vacuoles in cells and is one of the major virulence factors released by Helicobacter pylori (Rassow 2011). VacA toxin is the prominent mediator in H. pylori-induced apoptosis of cells (Cover and Blanke 2005; Boquet et al. 2003), and is also known to form anion channels (Iwamoto et al. 1999; Czajkowsky et al. 1999; Szabo et al. 1999) of low conductance of ~10 pS. This conductivity is completely inhibited by the chloride channel blocker NPPB (Szabo et al. 1999; Tombola et al. 2000). Previous studies also demonstrated that VacA intoxicates mammalian cells by reducing mitochondrial membrane potential and inducing apoptosis (Willhite and Blanke 2004). Based on these studies a model has been hypothesized to link the channel activity with its function: VacA after entering mammalian cells localizes to the mitochondria, and modulates mitochondrial membrane permeability by forming a channel resulting in cytochrome c release. Thus, VacA joins the ranks of anion channels playing a role in intrinsic pathway of apoptosis (Willhite and Blanke 2004).
5 Pharmacological Evidence of Cardiac Mitochondrial Anion Channels
Most of the chloride channels in mitochondria have been identified and characterized using pharmacological inhibitors. However the specificity of these pharmacological agents is not yet established, and is shown to be non-specific at different concentrations which is a limitation for their usage. Nevertheless, these pharmacological modulators have played a significant part in deciphering the molecular identity and physiological functions of these channels. Thus, in this section we investigated the presence of putative chloride channels in cardiac mitochondria of Rattus norvegicus and their respective roles in modulating mitochondrial ROS generation (Murphy 2009) using these pharmacological inhibitors of chloride channels.
Mitochondrial Ca2+ capacity and ROS generation play a key role in multiple pathologies, including cardiac IR injury, neurodegenerative diseases, diabetes, cancer, and premature aging (Sena and Chandel 2012). In a recent study, mitochondrial Ca2+ capacity was used to identify components of mPTP (Shanmughapriya et al. 2015). Here, we have incorporated widely used canonical anion channel blockers, and studied their impact on mitochondrial ROS generation to identify possible mitochondrial anion channels present in the heart. Cl− channel inhibitors used are IAA-94 (Landry et al. 1989; Weber-Schurholz et al. 1993), Anthracene-9-carboxylic acid (A9C) (Al Khamici et al. 2015), NPPB (Malekova et al. 2007), DIDS (Malekova et al. 2007), glibenclamide (Sheppard and Welsh 1992), and 4-[(2-Butyl-6,7-dichloro-2-cyclopentyl-2,3-dihydro-1-oxo-1H-inden-5-yl)oxy]butanoic acid (DCPIB) (Bourke et al. 1981; Decher et al. 2001) that are known blockers of CLICs (IAA-94 and A9C both), calcium-activated chloride channel, CLC’s, cystic fibrosis transmembrane conductance regulator (CFTR), and swelling-activated chloride channels, respectively. Further, earlier studies have shown the presence of these channels in the heart (Misak et al. 2013; Duan 2013; Baumgarten et al. 2005).
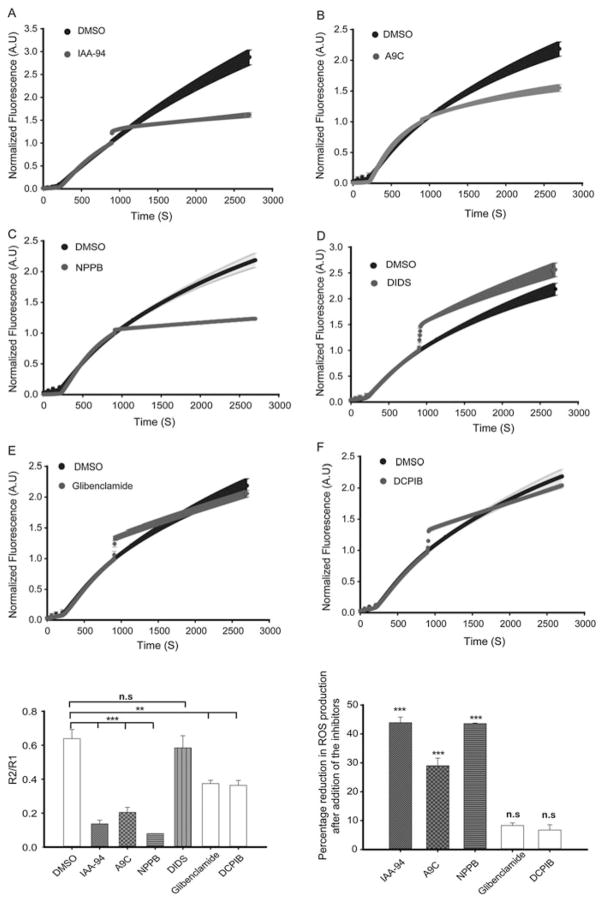
Electron transport chain complex II/III were activated with its substrate, succinate and the ROS generated was measured using amplex red as an indicator of H2O2 produced, in fluorescence spectrophotometer F-2710 as described previously (Singh et al. 2012b). It was observed that there was significant reduction in the rate as well as total ROS generation (relative to DMSO control) after addition of inhibitors such as A9C (100 μM), IAA-94 (100 μM), and NPPB (50 μM) (Fig. 4a–c, g and h). This further indicated the possible presence of CLICs, and calcium-activated chloride channel in the cardiac mitochondria isolated from R. norvegicus. There was no change in the rate of ROS production in case of 300 μM DIDS (CLC inhibitor) (Fig. 4d, g). Also, there was a non-specific immediate spike in the rate of ROS production observed for most of the inhibitors. 100 μM of each glibenclamide, and DCPIB showed significant reduction in the rate of ROS production (Fig. 4e–g), but no significant reduction in the total ROS produced was observed relative to vehicle control (DMSO) at the end of 45 min (Fig. 4h). This could be either due to reversible inhibitory effect of these channel inhibitors or unhealthy condition of the mitochondria in the presence of a drug. These results further strengthen the existence of Cl− channels in the mitochondria, and also provide evidence for their role in modulating mitochondrial function. Thus, molecular identity of other anion channels needs to be further elucidated, as they could be a probable candidate for developing new therapeutic tools.
Fig. 4.
Differential modulation of the rate of ROS generation in complex II/III of cardiac mitochondria by various chloride channel inhibitors. Cardiac mitochondria isolated from R. norvegicus and the ROS generated by complex II/III was measured as described earlier. Profile representing the rate of ROS production by cardiac mitochondria in the presence of DMSO (black) vs. 100 μM IAA-94 (gray, a), 100 μM A9C (gray, b), 50 μM NPPB (gray, c), 300 μM DIDS (gray, d), 100 μM glibenclamide (gray, e), and 100 μM DCPIB (gray, f). R1: Initial rate of ROS production which is 15 min prior to addition of the inhibitors, R2: rate of ROS production calculated after 15 min of addition of inhibitors. R2/R1 significantly decreased in presence of the chloride channel inhibitors IAA-94, A9C, NPPB, glibenclamide, and DCPIB (g). IAA-94, A9C, and NPPB showed significant reduction in the total ROS produced (relative to DMSO) at the end of 45 min whereas negligible change in ROS production relative to DMSO control was observed in case of glibenclamide and DCPIB (h). One way ANOVA was used to calculate the statistical significance (***p ≤ 0.0005, **p ≤ 0.005). IAA-94 R(+)-Methylindazone, R(+)-[(6,7-Dichloro-2-cyclopentyl-2,3-dihydro-2-methyl-1-oxo-1H-inden-5-yl)-oxy]acetic acid, A9C anthracene-9-carboxylic acid, NPPB (5-nitro-1-(3-phenylpropylamino)benzoic acid, DIDS 4,4′-Diisothiocyano-2,2′-stilbenedisulfonic acid, DCPIB 4-[(2-Butyl-6,7-dichloro-2-cyclopentyl-2,3-dihydro-1-oxo-1H-inden-5-yl)oxy]butanoic acid (n ≥ 4, independent preparations with each pharmacological agent used)
6 Concluding Remarks
Chloride channels always remained in rear seat as they are considered as “unimportant leaks” associated with cation channels in excitable cells (Jentsch et al. 2004; Ashley 2003; Jentsch et al. 2002). This is mainly due to lack of specific tools/ligands/modulators to characterize them. In spite of lack of efficient tool to identify channel recordings in intracellular organelles, several mitochondrial anion channel conductances (summarized in Table 1) have been reported but the molecular correlate of these channels is unknown or debatable. So far, molecular identities of only VDAC, CLIC4, and CLIC5 have been well established in mitochondria and many mitochondrial anion channels are yet to be identified.
Table 1.
Mitochondria ion channels, their localization, selectivity, and conductance
| Name | Location | Selectivity | References |
|---|---|---|---|
| VDAC1 | OMM | Cl selective at the open state of channel [PCa = 0.94 ± 0.05, PCl = 22.6 ±0.2(×10−16 L/s)], also permeable to calcium at the closed state [PCa = 4 ±1, PCl = 5 ±1(×10−16 L/s)], conductance of 2.4–4.3 nS at Vm = 10 mV | Szabo and Zoratti (2014), Tan and Colombini (1768), and De Pinto et al. (1987) |
| VDAC2 | OMM | Cl selective, conductance varied as it showed lower conductance (1.55 ±0.01 nS at 5 mV) and higher conductance (1.71 ±0.01 nS at 12 mV). On an average conductance reported were 3.79 ±0.10 nS. | Xu et al. (1999) and Menzel et al. (2009) |
| VDAC3 | OMM | Cl selective at a holding potential of 12 mV, a small conductance of 0.68 nS–0.71 nS at tenfold KCl gradient | Checchetto et al. (2014), Colombini (2004), and Xu et al. (1999) |
| CLIC4 | OMM | 15 pS (500/50 mM KCl), 30 pS (140 mM KCl), 1 pS (300:140 mM choline Cl), 43 (50 mM choline chloride) | Ponnalagu et al. (2016a), Duncan et al. (1997), Ponnalagu et al. (2016b), Singh and Ashley (2007), and Proutski et al. (2002) |
| IMAC | IMM | PCl/PK is 4.5, 107–110 pS in symmetrical 150 mM KCl. | Sorgato et al. (1987), Klitsch and Siemen (1991), and Sorgato et al. (1989) |
| CLIC5 | IMM | Varied from ~3–116 pS (500/50 mM KCl) when reconstituted in planar bilayers, 26 pS, 100 pS and 400 pS (140 mM KCl), 100/280 pS (140 mM), 42 pS (140 mM KCl) | Ponnalagu et al. (2016a), Ponnalagu et al. (2016b), Singh et al. (2007), Landry et al. (1989), Weber-Schurholz et al. (1993), and Edwards et al. (1998) |
| mPTP | IMM | 0.9–1.5 nS (150 mM KCl), average conductance of ~1.3 nS in patch clamp studies, low anion selectivity, and sometimes exhibit cation selectivity | Szabo and Zoratti (2014), De Marchi et al. (2006), and Petronilli et al. (1989) |
| Maxi Cl channel | IMM | 400 pS (fourfold KCl gradient) in patch clamp studies under reversal potentials of −26 to −31, PCl/PK ≈ 7 to 18 | De Marchi et al. (1777) |
| UCP | IMM | ~75 pS at positive holding potential (symmetrical 100 mM KCl) in patch clamp studies. ~84 pS at negative holding potential, inward rectifying Cl− currents were observed. | Huang and Klingenberg (1996) |
Moreover, ongoing and upcoming studies signify the potential role of mitochondrial ion channels as regulators of mitochondrial morphology as well as physiology, contributors of cancer and cardiac-related diseases, and key participants in cell life and death. Despite their significant functions in cell fate determination, little effort has been put in developing specifically targeted therapeutics agents to modulate their activity, mainly because of lack of molecular identity and structure of these channels. Therefore, efficient tools/modulators should be generated in identifying the molecular structure of these channels, as this will aid in providing specific targets for development of novel therapeutics.
Enormous evidence supports the existence of multiple physiologically relevant anion channel conductance in the mitochondria. Hence, these channels cannot be ignored as unimportant leaks and more efforts should be carried out towards understanding the molecular identity and physiological function of these channels.
Acknowledgments
We would like to thank Dr. Olimpia Meucci (DUCoM) for providing reagents, Jason Farber, Ahmed T. Hussain, and Kajol Shah for assistance with data analysis, and Dr. Shubha Gururaja Rao (DUCoM) for helpful comments and discussions. HS is supported by CTRI, CURE, AHA SDG (11SDG7230059), NIH NHLBI (1R01HL133050-01) and startup funds from DUCoM.
Contributor Information
Devasena Ponnalagu, Department of Pharmacology and Physiology, Drexel University College of Medicine, 245 N. 15th Street, Room 8154, Mail Stop 488, Philadelphia, PA 19102-1192, USA.
Harpreet Singh, Department of Pharmacology and Physiology, Drexel University College of Medicine, 245 N. 15th Street, Room 8154, Mail Stop 488, Philadelphia, PA 19102-1192, USA.
References
- Abu-Hamad S, Arbel N, Calo D, Arzoine L, Israelson A, Keinan N, Ben-Romano R, Friedman O, Shoshan-Barmatz V. The VDAC1 n-terminus is essential both for apoptosis and the protective effect of anti-apoptotic proteins. J Cell Sci. 2009;122:1906–1916. doi: 10.1242/jcs.040188. [DOI] [PubMed] [Google Scholar]
- Akar FG, Aon MA, Tomaselli GF, O’Rourke B. The mitochondrial origin of postischemic arrhythmias. J Clin Invest. 2005;115:3527–3535. doi: 10.1172/JCI25371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Khamici H, Brown LJ, Hossain KR, Hudson AL, Sinclair-Burton AA, Ng JP, Daniel EL, Hare JE, Cornell BA, Curmi PM, Davey MW, Valenzuela SM. Members of the chloride intracellular ion channel protein family demonstrate glutaredoxin-like enzymatic activity. PLoS One. 2015;10:e115699. doi: 10.1371/journal.pone.0115699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aon MA, Cortassa S, Akar FG, O’Rourke B. Mitochondrial criticality: a new concept at the turning point of life or death. Biochim Biophys Acta. 1762;2006:232–240. doi: 10.1016/j.bbadis.2005.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aon MA, Cortassa S, Marban E, O’Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem. 2003;278:44735–44744. doi: 10.1074/jbc.M302673200. [DOI] [PubMed] [Google Scholar]
- Arbel N, Ben-Hail D, Shoshan-Barmatz V. Mediation of the antiapoptotic activity of Bcl-xL protein upon interaction with VDAC1 protein. J Biol Chem. 2012;287:23152–23161. doi: 10.1074/jbc.M112.345918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley RH. Challenging accepted ion channel biology: P64 and the CLIC family of putative intracellular anion channel proteins (review) Mol Membr Biol. 2003;20:1–11. doi: 10.1080/09687680210042746. [DOI] [PubMed] [Google Scholar]
- Averaimo S, Abeti R, Savalli N, Brown LJ, Curmi PM, Breit SN, Mazzanti M. Point mutations in the transmembrane region of the CLIC1 ion channel selectively modify its biophysical properties. PLoS One. 2013;8:e74523. doi: 10.1371/journal.pone.0074523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi A, Azzone GF. Swelling and shrinkage phenomena in liver mitochondria. Ii. Low amplitude swelling-shrinkage cycles. Biochim Biophys Acta. 1965;105:265–278. doi: 10.1016/s0926-6593(65)80151-5. [DOI] [PubMed] [Google Scholar]
- Azzi A, Azzone GF. Swelling and shrinkage phenomena in liver mitochondria. Vi. -Metabolism-independent swelling coupled to ion movement. Biochim Biophys Acta. 1967;131:468–478. doi: 10.1016/0005-2728(67)90006-0. [DOI] [PubMed] [Google Scholar]
- Bahamonde MI, Fernandez-Fernandez JM, Guix FX, Vazquez E, Valverde MA. Plasma membrane voltage-dependent anion channel mediates antiestrogen-activated maxi cl- currents in c1300 neuroblastoma cells. J Biol Chem. 2003;278:33284–33289. doi: 10.1074/jbc.M302814200. [DOI] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballarin C, Sorgato MC. An electrophysiological study of yeast mitochondria. Evidence for two inner membrane anion channels sensitive to ATP. J Biol Chem. 1995;270:19262–19268. doi: 10.1074/jbc.270.33.19262. [DOI] [PubMed] [Google Scholar]
- Ballarin C, Sorgato MC. Anion channels of the inner membrane of mammalian and yeast mitochondria. J Bioenerg Biomembr. 1996;28:125–130. doi: 10.1007/BF02110642. [DOI] [PubMed] [Google Scholar]
- Banerjee J, Ghosh S. Bax increases the pore size of rat brain mitochondrial voltage-dependent anion channel in the presence of tBid. Biochem Biophys Res Commun. 2004;323:310–314. doi: 10.1016/j.bbrc.2004.08.094. [DOI] [PubMed] [Google Scholar]
- Banerjee J, Ghosh S. Phosphorylation of rat brain mitochondrial voltage-dependent anion as a potential tool to control leakage of cytochrome c. J Neurochem. 2006;98:670–676. doi: 10.1111/j.1471-4159.2006.03853.x. [DOI] [PubMed] [Google Scholar]
- Bates E. Ion channels in development and cancer. Annu Rev Cell Dev Biol. 2015;31:231–247. doi: 10.1146/annurev-cellbio-100814-125338. [DOI] [PubMed] [Google Scholar]
- Baumgarten CM, Browe DM, Ren Z. Swelling- and stretch-activated chloride channels in the heart: regulation and function. In: Kamkin A, Kiseleva I, editors. Mechanosensitivity in cells and tissues. Academia; Moscow: 2005. pp. 79–102. [PubMed] [Google Scholar]
- Bayrhuber M, Meins T, Habeck M, Becker S, Giller K, Villinger S, Vonrhein C, Griesinger C, Zweckstetter M, Zeth K. Structure of the human voltage-dependent anion channel. Proc Natl Acad Sci U S A. 2008;105:15370–15375. doi: 10.1073/pnas.0808115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavis AD. Properties of the inner membrane anion channel in intact mitochondria. J Bioenerg Biomembr. 1992;24:77–90. doi: 10.1007/BF00769534. [DOI] [PubMed] [Google Scholar]
- Beavis AD, Davatol-Hag H. The mitochondrial inner membrane anion channel is inhibited by dids. J Bioenerg Biomembr. 1996;28:207–214. doi: 10.1007/BF02110652. [DOI] [PubMed] [Google Scholar]
- Beavis AD, Vercesi AE. Anion uniport in plant mitochondria is mediated by a mg(2+)-insensitive inner membrane anion channel. J Biol Chem. 1992;267:3079–3087. [PubMed] [Google Scholar]
- Bera AK, Ghosh S. Dual mode of gating of voltage-dependent anion channel as revealed by phosphorylation. J Struct Biol. 2001;135:67–72. doi: 10.1006/jsbi.2001.4399. [DOI] [PubMed] [Google Scholar]
- Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di Lisa F, Forte MA. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 2006;273:2077–2099. doi: 10.1111/j.1742-4658.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- Berry KL, Bulow HE, Hall DH, Hobert O. A C. Elegans CLIC-like protein required for intracellular tube formation and maintenance. Science. 2003;302:2134–2137. doi: 10.1126/science.1087667. [DOI] [PubMed] [Google Scholar]
- Berryman M, Bruno J, Price J, Edwards JC. CLIC-5A functions as a chloride channel in vitro and associates with the cortical actin cytoskeleton in vitro and in vivo. J Biol Chem. 2004;279:34794–34801. doi: 10.1074/jbc.M402835200. [DOI] [PubMed] [Google Scholar]
- Betaneli V, Petrov EP, Schwille P. The role of lipids in VDAC oligomerization. Biophys J. 2012;102:523–531. doi: 10.1016/j.bpj.2011.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutner G, Ruck A, Riede B, Welte W, Brdiczka D. Complexes between kinases, mitochondrial porin and adenylate translocator in rat brain resemble the permeability transition pore. FEBS Lett. 1996;396:189–195. doi: 10.1016/0014-5793(96)01092-7. [DOI] [PubMed] [Google Scholar]
- Board PG, Coggan M, Watson S, Gage PW, Dulhunty AF. CLIC-2 modulates cardiac ryanodine receptor Ca2+ release channels. Int J Biochem Cell Biol. 2004;36:1599–1612. doi: 10.1016/j.biocel.2004.01.026. [DOI] [PubMed] [Google Scholar]
- Bonora M, Bononi A, De Marchi E, Giorgi C, Lebiedzinska M, Marchi S, Patergnani S, Rimessi A, Suski JM, Wojtala A, Wieckowski MR, Kroemer G, Galluzzi L, Pinton P. Role of the c subunit of the FO ATP synthase in mitochondrial permeability transition. Cell Cycle. 2013;12:674–683. doi: 10.4161/cc.23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boquet P, Ricci V, Galmiche A, Gauthier NC. Gastric cell apoptosis and h. Pylori: has the main function of VacA finally been identified? Trends Microbiol. 2003;11:410–413. doi: 10.1016/s0966-842x(03)00211-7. [DOI] [PubMed] [Google Scholar]
- Borecky J, Jezek P, Siemen D. 108-ps channel in brown fat mitochondria might be identical to the inner membrane anion channel. J Biol Chem. 1997;272:19282–19289. [PubMed] [Google Scholar]
- Bose T, Cieslar-Pobuda A, Wiechec E. Role of ion channels in regulating ca (2)(+) homeostasis during the interplay between immune and cancer cells. Cell Death Dis. 2015;6:e1648. doi: 10.1038/cddis.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke RS, Waldman JB, Kimelberg HK, Barron KD, San Filippo BD, Popp AJ, Nelson LR. Adenosine-stimulated astroglial swelling in cat cerebral cortex in vivo with total inhibition by a non-diuretic acylaryloxyacid derivative. J Neurosurg. 1981;55:364–370. doi: 10.3171/jns.1981.55.3.0364. [DOI] [PubMed] [Google Scholar]
- Brdiczka D, Kaldis P, Wallimann T. In vitro complex formation between the octamer of mitochondrial creatine kinase and porin. J Biol Chem. 1994;269:27640–27644. [PubMed] [Google Scholar]
- Brierley GP. Energy-linked alteration of mitochondrial permeability to anions. Biochem Biophys Res Commun. 1969;35:396–402. doi: 10.1016/0006-291x(69)90512-9. [DOI] [PubMed] [Google Scholar]
- Broekemeier KM, Dempsey ME, Pfeiffer DR. Cyclosporin a is a potent inhibitor of the inner membrane permeability transition in liver mitochondria. J Biol Chem. 1989;264:7826–7830. [PubMed] [Google Scholar]
- Buettner R, Papoutsoglou G, Scemes E, Spray DC, Dermietzel R. Evidence for secretory pathway localization of a voltage-dependent anion channel isoform. Proc Natl Acad Sci U S A. 2000;97:3201–3206. doi: 10.1073/pnas.060242297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checchetto V, Reina S, Magri A, Szabo I, De Pinto V. Recombinant human voltage dependent anion selective channel isoform 3 (hVDAC3) forms pores with a very small conductance. Cell Physiol Biochem. 2014;34:842–853. doi: 10.1159/000363047. [DOI] [PubMed] [Google Scholar]
- Chen Y, Craigen WJ, Riley DJ. Nek1 regulates cell death and mitochondrial membrane permeability through phosphorylation of VDAC1. Cell Cycle. 2009;8:257–267. doi: 10.4161/cc.8.2.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Gaczynska M, Osmulski P, Polci R, Riley DJ. Phosphorylation by nek1 regulates opening and closing of voltage dependent anion channel 1. Biochem Biophys Res Commun. 2010;394:798–803. doi: 10.1016/j.bbrc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- Chuang JZ, Milner TA, Zhu M, Sung CH. A 29 kDa intracellular chloride channel p64H1 is associated with large dense-core vesicles in rat hippocampal neurons. J Neurosci. 1999;19:2919–2928. doi: 10.1523/JNEUROSCI.19-08-02919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombini M. A candidate for the permeability pathway of the outer mitochondrial membrane. Nature. 1979;279:643–645. doi: 10.1038/279643a0. [DOI] [PubMed] [Google Scholar]
- Colombini M. VDAC: the channel at the interface between mitochondria and the cytosol. Mol Cell Biochem. 2004;256–257:107–115. doi: 10.1023/b:mcbi.0000009862.17396.8d. [DOI] [PubMed] [Google Scholar]
- Colombini M. Mitochondrial outer membrane channels. Chem Rev. 2012;112:6373–6387. doi: 10.1021/cr3002033. [DOI] [PubMed] [Google Scholar]
- Colombini M, Mannella CA. VDAC, the early days. Biochim Biophys Acta. 1818;2012:1438–1443. doi: 10.1016/j.bbamem.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover TL, Blanke SR. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat Rev Microbiol. 2005;3:320–332. doi: 10.1038/nrmicro1095. [DOI] [PubMed] [Google Scholar]
- Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin a of a Ca2 + –dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J. 1988;255:357–360. [PMC free article] [PubMed] [Google Scholar]
- Czajkowsky DM, Iwamoto H, Cover TL, Shao Z. The vacuolating toxin from helicobacter pylori forms hexameric pores in lipid bilayers at low pH. Proc Natl Acad Sci U S A. 1999;96:2001–2006. doi: 10.1073/pnas.96.5.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Wong R, Rajapakse N, Murphy E, Steenbergen C. Glycogen synthase kinase 3 inhibition slows mitochondrial adenine nucleotide transport and regulates voltage-dependent anion channel phosphorylation. Circ Res. 2008;103:983–991. doi: 10.1161/CIRCRESAHA.108.178970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AM, Halestrap AP. Partial inhibition by cyclosporin a of the swelling of liver mitochondria in vivo and in vitro induced by sub-micromolar [Ca2+], but not by butyrate. Evidence for two distinct swelling mechanisms. Biochem J. 1990;268:147–152. doi: 10.1042/bj2680147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marchi U, Szabo I, Cereghetti GM, Hoxha P, Craigen WJ, Zoratti M. A maxi-chloride channel in the inner membrane of mammalian mitochondria. Biochim Biophys Acta. 1777;2008:1438–1448. doi: 10.1016/j.bbabio.2008.08.007. [DOI] [PubMed] [Google Scholar]
- De Marchi U, Basso E, Szabo I, Zoratti M. Electrophysiological characterization of the cyclophilin D-deleted mitochondrial permeability transition pore. Mol Membr Biol. 2006;23:521–530. doi: 10.1080/09687860600907644. [DOI] [PubMed] [Google Scholar]
- De Pinto V, Ludwig O, Krause J, Benz R, Palmieri F. Porin pores of mitochondrial outer membranes from high and low eukaryotic cells: biochemical and biophysical characterization. Biochim Biophys Acta. 1987;894:109–119. doi: 10.1016/0005-2728(87)90180-0. [DOI] [PubMed] [Google Scholar]
- De Pinto V, Messina A, Lane DJ, Lawen A. Voltage-dependent anion-selective channel (VDAC) in the plasma membrane. FEBS Lett. 2010;584:1793–1799. doi: 10.1016/j.febslet.2010.02.049. [DOI] [PubMed] [Google Scholar]
- Decher N, Lang HJ, Nilius B, Bruggemann A, Busch AE, Steinmeyer K. DCPIB is a novel selective blocker of I(Cl, swell) and prevents swelling-induced shortening of guinea-pig atrial action potential duration. Br J Pharmacol. 2001;134:1467–1479. doi: 10.1038/sj.bjp.0704413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz RJ, Losito VA, Mao GD, Ford MK, Backx PH, Wilson GJ. Chloride channel inhibition blocks the protection of ischemic preconditioning and hypo-osmotic stress in rabbit ventricular myocardium. Circ Res. 1999;84:763–775. doi: 10.1161/01.res.84.7.763. [DOI] [PubMed] [Google Scholar]
- Diaz RJ, Hinek A, Wilson GJ. Direct evidence of chloride ion efflux in ischaemic and pharmacological preconditioning of cultured cardiomyocytes. Cardiovasc Res. 2010;87:545–551. doi: 10.1093/cvr/cvq084. [DOI] [PubMed] [Google Scholar]
- Duan DD. Phenomics of cardiac chloride channels. Compr Physiol. 2013;3:667–692. doi: 10.1002/cphy.c110014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan RR, Westwood PK, Boyd A, Ashley RH. Rat brain p64H1, expression of a new member of the p64 chloride channel protein family in endoplasmic reticulum. J Biol Chem. 1997;272:23880–23886. doi: 10.1074/jbc.272.38.23880. [DOI] [PubMed] [Google Scholar]
- Edwards JC, Kahl CR. Chloride channels of intracellular membranes. FEBS Lett. 2010;584:2102–2111. doi: 10.1016/j.febslet.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JC, Tulk B, Schlesinger PH. Functional expression of p64, an intracellular chloride channel protein. J Membr Biol. 1998;163:119–127. doi: 10.1007/s002329900376. [DOI] [PubMed] [Google Scholar]
- Elinder F, Akanda N, Tofighi R, Shimizu S, Tsujimoto Y, Orrenius S, Ceccatelli S. Opening of plasma membrane voltage-dependent anion channels (VDAC) precedes caspase activation in neuronal apoptosis induced by toxic stimuli. Cell Death Differ. 2005;12:1134–1140. doi: 10.1038/sj.cdd.4401646. [DOI] [PubMed] [Google Scholar]
- Elter A, Hartel A, Sieben C, Hertel B, Fischer-Schliebs E, Luttge U, Moroni A, Thiel G. A plant homolog of animal chloride intracellular channels (CLICs) generates an ion conductance in heterologous systems. J Biol Chem. 2007;282:8786–8792. doi: 10.1074/jbc.M607241200. [DOI] [PubMed] [Google Scholar]
- Fernandez-Salas E, Sagar M, Cheng C, Yuspa SH, Weinberg WC. P53 and tumor necrosis factor alpha regulate the expression of a mitochondrial chloride channel protein. J Biol Chem. 1999;274:36488–36497. doi: 10.1074/jbc.274.51.36488. [DOI] [PubMed] [Google Scholar]
- Fernandez-Salas E, Suh KS, Speransky VV, Bowers WL, Levy JM, Adams T, Pathak KR, Edwards LE, Hayes DD, Cheng C, Steven AC, Weinberg WC, Yuspa SH. MtCLIC/CLIC4, an organellular chloride channel protein, is increased by DNA damage and participates in the apoptotic response to p53. Mol Cell Biol. 2002;22:3610–3620. doi: 10.1128/MCB.22.11.3610-3620.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S, Wulff H, Skolnik EY. Ion channels in innate and adaptive immunity. Annu Rev Immunol. 2015;33:291–353. doi: 10.1146/annurev-immunol-032414-112212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier N, Ducet G, Crevat A. Action of cyclosporine on mitochondrial calcium fluxes. J Bioenerg Biomembr. 1987;19:297–303. doi: 10.1007/BF00762419. [DOI] [PubMed] [Google Scholar]
- Garlid KD, Beavis AD. Evidence for the existence of an inner membrane anion channel in mitochondria. Biochim Biophys Acta. 1986;853:187–204. doi: 10.1016/0304-4173(87)90001-2. [DOI] [PubMed] [Google Scholar]
- Gincel D, Zaid H, Shoshan-Barmatz V. Calcium binding and translocation by the voltage-dependent anion channel: a possible regulatory mechanism in mitochondrial function. Biochem J. 2001;358:147–155. doi: 10.1042/0264-6021:3580147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbole A, Varghese J, Sarin A, Mathew MK. VDAC is a conserved element of death pathways in plant and animal systems. Biochim Biophys Acta. 1642;2003:87–96. doi: 10.1016/s0167-4889(03)00102-2. [DOI] [PubMed] [Google Scholar]
- Gupta R, Ghosh S. Phosphorylation of voltage-dependent anion channel by c-jun n-terminal kinase-3 leads to closure of the channel. Biochem Biophys Res Commun. 2015;459:100–106. doi: 10.1016/j.bbrc.2015.02.077. [DOI] [PubMed] [Google Scholar]
- Halestrap AP. The C ring of the F1FO ATP synthase forms the mitochondrial permeability transition pore: a critical appraisal. Front Oncol. 2014;4:234. doi: 10.3389/fonc.2014.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion–a target for cardioprotection. Cardiovasc Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- Halle-Smith SC, Murray AG, Selwyn MJ. Palmitoyl-coa inhibits the mitochondrial inner membrane anion-conducting channel. FEBS Lett. 1988;236:155–158. doi: 10.1016/0014-5793(88)80305-3. [DOI] [PubMed] [Google Scholar]
- Hayman KA, Spurway TD, Ashley RH. Single anion channels reconstituted from cardiac mitoplasts. J Membr Biol. 1993;136:181–190. doi: 10.1007/BF02505762. [DOI] [PubMed] [Google Scholar]
- Herick K, Kramer R, Luhring H. Patch clamp investigation into the phosphate carrier from saccharomyces cerevisiae mitochondria. Biochim Biophys Acta. 1997;1321:207–220. doi: 10.1016/s0005-2728(97)00050-9. [DOI] [PubMed] [Google Scholar]
- Hiller S, Garces RG, Malia TJ, Orekhov VY, Colombini M, Wagner G. Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science. 2008;321:1206–1210. doi: 10.1126/science.1161302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang T, Matovic T, Parker J, Smith MD, Jelokhani-Niaraki M. Role of positively charged residues of the second transmembrane domain in the ion transport activity and conformation of human uncoupling protein-2. Biochemistry. 2015;54:2303–2313. doi: 10.1021/acs.biochem.5b00177. [DOI] [PubMed] [Google Scholar]
- Huang SG, Klingenberg M. Chloride channel properties of the uncoupling protein from brown adipose tissue mitochondria: a patch-clamp study. Biochemistry. 1996;35:16806–16814. doi: 10.1021/bi960989v. [DOI] [PubMed] [Google Scholar]
- Hunter DR, Haworth RA, Southard JH. Relationship between configuration, function, and permeability in calcium-treated mitochondria. J Biol Chem. 1976;251:5069–5077. [PubMed] [Google Scholar]
- Iwamoto H, Czajkowsky DM, Cover TL, Szabo G, Shao Z. VacA from helicobacter pylori: a hexameric chloride channel. FEBS Lett. 1999;450:101–104. doi: 10.1016/s0014-5793(99)00474-3. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ, Hubner CA, Fuhrmann JC. Ion channels: function unravelled by dysfunction. Nat Cell Biol. 2004;6:1039–1047. doi: 10.1038/ncb1104-1039. [DOI] [PubMed] [Google Scholar]
- Jezek P, Orosz DE, Garlid KD. Reconstitution of the uncoupling protein of brown adipose tissue mitochondria. Demonstration of GDP-sensitive halide anion uniport. J Biol Chem. 1990;265:19296–19302. [PubMed] [Google Scholar]
- Kerner J, Lee K, Tandler B, Hoppel CL. VDAC proteomics: post-translation modifications. Biochim Biophys Acta. 1818;2012:1520–1525. doi: 10.1016/j.bbamem.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnally KW, Antonsson B. A tale of two mitochondrial channels, MAC and PTP, in apoptosis. Apoptosis. 2007;12:857–868. doi: 10.1007/s10495-007-0722-z. [DOI] [PubMed] [Google Scholar]
- Kinnally KW, Zorov D, Antonenko Y, Perini S. Calcium modulation of mitochondrial inner membrane channel activity. Biochem Biophys Res Commun. 1991;176:1183–1188. doi: 10.1016/0006-291x(91)90410-9. [DOI] [PubMed] [Google Scholar]
- Kinnally KW, Antonenko YN, Zorov DB. Modulation of inner mitochondrial membrane channel activity. J Bioenerg Biomembr. 1992;24:99–110. doi: 10.1007/BF00769536. [DOI] [PubMed] [Google Scholar]
- Klitsch T, Siemen D. Inner mitochondrial membrane anion channel is present in brown adipocytes but is not identical with the uncoupling protein. J Membr Biol. 1991;122:69–75. doi: 10.1007/BF01872740. [DOI] [PubMed] [Google Scholar]
- Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano H, Shimizu S, Koya RC, Fujita H, Kamada S, Kuzumaki N, Tsujimoto Y. Human gelsolin prevents apoptosis by inhibiting apoptotic mitochondrial changes via closing VDAC. Oncogene. 2000;19:4807–4814. doi: 10.1038/sj.onc.1203868. [DOI] [PubMed] [Google Scholar]
- Landry DW, Akabas MH, Redhead C, Edelman A, Cragoe EJ, Jr, Al-Awqati Q. Purification and reconstitution of chloride channels from kidney and trachea. Science. 1989;244:1469–1472. doi: 10.1126/science.2472007. [DOI] [PubMed] [Google Scholar]
- Laus MN, Soccio M, Trono D, Cattivelli L, Pastore D. Plant inner membrane anion channel (PIMAC) function in plant mitochondria. Plant Cell Physiol. 2008;49:1039–1055. doi: 10.1093/pcp/pcn082. [DOI] [PubMed] [Google Scholar]
- Leanza L, Biasutto L, Manago A, Gulbins E, Zoratti M, Szabo I. Intracellular ion channels and cancer. Front Physiol. 2013;4:227. doi: 10.3389/fphys.2013.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AC, Xu X, Colombini M. The role of pyridine dinucleotides in regulating the permeability of the mitochondrial outer membrane. J Biol Chem. 1996;271:26724–26731. doi: 10.1074/jbc.271.43.26724. [DOI] [PubMed] [Google Scholar]
- Littler DR, Harrop SJ, Brown LJ, Pankhurst GJ, Mynott AV, Luciani P, Mandyam RA, Mazzanti M, Tanda S, Berryman MA, Breit SN, Curmi PM. Comparison of vertebrate and invertebrate CLIC proteins: the crystal structures of caenorhabditis elegans EXC-4 and drosophila melanogaster dmCLIC. Proteins. 2008;71:364–378. doi: 10.1002/prot.21704. [DOI] [PubMed] [Google Scholar]
- Littler DR, Harrop SJ, Goodchild SC, Phang JM, Mynott AV, Jiang L, Valenzuela SM, Mazzanti M, Brown LJ, Breit SN, Curmi PM. The enigma of the CLIC proteins: ion channels, redox proteins, enzymes, scaffolding proteins? FEBS Lett. 2010;584:2093–2101. doi: 10.1016/j.febslet.2010.01.027. [DOI] [PubMed] [Google Scholar]
- Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J. Molecular cell biology. 4. Scientific American Books; New York: 2000. Section 15.4 Intracellular ion environment and membrane electric potential. [Google Scholar]
- Machida K, Ohta Y, Osada H. Suppression of apoptosis by cyclophilin D via stabilization of hexokinase ii mitochondrial binding in cancer cells. J Biol Chem. 2006;281:14314–14320. doi: 10.1074/jbc.M513297200. [DOI] [PubMed] [Google Scholar]
- Malekova L, Tomaskova J, Novakova M, Stefanik P, Kopacek J, Lakatos B, Pastorekova S, Krizanova O, Breier A, Ondrias K. Inhibitory effect of DIDS, NPPB, and phloretin on intracellular chloride channels. Pflugers Arch. 2007;455:349–357. doi: 10.1007/s00424-007-0300-9. [DOI] [PubMed] [Google Scholar]
- Malia TJ, Wagner G. Nmr structural investigation of the mitochondrial outer membrane protein VDAC and its interaction with antiapoptotic bcl-xl. Biochemistry. 2007;46:514–525. doi: 10.1021/bi061577h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannella CA. Minireview: on the structure and gating mechanism of the mitochondrial channel, VDAC. J Bioenerg Biomembr. 1997;29:525–531. doi: 10.1023/a:1022489832594. [DOI] [PubMed] [Google Scholar]
- Menzel VA, Cassara MC, Benz R, de Pinto V, Messina A, Cunsolo V, Saletti R, Hinsch KD, Hinsch E. Molecular and functional characterization of VDAC2 purified from mammal spermatozoa. Biosci Rep. 2009;29:351–362. doi: 10.1042/BSR20080123. [DOI] [PubMed] [Google Scholar]
- Mertins B, Psakis G, Essen LO. Voltage-dependent anion channels: the wizard of the mitochondrial outer membrane. Biol Chem. 2014;395:1435–1442. doi: 10.1515/hsz-2014-0203. [DOI] [PubMed] [Google Scholar]
- Misak A, Grman M, Malekova L, Novotova M, Markova J, Krizanova O, Ondrias K, Tomaskova Z. Mitochondrial chloride channels: electrophysiological characterization and ph induction of channel pore dilation. Eur Biophys J. 2013;42:709–720. doi: 10.1007/s00249-013-0920-2. [DOI] [PubMed] [Google Scholar]
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann D, Buckers J, Kastrup L, Hell SW, Jakobs S. Two-color sted microscopy reveals different degrees of colocalization between hexokinase-i and the three human VDAC isoforms. PMC Biophys. 2010;3:4. doi: 10.1186/1757-5036-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG, Lindberg O. Brown-adipose-tissue mitochondria. The influence of albumin and nucleotides on passive ion permeabilities. Eur J Biochem. 1973;37:523–530. doi: 10.1111/j.1432-1033.1973.tb03014.x. [DOI] [PubMed] [Google Scholar]
- O’Rourke B. Pathophysiological and protective roles of mitochondrial ion channels. J Physiol. 2000;529(Pt 1):23–36. doi: 10.1111/j.1469-7793.2000.00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke B. Mitochondrial ion channels. Annu Rev Physiol. 2007;69:19–49. doi: 10.1146/annurev.physiol.69.031905.163804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke B, Cortassa S, Aon MA. Mitochondrial ion channels: gatekeepers of life and death. Physiology (Bethesda) 2005;20:303–315. doi: 10.1152/physiol.00020.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins G, Renken C, Martone ME, Young SJ, Ellisman M, Frey T. Electron tomography of neuronal mitochondria: three-dimensional structure and organization of cristae and membrane contacts. J Struct Biol. 1997;119:260–272. doi: 10.1006/jsbi.1997.3885. [DOI] [PubMed] [Google Scholar]
- Petronilli V, Szabo I, Zoratti M. The inner mitochondrial membrane contains ion-conducting channels similar to those found in bacteria. FEBS Lett. 1989;259:137–143. doi: 10.1016/0014-5793(89)81513-3. [DOI] [PubMed] [Google Scholar]
- Ponnalagu D, Gururaja Rao S, Farber J, Xin W, Hussain AT, Shah K, Tanda S, Berryman M, Edwards JC, Singh H. Molecular identity of cardiac mitochondrial chloride intracellular channel proteins. Mitochondrion. 2016a;27:6–14. doi: 10.1016/j.mito.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Ponnalagu D, Rao SG, Farber J, Xin W, Hussain AT, Shah K, Tanda S, Berryman MA, Edwards JC, Singh H. Data supporting characterization of CLIC1, CLIC4, CLIC5 and dmCLIC antibodies and localization of CLICs in endoplasmic reticulum of cardiomyocytes. Data Brief. 2016b;7:1038–1044. doi: 10.1016/j.dib.2016.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers MF, Beavis AD. Triorganotins inhibit the mitochondrial inner membrane anion channel. J Biol Chem. 1991;266:17250–17256. [PubMed] [Google Scholar]
- Powers MF, Smith LL, Beavis AD. On the relationship between the mitochondrial inner membrane anion channel and the adenine nucleotide translocase. J Biol Chem. 1994;269:10614–10620. [PubMed] [Google Scholar]
- Prindle A, Liu J, Asally M, Ly S, Garcia-Ojalvo J, Suel GM. Ion channels enable electrical communication in bacterial communities. Nature. 2015;527:59–63. doi: 10.1038/nature15709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proutski I, Karoulias N, Ashley RH. Overexpressed chloride intracellular channel protein CLIC4 (p64H1) is an essential component of novel plasma membrane anion channels. Biochem Biophys Res Commun. 2002;297:317–322. doi: 10.1016/s0006-291x(02)02199-x. [DOI] [PubMed] [Google Scholar]
- Rassow J. Helicobacter pylori vacuolating toxin a and apoptosis. Cell Commun Signal. 2011;9:26. doi: 10.1186/1478-811X-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez PFG, Wu Y, Singh H, Toro L, Stefani E. Building a fast scanning stimulated emission depletion microscope: a step by step guide. In: Méndez-Vilas A, editor. Current microscopy contributions to advances in science and technology. Formatex Research Center; 2012. pp. 791–800. [Google Scholar]
- Roelfsema MR, Hedrich R, Geiger D. Anion channels: master switches of stress responses. Trends Plant Sci. 2012;17:221–229. doi: 10.1016/j.tplants.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Rostovtseva TK, Bezrukov SM. VDAC regulation: role of cytosolic proteins and mitochondrial lipids. J Bioenerg Biomembr. 2008;40:163–170. doi: 10.1007/s10863-008-9145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabirov RZ, Sheiko T, Liu H, Deng D, Okada Y, Craigen WJ. Genetic demonstration that the plasma membrane maxianion channel and voltage-dependent anion channels are unrelated proteins. J Biol Chem. 2006;281:1897–1904. doi: 10.1074/jbc.M509482200. [DOI] [PubMed] [Google Scholar]
- Salvi M, Brunati AM, Toninello A. Tyrosine phosphorylation in mitochondria: a new frontier in mitochondrial signaling. Free Radic Biol Med. 2005;38:1267–1277. doi: 10.1016/j.freeradbiomed.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Schein SJ, Colombini M, Finkelstein A. Reconstitution in planar lipid bilayers of a voltage-dependent anion-selective channel obtained from paramecium mitochondria. J Membr Biol. 1976;30:99–120. doi: 10.1007/BF01869662. [DOI] [PubMed] [Google Scholar]
- Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci U S A. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz GE. The structure of bacterial outer membrane proteins. Biochim Biophys Acta. 2002;1565:308–317. doi: 10.1016/s0005-2736(02)00577-1. [DOI] [PubMed] [Google Scholar]
- Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmughapriya S, Rajan S, Hoffman NE, Higgins AM, Tomar D, Nemani N, Hines KJ, Smith DJ, Eguchi A, Vallem S, Shaikh F, Cheung M, Leonard NJ, Stolakis RS, Wolfers MP, Ibetti J, Chuprun JK, Jog NR, Houser SR, Koch WJ, Elrod JW, Madesh M. Spg7 is an essential and conserved component of the mitochondrial permeability transition pore. Mol Cell. 2015;60:47–62. doi: 10.1016/j.molcel.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon KL, Maldonado EN, Lemasters JJ, Rostovtseva TK, Bezrukov SM. Phosphorylation of voltage-dependent anion channel by serine/threonine kinases governs its interaction with tubulin. PLoS One. 2011;6:e25539. doi: 10.1371/journal.pone.0025539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard DN, Welsh MJ. Effect of ATP-sensitive K+ channel regulators on cystic fibrosis transmembrane conductance regulator chloride currents. J Gen Physiol. 1992;100:573–591. doi: 10.1085/jgp.100.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Ide T, Yanagida T, Tsujimoto Y. Electrophysiological study of a novel large pore formed by bax and the voltage-dependent anion channel that is permeable to cytochrome c. J Biol Chem. 2000;275:12321–12325. doi: 10.1074/jbc.275.16.12321. [DOI] [PubMed] [Google Scholar]
- Shoshan-Barmatz V, Keinan N, Abu-Hamad S, Tyomkin D, Aram L. Apoptosis is regulated by the VDAC1 n-terminal region and by VDAC oligomerization: release of cytochrome c, aif and smac/diablo. Biochim Biophys Acta. 1797;2010:1281–1291. doi: 10.1016/j.bbabio.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Shoshan-Barmatz V, Ben-Hail D, Admoni L, Krelin Y, Tripathi SS. The mitochondrial voltage-dependent anion channel 1 in tumor cells. Biochim Biophys Acta. 1848;2015:2547–2575. doi: 10.1016/j.bbamem.2014.10.040. [DOI] [PubMed] [Google Scholar]
- Shulga N, Wilson-Smith R, Pastorino JG. Sirtuin-3 deacetylation of cyclophilin D induces dissociation of hexokinase ii from the mitochondria. J Cell Sci. 2010;123:894–902. doi: 10.1242/jcs.061846. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Singh H. Two decades with dimorphic chloride intracellular channels (CLICs) FEBS Lett. 2010;584:2112–2121. doi: 10.1016/j.febslet.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Singh H, Ashley RH. Redox regulation of CLIC1 by cysteine residues associated with the putative channel pore. Biophys J. 2006;90:1628–1638. doi: 10.1529/biophysj.105.072678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Ashley RH. CLIC4 (p64H1) and its putative transmembrane domain form poorly selective, redox-regulated ion channels. Mol Membr Biol. 2007;24:41–52. doi: 10.1080/09687860600927907. [DOI] [PubMed] [Google Scholar]
- Singh H, Cousin MA, Ashley RH. Functional reconstitution of mammalian ‘chloride intracellular channels’ CLIC1, CLIC4 and CLIC5 reveals differential regulation by cytoskeletal actin. FEBS J. 2007;274:6306–6316. doi: 10.1111/j.1742-4658.2007.06145.x. [DOI] [PubMed] [Google Scholar]
- Singh H, Warburton S, Vondriska TM, Khakh BS. Proteomics to identify proteins interacting with p2x2 ligand-gated cation channels. J Vis Exp. 2009 doi: 10.3791/1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Stefani E, Toro L. Intracellular bk(ca) (ibk(ca)) channels. J Physiol. 2012a;590:5937–5947. doi: 10.1113/jphysiol.2011.215533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Lu R, Rodriguez PF, Wu Y, Bopassa JC, Stefani E, Toro L. Visualization and quantification of cardiac mitochondrial protein clusters with STED microscopy. Mitochondrion. 2012b;12:230–236. doi: 10.1016/j.mito.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollner K. A physicochemical cell model which simultaneously accumulates anions and cations against concentration gradients. Arch Biochem Biophys. 1955;54:129–134. doi: 10.1016/0003-9861(55)90015-1. [DOI] [PubMed] [Google Scholar]
- Sorgato MC, Keller BU, Stuhmer W. Patch-clamping of the inner mitochondrial membrane reveals a voltage-dependent ion channel. Nature. 1987;330:498–500. doi: 10.1038/330498a0. [DOI] [PubMed] [Google Scholar]
- Sorgato MC, Moran O, De Pinto V, Keller BU, Stuehmer W. Further investigation on the high-conductance ion channel of the inner membrane of mitochondria. J Bioenerg Biomembr. 1989;21:485–496. doi: 10.1007/BF00762520. [DOI] [PubMed] [Google Scholar]
- Stockdale M, Dawson AP, Selwyn MJ. Effects of trialkyltin and triphenyltin compounds on mitochondrial respiration. Eur J Biochem. 1970;15:342–351. doi: 10.1111/j.1432-1033.1970.tb01013.x. [DOI] [PubMed] [Google Scholar]
- Stoychev SH, Nathaniel C, Fanucchi S, Brock M, Li S, Asmus K, Woods VL, Jr, Dirr HW. Structural dynamics of soluble chloride intracellular channel protein CLIC1 examined by amide hydrogen-deuterium exchange mass spectrometry. Biochemistry. 2009;48:8413–8421. doi: 10.1021/bi9010607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh KS, Mutoh M, Nagashima K, Fernandez-Salas E, Edwards LE, Hayes DD, Crutchley JM, Marin KG, Dumont RA, Levy JM, Cheng C, Garfield S, Yuspa SH. The organellular chloride channel protein CLIC4/mtCLIC translocates to the nucleus in response to cellular stress and accelerates apoptosis. J Biol Chem. 2004;279:4632–4641. doi: 10.1074/jbc.M311632200. [DOI] [PubMed] [Google Scholar]
- Suh KS, Mutoh M, Mutoh T, Li L, Ryscavage A, Crutchley JM, Dumont RA, Cheng C, Yuspa SH. Clic4 mediates and is required for ca2 + –induced keratinocyte differentiation. J Cell Sci. 2007;120:2631–2640. doi: 10.1242/jcs.002741. [DOI] [PubMed] [Google Scholar]
- Sun Y, Olson R, Horning M, Armstrong N, Mayer M, Gouaux E. Mechanism of glutamate receptor desensitization. Nature. 2002;417:245–253. doi: 10.1038/417245a. [DOI] [PubMed] [Google Scholar]
- Szabo I, Zoratti M. The mitochondrial permeability transition pore may comprise VDAC molecules. I. Binary structure and voltage dependence of the pore. FEBS Lett. 1993;330:201–205. doi: 10.1016/0014-5793(93)80273-w. [DOI] [PubMed] [Google Scholar]
- Szabo I, Zoratti M. Mitochondrial channels: ion fluxes and more. Physiol Rev. 2014;94:519–608. doi: 10.1152/physrev.00021.2013. [DOI] [PubMed] [Google Scholar]
- Szabo I, De Pinto V, Zoratti M. The mitochondrial permeability transition pore may comprise VDAC molecules. Ii. The electrophysiological properties of VDAC are compatible with those of the mitochondrial megachannel. FEBS Lett. 1993;330:206–210. doi: 10.1016/0014-5793(93)80274-x. [DOI] [PubMed] [Google Scholar]
- Szabo I, Brutsche S, Tombola F, Moschioni M, Satin B, Telford JL, Rappuoli R, Montecucco C, Papini E, Zoratti M. Formation of anion-selective channels in the cell plasma membrane by the toxin VacA of helicobacter pylori is required for its biological activity. EMBO J. 1999;18:5517–5527. doi: 10.1093/emboj/18.20.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano K, Liu D, Tarpey P, Gallant E, Lam A, Witham S, Alexov E, Chaubey A, Stevenson RE, Schwartz CE, Board PG, Dulhunty AF. An x-linked channelopathy with cardiomegaly due to a CLIC2 mutation enhancing ryanodine receptor channel activity. Hum Mol Genet. 2012;21:4497–4507. doi: 10.1093/hmg/dds292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Colombini M. VDAC closure increases calcium ion flux. Biochim Biophys Acta. 1768;2007:2510–2515. doi: 10.1016/j.bbamem.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombola F, Del Giudice G, Papini E, Zoratti M. Blockers of VacA provide insights into the structure of the pore. Biophys J. 2000;79:863–873. doi: 10.1016/S0006-3495(00)76342-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonini R, Ferroni A, Valenzuela SM, Warton K, Campbell TJ, Breit SN, Mazzanti M. Functional characterization of the NCC27 nuclear protein in stable transfected CHO-K1 cells. FASEB J. 2000;14:1171–1178. doi: 10.1096/fasebj.14.9.1171. [DOI] [PubMed] [Google Scholar]
- Toro L, Li M, Zhang Z, Singh H, Wu Y, Stefani E. Maxik channel and cell signalling. Pflugers Arch. 2014;466:875–886. doi: 10.1007/s00424-013-1359-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulk BM, Kapadia S, Edwards JC. CLIC1 inserts from the aqueous phase into phospholipid membranes, where it functions as an anion channel. Am J Physiol Cell Physiol. 2002;282:C1103–C1112. doi: 10.1152/ajpcell.00402.2001. [DOI] [PubMed] [Google Scholar]
- Tung JJ, Hobert O, Berryman M, Kitajewski J. Chloride intracellular channel 4 is involved in endothelial proliferation and morphogenesis in vitro. Angiogenesis. 2009;12:209–220. doi: 10.1007/s10456-009-9139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujwal R, Cascio D, Colletier JP, Faham S, Zhang J, Toro L, Ping P, Abramson J. The crystal structure of mouse VDAC1 at 2.3 a resolution reveals mechanistic insights into metabolite gating. Proc Natl Acad Sci U S A. 2008;105:17742–17747. doi: 10.1073/pnas.0809634105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov B, Bruno J, Woost PG, Edwards JC. Tissue and subcellular distribution of CLIC1. BMC Cell Biol. 2007;8:8. doi: 10.1186/1471-2121-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela SM, Martin DK, Por SB, Robbins JM, Warton K, Bootcov MR, Schofield PR, Campbell TJ, Breit SN. Molecular cloning and expression of a chloride ion channel of cell nuclei. J Biol Chem. 1997;272:12575–12582. doi: 10.1074/jbc.272.19.12575. [DOI] [PubMed] [Google Scholar]
- Valenzuela SM, Mazzanti M, Tonini R, Qiu MR, Warton K, Musgrove EA, Campbell TJ, Breit SN. The nuclear chloride ion channel NCC27 is involved in regulation of the cell cycle. J Physiol. 2000;529(Pt 3):541–552. doi: 10.1111/j.1469-7793.2000.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkman AS, Galietta LJ. Chloride channels as drug targets. Nat Rev Drug Discov. 2009;8:153–171. doi: 10.1038/nrd2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma M, Shulga N, Pastorino JG. Sirtuin-3 modulates Bak- and Bax-dependent apoptosis. J Cell Sci. 2013;126:274–288. doi: 10.1242/jcs.115188. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang X, Cao Y, Shen M, Wang B, Zhang W, Liu Y, He X, Wang L, Xia Y, Ding M, Xu X, Ren J. Dids reduces ischemia/reperfusion-induced myocardial injury in rats. Cell Physiol Biochem. 2015;35:676–688. doi: 10.1159/000369728. [DOI] [PubMed] [Google Scholar]
- Warton K, Tonini R, Fairlie WD, Matthews JM, Valenzuela SM, Qiu MR, Wu WM, Pankhurst S, Bauskin AR, Harrop SJ, Campbell TJ, Curmi PM, Breit SN, Mazzanti M. Recombinant CLIC1 (NCC27) assembles in lipid bilayers via a ph-dependent two-state process to form chloride ion channels with identical characteristics to those observed in chinese hamster ovary cells expressing CLIC1. J Biol Chem. 2002;277:26003–26011. doi: 10.1074/jbc.M203666200. [DOI] [PubMed] [Google Scholar]
- Weber-Schurholz S, Wischmeyer E, Laurien M, Jockusch H, Schurholz T, Landry DW, al-Awqati Q. Indanyloxyacetic acid-sensitive chloride channels from outer membranes of skeletal muscle. J Biol Chem. 1993;268:547–551. [PubMed] [Google Scholar]
- Willhite DC, Blanke SR. Helicobacter pylori vacuolating cytotoxin enters cells, localizes to the mitochondria, and induces mitochondrial membrane permeability changes correlated to toxin channel activity. Cell Microbiol. 2004;6:143–154. doi: 10.1046/j.1462-5822.2003.00347.x. [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Abdul-Salam VB, Lao KH, Tsang H, Irwin DC, Lisk C, Loomis Z, Stenmark KR, Edwards JC, Yuspa SH, Howard LS, Edwards RJ, Rhodes CJ, Gibbs JS, Wharton J, Zhao L, Wilkins MR. Aberrant chloride intracellular channel 4 expression contributes to endothelial dysfunction in pulmonary arterial hypertension. Circulation. 2014;129:1770–1780. doi: 10.1161/CIRCULATIONAHA.113.006797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf A, Agnihotri S, Micallef J, Mukherjee J, Sabha N, Cairns R, Hawkins C, Guha A. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med. 2011;208:313–326. doi: 10.1084/jem.20101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Decker W, Sampson MJ, Craigen WJ, Colombini M. Mouse VDAC isoforms expressed in yeast: channel properties and their roles in mitochondrial outer membrane permeability. J Membr Biol. 1999;170:89–102. doi: 10.1007/s002329900540. [DOI] [PubMed] [Google Scholar]
- Xu Y, Kang J, Yuan Z, Li H, Su J, Li Y, Kong X, Zhang H, Wang W, Sun L. Suppression of CLIC4/mtCLIC enhances hydrogen peroxide-induced apoptosis in c6 glioma cells. Oncol Rep. 2013;29:1483–1491. doi: 10.3892/or.2013.2265. [DOI] [PubMed] [Google Scholar]
- Yamagata H, Shimizu S, Nishida Y, Watanabe Y, Craigen WJ, Tsujimoto Y. Requirement of voltage-dependent anion channel 2 for pro-apoptotic activity of bax. Oncogene. 2009;28:3563–3572. doi: 10.1038/onc.2009.213. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Jelokhani-Niaraki M, Kodama H. Second transmembrane domain of human uncoupling protein 2 is essential for its anion channel formation. FEBS Lett. 2004;577:299–304. doi: 10.1016/j.febslet.2004.09.070. [DOI] [PubMed] [Google Scholar]
- Zernig G, Graziadei I, Moshammer T, Zech C, Reider N, Glossmann H. Mitochondrial ca2+ antagonist binding sites are associated with an inner mitochondrial membrane anion channel. Mol Pharmacol. 1990;38:362–369. [PubMed] [Google Scholar]
- Zheng XB, Wang R, Yang HL, Sun XL. Effects of chloride ion channel and its blockers on myocardial ischemia reperfusion arrhythmias in rabbits. Zhonghua Yi Xue Za Zhi. 2013;93:1168–1173. [PubMed] [Google Scholar]
- Zizi M, Forte M, Blachly-Dyson E, Colombini M. Nadh regulates the gating of VDAC, the mitochondrial outer membrane channel. J Biol Chem. 1994;269:1614–1616. [PubMed] [Google Scholar]
- Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim Biophys Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]