Abstract
Please cite this paper as: Evans et al. (2011) Has estimation of numbers of cases of pandemic influenza H1N1 in England in 2009 provided a useful measure of the occurrence of disease? Influenza and Other Respiratory Viruses 5(6), e504–e512.
Background Surveillance indicators of influenza activity have generally provided robust comparative trend data for England. These indicators became less reliable, however, for monitoring trends in activity, or comparisons with previous years, during the influenza pandemic in 2009 because of changes in the perception of risk and changes in the systems of healthcare delivery. An approach was developed to estimate the number of cases of influenza‐like illness (ILI) occurring because of infection with pandemic influenza virus.
Methods and findings The number of cases was estimated each week in England on the basis of total number of patients consulting healthcare services with ILI; estimates of the proportion of individuals in the community experiencing an ILI‐seeking health care; and the proportion of these positive on laboratory testing.
Almost 800 000 cases (range 375 000–1·6 million) of symptomatic ILI cases were estimated to have occurred over the course of the two waves of pandemic activity in England. More cases were estimated to have occurred in the second wave than in the first.
Conclusions These results underestimate the total number of infections as they do not include asymptomatic infections nor those with mild illness not meeting the definition of a case of ILI. Nevertheless, the case number estimates provide a useful indicator of the trend in influenza activity and weekly data were extensively used in media reports. Although surveillance methods differ between countries, the approach of synthesising available data sources to produce an overall estimate of case numbers could be applied more widely to provide comparative data.
Keywords: Case number estimation, England, pandemic influenza
Introduction
During seasonal influenza epidemics in England, the Health Protection Agency (HPA) uses a variety of indicators to assess the level and impact of influenza virus activity. 1 This information is used for the development and revision of control and prevention policy and for healthcare planning. Influenza virus infection may cause a wide spectrum of clinical illness from asymptomatic infection through a typical influenza‐like illness (ILI) to severe and life‐threatening respiratory illness, and a wide range of respiratory viruses and other infections may cause an ILI. As influenza is a self‐limiting illness in the majority of people, and generally of mild (though sometimes greater) severity, most individuals with symptomatic infection manage their own illness without seeking formal health care. 2 , 3 Few of those who do seek health care have specimens taken for confirmation of infection. As a result, determining the number of people in the population who have had symptomatic illness caused by influenza during an epidemic is difficult. Routine surveillance has been developed to provide an assessment of the trends in the indicators of influenza activity and, by maintaining consistent surveillance systems over many years, comparisons of levels of such indicators are possible with previous seasons.
With the emergence of the pandemic influenza A H1N1 virus in 2009, and the first identification of a case in the United Kingdom (UK) in late April 2009 in a traveller returning from North America, 4 , 5 intensive surveillance activities were begun to identify and test all suspected cases in England. In common with other countries, a count of confirmed cases was published during the initial phase of the pandemic. It was recognised that these case numbers included only those identified and laboratory‐confirmed cases with symptomatic illness (conforming to the surveillance definition for ILI) and did not include either those with asymptomatic infection or those with very mild illness not meeting the definition of ILI.
Case numbers increased and sustained transmission was recognised to be occurring in the community beyond those cases and contacts identified as a result of intensive case finding. It was then no longer feasible to test all identified suspected cases and therefore to assume that those confirmed represented the total number of all symptomatic cases occurring. An estimate of the number of cases was considered to have the potential to provide a useful indicator of the trend of symptomatic cases and provide a continuation of the assessment of case numbers from the daily tally of laboratory‐confirmed cases.
A further reason for estimating symptomatic case numbers was that it could not be assumed that people would behave in the same way with illness caused by pandemic H1N1 2009 influenza in seeking health care as they usually do with seasonal influenza, for example, because of the intense media publicity during the pandemic. Additionally, in response to the pandemic in England, a telephone‐ and web‐ based service, the National Pandemic Flu Service (NPFS), 6 was established to reduce the pressure on general practitioners from patients with ILI and to issue antiviral medication. Patients were able, from 23 July 2009 to 11 February 2010, to contact NPFS by telephone or via the Internet in order to be assessed for their eligibility to receive antiviral medication. The introduction of this service further altered the pattern of contact with primary care, making comparison of consultation rates with previous years inappropriate.
Methods were therefore developed to estimate the numbers of cases of ILI caused by pandemic influenza infection based on the numbers of people seeking health care with a compatible clinical illness adjusted according to the proportion found to be positive for influenza virus infection on laboratory testing. This article describes the methodology employed and the results reported during the pandemic waves in England in 2009.
Methods
Surveillance methods
During the pandemic, there were two parallel healthcare mechanisms whereby symptomatic cases came into contact with healthcare services: primary care and the NPFS.
To estimate the numbers of pandemic influenza‐related ILI cases consulting health care through these two systems, three components were needed for each scheme: first the proportion of ILI cases found to be laboratory positive; secondly, the observed total number of patients consulting; and thirdly, the proportion of cases seeking health care.
(1) Estimating numbers consulting GPs with ILI and the proportion positive for pandemic influenza infection
Data on consultations with GPs for ILI were obtained from the HPA/QSurveillance scheme. 7 , 8 Data in this system are extracted from the computerised records of information systems of approximately 40% of all GPs. These data were used to provide the estimate in the whole English population of the number of patients consulting with ILI each week.
To obtain an estimate of the proportion of patients consulting with ILI whose illness was caused by pandemic influenza infection, data from two virological surveillance schemes in general practice were used. In the Royal College of General Practitioners (RCGP) sentinel surveillance scheme, 9 approximately 100 GPs report the number of cases of ILI consulting each week. A subset of 50% of these GPs obtain respiratory samples from a sample of patients presenting with ILI and submit them for investigation to the Respiratory Virus Unit of the HPA’s Centre for Infections. 10
In a complementary scheme in England, approximately 60 GPs submit respiratory specimens from patients presenting with ILI to one of the laboratories in the HPA’s Regional Microbiology Network (RMN). 11
Viral RNA was purified from the swabs, and multiplex real‐time PCR for pandemic H1N1, seasonal influenza H1N1, H3N2 and influenza B was performed. 12 There was a delay in testing some of the specimens for any particular week, so that, when tests were completed for that week, the positivity rate would be subsequently revised. The figure quoted for the previous week was the one given to the media. However, the model ran estimates based on each of the previous weeks in subsequent weeks, and hence, revised figures based on more accurate positivity rates were available (see Table 1).
Table 1.
Estimate and range of cases of pandemic (H1N1) 2009 by week
| Week ending | Laboratory‐confirmed cases* | Cases (range) in thousands** | Revised case numbers (range) in thousands*** |
|---|---|---|---|
| 7 June 2009 | 181 | 0·1 (0·0–0·1) | |
| 14 June 2009 | 385 | 0·4 (0·2–0·8) | |
| 21 June 2009 | 1316 | 2 (1–4) | |
| 28 June 2009 | 2841 | 8 (3–15) | |
| 5 July 2009 | 3544 | 15 (6–27) | |
| 12 July 2009 | 55 (30–85) | 25 (10–45) | |
| 19 July 2009 | 100 (60–140) | 75 (30–140) | |
| 26 July 2009 | 110 (60–160) | 65 (25–120) | |
| 2 August 2009 | 30 (15–85) | 45 (25–100) | |
| 9 August 2009 | 25 (15–60) | 28 (14–61) | |
| 16 August 2009 | 11 (6–25) | 12 (6–25) | |
| 23 August 2009 | 5 (3–12) | 6 (3–13) | |
| 30 August 2009 | 5 (3–10) | 4 (2–08) | |
| 6 September 2009 | 3 (2–7) | 5 (3–11) | |
| 13 September 2009 | 5 (3–11) | 8 (4–17) | |
| 20 September 2009 | 9 (5–20) | 11 (5–23) | |
| 27 September 2009 | 14 (7–30) | 16 (8–35) | |
| 4 October 2009 | 18 (9–38) | 28 (14–61) | |
| 11 October 2009 | 27 (13–58) | 34 (17–75) | |
| 18 October 2009 | 53 (27–115) | 56 (28–121) | |
| 25 October 2009 | 80 (39–169) | 78 (39–169) | |
| 1 November 2009 | 84 (42–181) | 70 (35–151) | |
| 8 November 2009 | 64 (32–140) | 53 (27–115) | |
| 15 November 2009 | 53 (26–114) | 50 (25–108) | |
| 22 November 2009 | 46 (23–99) | 39 (19–84) | |
| 29 November 2009 | 22 (11–47) | 23 (11–49) | |
| 6 December 2009 | 11 (6–24) | 14 (7–30) | |
| 13 December 2009 | 9 (5–19) | 11 (5–23) | |
| 20 December 2009 | 6 (3–13) | 6 (3–13) | |
| Cumulative | 845 (415–1662) | 788 (375–1644) |
*Laboratory‐confirmed case numbers during the period when almost all suspected cases were tested.
**Case numbers as published at the end of each week.
***Revised weekly estimates based on additional results available by 20 December.
(2) Estimating numbers consulting telephone (and Internet) advice lines and the proportion positive for pandemic influenza infection
Health telephone (and Internet) advice lines have also been in existence for the last 10 years [NHS Direct for England and Wales (http://www.nhsdirect.nhs.uk) and NHS24 in Scotland (http://www.nhs24.com)] and can be used for sampling for microbiological diagnosis. 13 , 14 The proportion of callers with ILI who were positive for pandemic H1N1 influenza was estimated by testing a subset of the ILI callers.
-
(a)
NHS Direct is a telephone helpline, established in 1999, for the general public wishing to seek advice on health problems. It is staffed by trained nurses who use structured algorithms to assess caller’s queries and determine appropriate management. Calls to NHS Direct for illness consistent with colds or ILI (‘cold/flu’) have been used to assess the trends in the occurrence of influenza in the population. 15 , 16 In response to the pandemic, callers aged 16 or over with ‘cold/flu’ were asked whether they would be willing to take a nasal swab from themselves and return the swab in the post to the HPA’s Centre for Infections for testing (as described for GP samples earlier in this article). A subset of those who said they would be willing to be approached were contacted.
-
(b)
The NPFS had been previously planned for a pandemic and was implemented in late July 2009. 6 Callers were asked to ring NPFS (or contact NPFS through an Internet site) if they had an ILI. Help‐line staff triaged calls and authorised antivirals to people fulfilling the algorithm for likely influenza. A ‘flu‐friend’ of the patient collected the antivirals from distribution points around the country. When this system was fully operational, callers to NHS Direct with ‘cold/flu’ were passed on to NPFS for assessment. An age‐group‐ and region‐stratified, random sample of those aged 16 years and older from the first 6000 individuals contacting NPFS each day, which were authorised for antivirals and not referred to their GP, were contacted with a letter and a self‐swabbing kit. Patients were asked to take a nasal swab from themselves and return it in a prepaid envelope. Self‐sampling through NPFS replaced sampling through NHS Direct from early August. From November 2009, parents of children with ILI between 5 and 12 years were asked to swab their children, and teenagers between 13 and 15 years were asked to self‐swab. 17
(3) Proportions seeking health care
Estimates of the proportion of patients with ILI who seek health care are difficult to obtain. 18 This proportion is likely to vary over time as the perception of risk and health‐seeking behaviour changes with media coverage of an influenza epidemic and seriousness of cases (especially press coverage of deaths caused by pandemic H1N1). Estimates of the proportion of ILI cases seeking health care were based on ‘expert opinion’. Initially, it was assumed that the proportion of ILI cases that would consult their GP would be within the range of 20–50%. Estimates from Flusurvey, an Internet‐based follow‐up of a cohort drawn from the general population (http://www.flusurvey.co.uk), indicated that this range was broadly correct (personal communication – John Edmunds). When the NPFS was implemented, the estimate of the proportion seeking health care (either by consulting their GP or by contacting the NPFS) was increased slightly to a range of 30–70% to reflect the increased availability of healthcare options. These two estimates were used during the course of the pandemic, and no further attempt was made to revise these for possible changes in health‐seeking behaviour during the course of the pandemic.
Statistical methods
The number of ILI cases consulting their GP or NPFS was adjusted with a scaling factor for those not seeing any health services and for the estimated proportion who genuinely had pandemic H1N1 infection. Estimates were made by age group and region. The resulting estimate is given as a range because of the range of uncertainty used for the proportion of those with ILI contacting healthcare services. The model used to obtain the estimated proportion positive for pandemic influenza provides some ‘smoothing’ as numbers by Strategic Health Authority (SHA) and age group are often small, and the variations seen from week to week are attributable, in part, to random variation as a result of these small numbers. Data for the most recent week were limited because of delays in receiving and processing samples. The results for previous weeks were revised to incorporate the results of tests that became available in the subsequent weeks. Further details of the statistical methods are given in the Appendix.
Results
A total of 788 000 symptomatic cases (range 375 000–1 644 000) were estimated to have occurred during 2009 (Table 1). These estimates represent the revised figures based on corrections following receipt of late results. The figures, as actually published at the end of each week, are also shown in Table 1 along with the laboratory‐confirmed case numbers to the end of June. The distribution of these cases over time is shown in Figure 1, by English region in Figure 2 and by age group in Figure 3.
Figure 1.
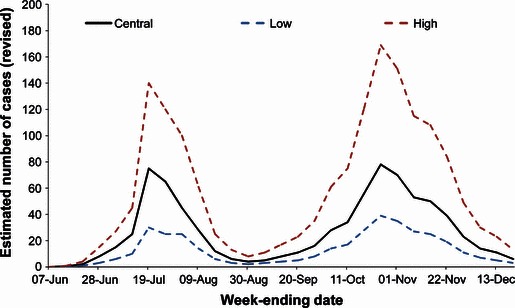
Revised estimations of cases of pandemic (H1N1) 2009 by week. Estimated numbers as recalculated on 20 December.
Figure 2.
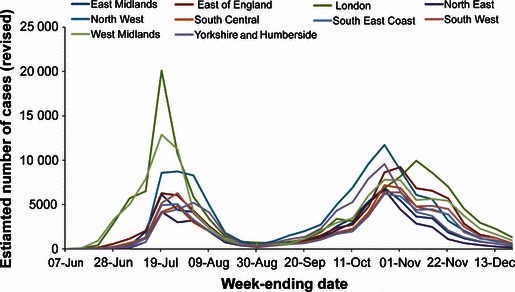
Revised estimations of cases of pandemic (H1N1) 2009 in each Strategic Health Authority (SHA) by week. Regional (SHA) figures are the estimated numbers as recalculated on 20 December.
Figure 3.
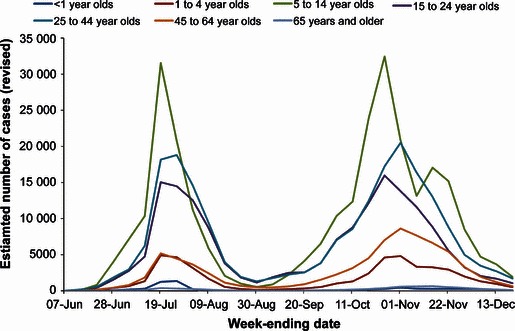
Revised estimations of cases of pandemic (H1N1) 2009 in each age group by week. Age figures are the estimated numbers as recalculated on 20 December.
Two waves of pandemic influenza cases are apparent, consistent with other data indicating two periods of clinical influenza activity in the community interrupted by the school summer holiday period. In contrast to the data from consultations for ILI with general practitioners, however, the estimated number of cases in the second wave is almost twice that in the first wave (502 000 versus 285 500). Rates of consultations with general practitioners in England were much higher in the first wave than in the second because of the introduction of the NPFS towards the latter part of the first wave and operational during the whole of the second wave. 19
The distribution of cases by geographical regions is very heterogeneous in the first wave with particularly high numbers estimated in London and the West Midlands regions. By contrast, during the second wave, the distribution of cases is much more homogenous.
The highest rate in both waves occurred in school‐age children, 5–14 years of age, followed by young adults aged 15–24. Numbers of cases in the over 65‐year age group were very low.
Discussion
A central estimate of around 800 000 people was considered to have contracted an ILI caused by infection with pandemic influenza H1N1 2009 in England during 2009 using the method described. A number of assumptions were used in producing this estimate and, in view of the uncertainties around these assumptions, a range is given around this central estimate (375 000–1·64 million cases). Subsequent information from serological surveys in the population of England 20 and estimates derived from alternative mathematical and statistical models suggest that a considerably higher proportion of the population was infected during this period. The apparent discrepancies in these estimates need to be understood to be able to assess the validity of the case number estimations developed during the pandemic and their usefulness as an indicator of the trends and distribution of the disease in England.
The estimates of the numbers of cases of disease caused by pandemic influenza infection have been based on the reports of patients presenting with an ILI and consulting healthcare services. This excludes three important groups: those infected but not developing any symptoms at all; those infected who developed a very mild illness that was not recognised or reported as an ILI; and those infected and who develop ILI, but who do not consult healthcare services (although a correction factor was introduced to try to take this group into account).
The occurrence of asymptomatic infection is well recognised with influenza virus infection in humans. 21 , 22 Mathematical models typically assume that 33–50% of influenza infections are asymptomatic or subclinical. 23 , 24 Influenza infection is also recognised to cause a range of illnesses in humans from mild coryza to fulminant pneumonia, as well as to cause exacerbations of underlying chronic conditions. Although an ILI is a common manifestation of symptomatic influenza virus infection, it is not seen in all cases. In an unpublished study of a clinical and serological follow‐up of pupils (aged 13–18) and staff at a large boarding school in England in 2009 in which an outbreak of pandemic influenza occurred, of those with serological evidence of infection, approximately a third had an ILI, a third had milder symptoms not reaching the definition of an ILI and a third had no reported symptoms at all (Chikwe Ihekweazu – personal communication). As a result, it is to be expected that estimates of the numbers of cases of ILI will be considerably lower than the numbers estimated to have been infected by the virus. As some parts of the country were recognised to have been impacted by the pandemic to a much greater extent than others, it is also to be expected that serological data from those areas will indicate higher levels of infection. 20
To reach the estimates of case numbers, the numbers of individuals reported to be consulting their GPs or NPFS with ILI were adjusted by two key factors: the estimated proportion of patients experiencing an ILI who seek health care and the proportion of people presenting with ILI whose illness is the result of pandemic influenza virus infection. Both of these estimates are subject to uncertainty. The likelihood of consulting with an ILI will be influenced by perception of the severity of the illness, perception of the subsequent risk to health, availability of effective interventions (accessible in the community or through healthcare consultation) and the accessibility of health care. It is likely that as media coverage of the threat of the pandemic varied, so too would the perception of risk on the part of sick individuals. Information from a community survey of symptomatic illness (FluSurvey) on the proportion of sick individuals seeking health care during the first wave was 0·3 with the scaling factor (3·5) adopted in these case estimates. In addition, a wide range was placed around this estimate to allow for the uncertainty. With the introduction of the NPFS, and ready availability of antiviral treatment for those with an ILI, it was assumed that the proportion of sick individuals seeking health care would increase slightly. It is highly likely, however, as the generally mild nature of the illness became apparent during the pandemic, that the likelihood of consulting healthcare services declined with time. This factor has not been taken into account in these estimates, with the effect that the assessment of the size of the second wave will be an underestimate. Further information on this will become available from sources such as FluSurvey. The accuracy of case number estimates would be increased with more robust information on consulting patterns according to the phase of the pandemic, and by geographical region, severity of illness and age group.
Subsamples of patients presenting to GPs or consulting NHS Direct or the NPFS were asked to provide specimens for laboratory testing to determine the proportion with evidence of true influenza virus infection. It is likely that the proportions found to be positive will have underestimated the true level of infection somewhat. The PCR test utilised has been demonstrated to be highly sensitive, 25 although no laboratory test for infection can be 100% sensitive. In addition, by the time samples were taken, a considerable period will have elapsed since the onset of illness in some patients. As swab positivity declines with longer delays between symptoms and swabbing, cases in which the specimen was taken 6 days or more after onset of illness were excluded from the calculation of the proportions positive.
As a consequence of these limitations, the estimates reported in this study are acknowledged to underestimate the absolute numbers of cases of symptomatic illness occurring in the population of England during 2009. Nevertheless, the use of this approach throughout the period of the pandemic provided estimates that made it possible to observe trends in the occurrence of illness, the distribution of illness by age group and geographical region and to compare the relative sizes of the two pandemic waves that occurred in England in 2009.
Both the distribution of the estimated case numbers by age group and geographical region were consistent with information from other sources about the distribution of pandemic influenza in England. 19 , 26 During the first wave, the London and West Midlands regions experienced large numbers of school outbreaks and considerable pressure on primary care, secondary care and public health services. The case number estimates reflect these reports well. However, a population‐based seroprevalence survey suggested that in these worst affected regions, around one in three children were infected in the first wave – 10 times the estimated numbers given in this paper. Rather than this being indicative of estimates being incorrect by a factor of 10, it illustrates the very high asymptomatic or very mildly symptomatic rates in children which do not fulfil the case definition for ILI. Numbers (Table 1 and 1, 2, 3) were the estimates of ILI cases caused by H1N1 not of the total number of infections. Also this 10‐fold difference is subject to uncertainty in the estimates of ILI case numbers and number infected such that it could be much lower or higher. It is also possible that differences could affected by the representativeness of both the biological and serological samplings. The case number estimates provide insight into the relative size of the waves in the pandemic. Data from sentinel general practitioner consultation schemes suggest that considerably higher numbers of people became ill during the first wave than the second. The case number estimates, however, indicate that the peak weekly numbers with true infection was similar in both waves and that, overall, the numbers of cases in the second wave was greater than in the first. A total of 286 000 cases were estimated to have occurred in the first wave compared with 502 000 in the second with a ratio of 1·8 times in the second wave compared with the first (on the assumption that healthcare‐seeking behaviours did not change, but in practice they are likely to have and this would increase the second wave compared with the first). Hospitalisations and deaths from confirmed pandemic influenza infection indicated that a much greater number of people were hospitalised (1·7 times, Colin Campbell – personal communication) or died (4·2 times) 27 in the second wave compared with the first.
A potential explanation of the apparent discrepancy in the levels of illness leading to healthcare seeking in the two waves is that levels of concern about the threat of pandemic influenza were greater in the first wave and had considerably subsided by the second wave. As influenza virus infection, even during a pandemic, is only one of a number of causes of an ILI, it is likely that a greater number and proportion of people with illness not caused by pandemic influenza consulted during the first wave. This is supported by the lower proportion of cases in the first wave being found to be positive on virological testing.
This approach provided an assessment of the number of cases of ILI that was, as far as possible, independent of changes in health‐seeking behaviour and took into account the proportion of presenting cases with true influenza virus infection. It gave a consistent and useful indicator of the trends in the occurrence of cases across the country and a representative assessment of the distribution of cases by geographical area and age group.
The limitations identified in this study highlight the problems associated with producing estimates of absolute numbers of cases of influenza in a population. It has not been standard practice in the past in England or other parts of the UK to provide the estimates of case numbers in annual epidemics. 1 A variety of indicators is used to provide trends in the population (usually expressed as rates per 100 000 population), which cannot only be used to indicate trends and distribution of disease, but can also be compared with data collected in the same way in previous years. During the pandemic in 2009, considerable emphasis was placed on the absolute numbers of cases estimated using the methods described in this report as comparison with previous years was subject to many differences in health seeking behaviour and change in service delivery (NPFS) in particular. The value of the estimates obtained using the method described lies in the ability to monitor trends from week to week in the year in question and distribution of disease across the country, rather than to provide estimated absolute numbers of ILI caused by pandemic H1N1. Although other countries will have different sources of data, the principle of synthesising to obtain an overall estimate of clinical case numbers is one which is applicable outside England. These data were very useful in describing trends to the media at a weekly press conference held by the Chief Medical Officer for England and were especially helpful when widespread testing ceased as the press had become accustomed to hearing weekly counts on how many people had tested positive for pandemic H1N1 2009. Further work is required to continue to synthesise data to be able to provide an overall estimate in future seasons when the parameters contributing to such an estimate may change, thus ensuring spatial and temporal trends can be monitored. A Bayesian synthesis of multiple sources of evidence is now being developed from which the symptomatic case count is one of several important parameters that the model estimates. This approach allows a much more robust estimate of the uncertainty to be obtained but requires serological data to provide independent validation for the estimates obtained.
Contributions/Acknowledgements
Barry Evans and Andre Charlett and Nick Andrews were involved in the weekly calculation of case numbers. BE wrote the first draft of the paper. John Watson and Richard Pebody oversaw the work and made many helpful comments on drafts of the paper. Cassandra Powers was involved in the production of the NPFS numbers and positivity rates each week. Estelle McLean and Hongxin Zhao were involved in the weekly production of situation reports, incorporating the estimated numbers. They also were involved in the production of diagrams and commentary on and drafting of this paper. Alison Bermingham and Tim Wreghitt were involved in testing specimens that formed the basis of rates used in the calculations of case numbers. Gillian Smith heads up the primary care surveillance unit within the HPA, was involved in data production used in the calculations and helpfully commented on drafts.
Appendix – statistical method
The method used provides an estimate of the number of symptomatic pandemic H1N1 cases, 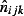 , occurring in age group i, SHA j and week k.
, occurring in age group i, SHA j and week k.
The estimate 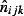 is obtained using Eqn 1
is obtained using Eqn 1
| (1) |
where 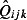 is the estimated GP ILI consultations from QSurveillance,
is the estimated GP ILI consultations from QSurveillance, 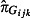 is the estimated positivity obtained from the RCGP and RMN swabbing schemes, F
ijk is the number of antiviral collections obtained from the NPFS, and
is the estimated positivity obtained from the RCGP and RMN swabbing schemes, F
ijk is the number of antiviral collections obtained from the NPFS, and 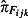 is the estimated positivity obtained from the NPFS swabbing scheme.
is the estimated positivity obtained from the NPFS swabbing scheme.
The estimated total cases of symptomatic pandemic (H1N1) 2009 is obtained using Eqn 2
| (2) |
where 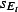 is a scaling factor. This scaling factor comprises of both an estimate for the proportion of symptomatic pandemic H1N1 cases that seek health services and the uncertainty in the estimated positivity. For the latter, the average of the median relative upper and lower half‐interval widths for both schemes was 0·3.
is a scaling factor. This scaling factor comprises of both an estimate for the proportion of symptomatic pandemic H1N1 cases that seek health services and the uncertainty in the estimated positivity. For the latter, the average of the median relative upper and lower half‐interval widths for both schemes was 0·3.
There were no data from which the proportion of symptomatic pandemic H1N1 cases that seek health services could be estimated from directly. Therefore, this was elicited from expert clinical opinion in the light of the information obtained from flu survey. The proportion seeking health services has been estimated for two periods, pre and post the inception of the NPFS. Owing to the uncertainty in the proportions seeking health care rather than using a single‐point estimate, the extremes of the likely range within which this proportion may lie was used, providing a range within which the total number of symptomatic cases each week may lie, with the midpoint of this range also being presented.
The scaling factors are subscripted by E to denote the 0 (mid), −1 (low) or 1 (high) estimates. Prior to NPFS being implemented, the range of proportions for symptomatic cases seeking health services was considered to be 0·2–0·5, i.e. for each cases seeking health care, there were between 1 and 4 additional cases not seeking health care. The proportions were increased to 0·3–0·7 once the NPFS was operational. To allow for the uncertainty in the estimated positivity, the average half confidence interval wide (30% of the point estimate) was used to inflate the upper number of cases by 30% and reduced the lower number of cases by 30%. The scaling factors used are given in Table 2.
Table 2.
Scaling factors 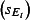
| Time period | Low estimate (E = −1) | Mid estimate (E = 0) | High estimate (E = 1) |
|---|---|---|---|
| Up to week ending 26th July (t = 0) |
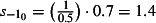
|
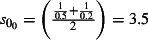
|
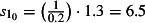
|
| Starting the week beginning 27th July (t = 1) |
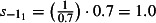
|
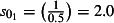
|
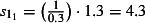
|
The scaling factors 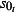 used in the estimation equate to 29% of symptomatic cases visiting their GP prior to the introduction of NPFS and 50% of symptomatic cases either visiting their GP or self‐referring to NPFS, once NPFS was established.
used in the estimation equate to 29% of symptomatic cases visiting their GP prior to the introduction of NPFS and 50% of symptomatic cases either visiting their GP or self‐referring to NPFS, once NPFS was established.
The estimated numbers of GP ILI consultations 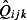 is obtained from Eqn 3
is obtained from Eqn 3
| (3) |
where Q
ijk is the total number of GP ILI consultations reported by QSurveillance, 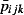 is the average daily total number of patients registered at the GP practices sending data to QSurveillance, and
is the average daily total number of patients registered at the GP practices sending data to QSurveillance, and 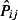 is the estimated 2007 population obtained from the Office of National Statistics. (http://www.statistics.gov.uk/statbase/Product.asp?vlnk=15106)
is the estimated 2007 population obtained from the Office of National Statistics. (http://www.statistics.gov.uk/statbase/Product.asp?vlnk=15106)
Both 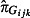 and
and 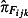 are estimated from the virological results obtained from the swabbing schemes using mixed effects logistic regression models. Because of the relatively small numbers of swabs taken, Strategic Health Authorities (SHAs) have been grouped into four regions (cGOR): North (East Midlands, north‐east, north‐west and Yorkshire and the Humber), South (east of England, south central, south‐east coast and south‐west), West Midlands and London. West Midlands and London were the SHAs most affected in the first wave. Equation 4 gives the model used to estimate the positivity from the GP swabbing schemes
are estimated from the virological results obtained from the swabbing schemes using mixed effects logistic regression models. Because of the relatively small numbers of swabs taken, Strategic Health Authorities (SHAs) have been grouped into four regions (cGOR): North (East Midlands, north‐east, north‐west and Yorkshire and the Humber), South (east of England, south central, south‐east coast and south‐west), West Midlands and London. West Midlands and London were the SHAs most affected in the first wave. Equation 4 gives the model used to estimate the positivity from the GP swabbing schemes
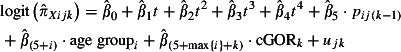 |
(4) |
where X is either G (RCGP) or F (NPFS) scheme, t is the centred week, and additional terms t i(i = 2,3,4) were included at points in time when this improved the model fit. p ij(k‐1)is the first‐order lagged observed positivity, and u jk is the random effect. Rather than including two independent random effects for region and week, these were cross‐classified to obtain a single random effect that allows estimates to vary over time by region. Separate models were fitted to those aged below 44 and 45 or older. These models were fitted using Stata 10.1.
References
- 1. McLean E, Murdoch H, Reynolds A et al. Surveillance of influenza and other respiratory viruses in the United Kingdom: October 2008 to April 2009. Health Protection Report. 2‐10‐2009. 10‐5‐2010.
- 2. Nicholson KG. Clinical features of influenza. Semin Respir Infect 1992; 7:26–37. [PubMed] [Google Scholar]
- 3. Nicholson KG, Wood JM, Zambon M. Influenza. Lancet 2003; 362:1733–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Health Protection Agency, Health Protection Scotland, National Public Health Service for Wales, and HPA Northern Ireland Swine influenza investigation teams . Epidemiology of new influenza A (H1N1) virus infection, United Kingdom, April–June 2009. Eurosurveillance 2009; 14:pii=19232. [PubMed] [Google Scholar]
- 5. Health Protection Agency, Health Protection Scotland, National Public Health Service for Wales, and HPA Northern Ireland Swine influenza investigation teams . Epidemiology of new influenza A(H1N1) in the United Kingdom, April–May 2009. Eurosurveillance 2009; 14:pii=19213. [Google Scholar]
- 6. Department of Health . A (H1N1) Swine Influenza: launch of the National Pandemic Flu Service. Dear Colleague letters. 23‐7‐2009. 23‐6‐2010.
- 7. Smith G, Hippisley‐Cox J, Harcourt S et al. Developing a national primary care‐based early warning system for health protection – a surveillance tool for the future? Analysis of routinely collected data. J Public Health (Oxf) 2007; 29:75–82. [DOI] [PubMed] [Google Scholar]
- 8. Health Protection Agency/QSurveillance National Surveillance System . HPA Website. 2010. 23‐6‐2010.
- 9. Fleming DM, Elliot AJ. Lessons from 40 years’ surveillance of influenza in England and Wales. Epidemiol Infect 2008; 136:866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zambon MC, Stockton JD, Clewley JP, Fleming DM. Contribution of influenza and respiratory syncytial virus to community cases of influenza‐like illness: an observational study. Lancet 2001; 358:1410–1416. [DOI] [PubMed] [Google Scholar]
- 11. Joseph CA. Virological surveillance of influenza in England and Wales: results of a two year pilot study 1993/94 and 1994/95. PHLS Influenza Subcommittee. Commun Dis Rep CDR Rev 1995; 5:R141–R145. [PubMed] [Google Scholar]
- 12. Ellis J, Iturriza M, Allen R et al. Evaluation of four real‐time PCR assays for detection of influenza A(H1N1)v viruses. Eurosurveillance 2009; 14:pii=19230. [DOI] [PubMed] [Google Scholar]
- 13. Elliot AJ, Powers C, Thornton A et al. Monitoring the emergence of community transmission of influenza A/H1N1 2009 in England: a cross sectional opportunistic survey of self sampled telephone callers to NHS Direct. BMJ 2009; 339:b3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cooper DL, Smith GE, Chinemana F et al. Linking syndromic surveillance with virological self‐sampling. Epidemiol Infect 2008; 136:222–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith GE, Cooper DL, Loveridge P, Chinemana F, Gerard E, Verlander N. A national syndromic surveillance system for England and Wales using calls to a telephone helpline. Euro Surveill 2006; 11:220–224. [PubMed] [Google Scholar]
- 16. Cooper DL, Verlander NQ, Elliot AJ, Joseph CA, Smith GE. Can syndromic thresholds provide early warning of national influenza outbreaks? J Public Health (Oxf) 2009; 31:17–25. [DOI] [PubMed] [Google Scholar]
- 17. Elliot A. Syndromic surveillance: the next phase of public health monitoring during the H1N1 influenza pandemic? Euro Surveill 2009; 14:pii=19391. [PubMed] [Google Scholar]
- 18. Fleming DM. The contribution of influenza to combined acute respiratory infections, hospital admissions, and deaths in winter. Commun Dis Public Health 2000; 3:32–38. [PubMed] [Google Scholar]
- 19. Health Protection Agency . National weekly influenza report. 1‐7‐2009. 23‐6‐2010.
- 20. Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, Zambon MC. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross‐sectional serological study. Lancet 2010; 375:1100–1108. [DOI] [PubMed] [Google Scholar]
- 21. Monto AS, Koopman JS, Longini IM Jr. Tecumseh study of illness. XIII. Influenza infection and disease, 1976‐1981. Am J Epidemiol 1985; 121:811–822. [DOI] [PubMed] [Google Scholar]
- 22. Carrat F, Vergu E, Ferguson NM et al. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol 2008; 167:775–785. [DOI] [PubMed] [Google Scholar]
- 23. Ferguson NM, Cummings DA, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature 2006; 442:448–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Longini IM Jr, Halloran ME, Nizam A, Yang Y. Containing pandemic influenza with antiviral agents. Am J Epidemiol 2004; 159:623–633. [DOI] [PubMed] [Google Scholar]
- 25. Ellis J, Iturriza M, Allen R et al. Evaluation of four real‐time PCR assays for detection of influenza A(H1N1)v viruses. Eurosurveillance 2009; 14:pii=19230. [DOI] [PubMed] [Google Scholar]
- 26. Health Protection Agency . Pandemic (H1N1) 2009 in England: an overview of initial epidemiological findings and implications for the second wave, 24 November 2009.
- 27. Pebody RG, McLean E, Zhao H et al. Pandemic Influenza A (H1N1) 2009 and mortality in the United Kingdom: risk factors for death, April 2009 to March 2010. Euro Surveill 2010; 15:pii=19571. [PubMed] [Google Scholar]


