Abstract
Objectives
This exploratory study aimed to investigate associations between neonatal brain volumes and visual–motor integration (VMI) and fine motor skills in children born extremely preterm (EPT) when they reached 6½ years of age.
Setting
Prospective population-based cohort study in Stockholm, Sweden, during 3 years.
Participants
All children born before gestational age, 27 weeks, during 2004–2007 in Stockholm, without major morbidities and impairments, and who underwent MRI at term-equivalent age.
Main outcome measures
Brain volumes were calculated using morphometric analyses in regions known to be involved in VMI and fine motor functions. VMI was assessed with The Beery-Buktenica Developmental Test of Visual–Motor Integration—sixth edition and fine motor skills were assessed with the manual dexterity subtest from the Movement Assessment Battery for Children—second edition, at 6½ years. Associations between the brain volumes and VMI and fine motor skills were evaluated using partial correlation, adjusted for total cerebral parenchyma and sex.
Results
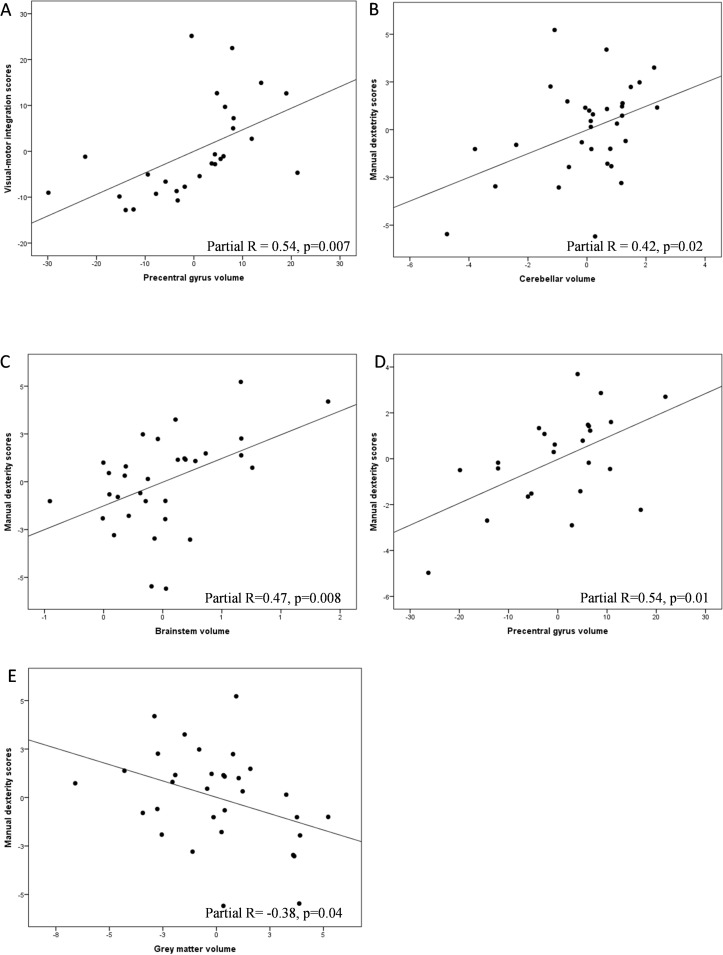
Out of 107 children born at gestational age <27 weeks, 83 were assessed at 6½ years and 66/83 were without major brain lesions or cerebral palsy and included in the analyses. A representative subsample underwent morphometric analyses: automatic segmentation (n=34) and atlas-based segmentation (n=26). The precentral gyrus was associated with both VMI (r=0.54, P=0.007) and fine motor skills (r=0.54, P=0.01). Associations were also seen between fine motor skills and the volume of the cerebellum (r=0.42, P=0.02), brainstem (r=0.47, P=0.008) and grey matter (r=−0.38, P=0.04).
Conclusions
Neonatal brain volumes in areas known to be involved in VMI and fine motor skills were associated with scores for these two functions when children born EPT without major brain lesions or cerebral palsy were evaluated at 6½ years of age. Establishing clear associations between early brain volume alterations and later VMI and/or fine motor skills could make early interventions possible.
Keywords: brain development, extremely preterm, outcome, school-age
Strengths and limitations of this study.
This was a robust population-based cohort study design that used advanced MRI techniques and established assessment tools.
It studied visual–motor integration and fine motor skills in extremely preterm born children at 6½ years, which is an important age for Swedish school children.
Although we had to exclude numerous children from the morphometric analyses, due to strict data quality criteria, the sample was representative of the cohort.
We were unable to include a control group of children born at term in the analyses.
Introduction
Visual–motor function is an important cross-modal ability that involves the integration of visual function and perception, eye–hand coordination, fine motor skills and visual–motor integration (VMI).1 Studies have shown that VMI can predict a child’s future hand-writing skills2–4 and academic performance in reading, writing and mathematics.5 Evaluating VMI performance is therefore an important component of the test batteries that are used to detect children who risk school problems. Fine motor skills are a fundamental component of hand and visual–motor function, which also contributes to school performance.6 Several reports have shown that children born preterm have poor VMI and motor function,7–10 and it is well known that this group of children risk poor school performance and learning disabilities.11–13
Although problems with VMI can stem from any of the underlying, contributing abilities listed above, when VMI and motor abilities have been investigated together, and perceptual and general cognitive abilities have been held constant, children with lower general motor scores are seen to have lower VMI scores.14 Children born with very low birth weight show a similar pattern15: VMI showing stronger relationships with motor ability than with visual perception, suggesting that motor skills and manual fine motor skills in particular are crucial components underlying VMI. On the other hand, visual perceptual deficits in children born extremely preterm (EPT) are well documented7 and their role in VMI should not be underestimated even though their contribution to VMI in children born EPT was not investigated in the present study. Therefore, a less-defined exploration of brain areas known to be associated with VMI is a useful approach, especially when investigating the neural associations of a multi-modal ability, in groups with potentially atypical brain development trajectories.16
It is largely unknown how the developmental alterations of the brain affect VMI and fine motor skills in children born preterm. However, we have previously reported that neonatal brain volumes are affected in preterm infants,17 and other researchers have shown that these alterations in preterm brain volumes persist until at least early childhood.18 Volumetric alterations in the brain have also been associated with neurodevelopmental outcomes in preterm born children.19 20 However, the relationship between volume alterations in the brains of children born extremely (EPT), at <27 weeks of gestation, and VMI and fine motor skills at school age remains largely unexplored.
It has been reported that several networks in the brain are involved in the mediation of VMI and fine motor skills: the visual, salience, sensory motor and default mode networks.21 Previous studies in adolescents born preterm have indicated that volumes of the cerebellum and thalamus,22 superior temporal gyrus, insula, medial occipital lobe and temporal lobe23 are associated to VMI scores. Also, a study looking at brain growth in preterm children reported that growth of the caudate and globus pallidus could predict VMI scores.24 Based on these previous reports, we hypothesised that early brain volume alterations in these regions and networks in the brain would be related to later VMI and fine motor skills in children born EPT. We used an exploratory approach to investigate these possible associations, using two separate analyses to measure the brain volumes at term-equivalent age: atlas-based segmentation and automatic segmentation, and carried out a clinical evaluation of VMI and fine motor skills at the age of 6½ years.
Methods
Study population
The study population was a subcohort of the Extremely Preterm Infants in Sweden Study (EXPRESS) cohort. EXPRESS was a prospective national population-based cohort study which invited all children born in Sweden at a gestational age (GA) of <27 weeks over a 3-year period to take part in clinical follow ups at 2½ and 6½ years of age.25 26 The present study included 107 children born in Stockholm between 1 January 2004 and 31 March 2007, without chromosomal aberrations, congenital malformations or infections, who had undergone MRI of the brain at term-equivalent age.
GA was assessed by maternal ultrasound at around a GA of 18 weeks and perinatal and neonatal data were prospectively collected from the children’s medical records. Cranial ultrasound was performed on a regular basis during the neonatal period up to a GA of 40 weeks, according to our clinical routine. Children were excluded from the analyses in the present study if they had major brain lesions defined as intraventricular haemorrhage, periventricular leukomalacia, severe white matter score according to Inder et al27 or hydrocephalus, or cerebral palsy (CP) as defined by the Surveillance of Cerebral Palsy in Europe Working Group.28 All children had been screened for retinopathy of prematurity, and evidence-based treatment had been administered.29
The parents of all the children gave their written informed consent before the study.
Assessment at 6½ years of age
VMI was assessed using the Beery-Buktenica Developmental Test of Visual–Motor Integration—sixth edition.30 This test consists of 30 geometrical shapes that the child is asked to copy with a pen and paper and it is terminated when three figures in a row have been incorrectly copied. The drawings are examined and acceptable approximations of the model drawings are each given one point. The raw score is the total number of correct drawings and this is then transformed to an age-corrected standard score. Standard scores were used in the analyses in this study. The normative mean score is 100 with an SD of 15.
Fine motor skills were assessed with the manual dexterity subtest of the Movement Assessment Battery for Children—second edition which measures unimanual speed, bimanual coordination and unimanual spatial accuracy.31 Age-adjusted standard scores were used for the analyses. The reference mean score is 10 with an SD of 3, and scores below 7 indicate definitive or borderline motor problems.
Binocular visual acuity was measured with habitual correction at 3 m with the Lea Hyvärinen chart.32 Visual impairment was defined as a visual acuity of <0.33 in the better eye.
MRI data acquisition and processing
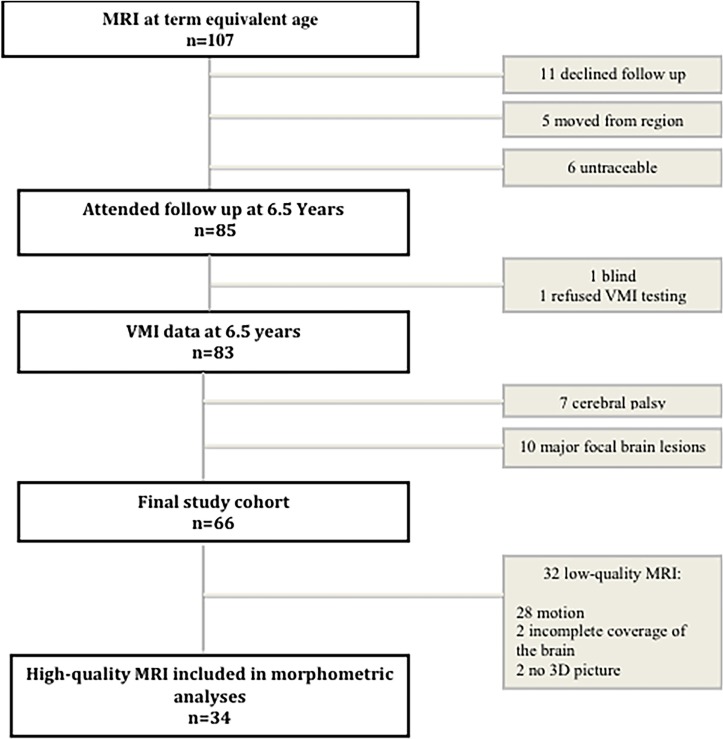
All the children were scanned using a Philips Intera 1.5 Tesla MRI system (Philips International, Amsterdam, The Netherlands) at term-equivalent age. The child was fed before the scanning procedure and given a low dose of chloral hydrate (30 mg/kg) orally or rectally, as previously described.33 A sagittal T1-weighted turbo spin echo sequence, an axial inversion recovery sequence and an axial T2-weighted sequence were run. The three-dimensional T1-weighted images were acquired with an echo time of 4.6 ms, a repetition time of 40 ms, a flip angle of 30°, a voxel size of 0.7×0.7×0.1 mm and a field of view of 180 mm. The MRI protocol has previously been reported.33 Quality assurance was considered important in order to obtain accurate data, even though this meant that we were left with a relatively small sample size. The imaging data was checked for quality, based on a visual inspection of the raw data sets, and the reasons for low imaging quality are presented in figure 1. MRI data went into two separate analyses, atlas-based segmentation and automatic segmentation; atlas-based segmentation to conduct the regional segmentation of specific regions34 and automatic segmentation to extract the mean volumes of the grey matter white matter, cerebrospinal fluid, basal ganglia, brainstem and cerebellum.35
Figure 1.
Study population. 3D, three dimensional; VMI, visual–motor integration.
Atlas-based segmentation
The whole brain of the included infants was divided into 90 anatomical regions, by using the automated anatomical labelling neonatal atlas,34 as previously described.36 Briefly, the intensity image that generated the neonatal atlas was registered to the T1-weighted image of each infant, then the generated deformation field was used to transform the label map with 90 regions from the atlas space to the subject space (online supplementary figure 1). A visual inspection was performed for each subject and each step. The volume of each region was determined by using a proper script written in MATLAB selecting the region of interest via its voxel value.
bmjopen-2017-020478supp001.jpg (1,016KB, jpg)
We selected brain regions that had been previously described as being in the networks that mediate VMI and fine motor skills, namely the visual, salience, sensory motor and default mode networks.22–24 We also considered the subcortical regions: pallidum, putamen, caudate and thalamus. The volume of each brain region was determined by adding together the volumes of their components.
Automatic segmentation
We first performed automatic segmentation of brain tissues (T1-weighted images) using SPM8 software (http://www.fil.ion.ucl.ac.uk/spm), running on MATLAB V.7.5 (MathWorks, Natick, Massachusetts, USA). This process focused on specific neonatal priors, including grey matter, white matter, cerebrospinal fluid, deep grey matter, the cerebellum and brainstem, which have previously been described in detail.17 35 We used the tissue class images created during segmentation to generate a custom template to improve coregistration using Diffeomorphic Anatomical Registration Through an Exponential Lie algebra algorithm.37 After this step the images were modulated via SPM8 software to improve the intersubject registration. The easy volume toolbox38 was used to extract the global brain tissue volumes from the segmented, normalised and modulated images of each child.
Statistical analyses
The analyses were carried out with SPSS for Windows, V.22.0 and the data were checked for normality, homogeneity and outliers. In order to compare the groups, we used the Student’s t-test and Mann-Whitney U test for continuous variables and the χ2 test or Fisher’s exact test for categorical data, as appropriate. The associations between brain volumes, VMI scores and fine motor skills scores were explored using partial correlation. Total cerebral parenchyma (CPAR), the sum of the grey matter and white matter, excluding cerebrospinal fluid, was used as a covariate to control for generalised scaling effects. Because sex, GA at birth and the GA at the time of the scan have previously been shown to influence brain volume size in preterm children,39 40 we performed the analyses with different covariate pairs. First we used CPAR and GA at birth, then we used CPAR and the GA at scan and finally we used CPAR and sex. GA at birth and GA at scan did not influence the results, but sex did, so CPAR and sex were chosen as the covariates in the final model. There was one multivariate outlier which was identified by the Mahalanobis distance when we investigated the atlas segmentation data in relation to the fine motor skills scores, and this child was excluded from those analyses. Bonferroni correction for multiple comparisons was not applied because of the exploratory nature of the study and the reduced sample size examined.41–43 The level of significance was set at a two-sided P value of <0.05.
Results
Study population
A summary of the study population is presented in figure 1 and their perinatal characteristics are presented in table 1.
Table 1.
Characteristics of the 66 children born at gestational age below 27 weeks and without major brain lesions or cerebral palsy (n=66)
| Gestational age at birth, weeks, median (range) | 25.6 (23.4–26.6) |
| Birth weight, g, mean (SD) | 839 (152) |
| Gender, girls/boys | 29/37 |
| Small for gestational age, n (%) | 4 (6) |
| Prenatal steroids, n (%) | 60 (91) |
| Premature rupture of the membranes, n (%) | 19 (29) |
| Caesarean section, n (%) | 32 (48) |
| Sepsis, n (%) | 51 (77) |
| Days of mechanical ventilation, median (range) | 7 (0–55) |
| Postnatal steroids, n (%) | 11 (17) |
| Bronchopulmonary dysplasia, oxygen at 36 weeks, n (%) | 26 (39) |
| Necrotising enterocolitis, n (%) | 6 (9) |
| Patent ductus arteriosus, ligation, n (%) | 20 (30) |
| Laser treatment for retinopathy of prematurity, n (%) | 8 (12) |
| Intraventricular haemorrhage grade I–II, n (%) | 23 (35) |
Sepsis was defined as positive blood culture or clinical picture of sepsis in association with elevated C reactive protein or leucocyte count. Data on small for gestational age was missing for four children.
At the time of the scan, the median (range) GA in the 66 children without major brain lesions or CP was 40.8 (39.1–45.3) weeks and for the assessment of VMI and fine motor skills it was 6 years 5 months (6 years 3 months–7 years 2 months).
The subsample of 34 children with high-quality MRI scans was representative of the whole cohort with regard to their perinatal and neonatal characteristics, findings on structural MRI, gestational age at MRI, age at assessment at 6½ years and mean VMI and fine motor score (online supplementary table 1). During the atlas-based segmentation process, eight subjects had coregistration failure, leaving 26 children in the final sample. These 26 children were also representative of the cohort of 66 children with regard to the above characteristics, except for days on mechanical ventilation, which was a median (range) of 3 (0–36) days for the subsample versus 10 (0–55) days for the other children (P=0.04) (online supplementary table 2).
bmjopen-2017-020478supp002.pdf (18.5KB, pdf)
bmjopen-2017-020478supp003.pdf (18.4KB, pdf)
Assessment at 6½ years of age
The mean (SD) VMI standard score for the 66 children without major brain lesions or CP was 93 (10), 7 points (0.5 SD) below the norm. The mean (SD) VMI scores for the 29 girls was higher than for the 37 boys: mean (SD) at 96 (10) versus 90 (8), respectively (P=0.02).
The mean (SD) fine motor skills score was 8 (3), which was 2 points (0.5 SD) below the norm, and there were no significant sex differences as the girls scored 8 (3) and the boys 7 (3) (P=0.16).
None of the children included in the study had a visual impairment.
Brain volumes and associations with VMI and fine motor skills
We identified a number of regions that demonstrated significant associations between brain volumes, VMI and fine motor skills scores. VMI performance showed a positive correlation with the volume of the precentral gyrus (partial r=0.54, P=0.007), whereas the fine motor skills scores showed a positive correlation with the volumes of the precentral gyrus (partial r=0.54, P=0.01), the cerebellum (partial r=0.42, P=0.02) and the brainstem (partial r=0.47 P=0.008) and a negative correlation with cortical grey matter volume (partial r=−0.38, P=0.04) (figure 2). All the correlation analyses can be found in online supplementary table 3.
Figure 2.
Illustrations of partial correlations between neonatal brain volumes, VMI and fine motor skills (assessed by manual dexterity scores on the Movement Assessment Battery for Children-2) at 6½ years of age, using plots of residuals. Analyses are adjusted for total cerebral parenchyma (grey matter plus white matter) and sex. (A) Correlations between the volume of the precentral gyrus and VMI scores. (B–E) Correlations between the volumes of the cerebellum, brainstem, precentral gyrus, grey matter and fine motor skills. Results are presented without correction for multiple comparisons. VMI, visual–motor integration.
bmjopen-2017-020478supp004.pdf (29.8KB, pdf)
Discussion
In this exploratory study, the associations between brain volumes at term-equivalent age and VMI and fine motor skills at 6½ years in children born EPT without major brain lesions or CP were explored in regions of the brain previously reported to be involved in those functions. The volume of the precentral gyrus showed a positive correlation with both VMI and fine motor skills, and the volumes of the cerebellum and the brainstem correlated positively to fine motor skills. Total cortical grey matter volume showed a negative correlation with fine motor skills.
The group scores for the children included in this study were below the test norms on both VMI and fine motor skills, which was in line with previous studies where children born preterm have consistently shown poorer VMI performance and fine motor skills compared with children born at term.7 44 However, the lower group performance is still considered to be within low average range. Within this relatively well-functioning group of children born EPT, we found a small, but statistically significant, difference between the sexes with regards to VMI performance, with girls outperforming boys. Sex differences in VMI performance have consistently been reported in children born preterm7 and MRI studies of children born preterm have revealed differences between the sexes in brain volumes and the microstructure of the brain,39 45 46 indicating altered early development of the brain in boys born preterm. This indicates that the brain of preterm born boys develops in an altered way compared with preterm born girls, affecting both the structure and the function of the brain.
Cerebellar underdevelopment, with reductions in cerebellar volume, and sustained white matter injuries in children born preterm have been suggested as possible mechanisms for poor visuomotor function,9 23 47 although many neural networks comprised of other neural structures have also been proposed to be involved in VMI including the visual, motor, sensory, salience and default mode networks, the subcortical regions and the brain stem.21 48 Even though this study is the first, to our knowledge, to explore the associations between brain volumes at term-equivalent age and VMI at 6½ years in a cohort of children born EPT, there have been previous reports of associations between brain volumes and VMI performance in more mature preterm children. In these reports, positive correlations were seen between the growth rate of the caudate and the thalamus during the neonatal period and VMI scores at 4 years of age,24 and between thalamic and cerebellar white matter volumes and VMI scores at 15 years of age.22 We did not find these associations in our cohort and this finding was in line with a previous study that evaluated 8-year-old children born preterm.49 One explanation for these different results could be individual childhood growth trajectories and different gestational ages in the various study cohorts. Additional studies using inductive (exploration) and deductive (hypotheses driven) approaches will be useful in determining the relationships between VMI and neural structure.
VMI performance is dependent on fine motor skills. And, fine motor skills have been shown to be a facilitator of visuomotor function even though the causality of this mediation is not known.47 The present study revealed positive correlations between better fine motor skills and larger volumes of the precentral gyrus, the cerebellum and the brainstem—motor areas of the brain that have been reported to be involved in fine motor skills.50 The precentral gyrus, which is located in the frontal lobe, is the origin of the corticospinal tracts known to affect motor functions. A smaller precentral gyrus could, therefore, be expected to be correlated with reduced motor performance and also to diminished VMI performance, in line with our findings, since fine motor skills have been shown to be a mediator of visuomotor function, even though the cause of this mediation is not known.47 Both the cerebellum and the brain stem are important for fine motor skills and VMI, as well as for other tasks that rely on the motor system,48 and our results support their important role in this cross-modal function. In addition, the cerebellum has long been known to be involved in movement modulation, balance and coordination, and there is now evidence that it is also important for a number of cognitive functions, including visuospatial attention.51 Finally, cerebellar growth has been shown to relate to visual perception at school age in children born very preterm52 and specific subregional volumes of the cerebellum have been shown to be related to VMI and influenced by other perinatal factors, such as pain and infections.52 Taken together, the literature suggests that neurocorrelates for fine motor skills are important for VMI performance as well, whether the neurocorrelates are contributing to VMI performance directly, or indirectly, through fine motor skills. The current findings suggest that the brain growth of the precentral gyrus, cerebellum and brainstem have already altered during the neonatal period and that this affects functions such as VMI and fine motor skills.
We were surprised to find a negative correlation between cortical grey matter volume and fine motor skills in our cohort, despite the fact that Keunen et al20 reported a similar finding. Those authors also reported an inverse relationship between cortical grey matter volume and fine motor function when children who were born preterm reached 2½ years. Measuring the cortical grey volume depends on the segmentation methods used to separate grey matter from the cerebellum which could make the classifying cortical grey matter volume imprecise. It is also possible that atypical patterns of brain development play a role in the grey matter volume and fine motor skills relationship, in the preterm brain.16
A strength of this study is its population-based prospective cohort study design. The study focused on children born EPT without major brain lesions, which meant that our cohort was relatively healthy. This was reflected in their average range performances on both the VMI and the fine motor skills tests, with a mean of only −0.5 SD below the norms, which is higher than previously reported in preterm populations. This study also has several limitations. We had to exclude many children from the morphometric analyses, due to the strict criteria for data quality, and this was a reflection of the well-known difficulties in processing neonatal MRI images in children born EPT. This left us with a relatively small sample size, although the children with morphometric data were largely representative of the whole cohort. This was due to rigorous entry and data quality criteria, as well as implicit methodological difficulties related to the scanning of preterm infants. Scanning preterm neonates is considered a challenging task due to their immature physiology and anatomy. Patient motion may occur more often thus patient preparation and image protocols should be modified and be dedicated for neonates. To minimise this limitation the development of novel pulse sequences to increase the speed of image acquisition, and MRI coils tailored to the head size of the subject, would have the potential to further increase success.53
Segmentation of cerebral tissues at term-equivalent age in children who were born extremely preterm is challenging due to the characteristics of the developing preterm brain. The segmentation can be limited in small structures of the brain since the volumes are smaller and there is also a lower signal-to-noise ratio in preterm children. To minimise this we used only high quality MRI. To guide segmentation we used a larger number of tissue probability maps from preterms35 with an extraclass tissue map for background, to provide a better modelling of the cerebrospinal fluid and other non-brain voxels and also ta aid further tissue classification. VMI was assessed with the main Beery’s VMI test, but did not include the supplementary tests of visual perception and motor coordination which could have enabled us to distinguish between visual perception and fine motor function with regard to the VMI function. We did not adjust for any diagnosis of autism or ADHD, which are reported to be common in children born extremely preterm54 and have been linked to altered brain development in preterms36 55 Finally, we were not able to include a control group of children born at term.
Conclusion
In summary, this study found positive correlations between VMI performance, fine motor skills and brain volumes at term-equivalent age in regions that were already known to be involved in these functions. To our knowledge, this is the first study to examine the relationship between neonatal brain volumes and VMI and fine motor skills at the age of 6½ years in a population-based cohort of children born EPT. Further studies including larger sample sizes are needed to confirm our results, to explore the relationships between the underlying visual and motor modalities and VMI in greater depth, and to examine the potential use of early regional brain volumes as imaging biomarkers related to VMI and fine motor skills. Since it has been reported that early interventions can improve VMI in general school populations56 and in children born preterm,57 a possibility of early identification of children at risk could diminish the impact of preterm birth on these functions.
Supplementary Material
Acknowledgments
We sincerely thank all the participating children and parents for making this study possible. We want to thank our research nurse Lena Swartling-Schlinzig and our psychologist Eva Eklöf, the NeoBIG group and the EXPRESS group.
Footnotes
Contributors: JB and NP designed the study, collected data, performed analyses, interpreted the data, drafted and revised the manuscript, and approved the final version. LB, LF and KH collected data, interpreted data, revised the manuscript and approved the final version. UÅ designed the study, collected data, interpreted the data, revised the manuscript and approved the final version.
Funding: This work was supported by the Swedish Medical Research Council (grant numbers 523-2011-3981), the regional agreement on medical training and clinical between Stockholm County Council and the Karolinska Institutet (grant number ALF-20140316, 20100646 and 20150876), the Marianne and Marcus Wallenberg foundation (grant number 2011.0085), the Swedish Order of Freemasons in Stockholm, the Swedish Medical Society, the Swedish Brain Foundation (grant number FP2017-0131), Linnéa and Josef Carlssons Stiftelse, Sachs’ Children and Youth Hospital, Stiftelsen Samariten, H.K.H. Kronprinsessan Lovisas Förening för barnasjukvård, Sällskapet Barnavård, the Sigvard & Marianne Bernadotte Research Foundation for Children Eye Care and the Märta and Gunnar Philipson foundation. UÅ was supported by Stockholm County Council (clinical research appointment).
Disclaimer: The study sponsors had no role in the study design, collection, analysis or interpretation of data, writing the report or the decision to submit the article for publication.
Competing interests: None declared.
Patient consent: Detail has been removed from this case description/these case descriptions to ensure anonymity. The editors and reviewers have seen the detailed information available and are satisfied that the information backs up the case the authors are making.
Ethics approval: The study was approved by the local ethics committees in Stockholm and Lund (Regionala etikprövningsnämnden in Stockholm, dnr 04-889/2, 2006/1217–32 and 2010/850-31/1; Regionala etikprövningsnämnden in Lund, dnr 42/2004, dnr 2009/9 and dnr 2016/104). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Any requests for data sharing should be addressed to author UÅ.
References
- 1.Aylward GP. Neurodevelopmental outcomes of infants born prematurely. J Dev Behav Pediatr 2005;26:427–40. 10.1097/00004703-200512000-00008 [DOI] [PubMed] [Google Scholar]
- 2.Daly CJ, Kelley GT, Krauss A. Relationship between visual-motor integration and handwriting skills of children in kindergarten: a modified replication study. Am J Occup Ther 2003;57:459–62. 10.5014/ajot.57.4.459 [DOI] [PubMed] [Google Scholar]
- 3.Volman MJ, van Schendel BM, Jongmans MJ. Handwriting difficulties in primary school children: a search for underlying mechanisms. Am J Occup Ther 2006;60:451–60. 10.5014/ajot.60.4.451 [DOI] [PubMed] [Google Scholar]
- 4.Weil MJ, Amundson SJ. Relationship between visuomotor and handwriting skills of children in kindergarten. Am J Occup Ther 1994;48:982–8. 10.5014/ajot.48.11.982 [DOI] [PubMed] [Google Scholar]
- 5.Sortor JM, Kulp MT. Are the results of the beery-buktenica developmental test of visual-motor integration and its subtests related to achievement test scores? Optom Vis Sci 2003;80:758–63. 10.1097/00006324-200311000-00013 [DOI] [PubMed] [Google Scholar]
- 6.Vuijk PJ, Hartman E, Mombarg R, et al. Associations between academic and motor performance in a heterogeneous sample of children with learning disabilities. J Learn Disabil 2011;44:276–82. 10.1177/0022219410378446 [DOI] [PubMed] [Google Scholar]
- 7.Geldof CJ, van Wassenaer AG, de Kieviet JF, et al. Visual perception and visual-motor integration in very preterm and/or very low birth weight children: a meta-analysis. Res Dev Disabil 2012;33:726–36. 10.1016/j.ridd.2011.08.025 [DOI] [PubMed] [Google Scholar]
- 8.Williams J, Lee KJ, Anderson PJ. Prevalence of motor-skill impairment in preterm children who do not develop cerebral palsy: a systematic review. Dev Med Child Neurol 2010;52:232–7. 10.1111/j.1469-8749.2009.03544.x [DOI] [PubMed] [Google Scholar]
- 9.Van Braeckel KN, Taylor HG. Visuospatial and visuomotor deficits in preterm children: the involvement of cerebellar dysfunctioning. Dev Med Child Neurol 2013;55(Suppl 4):19–22. 10.1111/dmcn.12301 [DOI] [PubMed] [Google Scholar]
- 10.Setänen S, Lehtonen L, Parkkola R, et al. The motor profile of preterm infants at 11 y of age. Pediatr Res 2016;80:389–94. 10.1038/pr.2016.90 [DOI] [PubMed] [Google Scholar]
- 11.Wong T, Taylor HG, Klein N, et al. Kindergarten classroom functioning of extremely preterm/extremely low birth weight children. Early Hum Dev 2014;90:907–14. 10.1016/j.earlhumdev.2014.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Twilhaar ES, de Kieviet JF, Aarnoudse-Moens CS, et al. Academic performance of children born preterm: a meta-analysis and meta-regression. Arch Dis Child Fetal Neonatal Ed 2017. doi: 10.1136/fetalneonatal-2017-312916. [Epub ahead of print 30 Aug 2017] 10.1136/archdischild-2017-312916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson S, Hennessy E, Smith R, et al. Academic attainment and special educational needs in extremely preterm children at 11 years of age: the EPICure study. Arch Dis Child Fetal Neonatal Ed 2009;94:F283–9. 10.1136/adc.2008.152793 [DOI] [PubMed] [Google Scholar]
- 14.Bonifacci P. Children with low motor ability have lower visual-motor integration ability but unaffected perceptual skills. Hum Mov Sci 2004;23:157–68. 10.1016/j.humov.2004.08.002 [DOI] [PubMed] [Google Scholar]
- 15.Goyen TA, Lui K, Woods R. Visual-motor, visual-perceptual, and fine motor outcomes in very-low-birthweight children at 5 years. Dev Med Child Neurol 1998;40:76–81. 10.1111/j.1469-8749.1998.tb15365.x [DOI] [PubMed] [Google Scholar]
- 16.Haartsen R, Jones EJH, Johnson MH. Human brain development over the early years. Curr Opin Behav Sci 2016;10:149–54. 10.1016/j.cobeha.2016.05.015 [DOI] [Google Scholar]
- 17.Padilla N, Alexandrou G, Blennow M, et al. Brain Growth Gains and Losses in Extremely Preterm Infants at Term. Cereb Cortex 2015;25:1897–905. 10.1093/cercor/bht431 [DOI] [PubMed] [Google Scholar]
- 18.Monson BB, Anderson PJ, Matthews LG, et al. Examination of the Pattern of Growth of Cerebral Tissue Volumes From Hospital Discharge to Early Childhood in Very Preterm Infants. JAMA Pediatr 2016;170:772–9. 10.1001/jamapediatrics.2016.0781 [DOI] [PubMed] [Google Scholar]
- 19.Loh WY, Anderson PJ, Cheong JLY, et al. Neonatal basal ganglia and thalamic volumes: very preterm birth and 7-year neurodevelopmental outcomes. Pediatr Res 2017;82:970–8. 10.1038/pr.2017.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keunen K, Išgum I, van Kooij BJ, et al. Brain Volumes at Term-Equivalent Age in Preterm Infants: Imaging Biomarkers for Neurodevelopmental Outcome through Early School Age. J Pediatr 2016;172:88–95. 10.1016/j.jpeds.2015.12.023 [DOI] [PubMed] [Google Scholar]
- 21.Sepulcre J. Integration of visual and motor functional streams in the human brain. Neurosci Lett 2014;567:68–73. 10.1016/j.neulet.2014.03.050 [DOI] [PubMed] [Google Scholar]
- 22.Martinussen M, Flanders DW, Fischl B, et al. Segmental brain volumes and cognitive and perceptual correlates in 15-year-old adolescents with low birth weight. J Pediatr 2009;155:848–53. 10.1016/j.jpeds.2009.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sripada K, Løhaugen GC, Eikenes L, et al. Visual-motor deficits relate to altered gray and white matter in young adults born preterm with very low birth weight. Neuroimage 2015;109:493–504. 10.1016/j.neuroimage.2015.01.019 [DOI] [PubMed] [Google Scholar]
- 24.Young JM, Powell TL, Morgan BR, et al. Deep grey matter growth predicts neurodevelopmental outcomes in very preterm children. Neuroimage 2015;111:360–8. 10.1016/j.neuroimage.2015.02.030 [DOI] [PubMed] [Google Scholar]
- 25.Serenius F, Ewald U, Farooqi A, et al. Neurodevelopmental Outcomes Among Extremely Preterm Infants 6.5 Years After Active Perinatal Care in Sweden. JAMA Pediatr 2016;170:954 10.1001/jamapediatrics.2016.1210 [DOI] [PubMed] [Google Scholar]
- 26.Serenius F, Källén K, Blennow M, et al. Neurodevelopmental outcome in extremely preterm infants at 2.5 years after active perinatal care in Sweden. JAMA 2013;309:1810–20. 10.1001/jama.2013.3786 [DOI] [PubMed] [Google Scholar]
- 27.Inder TE, Wells SJ, Mogridge NB, et al. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J Pediatr 2003;143:171–9. 10.1067/S0022-3476(03)00357-3 [DOI] [PubMed] [Google Scholar]
- 28.Surveillance of Cerebral Palsy in Europe. Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Surveillance of Cerebral Palsy in Europe (SCPE). Dev Med Child Neurol 2000;42:816–24. [DOI] [PubMed] [Google Scholar]
- 29.Jones JG, MacKinnon B, Good WV, et al. The early treatment for ROP (ETROP) randomized trial: study results and nursing care adaptations. Insight 2005;30:7–13. [PubMed] [Google Scholar]
- 30.Bna BKE. The Beery-Buktenica developmental test of visual motor integration: administration, scoring and teaching manual. 5th ed Minneapolis: NCS Pearson Inc, 2006. [Google Scholar]
- 31.Henderson SE SD, Barnett AL. Movement assessment battery for children-2 second edition [Movement ABC-2]. London, UK: The Psychological Corporation 2007. [Google Scholar]
- 32.Hyvärinen L, Näsänen R, Laurinen P. New visual acuity test for pre-school children. Acta Ophthalmol 1980;58:507–11. 10.1111/j.1755-3768.1980.tb08291.x [DOI] [PubMed] [Google Scholar]
- 33.Skiöld B, Horsch S, Hallberg B, et al. White matter changes in extremely preterm infants, a population-based diffusion tensor imaging study. Acta Paediatr 2010;99:842–9. 10.1111/j.1651-2227.2009.01634.x [DOI] [PubMed] [Google Scholar]
- 34.Shi F, Yap PT, Wu G, et al. Infant brain atlases from neonates to 1- and 2-year-olds. PLoS One 2011;6:e18746 10.1371/journal.pone.0018746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuklisova-Murgasova M, Aljabar P, Srinivasan L, et al. A dynamic 4D probabilistic atlas of the developing brain. Neuroimage 2011;54:2750–63. 10.1016/j.neuroimage.2010.10.019 [DOI] [PubMed] [Google Scholar]
- 36.Padilla N, Eklöf E, Mårtensson GE, et al. Poor Brain Growth in Extremely Preterm Neonates Long Before the Onset of Autism Spectrum Disorder Symptoms. Cereb Cortex 2015;16:bhv300 10.1093/cercor/bhv300 [DOI] [PubMed] [Google Scholar]
- 37.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage 2007;38:95–113. 10.1016/j.neuroimage.2007.07.007 [DOI] [PubMed] [Google Scholar]
- 38.Pernet C, Andersson J, Paulesu E, et al. When all hypotheses are right: a multifocal account of dyslexia. Hum Brain Mapp 2009;30:2278–92. 10.1002/hbm.20670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skiöld B, Alexandrou G, Padilla N, et al. Sex differences in outcome and associations with neonatal brain morphology in extremely preterm children. J Pediatr 2014;164:1012–8. 10.1016/j.jpeds.2013.12.051 [DOI] [PubMed] [Google Scholar]
- 40.Keunen K, Kersbergen KJ, Groenendaal F, et al. Brain tissue volumes in preterm infants: prematurity, perinatal risk factors and neurodevelopmental outcome: a systematic review. J Matern Fetal Neonatal Med 2012;25(Suppl 1):89–100. 10.3109/14767058.2012.664343 [DOI] [PubMed] [Google Scholar]
- 41.Perneger TV. What’s wrong with Bonferroni adjustments. BMJ 1998;316:1236–8. 10.1136/bmj.316.7139.1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990;1:43–6. 10.1097/00001648-199001000-00010 [DOI] [PubMed] [Google Scholar]
- 43.Nakagawa S. A farewell to Bonferroni: the problems of low statistical power and publication bias. Behavioral Ecology 2004;15:1044–5. 10.1093/beheco/arh107 [DOI] [Google Scholar]
- 44.de Kieviet JF, Piek JP, Aarnoudse-Moens CS, et al. Motor development in very preterm and very low-birth-weight children from birth to adolescence: a meta-analysis. JAMA 2009;302:2235–42. 10.1001/jama.2009.1708 [DOI] [PubMed] [Google Scholar]
- 45.Kesler SR, Reiss AL, Vohr B, et al. Brain volume reductions within multiple cognitive systems in male preterm children at age twelve. J Pediatr 2008;152:513–20. 10.1016/j.jpeds.2007.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Constable RT, Ment LR, Vohr BR, et al. Prematurely born children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: an investigation of group and gender effects. Pediatrics 2008;121:306–16. 10.1542/peds.2007-0414 [DOI] [PubMed] [Google Scholar]
- 47.Thomas AR, Lacadie C, Vohr B, et al. Fine Motor Skill Mediates Visual Memory Ability with Microstructural Neuro-correlates in Cerebellar Peduncles in Prematurely Born Adolescents. Cereb Cortex 201714 10.1093/cercor/bhw415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Braddick O, Atkinson J. Development of human visual function. Vision Res 2011;51:1588–609. 10.1016/j.visres.2011.02.018 [DOI] [PubMed] [Google Scholar]
- 49.Peterson BS, Vohr B, Staib LH, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA 2000;284:1939–47. 10.1001/jama.284.15.1939 [DOI] [PubMed] [Google Scholar]
- 50.Holmström L, de Manzano O, Vollmer B, et al. Dissociation of brain areas associated with force production and stabilization during manipulation of unstable objects. Exp Brain Res 2011;215:359–67. 10.1007/s00221-011-2903-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noroozian M. The role of the cerebellum in cognition: beyond coordination in the central nervous system. Neurol Clin 2014;32:1081–104. 10.1016/j.ncl.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 52.Ranger M, Zwicker JG, Chau CM, et al. Neonatal Pain and Infection Relate to Smaller Cerebellum in Very Preterm Children at School Age. J Pediatr 2015;167:292–8. 10.1016/j.jpeds.2015.04.055 [DOI] [PubMed] [Google Scholar]
- 53.Smyser CD, Kidokoro H, Inder TE. Magnetic resonance imaging of the brain at term equivalent age in extremely premature neonates: to scan or not to scan? J Paediatr Child Health 2012;48:794–800. 10.1111/j.1440-1754.2012.02535.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D’Onofrio BM, Class QA, Rickert ME, et al. Preterm birth and mortality and morbidity: a population-based quasi-experimental study. JAMA Psychiatry 2013;70:1231–40. 10.1001/jamapsychiatry.2013.2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bora S, Pritchard VE, Chen Z, et al. Neonatal cerebral morphometry and later risk of persistent inattention/hyperactivity in children born very preterm. J Child Psychol Psychiatry 2014;55:828–38. 10.1111/jcpp.12200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ohl AM, Graze H, Weber K, et al. Effectiveness of a 10-week tier-1 response to intervention program in improving fine motor and visual-motor skills in general education kindergarten students. Am J Occup Ther 2013;67:507–14. 10.5014/ajot.2013.008110 [DOI] [PubMed] [Google Scholar]
- 57.Van Hus JW, Jeukens-Visser M, Koldewijn K, et al. Sustained developmental effects of the infant behavioral assessment and intervention program in very low birth weight infants at 5.5 years corrected age. J Pediatr 2013;162:1112–9. 10.1016/j.jpeds.2012.11.078 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-020478supp001.jpg (1,016KB, jpg)
bmjopen-2017-020478supp002.pdf (18.5KB, pdf)
bmjopen-2017-020478supp003.pdf (18.4KB, pdf)
bmjopen-2017-020478supp004.pdf (29.8KB, pdf)