Abstract
Cache Valley virus, an orthobunyavirus, is an important cause of ovine neonatal malformations. Information on the seroprevalence of this virus in Saskatchewan livestock populations is lacking. The objectives of this study were to determine the seroprevalence of Cache Valley virus and closely related viruses in sheep, cattle, goats, horses, and mule deer in Saskatchewan by performing a plaque-reduction neutralization test using Cache Valley virus. In total, sera from 130 sheep from 50 flocks were tested. Seroprevalence in sheep was 64.6% (84/130) and 94.0% (47/50) of flocks had 1 or more seropositive sheep. Antibodies to Cache Valley virus or closely related viruses were also detected in serum samples collected from cattle, goats, horses, and mule deer with seroprevalences of 20.0% (5/25), 33.3% (8/24), 69.0% (40/58), and 50.8% (33/65), respectively. These results suggest widespread exposure to Cache Valley virus or closely related viruses in domestic animals and mule deer in Saskatchewan.
Résumé
Séroprevalence du virus de la Vallée Cache ou de virus connexes chez les moutons et d’autres animaux de cheptel en Saskatchewan, Canada. Le virus de la Vallée Cache, un orthobunyavirus, est une cause importante de malformations néonatales ovines. Il manque des renseignements sur la séroprévalence de ce virus dans les populations des cheptels de la Saskatchewan. Les objectifs de cette étude consistaient à déterminer la séroprévalence du virus de la Vallée Cache et des virus étroitement apparentés chez les moutons, les bovins, les chèvres, les chevaux et les cerfs mulets en Saskatchewan en réalisant un test de séro-neutralisation par réduction des plages en utilisant le virus de la Vallée Cache. Au total, le sérum provenant de 130 moutons dans 50 troupeaux a été testé. Chez les moutons, la séroprévalence était de 64,6 % (84/130) et 94,0 % (47/50) des troupeaux avaient un mouton ou plusieurs moutons séropositifs. Les anticorps pour le virus de la Vallée Cache ou les virus étroitement apparentés ont aussi été détectés dans les échantillons de sérum prélevés auprès des bovins, des chèvres, des chevaux et des cerfs mulets avec une séroprévalence de 20,0 % (5/25), de 33,3 % (8/24), de 69,0 % (40/58) et de 50,8 % (33/65), respectivement. Ces résultats suggèrent une vaste exposition au virus de la Vallée Cache ou à des virus étroitement apparentés chez les animaux domestiques et les cerfs mulets en Saskatchewan.
(Traduit par Isabelle Vallières)
Introduction
Cache Valley virus (CVV), an arbovirus belonging to the genus Orthobunyavirus (family Bunyaviridae, serogroup Bunyamwera (BUN)), was first isolated from mosquitoes in the Cache Valley, Utah, USA in 1956. This virus has since been isolated from caribou, horses, sheep, cattle, and other domestic and wild animals and is considered endemic in North America, the Caribbean, and Argentina (1–3). The virus is transmitted through Aedes and other non-Culex mosquitoes and is considered an indirect zoonosis in humans, in whom infection occurs through mosquito bites but not through direct contact with infected domestic or wild animals. Although other viruses belonging to the Bunyamwera serogroup occur in the United States, such as the Potosi virus, Main Drain virus, Lokern virus, Tensaw virus, and Northway virus, in terms of its impact on animal health, CVV is considered the most important in the USA (4). In humans, a diagnosis is rarely made, but limited surveys indicate that the seroprevalence may be as high as 18% (5–8). To date, only 3 cases of severe CVV disease have been reported in humans (5,9,10).
Clinically, CVV is of most significance in the sheep industry in which infection of pregnant ewes can result in pregnancy failure or fetal malformations and, therefore, significant economic losses (11). Infection in non-pregnant sheep tends to be subclinical, and there is 1 report of CVV in a clinically ill ram (12). Overall, reproductive losses are amongst the most significant economic burden to a sheep enterprise and there are no vaccinations or treatments available for CVV in sheep. Despite its importance to the sheep industry, systematic prevalence investigations are few and some of the available data are in part limited due to non-random sampling or small sample sizes. Recent investigations found a seroprevalence of 0.01% in Montana (13) and 28.3% in 22 states in the USA (4), while older surveys from Texas and Maryland reported seroprevalences of 19.1% (14) and 27.2% (6), respectively.
In Canada, the first definitive report of Cache Valley virus isolation from mosquitoes was made in Saskatchewan in 1973 (15). It has since been detected in mosquitoes from Alberta (16), Ontario (17), and Manitoba (18). Human disease due to CVV has not been definitively reported in Canada, although seroprevalence in West Nile Virus-suspect persons tested in Manitoba and Saskatchewan ranged from 5% to 16% (8). Furthermore, in a prioritization exercise conducted by provincial and territorial public stakeholders, the virus was identified as having a high risk of emergence in Canada (19). Information is scarce with regard to the prevalence or incidence of CVV in Canadian livestock. In 2012, Shapiro et al (20) for the first time reported CVV infections in malformed lambs from 2 flocks in Ontario and, in 2013, the virus was isolated from a flock in Quebec which presented with fetal malformations (21). Most recently, ovine fetal malformations appeared to have increased in Ontario during the 2015/16 lambing season, although the reasons for this emergence were unclear (22).
There is no information on the prevalence of orthobunyaviruses such as CVV in livestock, particularly sheep, in western Canada. In light of this, the primary objective of this study was to assay sera from sheep in Saskatchewan by a plaque neutralization reduction test (PRNT) using CVV in order to detect antibodies to CVV and closely related viruses. A secondary objective was to report PRNT serology results for CVV and closely related viruses from other domestic (cattle, goats, horses) and wild (mule deer) animals conveniently sampled in Saskatchewan.
Materials and methods
Sample population
A random number generator was used to select 130 serum samples for testing for antibodies against CVV and closely related viruses in sheep from an existing serum bank containing 2041 sheep sera from 68 flocks across Saskatchewan. Sample size was a function of logistic and financial constraints. This serum bank was established as part of a serosurveillance study for Maedi-visna in Saskatchewan sheep (23). Briefly, 75 meat and dairy sheep flocks from Saskatchewan were selected through simple random sampling. In each flock, serum samples were collected from 30 sheep. Private veterinarians collected the samples after consulting with flock owners but all sampling costs were paid for by the study.
All 980 flocks registered with the Saskatchewan Sheep Development Board as of December 2013 were eligible for inclusion in the study (23). Flock owners were contacted sequentially according to a list produced with a random number generator until the target of 75 participants was met. Producers with at least 30 sheep were included. In each flock, 30 sheep (rams and ewes; 2 y of age or older) were conveniently sampled by jugular venipuncture using standard techniques. Flocks were visited once between May 1, 2013 and May 31, 2014.
In addition to the systematic sampling of sheep serum, convenience samples of bovine (n = 25), caprine (n = 24), and equine (n = 58) sera from Saskatchewan were tested for the presence of CVV antibody in samples submitted to Prairie Diagnostic Services (PDS), Saskatoon, Saskatchewan, in 2012. Mule deer serum samples (n = 65) collected from southwestern Saskatchewan by the Canadian Cooperative Wildlife Health Centre from 2007 to 2011 were also analyzed. These samples had been collected for other studies or reasons unrelated to CVV.
Laboratory analysis
Sheep serum samples were submitted to PDS for testing as part of the Maedi-visna surveillance program. Aliquots of serum were stored at –20°C until they were forwarded to the National Microbiology Laboratory (NML), Winnipeg, Manitoba, for tests for CVV and closely related viruses antibodies. Bovine, caprine, and equine serum samples submitted to the laboratory for routine diagnostic testing were similarly stored and then forwarded.
To identify serum samples with specific antibodies against CVV or closely related viruses, a CVV-specific PRNT, which may exhibit some cross-reactivity to antibodies against related BUN-serogroup viruses, was performed as previously described (24). Briefly, for virus neutralization, several dilutions of test sera were placed in an incubator with a constant number of plaque forming units (PFU) of CVV for 1 h at 37°C in 5% CO2. Six-well plates with Vero E6 cell monolayers were then used for further incubation of each aliquot, again for 1 h at 37°C. A nutrient agar overlay was applied and the plates were incubated at 37°C in 5% CO2 for approximately 3 d. To demonstrate plaque formation, a vital stain (neutral red) was spread over the plates. A serum sample was considered positive for viral antibodies if at least 90% of possible plaque formation relative to virus controls was inhibited. For sheep sera, the titration endpoint was the highest serum diluation with a plaque reduction of at least 90%. The PRNT results were considered positive if the neutralizing antibody titre was ≥ 1:20. Further titrations were not performed for other livestock sera.
Statistical analysis
The overall seroprevalence and 95% confidence interval (CI) for CVV or closely related viruses for the sheep samples were estimated taking into account the probability for random flock selection within Saskatchewan and the probability for a sheep being sampled within each flock. Flock-level prevalence and 95% CI were calculated. Summary statistics were generated and descriptive statistics for number of animals tested per flock and flock sizes were depicted by median and interquartile range (IQR) due to nonparametric distribution of these variables. Percent positive and 95% CI were calculated for bovine, caprine, equine, and mule deer samples. The convenience sampling structure in these populations precluded calculations of sampling weights and simple proportions and 95% CI were calculated. A map was generated depicting the location of seropositive flocks in Saskatchewan. The information on location for the bovine, caprine, and equine samples was insufficient to allow the generation of a map. Deer samples all originated from within 1 rural municipality in southwestern Saskatchewan. All analyses were conducted using Stata IC 13.1 (StataCorp, College Station, Texas, USA).
Results
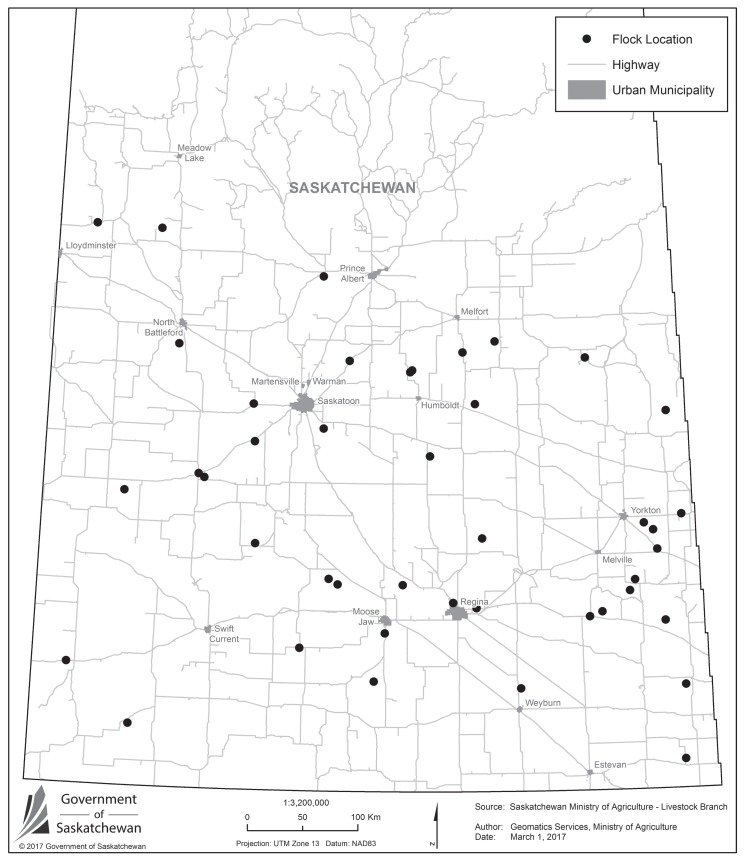
In total, 130 sheep sera were tested from 50 flocks across Saskatchewan; 84 sera tested positive for CVV or closely related antibodies. The PRNT titers for the seropositive sheep were as follows: > 80 (n = 55), 80 (n = 9), 40 (n = 13), 20 (n = 7). Overall sheep-level seroprevalence was 64.6% (95% CI: 51.3% to 72.2%). Of the flocks sampled, 94.0% (47/50; 95% CI: 82.4% to 98.1%) had at least 1 seropositive sheep. The number of randomly tested samples per flock ranged from 1 to 7 (median: 3; IQR: 2 to 6) and flock size ranged from 36 to 1004 (median: 92.5; IQR: 62 to 263) adult sheep (> 2 y old at the time of sampling). Flock size was not recorded in 4 instances. Figure 1 shows the locations of seropositive flocks in Saskatchewan and indicates widespread exposures to the virus in the areas from which sheep sera were tested.
Figure 1.
Map of Saskatchewan depicting the locations of sheep flocks seropositive for Cache Valley virus or closely related viruses (n = 47).
Table 1 shows the number of samples, number of sampling sites, and percent positive samples from cattle, goats, horses and mule deer. All 24 goat samples were collected at 1 production site, while the 25 cattle samples came from 20 farms (Table 1). Each of the 58 horse samples originated from a different site (Table 1). The 65 mule deer serum samples were obtained from 1 rural municipality in Saskatchewan (Table 1). Antibodies against Cache Valley virus or closely related viruses were detected in the sera of all species, with the highest seroprevalence found in the horse population (60.0%).
Table 1.
Number and proportion of serum samples from domestic and wild animals conveniently sampled in Saskatchewan that were positive for Cache Valley virus or closely related viruses.
| Species | Number of samples | Number of sampling sites | Number of positive samples (%; 95% CI) |
|---|---|---|---|
| Bovine | 25 | 20 | 5 (20.0; 8.0 to 41.7) |
| Caprine | 24 | 1 | 8 (33.3; 16.7 to 55.5) |
| Equine | 58 | 58 | 40 (69.0; 55.6 to 79.8) |
| Mule deer | 65 | 65 | 33 (50.8; 38.5 to 63.0) |
Most of the seropositive goats (7/8), cattle (3/5), horses (30/40), and mule deer (21/33) had CVV PRNT titers > 20.
Discussion
Documentation about CVV in sheep in Canada first became available in 2012 and 2013 when causes of malformed lambs were investigated in Ontario and Quebec (20,21); however, there has not previously been any information published on the seroprevalence of CVV (or closely related viruses) in sheep or other animal species in Canada. Therefore, this is the first study to report results from a seroprevalence survey of CVV or closely related viruses in sheep and other domestic and wild animals in western Canada.
Information on CVV seroprevalence in sheep worldwide is scarce. Compared to the limited available literature from North America, however, seroprevalence of CVV (or closely related viruses) was high (64.6%) in the sheep tested in this study. In Montana, only 1 of 104 sheep was seropositive for CVV (13), while 28.0% of 5150 sheep tested positive for CVV in a cross-sectional, multi-state study in the USA (4). In Texas, 19.1% of 366 sheep and 34 of 50 flocks had CVV-specific antibodies (14), while 12.9% of 31 sheep in the Yucatan Peninsula of Mexico tested positive (25). Although differences in sampling strategies and analyses may hamper direct comparison of prevalence data among studies, other factors must be considered such as flock management, environmental conditions, and regional differences influencing populations of transmitting mosquitoes and virus activity. Meyers et al (4) collected information on potential risk factors for CVV prevalence and found that older sheep and flocks with higher average age had a higher seroprevalence; animals from small (20 to 99) to medium-sized (100 to 499) flocks were significantly more positive compared with large flocks (500+); herded/open-range sheep were less likely to be positive compared to other management groups (fenced-range; pasture; feedlot), and lambing in an enclosed structure with door closed most of the time was associated with significantly higher seroprevalence compared with other management groups. The sampling strategy of the current study did not allow for more detailed analysis and, therefore, similar associations could not be investigated. Based on the high flock-level seroprevalence of 94.0%, differences in herd-level risk factors might not have been detectable. However, the results by Meyers et al (4) highlight important variables that must be considered when comparing CVV seroprevalence data between studies and among flocks. It is also interesting to note that Meyers et al (4) found a significantly higher seroprevalence in sheep from the eastern USA (96.4%) compared to central (53.3%) and western (58.9%) USA. If a similar geographic difference in CVV is present in Canada and, considering that the flock seroprevalence in the current study was 94.0%, the flock seroprevalence in sheep in eastern Canada could be as high as 100%. With regard to distribution within Saskatchewan, Figure 1 demonstrates that CVV circulates widely in southern Saskatchewan.
Antibodies to Cache Valley virus or closely related viruses were also detected from all other domestic animal species tested in this study. These results suggest a widespread high exposure of domestic animals to CVV or closely related viruses in Saskatchewan, although the convenient sampling structure applied to these populations impedes extrapolation of the results to their respective animal populations.
No comparative published literature was found on CVV seroprevalence in livestock in other parts of Canada and few reports from around the world have been published. However, limited comparisons show that the high seroprevalence in horses and the moderate seroprevalence in cattle in this study are similar to results from other investigations. In the USA, Buescher et al (6) first reported on the presence of CVV antibodies in other animals and identified 84/88 horses, 61/118 cattle, 3/13 goats, 6/22 sheep, and 2/10 pigs as testing seropositive. More recently, the distribution of Bunyamwera serogroup viruses in cattle was determined in a multi-state (n = 22) study in the USA (26). Cache Valley virus seroprevalence ranged from 4% to 56% and was present in cattle from 21/22 states. In the Yucatan Peninsula of Mexico, 48/184 (26.1%) horses were seropositive for CVV, while in Michigan, USA, 66.3% of 86 tested horses were CVV seropositive (25,27).
Other orthobunyaviruses from the BUN serogroup, such as Main Drain, Potosi, Lokern virus, Tensaw, and Northway viruses, occur in the USA (4). Of these, only Northway virus in mosquitoes from the Northwest Territories has been reported in Canada (28) and, compared with other BUN viruses, CVV has been predominantly isolated from mosquitoes in Canada (15–17,28,29). However, Bunyamwera serogroup viruses are antigenically closely related and cross-reactions may occur. Therefore, some caution is warranted when interpreting the results herein as they indicate exposure to all BUN serogroup viruses and not just CVV. Most of the sheep PRNT results using CVV, however, resulted in titers > 80 supporting exposure to CVV. Although not performed in this study, CVV-seropositive samples from sheep in Ontario and Quebec, which had given birth to CVV-infected malformed lambs, were tested for cross-reactivity to Potosi, Main Drain, Tensaw, Lokern, and Northway viruses. There was very little evidence of exposure to these BUN viruses based on a 4-fold or greater difference in PRNT titers to CVV (personal communication, M. Drebot, National Disease Laboratory, Winnipeg, Canada) and may further support that CVV exposure is more common than exposure to other BUN-serogroup viruses in sheep in Canada. More caution may be warranted in the interpretation of the titers from cattle, goats, horses, and mule deer as titrations beyond 1:20 were not performed. In addition to BUN serogroup viruses, other orthobunyaviruses, such as California serogroup viruses, circulate widely throughout Canada and have been shown to infect sheep, cattle, and other livestock (30). Therefore, it is possible that livestock may be exposed to different bunyaviruses during their lifetime and antibodies to several different viruses may be documented upon further testing. However, as part of another investigation, a subset of CVV positive sera from sheep and other livestock from the current study were also tested for the presence of antibodies to other bunyaviruses such as California serogroup viruses (e.g., Jamestown Canyon and snowshoe hare viruses) and no cross-reactive antibodies were detected using plaque reduction neutralization assays (personal communication, M. Drebot, National Disease Laboratory, Winnipeg, Canada; data not shown).
While significant economic impacts due to CVV in the sheep industry are characterized by fetal malformations (e.g., arthrogryposis, hydranencephaly), embryonic death, and mummification, severe clinical disease in other livestock and horses appears to be rare (6,11). Calisher and Sever (31) reported the presence of CVV antibodies in cattle that gave birth to calves with arthrogryposis and hydranencephaly in Saskatchewan; however, CVV prevalence in that cattle population was unknown and the association of CVV with those birth defects was unsubstantiated. In the apparent absence of considerable clinical and economic consequences in CVV-exposed domestic animals (other than sheep), the importance in quantifying sero-prevalence in these species may lie primarily in gathering surveillance information to document the risk of exposure and potential illness in humans and sheep.
The primary amplifying vertebrate host for CVV is unknown but white-tailed deer are believed to play a role as a reservoir and amplification host (32,33). Interestingly, there was no significant association with number of CVV isolations from mosquitoes and estimated white-tailed deer densities in a recent study (34). In the current study, only mule deer samples were analyzed, 50% of which contained antibodies against CVV or closely related viruses. Compared with other serological surveys in deer populations, the seroprevalence in the mule deer tested herein was markedly higher (32,33). It is unclear whether this is due to a true higher seroprevalence of CVV or closely related viruses in mule deer in Saskatchewan compared with other deer populations or whether these findings reflect the variations in virus prevalence at the time of testing due to other influencing factors such as geographic region, climate, and environmental conditions. Although mule deer differ from white-tailed deer with regard to their social behaviors and habitats, and, therefore, likely to the risk they may pose as reservoir hosts, this study suggests that further investigation into the role of mule deer in the epidemiology of CVV may be warranted.
A number of studies have addressed the presence and epidemiology of CVV in mosquitoes. In the northeastern USA, CVV isolation varied greatly from year to year irrespective of the concentration of mosquitoes and virus activity was highest during August to September (34). Increased CVV presence in mosquitoes during August to October was also found by Buescher et al (6). Additionally, the virus was found more often during years with above-average rainfall and resulting higher mosquito populations (34). These factors may translate into a higher CVV seroprevalence in domestic and wild animals and increased risk of infection in humans, but this has not been objectively documented to date. However, it was recently hypothesized that similar influences, including warm autumn weather resulting in higher mosquito populations, may have contributed to the increased incidence of clinical CVV in sheep in Ontario (22).
In Canada, the earliest documentation of CVV-positive mosquitoes (primarily Culiseta inornata) was reported in 1986 by Calisher et al (28) from Alberta, Saskatchewan, Manitoba, and Ontario. The presence of CVV was suggested from Alberta in 1968 and was subsequently demonstrated in mosquitoes from Saskatchewan in 1973 and again in 1979 (15,29,35). Based on their findings, Iversen et al (29) suggested that the prairie grasslands in Saskatchewan were enzootic for CVV and, similar to Calisher et al (28), the virus was also most commonly found in C. inornata mosquito species. In eastern Canada (Ontario), the virus was again reported in 1980 (17) and most recently, CVV was confirmed in mosquitoes in Alberta (16). Recent comprehensive information on the presence of CVV in mosquitoes in Canada was not available. However, changing climatic and environmental conditions affecting mosquito populations and habitats, and increasing mobility of humans and animals enhance the risk of virus introduction into naïve populations. Given the changing environmental and climatic landscape and the limited body of knowledge, a comprehensive, longitudinal study assessing flock-level risk factors and presence of CVV in mosquitoes in Canada would contribute to improved surveillance and risk assessments of this zoonotic pathogen.
A clinical diagnosis of Cache Valley virus is rarely made in humans, likely in part due to the lack of specific CVV testing, but limited surveys from the USA and Argentina indicate that the seroprevalence may be as high as 18% (2,5–7). To date, only 3 cases of severe CVV disease have been reported in humans (5,9,10) and, while more information is necessary to determine a potential association, CVV may be linked to macrocephaly in infants (31). In Canada, clinical CVV infection in humans has not been reported, but seroprevalence in West Nile virus-suspect humans tested in Manitoba and Saskatchewan ranged from 5% to 16% (8). Cache Valley virus is not a nationally notifiable disease, but CVV may increasingly become part of a potential differential diagnosis in patients presenting with fever and neurologic signs during the mosquito season.
In conclusion, the seroprevalence of CVV or closely related viruses in selected domestic and wild animals from Saskatchewan, Canada, was high, and seropositive sheep flocks were distributed widely throughout southern Saskatchewan. Our observations indicate that there is the potential for economic losses to the sheep industry due to CVV, in which clinical disease involving lambs can be significant. Although CVV-associated fetal malformations have been reported recently in Ontario and Quebec, the occurrence or the seasonal incidence of deformed lambs due to this virus in western provinces has not been well-documented. Further investigation of suspected CVV cases among sheep flocks in the prairies are justified to more fully define the risks of viral infection. In addition, the high seroprevalence for CVV or closely related viruses in mule deer, which are abundant in western Canada, highlights the importance of further investigating reservoir hosts for CVV in addition to white-tailed deer. Examination of infection rates among mosquito populations in Canada is also warranted to gain a better understanding of CVV epidemiology in this country.
Acknowledgments
The study was supported by funding from the Saskatchewan Agriculture Development Fund. The authors thank the Canadian Wildlife Health Cooperative Centre, Saskatoon, for the mule deer serum samples. The authors also acknowledge the expert technical assistance provided by Kristina Dimitrova and Maya Andonova during the testing of animal sera for CVV antibody. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Holden P, Hess AD. Cache Valley virus, a previously undescribed mosquito-borne agent. Science. 1959;130:1187–1188. doi: 10.1126/science.130.3383.1187. [DOI] [PubMed] [Google Scholar]
- 2.Tauro LB, Almeida FL, Contigiani MS. First detection of human infection by Cache Valley and Kairi viruses (Orthobunyavirus) in Argentina. Trans R Soc Trop Med Hyg. 2009;103:197–199. doi: 10.1016/j.trstmh.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Drebot M. Emerging mosquito-borne bunyaviruses in Canada. Can Commun Dis Rep. 2015;41:117–123. doi: 10.14745/ccdr.v41i06a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyers MT, Bahnson CS, Hanlon M, et al. Management factors associated with operation-level prevalence of antibodies to cache valley virus and other bunyamwera serogroup viruses in sheep in the United States. Vector Borne Zoonotic Dis. 2015;15:683–693. doi: 10.1089/vbz.2015.1810. [DOI] [PubMed] [Google Scholar]
- 5.Campbell GL, Mataczynski JD, Reisdorf ES, et al. Second human case of Cache Valley virus disease. Emerg Infect Dis. 2006;12:854–856. doi: 10.3201/eid1205.051625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buescher EL, Byrne RJ, Clarke GC, et al. Cache Valley virus in the Del Mar Va Peninsula. I. Virologic and serologic evidence of infection. Am J Trop Med Hyg. 1970;19:493–502. doi: 10.4269/ajtmh.1970.19.493. [DOI] [PubMed] [Google Scholar]
- 7.Blitvich BJ, Saiyasombat R, Talavera-Aguilar LG, et al. Orthobunyavirus antibodies in humans, Yucatan Peninsula, Mexico. Emerg Infect Dis. 2012;18:1629–1632. doi: 10.3201/eid1810.120492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimitrova K, Andonova M, Makowski K, et al. Preliminary evidence of Cache Valley virus infections and associated human illness in western Canada in 2009. Can J Infect Dis Med Microbiol. 2011;22:15A. [Google Scholar]
- 9.Sexton DJ, Rollin PE, Breitschwerdt EB, et al. Life-threatening Cache Valley virus infection. N Engl J Med. 1997;336:547–549. doi: 10.1056/NEJM199702203360804. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen NL, Zhao G, Hull R, et al. Cache valley virus in a patient diagnosed with aseptic meningitis. J Clin Microbiol. 2013;51:1966–1969. doi: 10.1128/JCM.00252-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards JF, Livingston CW, Chung SI, Collisson EC. Ovine arthrogryposis and central nervous system malformations associated with in utero Cache Valley virus infection: Spontaneous disease. Vet Pathol. 1989;26:33–39. doi: 10.1177/030098588902600106. [DOI] [PubMed] [Google Scholar]
- 12.McConnell S, Livingston C, Jr, Calisher CH, Crandell RA. Isolations of Cache Valley virus in Texas, 1981. Vet Microbiol. 1987;13:11–18. doi: 10.1016/0378-1135(87)90093-9. [DOI] [PubMed] [Google Scholar]
- 13.Johnson GD, Bahnson CS, Ishii P, et al. Monitoring sheep and Culicoides midges in Montana for evidence of Bunyamwera serogroup virus infection. Vet Rec Open. 2014;1:e000071. doi: 10.1136/vetreco-2014-000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung SI, Livingston CW, Jr, Jones CW, Collisson EW. Cache Valley virus infection in Texas sheep flocks. J Am Vet Med Assoc. 1991;199:337–340. [PubMed] [Google Scholar]
- 15.Burton AN, McLintock J, Francy DB. Isolation of St. Louis encephalitis and Cache Valley viruses from Saskatchewan mosquitoes. Can J Public Health. 1973;64:368–373. [PubMed] [Google Scholar]
- 16.Pabbaraju K, Ho KC, Wong S, et al. Surveillance of mosquito-borne viruses in Alberta using reverse transcription polymerase chain reaction with generic primers. J Med Entomol. 2009;46:640–648. doi: 10.1603/033.046.0332. [DOI] [PubMed] [Google Scholar]
- 17.Thorsen J, Artsob H, Spence L, Surgeoner G, Helson B, Wright R. Virus isolations from mosquitoes in southern Ontario, 1976 and 1977. Can J Microbiol. 1980;26:436–440. doi: 10.1139/m80-072. [DOI] [PubMed] [Google Scholar]
- 18.Makowski K, Dimitrova K, Andonova M, et al. The identification of probable cases of California serogroup virus infections in Manitoba in 2010. Can J Infect Dis Med Microbiol. 2011;22:15A–16A. [Google Scholar]
- 19.Kulkarni MA, Berrang-Ford L, Buck PA, Drebot MA, Lindsay LR, Ogden NH. Major emerging vector-borne zoonotic diseases of public health importance in Canada. Emerg Microbes Infect. 2015;4:e33. doi: 10.1038/emi.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shapiro J, Brooks A, Menzies P, et al. Cache Valley virus identified as a cause of malformed lambs in Ontario. AHL Newsl. 2012;16:15. [Google Scholar]
- 21.Côté G, Fournier D, Leboeuf A. Cas de malformations congénitales chez des ovins causées par le virus de la vallée cache (birth defect cases in sheep caused by Cache Valley virus) [monograph on the Internet] MAPAQ; QC, Canada: 2013. [Last accessed February 20, 2018]. Available from: http://www.cepoq.com/admin/useruploads/files/info_malformations_congenitales.pdf. [Google Scholar]
- 22.Jansen J, Spinato M, Menzies P. Cache Valley virus — A cause of birth defects in Ontario lambs [monograph on the Internet] 2016. [Last accessed February 20, 2018]. Available from: http://oahn.ca/resources/small-ruminants/cache-valley-virus-acause-of-birth-defects-in-ontario-lambs/
- 23.Heinrichs R, Wilkins W, Schroeder G, Campbell J. Prevalence of Maedivisna in Saskatchewan sheep. Can Vet J. 2017;58:183–186. [PMC free article] [PubMed] [Google Scholar]
- 24.Beaty BJ, Calisher CH, Shope RS. Diagnostic procedures for viral, rickettsial and chlamydial infections. Washington, USA: Am Public Health Assoc; 1989. pp. 797–856. [Google Scholar]
- 25.Blitvich BJ, Saiyasombat R, Travassos da Rosa A, et al. Orthobunyaviruses, a common cause of infection of livestock in the Yucatan peninsula of Mexico. Am J Trop Med Hyg. 2012;87:1132–1139. doi: 10.4269/ajtmh.2012.12-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sahu SP, Pedersen DD, Ridpath HD, Ostlund EN, Schmitt BJ, Alstad DA. Serologic survey of cattle in the northeastern and north central United States, Virginia, Alaska, and Hawaii for antibodies to Cache Valley and antigenically related viruses (Bunyamwera serogroup virus) Am J Trop Med Hyg. 2002;67:119–122. doi: 10.4269/ajtmh.2002.67.119. [DOI] [PubMed] [Google Scholar]
- 27.McLean RG, Calisher CH, Parham GL. Isolation of Cache Valley virus and detection of antibody for selected arboviruses in Michigan horses in 1980. Am J Vet Res. 1987;48:1039–1041. [PubMed] [Google Scholar]
- 28.Calisher CH, Francy DB, Smith GC, et al. Distribution of Bunyamwera serogroup viruses in North America, 1956–1984. Am J Trop Med Hyg. 1986;35:429–443. doi: 10.4269/ajtmh.1986.35.429. [DOI] [PubMed] [Google Scholar]
- 29.Iversen JO, Wagner RJ, Leung MK, Hayles LB, McLintock JR. Cache Valley virus: Isolations from mosquitoes in Saskatchewan, 1972–1974. Can J Microbiol. 1979;25:760–764. doi: 10.1139/m79-110. [DOI] [PubMed] [Google Scholar]
- 30.Goff G, Whitney H, Drebot MA. Roles of host species, geographic separation, and isolation in the seroprevalence of Jamestown Canyon and snowshoe hare viruses in Newfoundland. Appl Environ Microbiol. 2012;78:6734–6740. doi: 10.1128/AEM.01351-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calisher CH, Sever JL. Are North American Bunyamwera serogroup viruses etiologic agents of human congenital defects of the central nervous system? Emerg Infect Dis. 1995;1:147–151. doi: 10.3201/eid0104.950409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell GL, Eldridge BF, Hardy JL, Reeves WC, Jessup DA, Presser SB. Prevalence of neutralizing antibodies against California and Bunyamwera serogroup viruses in deer from mountainous areas of California. Am J Trop Med Hyg. 1989;40:428–437. doi: 10.4269/ajtmh.1989.40.428. [DOI] [PubMed] [Google Scholar]
- 33.Neitzel DF, Grimstad PR. Serological evidence of California group and Cache Valley virus infection in Minnesota white-tailed deer. J Wildl Dis. 1991;27:230–237. doi: 10.7589/0090-3558-27.2.230. [DOI] [PubMed] [Google Scholar]
- 34.Andreadis TG, Armstrong PM, Anderson JF, Main AJ. Spatial-temporal analysis of Cache Valley virus (Bunyaviridae: Orthobunyavirus) infection in anopheline and culicine mosquitoes (Diptera: Culicidae) in the northeastern United States, 1997–2012. Vector Borne Zoonotic Dis. 2014;14:763–773. doi: 10.1089/vbz.2014.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall RR, McKiel JA, Brown JH. Isolation of Turlock virus and a member of the Bunyamwera group, probably Cache Valley virus, from Alberta mosquitoes. Can J Public Health. 1968;59:159–160. [PubMed] [Google Scholar]