Congenital tremor, also referred to as dancing pigs (1), shivers (2), or myoclonia congenita (3,4), is a well-recognized, usually sporadic phenomenon described in neonatal piglets (5,6). The condition, first described in the United States by Kinsley (1), is characterized by local or systemic muscle spasms appearing within hours of birth, ranging from mild tremors of the ears, flank, and hind leg, to severe systemic tremors resulting in difficulty standing and walking (5). The rhythmic tremors worsen with excitement but cease with sleep (6). Morbidity and mortality may result from difficulties or an inability to nurse.
Congenital tremor is not specific to any one cause and has been classified according to the presence (Type A) or absence (Type B) of lesions, mainly hypomyelination, in the central nervous system (7). Congenital tremor Type A is subdivided into 5 subtypes, AI to AV, with distinctive causes (6). Type AI is caused by transplacental infection with the pestivirus classical swine fever virus (CSFV) (7), while Types AIII and AIV are inherited, and Type AV is of toxic origin (6–9). Congenital tremor Type AII was considered infectious in origin (5,7,10), and recently a link was established between congenital tremor Type AII and a newly identified pestivirus tentatively named atypical porcine pestivirus (APPV) (11–14).
There is no apparent breed predilection with Congenital tremor Type AII, but the sporadic disease is more frequent in litters of gilts (5). Also, the high prevalence of the disease, with increased mortality and morbidity in piglets, makes it of great clinical interest. Moreover, the clinical resemblance between congenital tremor Type AI (caused by CSFV) and Type AII (caused by APPV), makes it essential to differentiate between the 2 conditions.
Atypical porcine pestivirus has been identified in several countries around the world (11–18), but to the authors’ knowledge, the infection has never been identified in Canada. Here we present the first report of APPV infection in piglets with congenital tremor Type AII in Canada.
Case description
Two 2-day-old, F1 Yorkshire-Landrace piglets from a farrow-to-finish multiplier farm with a high health status were submitted for postmortem examination. The clinical history was of congenital tremor. The farm, located in eastern Canada, housed 400 Landrace breeding sows in sanitized facilities equipped with air filtration system. A vaccination program for parvovirus, leptospirosis, and Erysipelothrix rhusiopathiae was in place and the sows were in good health. The herd was negative for the infectious agents porcine reproductive and respiratory syndrome virus (PRRSV), influenza, Mycoplasma hyopneumoniae, Actinobacillus pleuropneumoniae, and transmissible gastroenteritis coronavirus.
An outbreak of congenital tremor was observed at birth in piglets of gilts, while piglets of multiparous sows showed no clinical signs. Prior to this outbreak, congenital tremor had never been reported in the herd, which had produced 25 weaned piglets per sow over the previous 12 months. The clinician also reported normal mortality rates in piglets and sows before the outbreak, and the herd was in good health.
Replacement rate for this herd is around 40%, and groups of approximately 20 gilts are introduced every 8 wk from a high health nucleus herd. Before or after farrowing, no clinical signs were reported from a group of 20 new gilts introduced to the herd 6 mo earlier or from other sows within the herd. Five of the new gilts produced litters affected by congential tremor, with 100% of piglets within each litter displaying systemic tremors and difficulty standing or walking from birth (N = 61 piglets affected). Clinical signs were severe enough to impair nursing and cause mortality. During the same period, 14 other gilts had farrowed but none had piglets with congenital tremor. The average litter size was slightly smaller in congenital tremor-affected litters (12.2 piglets per litter) compared with other gilt litters (13.3 piglets per litter). There were no stillborns in the congenital tremor-affected litters. At 3 wk of age (time of weaning) the mortality rate in congenital tremor-affected litters had reached an average of 24.6% (15/61), varying from 13.3% (2/15) to 41.2% (7/17) between litters with congenital tremor, compared with an average mortality rate of 12.7% piglets per litter in the other gilts. In surviving piglets, clinical signs of congenital tremor had completely vanished by 3 wk of age.
Body condition score was within normal limits at postmortem examination of the 2 piglets with congenital tremor. The stomach of 1 piglet, a 1.5-kg female, contained partly digested milk, while the stomach of the other, a 1.2-kg male, was empty. Meconium was still present in the colon of this piglet. No macroscopic lesions were noted in the central nervous system (CNS).
Most of the brain, the entire spinal cord, and sections of lungs, heart, liver, spleen, kidneys, skeletal muscles, small intestine, large intestine, tonsils, and thymus were fixed in 10% buffered formalin for 24 h, processed routinely for histological examination, and stained with hematoxylin phloxine saffron (HPS) stain. The same process was applied on the spinal cord of a control subject, a healthy 2-day-old piglet from another farm free of congenital tremor. Sections of thoracic spinal cord of the control and affected piglets were also stained with luxol fast blue (LFB). Fresh specimens of lungs, ileum, and spiral colon from piglets with congenital tremor were submitted for aerobic culture. Pools of tissues were frozen at –20°C and later tested by reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR) for PRRSV (single pool of lungs, tonsils, thymus, and inguinal lymph nodes from both piglets), porcine circovirus type 2 (PCV2; single pool of lungs, tonsils, thymus, and inguinal lymph nodes from both piglets) and APPV (pool A with lungs, tonsils, thymus, and inguinal lymph node from both subjects; pool B, 1 per piglet, with thymus, spleen, kidney, ileum, cortex, cerebellum, and brainstem). Sections of formalin-fixed and paraffin-embedded thoracic spinal cord from the control piglet and the piglets with congenital tremor were also tested for APPV by RT-qPCR. The atypical porcine pestivirus RT-qPCR assay used was adapted from the report of Arruda et al (12). Briefly, this RT-qPCR assay targets the NS3 gene of the virus and produces an amplicon of 123 nucleotides in length.
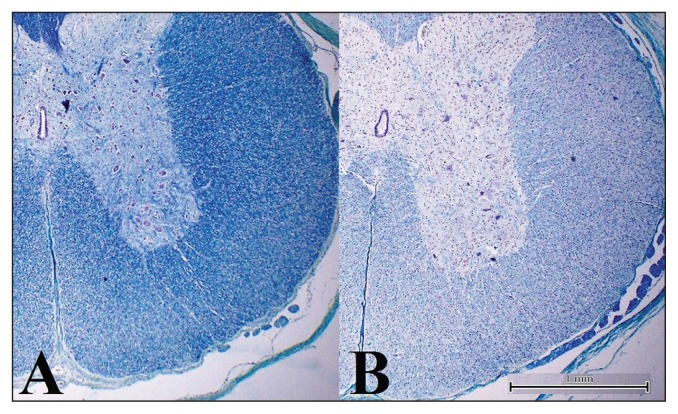
Microscopically, significant lesions were noted only in affected animals. In HPS-stained sections of cervical, thoracic, and lumbar spinal cord, a few random vacuoles were seen in both lateral and ventral funiculi (white matter), and oligodendrocytes appeared more prominent compared with the control piglet. Hypomyelination was evident as a lack of staining with the LFB stain in the white matter of the spinal cord of piglets with congenital tremor, but not the healthy control (Figure 1). In comparison, the amount of LFB-stained material (myelination) in the adjacent nerve roots (internal control) and in the spinal cord of the healthy subject (external control) appeared normal. No changes were noted in the cerebral cortex, cerebellum, and brainstem of affected piglets. No bacterial pathogens were isolated from culture.
Figure 1.
Luxol Fast Blue staining of the thoracic spinal cord of 2-day-old piglets. A — Unaffected piglet; there is regular myelination. B — Congenital tremor-affected piglets, there is an important loss of myelin from the periphery of the cord, more severe in the lateral and ventral funiculi. Notice the normal myelination in the nerve roots (internal control). Scale bars = 1 mm.
Molecular diagnostic results for PRRSV and PCV2 were negative. All frozen samples tested for APPV by RT-qPCR were positive, with Ct values of 26.09 for pool A, and 31.34 (subject 1) and 28.39 (subject 2) for pool B. Sections of formalin-fixed and paraffin-embedded thoracic spinal cord from the control piglet were negative for APPV by RT-qPCR, but sections from diseased piglets were positive, with Ct values of 33.68 (subject 1) and 34.90 (subject 2). To confirm the APPV RT qPCR results, the PCR amplicon was sequenced and compared to one recently reported in United States reference strain (GenBank accession numbers: KU194229). Interestingly, the nt identity of our sequence compared to that APPV reference strain was relatively low, with 87.9%.
Discussion
The clinical signs, postmortem and histopathology findings in the 2 pigs are consistent with CT type AII caused by APPV infection. Atypical porcine pestivirus is a recently discovered porcine pestivirus, first described by Hause et al (11) following high throughput sequencing. Pestiviruses are enveloped, highly variable RNA viruses belonging to the family Flaviviridae (19). Several pestiviruses are of great socioeconomic relevance, notably CSFV in pigs, bovine viral diarrhea virus type 1 (BVDV-1) and type 2 (BVDV-2) in cattle, and border disease virus (BDV) in sheep and goats (20). Fetal disease is a common feature of intrauterine pestivirus infections and in pigs, highly virulent strains of CSFV cause severe clinical signs and high mortality resulting in a significant decrease in productivity (19,20). It was believed that pestiviruses only affected ungulate species, but recently several novel pestiviruses were identified in various domestic and wild species including non-ungulate species such as the rat (21) and bat (22).
Atypical porcine pestivirus is one of the novel emerging pestiviruses identified in pigs. Others are Bungowannah virus (23) and Linda virus (24). Bungowannah virus causes stillbirths or heart disease and sudden death in piglets in Australia (25), while Linda virus was identified in Austria from piglets with congenital tremor and severe hypomyelination (24). The clinical signs and lesions observed in piglets infected with Linda virus are quite similar to those of APPV infection, but phylogenic analysis revealed that Linda virus has closer identity with Bungowannah virus than with APPV.
Phylogenic analysis revealed only a distant genetic relationship between APPV and ruminant pestiviruses and CSFV, with < 50% nucleotide identity with CSFV (11). A distant genetic relationship was also noted between APPV and Bungowannah virus and the rat pestivirus, while the bat pestivirus appears to be more closely related (11).
Like other pestiviruses, APPV displays great genetic variation, and a large number of strains (13–18,26). Reports of these various strains in commercial pig populations in several countries including Austria, China, Germany, Great Britain, Italy, Netherlands, Serbia, Spain, Switzerland, and the USA, indicate it is widely distributed (12–18,26). The prevalence reported in herds is variable but can be high (13,15,18).
A strong association between CT type AII and APPV was observed by several authors (12–14,16,17) and reproduction of CT in piglets was achieved experimentally following inoculation of APPV into pregnant gilts at day 32 of gestation (14), and following inoculation of APPV into pregnant gilts and fetal amniotic vesicle at day 45 or 62 of gestation (12). In both studies, the inoculation times were established with the knowledge of fetal development of immunocompetence (around 70 d of gestation; 5,27–29) and of the CNS (at 62 d of gestation; 30,31) in pigs.
Congenital infection with APPV is typically associated with outbreaks of CT in litters of gilts (13,14,18), although piglets born from higher parity sows can also be affected. While the infection does not affect the number of piglets born per litter, the prevalence of CT within a litter, which is highly variable, can reach 100% (14). In the present case, the cause for a slightly lower number of piglets per litter within congenital tremor-affected litters compared with other litters remains unknown. Morbidity is typically increased with congenital tremor, resulting frequently in growth retardation, but mortality can be normal or increased (13,16,17).
The intensity of signs of congenital tremor can be variable (12,14,17), and mild tremor could be unnoticed or mistaken for shivering from cold. While piglets will typically recover completely within 2 to 3 wk (13), some authors (16) report the persistence of mild tremor of ear tips and flank for up to 14 wk, after which it disappears completely. As with other types of congenital tremor, no shaking is noted in these piglets during relaxation or sleep, but tremors are increased or induced by stress.
Congenital tremor was the only clinical sign reported here. Other changes such as splay leg (12,14,16), which reflects muscle weakness, or an abnormal posture with xyphosis and ears on the neck (14) are reported with APPV infection.
The histological changes observed here are consistent with lesions reported by previous authors (13,16). Congenital tremor due to APPV infection is typically caused by hypomyelination in the lateral white matter of the spinal cord only (13) or in the white matter of the cerebellum and spinal cord (16). By electron microscopy (16), hypomyelination and myelin disruption and myelin breakdown were described, while no detectable loss of oligodendrocytes was noted by immunoperoxidase staining (16). Such findings suggest that APPV has deleterious functional effects on fetal oligodendrocytes, likely reducing myelin development and function, or leading to degeneration of myelin.
The presence of APPV detected by fluorescent in-situ hybridization is reported in the inner granular layer of the cerebellum and neurones of spinal ganglia, the epithelium of tonsils, and follicular centers of mandibular lymph nodes, but not in the thymus of piglets with congenital tremor type AII (13).
Apart from the CNS (brain, cerebellum, brainstem, spinal cord, and trigeminal ganglia) and the lymphoid organs (thymus, tonsils, spleen, and mandibular, tracheal, inguinal, or mesenteric lymph nodes), the virus has also been identified by PCR from peripheral nerves, heart, lungs, liver, kidney, bladder, pancreas, small intestine, colon, salivary glands, skeletal muscle, umbilical blood, serum, cerebrospinal fluid, saliva, nasal swabs, rectal swabs, and even semen (12–14,16,18,32). The frequency of identification and the genome load vary among samples and among studies; however, lymphoid organs seem to be the most frequently positive samples and usually with a higher viral genome load (13,14,17,18). The pathogenesis is still unclear and the presence of APPV in central nervous system may explain neurological symptoms. The presence of APPV in a wide variety of other tissues suggests it replicates systemically.
No relationship has been established between the virus concentration in blood and severity of the disease (14), but the presence of APPV in samples such as rectal and nasal swabs, salivary glands, saliva, intestines, and semen represent potential sources for viral shedding into the environment.
One important observation in the literature is the high prevalence of APPV infection in herds or individuals with no obvious clinical disease (13–15,26) and studies suggest that congenital tremor type AII only occurs when naïve sows are infected at a specific time in gestation, before the development of immunocompetence of the fetus (12,14, 6). Infection may not induce clinical signs in an immune herd. With clinical outbreaks of congenital tremor, asymptomatic viremic piglets are reported (17) and persistent shedding of the virus after the resolution of clinical disease is observed (14,16,17). The high prevalence of APPV in tonsils or serum from apparently healthy pigs supports the presence of persistently infected animals and may explain why congenital tremor can be recurrent (14). In such cases, congenital tremor will usually reappear after the introduction of new gilts, allowing clinically healthy but chronically or persistently infected pigs to infect naïve subjects (14,16).
Detection of the virus, sometimes with prolonged shedding (14,16), from feces, saliva, intestines, pancreas, salivary glands (14,16,18) of pigs suggests that the transmission of the virus in herd is fecal-oral, and its presence in semen (32) suggests it is also venereally transmitted. Higher viral load in lymphoid tissues suggests an immunosuppressive capacity. In piglets, transplacental transmission of the virus is the most likely transmission route. However, the sera of sows are usually negative for APPV at time of delivery (13,14).
Cell culture isolation was first achieved by Beer et al (15), but several authors have failed to replicate the virus (11–14,18), suggesting that in vitro infectivity is limited. Isolation and passage on different porcine cells was recently achieved (16,33), but infection of subjects with isolated virus remains to be done.
The present case appears to be the first report of APPV in piglets with congenital tremor in Canada. The case history here suggests the newly introduced gilts were infected after entry into this herd; the exact prevalence of the virus in Canadian herds, the route of transmission of the infection, and its pathogenesis in the Canadian context are unknown. A better understanding of APPV pathogenicity, epidemiology, and immunobiology is required to detect affected herds and develop a vaccine and other prevention strategies.
Finally, the diagnosis of APPV is particularly important because CSFV, a federally reportable disease in Canada, is a clinical differential diagnosis for congenital tremor in piglets. Congenital tremor type AI and type AII cannot be clinically distinguished and adequate diagnostic tools are essential to distinguish between them. Fortunately, there is no evidence of cross-reactivity with diagnostic tests routinely used for the diagnosis of classical swine fever (32). It is unlikely that APPV could negatively impact the diagnosis and surveillance programs in place for CSFV.
Acknowledgment
We thank Dr. Lalitha Peddireddi for the confirmation of our atypical porcine pestivirus positive samples by her diagnostic laboratory at Kansas State Veterinary Diagnostic Laboratory.
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
This work was funded by Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec (MAPAQ) and Faculté de médecine vétérinaire, Université de Montréal. Carl A. Gagnon was financially supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) discovery grant.
References
- 1.Kinsley AT. Dancing pigs. Vet Med. 1922;17:123. [Google Scholar]
- 2.Nissley SM. Shivers in pigs. J Am Vet Med Assoc. 1932;81:551. [Google Scholar]
- 3.Kernkamp HCH. Myoclonia congenita: A disease of newborn pigs. Vet Med. 1950;45:189. [PubMed] [Google Scholar]
- 4.Maplesden DC, Brown GCT. Myoclonia congenital in a litter of young pigs. Can J Vet Res. 1957;21:170–172. [PMC free article] [PubMed] [Google Scholar]
- 5.Done S, Williamson SM, Strugnell BW. Nervous and locomotor systems. In: Zimmerman JJ, Karriker LA, Ramirez A, Schwarts KJ, Stevenson GW, editors. Diseases of Swine. 10th ed. Ames, Iowa: Wiley-Blackwell; 2012. pp. 303–305. [Google Scholar]
- 6.Cantile C, Youssef S. Nervous system. In: Maxie MG, editor. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. 6th ed. Vol. 1. London, UK: Saunders Elsevier; 2016. pp. 336–338. [Google Scholar]
- 7.Done JT. Congenital nervous diseases of pigs: A review. Lab Animal. 1968;2:207–217. [Google Scholar]
- 8.Blakemore WF, Harding JD, Done JT. Ultrastructural observations on the spinal cord of a Landrace pig with congenital tremor type AIII. Res Vet Sci. 1974;17:174–178. [PubMed] [Google Scholar]
- 9.Blakemore WF, Harding JD. Ultrastructural observations on the spinal cords piglets with congenital tremor type AIV. Res Vet Sci. 1974;17:248–255. [PubMed] [Google Scholar]
- 10.Blomström A-L, Cecilia Ley, Jacobson M. Astrovirus as a possible cause of congenital tremor type AII in piglets? Acta Vet Scand. 2014;56:82. doi: 10.1186/s13028-014-0082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hause BM, Collin EA, Peddireddi L, et al. Discovery of a novel putative atypical porcine pestivirus in pigs in the USA. J Gen Virol. 2015;96:2994–2998. doi: 10.1099/jgv.0.000251. [DOI] [PubMed] [Google Scholar]
- 12.Arruda BL, Arruda PH, Magstadt DR, et al. Identification of a divergent lineage porcine pestivirus in nursing piglets with congenital tremors and reproduction of disease following experimental inoculation. PLoS ONE. 2016 doi: 10.1371/journal.pone.0150104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Postel A, Hansmann F, Baechlein C, et al. Presence of atypical porcine pestivirus (APPV) genomes in newborn piglets correlated with congenital tremor. Sci Rep. 2016 doi: 10.1038/srep27735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Groof A, Deij M, Guelen L, et al. Atypical porcine pestivirus: A possible cause of congenital tremor type A-II in newborn piglets. Viruses. 2016;8:271. doi: 10.3390/v8100271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beer M, Wernike K, Dräger C, et al. High prevalence of highly variable atypical porcine pestiviruses found in Germany. Transbound Emerg Dis. 2016;64:e22–e26. doi: 10.1111/tbed.12532. [DOI] [PubMed] [Google Scholar]
- 16.Schwarz L, Riedel C, Högler S, et al. Congenital infection with atypical porcine pestivirus (APPV) is associated with disease and viral persistence. Vet Res. 2017;48:1. doi: 10.1186/s13567-016-0406-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muñoz-González S, Canturri A, Pérez-Simó M, et al. First report of the novel atypical porcine pestivirus in Spain and a retrospective study. Transbound Emerg Dis. 2017:1–5. doi: 10.1111/tbed.12699. [DOI] [PubMed] [Google Scholar]
- 18.Yuan J, Han Z, Li J, et al. Atypical porcine pestivirus as a novel type of pestivirus in pigs in China. Front Microbiol. 2017 doi: 10.3389/fmicb.2017.00862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy FA, Gibbs EPJ, Horzinek MC, Studdert MJ, editors. Veterinary Virology. 3rd ed. San Diego, California: Academic Press; 1999. Flaviviridae; pp. 555–569. [Google Scholar]
- 20.Kirkland PD, Le Potier M-F, Vannier P, Finlaison D. Pestiviruses. In: Zimmerman JJ, Karriker LA, Ramirez A, Schwarts KJ, Stevenson GW, editors. Diseases of Swine. 10th ed. Ames, Iowa: Wiley-Blackwell; 2012. pp. 538–553. [Google Scholar]
- 21.Firth C, Bhat M, Firth MA, et al. Detection of zoonotic pathogens and characterization of novel viruses carried by commensal Rattus norvegicus in New York City. mBio. 2014;5:e01933–14. doi: 10.1128/mBio.01933-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Z, Ren X, Yang L, et al. Virome analysis for identification of novel mammalian viruses in bat species from Chinese provinces. J Virol. 2012;86:10999–11012. doi: 10.1128/JVI.01394-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirkland PD, Frost MJ, Finlaison DS, King KR, Ridpath JF, Gu X. Identification of a novel virus in pigs — Bungowannah virus: A possible new species of pestivirus. Virus Res. 2007;129:26–34. doi: 10.1016/j.virusres.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Lamp B, Schwarz L, Högler S, et al. Novel pestivirus species in pigs, Austria, 2015. Emerg Infect Dis. 2017;23:1176–1179f. doi: 10.3201/eid2307.170163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirkland PD, Read AJ, Frost J, Finlaison DS. Bungowannah virus — A probable new species of pestivirus — What have we found in the last 10 years? Anim Health Res Rev. 2015;16:60–63. doi: 10.1017/S1466252315000031. [DOI] [PubMed] [Google Scholar]
- 26.Postel A, Meyer D, Cagatay G, et al. High abundance and genetic variability of atypical porcine pestivirus in pigs from Europe and Asia. Emerg Infect Dis. 2017;23:2104–2107. doi: 10.3201/eid2312.170951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salmon H. Immunité chez le foetus et le nouveau-né : modèle porcin. Reprod Nutr Dévelop. 1984;24:197–206. [PubMed] [Google Scholar]
- 28.Nielsen J, Rønsholt L, Sørensen KJ. Experimental in utero infection of pig foetuses with porcine parvovirus (PPV) Vet Microbiol. 1991;28:1–11. doi: 10.1016/0378-1135(91)90095-w. [DOI] [PubMed] [Google Scholar]
- 29.Sinkora M, Butler JE. The ontogeny of the porcine immune system. Dev Comp Immunol. 2009;33:273–283. doi: 10.1016/j.dci.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pond WG, Boleman SL, Fiorotto ML, et al. Perinatal ontogeny of brain growth in the domestic pig. Proc Soc Exp Biol Med. 2000;223:102–108. doi: 10.1177/153537020022300114. [DOI] [PubMed] [Google Scholar]
- 31.Sweasey D, Patterson DSP, Glancy EM. Biphasic myelination and the fatty acid composition of cerebrosides and cholesterol esters in the developing central nervous system of the domestic pig. J Neurochem. 1976;27:375–380. doi: 10.1111/j.1471-4159.1976.tb12256.x. [DOI] [PubMed] [Google Scholar]
- 32.Gatto IRH, Arruda PH, Visek CA, et al. Detection of atypical porcine pestivirus in semen from commercial boar studs in the United States. Transbound Emerg Dis. 2017:1–5. doi: 10.1111/tbed.12759. [DOI] [PubMed] [Google Scholar]
- 33.Postel A, Meyer D, Petrov A, Becher P. Recent emergence of a novel porcine pestivirus: Interference with classical swine fever diagnosis? Emerg Microbes Infect. 2017:6. doi: 10.1038/emi.2017.5. [DOI] [PMC free article] [PubMed] [Google Scholar]