Abstract
Background:
The range of thermoneutral zone of chickens is narrow, and they become easily susceptible to environmental stress, a common and major concern for poultry causing a production loss.
Objective:
The present study was designed to comparatively evaluate anti-stress activity of Phytocee™ and Vitamin C in chickens reared under heat stress.
Materials and Methods:
A total of 600-day-old chicks of Cobb 400 were randomly assigned to 4 groups with 6 replicates comprising 25 birds each (n = 150). G1 served as a normal control (NC) and supplemented with Vitamin C at 100 g/ton of feed. G2 served as a heat stress control (HSC), subjected to heat stress (34°C–36°C) without Vitamin C supplementation. G3 and G4 served as positive control and treatment group (TC), subjected to heat stress and supplemented with Vitamin C and Phytocee™ at 100 g/ton of feed, respectively. The impact on zootechnical parameters and cloacal temperature was assessed at regular intervals, and blood was collected at the end of the experiment for evaluation of stress parameters, namely heterophil lymphocyte ratio (H:L ratio) and serum corticosterone.
Results:
Exposure of chickens to heat stress caused a significant decrease in body weight, worsening of feed conversion ratio, higher mortality, and poor production efficiency. Moreover, serum corticosterone level, H:L ratio, and cloacal temperature were significantly increased in HSC as compared to NC. However, supplementation of Phytocee™ in feed significantly ameliorated the negative impact of heat stress in broiler birds.
Conclusion:
The supplementation of Phytocee™ demonstrated an anti-stress effect in chickens through restoration of serum corticosterone level, H:L ratio, and thermoregulatory mechanism.
SUMMARY
Combating heat stress remains a challenge for the broiler industry in the tropics and subtropics, which is even aggravated by the changing climatic conditions
The present study was designed to evaluate the anti-stressor activity of Phytocee™, a polyherbal formulation containing Emblica officinalis, Ocimum sanctum, and Withania somnifera in broiler using heat stress model in comparison with Vitamin C
Phytocee™ demonstrated an anti-stress effect in the current study by ameliorating the negative effects of heat stress on zootechnical parameters, serum corticosterone, heterophil lymphocyte ratio, and cloacal temperature of broilers through modulating the hypothalamic–pituitary–adrenal axis and thermoregulatory mechanism
Hence, Phytocee™ could be recommended in broilers and livestock animals for modulating and combating adverse effects of heat stress and thereby reducing the economic losses incurred by farmers.
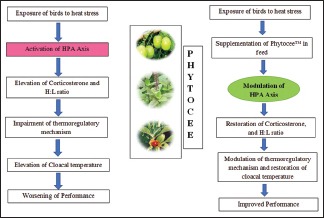
Abbreviations Used: HPA axis: Hypothalamic pituitary adrenal axis.
Key words: Anti-stress, corticosterone, heat stress, heterophil lymphocyte ratio, Phytocee™, poultry
INTRODUCTION
In many parts of the world, poultry farming occupies a significant position among agricultural industries since it contributes major portion of the animal protein to human population. The foremost drawback of poultry production in tropical and subtropical countries is high environmental temperature and humidity as it induces a heat stress to the birds by altering its thermoregulatory mechanisms. Birds have a greater challenge in maintaining homoeothermic body temperature during heat stress because of the absence of the sweat glands and higher body temperature when compared to other domestic animals.[1] Earlier studies reported that an increase in temperature above 25°C significantly lowers the growth rate of broilers.[2,3] Typically, birds can tolerate and adapt to the temperature of about 20°C–25°C, and beyond this, it turns down the birds' immunity, feed intake, weight gain, egg production, hatchability of fertile eggs, number of eggs and carcass quality, mineral balance, semen quality, and fertility in male birds.[4]
As the ratio of humidity and temperature overdoes the thermal comfort zone, the birds start to exhibit the signs of heat stress. The cardinal symptoms exhibited by birds during heat stress are gasping, panting, pale comb and wattle, dull and depressed, decreased appetite, and increased cannibalism resulting in production loss.[5] During the heat stress condition, birds would be panting excessively by keeping the mouth wide opened, spreading the wings, and squatting close to the ground and more blood flow gets diverted from internal organs to skin which leads to dark skin color.[6] Hence, during the period of heat stress, most of the production energy would be engaged toward the thermoregulatory adaptations which results in induction of oxidative stress in birds. Birds of all ages could be susceptible to heat stress but it is more common in meat-type rather than egg-type birds that is why raising broilers under increased environmental temperature had gained much attention.[7]
Combating heat stress remains a challenge for the broiler industry in the tropics and subtropics, which is even aggravated by the changing climatic conditions. Heat stress exhibits a negative response on body weight, plasma glucose, protein and cortisone level, heterophil lymphocyte ratio (H:L ratio), and sometimes even lead to increased mortality. The development of novel dietary measures such as supplementation of anti-stress agents, vitamins, and minerals may be beneficial in ameliorating heat stress and enhancing optimum performance in broiler chickens.[8,9] Of several strategies, the dietary modification is one of the most preferred which could be accomplished using traditional antioxidants such as Vitamin C, Vitamin E, and acetylsalicylic acid to attenuate the effects of heat stress on performance of birds.[10] Moreover, supplementation with Vitamin C is the most beneficial among other vitamins throughout the heat stress period.[11] Vitamin C or L-ascorbic acid is nonessential vitamin in poultry diet as the birds possess the enzyme gluconolactone oxidase required for its biosynthesis in kidneys;[12,13,14] however, the ability to synthesize Vitamin C becomes inadequate during heat stress.[15]
Phytocee™ (M/s Natural Remedies Private Limited, Bengaluru, India) is a polyherbal supplement for poultry and other animal species, a combination of herbs, namely Emblica officinalis, Ocimum sanctum, and Withania somnifera. All these plants are reported for their antioxidant property.[16,17,18] Phytocee™ was extensively studied for its efficacy and safety in in vitro and in vivo models; it demonstrated a significant antioxidant and protective effect against carbon tetrachloride-induced oxidative stress in albino Wistar rats.[19] In addition, cellular antioxidant potential of Phytocee™ was demonstrated against AAPH-induced oxidative stress using HepG2 cells and reported to be safe and free from causing toxic effects in in vitro systems.[20]
In the traditional system of Indian medicine, polyherbal formulations and combined extracts of plants are used as a drug of choice rather than individual plants and extracts.[21] This therapeutic approach is not considered to be an alternative to conventional medicine by others due to lack of scientific validation of efficacy and safety.[22] An editorial in Journal of American Medical Association emphasizes that the fundamental issue is not traditional medicine versus alternative medicine, but medical practice supported by clinical and scientific evidence.[23] Hence, there is an obvious need for establishing the proof of efficacy of these polyherbal formulations in target animal species. Although scientific evidence is available for antioxidant and anti-stressor activity in in vitro and in vivo models, Phytocee™ is not scientifically validated for its efficacy as an anti-stressor in broiler chickens exposed to heat stress. Therefore, this study was designed to evaluate the anti-stressor activity of Phytocee™ in broiler using heat stress model in comparison with Vitamin C.
MATERIALS AND METHODS
Phytocee™
Phytocee™ is a proprietary polyherbal formulation developed by M/s. Natural Remedies Private Limited, Bengaluru, India, containing E. officinalis fruits (70% w/w), O. sanctum whole plant (20% w/w), and W. somnifera roots (10% w/w).
Ethical approval
The study was conducted by authorized, qualified, and trained veterinarians, scientists, and technicians, in compliance with the guidelines of the Institutional Animal Ethics Committee approved by Committee for the Purpose of Control and Supervision of Experiments on Animals, India. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.[24]
Study design
A total of 600-day-old male Cobb 400 broiler chicks were used in this study. The experiment was carried out in poultry research station, recognized by Department of Scientific and Industrial Research, India; DSIR Reg No.: TU/IV-RD/2000/2016, located in Anniyalam, Tamil Nadu for a period of 42 days. Chicks were individually weighed, tagged with a wing band bearing an identification number and randomly assigned to G1, G2, G3, and G4 with 6 replicates comprising of 25 birds.
Housing of birds
The design of this study was completely randomized block design. The chicks were housed in semi-closed house being divided into pens with floor space of 25 square feet. The approximate size of the individual pen was 4.25” × 6” × 2” (Length × Width × Height). Each individual pen was provided with facilities for brooder, a bell drinker, chick feeder, and/or jumbo feeders. The size and floor space of the pen were modified according to number of chicks housed in it with the help of polyvinyl chloride sheet.
Environmental conditions
The optimum temperature was maintained with the use of brooding lamps and fiery charcoal fumes depending on the season and time of the day. The actual temperature and relative humidity at room and chick level was measured using a digital thermohygrometer [Tables 1 and 2].
Table 1.
Normal control

Table 2.
Heat stress control
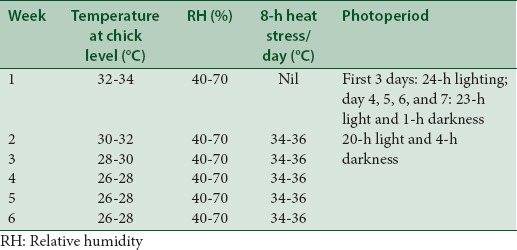
Management of birds
The chicks were vaccinated against Marek's disease immediately after hatching. On arrival at poultry research farm, chicks were provided with 4% sugar solution for first 4 h to replenish the depleted energy and stimulate the feed consumption. All chicks were vaccinated against Newcastle disease vaccine (LaSota strain) and infectious bursal disease as eye drops on day 5 and day 14, respectively. The chlorinated potable drinking water (Innoclean™, Natural Remedies Pvt. Ltd., 1 tablet/500 L of water) was provided ad libitum to chicks.
Feed
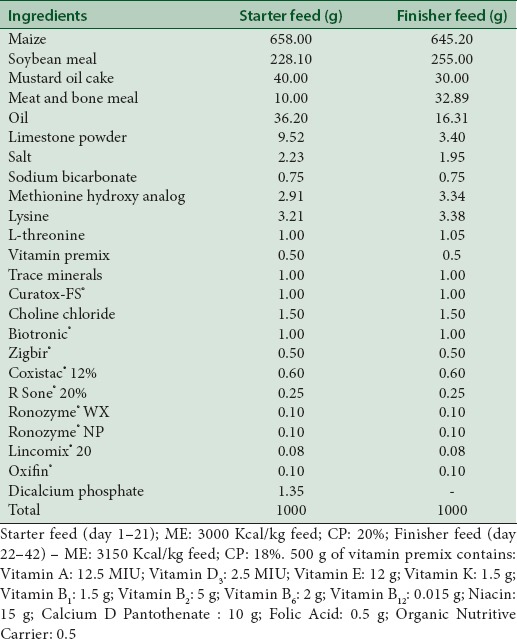
The chicks of different treatment groups were provided with respective poultry crumble feed ad libitum manufactured by Biogain Feeds Pvt. Ltd., Bangalore. Phytocee™ and coated Vitamin C were thoroughly mixed with feed at the rate of 100 g/ton and subjected to pelleting. The pellets were then crumbled to desired particle sizes for feeding the chicks. The feed ingredients and composition of starter and finisher feed are detailed in Table 3.
Table 3.
Feed ingredients and composition of diets
Experimental design and feeding level
G1: Normal control (NC; no heat stress + Vitamin C at 100 g/ton of feed)
G2: Heat stress control (HSC; heat stress – Vitamin C)
G3: Positive control (PC; heat stress + Vitamin C coated at 100 g/ton of feed)
G4: Treatment (TC; heat stress + Phytocee™ at 100 g/ton of feed).
Determination of heterophil lymphocyte ratio
Whole blood samples were collected from 12 birds per group (2 birds/replicate) on day 42 in the heparinized centrifuge tubes. The blood smears were prepared using wedge slide technique, air dried, fixed in methanol, and stained with May–Grunwald–Giemsa stain. One hundred leukocytes including granular (heterophil, eosinophil, and basophil) and nongranular (lymphocyte and monocyte) cells were counted on duplicates slide per bird, and the H: L ratio was calculated using the average counts of these cell types.[25]
Determination of serum corticosterone level
On day 42, the blood samples (approximately 2 ml) were collected from 12 birds per group (2 birds/replicate) in plain centrifuge tubes (BD Vacutainer tube, India). The blood was collected from brachial vein of chicks and serum was separated by centrifugation and stored at −80°C until analysis. The serum corticosterone level was measured using a commercially available competitive enzyme-linked immunosorbent assay kit (Cayman Chemical Co., Ann Arbor, MI, USA) as per the manufacturer's instructions. Briefly, this assay is based on the competition between corticosterone and a corticosterone-acetylcholinesterase conjugate (conjugate tracer) for a limited amount of corticosterone antiserum. Because the concentration of the corticosterone varies, the amount of corticosterone tracer that binds to the corticosterone antiserum is inversely proportional to the concentration of corticosterone in the well. This antiserum-corticosterone (either free or tracer) complex binds to the mouse anti-rabbit IgG that has been previously attached to the well. The plate is washed to remove any unbound reagents and then Ellman's reagent is added to the well. The product of this enzymatic reaction has a distinct yellow color and absorbs strongly at 412 nm. The intensity of this color, determined spectrophotometrically, is proportional to the amount of corticosterone tracer bound to the well, which is inversely proportional to the amount of free corticosterone present in the well during the incubation. The assay has a range of 8.2–5000 pg/ml and a sensitivity of approximately 30 pg/ml. NC, HSC, PC, and TC birds were alternated for blood collection, and their blood was taken at the same time of the day (i.e., between 0800 and 1000 h) to decrease data variability of serum corticosterone levels.
Assessment of zootechnical parameters
The chicks in the individual pen were observed for mortality, three times a day throughout the experimental period. The body weight of individual chicks was recorded on day 1 and thereafter on day 7, 21, and 42. Feed intake was calculated by subtracting the amount of left-over feed from the total amount of feed offered per replicate, and it was measured on day 21 and 42. Feed conversion ratio (FCR) was calculated as feed intake divided by body weight. The European Production Efficiency Factor (EPEF) was calculated according to the equation: Body weight (kg) × livability (%) × 100/FCR × age (days).
Assessment of cloacal temperature
On day 21, 28, 35, and 42, the cloacal temperature was recorded from 5 birds per replicate (n = 30 per group) during peak temperature of the day (between 1100 and 1500 h) using digital thermometer (Medigold™; ±0.1°C accuracy) inserted into the cloaca of the birds. Briefly, birds were selected randomly from each replicate of groups and were gently held by an assistant with back of bird facing toward the veterinarian. The probe of the digital thermometer was gently inserted 3 cm into the cloaca and maintained in steady position until the thermometer beeps and shows the stable temperature reading.
Statistical analysis
On completion of the trial, the raw data were compiled, processed, and expressed as mean ± standard error of the mean. Because a randomized complete block experimental design was applied, the data thus generated were analyzed by one-way analysis of variance according to Latham et al.,[26] using replicate as a blocking factor to determine if blocking was effective at reducing the random error. In case of significant differences among treatments (P < 0.05), means were subjected to Dunnett's multiple comparison test (IBM SPSS Statistics Version. 21.0; SPSS Inc., Chicago, IL, USA) and differences were considered statistically significant at P < 0.05.
RESULTS
Effect of Phytocee™ on zootechnical parameters of chickens under heat stress
There were no differences in initial live weight among the dietary treatment groups indicating the homogeneity in the weight of animals used in the study. The birds exposed to heat stress (HSC) displayed a drastic reduction (P < 0.01) in growth performance. The HSC group had the lower body weight, body weight gain, feed intake, and the higher and poorer FCR as well as the higher mortality rate compared with NC group. However, on days 21 and 42, Phytocee™ and Vitamin C supplemented birds showed significant and nonsignificant increase of body weight and body weight gain, respectively [Table 4].
Table 4.
Effect of Phytocee™ on body weight, body weight gain, and cumulative mortality
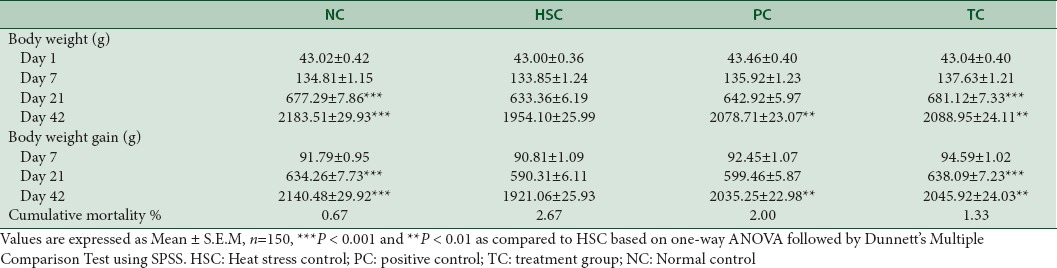
The feed intake of birds kept under heat stress was significantly (P < 0.05) decreased [Figure 1]. FCR of HSC birds fed basal diet without Vitamin C, gained significantly lesser body weight with reduced feed consumption. Improved FCR was observed in PC and TC where birds gained more body weight with less amount of feed consumption (30 g and 50 g per kg body weight respectively; P < 0.05) as compared to HSC. In addition, FCR of TC was at par with NC, but the FCR of PC was relatively better than NC [Table 5]. The EPEF of HSC was worsened when compared to NC (286.61 vs. 324.89), whereas supplementation with Vitamin C and Phytocee™ improved the EPEF and production efficiency of TC is found to be marginally better than PC (311.72 vs 310.87) [Figure 2]. Similarly, high mortality rate was observed in HSC as compared to NC group, whereas reduced mortality rate was observed in Vitamin C and Phytocee™ supplemented birds subjected to heat stress.
Figure 1.
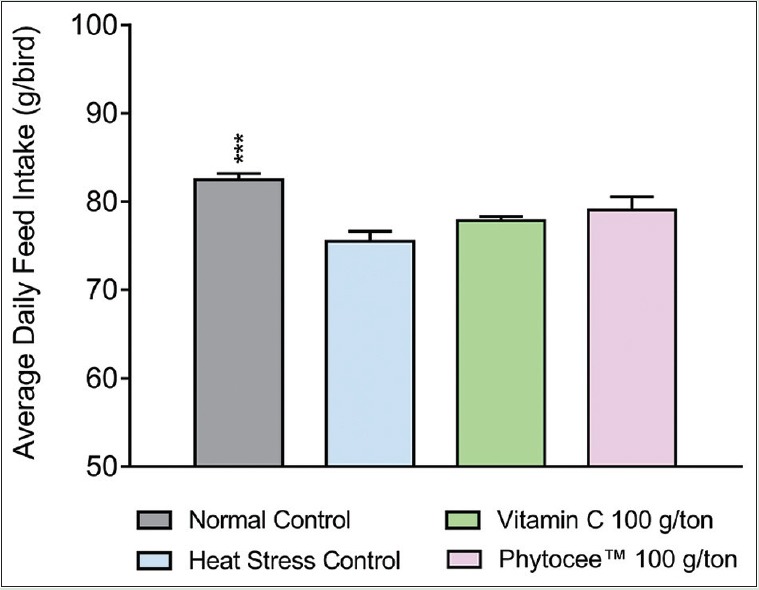
Effect of Phytocee™ on average daily feed intake values is expressed as mean ± standard error of the mean; n = 6 ***P < 0.001 as compared to heat stress control based on one-way analysis of variance followed by Dunnett's multiple comparison test using SPSS
Table 5.
Effect of Phytocee™ on feed intake and feed conversion ratio
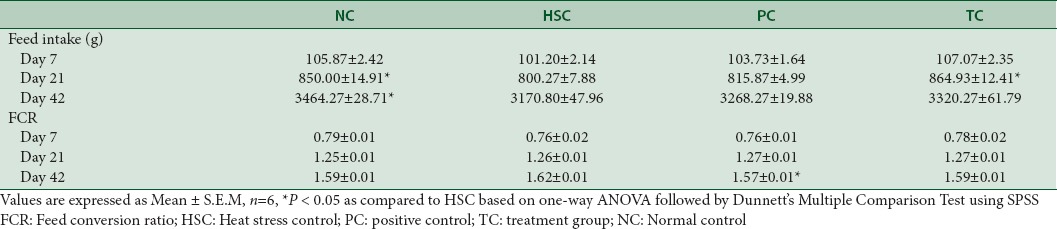
Figure 2.
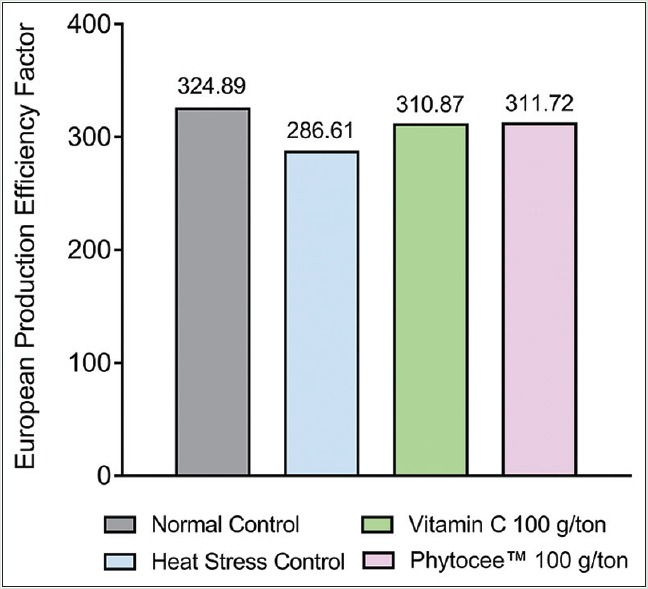
Effect of Phytocee™ on European Production Efficiency Factor
Effect of Phytocee™ on blood heterophil lymphocyte ratio and serum corticosterone level of chickens under heat stress
H:L ratio and corticosterone are indicators of stress. Heat stress caused a significant increase in H:L ratio and corticosterone level in birds subjected to high temperature when compared to NC group. However, supplementation of Vitamin C and Phytocee™ modulated the serum corticosterone level and H:L ratio to normal, but significant reduction (P < 0.05) was observed in Phytocee™ supplemented group as compared to HSC group [Figures 3 and 4].
Figure 3.
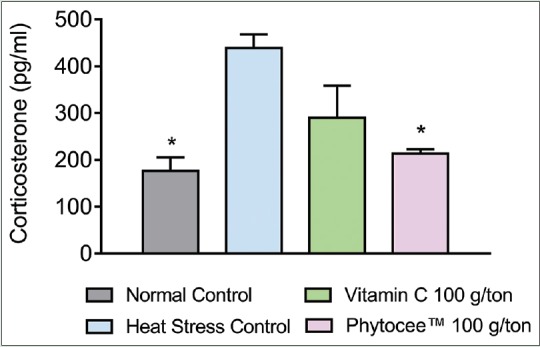
Effect of Phytocee™ on serum corticosterone level values s expressed as mean ± standard error of the mean; n = 12 *P < 0.05 as compared to heat stress control based on one-way analysis of variance followed by Dunnett's multiple comparison test using SPSS
Figure 4.
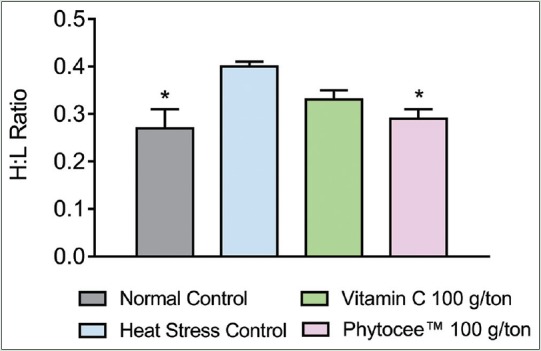
Effect of Phytocee™ on heterophil lymphocyte ratio values is expressed as mean ± standard error of the mean; n = 12 *P < 0.05 as compared to heat stress control based on one-way analysis of variance followed by Dunnett's multiple comparison test using SPSS
Effect of Phytocee™ on cloacal temperature of chickens under heat stress
Cloacal temperature was significantly increased in HSC group on all days as compared to NC group. However, cloacal temperature of birds supplemented with Vitamin C was significantly decreased on day 28, whereas supplementation of Phytocee™ showed a significant improvement on day 28 and day 42 when compared to HSC birds [Figure 5].
Figure 5.
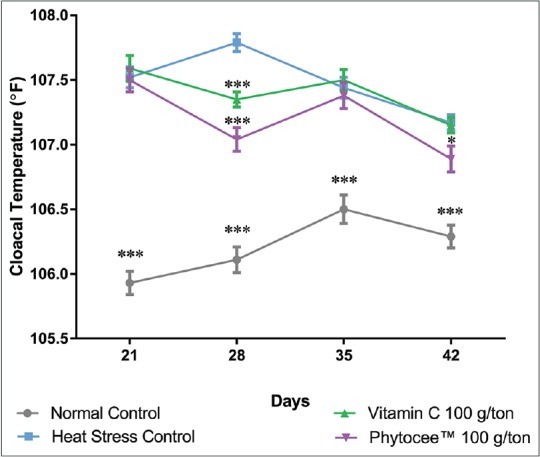
Effect of Phytocee™ on cloacal temperature values is expressed as mean ± standard error of the mean; n = 30 ***P < 0.001 and *P < 0.05 as compared to heat stress control based on one-way analysis of variance followed by Dunnett's multiple comparison test using SPSS
DISCUSSION
Stress plays a major role in broilers' welfare as birds are exposed continuously to various stressors such as environmental temperature, humidity, cold, vaccination, beak trimming, and holding. Stress due to high ambient temperature is especially of major concern in tropical and subtropical countries influencing the performance of broiler chickens negatively.[27,28] Heat stress results from a negative balance between the net amount of energy flowing from the animal's body to its surrounding environment and the amount of heat energy produced by the animal. It has been suggested that modern poultry genotypes produce more body heat, due to their greater metabolic activity.[5,6] Hence, alleviation of stress is essential for maintaining the health, productivity, and reproductive performance in birds. Several methods are available to alleviate the negative effects of high environmental temperature on performance of poultry. However, of the high cost and impracticality of cooling animal buildings, interest on dietary manipulations has increased in recent times.[29] The propensity for natural anti-stressors is growing because of the global trend of restricting the use of synthetic substances in feed. In pursuit of the above, the present study investigated the anti-stress potential of Phytocee™, a novel polyherbal formulation using a heat stress model as it is one of the well-recognized and widely used models to study the stress in broilers. In addition, the study also aimed at evaluation of efficacy of Phytocee™ in comparison with Vitamin C and at elucidation of the possible mechanism for anti-stress activity of Phytocee™ on alleviation of heat stress.
In the present study, the chronic heat stress model was employed for comparative evaluation of Phytocee™ and Vitamin C; the stress was induced in chicks by exposing them to high temperature (34°C–36°C) for 8 h per day from day 8 to day 42. The chicks were subjected to heat stress from day 8 because the broiler chick seems anatomically complete after hatching, but thermoregulatory systems need further development and maturation and become fully homeotherm about 7 days after hatching.[30] Moreover, the heat stress during the 1st week may lead to greater adaptability to high temperatures due to high metabolic rate of broiler chickens[31] and may not cause changes in markers of stress such as corticosterone, H:L ratio, and cloacal temperature.
The heat stress or any kind of homeostatic disturbances alter the activity of the neuroendocrine system of poultry, resulting in activation of the hypothalamic–pituitary–adrenal axis; it is an important system for integration of body functions and activation of which causes release of Adrenocorticotropic hormone (ACTH) from anterior pituitary and subsequent elevation in plasma corticosterone levels and deterioration of the health status of animals.[32,33] Broilers with elevated corticosterone levels have demonstrated a depressed lymphocyte numbers culminating in a higher H:L ratio.[28] The concentration of blood corticosterone and H:L ratio are the most sensitive indicators of stress in broilers[34] and are commonly used as tools to measure the physiological stress. Gross and Siegel[35] reported that H:L ratio only measures the physiological change but the concentration of blood corticosteroids alters before physiological change occurs and concluded that the H:L ratio and the concentration of glucocorticoids are the better measure of long-term changes and short-term changes, respectively, in the environment. The results of the present study indicate that exposure of broilers to high temperature induced a long-term heat stress; as a consequence, there was activation of HPA axis followed by a significant elevation in both serum corticosterone level and H:L ratio, whereas supplementation of Phytocee™ and Vitamin C in the diet was shown to restore the serum corticosterone level and H:L ratio toward normal. However, inhibitory effect of Phytocee™ on serum corticosterone level and H:L ratio was statistically significant as compared to HSC, superior to Vitamin C and comparable to NC. Hence, the present study clearly indicates that Phytocee™ could modulate HPA-induced stimulation of corticosterone release and subsequent elevation in blood H:L ratio. In contrary to earlier report, our study findings suggest that both serum corticosterone level and H:L ratio were significantly increased in heat stress and could be used as reliable measures of chronic stress in broiler chickens.
The corticosterone is the principal glucocorticoid involved in the regulation of feed intake, body weight gain, relative immune organ weight, and innate immunity in poultry.[36,37] The chronic heat stress is reported to cause a significant reduction in feed intake, body weight, higher FCR,[38] and a higher mortality rates in broilers.[39] The supplementation of Vitamin C aids in attenuation of elevated corticosterone level and H:L ratio[40] and improves the body weight in broilers exposed to heat stress.[41,42,43] In the present study, the elevated serum corticosterone level and H:L ratio observed in HSC displayed a significant negative impact on body weight, body weight gain, feed intake, and mortality rate, whereas the supplementation of Phytocee™ or Vitamin C was able to reverse the heat stress-induced serum corticosterone level and H:L ratio, thereby restoring the body weight and FCR. This result also corroborates with Attia et al.[44] who reported the feeding of Vitamin C to heat-stressed birds, relieves the undesirable effect, and improves the feed intake, protein digestibility, dressing percentage, and FCR. In addition, Phytocee™ group exhibited a lower mortality rate than Vitamin C group (1.33% vs. 2.00%) suggesting a possible immunomodulatory effect attributable to the bioactive compounds present in the Phytocee™.
Beside serum corticosterone and H:L ratio, the cloacal temperature is also one of the indices used to evaluate the degree of heat stress in broilers, reflecting the core body temperature;[45,46] it will be altered when the thermoregulatory mechanisms of birds are compromised. The supplementation of Vitamin C was reported to decrease the cloacal temperature and ameliorate the ability of the birds to discern higher heat loads in broiler chickens during the heat stress.[47] Moreover, the optimum recommended dose of Vitamin C was found to be 100–200 ppm for broilers under stress conditions such as beak trimming, disease, and heat stress.[48] In the present study, HSC group presented a statistically significant increase in cloacal temperature on days 21, 28, 35, and 42 indicating that the thermoregulatory mechanism of the birds was adversely challenged, apparently due to heat stress and concomitant increase in metabolic rate. Furthermore, supplementation of Phytocee™ caused a reduction in cloacal temperature on all time points, but statistical significant (P < 0.05) was observed on days 28 and 42. Although Vitamin C supplementation reduced the cloacal temperature on all time points, statistical significant (P < 0.001) was noticed only on day 28. It signifies that Phytocee™ supplementation is comparable or relatively superior to Vitamin C in alleviation of body temperature toward normal in broilers during heat stress. These findings are in line with previous reports wherein supplementation of Vitamin C was reported to reduce the rectal and skin temperatures as well as oxygen consumption in heat-stressed birds.[49,50]
Phytocee™ is an unique polyherbal formulation containing W. somnifera, O. sanctum, and E. officinalis. O. sanctum was reported to improve body weight in immunosuppressed birds through enhancing the immune status[51] and displayed an anti-stress effect by directly inhibiting cortisol secretions and indirectly having an antagonist effect on CRHR1 receptor.[52] Similarly, E. officinalis and W. somnifera were also known to improve the body weight and FCR in broilers attributable to anabolic effect[53,54] and possess an anti-stress effect and antioxidant activities.[19,55] These findings strongly indicate that improvement in body weight and FCR of Phytocee™ supplemented group could be attributed to its anti-stress effect.
The results of the present study also indicates, for the first time, that the polyherbal formulation Phytocee™ has tendency to decrease the cloacal temperature of the broiler chickens possibly through modulating the thermoregulatory mechanisms. Phytocee™ was shown to decrease the serum corticosterone level and H:L ratio probably by modulating the HPA axis and having an antagonist effect on CRHR1 receptor that could have contributed to its anti-stress effect and further improvement in zootechnical parameters in broilers exposed to heat stress.
CONCLUSION
Phytocee™ demonstrated an anti-stress effect in the current study by ameliorating the negative effects of heat stress on zootechnical parameters, serum corticosterone, H:L ratio, and cloacal temperature of broilers through modulating the HPA axis and thermoregulatory mechanism. Hence, Phytocee™ could be recommended in broilers and livestock animals for modulating and combating adverse effects and thereby reducing the economic losses incurred by farmers due to heat stress. However, the phytoactives of Phytocee™ are yet to be explored, and further investigations are required at molecular level to elucidate the concrete mechanism of action of Phytocee™ on alleviation of heat stress in broiler chickens.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sahin K, Sahin N, Kucuk O, Hayirli A, Prasad AS. Role of dietary zinc in heat-stressed poultry: A review. Poult Sci. 2009;88:2176–83. doi: 10.3382/ps.2008-00560. [DOI] [PubMed] [Google Scholar]
- 2.Njoya J. Effect of diet and natural variations in climates on the performance of laying hens. Br Poult Sci. 1995;36:537–54. doi: 10.1080/00071669508417800. [DOI] [PubMed] [Google Scholar]
- 3.Yahav S, Straschnow A, Plavnik I, Hurwitz S. Effects of diurnally cycling versus constant temperatures on chicken growth and food intake. Br Poult Sci. 1996;37:43–54. doi: 10.1080/00071669608417835. [DOI] [PubMed] [Google Scholar]
- 4.Ayo JO, Obidi JA, Rekwot PI. Effects of heat stress on the well-being, fertility, and hatchability of chickens in the Northern Guinea Savannah Zone of Nigeria: A review. Int Sch Res Netw. 2011:1–10. doi: 10.5402/2011/838606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Safdar AH, Maghami SP. Heat stress in poultry: Practical tips. Eur J Exp Biol. 2014;4:625–31. [Google Scholar]
- 6.Nardone A, Ronchi B, Lacetera N, Ranieri MS, Bernabucci U. Effects of climate changes on animal production and sustainability of livestock systems. Livest Sci. 2010;130:57–69. [Google Scholar]
- 7.Diarra SS, Tabuaciri P. Feeding management of poultry in high environmental temperature. Int J Poult Sci. 2014;13:657–61. [Google Scholar]
- 8.Erwan E, Tomonaga S, Yoshida J, Nagasawa M, Ogino Y, Denbow DM, et al. Central administration of L- and D-aspartate attenuates stress behaviors by social isolation and CRF in neonatal chicks. Amino Acids. 2012;43:1969–76. doi: 10.1007/s00726-012-1272-4. [DOI] [PubMed] [Google Scholar]
- 9.Whitehead CC, Keller T. An update on ascorbic acid in poultry. Worlds Poult Sci J. 2003;59:161–84. [Google Scholar]
- 10.Stilborn HL, Harris GC, Jr, Bottje WG, Waldroup PW. Ascorbic acid and acetylsalicylic acid (aspirin) in the diet of broilers maintained under heat stress conditions. Poult Sci. 1988;67:1183–7. doi: 10.3382/ps.0671183. [DOI] [PubMed] [Google Scholar]
- 11.Suganya T, Senthilkumar S, Deepa K, Amutha R. Nutritional management to alleviate heat stress in broilers. Int J Sci Environ Technol. 2015;4:661–6. [Google Scholar]
- 12.Elkheir S, Mohammed MK, Ahmed HM, Abdelgadir SM. Effect of feed restriction and ascorbic acid supplementation on performance of broiler chicks reared under heat stress. Res J Anim Vet Sci. 2008;3:1–8. [Google Scholar]
- 13.Lin H, Jiao HC, Buyse J, Decuypere E. Strategies for preventing heat stress in poultry. Worlds Poult Sci J. 2006;62:71–86. [Google Scholar]
- 14.Khan RU. Antioxidants and poultry semen quality. Worlds Poult Sci J. 2011;67:297–308. [Google Scholar]
- 15.Pardue SL, Thaxton JP, Brake J. Influence of supplemental ascorbic acid on broiler performance following exposure to high environmental temperature. Poult Sci. 1985;64:1334–8. doi: 10.3382/ps.0641334. [DOI] [PubMed] [Google Scholar]
- 16.Fujii T, Wakaizumi M, Ikami T, Saito M. Amla (Emblica officinalis Gaertn.) extract promotes procollagen production and inhibits matrix metalloproteinase-1 in human skin fibroblasts. J Ethnopharmacol. 2008;119:53–7. doi: 10.1016/j.jep.2008.05.039. [DOI] [PubMed] [Google Scholar]
- 17.Kath RK, Gupta RK. Antioxidant activity of hydroalcoholic leaf extract of Ocimum sanctum in animal models of peptic ulcer. Indian J Physiol Pharmacol. 2006;50:391–6. [PubMed] [Google Scholar]
- 18.Bhattacharya A, Ghosal S, Bhattacharya SK. Anti-oxidant effect of Withania somnifera glycowithanolides in chronic footshock stress-induced perturbations of oxidative free radical scavenging enzymes and lipid peroxidation in rat frontal cortex and striatum. J Ethnopharmacol. 2001;74:1–6. doi: 10.1016/s0378-8741(00)00309-3. [DOI] [PubMed] [Google Scholar]
- 19.Joseph JA, Uma R, Sridhar M, Krishnagouda SG, Usha PT, Amit A. Ameliorative effect of phytocee™ against carbon tetrachloride-induced oxidative stress. Pharmacogn Res. 2015;6:183–7. [Google Scholar]
- 20.Chandrasekaran CV, Sundarajan K, David K, Agarwal A. In vitro efficacy and safety of poly-herbal formulations. Toxicol In Vitro. 2010;24:885–97. doi: 10.1016/j.tiv.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 21.Ansarullah A, Jadeja RN, Thounaojam MC, Patel V, Devkar RV, Ramachandran AV. Antihyperlipidemic potential of a polyherbal preparation on triton WR 1339 (Tyloxapol) induced hyperlipidemia: A comparison with lovastatin. Int J Green Pharm. 2009;3:119–24. [Google Scholar]
- 22.Yuan R, Lin Y. Traditional Chinese medicine: An approach to scientific proof and clinical validation. Pharmacol Ther. 2000;86:191–8. doi: 10.1016/s0163-7258(00)00039-5. [DOI] [PubMed] [Google Scholar]
- 23.Fontanarosa PB, Lundberg GD. Alternative medicine meets science. JAMA. 1998;280:1618–9. doi: 10.1001/jama.280.18.1618. [DOI] [PubMed] [Google Scholar]
- 24.Selvam R, Saravanakumar M, Sureshbabu G. A liquid multivitamin and amino acid supplement, Easygrow™ on growth performance in Vencobb 400 broiler chickens. Int J Adv Sci Technol Res. 2015;5:72–7. [Google Scholar]
- 25.Onbaşılar EE, Erol1 H, Cantekin Z, Kaya U. Influence of intermittent lighting on broiler performance, incidence of tibial dyschondroplasia, tonic immobility, some blood parameters and antibody production. Asian Aust J Anim Sci. 2007;20:550–5. [Google Scholar]
- 26.Latham RE, Williams M, Smith K, Stringfellow K, Clemente S, Brister R, et al. Effect of β-mannanase inclusion on growth performance, ileal digestible energy, and intestinal viscosity of male broilers fed a reduced-energy diet. J Appl Poult Res. 2015;25:40–7. [Google Scholar]
- 27.Donkoh A. Ambient temperature: A factor affecting performance and physiological response of broiler chickens. Int J Biometeorol. 1989;33:259–65. doi: 10.1007/BF01051087. [DOI] [PubMed] [Google Scholar]
- 28.Siegel HS. Gordon memorial lecture. Stress, strains and resistance. Br Poult Sci. 1995;36:3–22. doi: 10.1080/00071669508417748. [DOI] [PubMed] [Google Scholar]
- 29.Njoku PC. Effect of dietary ascorbic acid (Vitamin C) supplementation on the performance of broiler chickens in a tropical environment. Anim Feed Sci Technol. 1986;16:17–24. [Google Scholar]
- 30.van den Brand H, Molenaar R, van der Star I, Meijerhof R. Early feeding affects resistance against cold exposure in young broiler chickens. Poult Sci. 2010;89:716–20. doi: 10.3382/ps.2009-00432. [DOI] [PubMed] [Google Scholar]
- 31.Tan GY, Yang L, Fu YQ, Feng JH, Zhang MH. Effects of different acute high ambient temperatures on function of hepatic mitochondrial respiration, antioxidative enzymes, and oxidative injury in broiler chickens. Poult Sci. 2010;89:115–22. doi: 10.3382/ps.2009-00318. [DOI] [PubMed] [Google Scholar]
- 32.Costa-Pinto FA, Palermo-Neto J. Neuroimmune interactions in stress. Neuroimmunomodulation. 2010;17:196–9. doi: 10.1159/000258722. [DOI] [PubMed] [Google Scholar]
- 33.Quinteiro-Filho WM, Righi DA, Palermo-Neto J. Effect of cyhalothrin on Ehrlich tumor growth and macrophage activity in mice. Braz J Med Biol Res. 2009;42:912–7. doi: 10.1590/s0100-879x2009001000006. [DOI] [PubMed] [Google Scholar]
- 34.Thaxton JP, Puvadolpirod S. Model of physiological stress in chickens 5.Quantitative evaluation. Poult Sci. 2000;79:391–5. doi: 10.1093/ps/79.3.391. [DOI] [PubMed] [Google Scholar]
- 35.Gross WB, Siegel HS. Evaluation of the heterophil/lymphocyte ratio as a measure of stress in chickens. Avian Dis. 1983;27:972–9. [PubMed] [Google Scholar]
- 36.Post J, Rebel JM, ter Huurne AA. Physiological effects of elevated plasma corticosterone concentrations in broiler chickens. An alternative means by which to assess the physiological effects of stress. Poult Sci. 2003;82:1313–8. doi: 10.1093/ps/82.8.1313. [DOI] [PubMed] [Google Scholar]
- 37.Zulkifli I, Sti Nor Azah A. Fear and stress reactions and the performance of commercial broiler chickens subjected to regular pleasant and unpleasant contacts with human being. Appl Anim Behav Sci. 2004;88:77–87. [Google Scholar]
- 38.Sohail MU, Hume ME, Byrd JA, Nisbet DJ, Ijaz A, Sohail A, et al. Effect of supplementation of prebiotic mannan-oligosaccharides and probiotic mixture on growth performance of broilers subjected to chronic heat stress. Poult Sci. 2012;91:2235–40. doi: 10.3382/ps.2012-02182. [DOI] [PubMed] [Google Scholar]
- 39.Warriss PD, Pagazaurtundua A, Brown SN. Relationship between maximum daily temperature and mortality of broiler chickens during transport and lairage. Br Poult Sci. 2005;46:647–51. doi: 10.1080/00071660500393868. [DOI] [PubMed] [Google Scholar]
- 40.Satterlee DG, Aguilera-Quintana I, Munn BJ, Krautmann BA. Vitamin C amelioration of the adrenal stress response in broiler chickens being prepared for slaughter. Comp Biochem Physiol A Comp Physiol. 1989;94:569–74. doi: 10.1016/0300-9629(89)90595-1. [DOI] [PubMed] [Google Scholar]
- 41.Jaffar GH, Bláha J. Effect of ascorbic acid supplementation in drinking water on growth rate, feed consumption and feed efficiency of broiler chickens maintained under acute heat stress conditions. 1. Vol. 41. Universitas Agric Praga Press; 1996. pp. 485–90. [Google Scholar]
- 42.Bláha J, Kroesna K. Effect of vitamin and electrolytes supplements on broilers performance, slaughter value and chemical composition of meat during the heat stress. Vol. 30. Universitäs Agric Praga Press; 1997. pp. 103–13. [Google Scholar]
- 43.Vathana S, Kang K, Loan CP, Thinggaard G, Kabasa JD, ter Meulen U. Effect of Vitamin C Supplementation on Performance of Broiler Chicken in Cambodia. Conference on International Agricultural Research for Development Deutscher Tropentag. 2002 [Google Scholar]
- 44.Attia YA, Hassan RA, Qota EM. Recovery from adverse effects of heat stress on slow-growing chicks in the tropics 1: Effect of ascorbic acid and different levels of betaine. Trop Anim Health Prod. 2009;41:807–18. doi: 10.1007/s11250-008-9256-9. [DOI] [PubMed] [Google Scholar]
- 45.Edgar JL, Nicol CJ, Pugh CA, Paul ES. Surface temperature changes in response to handling in domestic chickens. Physiol Behav. 2013;119:195–200. doi: 10.1016/j.physbeh.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 46.Chen XY, Wei PP, Xu SY, Geng ZY, Jiang RS. Rectal temperature as an indicator for heat tolerance in chickens. Anim Sci J. 2013;84:737–9. doi: 10.1111/asj.12064. [DOI] [PubMed] [Google Scholar]
- 47.Egbuniwe CI, Ayo JO, Kawu UM, Mohammed A. Cloacal temperature responses of broiler chickens administered with betaine and ascorbic acid during the hot dry season. Biol Rhythm Res. 2015;46:207–19. [Google Scholar]
- 48.Krautmann BA, Gwynther MJ, Peterson LA. Ascorbic Acid in Domestic Animals: Symposium; Kartause Ittingen. Vol. 2. Switzerland: 1990. Practical Applications of Ascorbic Acid for Poultry; pp. 292–313. [Google Scholar]
- 49.Attia M. Effect of different levels of Vitamin C on body temperature of white Russian birds during heat stress. Egypt Vet Med J. 1976;24:111. [Google Scholar]
- 50.Kutlu HR, Forbes JM. Changes in growth and blood parameters in heat stressed broiler chicks in response to dietary ascorbic acid. Livest Prod Sci. 1993;36:335–50. [Google Scholar]
- 51.Mode SG, Funde ST, Waghmare SP, Kolte AY. Effect of herbal immuno modulator on body weight gain in immunosuppressed broiler birds. Vet World. 2009;2:269–70. [Google Scholar]
- 52.Jothie Richard E, Illuri R, Bethapudi B, Anandhakumar S, Bhaskar A, Chinampudur Velusami C, et al. Anti-stress activity of Ocimum sanctum: Possible effects on hypothalamic-pituitary-adrenal axis. Phytother Res. 2016;30:805–14. doi: 10.1002/ptr.5584. [DOI] [PubMed] [Google Scholar]
- 53.Patel AP, Bhagwat SR, Pawar MM, Prajapati KB, Chauhan HD, Makwana RB, et al. Evaluation of Emblica officinalis fruit powder as a growth promoter in commercial broiler chickens. Vet World. 2016;9:207–10. doi: 10.14202/vetworld.2016.207-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akotkar NS, Sarag AN, Rakhate DH, Dhok AP. Effect of supplementation of Ashwagandha (Withania somnifera) on performance of broilers. Indian J Poult Sci. 2007;42:92–4. [Google Scholar]
- 55.Kaur P, Mathur S, Sharma M, Tiwari M, Srivastava KK, Chandra R, et al. A biologically active constituent of Withania somnifera (Ashwagandha) with antistress activity. Indian J Clin Biochem. 2001;16:195–8. doi: 10.1007/BF02864860. [DOI] [PMC free article] [PubMed] [Google Scholar]


