Abstract
Background:
Oxidative stress is frequently identified as a key element in the pathophysiology of many complications of diabetes mellitus, including reproductive complications. The antioxidant potential of medicinal plants have been suggested for therapeutic focus of diseases in recent reports.
Objective:
To investigate the effect of Basella alba (Ba) aqueous leave extract on diabetes-induced oxidative stress.
Materials and Methods:
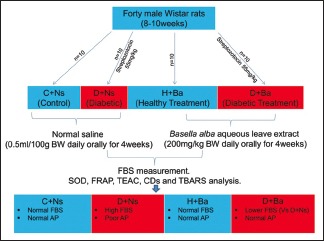
Forty male Wistar rats (8–10 weeks) were randomly divided into four groups (n = 10) and treated as follows; Control (C + Ns) and Diabetic (D + Ns) animals received oral normal saline 0.5 ml/100 g body weight daily, while Healthy Treatment (H + Ba) and Diabetic Treatment (D + Ba) rats were given Ba extract at an oral dose of 200 mg/kg body weight daily. Treatment was by gavage and lasted 4 weeks in all groups. Diabetes was induced in D + Ns and D + Ba rats by single intraperitoneal injection of streptozotocin (55 mg/kg) and fasting blood sugar (FBS) recorded weekly in all rats afterwards. Animals were euthanized at the end of the experiment and blood samples, pancreas, testes, and epididymis were preserved for analysis of oxidative stress biomarkers.
Results:
Oral administration of aqueous leave extract of Ba significantly (P < 0.0001) lowered FBS in D + Ba rats. There was significantly higher blood superoxide dismutase activity and serum ferric reducing antioxidant power, but lower serum concentration of conjugated dienes and thiobarbituric acid reactive substances in D + Ba compared to D + Ns rats (P < 0.05).
Conclusion:
Ba exerts antioxidant effects in the gonads by enhancing antioxidant parameters in circulating blood, but not necessarily in the gonadal tissues.
SUMMARY
Oral treatment of diabetic rats with aqueous leave extract of Basella alba exerts antioxidant effects in the gonads by enhancing antioxidant parameters in circulating blood, but not necessarily in the gonadal tissues.
Abbreviations Used: AP – Antioxidant parameters, Ba – Basella alba, CAT – Catalase, CDs - Conjugated dienes, DM – Diabetes mellitus, FBS – Fasting blood sugar, FRAP - Ferric reducing antioxidant power, GSH - reduced glutathione, Ns – Normal saline, ORAC - oxygen radical antioxidant capacity, RNS - reactive nitrogen species, ROS - reactive oxygen species, SOD - superoxide dismutase, TAC - Total antioxidant capacity, TBARS - thiobarbituric acid reactive substances, TEAC - trolox equivalent antioxidant capacity.
Key words: Antioxidant, Basella alba, blood, gonads, oxidative stress, pancreas
INTRODUCTION
Oxidative stress as a result of increased production of free radicals is becoming more frequently identified as a key pathophysiological element in most complications of diabetes mellitus (DM).[1,2,3,4] The body is equipped with a number of enzymatic and nonenzymatic antioxidant systems with the primary role of neutralizing free radicals such as reactive oxygen species (ROS) and reactive nitrogen species (RNS), thereby rendering them nontoxic to body cells.[5] ROS are inevitable by-products of normal physiological processes in the body and are required to an extent in the immune system for antimicrobial actions and also function as signaling molecules in the maintenance of intracellular homeostasis.[6] In conditions where the rate of generation of these free radicals is increased or the protective antioxidant mechanism is reduced, an imbalance occurs in favor of free radicals leading to increased oxidative stress and subsequent tissue damage.[7] The resultant tissue damage is caused by the tendency for these unstable oxygen/nitrogen species to enter into reactions with cellular component by donating free electrons which results in DNA damage and peroxidative changes in cell membrane.[8] This is the basis of most health complications of diseases that trigger increased generation of systemic oxidative stress, including DM.
DM is a metabolic disorder that is characterized by chronic hyperglycemia due to relative or absolute insulin deficiency.[9] The two major classes of DM are insulin dependent or type 1 DM (insulin deficiency due to auto-immune β-islet cell damage) and noninsulin dependent or type 2 (target organ resistance to insulin).[10] Both classes essentially encompass abnormalities in carbohydrate, fat, and protein metabolism due to deficient insulin action.[11] The persistent hyperglycemia in DM stimulate increased generation of ROS, which coupled with the weakened body defense system, result in an imbalance between ROS and antioxidants that culminates in oxidative stress.[12] The generation of oxidative stress in DM occurs through a number of pathophysiological routes. Chronic hyperglycemia might result in auto-oxidation of glucose, a shift in redox balance, limited activities of antioxidant enzymes such as catalase (CAT) and superoxide dismutase (SOD) or depletion of low-molecular-weight antioxidants like Vitamin E and reduced glutathione (GSH).[13] In addition, metabolic modification of proteins and/or lipids in DM leads to the formation of advanced glycation end products, which is said to increase generation of ROS as well as destroys the antioxidant enzyme system.[14]
Dysfunctional free radical scavenging and increased production of ROS/RNS have been frequently implicated in the pathophysiology of male reproductive complications of DM,[15,16] as well as other systemic complications. The use of medicinal plants as alternative therapies in the management of DM and its complications is therefore largely hinged on both the free radical scavenging and anti-hyperglycemic potentials of the phytochemical components of such plants.[17] Basella alba (Ba), also called Malabar or Indian spinach, is a creeping mucilaginous green vegetable that have been reported for its anti-hyperglycemic activity and successfully used in the treatment of experimental DM in rats.[18] Ba is believed to originate from Asia but is widely cultivated and consumed as vegetable in the humid tropical regions of Africa and have been reportedly used to relieve sexual asthenia in Cameroonian men.[19] Previous studies have also confirmed the androgenic properties of the plant[20,21] and unpublished experimental data by our research group demonstrated the value of Ba in alleviating some diabetes-induced abnormalities in sperm parameters. The antioxidant properties of some of the phytochemical components of the plant have been suggested as a possible reason for the antidiabetic and profertility effects. This present study aimed at investigating the concise in vivo effect of orally administered aqueous leave extract of Ba on the various biomarkers of oxidative stress in the systemic circulation, pancreatic tissues as well as in the gonadal tissues of diabetic male rats.
MATERIALS AND METHODS
Plant material and extract preparation
Fresh Ba was collected from multiple locations within Osun state, southwestern Nigeria and authenticated by the Department of Botany, University of Ibadan, Nigeria under voucher number UIH-22391. The leaves were removed from the stem, washed, and then dried at room temperature for about 4 weeks. This was macerated into fine powdery form and 100 g extracted in 1000 ml distilled water using the method of Iloki-Assanga et al., 2015.[22] The product of extraction was filtered through a muslin cloth and freeze-dried into a powdery extract which was dissolved in normal saline to form the stock solution that was administered to rats by oral gavage during this study.
Animal care and ethical considerations
Male Wistar rats were obtained from the animal unit at Stellenbosch University and acclimatized in the animal experimental laboratory of the Faculty of Medicine and Health Sciences, Stellenbosch University, Tygerberg, South Africa. Animals had free access to food (standard rat chow) and water with the exception of periods before fasting blood sugar (FBS) sample collection when they were fasted overnight. Housing was under standard atmospheric conditions and animals were exposed to a light/dark cycle of 12 h. Compliance with the National Institutes of Health Guidelines (National Institutes of Health Publication No. 80-23, revised 1978) for the handling of Laboratory animals was ensured. The Health and Wellness Sciences Research Ethics Committee (HWS-REC) of Cape Peninsula University of Technology, Cape Town, South Africa, granted ethical approval for this study with reference number; CPUT/HWS-REC 2015/A04.
Study design
A total of forty male Wistar rats, 8–10 weeks of age were used for this study. These were randomly divided into four groups of ten rats each and treated as follows; Control (C + Ns) and Diabetic (D + Ns) animals received oral normal saline 0.5 ml/100 g body weight daily, and Healthy Treatment (H + Ba) and Diabetic Treatment (D + Ba) rats were given Ba extract at an oral dose of 200 mg/kg body weight daily. Administration of the extract and normal saline was done by gavage using a metal endotracheal tube.
Induction of diabetes mellitus and measurement of fasting blood sugar
DM was induced in rats from diabetic control and diabetic treatment groups by single intraperitoneal injection of streptozotocin (STZ), 55 mg/kg subsequent to an overnight fast. STZ was prepared fresh just before each administration by dissolving in ice cold citrate buffer (0.1M) at a pH of 4.5. Animals were allowed access to food and water afterward, and FBS was recorded after 72 h to confirm diabetes. Rats with FBS above 11.1 mmol/L (200 mg/dL) were considered diabetic and included in this study.[23] FBS was subsequently recorded weekly in all animals from the four experimental groups after the commencement of treatment. Blood sample was taken from tail capillaries to determine FBS using a standard glucometer (ONETOUCH® Ultra2).
Preparation of serum and whole blood samples
At the completion of 4 weeks of treatment, all animals were euthanized through exsanguination under high dose (100 mg/kg) intraperitoneal sodium pentobarbital and blood was collected through cardiac puncture. For serum, blood was collected into serum clot activator tubes (VACUETTE®) and centrifuged at 4000 rpm in 4°C for 10 min to get the supernatant. Whole blood samples were dispensed into ethylenediaminetetraacetic acid tubes and all samples were stored at −80°C until further biochemical analysis.
Preparation of tissue homogenates (pancreas, testes, and epididymis)
All animals in the four experimental groups were euthanized and dissected after blood sample collection. The pancreas, testes, and epididymis were excised, washed, and preserved at −80°C. Frozen samples of these tissues were weighed and homogenized in glass tubes on ice, centrifuged at 15,000 rpm for 10 min at 4°C and the supernatant kept at −80°C for further analysis.
Analysis of reduced glutathione and antioxidant enzymes
The concentration of reduced GSH was determined in whole blood samples and tissue homogenates according to the method of Asensi et al.[24]
SOD and CAT activities were determined in whole blood and tissue homogenates using the method described by Ellerby and Bredesen.[25]
Determination of antioxidant capacities
Antioxidant capacity was measured in all serum samples by three different methods; ferric reducing antioxidant power (FRAP) was determined according to the method of Benzie and Strain,[26] trolox equivalent antioxidant capacity (TEAC) was determined using the method described by Re et al.,[27] and oxygen radical antioxidant capacity (ORAC) according to the method of Cao and Prior.[28]
Estimation of lipid peroxidation
Lipid peroxidation was quantified through two methods; Initiation of peroxidation was assessed by measuring conjugated dienes (CDs) concentration in serum samples according to the method of Recknagel and Glende.[29] Termination of lipid peroxidation was assessed by determining the concentration of thiobarbituric acid reactive substances (TBARS) in serum samples according to the method of Esterbauer and Cheeseman.[30]
Statistical analysis
Data for all parameters are expressed as mean ± standard error of the mean. GraphPad Prism version 5.0 (GraphPad Software, Inc. USA) was used for the data analysis employing analysis of variance for multiple comparisons with Bonferroni's posttest. Confidence interval was placed at 95% (P < 0.05).
RESULTS
Effect of Basella alba on fasting blood sugar
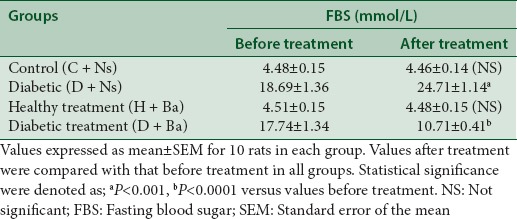
Ba significantly lowered FBS in rats from the diabetic treatment group after 4 weeks of treatment as opposed to the diabetic control group where FBS was significantly higher after the experimental period (P < 0.0001 and P < 0.001 respectively versus FBS before treatment). This is shown in Table 1.
Table 1.
Fasting blood sugar before commencement and after the completion of treatment in control and experimental groups
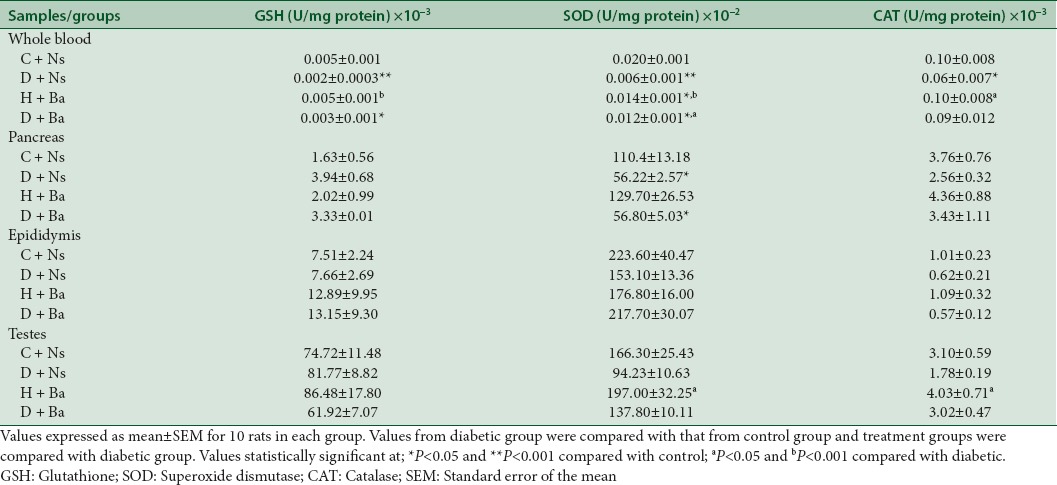
Effect of Basella alba on enzymatic and nonenzymatic antioxidants
As demonstrated in Table 2, the blood concentration of nonenzymatic antioxidant GSH and antioxidant enzymes SOD and CAT were significantly lower in samples from rats in the D + Ns group when compared to C + Ns and H + Ns rats (P < 0.001 for GSH and SOD, P < 0.05 for CAT). However, apart from the lower concentration of SOD in pancreas homogenates (P < 0.05), the differences in concentration of these antioxidants in tissue homogenates were not statistically significant when the D + Ns group was compared to C + Ns group. SOD concentration in blood was also observed to be significantly higher (P < 0.05) in diabetic rats treated with the plant extract (D + Ba) when compared with the untreated diabetic (D + Ns) counterpart. However, all other differences observed in blood and tissue concentrations of the three antioxidant parameters between the D + Ba and D + Ns groups were not statistically significant.
Table 2.
Effect of treatments on enzymatic and nonenzymatic antioxidant activities in blood and tissue homogenates of rats from control and experimental groups
Effect of Basella alba on the antioxidant capacities of serum
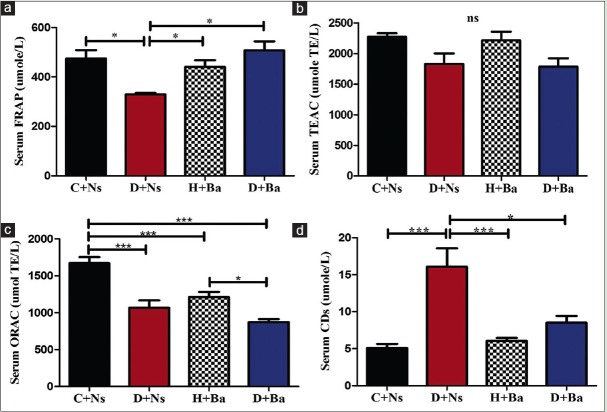
Figure 1a demonstrates a significantly (P < 0.05) lower FRAP in the serum samples from diabetic rats when compared to the remaining three groups. There was no significant difference in TEAC among the four experimental group [Figure 1b], but ORAC was significantly higher in the control rat's serum compared to the other experimental groups and in H + Ba compared to D + Ba as shown in Figure 1c (P < 0.0001 and P < 0.05, respectively).
Figure 1.
Antioxidant capacities FRAP, TEAC and ORAC (a, b and c respectively) and concentration of conjugated dienes (d) in serum of rats from control and experimental groups. (*P<0.05, **P<0.001, ***P<0.0001, ns = Differences not statistically significant)
Effect of Basella alba on serum concentration of conjugated dienes
The level of CDs in the serum samples of rats in the experimental groups was demonstrated in Figure 1d. CDs concentration was significantly (P < 0.001) higher in the diabetic group of rats compared to the healthy rats (C + Ns and H + Ba), but significantly (P < 0.05) lower in the diabetic rats treated with the study extract (D + Ba) when compared with the untreated counterpart (D + Ns).
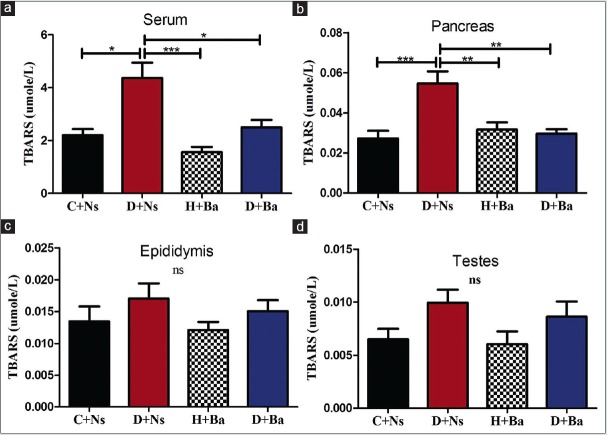
Effect of Basella alba on concentration of thiobarbituric acid reactive substances
As shown in Figure 2a, serum concentration of TBARS was significantly higher in rats from D + Ns group when compared to the other groups (P < 0.05 vs. C + Ns, D + Ba, and P < 0.0001 vs. H + Ba). Similarly, as demonstrated in Figure 2b, TBARS concentration was significantly higher in pancreas homogenates of rats from D + Ns when compared to the other three groups (P < 0.0001 vs. C + Ns and P < 0.001 vs. H + Ba, D + Ba). Despite equally higher concentrations of TBARS in both epididymis and testes homogenates of rats in the diabetic group, the observed differences were not statistically significant [Figure 2c and d respectively].
Figure 2.
Concentration of thiobarbituric acid reactive substances (TBARS) in serum (a), Pancreas (b), Epididymis (c) and Testes (d) of rats from control and experimental groups. (*P<0.05, **P<0.001, ***P<0.0001, ns = Differences not statistically significant)
DISCUSSION
In line with previous reports, findings in this study demonstrated the antihyperglycemic ability of Ba as confirmed by the lower FBS in D + Ba rats after 4 weeks of treatment [Table 1]. This antihyperglycemic effect may be sufficient in bringing about significant correction in the oxidative stress status of the animals since prolonged hyperglycemia in itself have been said to induce oxidative stress.[12,13] However, the possibility of the lowering of blood sugar being a direct consequence of antioxidant activity of some phytochemical components of the plant have also been suggested.[18,31] The findings in this study confirmed both the antioxidant and antihyperglycemic effects of Ba, but it is not exactly certain if one occurred as a direct sequel of the other or both effects were achieved via separate mechanisms. Both events however appear to occur in a mutually synergistic cycle.
Changes in concentration and activities of enzymatic and nonenzymatic antioxidants in biological systems following an in vivo oxidative insult have been a subject of research controversy over the years. Whereas some studies have reported a decrease in the activity of endogenous antioxidants such as GSH, SOD, and CAT following exposure to oxidative stress,[5,32,33] others have reported increased activities of the antioxidant enzymes in the face of persistent exposure to oxidative stress.[34,35,36,37] It is therefore imperative to acknowledge some of the factors that may determine the level of antioxidant enzyme activities in biological tissues when subjected to oxidative stress. The duration and severity of oxidative stress is an important factor since antioxidant enzyme activity is presumed to increase initially in response to oxidative stressors, but as the stress process become persistent, the enzyme activities may decrease due to damage to the proteins and genes responsible for the antioxidant enzyme expression. There is also a variation in the enzymatic activities in the various tissues of the body and for instance, pancreatic islets have been described as one of the tissues with the lowest intrinsic antioxidant defenses.[7] In this present study, the result revealed a significant reduction in the concentration of GSH and activities of SOD and CAT in the blood samples of diabetic control rats, whereas, these animals showed no significant change in the expression of the antioxidants in the gonads and pancreas except for the reduced SOD activity observed in pancreas homogenate. This suggests worse antioxidant status in the systemic circulation compared to the tissues, and expectedly, the pancreas being the primary organ of insult in STZ diabetes showed significantly reduced SOD activity. Furthermore, the dismutation of superoxide anions (O2–) into molecular oxygen (O2) and hydrogen peroxide (H2O2) by SOD is the first line of defense against ROS-induced tissue damage,[10] therefore, generation of a greater volume of O2– in the pancreatic islet may explain why SOD activity is more significantly reduced in the pancreatic tissues of the diabetic rats. Ba appears to offer some form of protection against this imbalance in the treated diabetic group of rats as evidenced by the higher SOD activity when they were compared with rats from the untreated diabetic group. We hypothesized that this protective action of Ba leave extract may be due to its ability to directly inactivate superoxide anions thereby sparing SOD, or due to a component of the extract that mimics SOD activity by enhancing dismutation of the anions into oxygen and hydrogen peroxide.
The more obvious confirmation of the antioxidant properties of Ba in our result was displayed in the effects it exerted on serum total antioxidant capacity (TAC) and lipid peroxidation in both serum and the tissues.
TAC is a term that is used in attempts to describe the cumulative effect of the various intrinsic antioxidants in body fluids[38] and is said to increase in vivo after the consumption of foods rich in phenolic compounds[39] and flavonoids.[38] In this study, TAC was assessed via three methods (FRAP, TEAC, and ORAC), each of which employs three different mechanisms. TEAC and ORAC are direct assays that are based on the ability of the samples to inhibit substance oxidation while FRAP is indirect and based on evaluating the ability of the samples to reduce iron-complex from ferric to ferrous state.[40] FRAP was significantly increased in the group of diabetic rats treated with Ba when compared with the untreated counterpart, but TEAC and ORAC values were not significantly different between the two groups. This is a confirmation of the fact that TAC varies considerably depending on the assay method used since the various methods actually measure different components of TAC.[41] In human serum, FRAP is said to measure mainly uric acid while TEAC measures mainly albumin but in addition, both assays also measure α-tocopherol, bilirubin, and ascorbic acid.[40]
The accumulation of products of lipid peroxidation, CDs, and TBARS, was highly significant in the serum and pancreatic tissues of the untreated diabetic rats, but not in the testis and epididymis. This notwithstanding, data not published in the present study suggested gonadal complications of DM as evidenced by subnormal sperm parameters in the diabetic animals. This means systemic oxidative stress induced by DM can impact negatively on gonadal functions without necessarily causing significant local oxidative damage in the gonads. The lower concentration of TBARS observed in the diabetic rats that were treated with Ba for 4 weeks was equally significant in the serum and pancreas but not in the gonadal tissues. This is an indication that the antioxidant effect of Ba may be more of a systemic rather than localized tissue action and therefore, the beneficial effect in ameliorating diabetic complications is expected to be multisystemic.
CONCLUSION
Ba ameliorate diabetes-induced oxidative stress and some of the complications associated with it. The antioxidant effect of the plant in diabetic rats is closely related to the antihyperglycemic effect, and the two actions appear to enhance each other mutually. This study also establishes the predominantly systemic nature of the antioxidant activities of Ba rather than localized tissue effects, but further studies involving a wider variety of tissues will be needed to fully substantiate this observation.
Financial support and sponsorship
The work was partly supported by the University Research Fund (URF) awarded to Dr YG Aboua by Cape Peninsula University of Technology, Cape Town, South Africa.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: A new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Ceriello A, Morocutti A, Mercuri F, Quagliaro L, Moro M, Damante G, et al. Defective intracellular antioxidant enzyme production in type 1 diabetic patients with nephropathy. Diabetes. 2000;49:2170–7. doi: 10.2337/diabetes.49.12.2170. [DOI] [PubMed] [Google Scholar]
- 3.Telci A, Cakatay U, Salman S, Satman I, Sivas A. Oxidative protein damage in early stage type 1 diabetic patients. Diabetes Res Clin Pract. 2000;50:213–23. doi: 10.1016/s0168-8227(00)00197-2. [DOI] [PubMed] [Google Scholar]
- 4.Matough FA, Budin SB, Hamid ZA, Alwahaibi N, Mohamed J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ Med J. 2012;12:5–18. doi: 10.12816/0003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taheri E, Djalali M, Saedisomeolia A, Moghadam AM, Djazayeri A, Qorbani M, et al. The relationship between the activates of antioxidant enzymes in red blood cells and body mass index in Iranian type 2 diabetes and healthy subjects. J Diabetes Metab Disord. 2012;11:3. doi: 10.1186/2251-6581-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halliwell B. Antioxidants in human health and disease. Annu Rev Nutr. 1996;16:33–50. doi: 10.1146/annurev.nu.16.070196.000341. [DOI] [PubMed] [Google Scholar]
- 7.Tiwari BK, Pandey KB, Abidi AB, Rizvi SI. Markers of oxidative stress during diabetes mellitus. J Biomark. 2013;2013:378790. doi: 10.1155/2013/378790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matés JM, Pérez-Gómez C, Núñez de Castro I. Antioxidant enzymes and human diseases. Clin Biochem. 1999;32:595–603. doi: 10.1016/s0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35(Suppl 1):S64–71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asmat U, Abad K, Ismail K. Diabetes mellitus and oxidative stress - A concise review. Saudi Pharm J. 2016;24:547–53. doi: 10.1016/j.jsps.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(Suppl 1):S62–7. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandey KB, Mishra N, Rizvi SI. Protein oxidation biomarkers in plasma of type 2 diabetic patients. Clin Biochem. 2010;43:508–11. doi: 10.1016/j.clinbiochem.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Haskins K, Bradley B, Powers K, Fadok V, Flores S, Ling X, et al. Oxidative stress in type 1 diabetes. Ann N Y Acad Sci. 2003;1005:43–54. doi: 10.1196/annals.1288.006. [DOI] [PubMed] [Google Scholar]
- 14.Nowotny K, Jung T, Höhn A, Weber D, Grune T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules. 2015;5:194–222. doi: 10.3390/biom5010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.La Vignera S, Condorelli R, Vicari E, D'Agata R, Calogero AE. Diabetes mellitus and sperm parameters. J Androl. 2012;33:145–53. doi: 10.2164/jandrol.111.013193. [DOI] [PubMed] [Google Scholar]
- 16.Jain GC, Jangir RN. Modulation of diabetes-mellitus-induced male reproductive dysfunctions in experimental animal models with medicinal plants. Pharmacogn Rev. 2014;8:113–21. doi: 10.4103/0973-7847.134245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan CH, Ngoh GC, Yusoff R. A brief review on anti diabetic plants: Global distribution, active ingredients, extraction techniques and acting mechanisms. Pharmacogn Rev. 2012;6:22–8. doi: 10.4103/0973-7847.95854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bamidele O, Arokoyo DS, Akinnuga AM, Oluwarole AO. Antidiabetic effect of aqueous extract of Basella alba leaves and metformin in alloxan-induced diabetic albino rats. Afr J Biotechnol. 2014;13:2455–8. [Google Scholar]
- 19.Adhikari R, Kumar HN, Shruthi SD. A review of medicinal importance of Basella alba L. Int J Pharm Sci Drug Res. 2012;4:110–4. [Google Scholar]
- 20.Nantia EA, Moundipa PF, Monsees TK, Carreau S. Medicinal plants as potential male anti-infertility agents: A review. Andrologie. 2009;19:148–58. [Google Scholar]
- 21.Nantia EA, Travert C, Manfo FP, Carreau S, Monsees TK, Moundipa PF, et al. Effects of the methanol extract of Basella alba L (Basellaceae) on steroid production in Leydig cells. Int J Mol Sci. 2011;12:376–84. doi: 10.3390/ijms12010376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iloki-Assanga SB, Lewis-Luján LM, Lara-Espinoza CL, Gil-Salido AA, Fernandez-Angulo D, Rubio-Pino JL, et al. Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. And Phoradendron californicum. BMC Res Notes. 2015;8:396. doi: 10.1186/s13104-015-1388-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pournaghi P, Sadrkhanlou RA, Hasanzadeh S, Foroughi A. An investigation on body weights, blood glucose levels and pituitary-gonadal axis hormones in diabetic and metformin-treated diabetic female rats. Vet Res Forum. 2012;3:79–84. [PMC free article] [PubMed] [Google Scholar]
- 24.Asensi M, Sastre J, Pallardo FV, Lloret A, Lehner M, Garcia-de-la Asuncion J, et al. Ratio of reduced to oxidized glutathione as indicator of oxidative stress status and DNA damage. Methods Enzymol. 1999;299:267–76. doi: 10.1016/s0076-6879(99)99026-2. [DOI] [PubMed] [Google Scholar]
- 25.Ellerby LM, Bredesen DE. Measurement of cellular oxidation, reactive oxygen species, and antioxidant enzymes during apoptosis. Methods Enzymol. 2000;322:413–21. doi: 10.1016/s0076-6879(00)22040-5. [DOI] [PubMed] [Google Scholar]
- 26.Benzie IF, Strain JJ. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999;299:15–27. doi: 10.1016/s0076-6879(99)99005-5. [DOI] [PubMed] [Google Scholar]
- 27.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C, et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–7. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 28.Cao G, Prior RL. Measurement of oxygen radical absorbance capacity in biological samples. Methods Enzymol. 1999;299:50–62. doi: 10.1016/s0076-6879(99)99008-0. [DOI] [PubMed] [Google Scholar]
- 29.Recknagel RO, Glende EA., Jr Spectrophotometric detection of lipid conjugated dienes. Methods Enzymol. 1984;105:331–7. doi: 10.1016/s0076-6879(84)05043-6. [DOI] [PubMed] [Google Scholar]
- 30.Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–21. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 31.Olajire AA, Azeez L. Total antioxidant activity, phenolic, flavonoid and ascorbic acid contents of Nigerian vegetables. Afr J Food Sci Technol. 2011;2:22–9. [Google Scholar]
- 32.Pari L, Umamaheswari J. Antihyperglycaemic activity of Musa sapientum flowers: Effect on lipid peroxidation in alloxan diabetic rats. Phytother Res. 2000;14:136–8. doi: 10.1002/(sici)1099-1573(200003)14:2<136::aid-ptr607>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 33.Bajaj S, Khan A. Antioxidants and diabetes. Indian J Endocrinol Metab. 2012;16:S267–71. doi: 10.4103/2230-8210.104057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shull S, Heintz NH, Periasamy M, Manohar M, Janssen YM, Marsh JP, et al. Differential regulation of antioxidant enzymes in response to oxidants. J Biol Chem. 1991;266:24398–403. [PubMed] [Google Scholar]
- 35.Michiels C, Raes M, Toussaint O, Remacle J. Importance of se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free Radic Biol Med. 1994;17:235–48. doi: 10.1016/0891-5849(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 36.Dias JP, Talbot S, Sénécal J, Carayon P, Couture R. Kinin B1 receptor enhances the oxidative stress in a rat model of insulin resistance: Outcome in hypertension, allodynia and metabolic complications. PLoS One. 2010;5:e12622. doi: 10.1371/journal.pone.0012622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zelen I, Djurdjevic P, Popovic S, Stojanovic M, Jakovljevic V, Radivojevic S, et al. Antioxidant enzymes activities and plasma levels of oxidative stress markers in B-chronic lymphocytic leukemia patients. J BUON. 2010;15:330–6. [PubMed] [Google Scholar]
- 38.Sies H. Total antioxidant capacity: Appraisal of a concept. J Nutr. 2007;137:1493–5. doi: 10.1093/jn/137.6.1493. [DOI] [PubMed] [Google Scholar]
- 39.Cao G, Russell RM, Lischner N, Prior RL. Serum antioxidant capacity is increased by consumption of strawberries, spinach, red wine or Vitamin C in elderly women. J Nutr. 1998;128:2383–90. doi: 10.1093/jn/128.12.2383. [DOI] [PubMed] [Google Scholar]
- 40.Rubio CP, Hernández-Ruiz J, Martinez-Subiela S, Tvarijonaviciute A, Ceron JJ. Spectrophotometric assays for total antioxidant capacity (TAC) in dog serum: An update. BMC Vet Res. 2016;12:166. doi: 10.1186/s12917-016-0792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winter JL, Barber LG, Freeman L, Griessmayr PC, Milbury PE, Blumberg JB, et al. Antioxidant status and biomarkers of oxidative stress in dogs with lymphoma. J Vet Intern Med. 2009;23:311–6. doi: 10.1111/j.1939-1676.2009.0273.x. [DOI] [PubMed] [Google Scholar]