Abstract
Introduction
As many as 250 million children under the age of 5 may not be reaching their full developmental potential partly due to poor nutrition during pregnancy and the first 2 years of life. Micronutrients, including vitamin B12, are important for the development of brain structure and function; however, the timing, duration and severity of deficiencies may alter the impact on functional development outcomes. Consequently, to fully explore the effect of vitamin B12 on cognitive function, it is crucial to measure neurodevelopment at different ages, in different populations and with vitamin B12 supplementation at different times during the critical periods of neurodevelopment.
Methods and analysis
In this project, we follow up children from four recently completed randomised placebo-controlled trials of oral vitamin B12 supplementation, two in India and two in Tanzania, to explore the long-term effects on neurodevelopmental outcomes and growth. All the included trials provided at least two recommended dietary allowances of oral vitamin B12 daily for at least 6 months. Vitamin B12 was supplemented either during pregnancy, early infancy or early childhood. Primary outcomes are neurodevelopmental status, cognitive function and growth later in childhood. We apply validated and culturally appropriate instruments to identify relevant developmental outcomes. All statistical analyses will be done according to intention-to-treat principles. The project provides an excellent opportunity to examine the effect of vitamin B12 supplementation in different periods during early life and measure the outcomes later in childhood.
Ethics and dissemination
The study has received ethical approvals from all relevant authorities in Norway, USA, Tanzania and India and complies fully with ethical principles for medical research. Results will be presented at national and international research and policy meetings and published in peer-reviewed scientific journals, preferably open access.
Trial registration number
NCT00641862 (Bangalore); NCT00717730, updated CTRI/2016/11/007494 (Delhi); NCT00197548 and NCT00421668 (Dar es Salaam).
Keywords: vitamin B12, neurodevelopment, cognitive function, event related potential, executive function, growth
Strengths and limitations of this study.
The current project takes advantage of recently completed randomised placebo-controlled trials to study long-term effects of oral vitamin B12 supplementation on neurodevelopmental outcomes and growth in children in Tanzania and India.
The project provides an excellent opportunity to examine the effect of vitamin B12 supplementation during different periods of the critical first 1000 days and measure the outcomes later in childhood.
The studies were not designed to follow the children up beyond the time of supplementation, which may lead to a somewhat high rate of loss to follow-up.
The effect measures may be difficult to standardise across the different studies.
Introduction
New estimates based on proxy measures of stunting and poverty indicate that as many as 250 million children under the age of 5 from low-income and middle-income countries (LMICs) are at risk of not fulfilling their developmental potential partly because of poor nutrition during pregnancy and the first 2 years of life.1 Malnutrition and micronutrient deficiencies represent a major challenge to child health in many LMICs and are associated with suboptimal cognitive function and poor growth.2 Many children in South Asia and Africa suffer from deficiencies of several nutrients including vitamin B12.3–7
Why vitamin B12 may be important for the developing brain
Vitamin B12 plays a key role in normal brain development and function and is required for the folate-dependent enzyme, methionine synthase, which is necessary for the synthesis of methionine from homocysteine.8 Methionine in its activated form, S-adenosylmethionine, is the major methyl group donor used in human methylation reactions, including methylation of DNA and RNA. Deficient methylation reactions in the central nervous system can impair the methylation of myelin basic protein in the central as well as peripheral nervous system.9 The production of myelin is a key component of brain development from gestation, throughout childhood and well into middle age.10 The myelination of the brain is of importance for multiple brain systems and is highly related to neurodevelopment and subsequent cognitive functioning.11 Vitamin B12 also serves as a cofactor in numerous catalytic reactions in the human body, which are required for neurotransmitter synthesis and functioning. Vitamin B12 deficiency may cause pernicious anaemia with similar effects on cognitive development and functioning as anaemia caused by iron deficiency.12 Vitamin B12 deficiency can also result in neuropathy through degeneration of nerve fibres and irreversible brain damage.13 However, although subtle vitamin B12 deficiency is very common, the degree to which it has significant consequences for neurodevelopment and cognitive function is not established.14 15
Vitamin B12 and neurodevelopment
There is ample evidence that vitamin B12 is important for cognitive development.11 In observational studies among adults and elderly, low levels of vitamin B12 are associated with cognitive decline and dementia.16 Although there are fewer observational studies in children, the association between vitamin B12 status and cognitive functioning has also been documented from early infancy to adolescence. Neonatal severe vitamin B12 deficiency causes failure to thrive and possible irreversible neurological manifestations.12 17 A study in the Netherlands showed that infants fed on a macrobiotic diet, which is low on vitamin B12, had delayed gross motor, speech and language development compared with infants on an omnivore diet.18 In adolescence, these same children who were fed a macrobiotic diet for the six first years of life had poorer performance on cognitive tests, independent of their current vitamin B12 status, compared with adolescents who were fed on an omnivorous diet.19 In an Indian infant cohort, we also demonstrated that poor vitamin B12 status was associated with lower scores on the Mental Development Index in the Bayley Scales of Infant and Toddler Development second edition.4 In a study from Nepal, we were able to demonstrate associations between early vitamin B12 status (2–12 months) and cognitive functioning 5 years later.20 These long-term associations remained strong, even after adjusting for several potential confounders. There is also evidence for the significance of vitamin B12 to the developing brain in a few clinical trials. In a randomised controlled trial (RCT) Indian children aged 6–30 months were administered placebo or approximately twice the recommended daily allowances of vitamin B12 and/or folic acid for 6 months. Children provided with vitamin B12 and folic acid scored higher on a neurodevelopment assessment compared with the placebo group.21 The effects were most prominent in stunted children or children less than 2 years of age. A favourable effect of vitamin B12 was also demonstrated on motor development in two RCTs involving Norwegian infants with developmental regressions and signs of vitamin B12 deficiency 1 and 6 months following a 400 µg injection of vitamin B12.22 23
Knowledge gaps
There is a need to clarify to what extent improving vitamin B12 status impacts developmental outcomes in children in LMICs. It is also important to identify populations in which such interventions can be beneficial and at what age vitamin B12 administration is most effective.11 Most studies in this field have used only motor function tests or general neurodevelopment assessments,21 22 24 while less is known on the effect of vitamin B12 on specific cognitive functions such as executive functions, attention and sensorimotor and visuospatial abilities.20 The functions and areas under development are most sensitive to negative influences and will provide the most specific outcomes in assessments.25 26 Consequently, to fully understand findings in developmental assessments, we must consider the developmental timing of an exposure, in this case, vitamin B12 supplementation (or lack thereof), as well as the timing of the assessment.25 Measuring neurodevelopment at different ages, in different populations and the effect of exposure at different times during the critical periods of neurodevelopment is needed to understand the role of vitamin B12 in cognition.
Research on the impact of vitamin B12 on the neurophysiological outcomes in children is scarce. Event-related potential (ERP) are non-invasive, reliable measures of neurophysiological brain function27 that provide direct measure of underlying neural activity of higher mental processes. Behavioural assessments may sometimes not be sensitive enough to detect these small changes in brain functions28 and these neurophysiological processes may be important correlates of the cognitive abilities and give new insight into the nutritional impact on the developing brain.29
Study objectives
The aim of the current study, the Vitabeginning project, is to determine the long-term effects of vitamin B12 supplementation given during the first 1000 days of life on neurodevelopmental outcomes and growth.
General objective
To provide evidence for the role of vitamin B12 supplementation in pregnancy or early childhood on neurodevelopment in vulnerable children in LMICs.
Specific objectives and objectives of the separate studies
In North Indian children aged 6 to 30 months at enrolment, measure to what extent 6 months’ administration of vitamin B12 (1.8 µg) with or without folic acid (150 µg) improves neurodevelopment and cognitive function 5 years after the end of supplementation. Age range at follow-up is 7–9 years.
In South Indian children, measure to what extent administration of daily vitamin B12 (50 µg) in combination with iron and folic acid from 14 weeks’ gestational age through 6 weeks postpartum improves neurodevelopment, cognition and neurophysiological function in childhood. Age range at follow-up is 5–6.5 years.
In Tanzanian children, measure to what extent administration of vitamin B12 (50 µg) given with other vitamins from 11 to 17 weeks’ gestational age until delivery improves neurodevelopment and cognitive function up to school age. Age range at follow-up is 11–15 years.
In Tanzanian children, measure to what extent administration of vitamin B12 (1 mg) given with other vitamins from week 6 to 18 months of age improves neurodevelopment and cognitive function up to school age. Age range at follow-up is 7–10 years.
To estimate the effect of vitamin B12 administration on certain domains of neurodevelopment using pooled data from all of the above studies.
Methods and analysis
Study design and interventions
This study is a follow-up of four recently completed double-blind randomised placebo-controlled trials conducted in lower socioeconomic, semiurban and urban populations in Delhi (India),21 30 Bangalore (India)31 and Dar es Salaam (Tanzania)32 33 in 2001– 2011. All trials provided at least two recommended dietary allowances (RDA) of vitamin B12 daily (2×2.6 µg for adults and 9 µg for small children) for at least 6 months, with no vitamin B12 supplementation in the placebo groups. The two studies that enrolled pregnant women provided the highest dose of vitamin B12 (50 µg daily, which is approximately 20 times the RDA). An overview of the original trials and the follow-ups is presented in table 1.
Table 1.
Overview of study populations, study period and exposure in the original trials and in the Vitabeginning follow-up study
| Characteristics | Bangalore | Delhi | Dares Salaam | |
| Original trials | ||||
| Trial registration number | NCT00641862 | NCT00717730 | NCT00197548 | NCT00421668 |
| Intervention period | 09.2010–08.2011 | 01.2010–03.2011 | 08.2001–02.2005 | 07.2007–05. 2011 |
| Original sample size | 366 | 1000 | 8468 | 2400 |
| Exposure | Vitamin B12* | Vitamin B12 and/or folic acid† | Maternal multivitamins‡ | Multivitamins and/or zinc§ |
| Timing of exposure | Pregnancy (daily supplement from <14 weeks gestational age through 6-week postpartum) | Infancy/early childhood (daily supplement for 6 months from 6 to 30 months) | Pregnancy (daily supplement from 11-17 weeks until delivery) | Infancy (daily supplement from 6 weeks to 18 months) |
| Vitabeginning follow-ups | ||||
| Expected sample size | 230 | 800–900 | 366 | 446 |
| Age range at follow-up | 5–6.5 years | 7–9 years | 11–15 years | 7–10 years |
| Start date of enrolment | 01.2015 | 10.2016 | 07.2015 | 07.2015 |
| Expected date for study completion | Estimated 07.2017 | Estimated 11.2017 | Estimated 11.2017 | Estimated 11.2017 |
*From <14 weeks gestational age to 6 weeks postpartum vitamin B12 50 µg daily. In addition, all women were given iron and folic acid supplementation throughout pregnancy.
†From 6 to 30 months for 6 months; four groups: vitamin B12, vitamin B12 +folic acid, folic acid and placebo, doses >12 months: vitamin B12 1.8 µg and folic acid 150 µg (half doses for <12 months).
‡From 12 to 27 weeks gestational age until delivery: multivitamin B12 50 µg, vitamin B1 20 mg, vitamin B2 20 mg, vitamin B6 25 mg, niacin 100 mg, vitamin C 500 mg, vitamin E 30 mg, folic acid 0.8 mg.
§From 6 weeks to 18 months, four groups; multivitamin + zinc, vitamin Z and placebo. Multivitamin <6m: vitamin B12 1 mg, vitamin B1 0.5 mg, vitamin B2 0.6 mg, vitamin B 60.6mg, niacin 4 mg, folic acid 130 µg, vitamin C 60 mg, vitamin E 8 mg. Zinc 5 mg >6 months multivitamins and zinc doses were double.
In Bangalore, South India, HIV-negative pregnant women recruited before or at 14 weeks gestational age were randomised to receive a daily dose of oral vitamin B12 (50 µg) or a placebo through 6 weeks postpartum. The primary objective was to determine the effect of vitamin B12 supplementation in improving maternal vitamin B12 status. Enrolment was completed in September 2010, and the last enrolled infant was born in August 2011. Oral vitamin B12 supplementation of women throughout pregnancy and early lactation, in combination with standard prenatal care with routine supplementation of iron and folate, significantly increased the vitamin B12 status of women and their offspring.31 Neurodevelopment was measured at 9 and 30 months. In this follow-up study, we will measure the effect of maternal vitamin B12 supplementation on neurodevelopment and cognitive function several times 5–6.5 years after supplementation and on neurophysiological outcomes using ERP.
In Delhi, North India, children 6–30 months of age were randomised to receive daily (1) vitamin B12, (2) vitamin B12 and folate, (3) folate or (4) placebo for 6 months. The supplementation included vitamin B12 (1.8 µg) and/or folate (150 µg) and with half doses for children <12 months. The primary objective was to measure the effect of these interventions on the incidence of diarrhoea and pneumonia. Neurodevelopment was a predefined secondary outcome and was measured in a subsample of 422 children. In total, 1000 children were enrolled between January 2010 and September 2011. Vitamin B12 and folic acid-supplemented children scored significantly higher on neurodevelopment scores at the age of 12–36 months, compared with those who received placebo.21 In this follow-up study, we will measure to what extent early supplementation of folic acid and/or vitamin B12 improves neurodevelopment and cognitive function 5–6 years after supplementation. The study is powered to measure the effect of vitamin B12, folic acid and the two vitamins combined on neurodevelopmental outcomes. The follow-up study will also measure to what extent vitamin D status in early life is associated with neurodevelopmental scores in early school years.
In Dar es Salaam, Tanzania, infants 6–10 weeks of age born to HIV-negative mothers were randomised to receive daily (1) zinc, (2) multivitamins, (3) zinc + multivitamin or (4) placebo. Multivitamins included vitamin B121 mg, B10.5 mg, B20.6 mg, B60.6 mg, niacin 4 mg, folic acid 130 µg, vitamin C 60 mg and vitamin E 8 mg and was provided alongside zinc 5 mg. Doses were doubled after 6 months.
Children were followed for 18 months (ie, until age 19.5 months). The primary objective was to measure the incidence of diarrhoea and respiratory tract infections. Enrolment was completed in December 2009, and follow-up ended in May 2011. Neurodevelopmental outcomes were assessed in a subset of children at 15 months.32 Daily zinc supplementation lowered the burden of diarrhoea and respiratory tract infections. No added benefit was seen from the provision of multivitamins.33
In another trial in Dar es Salaam, Tanzania on micronutrients and adverse pregnancy outcomes, HIV-negative pregnant women at 12–27 weeks gestational age were randomised to receive daily oral supplementation of multivitamins including vitamin B12 or placebo. Multivitamins included vitamin B1250 µg, vitamin B120 mg, vitamin B220 mg, vitamin B625 mg, niacin 100 mg, vitamin C 500 mg, vitamin E 30 mg and folic acid 0.8 mg. All women received prenatal iron and folate supplementation. The primary objective was to measure the effect of vitamin supplementation on fetal loss, low birth weight and severe preterm birth. Enrolment was completed in July 2004, and the last enrolled infant was born in February 2005. Multivitamin supplementation reduced the incidence of low birth weight and small for gestational age births but had no significant effects on prematurity or fetal death.34 The two studies from Tanzania are well suited for follow-up studies on potential impact of micronutrient supplementation on child health and neurodevelopmental outcomes in older children.
Outcomes
Neurodevelopment, cognitive function and linear growth will be key outcomes in the different studies.
Neurodevelopment
Neurodevelopmental status in young children has been assessed by the comprehensive assessment tool Bayley Scales of Infant and Toddler Development third edition35 and the easily administered screening tool Ages and Stages Questionnaire third edition in the original studies.21 36
In the follow-up, each site use their own unique collections of tests and questionnaires (table 2). General intellectual functioning is assessed by a modified Kaufmann ABC II (Bangalore and Dar es Salaam), the Wechsler Preschool and Primary Scale of Intelligence–III (Bangalore) or the Wechsler Intelligence Scale for Children VI, Indian version (Delhi). These are complemented by tests on specific cognitive functions by subtests from the developmental neuropsychological test battery A Developmental NEuroPSYchological Assessment, version 2 (NEPSY-II) including attention and executive functioning, language, social perception, sensorimotor and visuospatial processing (Delhi) and adaptive functioning by the Vineland Social Maturity Scale (Bangalore). Mental health and behaviour problems are measured by the parent-reported screening instrument Strengths and Difficulties Questionnaire and executive functions by the parent-reported questionnaire Behaviour Rating Inventory of Executive Function (all sites). Finally, in one study (Bangalore), we measure neurophysiological functions using event-related potentials as this may yield additional information on the effects of nutritional deficiencies on brain function. In the present study, we propose to use two well-characterised ERPs, P-300 and Mis-match Negativity that are known to reflect higher cognitive functions of attention and memory (see table 2 for details on the assessments at each site).
Table 2.
Overview over inventories and data collection tools used in the different studies and the age of assessment
| Outcomes to be measured | Bangalore | Delhi | Dar es Salaam | |
| Vitamin B12 exposure | Pregnancy | Child | Pregnancy | Child |
| Outcomes to be measured | Age (year) | Age (year) | Age (year) | Age (year) |
| General abilities | ||||
| Modified KABC-II* EACABT | 5, 6 | 11–15 | 7–10 | |
| WISC-IVINDIA | 7–9 | |||
| Verbal abilities | ||||
| Crichton Vocabulary Scale, Hindi edition | 7–9 | |||
| Neurophysiological tests | ||||
| Event-related potential† | 6 | |||
| Neuropsychological tests | ||||
| NEPSY-II | 7–9 | |||
| Questionnaires | ||||
| Brief‡ | 5.5 | 7–9 | 11–15 | 7–10 |
| SDQ | 6.5 | 7–9 | 11–15 | 7–10 |
| VSMS | 5 | |||
| Maternal assessment | ||||
| Demography | 5 | 7–9 | 11–15 | 7–10 |
| Anthropometry | 5, 5.5, 6, 6.5 | 11–15 | 7–10 | |
| Home environment | 5.5 | 11–15 | 7–10 | |
| Nutrition | ||||
| 24-hour diet recall form | 5, 5.5, 6, 6.5 | 7–9 | ||
| Anthropometry | 5, 5.5, 6, 6.5 | 7–9 | 11–15 | 7–10 |
| Household food security questionnaire | 5, 6 | |||
| Medical morbidity | ||||
| Morbidity questionnaire | 5, 5.5, 6, 6.5 | 11–15 | 7–10 | |
| Biomarkers | ||||
| Vitamin B12 | 5, 6 | 7–9 | 6–24 m§ | |
| CBC, MMA, HcY, RBC folate, CRP | 5, 6 | 7–9 | ||
| Haemoglobin | 5, 6 | 7–9 | 11–15 | 7–10 |
*In Bangalore: Atlantis, number recall, word order, pattern reasoning and triangles from KABC and Koh’s Block Design Test and Verbal Fluency in addition; in Dar es Salaam: the EACABT with permission from Savings Brain Multisite Study (WHO/BMGF) including Atlantis, hand movements, footsteps, story completion, Kilifi Naming Test, ROCF, NOGO, shift, people search, literacy and numeracy, HOME, Brief-P (modified from MAL-ED), SDQ, BQP, in addition to verbal fluency and Koh’s Block.
†ENOBIO 32 (Device name)—brain monitoring and stimulation technologies, mismatch negativity, P300 (Neuroelectrics, Boston, MA).
‡Different versions used in different sites: in Bangalore: Brief Parent; in Delhi: Brief Second Edition; in Dares es Salaam: Saving Brains/Gates/WHO modified version from the malaria study.
§On stored serum samples from Child II cohort (subject to funding).
BMGF, Bill & Melinda Gates Foundation; BQP, Behavior Questionnaire for Parents; CBC, complete blood count; CRP, C reactive protein; EACBT, African Cognitive Assessment Battery; HcY, homocysteine; HOME, Home Observation Measurement of the Environment; KABC, Kaufman Assessment Battery for Children; MAL-ED, Etiology, Risk Factors and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development, MMA, methylmalonic acid; NEPSY-II, A Developmental NEuroPSYchological Assessment; NOGO, go/no go test for sustained attention and response control; RBC folate, red blood cell folate analysis; ROCF, Rey–Osterrieth complex figure; SDQ, Strengths and Difficulties Questionnaire; VSMS, Vineland Social Maturity Scale; WISC-IVINDIA, Wechsler Intelligence Scale for Children VI, Indian version.
Anthropometry
Weight and height are measured using standard methods. In Delhi, height, weight and skin fold thickness were measured in children, using Seca scales (hight/weight) and Holtain Calipers. In Bangalore, weights of mothers and children were recorded using a digital balance (Salter’s 9016; Tonbridge) to the nearest 100 g, and the heights were measured using a stadiometer to the nearest 0.1 cm.
Sample size calculation
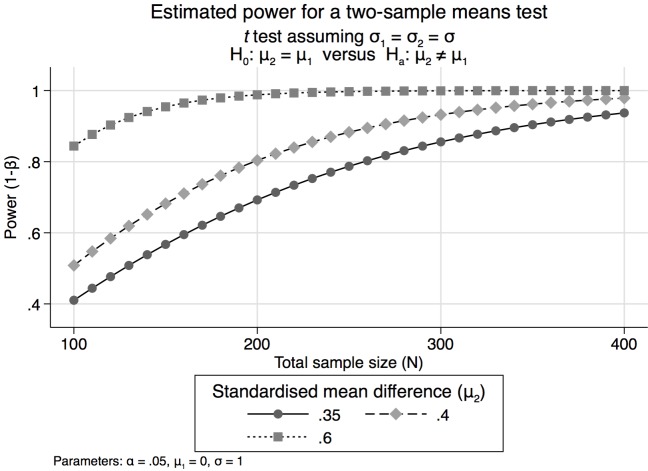
The data for our most relevant tests are expected to be normally distributed. We have decided that a standardised effect size of 0.35 SD is the minimally clinically relevant effect for the main psychometric tests. If the smallest group in any of our comparisons includes at least 130 children in each arm, and assuming a two-sided alpha error of 0.05 we will have 81%, 89% and 98% power to detect differences for effect sizes of 0.35, 0.4 and 0.6, respectively. As a result, all of the described studies have sufficient power to detect important differences between vitamin B12 and placebo recipients between individuals with poor and adequate vitamin B12 status or between other groups of other exposures as long as each of the groups contain at least 130 children. Statistical powers according to standardised mean effect sizes of .35, 0.4 and 0.6 and total sample sizes are depicted in figure 1. This graph was generated using the ‘power two means’ command in STATA V.13; it assumes equal group sizes and equal variances and a significance level of 0.05.
Figure 1.
Estimated required total sample sizes based on relevant effect sizes.
Data collection
Data collection for the primary and secondary outcomes are synchronised and selected to capture the same domains of development using different tools. Assessment tools for cognitive functions such as general abilities, achievement, verbal, visual spatial and sensorimotor skills, memory, executive functioning, general behaviour and social perception and maturity are dependent on the child’s age. We have carefully selected well-validated and developmentally sensitive instruments to ensure detection of the relevant predictors according to age and research questions. Instruments are adapted to ensure psychometric qualities, as well as cultural and linguistic appropriateness of the test at each site. Clinically useful tests will be prioritised to improve sustainability of test material and knowledge of developmental assessment at the specific sites. Assessments are administered by trained psychologists in the Indian sites and by trained healthcare workers in the Tanzania site. A group of experienced scientists with expertise in developmental, neuropsychological and neurophysiological assessments are responsible for the training and standardisation in the different sites.
Analysis plan
Several domains will be measured and compared between the study groups within each of the studies. In these analyses, we will initially use the Student’s t-test for the crude analyses and multiple linear regression models to adjust for potential baseline differences and when measuring effect modification.
Each of the studies has several outcomes as we will compare both linear and ponderal growth, in addition to all the above-mentioned neurodevelopmental measures between the study groups. Thus, there will be several comparisons from each study, and negative and positive effects will be reported to avoid focusing on spurious positive findings.
For each of the planned publications, we will make a detailed plan of analysis before commencing the analysis. In these plans, we will include sections on how to deal with multiple comparisons and whether post hoc adjustments will be done. In addition to these standard per-protocol analysis, we will consider instrumental variable analysis in an attempt to estimate the true effect of vitamin B12 had it been given to all participants in the scheduled doses and intervals. The random allocation will be the instrument in these analyses. For per-protocol analysis, participants who received less than 50% of the projected doses during the period of intervention will not be included in the analyses, well acknowledging that the ensuing effect estimates may not only be biased but will certainly represent an effect higher than what can be achieved even in our well-resourced study setting.
For our subgroup analyses, we will include interaction terms to measure whether or not the subgrouping variable significantly modifies the effect of the exposure of interest. All of our analyses will initially be done according to intent to treat.
We will not be able to retain the complete number of children from these studies. We will compare the features of the population that is included in this analysis with the population that we failed to re-enrol into the study. We will also detect risk factors for poor neurodevelopment in multiple linear or binomial regression models. We will include socioeconomic and seasonal factors and dietary intake as exposures in the analyses. A significance level of 0.05 will be used.
Ethics and dissemination
Ethical and safety considerations
The exposures under investigation in this study were included in the original trials. Informed written parental consent will be taken from one or both parents of participating children prior to enrolment in the follow-up study and assent will be obtained from children older than 7 years in the Delhi site. Parents unable to read or write will be encouraged to bring along a literate relative or neighbour as an impartial witness.
Relevance and benefit to society
The current project takes advantage of four recently completed randomised placebo-controlled trials to study the long-term effects of vitamin B12 supplementation on neurodevelopmental outcomes and growth in children in low- and middle-income countries. When measuring the effect of vitamin B12 on growth and development, long-term follow-up is important: vitamin B12 can be stored for years in the body, and the effect of an increased intake for a relatively short period such as 6 months may accordingly last much longer. Furthermore, for many of the neurodevelopmental outcomes, it might not be possible to estimate the effect of early life exposures until later in childhood because of limitations in the assessment tools. Thus, to follow children for a long time, as in this project, is important to fully understand the role of vitamin B12 for brain development and growth.
In most studies, follow-up for several years is not possible, so the results from our studies will be of great importance. Since none of the RCTs originally were designed for such long-term follow-up, there is risk that loss to follow-up can bias our effect estimates. There is, however, no reason to believe that the loss to follow-up rate will be different according to randomised regimen in any of the described studies. The risk of confounding of the main exposures (Vitamin B12 supplementation) is small because of the RCT design and the large number of randomization units. Substantial imbalances of potential confounding factors across study groups are unlikely.
If positive effects of supplementation are observed, this may constitute important contributions to improve childhood nutrition in many LMICs. However, several factors may have profound impact on long-term neurodevelopment outcomes and growth and potentially dilute the effect of optimising vitamin B12 status in early life. Our findings, however, must be interpreted in light of the results from other RCTs that are measuring the effect of vitamin B12 supplementation on growth and development. If anything, our findings will guide our next step in understanding the role of vitamin B12 nutrition on child growth and development: Should there be additional follow-ups in the ongoing studies or will the results from our studies discourage further studies on vitamin B12 and child health?
If none, or very few, of our several outcomes respond to vitamin B12 supplementation, alone or when given in combination with several other micronutrients, then poor vitamin B12 status is likely not an important contributor to impaired neurodevelopment. Thus, we believe that a negative result of our studies is also of substantial public health importance.
Millions of children across the world grow up malnourished lacking essential nutrients, with high burden of infectious diseases and where parents may not have the resources to provide an optimal environment for nurturing care. Acting in early childhood, these factors may result in poorer chances for later success in school and work. The results from the RCTs can lead to dietary recommendations that can improve learning and academic achievements, which again can lift individuals from the vicious cycle of poverty and malnutrition. Programme designed to prevent or treat micronutrient deficiencies can be targeted towards specific recommendations. Any further evidence for the long-term effect of dietary supplements has potentially high impact and may provide sustainable improvements in health and equity.
The suggested studies are geared towards rapid dissemination of results into national and international child health promotion programme. We will actively use the potential influence of the international collaborators to ensure that our results reach the relevant health authorities.
Planned publications
Study results will be presented at national and international research and policy meetings, and published in peer-reviewed scientific journals, preferably open access. We will also discuss alternative strategies to inform the public. We will publish scientific papers as a consortium, specifically directed towards developmental and neuropsychological assessment methodology and site-specific publications. We expect each of the sites to generate approximately five publications in high-ranked international peer-reviewed journals.
Supplementary Material
Footnotes
Contributors: TAS, IK, CPD and WF took the initiative for the study. TAS, IK, CPD, WF, BAW, SK, KM, ST, MH, NB and ES were involved in developing the design and the study protocol. ST, SK and KM were responsible for setting up the study conduct in each site, with support from AMD, ST, TK, AK and SB. The statistical approach of the study was drafted by CRS, MH, TAS and WF. MH, IK, ES, DCB, and SK were responsible for the cognitive assessments. All authors approved the final version of the protocol. BAW, IK and TAS drafted the current manuscript. All authors have reviewed and accepted the final version of the manuscript.
Funding: The Vitabeginning study was funded by the Norwegian Research Council Grant number 234495. The original trial in Bangalore was funded by the Indian Council of Medical Research (grant 5/7/192/06-RHN) and Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant R03 HD054123). The study in Delhi was funded by the Thrasher Research Fund (grant no. 02827) and the Research Council of Norway (grant 172226). The trials in Tanzania were funded by National Institute of Child Health and Human Development (NICHD R01 37701) and the Eunice Kennedy Shriver NIH grant (R01 HD048969-01). CPD is supported by NIH (grants K24DK104676 and P30 DK040561).
Competing interests: None declared.
Patient consent: Detail has been removed from this/these case description/s to ensure anonymity. The editors and reviewers have seen the detailed information available and are satisfied that the information backs up the case the authors are making.
Ethics approval: Ethical Review Board in Norway (2014/1359, 2015/640), in addition to approvals from partners in USA, Tanzania and India.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Black MM, Walker SP, Fernald LCH, et al. Early childhood development coming of age: science through the life course. Lancet 2017;389:77–90. 10.1016/S0140-6736(16)31389-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black MM. Micronutrient deficiencies and cognitive functioning. J Nutr 2003;133(11 Suppl 2):3927S–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhan MK, Sommerfelt H, Strand T. Micronutrient deficiency in children. Br J Nutr 2001;85(Suppl 2):S199–203. 10.1079/BJN2001315 [DOI] [PubMed] [Google Scholar]
- 4.Taneja S, Bhandari N, Strand TA, et al. Cobalamin and folate status in infants and young children in a low-to-middle income community in India. Am J Clin Nutr 2007;86:1302–9. [DOI] [PubMed] [Google Scholar]
- 5.Allen LH. How common is vitamin B-12 deficiency? Am J Clin Nutr 2009;89:693S–6. 10.3945/ajcn.2008.26947A [DOI] [PubMed] [Google Scholar]
- 6.Ulak M, Chandyo RK, Adhikari RK, et al. Cobalamin and folate status in 6 to 35 months old children presenting with acute diarrhea in Bhaktapur, Nepal. PLoS One 2014;9:e90079 10.1371/journal.pone.0090079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ulak M, Chandyo RK, Thorne-Lyman AL, et al. Vitamin status among breastfed infants in Bhaktapur, Nepal. Nutrients 2016;8:149 10.3390/nu8030149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ueland PM, Monsen AL. Hyperhomocysteinemia and B-vitamin deficiencies in infants and children. Clin Chem Lab Med 2003;41:1418–26. 10.1515/CCLM.2003.218 [DOI] [PubMed] [Google Scholar]
- 9.Weir DG, Scott JM. Brain function in the elderly: role of vitamin B12 and folate. Br Med Bull 1999;55:669–82. 10.1258/0007142991902547 [DOI] [PubMed] [Google Scholar]
- 10.Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev 2010;20:327–48. 10.1007/s11065-010-9148-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Black MM. Effects of vitamin B12 and folate deficiency on brain development in children. Food Nutr Bull 2008;29(2 Suppl):S126–31. 10.1177/15648265080292S117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dror DK, Allen LH. Effect of vitamin B12 deficiency on neurodevelopment in infants: current knowledge and possible mechanisms. Nutr Rev 2008;66:250–5. 10.1111/j.1753-4887.2008.00031.x [DOI] [PubMed] [Google Scholar]
- 13.Calvaresi E, Bryan J. B vitamins, cognition, and aging: a review. J Gerontol B Psychol Sci Soc Sci 2001;56:P327–39. 10.1093/geronb/56.6.P327 [DOI] [PubMed] [Google Scholar]
- 14.van de Rest O, van Hooijdonk LW, Doets E, et al. B vitamins and n-3 fatty acids for brain development and function: review of human studies. Ann Nutr Metab 2012;60:272–92. 10.1159/000337945 [DOI] [PubMed] [Google Scholar]
- 15.Finkelstein JL, Layden AJ, Stover PJ. Vitamin B-12 and perinatal health. Adv Nutr 2015;6:552–63. 10.3945/an.115.008201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selhub J, Morris MS, Jacques PF, et al. Folate-vitamin B-12 interaction in relation to cognitive impairment, anemia, and biochemical indicators of vitamin B-12 deficiency. Am J Clin Nutr 2009;89:702S–6. 10.3945/ajcn.2008.26947C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bjørke Monsen AL, Ueland PM. Homocysteine and methylmalonic acid in diagnosis and risk assessment from infancy to adolescence. Am J Clin Nutr 2003;78:7–21. [DOI] [PubMed] [Google Scholar]
- 18.Schneede J, Dagnelie PC, van Staveren WA, et al. Methylmalonic acid and homocysteine in plasma as indicators of functional cobalamin deficiency in infants on macrobiotic diets. Pediatr Res 1994;36:194–201. 10.1203/00006450-199408000-00010 [DOI] [PubMed] [Google Scholar]
- 19.Louwman MW, van Dusseldorp M, van de Vijver FJ, et al. Signs of impaired cognitive function in adolescents with marginal cobalamin status. Am J Clin Nutr 2000;72:762–9. [DOI] [PubMed] [Google Scholar]
- 20.Kvestad I, Hysing M, Shrestha M, et al. Vitamin B-12 status in infancy is positively associated with development and cognitive functioning 5 y later in Nepalese children. Am J Clin Nutr 2017;105:1122–31. 10.3945/ajcn.116.144931 [DOI] [PubMed] [Google Scholar]
- 21.Kvestad I, Taneja S, Kumar T, et al. Vitamin B12 and folic acid improve gross motor and problem-solving skills in young North Indian children: a randomized placebo-controlled trial. PLoS One 2015;10:e0129915 10.1371/journal.pone.0129915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torsvik I, Ueland PM, Markestad T, et al. Cobalamin supplementation improves motor development and regurgitations in infants: results from a randomized intervention study. Am J Clin Nutr 2013;98:1233–40. 10.3945/ajcn.113.061549 [DOI] [PubMed] [Google Scholar]
- 23.Torsvik IK, Ueland PM, Markestad T, et al. Motor development related to duration of exclusive breastfeeding, B vitamin status and B12 supplementation in infants with a birth weight between 2000-3000 g, results from a randomized intervention trial. BMC Pediatr 2015;15:218 10.1186/s12887-015-0533-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strand TA, Taneja S, Ueland PM, et al. Cobalamin and folate status predicts mental development scores in North Indian children 12-18 mo of age. Am J Clin Nutr 2013;97:310–7. 10.3945/ajcn.111.032268 [DOI] [PubMed] [Google Scholar]
- 25.Walder D, Jc S, Pulsifer M. Evidence-based practice in infant and early childhood psychology. Neurodevelopmental Assessment 2009:1 67–205. [Google Scholar]
- 26.Thompson RA, Nelson CA. Developmental science and the media. Early brain development. Am Psychol 2001;56:5–15. 10.1037/0003-066X.56.1.5 [DOI] [PubMed] [Google Scholar]
- 27.Light GA, Willians LE, Minow F, et al. Electroencephalography (EEG) and Event-Related Potentials (ERPs) with Human Participants. Curr Protoc Neurosci 2010;6:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uauy R, Peirano P. Breast is best: human milk is the optimal food for brain development. Am J Clin Nutr 1999;70:433–4. [DOI] [PubMed] [Google Scholar]
- 29.deRegnier RA, Long JD, Georgieff MK, et al. Using event-related potentials to study perinatal nutrition and brain development in infants of diabetic mothers. Dev Neuropsychol 2007;31:379–96. 10.1080/87565640701229524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taneja S, Strand TA, Kumar T, et al. Folic acid and vitamin B-12 supplementation and common infections in 6-30-mo-old children in India: a randomized placebo-controlled trial. Am J Clin Nutr 2013;98:731–7. 10.3945/ajcn.113.059592 [DOI] [PubMed] [Google Scholar]
- 31.Duggan C, Srinivasan K, Thomas T, et al. Vitamin B-12 supplementation during pregnancy and early lactation increases maternal, breast milk, and infant measures of vitamin B-12 status. J Nutr 2014;144:758–64. 10.3945/jn.113.187278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Locks LM, Manji KP, McDonald CM, et al. The effect of daily zinc and/or multivitamin supplements on early childhood development in Tanzania: results from a randomized controlled trial. Matern Child Nutr 2017;13:e12306 10.1111/mcn.12306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDonald CM, Manji KP, Kisenge R, et al. Daily zinc but not multivitamin supplementation reduces diarrhea and upper respiratory infections in tanzanian infants: a randomized, double-blind, placebo-controlled clinical trial. J Nutr 2015;145:2153–60. 10.3945/jn.115.212308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fawzi WW, Msamanga GI, Urassa W, et al. Vitamins and perinatal outcomes among HIV-negative women in Tanzania. N Engl J Med 2007;356:1423–31. 10.1056/NEJMoa064868 [DOI] [PubMed] [Google Scholar]
- 35.Srinivasan K, Thomas T, Kapanee AR, et al. Effects of maternal vitamin B12 supplementation on early infant neurocognitive outcomes: a randomized controlled clinical trial. Matern Child Nutr 2017;13:e12325 10.1111/mcn.12325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kvestad I, Taneja S, Kumar T, et al. The assessment of developmental status using the ages and stages questionnaire-3 in nutritional research in north Indian young children. Nutr J 2013;12:50 10.1186/1475-2891-12-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.