Abstract
Background:
Plasmacytomas are monoclonal proliferations of plasma cells that typically affect the intramedullary axial skeleton. Imaging findings of an extramedullary plasmacytoma on radiograph and computed tomography can be nonspecific and can resemble other entities such as lymphoma, metastases, chondrosarcomas, or giant cell tumors.
Case Report:
A 60-year-old female with a medical history of partial complex seizures, hypertension, diabetes, glaucoma, and hyperlipidemia presented with complaints of superficial abdominal pain associated with erythema and swelling for 3 weeks. Computed tomography of her abdomen at time of presentation revealed a 5.8 × 2.7-cm irregularly marginated soft-tissue density just below the umbilicus with an adjacent defect in the midline rectus abdominis. The final pathologic diagnosis was extramedullary plasmacytoma. Treatment during the next year included local radiation, systemic chemotherapy, and an autologous peripheral blood stem cell transplant. Three years after initial diagnosis, the patient presented to the emergency department, and testing revealed new plasmacytomas. The decision was made to proceed with palliative care.
Conclusion:
This case is a unique example of a patient with an extramedullary plasmacytoma with no diagnostic signs of multiple myeloma.
Keywords: Multiple myeloma, plasmacytoma, soft tissue neoplasms
INTRODUCTION
Plasmacytomas are monoclonal proliferations of plasma cells that typically affect the intramedullary axial skeleton.1 In rare cases, they may occur first in soft tissues as focal proliferations of tissue and, in even rarer cases, may present as multiple nodules of soft-tissue density.2 Typically, multiple myeloma presents with cutaneous manifestations rather than cutaneous plasmacytomas degenerating into multiple myeloma.3 Imaging findings of an extramedullary plasmacytoma on radiograph and computed tomography (CT) can be nonspecific and can resemble other entities such as lymphoma, metastases, chondrosarcomas, or giant cell tumors.
CASE REPORT
A 60-year-old female with a medical history of partial complex seizures, hypertension, diabetes, glaucoma, and hyperlipidemia presented with complaints of superficial abdominal pain associated with erythema and swelling for 3 weeks. Physical examination revealed a scaly, indurated 9-cm diameter plaque with 4.5-cm central nodular erythematous tissue in the periumbilical abdomen. Laboratory data showed normal calcium (10.1 mg/dL), normal blood urea nitrogen and creatinine (18 mg/dL and 0.75 mg/dL, respectively), and no evidence of anemia.
CT scan of the patient's abdomen at the time of presentation revealed a 5.8 × 2.7-cm irregularly marginated soft-tissue density just below the umbilicus with an adjacent defect in the midline rectus abdominis (Figure 1). Preliminary pathology suggested lymphoma. Histologically, atypical mononuclear infiltrating fibroadipose and fibrocollagenous tissue and cells demonstrated foamy eosinophilic cytoplasm and oval to slightly irregular nuclear contours with rare Dutcher bodies. Cells were positive for CD79a, CD138, MUM1, and vimentin with kappa immunoglobulin (Ig) light chain restriction. Stain for Ki-67 showed a proliferation index within the tumor cells of 30%. Final pathologic diagnosis was extramedullary plasmacytoma.
Figure 1.
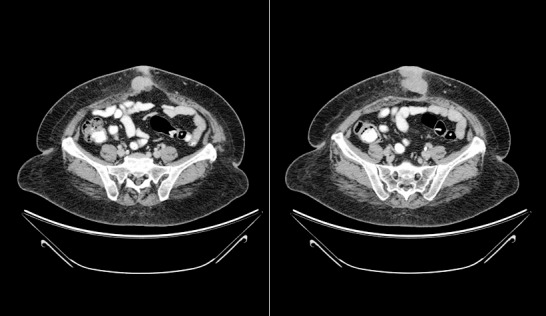
Contrast-enhanced axial computed tomography images show a well-defined soft tissue mass just below the umbilicus.
Further workup, including skeletal survey radiograph and spine magnetic resonance imaging (MRI), demonstrated no evidence of metastases. Left iliac crest bone marrow biopsy was negative for multiple myeloma (12% plasma cells). The patient was started on focal radiation therapy (21 cycles) of the abdomen.
Approximately 6 months after completing radiation therapy for the abdominal mass, the patient developed a focal mass-like swelling on her right neck. Following ultrasound-guided biopsy, pathology returned another diagnosis of plasmacytoma. Positron emission tomography (PET)-CT identified an additional pathology-proven medullary plasmacytoma in the left femur that corresponded to a lucency noted on radiograph (Figure 2).
Figure 2.
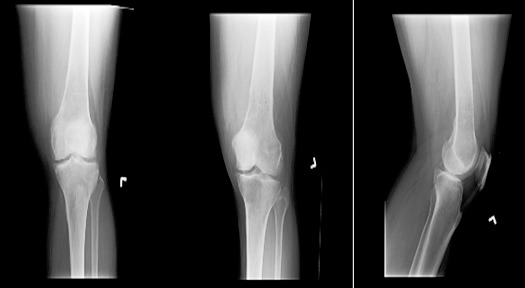
Radiographs demonstrate a moth-eaten/permeative pattern medially in the distal left femoral metadiaphysis with overlying cortical erosions and periosteal reaction.
During the next year, the patient was treated with local radiation to the left femur, systemic chemotherapy, and an autologous peripheral blood stem cell transplant. The patient's clinical course was complicated by the development of a pathologic fracture of the left femur treated with surgical hardware and multiple lower extremity deep vein thromboses treated with long-term oral anticoagulation. During this time, the patient's laboratory data showed normal beta-2 microglobulin and normal serum protein electrophoresis. Repeat bone marrow biopsies revealed normal hematopoiesis with plasma cells ranging from 7%-15%.
Three years after her initial diagnosis, the patient again presented to the emergency department complaining of multiple subcutaneous masses of the bilateral lower extremities. Evaluation with radiographs, CT, and biopsy revealed multiple new plasmacytomas in both thighs. Given the patient's poor functional status, multiple comorbid conditions, and progression of disease despite radiation, chemotherapy, and stem cell transplant, the decision was made to proceed with palliative care.
DISCUSSION
Plasmacytomas are differentiated into 4 basic categories: multiple myeloma, solitary bone plasmacytomas, extramedullary plasmacytomas, and extramedullary plasmacytomas in diffuse multiple myeloma.4 Multiple myeloma and solitary plasmacytoma are B-cell lymphoproliferative diseases composed of abnormal aggregates of plasma cells. The peak occurrence is in adults 50-60 years of age, and the male to female ratio is approximately 1.82:1 for solitary plasmacytoma.5 The annual incidence of myeloma is approximately 2-3 per 100,000 in the general population. Risk factors include exposure to ionizing radiation, herbicides, insecticides, and benzene.5 Solitary plasmacytoma is much less common; risk factors include exposure to ionizing radiation, herbicides, insecticides, and benzene.5 Solitary extramedullary plasmacytomas occur around the upper digestive tract in up to 85% of cases but can occur virtually anywhere.5,6 Incidence data for plasmacytoma, and more specifically solitary extramedullary plasmacytoma, are sparse given the rarity of the disease. Fewer than 30% of patients will progress to multiple myeloma, and approximately 37% disseminate past a single soft-tissue lesion.5,6 Genetic analysis of the tumor cells demonstrates abnormalities in band q32 of chromosome 14.7 Extramedullary plasmacytomas are commonly associated with polysomy and in approximately one-third of cases occur with a break in the long arm of chromosome 14, a slightly lower rate than in multiple myeloma. Ten percent to 20% of cases of extramedullary plasmacytoma can occur with a translocation at the 14th chromosome, t(4;14).8
Symptomatic plasmacytoma/myeloma presents with several common symptoms and laboratory findings. These findings include bone pain (65%), weakness and fatigue (50%), anemia (65%), renal insufficiency (20%), hypercalcemia (20%), marrow plasmacytosis >10% (90%), serum monoclonal Ig peak on standard electrophoresis (80%), or monoclonal Ig peak on immunofixation of serum or urine (97%).9
Diagnosis of myeloma is defined by major and minor criteria according to the Williams Manual of Hematology.9 Major criteria include plasmacytomas on tissue biopsy; marrow plasmacytosis with >30% plasma cells; monoclonal globulin spike on serum electrophoresis >3.5 g/dL for IgG or >2.0 g/dL for IgA; and 1.0 g/24 h of light-chain excretion on urine electrophoresis in the absence of amyloidosis. Minor criteria include marrow plasmacytosis 10%-30%; monoclonal globulin spike present but less than the levels defined in major criteria; lytic bone lesions; and normal IgM <0.05 g/dL, IgA <0.1 g/dL, or IgG <0.6 g/dL. Diagnosis of plasma cell myeloma is confirmed when one major and one minor criterion or 3 minor criteria are present in symptomatic patients with progressive disease. The presence of findings not specific for the disease, such as anemia, hypercalcemia, azotemia, bone demineralization, or hypoalbuminemia, supports the diagnosis.9 Multiple myeloma must be ruled out before making a diagnosis of solitary plasmacytoma and should be ruled out with a bone biopsy.
On initial CT imaging, the differential diagnosis includes lymphoma, chondrosarcoma, brown tumors, and giant cell tumors.10 General imaging considerations focus on the early use of MRI in evaluation of biopsy-proven lesions to exclude systemic disease. A finding of systemic spread would shift the diagnosis to multiple myeloma and require systemic chemotherapy for treatment.11
CT imaging is often indeterminate and shows a contrast-enhanced soft-tissue density mass with no identifiable matrix.10 The most helpful information is often location, as extramedullary plasmacytomas have a predilection for the upper gastrointestinal tract.10
Initial treatment of myeloma consists of chemotherapy and irradiation. Chemotherapy is an effective means of controlling the advancement of the disease process but may increase the risk of secondary leukemia. The commonly employed agents include melphalan and prednisone. Radiation to affected osseous sites may reduce pain and prevent vertebral collapse, deformity, and neural compression.7 In the absence of systemic disease, treatment includes 40-50 Gy radiotherapy in 20 fractions.12 Most patients are cured with local radiotherapy; however, lesions >5 cm are more likely to progress to systemic disease.13,14 Patients with solitary plasmacytoma have a 5-year survival rate of 60%. In contrast, patients with multiple myeloma have a 5-year survival rate of 18% and a median survival of 24 months.7
CONCLUSION
This case is a rare example of a patient with an extramedullary plasmacytoma with no diagnostic signs of multiple myeloma.
ACKNOWLEDGMENTS
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.
REFERENCES
- 1. Knowling MA., Harwood AR., Bergsagel DE. Comparison of extramedullary plasmacytomas with solitary and multiple plasma cell tumors of bone. J Clin Oncol. 1983. April; 1 4: 255- 262. [DOI] [PubMed] [Google Scholar]
- 2. Alvarez-Twose I., Vano-Galvan S., Calvo-Villas JM., Carreter E., Piqué E., Palacios S. Metastatic cutaneous plasmacytoma presenting as a perianal giant mass. Dermatol Online J. 2008. September 15; 14 9: 17. [PubMed] [Google Scholar]
- 3. Varettoni M., Corso A., Pica G., Mangiacavalli S., Pascutto C., Lazzarino M. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Ann Oncol. 2010. February; 21 2: 325- 330. 10.1093/annonc/mdp329. [DOI] [PubMed] [Google Scholar]
- 4. Dimopoulos MA., Kiamouris C., Moulopoulos LA. Solitary plasmacytoma of bone and extramedullary plasmacytoma. Hematol Oncol Clin North Am. 1999. December; 13 6: 1249- 1257. [DOI] [PubMed] [Google Scholar]
- 5. Knobel D., Zouhair A., Tsang RW., et al. Rare Cancer Network. Prognostic factors in solitary plasmacytoma of the bone: a multicenter Rare Cancer Network study. BMC Cancer. 2006. May 5; 6: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dores GM., Landgren O., McGlynn KA., Curtis RE., Linet MS., Devesa SS. Plasmacytoma of bone, extramedullary plasmacytoma, and multiple myeloma: incidence and survival in the United States, 1992-2004. Br J Haematol. 2009. January; 144 1: 86- 94. 10.1111/j.1365-2141.2008.07421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Skinner HB., McMahon PJ. Current Diagnosis & Treatment in Orthopedics. 5th ed. New York, NY: McGraw-Hill Education/Medical; 2013. [Google Scholar]
- 8. Bink K., Haralambieva E., Kremer M., et al. Primary extramedullary plasmacytoma: similarities with and differences from multiple myeloma revealed by interphase cytogenetics. Haematologica. 2008. April; 93 4: 623- 626. 10.3324/haematol.12005. [DOI] [PubMed] [Google Scholar]
- 9. Lichtman MA., Kaushanksy K., Kipps TJ., Prchal JT., Levi MM. Williams Manual of Hematology. 8th ed. New York, NY: McGraw-Hill Education/Medical; 2011. [Google Scholar]
- 10. Hall MN., Jagannathan JP., Ramaiya NH., Shinagare AB., Van den Abbeele AD. Imaging of extraosseous myeloma: CT, PET/CT, and MRI features. AJR Am J Roentgenol. 2010. November; 195 5: 1057- 1065. 10.2214/AJR.10.4384. [DOI] [PubMed] [Google Scholar]
- 11. Moulopoulos LA., Granfield CA., Dimopoulos MA., Kim EE., Alexanian R., Libshitz HI. Extraosseous multiple myeloma: imaging features. AJR Am J Roentgenol. 1993. November; 161 5: 1083- 1087. [DOI] [PubMed] [Google Scholar]
- 12. Kumar S. Solitary plasmacytoma: is radiation therapy sufficient? Am J Hematol. 2008. September; 83 9: 695- 696. 10.1002/ajh.21248. [DOI] [PubMed] [Google Scholar]
- 13. Ozsahin M., Tsang RW., Poortmans P., et al. Outcomes and patterns of failure in solitary plasmacytoma: a multicenter Rare Cancer Network study of 258 patients. Int J Radiat Oncol Biol Phys. 2006. January 1; 64 1: 210- 217. [DOI] [PubMed] [Google Scholar]
- 14. Soutar R., Lucraft H., Jackson G., et al. Guidelines Working Group of the UK Myeloma Forum; British Committee for Standards in Haematology; British Society for Haematology. Guidelines on the diagnosis and management of solitary plasmacytoma of bone and solitary extramedullary plasmacytoma. Br J Haematol. 2004. March; 124 6: 717- 726. [DOI] [PubMed] [Google Scholar]


