Abstract
Background:
Inflammatory bowel disease (IBD) can disrupt normal sleep physiology and amplify a negative perception about quality of life. Evidence suggests increased circulation of inflammatory cytokines, such as tumor necrosis factor–alpha and interleukin-1, may play a role.
Methods:
A total of 56 patients completed the Pittsburgh Sleep Quality Index (PSQI) to measure 7 sleep domains: sleep quality, sleep latency, sleep duration, sleep efficacy, sleep disturbance, sleep medications, and daytime dysfunction. Domain scores were summed to determine the presence or absence of sleep impairment. We compared patients taking immunomodulators or biologic agents to patients not on immunomodulator or biologic agent therapy. Demographics and IBD-related clinical information were collected to adjust for potential confounders that may secondarily affect sleep, such as body mass index, depression/anxiety, and sleep-affecting medications.
Results:
The majority of patients with IBD (46 [82%]) reported poor sleep quality; 22 (79%) of the patients taking immunomodulators or biologic agents and 24 (86%) of the patients not on these therapies had a global PSQI score ≥5, suggestive of poor sleep quality. However, we found no significant difference between the 2 groups. When we analyzed the 7 PSQI sleep domains individually, we found improved sleep duration in the group taking immunomodulators or biologic agents compared to the group not on therapy, although the difference was not statistically significant.
Conclusion:
The majority of patients with IBD experience some degree of sleep impairment, and treatment with immunomodulators and biologic agents does not appear to improve sleep quality. A multicenter study with a larger sample size is warranted to better assess the diverse population of patients with IBD and the factors that impact their sleep. Routine assessment of sleep quality during IBD clinical encounters is recommended.
Keywords: Immunologic factors, inflammatory bowel disease, sleep medicine
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic relapsing-remitting disease that requires lifelong management. IBD is classified as ulcerative colitis (UC), Crohn disease (CD), or indeterminate colitis. Affected patients present with various clinical symptoms such as abdominal pain, diarrhea, and rectal bleeding. Some patients also present with extraintestinal manifestations, including arthropathy (eg, ankylosing spondylitis, sacroiliitis, peripheral arthritis), ophthalmologic disease (eg, episcleritis, uveitis), dermatologic manifestations (eg, erythema nodosum, pyoderma gangrenosum), and hepatobiliary disease (eg, primary sclerosing cholangitis). The different manifestations of IBD can impact patients' quality of life, particularly sleep health.
IBD is a known catalyst for sleep disturbance.1-4 With varying disease activity, frequent awakening because of abdominal pain and nocturnal stooling can cause sleep deprivation and amplify a negative perception about quality of life.5 Studies have shown a similar association between sleep quality and clinically inactive disease.4,6,7 In a 2006 study of 16 patients with biopsy-proven inactive IBD, the assessment of sleep by a single overnight polysomnogram demonstrated an overall decrease in sleep efficiency.7 Although the precise underlying pathophysiology remains in question, evidence supports the upregulation of inflammatory cytokines such as tumor necrosis factor–alpha (TNFα), interleukin-1 (IL-1), and interleukin-6 playing a key role in altering the normal sleep cycle.8,9 An increase in these cytokine levels has also been associated with sleep impairment in other chronic inflammatory conditions, such as rheumatoid arthritis, systemic lupus erythematosus, and multiple sclerosis.10-12 Follow-up studies in patients with rheumatoid arthritis, idiopathic juvenile arthritis, and ankylosing spondylitis have found that treatment with anti-TNFα therapy improved sleep patterns.13-15 Conversely, studies have found that a primary sleep disorder can alter immune function by activating the same inflammatory cytokines, further supporting the role of sleep disruption in triggering disease relapse or worsening symptom severity in patients with ongoing disease activity.2,16 The bidirectional relationship between IBD activity and sleep dysfunction poses the question of whether medical treatment with an immunologic agent could improve sleep quality in patients with IBD in addition to achieving disease remission.
To our knowledge, no study has evaluated the effects of specific drug therapy on sleep quality in patients with IBD. The purpose of our study was to assess the effect of immunomodulator and anti-TNFα therapy on sleep quality using a validated measure of sleep. Our overall goal is to improve the management of IBD beyond treating the somatic symptoms and address the psychosocial consequences leading to detrimental effects on quality of life.
METHODS
This study was a single-center prospective cohort study conducted at University of Florida Health Jacksonville. All patients were aged ≥18 years and provided informed consent prior to enrollment. The study was approved by the University of Florida College of Medicine–Jacksonville Institutional Review Board.
Subjects
Study participants (n=56) were recruited from the general gastroenterology and IBD specialty clinic between November 2013 and May 2015. Consecutive patients with a diagnosis of either CD or UC, confirmed by clinical, radiologic, laboratory, endoscopic, and histologic assessment, were eligible for enrollment.
Data Collection
We reviewed the electronic medical record of each patient to obtain demographic data and other relevant clinical information: age; sex; body mass index (BMI); smoking history; presence of chronic medical conditions other than gastrointestinal disease such as obstructive sleep apnea, rheumatologic disorders (eg, systemic lupus erythematosus, rheumatoid arthritis), mood or psychiatric disorders (eg, depression, anxiety, bipolar disorder, schizophrenia), and home medications (eg, antidepressants, anxiolytics, narcotics). Additional variables of interest were IBD-related history such as the type of inflammatory bowel disease (UC or CD), location of disease (eg, small bowel, colon, or small bowel and colon), presence of extraintestinal manifestations (uveitis, ankylosing spondylitis, peripheral arthritis, pyoderma gangrenosum), history of IBD-related surgery (small bowel resection, partial or total colectomy, proctocolectomy), and medication for IBD treatment (eg, aminosalicylate, steroids, azathioprine, mercaptopurine, infliximab, adalimumab).
We assessed disease severity using 2 validated, clinician-completed scales: the Lichtiger symptom score (LSS) for patients with UC and the Harvey-Bradshaw index (HBI) for patients with CD. The LSS has a score range of 0-21, with higher scores correlating with higher disease severity; patients with an LSS >3 are considered to have active disease. The HBI is scored from 1 to no endpoint because of the number of diarrhea stools, with higher scores correlating with higher disease severity; an HBI score <5 represents clinical remission, and an HBI score ≥5 represents active disease (scores 5-7 represent mild disease, scores 8-16 represent moderate disease, and scores >16 represent severe disease).
Sleep Quality Assessment
We assessed the quality and pattern of sleep in patients with IBD using the Pittsburgh Sleep Quality Index (PSQI),17 a validated questionnaire that consists of 19 patient-reported questions. The PSQI measures 7 domains of sleep in the previous 1 month: sleep quality, sleep latency, sleep duration, sleep efficacy, sleep disturbance, sleep medications, and daytime dysfunction. Each of these 7 domains is rated on a scale of 0-3, with 3 reflecting the negative extreme on the Likert scale. The sum of these 7 domain scores generates a global score that differentiates poor from good sleep quality. A global score ≥5 is consistent with poor sleep quality. Study participants completed the PSQI questionnaire at the time of their scheduled outpatient visit. Brief instructions were provided, and all questions concerning the study were answered prior to obtaining the informed consent. Patients were asked to provide the best assessment of their symptoms when answering each question. Patients on immunomodulator or biologic agent therapy were included only if they had been on their respective therapy for at least 1 month.
Statistical Analysis
Frequencies and percentages are presented for categorical variables, and means ± standard deviations (medians) are presented for numeric variables. The 2 groups (patients taking immunomodulators or biologic agents vs patients not taking these therapies) were compared using the Pearson chi-square test (or Fisher exact test if cell frequencies were small) for categorical data or the Wilcoxon rank sum test for numeric data. The level of significance is Bonferroni-adjusted for multiple tests to preserve a family-wise level of significance of 5%. That is, a P value is significant if it is <0.0063 (0.05/8 tests for outcomes). All analyses were performed with SAS v.9.4 (SAS Institute, Inc.).
RESULTS
Demographic and Clinical Characteristics
A total of 56 patients completed the PSQI questionnaire, with both comparison groups equally represented. Table 1 shows demographic and baseline characteristics. The mean age of the cohort was 45 years (±13 years), and the majority of patients were female (66%). We found no differences between the groups in the baseline characteristics with the exception of BMI (mean BMI 30 kg/m2 in the no therapy group vs 26 kg/m2 in the group taking an immunomodulator or biologic agent; P=0.029).
Table 1.
Demographic and Baseline Characteristics
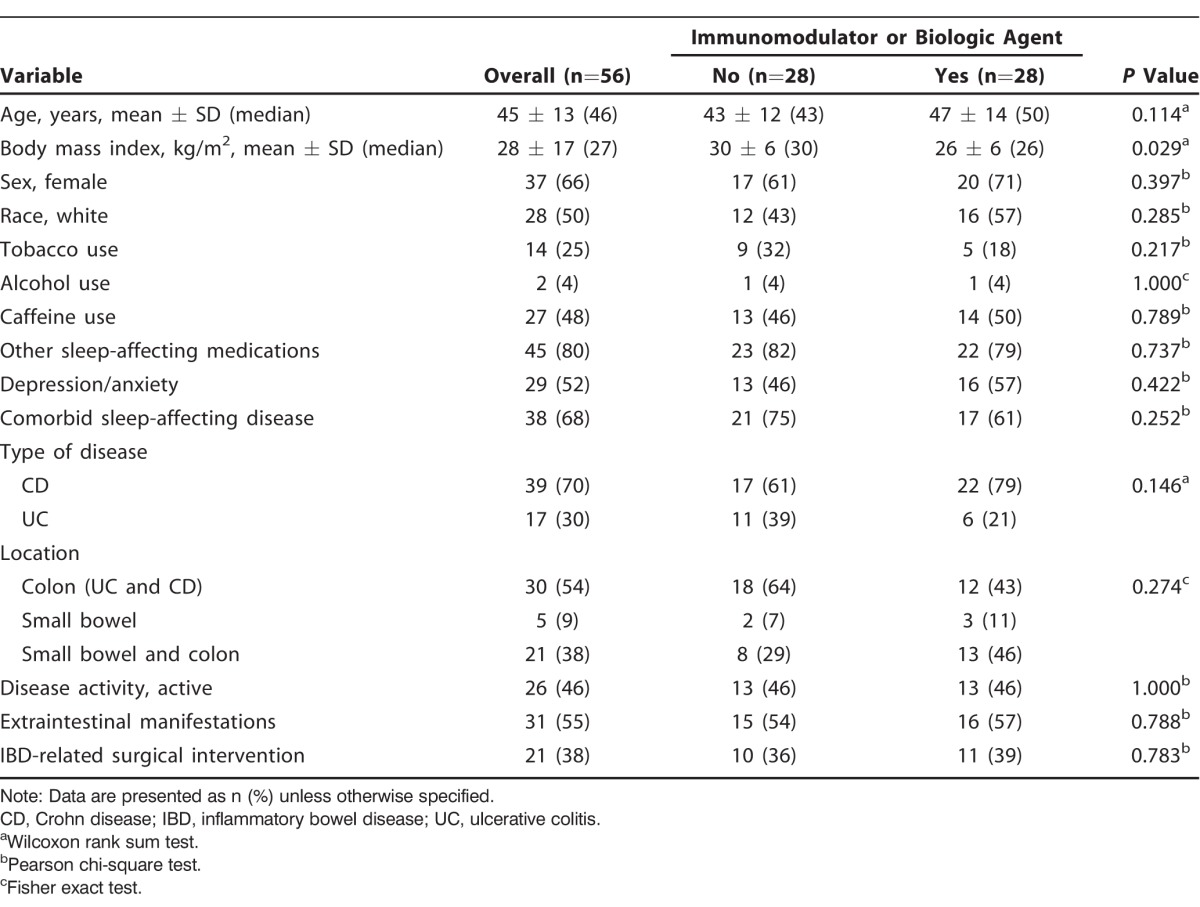
A total of 17 patients had UC (30%), and 39 patients had CD (70%). We found no significant difference in the remaining IBD-related characteristics such as location of disease (54% had colon disease only, 9% had small bowel disease only, and 38% had combined small bowel and colon disease), presence of extraintestinal manifestations (55%), and history of IBD-related surgery (38%). In both groups, disease activity was reported with the same frequency. Overall, 46% of the patient cohort had disease activity based on an LSS >3 in patients with UC and an HBI ≥5 in patients with CD.
Sleep Assessment
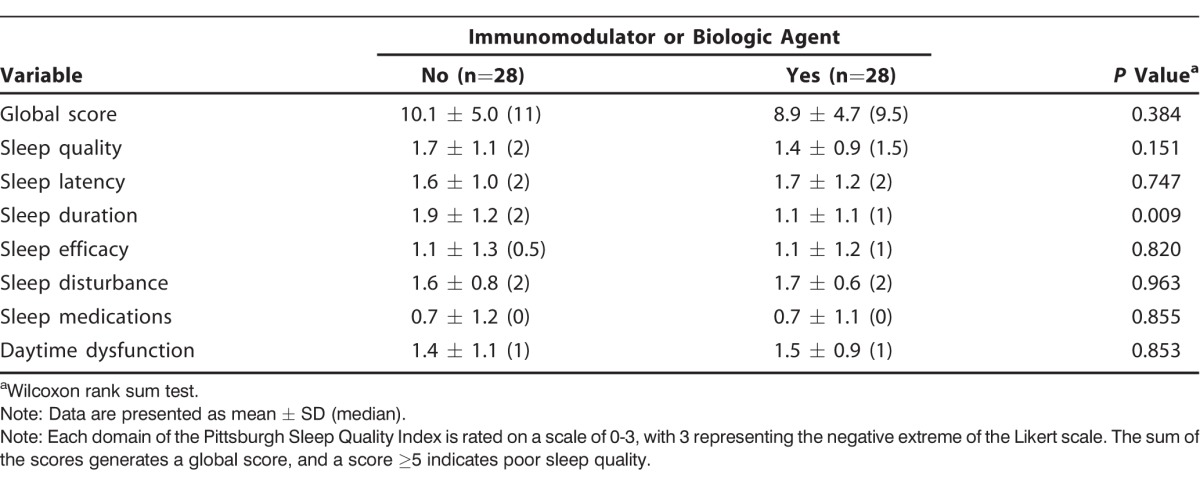
Overall, the majority of patients with IBD (82%) reported poor sleep quality (Table 2). Of the 56 patients, 22 (79%) of the 28 patients taking immunomodulators or biologic agents and 24 (86%) of the 28 patients not taking these medications had a global PSQI score ≥5, suggestive of poor sleep quality. However, we found no significant difference between the 2 groups. When the 7 sleep domains were analyzed individually, the group taking immunomodulators or biologic agents had improved sleep duration compared to the group not on therapy (1.1 ± 1.1 vs 1.9 ± 1.2, respectively; P=0.009) as shown in Table 3. Although the P value approached significance, it was not statistically significant after adjusting for multiple tests (Bonferroni adjusted significance level of 0.0063). We found varying deficiencies in the remaining sleep domains, but the differences between the groups were not statistically significant. Few patients reported the use of sleep medications (median of 0 in both groups).
Table 2.
Patients With Poor Sleep Quality According to the Pittsburgh Sleep Quality Index (PSQI) Global Score
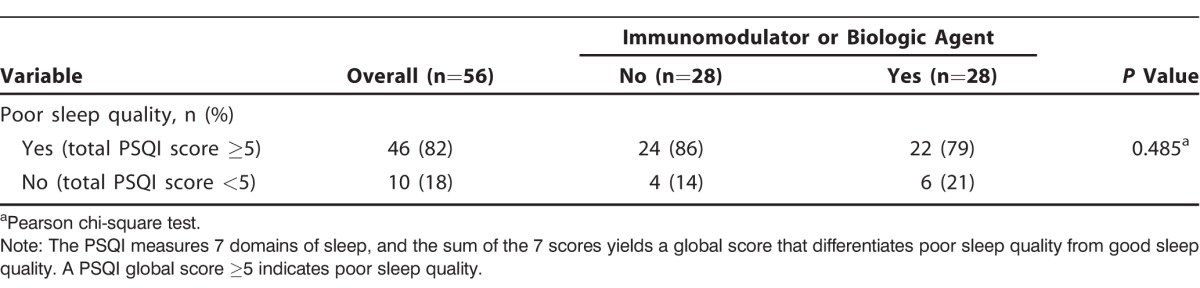
Table 3.
Pittsburgh Sleep Quality Index Global and Domain Scores
DISCUSSION
Sleep disturbances are not uncommon in patients with chronic inflammatory diseases.10-15 Sleep impairment has been demonstrated in patients with IBD during active disease and clinical remission phases. Active IBD causes physical suffering and psychosocial sequelae, disrupting the circadian rhythm and altering the normal sleep cycle.7-9 A primary sleep disorder can also increase the risk of disease relapse or affect the intensity of disease activity by altering the immune system.2,16 Our study investigated the possible effects of treatment intervention with an immune system modifying agent (immunomodulators or anti-TNFα biologic therapy) on sleep physiology.
We found that the majority of patients with IBD reported some degree of sleep abnormality. The mean global PSQI score in patients who were taking immunomodulators or biologic agents was similar to the score in patients who were not on therapy, suggesting a minimal to nonsignificant influence of treatment selection on sleep pattern. Both groups shared similar concerns across the 7 PSQI sleep domains with varying degrees of severity, and we found no statistically significant difference between the 2 groups. Patients in both groups reported prolonged sleep onset (sleep latency); increased sleep disturbance because of frequent nocturnal awakening for pain, bathroom use, difficulty breathing, feeling hot/cold, or having bad dreams; and a shorter total sleep duration. Despite the observed impairment in sleep quality, few patients reported using adjunct sleep medications. Whether this apparent undertreatment of impaired sleep quality is a result of the overshadowing effect of the IBD-related intestinal symptoms or the lack of physician familiarity with sleep disorders in patients with IBD is uncertain. Clinicians should consider incorporating a routine assessment of sleep quality as part of the IBD management algorithm. A proactive role by the treating physician may lead to early recognition and medical intervention, further influencing the quality of life of patients with IBD and minimizing IBD morbidity.
Our study has several limitations. First, the number of participants was small, lowering the statistical power so that subtle differences between the 2 groups may not have been detected. Our study was conducted at a single center in a metropolitan area with a high rate of poverty and limited access to healthcare. Patients may have had undiagnosed illnesses contributing to disturbed sleep for which the final data analysis was not adjusted. In addition, depression and anxiety, independent risk factors for sleep disorder, were self-reported diagnoses.
The treatment regimen was assigned prior to patient enrollment in this study, so other factors may have influenced treatment regimen assignment that we did not account for. Such additional factors, if they exist, may confound the study results. Further studies may design a more robust control group consisting of patients before and after treatment with biologic agents and include measurement of TNFα levels to support the hypothesis of the interaction between sleep disturbance and inflammatory cytokine upregulation. Because of our small sample size, we did not account for concomitant steroid use, which may be an important contributor to sleep, in the analysis. Sleep assessment was based on a self-reported questionnaire that is subject to recall bias. An objective measurement of sleep such as polysomnography may be necessary to accurately assess patients' sleep patterns.
CONCLUSION
To our knowledge, our study is the first to assess the effects of IBD treatment with immunomodulators or biologic agents on sleep physiology. The group receiving immunomodulators or biologic agents experienced a nonsignificant improvement in sleep duration. Furthermore, we found a high prevalence of sleep disorder in all patients with IBD, suggesting the potential need to routinely assess sleep habits during IBD clinical encounters.
ACKNOWLEDGMENTS
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care, Medical Knowledge, and Practice-Based Learning and Improvement.
REFERENCES
- 1. Zimmerman J. Extraintestinal symptoms in irritable bowel syndrome and inflammatory bowel diseases: nature, severity, and relationship to gastrointestinal symptoms. Dig Dis Sci. 2003. April; 48 4: 743- 749. [DOI] [PubMed] [Google Scholar]
- 2. Ananthakrishnan AN., Long MD., Martin CF., Sandler RS., Kappelman MD. Sleep disturbance and risk of active disease in patients with Crohn's disease and ulcerative colitis. Clin Gastroenterol Hepatol. 2013. August; 11 8: 965- 971. 10.1016/j.cgh.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ali T., Madhoun MF., Orr WC., Rubin DT. Assessment of the relationship between quality of sleep and disease activity in inflammatory bowel disease patients. Inflamm Bowel Dis. 2013. October; 19 11: 2440- 2443. 10.1097/MIB.0b013e3182a0ea54. [DOI] [PubMed] [Google Scholar]
- 4. Gingold-Belfer R., Peled N., Levy S., et al. Impaired sleep quality in Crohn's disease depends on disease activity. Dig Dis Sci. 2014. January; 59 1: 146- 151. 10.1007/s10620-013-2890-8. [DOI] [PubMed] [Google Scholar]
- 5. Pace F., Molteni P., Bollani S., et al. Inflammatory bowel disease versus irritable bowel syndrome: a hospital-based, case-control study of disease impact on quality of life. Scand J Gastroenterol. 2003. October; 38 10: 1031- 1038. [DOI] [PubMed] [Google Scholar]
- 6. Ranjbaran Z., Keefer L., Farhadi A., Stepanski E., Sedghi S., Keshavarzian A. Impact of sleep disturbances in inflammatory bowel disease. J Gastroenterol Hepatol. 2007. November; 22 11: 1748- 1753. [DOI] [PubMed] [Google Scholar]
- 7. Keefer L., Stepanski EJ., Ranjbaran Z., Benson LM., Keshavarzian A. An initial report of sleep disturbance in inactive inflammatory bowel disease. J Clin Sleep Med. 2006. October 15; 2 4: 409- 416. [PubMed] [Google Scholar]
- 8. Uthgenannt D., Schoolmann D., Pietrowsky R., Fehm HL., Born J. Effects of sleep on the production of cytokines in humans. Psychosom Med. 1995. Mar-Apr; 57 2: 97- 104. [DOI] [PubMed] [Google Scholar]
- 9. Shoham S., Davenne D., Cady AB., Dinarello CA., Krueger JM. Recombinant tumor necrosis factor and interleukin 1 enhance slow-wave sleep. Am J Physiol. 1987. July; 253 1 Pt 2: R142- R149. [DOI] [PubMed] [Google Scholar]
- 10. Abad VC., Sarinas PS., Guilleminault C. Sleep and rheumatologic disorders. Sleep Med Rev. 2008. June; 12 3: 211- 28. 10.1016/j.smrv.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 11. Chandrasekhara PK., Jayachandran NV., Rajasekhar L., Thomas J., Narsimulu G. The revalence and associations of sleep disturbances in patients with systemic lupus erythematosus. Mod Rheumatol. 2009; 19 4: 407- 415. 10.1007/s10165-009-0185-x. [DOI] [PubMed] [Google Scholar]
- 12. Brass SD., Duquette P., Proulx-Therrien J., Auerbach S. Sleep disorders in patients with multiple sclerosis. Sleep Med Rev. 2010. April; 14 2: 121- 129. 10.1016/j.smrv.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 13. Wells G., Li T., Maxwell L., Maclean R., Tugwell P. Responsiveness of patient reported outcomes including fatigue, sleep quality, activity limitation, and quality of life following treatment with abatacept for rheumatoid arthritis. Ann Rheum Dis. 2008. February; 67 2: 260- 265. [DOI] [PubMed] [Google Scholar]
- 14. Ruperto N., Lovell DJ., Li T., et al. Paediatric Rheumatology International Trials Organisation (PRINTO); Pediatric Rheumatology Collaborative Study Group (PRCSG). Abatacept improves health-related quality of life, pain, sleep quality, and daily participation in subjects with juvenile idiopathic arthritis. Arthritis Care Res (Hoboken). 2010. November; 62 11: 1542- 1551. 10.1002/acr.20283. [DOI] [PubMed] [Google Scholar]
- 15. Karadağ O., Nakas D., Kalyoncu U., Akdoğan A., Kiraz S., Ertenli I. Effect of anti-TNF treatment on sleep problems in ankylosing spondylitis. Rheumatol Int. 2012. July; 32 7: 1909- 1913. 10.1007/s00296-011-1907-x. [DOI] [PubMed] [Google Scholar]
- 16. Vgontzas AN., Zoumakis E., Bixler EO., et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004. May; 89 5: 2119- 2126. [DOI] [PubMed] [Google Scholar]
- 17. Buysse DJ., Reynolds CF, 3rd, Monk TH., Berman SR., Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989. May; 28 2: 193- 213. [DOI] [PubMed] [Google Scholar]


