Abstract
Flow cytometry was used to study signaling events in individual CD4 T cells after antigen recognition in the body. Phosphorylation of c-jun and p38 mitogen-activated protein kinase was detected within minutes in all antigen-specific CD4 T cells in secondary lymphoid tissues after injection of peptide antigen into the bloodstream. The remarkable rapidity of this response correlated with the finding that most naive T cells are in constant contact with dendritic antigen-presenting cells. Contrary to predictions from in vitro experiments, antigen-induced c-jun and p38 mitogen-activated protein kinase phosphorylation did not depend on CD28 signals and was insensitive to inhibition by cyclosporin A. Our results highlight the efficiency of the in vivo immune response and underscore the need to verify which signaling pathways identified in vitro actually operate under physiological conditions.
Maximal production of IL-2 by T cells depends on signals transduced through the T-cell receptor (TCR) and costimulatory receptors such as CD28 (1). Studies on transformed T cells and long-term T-cell clones have produced a consensus pathway in which signals transduced by each receptor are necessary to activate the nuclear factor of activated T cells (NFAT), NFκB, and AP-1 transcription factors that bind to distinct sites in the IL-2 gene enhancer and are essential for maximal transcription of the IL-2 gene (2–4). In this model, TCR aggregation initiates a tyrosine kinase cascade that ultimately results in the nuclear translocation and DNA binding of NFAT and NFκB. Transcriptionally active AP-1, which is composed of fos and phosphorylated jun components, is produced by signals from the TCR and CD28. TCR signaling activates extracellular signal-regulated kinases (ERK) 1 and ERK 2, which stimulate the production of c-fos via activation of the Elk-1 transcription factor (5). TCR and CD28 signals cooperate through effects on Vav1 (6) and protein kinase C-θ (7) to phosphorylate and activate jun N-terminal kinase (JNK), which in turn phosphorylates jun family proteins and enhances their transcription-promoting activity (8–10). The immunosuppressive drug cyclosporin A inhibits IL-2 production by preventing JNK activation (8) and translocation of NFAT into the nucleus (4).
Recent studies indicate that aspects of this pathway concerning several stress-activated protein kinases may not apply to nontransformed T cells. Freshly isolated T cells from protein kinase C-θ-deficient mice exhibit normal JNK activation after stimulation with anti-CD3 and -CD28 mAbs (11). In addition, Flavell and coworkers reported that JNK is poorly expressed in naive T cells (12) and is not necessary for IL-2 production (13), although another group reported that IL-2 production was defective in JNK-2-deficient mice (14). Other work has shown that p38 mitogen-activated protein kinase (MAPK), not JNK, transduces the CD28-mediated signal that cooperates with TCR signaling to enhance IL-2 production in nontransformed T-cell clones (15).
These discrepancies point to a need for new assays that can be used to study signal transduction in T cells under physiological conditions. Here we describe such an assay and report on its use to detail the rapidity and efficiency of c-jun and p38 MAPK phosphorylation in naive CD4 T cells stimulated by antigen in the lymphoid tissues. Importantly, our results also show that the in vivo activation requirements for c-jun and p38 MAPK phosphorylation differ from those predicted by in vitro studies with cell lines.
Materials and Methods
Mice.
BALB/c mice were purchased from the National Cancer Institute (Frederick, MD). The DO11.10 TCR transgenic mice (16) were bred and screened as described previously (17). These mice contain a large population of CD4 T cells that express a TCR specific for chicken ovalbumin peptide 323–339-I-Ad complexes. This TCR is uniquely recognized by the KJ1–26 anticlonotypic mAb (18). BALB/c SCID, BALB/c RAG2−/−, and BALB/c CD28−/− mice were obtained from The Jackson Laboratory and crossed with DO11.10 BALB/c mice to produce DO11.10 SCID, RAG2−/−, and CD28−/− BALB/c mice.
Adoptive Transfer of TCR Transgenic T Cells.
Naive DO11.10 CD4 T cells were studied by transferring DO11.10 SCID, DO11.10 RAG2−/−, or DO11.10 CD28−/− spleen and lymph node cells containing 2.5–5 × 106 CD4+, KJ1–26+ cells into unirradiated BALB/c mice by i.v. injection, as previously described (17). In some cases, spleen and lymph node cells from DO11.10 mice were treated with anti-CD8 and anti-B220 mAbs and rabbit complement to eliminate CD8 T cells and B cells. The surviving cells, which were highly enriched for CD4+, KJ1–26+ cells, were labeled with carboxyfluorescein diacetate succinimidyl ester (Molecular Probes) by using a modification of a previously described technique (19, 20), and then transferred into normal recipient mice.
Abs and Reagents.
The following Abs were purchased from PharMingen: CyChrome-labeled anti-CD4, phycoerythrin-labeled anti-IL-2, FITC-labeled anti-B7–1, FITC-labeled anti-B7–2, phycoerythrin- or biotin-labeled anti-CD11c (clone N418), biotin-labeled anti-I-Ad, purified anti-CD28, and biotin-labeled anti-mouse IgG1. Antiphospho-c-jun mAb (KM1) specific for c-jun p39 phosphorylated on serine-63, was purchased from Santa Cruz Biotechnology. Antiphospho-p38 MAPK Ab was purchased from New England Biolabs. FITC-labeled KJ1–26, biotin-labeled anti-rabbit IgG, and streptavidin-labeled phycoerythrin were obtained from Caltag. The anti-CD3ɛ hybridoma was obtained from J. A. Bluestone (University of Chicago) (21). Cyclosporin A (Calbiochem) was dissolved in olive oil and administered in a 0.2-ml volume (25 mg/kg/day) by i.p. injection.
DO11.10 T Cell Purification and in Vitro Stimulation with Abs.
Lymph nodes and spleens were harvested from DO11.10 BALB/c mice and CD4 T cells were bound to, and removed from, anti-CD4-coated magnetic beads according to the manufacturer's protocol (Dynal). Goat-anti-hamster IgG (Southern Biotechnology Associates) (1 μg/ml) was incubated overnight in 15 ml of polystyrene tubes at 4°C. After three washes in PBS, 1 μg/ml anti-CD3 mAb with or without 1 μg/ml anti-CD28 mAb was incubated in the tubes for 90 min at 37°C. The tubes were washed three times with PBS and T cells were added, centrifuged, and incubated at 37°C for various periods of time. The cells were removed from the tubes with 0.1 M EDTA and either stained for intracellular phospho-c-jun as described below or lysed and assayed for JNK enzymatic activity by using glutathione S-transferase-c-jun-Sepharose beads as a substrate as described previously (9). Phosphorylated glutathione S-transferase-c-jun was visualized after SDS/PAGE. The band corresponding to phosphorylated glutathione S-transferase-c-jun was quantified by densitometry.
In Vitro Stimulation of DO11.10 T Cells with Antigen.
Spleens from recipients of DO11.10 T cells were removed and incubated in collagenase D (400 units/ml) (Sigma) at 37°C for 25 min. Cells were centrifuged and resuspended at 20 × 106 cells/ml in complete Eagle's Hanks' amino acids medium (Biofluids) supplemented with 10% FCS/2 mM glutamine/100 units/ml penicillin/100 units/ml streptomycin/20 μg/ml gentamicin sulfate/5 × 10−5 M 2-mercaptoethanol. The cells were then incubated with 20 μg/ml of ovalbumin peptide for 60 min on ice. Aliquots containing 5 × 106 cells were then centrifuged and warmed to 37°C for various periods of time and fixed immediately in PBS containing 2% formaldehyde before intracellular staining.
In Vivo Stimulation of DO11.10 T Cells with Antigen.
Recipients of naive DO11.10 T cells were injected i.v. with ovalbumin peptide (100 μg). In some experiments, recipients were injected s.c. with 25 μg of lipopolysaccharide 6 h before peptide injection or daily with cyclosporin A (25 mg/kg/day i.p.), for 4 days before peptide injection. Spleens were removed at various times after peptide injection and fixed immediately by preparing a single cell suspension in PBS containing 2% formaldehyde before intracellular staining.
In Vitro Stimulation of Fibroblasts.
Immortalized mouse fibroblasts from wild-type or c-jun-deficient embryos (22) were cultured in DMEM supplemented with 10% FCS/2 mM glutamine/100 units/ml penicillin/100 units/ml streptomycin/20 μg/ml gentamicin sulfate/5 × 10−5 M 2-mercaptoethanol. Cells were cultured overnight in the absence of serum, removed from culture dishes by trypsinization, and stimulated with 15 ng/ml of epidermal growth factor (EGF) (Calbiochem) in serum-free DMEM for 10 min at 37°C. After stimulation, cells were fixed immediately in PBS containing 2% formaldehyde before intracellular staining.
Intracellular Staining and Flow Cytometric Analysis.
After fixation, cells were washed twice in PBS and incubated for 15 min in 1% mouse serum, 1% rat serum, and 10% spent culture medium containing anti-Fc receptor mAb 24G2. Cells were then stained with anti-CD4 CyChrome and FITC-labeled KJ1–26 mAb (specific for the DO11.10 TCR) in staining buffer (PBS plus 2% FCS and 0.2% sodium azide) for 15 min at room temperature. After a wash in staining buffer, cells were permeabilized with two washes in staining buffer containing 0.5% saponin (Sigma) and incubated for 30 min with phycoerythrin-labeled anti-IL-2 Ab, antiphospho c-jun mAb, or antiphospho p38 MAPK polyclonal Ab. Anti-IL-2 stained cells were then washed sequentially with saponin buffer, PBS, and staining buffer. Antiphospho c-jun- or antiphospho p38 MAPK-stained cells were washed in saponin buffer and then incubated for 30 min with biotin-labeled anti-mouse IgG1 or biotin-labeled anti-rabbit IgG Abs, washed, and incubated for 30 min with streptavidin-phycoerythrin. Cells were then washed as described above before analysis. The fluorescence intensities in the FL1, FL2, and FL3 channels of at least 1,000 CD4+, KJ1–26+, and CD4+, KJ1–26− events were collected for each sample on a FACScan flow cytometer.
Immunofluorescent Microscopy.
Spleens from mice that received carboxyfluorescein diacetate succinimidyl ester-labeled DO11.10 CD4 T cells 1 day earlier were frozen in isopentane. Ten-micrometer sections were cut on a cryotstat, dehydrated in acetone, and rehydrated in PBS. Sections were then blocked with anti-FcR mAb (24G2; American Type Culture Collection), avidin and biotin (Vector Laboratories), and stained sequentially with biotin-labeled anti-CD11c or biotin-labeled anti-I-Ad mAb, streptavidin-labeled horse radish peroxidase (NEN), and tyramide-labeled Cy5 (NEN). Confocal microscopy and image analyses were performed as previously described (23). Briefly, sections were analyzed by using a Bio-Rad MRC-1000 confocal microscope equipped with a krypton/argon laser. Separate images were captured for the carboxyfluorescein diacetate succinimidyl ester and Cy5 signals from each section. Adobe photoshop (Adobe Systems, Mountain View, CA) software was used to pseudocolor the carboxyfluorescein diacetate succinimidyl ester images green and the Cy5 images red. The corresponding red and green images were overlaid in photoshop to produce the final dual-color images of each section. Interactions between DO11.10 T cells and cells expressing I-Ad or CD11c were quantified by highlighting areas of overlap on the dual color images in photoshop by using the yellow Color Range function. The percentage of interacting DO11.10 T cells was calculated by dividing the number of DO11.10 T cells that had yellow color on any edge by the total number of DO11.10 T cells counted.
Results
Correlation of JNK Activation and c-jun Phosphorylation in Vitro.
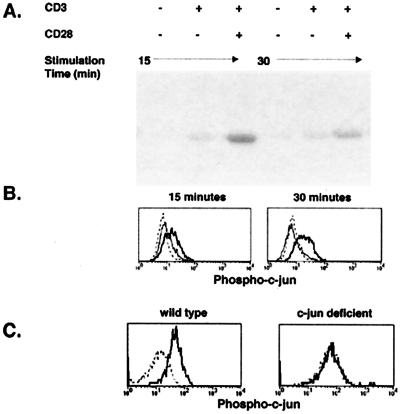
Initial experiments were designed to test the feasibility of detecting c-jun phosphorylation in individual naive T cells by flow cytometry. This was done by stimulating purified naive DO11.10 CD4 T cells in vitro and comparing c-jun phosphorylation activity in lysates by using recombinant c-jun as a substrate, with the ability to detect phosphorylated c-jun in permeabilized cells by using an mAb specific for c-jun phosphorylated at serine 63. The ability of c-jun to act as an optimal activator of transcription depends on phosphorylation of this serine residue (24, 25). As shown in Fig. 1A, weak c-jun phosphorylation activity was detected in lysates from DO11.10 T cells 15 or 30 min after stimulation with anti-CD3 mAb alone, and this activity was enhanced by stimulation with anti-CD28 mAb. Similarly, weak staining with the antiphospho-c-jun mAb was detected by flow cytometry 15 or 30 min after stimulation with anti-CD3 alone, and the amount of staining increased after stimulation with anti-CD3 plus anti-CD28 mAbs (Fig. 1B). The induction of phospho-c-jun staining in stimulated DO11.10 T cells was completely inhibited in the presence of 40 μM SB202190 (data not shown), which inhibits JNK and p38 MAPK but not 4 μM SB202190, which inhibits only p38 MAPK (26). These results confirm earlier work with cell lines (8) by showing that the induction of c-jun phosphorylation depends on the TCR and CD28 in naive CD4 T cells.
Figure 1.
Comparison of phospho-c-jun detection by flow cytometry and JNK enzymatic activity in naive T cells stimulated in vitro with mAbs. Purified DO11.10 T cells were stimulated for 15 or 30 min with control hamster IgG, anti-CD3, or anti-CD3 and anti-CD28 mAbs. (A) Whole-cell lysates from stimulated cells were incubated with GST-c-jun beads and analyzed for JNK activity. (B) Intracellular staining of phosphorylated c-jun protein in cells stimulated with control IgG (dashed line), anti-CD3 (thin line), or anti-CD3 and anti-CD28 mAbs (bold line). (C) Intracellular staining of phosphorylated c-jun protein in normal or c-jun-deficient mouse embryo fibroblasts stimulated for 10 min with EGF (bold line) or nothing (dashed line).
Because flow cytometry was a novel way to detect c-jun-phosphorylation, it was important to confirm the specificity of this method. Fibroblast cell lines from either normal or c-jun-deficient mouse embryos (22) were stimulated with EGF, a known stimulus of c-jun phosphorylation (27), and stained with antiphospho-c-jun mAb. As shown in Fig. 1C, wild-type fibroblasts stimulated with EGF showed a greater degree of intracellular staining with the antiphospho-c-jun mAb than wild-type fibroblasts cultured with medium alone. For unknown reasons, c-jun-deficient fibroblasts showed a slightly higher level of staining in the absence of EGF stimulation than did wild-type fibroblasts. Importantly, however, c-jun-deficient fibroblasts showed no EGF-dependent increase in antiphospho-c-jun mAb staining over that observed in response to medium alone. Therefore, although c-jun-deficient fibroblasts showed elevated staining in the absence of stimulation, perhaps because of their transformed state, the lack of staining of these cells with antiphospho-c-jun mAb after EGF stimulation is evidence that the antiphospho-c-jun mAb recognizes phospho-c-jun.
Comparison of Antigen-Induced c-jun Phosphorylation in Vitro and in Vivo.
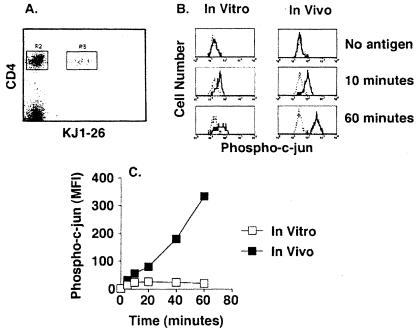
Flow cytometry was next used to measure c-jun phosphorylation in naive DO11.10 T cells stimulated by antigen in vitro or in vivo. DO11.10 T cells were activated under conditions where they represented less than 2% of the cells to mimic the physiological situation where T cells of differing specificities compete for space in lymphoid tissue. This was accomplished by transferring several million naive CD4 T cells from DO11.10 donor mice into normal recipient mice. For in vitro stimulation, spleens from recipient mice were removed and processed under conditions that release dendritic antigen-presenting cells as well as lymphocytes. Single-cell suspensions were incubated with ovalbumin peptide on ice and then warmed to 37°C to initiate activation of the DO11.10 T cells. For in vivo stimulation, the ovalbumin peptide was injected i.v. into recipient mice. The peptide was injected directly into the bloodstream to ensure immediate access to splenic antigen-presenting cells and synchronous activation of the DO11.10 T cells. At various times after culture initiation or peptide injection, spleens were processed quickly in formaldehyde to prevent signal decay during the intracellular staining procedure. As shown in Fig. 2A, the transferred DO11.10 T cells (KJ1–26+, R3 gate) were easily distinguished from the CD4 T cells of the recipient (KJ1–26−, R2 gate). Phosphorylation of c-jun was induced in DO11.10 CD4 T cells but not CD4 T cells of the recipient by 10 min after initiation of in vitro culture or i.v. injection of ovalbumin peptide (Fig. 2B). The amount of c-jun phosphorylation did not increase further by 60 min in DO11.10 cells that were stimulated in vitro but continued to increase in DO11.10 cells that were stimulated in vivo (Fig. 2 B and C). The amount of total c-jun detected by intracellular staining did not change over time (data not shown), confirming that the increased antiphospho-c-jun signal reflected increased phosphorylation of existing c-jun protein. These results show that signal transduction stimulated by antigen presentation in vivo occurs very rapidly and is more efficient than signaling induced in vitro.
Figure 2.
Comparison of c-jun phosphorylation after in vitro or in vivo stimulation of naive T cells with peptide antigen. (A) A representative flow cytometry dot plot showing CD4 and KJ1–26 staining of spleen cells from transferred recipients. The gating strategy discriminates transferred DO11.10 CD4+, KJ1–26+ cells (R3) from the recipient's CD4+, KJ1–26− cells (R2). (B) Antiphospho c-jun staining of DO11.10 (bold line) or recipient CD4 T cells (dashed line) from the same sample after initiation of in vitro culture with ovalbumin peptide-pulsed splenocytes (Left) or i.v. injection of ovalbumin peptide (Right). (C) Time course of c-jun phosphorylation after in vitro (open squares) or in vivo (filled squares) stimulation with ovalbumin peptide. The difference between the phospho-c-jun signal (mean fluorescence intensity, MFI) in DO11.10 and recipient CD4 T cells is shown.
The Rapidity of in Vivo T-Cell Activation Correlates with the Proximity of Naive T Cells and Antigen-Presenting Cells.
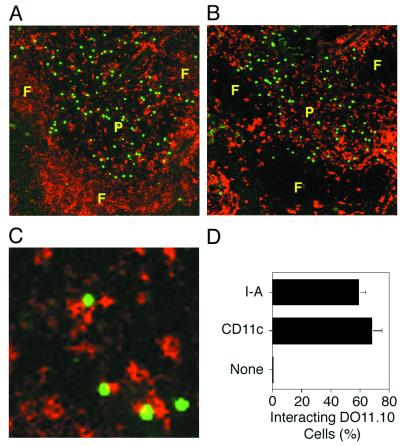
The phosphorylation of c-jun in virtually all DO11.10 T cells within 10 min of ovalbumin peptide injection implied that naive T cells must reside in close proximity to antigen-presenting cells in vivo. As shown in Fig. 3 A and B, DO11.10 T cells were found only in the periarteriolar lymphoid sheaths, also known as the T-cell zones, of the spleen after transfer into recipient mice. This region was also densely occupied by cells expressing high levels of I-Ad molecules (Fig. 3A), which are required for presentation of the ovalbumin peptide (28), and the dendritic cell marker CD11c (Fig. 3B). If the green-labeled DO11.10 T cells were in physical contact with the red-labeled I-Ad- and/or CD11c-expressing cells, then a unique yellow color would be present in the area of contact (23). As shown in Fig. 3, 60–70% of the naive DO11.10 T cells showed this evidence of physical contact with cells expressing I-Ad (Fig. 3 C and D) and CD11c (Fig. 3D). These results are consistent with the possibility that naive DO11.10 T cells are constantly interacting with dendritic cells in the T-cell zones in the absence of antigen, providing an explanation for the rapid signaling that occurred after injection of ovalbumin peptide.
Figure 3.
Naive CD4 T cells are located near I-Ad-expressing cells in vivo. The locations of DO11.10 CD4 T cells (shown green) and cells expressing anti-I-Ad (shown red in A) or anti-CD11c (shown red in B) in thin sections of the spleen are shown. A and B show adjacent sections containing the same white pulp chord with a perimeter of B-cell-rich follicles (F) surrounding a central periarteriolar lymphoid sheath (P). A magnified view of a periarteriolar lymphoid sheath is shown in C with DO11.10 T cells shown green and I-Ad-expressing cells shown red. The areas of yellow color indicate significant overlap between the two cell types. D shows the mean percentage of DO11.10 T cells (± the standard deviation) found interacting with cells stained with I-Ad, CD11c, or control Ig, as evidenced by the presence of yellow color. At least 200 cells from two to four white pulp chords were counted in each group.
Phosphorylation of c-jun and p38 MAP Kinase Does Not Depend on CD28 Costimulation in Antigen-Stimulated Naive CD4 T Cells.
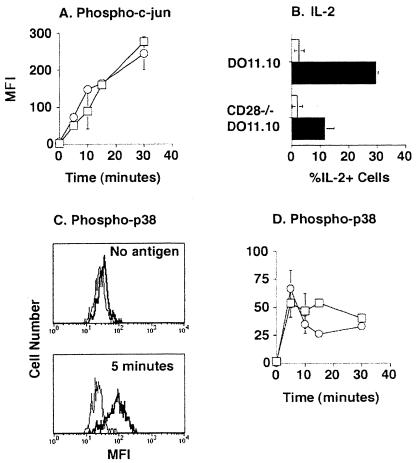
Because dendritic cells constitutively express B7–2 molecules (29), and c-jun phosphorylation was increased by anti-CD28 Ab stimulation in vitro (Fig. 1), it was of interest to assess the role of CD28 in c-jun phosphorylation stimulated by antigen presentation in vivo. As shown in Fig. 4A, the rate and magnitude of c-jun phosphorylation was identical in normal and CD28-deficient naive DO11.10 T cells in mice injected with ovalbumin peptide. In the same experiment, CD28-deficient DO11.10 T cells produced less IL-2 than wild-type DO11.10 T cells (Fig. 4B), demonstrating the CD28 dependence of this response.
Figure 4.
Phosphorylation of c-jun and p38 MAPK is CD28-independent in DO11.10 T cells. Mice were transferred with either naïve DO11.10 (squares) or CD28-deficient (circles) DO11.10 T cells. Phosphorylation of c-jun (A) or p38 MAPK (D) was assessed at the indicated times after i.v. injection of ovalbumin peptide. The difference between the signal (mean fluorescence intensity, MFI) in DO11.10 and recipient CD4 T cells is shown. C shows histograms of phospho-p38 MAPK staining in DO11.10 (bold line) or recipient (thin line) CD4 T cells at the indicated times after i.v. injection of ovalbumin peptide. Intracellular IL-2 was measured 2.5 h after i.v. injection of ovalbumin peptide (closed bars) or nothing (open bars) (B). These data are representative of two independent experiments.
The CD28 independence of c-jun phosphorylation could have been related to the fact that B7 molecules are weakly expressed on dendritic cells in the lymphoid tissues under normal conditions (30). This possibility was addressed by treating animals with lipopolysaccharide 6 h before ovalbumin peptide injection, which, as described by DeSmedt et al. (30), increased B7–1 levels on dendritic cells by 2-fold and B7–2 levels by 5-fold (data not shown). The presence of high levels of B7 molecules on dendritic cells at the time of ovalbumin peptide injection did not augment antigen-induced phosphorylation of c-jun in wild-type DO11.10 cells, although the cells produced more IL-2 in these recipients than they did in nonlipopolysaccharide-treated recipients (data not shown). This result and that obtained with CD28-deficient T cells show that, in contrast to Ab stimulation, phosphorylation of c-jun in response to antigenic stimulation did not depend on CD28.
It has been proposed that p38 MAPK is involved in CD28 signal transduction in naive T cells, at least after in vitro stimulation (15). As shown in Fig. 4 C and D, p38 MAPK phosphorylation was rapidly induced in naive DO11.10 T cells after ovalbumin peptide injection, achieving a maximum at 5 min and falling thereafter. Essentially all DO11.10 T cells contained phosphorylated p38 MAPK at the 5-min time point, whereas CD4 T cells of the recipient did not (Fig. 4C). An identical rate and magnitude of antigen-stimulated p38 MAPK phosphorylation was observed in CD28-deficient DO11.10 T cells in normal recipients (Fig. 4D) or in wild-type DO11.10 T cells in lipopolysaccharide-treated recipients containing B7high dendritic cells (data not shown). Therefore, CD28 was not essential for p38 MAPK phosphorylation in naive CD4 T cells stimulated by antigen in vivo.
Effects of Cyclosporin A on in Vivo Signaling in Naive T Cells.
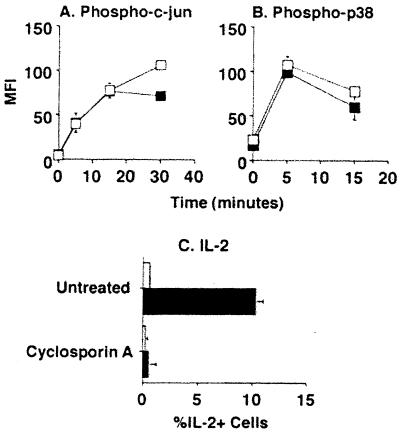
The finding that c-jun phosphorylation was not CD28-dependent in naive T cells warranted testing of another feature of c-jun phosphorylation that was defined in transformed T cells in vitro, that is, its sensitivity to inhibition by cyclosporin A (8). Cyclosporin A treatment of recipient mice did not affect the phosphorylation of c-jun (Fig. 5A) or p38 MAPK (Fig. 5B) in naive DO11.10 T cells after injection of ovalbumin peptide, although IL-2 production was completely blocked (Fig. 5C). Therefore, naive T cells differ from transformed T cells in that cyclosporin A does not inhibit phosphorylation of c-jun or p38 MAPK.
Figure 5.
Effect of cyclosporin A treatment on c-jun and p38 MAPK phosphorylation. Recipients of naive DO11.10 CD4 T cells were either untreated (open squares) or treated with cyclosporin A for 4 days to achieve a pharmacological level of drug (closed squares). Phosphorylation of c-jun (A) and p38 MAPK (B) was then measured at the indicated times after i.v. injection of ovalbumin peptide. The difference between the signal (mean fluorescence intensity, MFI) in DO11.10 and recipient CD4 T cells ± SD is shown (n = 2). (C) IL-2 was measured 3 h after i.v. injection of ovalbumin peptide (closed bars) or nothing (open bars). These data are representative of two independent experiments.
Discussion
The use of intracellular staining and flow cytometry allowed us to discover several aspects of in vivo T-cell signal transduction that were not appreciated from in vitro studies. One of these was the rapid and highly efficient nature of in vivo T-cell activation. Virtually all peptide-I-Ad-specific CD4 T cells in the spleen were stimulated within 5 min of i.v. injection of the relevant peptide. This rapid response is likely explained by the fact that the majority of naive CD4 T cells are in contact with I-Ad-expressing cells, probably dendritic cells, at all times. In this case, the only limitation to T-cell activation after peptide injection would be the production and display of surface class II MHC-peptide complexes. This could occur within minutes in our experiments, because i.v. injection delivers the peptide directly to splenic dendritic cells. Because this peptide does not require antigen processing (28), it would then be able to immediately bind to I-Ad molecules. Exclusive presentation of peptide–MHC complexes by dendritic cells may also explain the finding that signal transduction in specific T cells was more efficient in vivo than in vitro. Dendritic cells express higher levels of class II MHC and adhesion molecules than B cells (31), which by virtue of their abundance in dissociated lymphoid tissue are probably the major antigen-presenting cells for CD4 T cells in in vitro cultures. Alternatively, the diminished capacity to maintain T-cell signal transduction in vitro could be related to the accumulation of inhibitory factors that are removed by normal circulation in vivo.
It was surprising, on the basis of previous studies of JNK activation in cell lines (8, 9) and our own results with Ab stimulation (Fig. 1), to find that c-jun phosphorylation in antigen-stimulated naive CD4 T cells did not depend on CD28 in vivo. The discrepancy may be explained by the fact that CD28 is capable of augmenting TCR-mediated JNK activation and c-jun phosphorylation but is not unique in this regard. Thus, CD28 appears to play an important role in JNK activation in Ab stimulation experiments, where it is the only costimulatory receptor available. However, antigen-presenting cells may express ligands for other receptors on T cells that play an even greater role than CD28. If this were the case, then CD28 would play a minor role in c-jun phosphorylation under physiological conditions where T cells are stimulated by antigen-presenting cells. In addition, the putative non-CD28 receptors that stimulate c-jun phosphorylation in T cells in vivo may signal through a cyclosporin A-insensitive pathway, explaining our failure to observe an effect of this drug in our in vivo experiments.
Alternatively, although we found a correlation between JNK activation and c-jun phosphorylation in vitro, we cannot rule out the possibility that an unknown CD28-independent cyclosporin A-insensitive kinase phosphorylates c-jun in naive T cells in vivo. The finding of Flavell and coworkers that JNK is poorly expressed in naive T cells (12) and is not critical for IL-2 production (13) lends credence to this possibility. However, this point is controversial, as Karin and colleagues have shown that JNK2-deficient T cells are defective with respect to IL-2 production (14). If an unknown kinase is responsible for c-jun phosphorylation in naive T cells in vivo, then our results indicate that it has the same sensitivity to inhibition by the drug SB202190 as JNK.
In contrast to earlier findings on Ab-mediated stimulation of long-term T cell clones (15), CD28 signaling was not required for phosphorylation of p38 MAPK in naive CD4 T cells stimulated by antigen in vivo. This discrepancy could represent a difference in signal transduction between these cell types or may be related to differences in T cell stimulation by anti-CD28 Ab versus B7 molecules on antigen-presenting cells, as described in another system (32).
In vitro approaches have played a critical role in defining the potential signaling pathways in activated T cells. However, the microenvironmental conditions under which T-cell activation normally occurs in vivo may be difficult to replicate with in vitro systems that rely on transformed cell lines, Ab stimulation, and antigen-presenting cells other than dendritic cells. Therefore, it is crucial that sensitive methods like the one described here be used to study signal transduction in antigen-specific T cells undergoing primary and secondary immune responses in the body.
Acknowledgments
We thank Jennifer Walter for technical assistance and Drs. Yoji Shimizu, Stephen Jameson, and Matthew Mescher for critical review of the manuscript. This work was supported by grants from the National Institutes of Health (AI27998, AI35296, and AI39614 to M.K.J., GM54706 and AI35296 to D.L.M., and AI07313 to J.L.B.), the Howard Hughes Medical Institute (A.K.), and the Cancer Research Institute (T.Z.)
Abbreviations
- EGF
epidermal growth factor
- JNK
jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- NFAT
nuclear factor of activated T cells
- TCR
T-cell receptor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Chambers C A, Allison J P. Curr Opin Cell Biol. 1999;11:203–210. doi: 10.1016/s0955-0674(99)80027-1. [DOI] [PubMed] [Google Scholar]
- 2.van Leeuwen J E, Samelson L E. Curr Opin Immunol. 1999;11:242–248. doi: 10.1016/s0952-7915(99)80040-5. [DOI] [PubMed] [Google Scholar]
- 3.Crabtree G R, Clipstone N A. Annu Rev Biochem. 1994;63:1045–1083. doi: 10.1146/annurev.bi.63.070194.005145. [DOI] [PubMed] [Google Scholar]
- 4.Jain J, Loh C, Rao A. Curr Opin Immunol. 1995;7:333–342. doi: 10.1016/0952-7915(95)80107-3. [DOI] [PubMed] [Google Scholar]
- 5.Davis R J. Mol Reprod Dev. 1995;42:459–467. doi: 10.1002/mrd.1080420414. [DOI] [PubMed] [Google Scholar]
- 6.Moller A, Dienz O, Hehner S P, Droge W, Schmitz M L. J Biol Chem. 2001;276:20022–20028. doi: 10.1074/jbc.M011139200. [DOI] [PubMed] [Google Scholar]
- 7.Villalba M, Coudronniere N, Deckert M, Teixeiro E, Mas P, Altman A. Immunity. 2000;12:151–160. doi: 10.1016/s1074-7613(00)80168-5. [DOI] [PubMed] [Google Scholar]
- 8.Su B, Jacinto E, Hibi M, Kallunki T, Karin M, Ben-Neriah Y. Cell. 1994;77:727–736. doi: 10.1016/0092-8674(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 9.Li W, Whaley C D, Mondino A, Mueller D L. Science. 1996;271:1272–1275. doi: 10.1126/science.271.5253.1272. [DOI] [PubMed] [Google Scholar]
- 10.Nunes J A, Battifora M, Woodgett J R, Truneh A, Olive D, Cantrell D A. Mol Immunol. 1996;33:63–70. doi: 10.1016/0161-5890(95)00121-2. [DOI] [PubMed] [Google Scholar]
- 11.Sun Z, Arendt C W, Ellmeier W, Schaeffer E M, Sunshine M J, Gandhi L, Annes J, Petrzilka D, Kupfer A, Schwartzberg P L, Littman D R. Nature (London) 2000;404:402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 12.Weiss L, Whitmarsh A J, Yang D D, Rincon M, Davis R J, Flavell R A. J Exp Med. 2000;191:139–146. doi: 10.1084/jem.191.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong C, Yang D D, Tournier C, Whitmarsh A J, Xu J, Davis R J, Flavell R A. Nature (London) 2000;405:91–94. doi: 10.1038/35011091. [DOI] [PubMed] [Google Scholar]
- 14.Sabapathy K, Hu Y, Kallunki T, Schreiber M, David J P, Jochum W, Wagner E F, Karin M. Curr Biol. 1999;9:116–125. doi: 10.1016/s0960-9822(99)80065-7. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Salojin K V, Gao J X, Cameron M J, Bergerot I, Delovitch T L. J Immunol. 1999;162:3819–3829. [PubMed] [Google Scholar]
- 16.Murphy K M, Heimberger A B, Loh D Y. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 17.Kearney E R, Pape K A, Loh D Y, Jenkins M K. Immunity. 1994;1:327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 18.Haskins K, Kubo R, White J, Pigeon M, Kappler J, Marrack P. J Exp Med. 1983;157:1149–1169. doi: 10.1084/jem.157.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyons A B, Parish C R. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 20.Merica R, Khoruts A, Pape K A, Reinhardt R L, Jenkins M K. J Immunol. 2000;164:4551–4557. doi: 10.4049/jimmunol.164.9.4551. [DOI] [PubMed] [Google Scholar]
- 21.Leo O, Foo M, Sachs D H, Samelson L E, Bluestone J A. Proc Natl Acad Sci USA. 1987;84:1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schreiber M, Kolbus A, Piu F, Szabowski A, Mohle-Steinlein U, Tian J, Karin M, Angel P, Wagner E F. Genes Dev. 1999;13:607–619. doi: 10.1101/gad.13.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ingulli E, Mondino A, Khoruts A, Jenkins M K. J Exp Med. 1997;185:2133–2141. doi: 10.1084/jem.185.12.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pulverer B J, Kyriakis J M, Avruch J, Nikolakaki E, Woodgett J R. Nature (London) 1991;353:670–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- 25.Smeal T, Binetruy B, Mercola D, Grover B A, Heldecker G, Rapp U R, Karin M. Mol Cell Biol. 1992;12:3507–3513. doi: 10.1128/mcb.12.8.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le-Niculescu H, Bonfoco E, Kasuya Y, Claret F X, Green D R, Karin M. Mol Cell Biol. 1999;19:751–763. doi: 10.1128/mcb.19.1.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minden A, Lin A, McMahon M, Lange-Carter C, Derijard B, Davis R J, Johnson G L, Karin M. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- 28.Sette A, Buus S, Colon S, Miles C, Grey H M. J Immunol. 1988;141:45–48. [PubMed] [Google Scholar]
- 29.Inaba K, Witmer-Pack M, Inaba M, Hathcock K S, Sakuta H, Azuma M, Yagita H, Okumura K, Linsley P S, Ikehara S, et al. J Exp Med. 1994;180:1849–1860. doi: 10.1084/jem.180.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeSmedt T, Pajak B, Muraille E, Lespagnard L, Heinen E, Baetselier P D, Urbain J, Leo O, Moser M. J Exp Med. 1996;184:1413–1424. doi: 10.1084/jem.184.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nussenzweig M C, Steinman R M, Unkeless J C, Witmer M D, Gutchinov B, Cohn Z A. J Exp Med. 1981;154:168–187. doi: 10.1084/jem.154.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nunes J A, Collette Y, Truneh A, Olive D, Cantrell D A. J Exp Med. 1994;180:1067–1076. doi: 10.1084/jem.180.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]