Abstract
Plants facing the seasonal variations always need a growth restraining mechanism when temperatures turn down. C-repeat binding factor (CBF) genes work essentially in the cold perception. Despite lots of researches on CBFs, the multiple crosstalk is still interesting on their interaction with hormones and dormancy-associated MADS (DAM) genes in the growth and dormancy control. Therefore, this study highlights roles of PmCBFs in cold-induced dormancy from different orgens. And a sense-response relationship between PmCBFs and PmDAMs is exhibited in this process, jointly regulated by six PmCBFs and PmDAM4–6. Meantime, GA3 and ABA showed negative and positive correlation with PmCBFs expression levels, respectively. We also find a high correlation between IAA and PmDAM1–3. Finally, we display the interaction mode of PmCBFs and PmDAMs, especially PmCBF1-PmDAM1. These results can disclose another view of molecular mechanism in plant growth between cold-response pathway and dormancy regulation together with genes and hormones.
Keywords: CBF genes, DAM genes, Prunus mume, stem growth, expression analysis, Phytohormone assays
1. Introduction
Prunus mume was domesticated in northern China due to its cold resistance and adaptability to severe conditions. Bud dormancy is a crucial capacity of perennial plants that ensures survival during hard seasons. Recent genomic and transcriptomic investigations have brought to light fundamental mechanisms of molecular dormancy regulations [1,2]; however, the involvement of multiple hormones remains pluralistic, particularly in the fruit-bearing plants [3].
For woody plants, stem growth is significantly affected by low temperatures. Plants coordinate lifecycles in accordance with the seasonal shifts. Thus, they are affected by changes in temperatures. Central to this process is the ability to integrate environmental information into the vegetative growth rhythm. Understanding how plants regulate the timing of stem development has been given much attention, especially among deciduous trees, such as Prunus. Evidences indicate that some aspects of plant development are regulated by the MADS-box genes (Minichromosome maintenance 1 in yeast, AGAMOUS in Arabidopsis, DEFICIENS in snapdragon and serum response factor from human) and APETALA2/ethylene response factor (AP2/ERF) gene families, which integrate hormonal signals such as auxin, gibberellin, cytokinin and abscisic acid [4,5,6,7]. According to recent studies, ERF genes increase cell division and are important for ethylene signalling in vascular tissues. ERF family members also play vital roles in many other processes, including growth and development, dormancy and dormancy release, and biotic and abiotic stress responses [8,9,10]. Thus, combining analysis of genes expression and hormone levels may provide deeper understanding of the process of stem development. Additionally, acclimation enables plants to endure cold stress and is controlled by a mass of genes. AtCBFs (1–3) are key transcription factors directing the expression of specific groups of genes [11]. In cold climates, CBF genes play a prominent role in cold-response pathways, while DAM genes are related to plant dormancy and dormancy releasing. Because of their significant effects on plant development control, understanding their functions in stem development in Prunus can provide further value.
CBF genes, also named DREB genes, belong to AP2/ERF gene family [12,13]. CBF/DREB1 genes have been studied in various plant species, such as Arabidopsis thaliana, Oryza sativa, soybean (Glycine max), Chrysanthemum, Vitis vinifera, Malus domestica, Prunus persica and Prunus mume [14,15,16,17,18,19]. There are six CBF/DREB1 genes in the model plant Arabidopsis, CBF3/DREB1A regulates many genes related to cold response, and hormone metabolism [20]. DREB1 (AtCBF1–3) and DREB2 also function in the signal pathway under low-temperature conditions [21]. The transgenic plants overexpressing AtCBF1 and 3 show strong tolerance to cold stresses [22,23]. Unlike AtCBF1–3, the expression of AtCBF4 is induced by abscisic acid (ABA) and is not influenced by low temperatures [24]. What’s more, DDF1 and DDF2 (AtCBF5 and AtCBF6) are induced by salinity stress, which is connected to gibberellic acid (GA) levels and results in dwarfism and delayed flowering [25,26]. Cold hardiness has also been observed when a PpCBF from peach (Prunus persica) is overexpressed in apple [27]. In peach, there are five CBF genes, which are highly induced by 4 °C in leaves and this depends on specific CBF gene [27,28,29,30]. For other species, such as Triticum aestivum and O. sativa, the dwarf phenotype is observed in transgenic plants [14,31].
DAM genes act downstream for CBF genes and play a positive role in plant dormancy induction [8,32,33]. In P. mume, there are six DAM genes [34,35,36]. PmDAMs participate in endodormancy-associated expression [37,38]. The transcript accumulations of DAM5 and DAM6 in peach reach peaks, coinciding with the winter solstice and may be regulated by cold exposure [39]. In Japanese pear (Pyrus pyrifolia), PpCBF2 regulates the expression of PpMADS13–1, which is a DAM gene [40]. Therefore, analyzing the relationship between CBF and DAM genes could aid research for stem growth in Prunus.
In this study, six PmCBFs were cloned and the phylogenetic relationships between them were investigated. Then expression profiles for these genes in seven types of tissues were generated. To assess the interactive roles of PmCBFs and PmDAMs, and phytohormones in different processes of stem growth, the gene expression levels and varying hormonal concentrations were examined in stems. Finally, we reported the interaction modes between PmCBFs and PmDAMs in the dormancy of stem. The results of this study will contribute to a clearer understand of cold-response pathway and dormancy in plant.
2. Results
2.1. Identification and Cloning of CBF Genes in P. mume
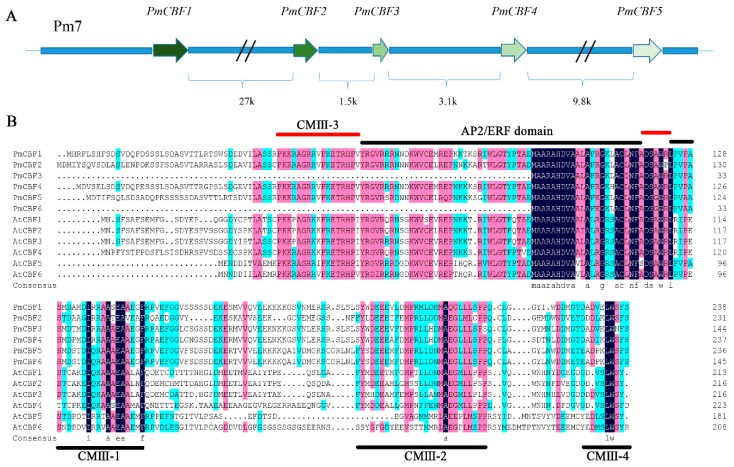
Six PmCBFs were identified from the Prunus mume protein database (Supplementary Table S1). And PmCBFs1–5 were found as a tandem array on Chromosome 7 (Figure 1A). The lengths of the sequences for PmCBF1–6 were 720 bp, 699 bp, 438 bp, 717 bp, 714 bp, and 441 bp, and encoded 239, 232, 145, 238, 237, and 146 amino acids, respectively (Supplementary Data S1). These PmCBFs were highly consistent among their homologous genes with conserved AP2/ERF domains (Supplementary Figure S1). Then, sequences and conserved domains were verified by the InterPro website.
Figure 1.
CBF genes in P. mume. (A) Gene locations of PmCBFs on chromosome 7; (B) Multiple sequences alignment of CBF proteins from P. mume and other species. Multiple sequence alignment of the CBF genes was applied using DNAMAN 7.0 program (Lynnon Corp., Quebec, QC, Canada). The AP2/ERF domain and CMIII domain (CMIII-1, CMIII-2, and CMIII-4) were shown by lines on bottom of the alignment. CMIII-3 was indicated by the red lines. The protein sequences of these CBF genes were shown in Supplementary Data S3.
2.2. Multiple Sequence Alignments and Phylogenetic Analysis
To carry out multiple sequence alignments, the protein sequences of six PmCBFs and 29 CBF genes from other plants were analysed. The genomic arrangement and related sequence structures were displayed in Figure 1. Especially the conserved domains, together with six AtCBFs, were marked (Figure 1B). At the N-terminals of the PmCBFs, the AP2/ERF domain exhibited high conservation, but the AP2/ERF domains of PmCBF3 and PmCBF6 were incomplete. In PmCBFs, CMIII domains, containing CMIII-1, CMIII-2, CMIII-3, and CMIII-4 motifs, were conserved. As shown in Figure 1B, CMIII-1 was behind the AP2/ERF domain; CMIII-3 had two regions on the both sides of the AP2/ERF domain; and CMIII-2 and CMIII-4 were at the C-terminals of protein sequences.
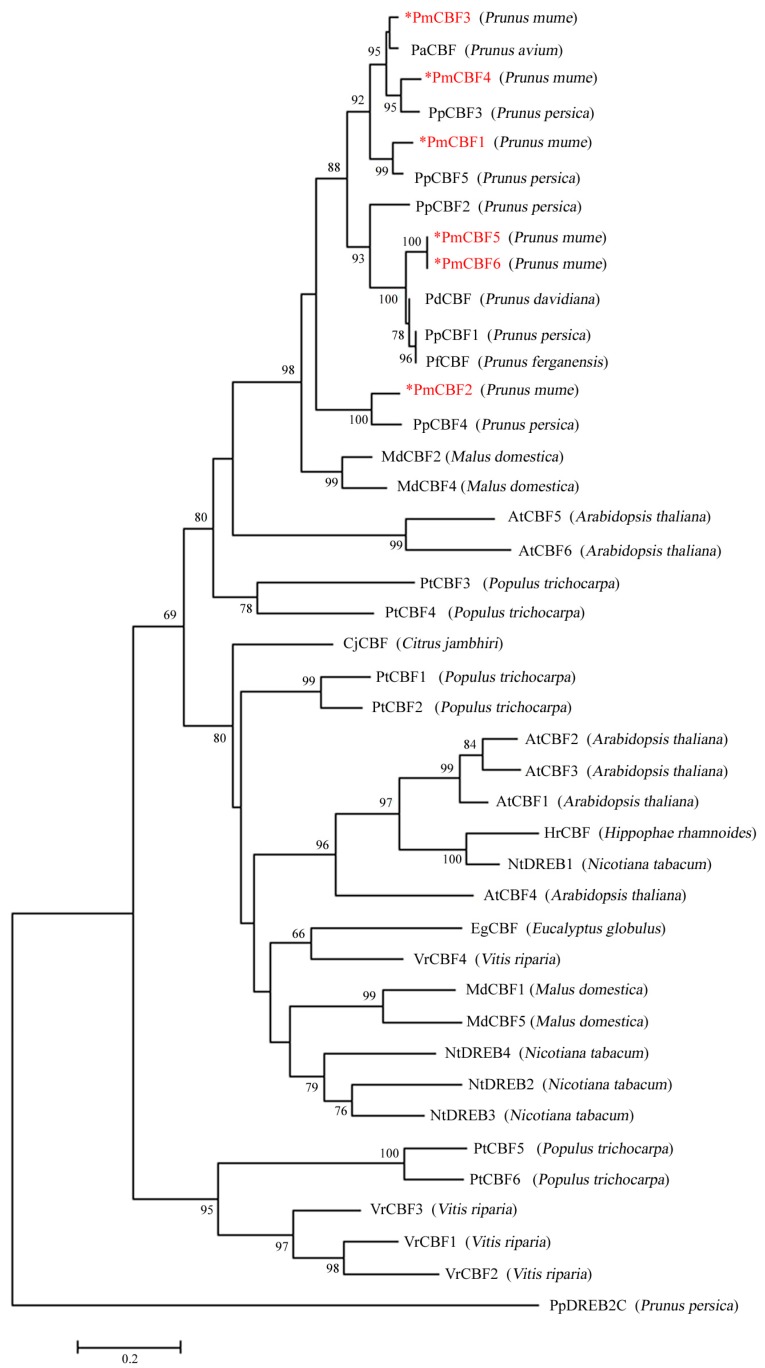
To elucidate the relationship among CBF proteins, a phylogenetic tree was generated (Figure 2). In this dendrogram, six PmCBFs were first gathered with CBF genes from other Prunus plants (i.e., P. persica, P. ferganensis, P. davidiana, and P. avium), and then clustered with genes from other species (Figure 2). Six PmCBFs were converged with other Prunus CBF genes and formed three different evolutionary branches. PmCBF5 and PmCBF6 were gathered in one branch, while PmCBF1, PmCBF3 and PmCBF4 were in another branch. All of these genes were clustered with PmCBF2. Main CBFs in Prunus gathered in one branch from the branch of AtCBF5–6. But other CBFs from Nicotiana, Populus tricocapa, Vitis riparia obtained a closer relationship with AtCBF1–4.
Figure 2.
Phylogenetic tree of CBF proteins from P. mume and other species. This tree was constructed using MEGA7.1 with the Maximum-likelihood (ML) method. The sequences of these proteins were provided in Supplementary Data S3. Numbers above branches represented bootstrap values and the bootstrap values which below 60 were not shown. Stars (*) presented the CBF genes in Prunus mume.
2.3. Expressions of PmCBFs in Different Organs
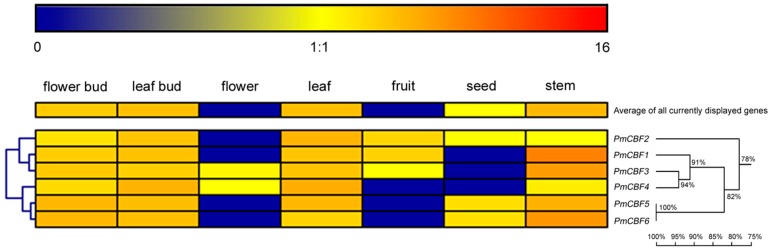
To measure the roles of PmCBFs in different organs (flower bud, leaf bud, flower, leaf, fruit, seed, and stem), the expression patterns were observed using the real-time quantitative PCR (RT-qPCR) and displayed by a heat map (Figure 3). Six PmCBFs were expressed in seven organs. These genes were highly detected in stem, moderately expressed in flower bud, leaf bud, and leaf, and poorly expressed in flower, fruit, and seed. According to the hierarchical clustering performed by Genesis software, PmCBFs were divided into two groups. PmCBF5 and PmCBF6 were gathered in one group. These two genes were predominantly detected in flower bud, leaf bud, leaf, and stem; and were slightly expressed in flower, fruit, and seed. The other group contained PmCBF1, PmCBF2, PmCBF3, and PmCBF4. The expression levels of these four genes were notable in flower bud, leaf bud, leaf, and stem. However, there were bare expressions for PmCBF4–6 in fruit, PmCBF1–2 and 5–6 in flower, PmCBF1 and 3–4 in seed.
Figure 3.
Expression profiles of CBF genes in different organs of P. mume. The expression levels of PmCBFs were obtained using RT-qPCR method. The colour scale represented the log2-transformed counts of the expression levels of PmCBFs. Blue indicated low expression levels and Red suggested high levels. Six PmCBFs were used to build the homology tree. Numbers above branches represented bootstrap value.
2.4. Sectionalization of PmCBFs and PmDAMs during the Expression in the Stem
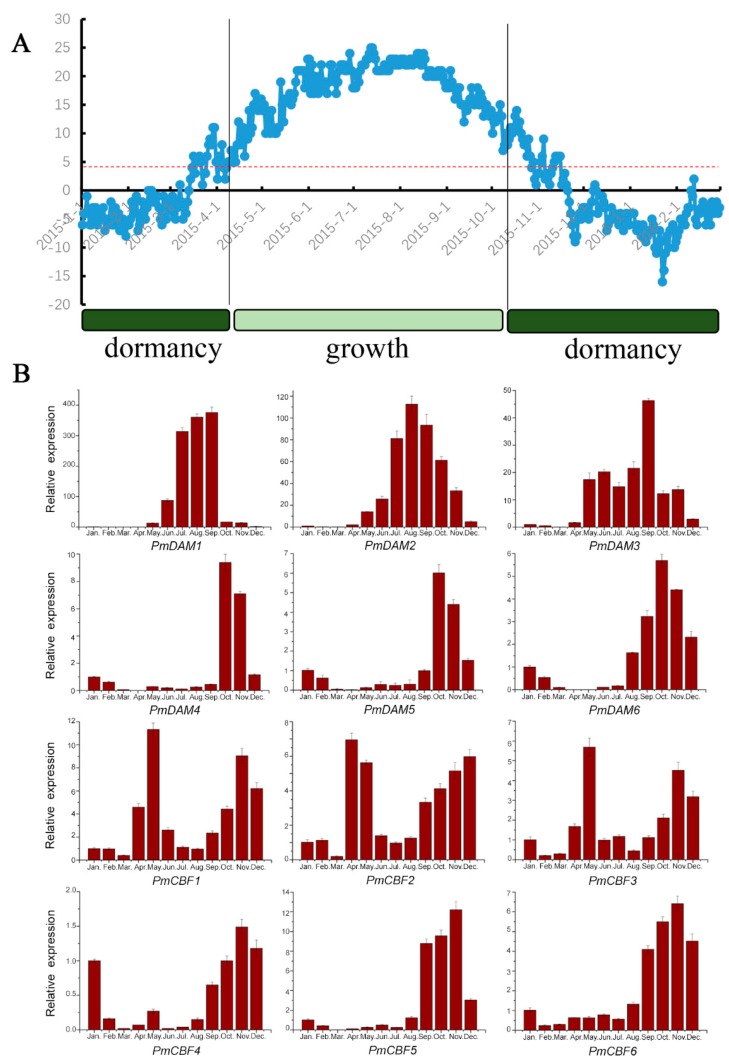
In Beijing (40°00′ N, 116°18′ E), the vegetation process occurs from April to September. The stem of P. mume ‘Sanlun Yudie’ grows rapidly in April, May, and June, becomes dormant in October and November, enters endo-dormancy from December to January and experiences dormancy release in February and March (Figure 4A). And six PmCBFs and six PmDAMs exhibited different expression levels in response to the cold stress from September to October when the stem fell into dormancy (Figure 4B).
Figure 4.
(A) The lowest day temperatures and growth statuses of Prunus mume stems; (B) Corresponding expression patterns of PmCBFs and PmDAMs during different periods of stem development. All RT-qPCR experiments were employed with three biological duplications, and each duplication was repeated in triplicate. The standard deviation of the results was shown by the error bars.
During the annual stem-growth process, the expression patterns of PmCBF genes showed significant specificity in the low-temperature climates (Figure 4B). The transcripts of PmCBFs manifested two similar expression trends and formed two groups. One group (PmCBF1, PmCBF2, and PmCBF3) was notably detected twice in the whole year: one expression peak occurred in May and the other in November, the appearance of expression peaks may be affected by the short coldness in the early spring and continuous low-temperature climates in winter. The other group, PmCBF4–6, established one expression peak together with the first group. All the expression of PmCBFs remarkably increased from August to November and decreased from December to March, indicating an active character in cold-response and dormancy control. PmCBF1–3 acted more sensitively to the stress of cold than PmCBF4–6.
With high inner correlations of PmDAM1–3 and PmDAM4–6, respectively, PmDAMs were divided into two groups based on different expression profiles (Figure 4B). The group consisting of PmDAM1–3 had significant expression levels in August and September. The expressions of these three genes were intense in May to November and had low levels from January to April. The transcripts of these genes were up-regulated from May to September, but down-regulated in September to December. PmDAM4–6 formed the second group, which showed strong expressions from September to December, with the highest expression level occurring in October. These genes also exhibited a rising expression trend from August to October, and gradually declined from October to February. The same genes were minimally expressed from March to July. These results demonstrated that these three genes might have positive correlations during dormancy induction and play a negative role in dormancy releasing.
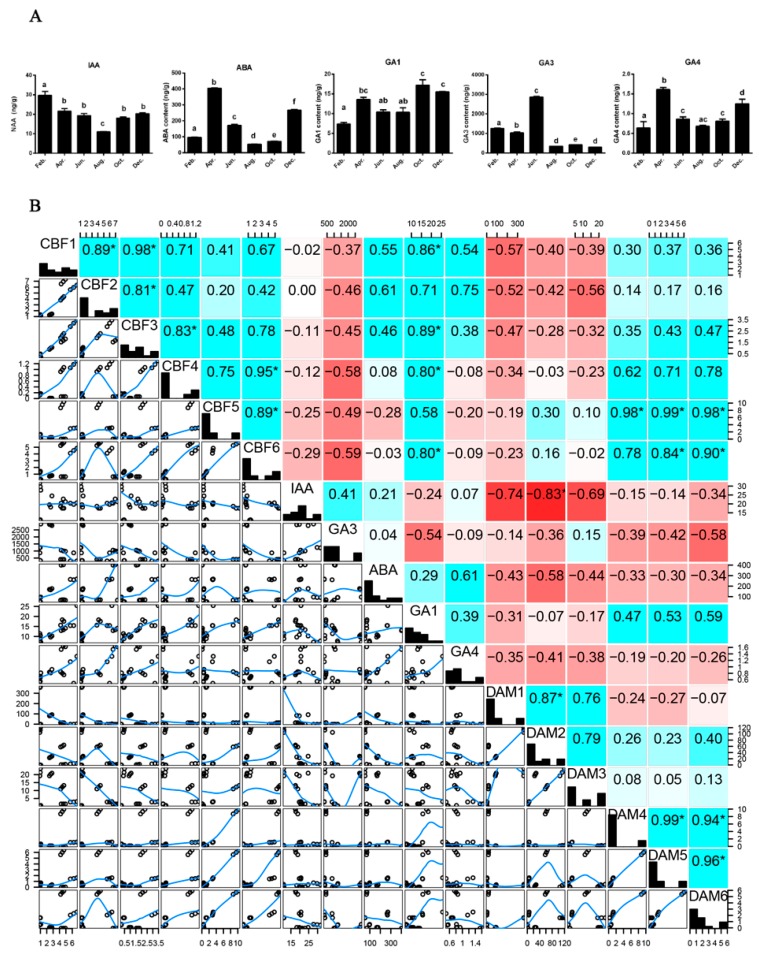
2.5. Seasonal Changes in Hormones Synthesis and Correlations with Gene Expression during Stem Development
Hormones like GAs, ABA, and IAA regulate plant development and dormancy, especially when the plants experience the change of climates. The growing cycle of P. mume stem occurs alongside seasonal changes, which may be affected by gene expression and hormonal content. Correlation assays were performed to explore the relationships between PmDAMs, PmCBFs and hormones. In February and August, IAA exhibited the highest and the lowest levels, respectively (Figure 5A). Both ABA and GA3 levels were significantly different at each stage of the growth cycle. ABA showed high levels in April and December, and the lowest in August. As for GA3, the highest level appeared in June with 10 or 100 times than that of GA1 or GA4.
Figure 5.
Hormone contents in stems and correlation matrix between hormones and genes. (A) the levels of IAA, ABA, GA1, GA3, GA4 in stems and over a time course of a year during which stems were experienced dormancy and dormancy release to growth and development; (B) correlation matrix between hormones and genes expression levels. The correlation values were calculated by spearman method and established by red (negative correlations) and blue (positive correlations) blocks. Stars above the numbers (*) means significant correlations.
The correlation matrix displayed the whole interaction among genes and hormones (Figure 5B). PmCBF1–3 displayed an obvious negative correlation with PmDAM1–3 and PmCBF4–6 showed a significant positive correlation with PmDAM4–6. IAA negatively correlated with PmDAM1–3. In contrast, the other genes seem not to relate with IAA. GA3 was negatively controlled by two types of genes, especially PmCBFs. PmCBF1–3 were positively correlated with the expression of ABA, and PmDAM1–6 performed antipodally with ABA. The relative low values in correlation might infer the indirect regulation model for PmCBFs and PmDAMs.
2.6. Subcellular Localization Assessment
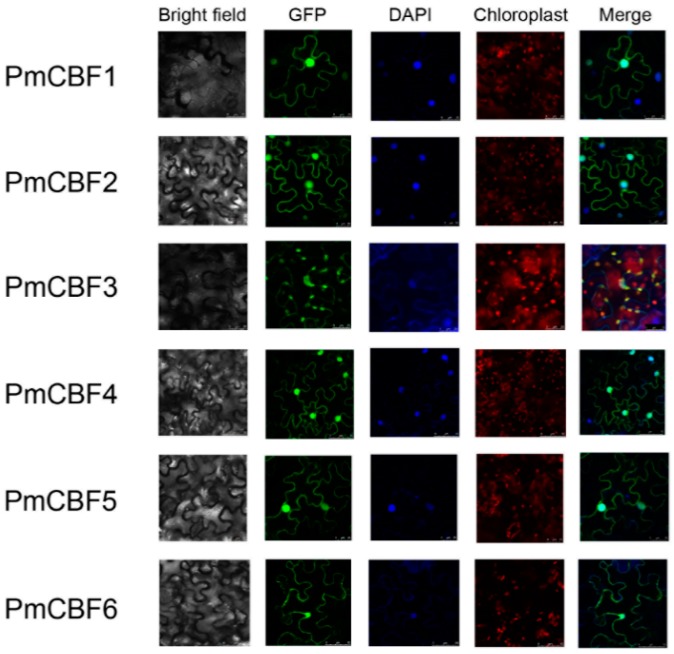
Controlled by a 35S promoter, the PmCBF with GFP was expressed in N. benthamiana leaves. All the PmCBFs were co-located alongside 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) in the parenchyma cells of leaves’ abaxial epidermis in N. benthamiana, suggesting that PmCBFs are primarily localized to the cell nucleus (Figure 6). PmCBF3 was also detected in chloroplasts.
Figure 6.
Subcellular localization of six PmCBFs. To determine the exact position of PmCBFs within the cell, subcellular localization experiments were performed using leaf tissues of N. benthamiana. The green fluorescent showed protein position. The blue fluorescent presented the nuclei position. The red fluorescent indicated the chloroplast position. The merge pictures of PmCBFs were formed by the pictures of GFP and DAPI except that PmCBF3 was formed by GFP, DAPI, and Chloroplast. For PmCBF1, 2, 3, and 5, the scale bar is 25; for PmCBF4 and 6, the scale bar is 50.
2.7. Protein-Protein Interactions between CBF and DAM Genes in P. mume
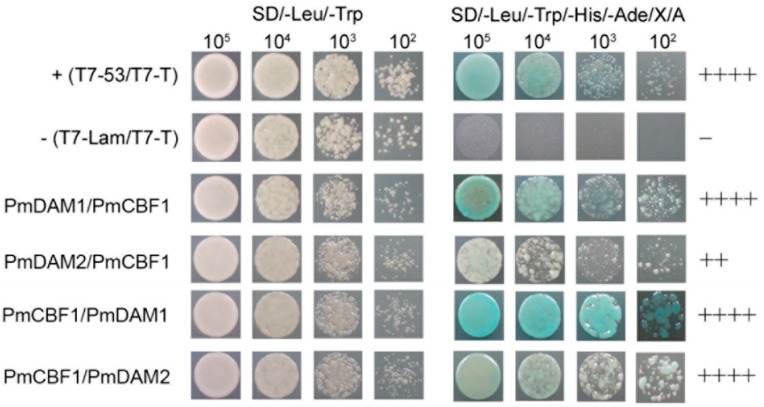
Yeast two-hybrid assays were performed to determine protein complexes formed by PmCBFs and PmDAMs. PmCBF2, PmCBF3, PmCBF4, PmCBF5, and PmCBF6 had auto activation activity and cannot be used as baits. PmCBF1 and all PmDAMs were reconstructed in bait vectors. None of the experimental proteins had toxicity. Consequently, only the interaction profiles of PmCBF1 and six PmDAMs could be obtained by yeast two-hybrid assay.
The dimerizations between PmCBF1 and PmDAMs are shown in Figure 7. PmDAM3, PmDAM4, and PmDAM5 could not form heterodimers with PmCBF1. PmDAM1 could dimerize strongly with PmCBF1, no matter used as a bait or a prey. While PmDAM2 and PmDAM6 showed poor interactions with PmCBF1. In addition, only acted as a bait, PmCBF1 interacted with PmDAM2, and PmDAM6 even stronger than the positive control.
Figure 7.
Protein-protein interactions between PmCBF1 and PmDAMs. T7–53/T7-T was positive control, and T7-Lam/T7-T was negative control. The symbol (+) was represented the capacity of the reaction. The more numbers of the symbol (+), stronger capacity of the reaction. The symbol (−) meant there were no interactions between proteins.
2.8. BiFC Assay
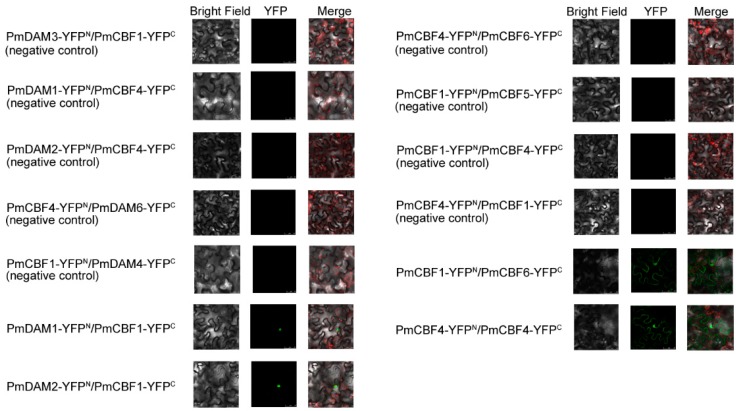
Inter-protein interactions between PmCBF1 and PmDAMs and among PmCBFs were confirmed by BiFC assays using a separated yellow fluorescent protein (YFP). In each interaction, two proteins were fused with either C or N terminus of YFP designated as YFPC or YFPN, respectively. There was no interaction in PmDAMs-YFPC/YFPN or YFPC/PmDAMs-YFPN, and no interaction in PmCBFs-YFPC/YFPN or YFPC/PmCBFs-YFPN (Supplementary Figure S2). In P. mume, the PmCBFs are six homologous genes and the PmDAMs are six homologous genes as well. For PmCBFs, the protein sequences of PmCBF1, PmCBF3, and PmCBF4 are similar, while PmCBF5 and PmCBF6 have high homology. For PmDAMs, PmDAM1, PmDAM2, and PmDAM3 possess similar protein sequences, while PmDAM4, PmDAM5, and PmDAM6 have high homology. In BiFC assays, a member of the same protein family can provide a negative control. Thus, the interactions of PmDAM3-YFPN/PmCBF1-YFPC and PmDAM1-YFPN/PmCBF4-YFPC were used as the negative controls of PmDAM1-YFPN/PmCBF1-YFPC; the interactions of PmDAM3-YFPN/PmCBF1-YFPC and PmDAM2-YFPN/PmCBF4-YFPC were used as the negative controls of PmDAM2-YFPN/PmCBF1-YFPC; the interactions of PmCBF4-YFPN/PmDAM6-YFPC and PmCBF1-YFPN/PmDAM4-YFPC were used as the negative controls of PmCBF1-YFPN/PmDAM6-YFPC; the interactions of PmCBF4-YFPN/PmCBF6-YFPC and PmCBF1-YFPN/PmCBF5-YFPC were used as the negative controls of PmCBF1-YFPN/PmCBF6-YFPC, the interactions of PmCBF1-YFPN/PmCBF4-YFPC and PmCBF4-YFPN/PmCBF1-YFPC were used as the negative controls of PmCBF4-YFPN/PmCBF4-YFPC. The YFP signals were not detected in any negative control tests (Figure 8).
Figure 8.
Bimolecular fluorescence complementation (BiFC) analysis of the protein interactions among PmCBFs and the reactions between PmCBFs and PmDAMs. Different combinations of the fused constructs were co-transformed into leaf cell of N. benthamiana, and then the cells were observed by confocal microscopy as described in “Materials and Methods”. In BiFC assays, a member of the same protein family can provide a negative control. The descriptions of the negative control tests were detail in “Results”. Bright field and YFP were excited at 514 nm. The green fluorescent presented protein position. The red fluorescent showed the chloroplast position.
The protein-protein interactions between PmCBFs and PmDAMs were further verified through BiFC. The reactions (i.e., PmCBF1 and PmDAM1, PmCBF1 and PmDAM2) showed the YFP fluorescence in the nucleus, indicating that PmCBF1 could form dimers with PmDAM1, PmDAM2. The localization of YFP fluorescence in the nucleus revealed the significant interactive trend among PmCBFs. For PmCBFs, PmCBF4 could form a homodimer, and PmCBF1 could dimerize with PmCBF6 (Figure 8).
3. Discussion
3.1. CBF Genes in P. mume and Their Evolution
AtCBF1–3 are located in a tandem array and show similar functions in cold response pathways. In P. mume, there are six CBF genes. Similar to P. persica, several of them are located in tandem arrays [29]. Each PmCBFs contain an AP2/ERF domain comprised of 58 amino acids. In the middle regions of these protein sequences, a CMIII-1 motif is found conserved among different species. For many plants, CBF/DREB1 proteins have two conservative sequences, PKK/RPAGRxKFxETRHP and DSAWR, that are distributed in the left and right of the AP2/ERF domain [41]. These CMIII-3 sequences help to distinguish CBF/DREB1 from other genes in AP2/ERF family. In the C-terminals of CBF proteins, there are two conserved motifs (CMIII-2 and CMIII-4) [13], which are both located in PmCBFs and more weakly conserved than other motifs. In the phylogenetic tree, six PmCBFs might have undergone different evolutionary processes because of their different evolutionary branches (Figure 2). These PmCBFs were clustered with the CBF proteins from other Prunus plants, suggesting that Prunus CBFs might experience a similar evolutionary process.
However, all PmCBFs had bias relationships with DDF1 and DDF2 (AtCBF5 and AtCBF6) in Arabidopsis. Overexpression of AtCBF5 and AtCBF6 can cause dwarfism and delayed flowering [25], resulting from the repression of GA biosynthesis by CBF. This may provide extra explanations for the function diversity, even infer that CBF genes may appear after the specie formation.
3.2. Positive Response to the Coldness by PmCBFs and PmDAM4–6
The CBF cold-response pathway is conserved in plants and contributes to plant dormancy and flowering time in previous studies [30,42,43,44]. Indeed, when low temperature occurred in October, all the PmCBFs were actively expressed (Figure 4B). This also confirms the upstream role of PmCBFs responding to low temperature. In particular, the possible relationship of CBF genes to dormancy in plants is highlighted [45]. In Arabidopsis thaliana, AtCBF1–3 play an essential role in cold acclimation [23]. Overexpression of a peach CBF gene (PpCBF1) in apple inhibits plant growth, induces leaf senescence, causes early bud set, and delays bud break [27]. In apple, MdDAM1–4 show specific expressions in dormant buds and are highly expressed during bud dormancy induction [46]. Moreover, during dormancy induction, PpCBF in Pyrus pyrifolia can enhance the transcriptional activity of PpDAM1 and PpDAM3 [3].
However, considering annual expression levels, the pattern of PmCBF1–3 corresponded negatively to PmDAM1–3. During the growing stage from May to September when the expressions of PmCBFs were depressed to relatively low levels, the PmDAM1–3 seem to be released, showing peak expressions in this stage. Therefore, a hypothesis is generated that PmDAM genes might function in stem for its growth. However, there are still no evidence for the growth maintenance, further researches would be required to analyze the unusual results.
3.3. The Interactive Roles of Major Hormones
Developmental transitions in deciduous trees rely on endogenous hormones and genes expression changes that are triggered by environmental factors [47]. It becomes clear that the CBF genes constitute a central node of hormone cross-talk during cold stress response [48]. So far, it has been demonstrated that CBF expression is modulated by gibberellins, ABA, jasmonate, etc. For example, in trees, gibberellin s are particularly important because they may establish dormancy to help the plants avoid injuries due to cold. In the previous studies, the accumulation of CBF reduces bioactive GA to benefit the growth restraint by affecting key enzymes in the GA pathway [49]. In the stem growing stage of P. mume, GA3 owns a content more than 100 times than that of GA1 or GA4. The negative influence on GA3 also explains the function of CBF by the side. On the other hand, GA3 may be the major valid member in the growth of the stem by growth repressing DELLA proteins [50,51]. Jasmonate functions in the upstream of CBFs and positively regulate Arabidopsis freezing tolerance [52]. However, transcriptome of alfalfa shows us an overview that in response to freezing stress, transcripts of JAZ are up-regulated, which interact physically and repress the transcriptional function of ICE (inducer of CBF expresion) [53]. Further investigation may provide more functional mechanism for jasmonate. Additionally, ABA is produced by plants under cold conditions and plays an important role in mediating stress tolerance [54]. Recent studies report that ABA activates the CBF and corresponding genes in this pathway [55]. Interestingly, there exists a feedback regulation between PpDAM1 and ABA via upstream promoter binding sites, CArG for PpNCED3 and ABRE motifs for PpDAM1 [56]. Our results are also consistent with the previous confirmation that a positive correlation appear between PmCBF1–3 and ABA in the stem, though more complex controls may exist in the downstream of PmCBFs.
In P. mume, there exists a mixed correlation for hormones around the PmCBFs, shown in Figure 5. There is another interesting observation in that PmDAM1–3 shows high correlation with IAA. The special expression patterns drive us to speculate the involvement of PmDAM1–3 in growth induction. But further studies are needed to decode the other identities of PmDAM1–3.
3.4. Proposed Regulation Network between Hormones and PmCBF and PmDAMs in Stem Cold Response
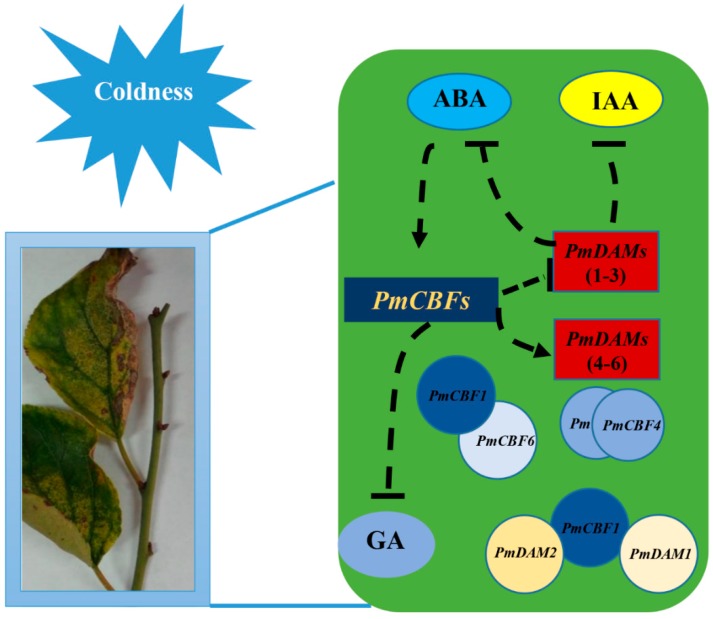
The present studies have shown that DAM genes have relationships with plant dormancy induction and release [36,57,58,59]. During dormancy process, PmCBF1–3 display negative correlation with PmDAM1–3 and PmCBF4–6 establish opposite roles with PmDAM4–6. The expressions of CBF genes can be induced by low temperatures [19,27]. In soybeans, most GmDREB1s are strongly influenced by various abiotic stresses, including low temperatures, high salt contents, drought, and heat [60]. The expressions of CBF genes in plants are associated with not only abiotic stresses but also hormones. PnDREB1 in Papaver nudicaule is involved in both GA and abiotic stresses pathways [61]. Furthermore, SwDREB1 from sweet potato participates in the ABA-independent pathway [62]. In this case, five PmCBFs are primarily expressed in the cell nucleus, which create possibilities for protein interactions among these proteins. Taken together, a hypothesis of molecular model for hormones, PmCBFs, and PmDAMs during the stem-growth process was proposed (Figure 9). For P. mume, six PmCBFs and PmDAM4–6 join in the regulation of cold-response and dormancy actively, and formed different types of protein complexes, especially PmCBF1-PmDAM1 and PmCBF1-PmDAM2. However, it remains unclear why CBFs and DAMs has to function as homodimers or heterodimers. The research in Arabidopsis demonstrate AtCBFs functioning as vital signal proteins link multiple pathway against abiotic stress [63]. The loss-function mutants of AtCBF1–3 created by CRISPER system also display the major function of AtCBFs in cold acclimation and freezing tolerance, even in salt tolerance and seedling development [64]. This throw a light in the exploration of genes that the regulation mechanisms seem different with Arabidopsis.
Figure 9.
Schematic representation of molecular regulation model of hormones, PmCBFs, and PmDAMs during stem growth and dormancy. PmCBF1 could make heterogeneous dimers with PmDAM1, PmDAM2, and PmCBF6. PmCBF4 formed homologous dimers. The dotted arrows mean potential positive regulations. And the dotted crosses mean potential negative regulations.
In the present study, the patterns of expression for both hormones and genes were analyzed to increase knowledge of cold-induced stem dormancy in this woody plant. Additionally, we introduced the inner interaction mode between PmCBFs and PmDAMs. This discovery may provide new insights into the roles that PmCBFs, PmDAMs and hormones play in regulating tree dormancy and growth.
4. Material and Methods
4.1. Plant Materials
P. mume “Sanlun Yudie” was selected from Beijing Forestry University, Beijing, China (40°00′ N, 116°18′ E). All three independent individuals for sampling were grown under similar environments. Each biological repeat was mixed from same organs or tissues, and separated into three tubes to conduct technical repeats. The flower buds were collected to clone genes. The samples of seven different organs were obtained to study expression patterns of PmCBFs, including flower bud (10 October 2015), leaf bud (10 October 2015), flower (full blooming; 22 March 2015), leaf (10 May 2015), fruit (10 June 2015), seed (10 June 2015) and stem (10 October 2015). The upper halves of the stems during different periods (from 10 January 2015 to 10 December 2015) were sampled to examine the expressions of PmCBFs and PmDAMs. These stem samples included bark tissues and vascular cambium and were taken to extract RNA and phytohormone using liquid nitrogen.
4.2. Identification and Cloning of CBF Genes
PmCBF (GeneBank: JX846908.1) was used as a query to identify additional CBFs in the P. mume protein database (http://prunusmumegenome.bjfu.edu.cn/) with an e-value set to 1e-3 by blastp. Seven PmCBFs were obtained, but one lacked the AP2/ERF domain, according to a domain analysis of these genes in InterPro (http://www.ebi.ac.uk/interpro/). Consequently, six CBF genes in P. mume were identified (Supplementary Table S1), and specific primers were designed based on CDS sequences (Supplementary Data S1). To clone these PmCBFs, total RNA was isolated from flower buds with TRIzol reagent (Aidlab, Beijing, China) following the manufacturer’s instructions. To synthesize first-strand cDNA, 2 µg of total RNA was used with TIANScript First Strand cDNA Synthesis Kit (Tiangen, Beijing, China). PCR reactions using Prime STAR HS DNA polymerase (Takara, Dalian, China) were performed to obtain cDNA of PmCBFs and PmDAMs. A 50 μL mix was used in these reactions, containing 10 μL of 5 × Prime STAR buffer, 4 μL of dNTP (10 mM), 2 μL of each primer (10 μM, Supplementary Table S2), 0.5 μL of cDNA, and 0.5 μL of Prime STAR polymerase. These PCR reactions were fulfilled in the following conditions: 94 °C for 2 min; 30 cycles of 98 °C for 10 s, annealing temperature (Supplementary Table S2) for 5 s, and 72 °C for 1 min; 72 °C for 5 min; and holding at 4 °C. To recover fragments, Gel Extraction Kit (Biomiga, San Diego, CA, USA) was employed. And then these fragments were cloned into pMDTM18-T vectors (TaKaRa, China) and transformed into DH5α (Tiangen, China). After sequencing the PCR-positive colonies (Taihe, Beijing, China), the cDNA sequences of PmCBFs (Supplementary Data S2) were obtained. The plasmids of these genes were extracted using Plasmid Miniprep Kit I (Biomiga, San Diego, CA, USA).
4.3. Phylogenetic Analyses
To execute multiple sequence alignment, the CBF protein sequences of P. mume and other species (one CBF protein each from Prunus persica, Prunus davidiana, Prunus ferganensis, Prunus avium, Eucalyptus globulus, Hippophae rhamnoides, and Citrus jambhiri, three Malus domestica CBF proteins, four CBF proteins each from Nicotiana tabacum and Vitis riparia, five Populus trichocarpa CBF proteins, and six Arabidopsis thaliana CBF proteins) were exploited using DNAMAN 7.0 software (Lynnon Corp., Quebec, QC, anada) by default parameters. To construct a phylogenetic tree of these CBF proteins (Supplementary Data S3), MEGA7.1 software was performed with the ML method. For this tree, the parameters were set to default and the bootstrap values were set to 1000.
4.4. RT-qPCR
PikoReal real-time PCR system (Thermo Fisher Scientific, Darmstadt, Germany) was used to conduct the RT-qPCR experiments with a 10 μL mix, including 5 μL of SYBR Premix ExTaqII (Takara, Dalian, China), 0.5 μL of each primer (10 μM, Supplementary Table S3), and 2 μL of cDNA (30 times dilution of 2 μg cDNA). PmPP2A was regarded as the reference gene [65], and the 2–ΔΔCt method was used to calculate relative expression levels. Three biological replicates (each including three technical replicates) were performed to calculate the standard deviation. The correlations between gene expressions and hormones were done by Spearman method, and significant was analysed with kruskal-wallis test in R.
4.5. Subcellular Localization Assessments
The sequences coding for PmCBFs were cloned into supper1300-GFP plasmid using specific primers (Supplementary Table S4) and In-Fusion HD Cloning Kit System (Clonetech, Mountain View, CA, USA) to obtain 35S::PmCBF::GFP fusion. The GFP construct (supper1300-PmCBF), checked after sequencing, was transferred to Nicotiana benthamiana following agroinfiltration. These plasmids were transformed into GV3101, Agrobacterium tumefaciens strains, followed by culturing on LB medium bearing rifampicin (50 μg/mL), kanamycin (50 μg/mL) and gentamicin (50 μg/mL) at 28 °C. Agrobacteria were harvested and suspended twice in an infiltration buffer with 150 µM acetosyringone, 10 mM MES and 10 mM MgCl2. Bacterial suspension culture concentrations were measured using spectroscopy at an optical density of 600 nm and the concentrations were adjusted at 0.5–0.8. The mixture was maintained at room temperature for 2–3 h in the dark. Using syringes, the bacterial culture was infiltrated on the abaxial surfaces of the leaves and sampling was performed after two days for location assessment. Nuclear position was precisely assessed by incubating N. benthamiana leaf tissues with the nucleus marker DAPI. Finally, infiltrated leaves were observed under Leica TCS SP8 Confocal Laser Scanning Platform (excitation/emission settings: 405 nm for DAPI and 488 nm for GFP).
4.6. Yeast Two-Hybrid Assays
Full-length cDNA sequences of six P. mume CBF genes and six P. mume DAM genes were amplified by PCR experiments with specific primers (Supplementary Table S5). In-Fusion HD Cloning Kit System (Clonetech, Mountain View, CA, USA) was utilised to clone these sequences into pGBKT7 vectors (bait; Clonetech, Mountain View, CA, USA) and pGADT7 vectors (prey; Clonetech, Mountain View, CA, USA) at the EcoRI and BamHI sites, respectively. The Yeastmaker Yeast Transformation System 2 (Clonetech, Mountain View, CA, USA) was used to transform the bait plasmids into Y2H gold yeast strains (Clonetech, Mountain View, CA, USA) and the prey plasmids into Y187 yeast strains (Clonetech, Mountain View, CA, USA), before culturing on SD/-Trp plates and SD/-Leu plates, respectively. Single colonies of each transformant were cultured for 16 h (30 °C, 250 rpm). For subsequent interactive tests, auto activation and toxicity of bait strains were tested, and two selective yeast strains (bait and prey) were mated using YPDA liquid medium (Clonetech, Mountain View, CA, USA) for 20 h (30 °C, 80 rpm). After mating, the diploid yeast strains, which had a cloverleaf structure observed by 40 × microscope, were cultured on DDO (SD/-Trp/-Leu) solid medium at 30 °C for 3–5 days. The single colonies were continually cultured in DDO liquid medium for 20–24 h (30 °C, 250 rpm). Then, these samples were centrifuged to obtain the yeast strains, 700 g for 2 min. The supernatant liquid was discarded. Subsequently, 1.5 mL aseptic ddH2O was added to suspended strains, and the previous centrifugal work was repeated. To ensure the OD600 values of yeast strains were approximately 0.8, the appropriate aseptic ddH2O was added. Finally, 100 μL of these yeast samples (1, 1/10, 1/100, and 1/1000) was severally cultured at 30 °C for 3–5 days using DDO solid medium and SD/-Leu/-Trp/-His/-Ade/X-α-Gal/Aba plates. The analysis of protein-protein interactions was viewed in triplicate.
4.7. BiFC Assays
Pair-wise cloning of the full-length cDNA sequences of PmCBFs into pCambia1300-YFP-C and pCambia1300-YFP-N was performed to obtain BiFC constructs. Co-expression was executed on N. benthamiana leaves, as described in subcellular localization assessments. Chimeric fluorescence from expressed fusion proteins was observed 2–3 days post-infiltration using Leica TCS SP8 Confocal Laser Scanning Platform (YFPs excited at 514 nm). Specific primers were used for BiFC assays (Supplementary Table S6).
4.8. Phytohormone Assays
IAA, GAs and ABA were quantifled from 100 mg samples from the stems of P. mume ‘Sanlun Yudie’ in February, April, June, August, October, and December, which were same in gene expression analyses. Every test had been taken in three biological replicates and flash frozen in liquid nitrogen and stored at −80 °C until analysis. Stems (80–100 mg) were ground and extracted overnight at 4 °C with 2 mL of 99:1 isopropanol–acetic acid with 50 ng of d2-GA1, d2-GA3, d2-GA4, d5-IAA, and d6-ABAadded as internal standards. Hormone determination was performed using high-performance liquid chromatography–mass spectrometry (HPLC-MS) analysis of citrate-buffered acetone extracts as described previously. Then each plant hormone underwent quantitative analysis. The molar amount of plant hormone = (signal of plant hormone × the molar amount of corresponding internal standard) × (correction factor)/(signal of corresponding internal standard in the same sample).
Acknowledgments
Acknowledgments: The research was supported by the program for Science and Technology of Beijing (No. Z171100002217005), National Natural Science Foundation of China (Grant No. 31471906), Fundamental Research Funds for the Central Universities (No. 2016ZCQ02), Special Fund for Beijing Common Construction Project.
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/19/2/15/s1.
Author Contributions
Yuzhen Zhou and Qixiang Zhang designed the experiments; Yuzhen Zhou completed the experiments; Yuzhen Zhou and Kai Zhao wrote the manuscript; Sagheer Ahmad improved the manuscript. Kai Zhao, Yuzhen Zhou, Xiaokang Zhuo, Yu Han, Xue Yong and Yushu Li analyzed the data. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare that this research is carried on the absence of any financial or commercial relationships that could be interpreted to a potential conflict of interest.
References
- 1.Liu G., Li W., Zheng P., Xu T., Chen L., Liu D., Hussain S., Teng Y. Transcriptomic analysis of ‘suli’ pear (Pyrus pyrifolia white pear group) buds during the dormancy by rna-seq. BMC Genom. 2012;13:700. doi: 10.1186/1471-2164-13-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai S., Saito T., Sakamoto D., Ito A., Fujii H., Moriguchi T. Transcriptome analysis of japanese pear (Pyrus pyrifolia nakai) flower buds transitioning through endodormancy. Plant Cell Physiol. 2013;54:1132–1151. doi: 10.1093/pcp/pct067. [DOI] [PubMed] [Google Scholar]
- 3.Niu Q., Li J., Cai D., Qian M., Jia H., Bai S., Hussain S., Liu G., Teng Y., Zheng X. Dormancy-associated mads-box genes and micrornas jointly control dormancy transition in pear (Pyrus pyrifolia white pear group) flower bud. J. Exp. Bot. 2016;67:239–257. doi: 10.1093/jxb/erv454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang H.B., Yang Y.H., Zhang Z.J., Chen J., Wang X.C., Huang R.F. Expression of the ethylene response factor gene tsrf1 enhances abscisic acid responses during seedling development in tobacco. Planta. 2008;228:777–787. doi: 10.1007/s00425-008-0779-0. [DOI] [PubMed] [Google Scholar]
- 5.Rashotte A.M., Goertzen L.R. The crf domain defines cytokinin response factor proteins in plants. BMC Plant Biol. 2010;10:74. doi: 10.1186/1471-2229-10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finkelstein R.R., Wang M.L., Lynch T.J., Rao S., Goodman H.M. The arabidopsis abscisic acid response locus abi4 encodes an apetala 2 domain protein. Plant Cell. 1998;10:1043–1054. doi: 10.1105/tpc.10.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirota A., Kato T., Fukaki H., Aida M., Tasaka M. The auxin-regulated ap2/erebp gene puchi is required for morphogenesis in the early lateral root primordium of arabidopsis. Plant Cell. 2007;19:2156–2168. doi: 10.1105/tpc.107.050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horvath D.P., Chao W.S., Suttle J.C., Thimmapuram J., Anderson J.V. Transcriptome analysis identifies novel responses and potential regulatory genes involved in seasonal dormancy transitions of leafy spurge (Euphorbia esula L.) BMC Genom. 2008;9:536. doi: 10.1186/1471-2164-9-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pre M., Atallah M., Champion A., De Vos M., Pieterse C.M., Memelink J. The ap2/erf domain transcription factor ora59 integrates jasmonic acid and ethylene signals in plant defense. Plant Physiol. 2008;147:1347–1357. doi: 10.1104/pp.108.117523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Licausi F., van Dongen J.T., Giuntoli B., Novi G., Santaniello A., Geigenberger P., Perata P. Hre1 and hre2, two hypoxia-inducible ethylene response factors, affect anaerobic responses in Arabidopsis thaliana. Plant J. 2010;62:302–315. doi: 10.1111/j.1365-313X.2010.04149.x. [DOI] [PubMed] [Google Scholar]
- 11.McKhann H.I., Gery C., Berard A., Leveque S., Zuther E., Hincha D.K., De Mita S., Brunel D., Teoule E. Natural variation in CBF gene sequence, gene expression and freezing tolerance in the versailles core collection of Arabidopsis thaliana. BMC Plant Biol. 2008;8:105. doi: 10.1186/1471-2229-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Q., Kasuga M., Sakuma Y., Abe H., Miura S., Yamaguchi-Shinozaki K., Shinozaki K. Two transcription factors, dreb1 and dreb2, with an erebp/ap2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in arabidopsis. Plant Cell. 1998;10:1391–1406. doi: 10.1105/tpc.10.8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakano T., Suzuki K., Fujimura T., Shinshi H. Genome-wide analysis of the erf gene family in arabidopsis and rice. Plant Physiol. 2006;140:411–432. doi: 10.1104/pp.105.073783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubouzet J.G., Sakuma Y., Ito Y., Kasuga M., Dubouzet E.G., Miura S., Seki M., Shinozaki K., Yamaguchi-Shinozaki K. Osdreb genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J. 2003;33:751–763. doi: 10.1046/j.1365-313X.2003.01661.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen M., Xu Z., Xia L., Li L., Cheng X., Dong J., Wang Q., Ma Y. Cold-induced modulation and functional analyses of the dre-binding transcription factor gene, gmdreb3, in soybean (Glycine max L.) J. Exp. Bot. 2009;60:121–135. doi: 10.1093/jxb/ern269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tong Z., Hong B., Yang Y., Li Q., Ma N., Ma C., Gao J. Overexpression of two chrysanthemum dgdreb1 group genes causing delayed flowering or dwarfism in arabidopsis. Plant Mol. Boil. 2009;71:115–129. doi: 10.1007/s11103-009-9513-y. [DOI] [PubMed] [Google Scholar]
- 17.Navarro M., Ayax C., Martinez Y., Laur J., El Kayal W., Marque C., Teulieres C. Two egucbf1 genes overexpressed in Eucalyptus display a different impact on stress tolerance and plant development. Plant Biotechnol. J. 2011;9:50–63. doi: 10.1111/j.1467-7652.2010.00530.x. [DOI] [PubMed] [Google Scholar]
- 18.Siddiqua M., Nassuth A. Vitis cbf1 and vitis cbf4 differ in their effect on Arabidopsis abiotic stress tolerance, development and gene expression. Plant Cell Environ. 2011;34:1345–1359. doi: 10.1111/j.1365-3040.2011.02334.x. [DOI] [PubMed] [Google Scholar]
- 19.Yang W., Liu X.D., Chi X.J., Wu C.A., Li Y.Z., Song L.L., Liu X.M., Wang Y.F., Wang F.W., Zhang C., et al. Dwarf apple mbdreb1 enhances plant tolerance to low temperature, drought, and salt stress via both aba-dependent and aba-independent pathways. Planta. 2011;233:219–229. doi: 10.1007/s00425-010-1279-6. [DOI] [PubMed] [Google Scholar]
- 20.Stockinger E.J., Gilmour S.J., Thomashow M.F. Arabidopsis thaliana cbf1 encodes an ap2 domain-containing transcriptional activator that binds to the c-repeat/dre, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc. Natl. Acad. Sci. USA. 1997;94:1035–1040. doi: 10.1073/pnas.94.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maruyama K., Sakuma Y., Kasuga M., Ito Y., Seki M., Goda H., Shimada Y., Yoshida S., Shinozaki K., Yamaguchi-Shinozaki K. Identification of cold-inducible downstream genes of the Arabidopsis dreb1a/cbf3 transcriptional factor using two microarray systems. Plant J. 2004;38:982–993. doi: 10.1111/j.1365-313X.2004.02100.x. [DOI] [PubMed] [Google Scholar]
- 22.Novillo F., Alonso J.M., Ecker J.R., Salinas J. Cbf2/dreb1c is a negative regulator of cbf1/dreb1b and cbf3/dreb1a expression and plays a central role in stress tolerance in arabidopsis. Proc. Natl. Acad. Sci. USA. 2004;101:3985–3990. doi: 10.1073/pnas.0303029101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Novillo F., Medina J., Salinas J. Arabidopsis cbf1 and cbf3 have a different function than cbf2 in cold acclimation and define different gene classes in the CBF regulon. Proc. Natl. Acad. Sci. USA. 2007;104:21002–21007. doi: 10.1073/pnas.0705639105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haake V., Cook D., Riechmann J.L., Pineda O., Thomashow M.F., Zhang J.Z. Transcription factor cbf4 is a regulator of drought adaptation in arabidopsis. Plant Physiol. 2002;130:639–648. doi: 10.1104/pp.006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magome H., Yamaguchi S., Hanada A., Kamiya Y., Oda K. Dwarf and delayed-flowering 1, a novel arabidopsis mutant deficient in gibberellin biosynthesis because of overexpression of a putative ap2 transcription factor. Plant J. 2004;37:720–729. doi: 10.1111/j.1365-313X.2003.01998.x. [DOI] [PubMed] [Google Scholar]
- 26.Magome H., Yamaguchi S., Hanada A., Kamiya Y., Oda K. The ddf1 transcriptional activator upregulates expression of a gibberellin-deactivating gene, ga2ox7, under high-salinity stress in arabidopsis. Plant J. 2008;56:613–626. doi: 10.1111/j.1365-313X.2008.03627.x. [DOI] [PubMed] [Google Scholar]
- 27.Wisniewski M., Norelli J., Artlip T. Overexpression of a peach CBF gene in apple: A model for understanding the integration of growth, dormancy, and cold hardiness in woody plants. Front. Plant Sci. 2015;6:85. doi: 10.3389/fpls.2015.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Artlip T.S., Wisniewski M.E., Arora R., Norelli J.L. An apple rootstock overexpressing a peach CBF gene alters growth and flowering in the scion but does not impact cold hardiness or dormancy. Hortic. Res. 2016;3:16006. doi: 10.1038/hortres.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Artlip T.S., Wisniewski M.E., Bassett C.L., Norelli J.L. Cbf gene expression in peach leaf and bark tissues is gated by a circadian clock. Tree Physiol. 2013;33:866–877. doi: 10.1093/treephys/tpt056. [DOI] [PubMed] [Google Scholar]
- 30.Wisniewski M., Norelli J., Bassett C., Artlip T., Macarisin D. Ectopic expression of a novel peach (Prunus persica) CBF transcription factor in apple (malus × domestica) results in short-day induced dormancy and increased cold hardiness. Planta. 2011;233:971–983. doi: 10.1007/s00425-011-1358-3. [DOI] [PubMed] [Google Scholar]
- 31.Shen Y.G., Zhang W.K., He S.J., Zhang J.S., Liu Q., Chen S.Y. An erebp/ap2-type protein in triticum aestivum was a dre-binding transcription factor induced by cold, dehydration and aba stress. Theor. Appl. Genet. 2003;106:923–930. doi: 10.1007/s00122-002-1131-x. [DOI] [PubMed] [Google Scholar]
- 32.Mazzitelli L., Hancock R.D., Haupt S., Walker P.G., Pont S.D., McNicol J., Cardle L., Morris J., Viola R., Brennan R., et al. Co-ordinated gene expression during phases of dormancy release in raspberry (Rubus idaeus L.) buds. J. Exp. Bot. 2007;58:1035–1045. doi: 10.1093/jxb/erl266. [DOI] [PubMed] [Google Scholar]
- 33.Kumar G., Arya P., Gupta K., Randhawa V., Acharya V., Singh A.K. Comparative phylogenetic analysis and transcriptional profiling of mads-box gene family identified dam and flc-like genes in apple (Malusx domestica) Sci. Rep. 2016;6:20695. doi: 10.1038/srep20695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Q., Chen W., Sun L., Zhao F., Huang B., Yang W., Tao Y., Wang J., Yuan Z., Fan G., et al. The genome of Prunus mume. Nat. Commun. 2012;3:1318. doi: 10.1038/ncomms2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Z., Zhang Q., Sun L., Du D., Cheng T., Pan H., Yang W., Wang J. Genome-wide identification, characterisation and expression analysis of the mads-box gene family in Prunus mume. Mol. Genet. Genom. 2014;289:903–920. doi: 10.1007/s00438-014-0863-z. [DOI] [PubMed] [Google Scholar]
- 36.Sasaki R., Yamane H., Ooka T., Jotatsu H., Kitamura Y., Akagi T., Tao R. Functional and expressional analyses of pmdam genes associated with endodormancy in japanese apricot. Plant Physiol. 2011;157:485–497. doi: 10.1104/pp.111.181982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamane H., Kashiwa Y., Ooka T., Tao R., Yonemori K. Suppression subtractive hybridization and differential screening reveals endodormancy-associated expression of an svp/agl24-type mads-box gene in lateral vegetative buds of japanese apricot. J. Am. Soc. Hortic. Sci. 2008;133:708–716. [Google Scholar]
- 38.Kitamura Y., Takeuchi T., Yamane H., Tao R. Simultaneous down-regulation of dormancy-associated mads-box6 and soc1 during dormancy release in japanese apricot (Prunus mume) flower buds. J. Hortic. Sci. Biotechnol. 2016;91:1–7. doi: 10.1080/14620316.2016.1173524. [DOI] [Google Scholar]
- 39.Li Z., Reighard G.L., Abbott A.G., Bielenberg D.G. Dormancy-associated mads genes from the evg locus of peach [Prunus persica (L.) batsch] have distinct seasonal and photoperiodic expression patterns. J. Exp. Bot. 2009;60:3521–3530. doi: 10.1093/jxb/erp195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saito T., Bai S., Imai T., Ito A., Nakajima I., Moriguchi T. Histone modification and signalling cascade of the dormancy-associated mads-box gene, ppmads13-1, in japanese pear (Pyrus pyrifolia) during endodormancy. Plant Cell Environ. 2015;38:1157–1166. doi: 10.1111/pce.12469. [DOI] [PubMed] [Google Scholar]
- 41.Jaglo K.R., Thomashow M.F. Components of the arabidopsis c-repeat/dehydration-responsive element binding factor cold-response pathway are conserved in brassica napus and other plant species. Plant Physiol. 2001;127:910–917. doi: 10.1104/pp.010548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seo E., Lee H., Jin J., Park H., Kim J., Noh Y.S., Lee I. Crosstalk between cold response and flowering in arabidopsis is mediated through the flowering-time gene soc1 and its upstream negative regulator flc. Plant Cell. 2009;21:3185–3197. doi: 10.1105/tpc.108.063883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ibanez C., Kozarewa I., Johansson M., Ogren E., Rohde A., Eriksson M.E. Circadian clock components regulate entry and affect exit of seasonal dormancy as well as winter hardiness in populus trees. Plant Physiol. 2010;153:1823–1833. doi: 10.1104/pp.110.158220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ebrahimi M., Abdullah S.N., Abdul Aziz M., Namasivayam P. Oil palm egcbf3 conferred stress tolerance in transgenic tomato plants through modulation of the ethylene signaling pathway. J. Plant Physiol. 2016;202:107–120. doi: 10.1016/j.jplph.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Wisniewski M., Nassuth A., Teulières C., Marque C., Rowland J., Cao P.B., Brown A. Genomics of cold hardiness in woody plants. Crit. Rev. Plant Sci. 2014;33:92–124. doi: 10.1080/07352689.2014.870408. [DOI] [Google Scholar]
- 46.Porto D.D., Falavigna V.D.S., Arenhart R.A., Perini P., Buffon V., Anzanello R., Santos H.P.D., Fialho F.B., Oliveira P.R.D.D., Revers L.F. Structural genomics and transcriptional characterization of the dormancy-associated mads-box genes during bud dormancy progression in apple. Tree Genet. Genom. 2016;12:46. doi: 10.1007/s11295-016-1001-3. [DOI] [Google Scholar]
- 47.Kurepin L.V., Dahal K.P., Savitch L.V., Singh J., Bode R., Ivanov A.G., Hurry V., Huner N.P. Role of cbfs as integrators of chloroplast redox, phytochrome and plant hormone signaling during cold acclimation. Int. J. Mol. Sci. 2013;14:12729–12763. doi: 10.3390/ijms140612729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barrero-Gil J., Salinas J. Cbfs at the crossroads of plant hormone signaling in cold stress response. Mol. Plant. 2017;10:542–544. doi: 10.1016/j.molp.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 49.Niu S., Gao Q., Li Z., Chen X., Li W. The role of gibberellin in the cbf1-mediated stress-response pathway. Plant Mol. Biol. Rep. 2014;32:852–863. doi: 10.1007/s11105-013-0693-x. [DOI] [Google Scholar]
- 50.Achard P., Genschik P. Releasing the brakes of plant growth: How gas shutdown della proteins. J. Exp. Bot. 2009;60:1085–1092. doi: 10.1093/jxb/ern301. [DOI] [PubMed] [Google Scholar]
- 51.Achard P., Gong F., Cheminant S., Alioua M., Hedden P., Genschik P. The cold-inducible cbf1 factor-dependent signaling pathway modulates the accumulation of the growth-repressing della proteins via its effect on gibberellin metabolism. Plant Cell. 2008;20:2117–2129. doi: 10.1105/tpc.108.058941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu Y., Jiang L., Wang F., Yu D. Jasmonate regulates the inducer of CBF expression-c-repeat binding factor/dre binding factor1 cascade and freezing tolerance in arabidopsis. Plant Cell. 2013;25:2907–2924. doi: 10.1105/tpc.113.112631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shu Y., Li W., Zhao J., Zhang S., Xu H., Liu Y., Guo C. Transcriptome sequencing analysis of alfalfa reveals CBF genes potentially playing important roles in response to freezing stress. Genet. Mol. Biol. 2017;40:824–833. doi: 10.1590/1678-4685-gmb-2017-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knight H., Zarka D.G., Okamoto H., Thomashow M.F., Knight M.R. Abscisic acid induces CBF gene transcription and subsequent induction of cold-regulated genes via the crt promoter element. Plant Physiol. 2004;135:1710–1717. doi: 10.1104/pp.104.043562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim Y.S., Lee M., Lee J.H., Lee H.J., Park C.M. The unified ice-CBF pathway provides a transcriptional feedback control of freezing tolerance during cold acclimation in arabidopsis. Plant Mol. Biol. 2015;89:187–201. doi: 10.1007/s11103-015-0365-3. [DOI] [PubMed] [Google Scholar]
- 56.Tuan P.A., Bai S., Saito T., Ito A., Moriguchi T. Dormancy-associated mads-box (dam) and the abscisic acid pathway regulate pear endodormancy through a feedback mechanism. Plant Cell Physiol. 2017;58:1378–1390. doi: 10.1093/pcp/pcx074. [DOI] [PubMed] [Google Scholar]
- 57.Etchells J.P., Provost C.M., Turner S.R. Plant vascular cell division is maintained by an interaction between pxy and ethylene signalling. PLoS Genet. 2012;8:e1002997. doi: 10.1371/journal.pgen.1002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Campbell M., Segear E., Beers L., Knauber D., Suttle J. Dormancy in potato tuber meristems: Chemically induced cessation in dormancy matches the natural process based on transcript profiles. Funct. Integr. Genom. 2008;8:317–328. doi: 10.1007/s10142-008-0079-6. [DOI] [PubMed] [Google Scholar]
- 59.Ruttink T., Arend M., Morreel K., Storme V., Rombauts S., Fromm J., Bhalerao R.P., Boerjan W., Rohde A. A molecular timetable for apical bud formation and dormancy induction in poplar. Plant Cell. 2007;19:2370–2390. doi: 10.1105/tpc.107.052811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kidokoro S., Watanabe K., Ohori T., Moriwaki T., Maruyama K., Mizoi J., Myint Phyu Sin Htwe N., Fujita Y., Sekita S., Shinozaki K., et al. Soybean DREB1/CBF-type transcription factors function in heat and drought as well as cold stress-responsive gene expression. Plant J. 2015;81:505–518. doi: 10.1111/tpj.12746. [DOI] [PubMed] [Google Scholar]
- 61.Huang Z., He J., Zhong X.J., Guo H.D., Jin S.H., Li X., Sun L.X. Molecular cloning and characterization of a novel freezing-inducible dreb1/CBF transcription factor gene in boreal plant Iceland poppy (Papaver nudicaule) Genet. Mol. Boil. 2016;39:616–628. doi: 10.1590/1678-4685-gmb-2015-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim Y.H., Yang K.S., Ryu S.H., Kim K.Y., Song W.K., Kwon S.Y., Lee H.S., Bang J.W., Kwak S.S. Molecular characterization of a cdna encoding dre-binding transcription factor from dehydration-treated fibrous roots of sweetpotato. Plant Physiol. Biochem. 2008;46:196–204. doi: 10.1016/j.plaphy.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 63.Zhao C., Zhu J.K. The broad roles of CBF genes: From development to abiotic stress. Plant Signal. Behav. 2016;11:e1215794. doi: 10.1080/15592324.2016.1215794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao C., Zhang Z., Xie S., Si T., Li Y., Zhu J.K. Mutational evidence for the critical role of CBF transcription factors in cold acclimation in arabidopsis. Plant Physiol. 2016;171:2744–2759. doi: 10.1104/pp.16.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang T., Hao R., Pan H., Cheng T., Zhang Q. Selection of suitable reference genes for quantitative real-time polymerase chain reaction in Prunus mume during flowering stages and under different abiotic stress conditions. J. Am. Soc. Hortic. Sci. 2014;139:113–122. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.