Abstract
Banana Fusarium wilt caused by Fusarium oxysporum f. sp. cubense (Foc) is one of the most destructive soil-borne diseases. In this study, young tissue-cultured plantlets of banana (Musa spp. AAA) cultivars differing in Foc susceptibility were used to reveal their differential responses to this pathogen using digital gene expression (DGE). Data were evaluated by various bioinformatic tools (Venn diagrams, gene ontology (GO) annotation and Kyoto encyclopedia of genes and genomes (KEGG) pathway analyses) and immunofluorescence labelling method to support the identification of gene candidates determining the resistance of banana against Foc. Interestingly, we have identified MaWRKY50 as an important gene involved in both constitutive and induced resistance. We also identified new genes involved in the resistance of banana to Foc, including several other transcription factors (TFs), pathogenesis-related (PR) genes and some genes related to the plant cell wall biosynthesis or degradation (e.g., pectinesterases, β-glucosidases, xyloglucan endotransglucosylase/hydrolase and endoglucanase). The resistant banana cultivar shows activation of PR-3 and PR-4 genes as well as formation of different constitutive cell barriers to restrict spreading of the pathogen. These data suggest new mechanisms of banana resistance to Foc.
Keywords: banana Fusarium wilt, DGE, Venn diagram, GO annotation, KEGG pathways, resistance genes
1. Introduction
Banana (Musa spp.) is one of the most important fruit and food crops in the world with annual production of more than 100 Mt [1]. Fusarium wilt caused by Fusarium oxysporum f. sp. cubense (Foc) is one of the most destructive diseases substantially reducing the production of banana in the world [2,3]. Foc has been classified into four physiological races according to the symptoms of different banana cultivars [4]. Foc race 4 (Foc4) is the most virulent one and affects many Musa spp. AAA Cavendish cultivars [3]. Unfortunately, current methods for the management of this disease are not efficient. Therefore, it is of both biological and agricultural importance to understand the molecular mechanism of banana resistance to Foc4.
Banana adopted several strategies to cope with Foc [5]. Resistant cultivars can avoid the root colonization by inhibiting germination of Foc spores [6]. Cell walls of banana root cells undergo reorganization upon Foc attack, including pectin methylesterification and changes in the distribution and in the abundance of arabinogalactan proteins and extensins. These dynamic cell wall changes have significant impact on the resistance of banana against Foc [7,8]. In addition, genes involved in hormone (jasmonic acid, salicylic acid and ethylene) signalling, canonical pathogen defense genes and antioxidant defense genes have been proposed as candidate genes of banana resistance against Foc4 [9,10,11]. As recently suggested, DNA methylation may also contribute to such resistance [12].
Modern bio-techniques, including high-throughput RNA sequencing, are widely employed to study molecular mechanisms of plant growth and development [13], and plant stress defense [14,15]. So far, only a very limited number of studies attempted to reveal the molecular mechanism of banana response to Foc using these techniques. For example, general responses of Cavendish banana to Foc4 [16] and Foc1 were investigated [17] previously. However, a comparison of resistant and susceptible cultivars provides more efficient way to determine the resistance mechanism. Li and coauthors [18] selected a moderately resistant cultivar “Nongke No. 1” and highly susceptible “Brazilian” (in the stage of approximately 30 cm in height) for comparative gene expression analysis focused on metabolic pathways related to the plant immunity. In a similar study, 8-week-old seedlings of highly resistant cultivar “Yueyoukang 1” and highly susceptible “Brazilian” were used by Bai et al. [19] profiting from the release of banana draft genome sequence [20]. They evaluated differential gene expressions in both cultivars after five consecutive infection stages focusing on plant-pathogen interaction and plant hormone signal transduction pathways employing Kyoto encyclopedia of genes and genomes (KEGG) analysis. Both above-mentioned studies highlighted important roles of pathogenesis-related proteins (PRs), signalling, cell wall lignification, hypersensitive response and senesce in the banana resistance against Foc4. Nevertheless, detailed information about differences in the gene expression, gene ontology (GO) annotation items and KEGG pathways between resistant and susceptible cultivars was not reported yet.
In this study, tissue-cultured banana plantlets of a highly resistant (“Yueyoukang 1”, Musa spp. AAA Cavendish, abbreviated as YK in this study) and susceptible (“Baxijiao”, Musa spp. AAA Cavendish, abbreviated as BX in this study) cultivars were subjected to digital gene expression (DGE) analysis before and after Foc4 infection. It is known that micropropagated bananas are more susceptible to Fusarium wilt than plants grown from conventional material [21]. For this reason, a comparative DGE analysis, exploiting the above mentioned bioinformatic tools, might be more efficient to determine banana resistance mechanisms against Foc4. Thus, our approach provides new genes, GO annotation items and KEGG pathways possibly involved in the resistance or susceptibility of banana to Foc4. In additional, immunofluorescence labelling method was employed to investigate the changes in the spatio-temporal distribution of xyloglucan in the Foc-infected bananas. Most importantly, we have found that enhanced expression of specific transcription factors, receptor-like kinases and genes involved in the cell wall metabolism play a substantial role in the banana resistance to Foc.
2. Results
2.1. Histological Observation of Fusarium Spreading in Root Tissues of Banana Cultivars
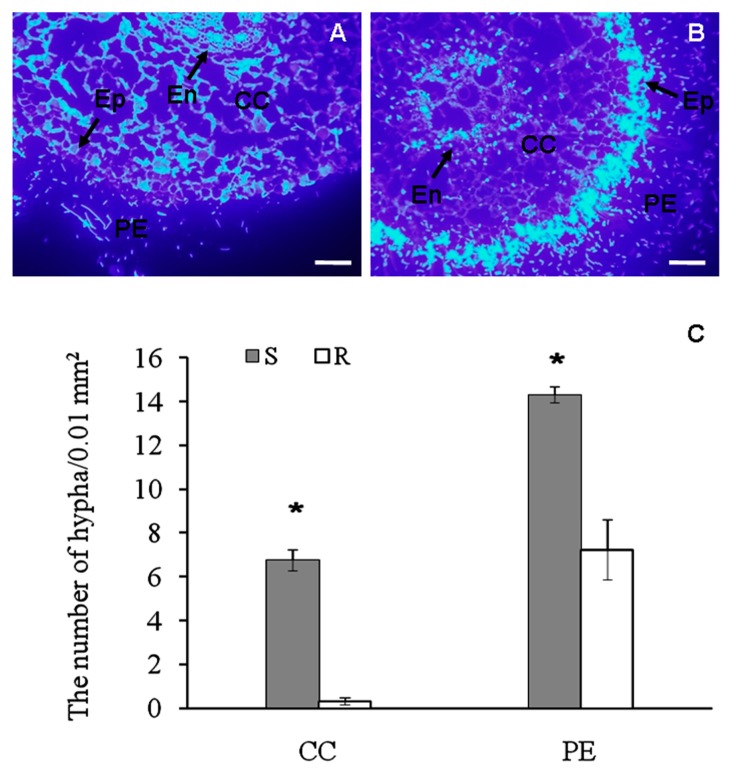
Using calcofluor white staining, we attempted to evaluate spreading of Fusarium in the inoculated roots of banana seedlings. We observed high density of hyphae at the periphery of epidermis in the resistant cultivar, while it was much less abundant in the endodermis and cortical cells (Figure 1A,C). In contrast, an intense pathogen-derived fluorescent signal was observed in the endodermis and cortical cells of the susceptible cultivar (Figure 1B,C). These results illustrate the resistance of the “Yueyoukang 1” and the susceptibility of “Baxijiao” banana cultivar to Foc4 by spatial restriction of the pathogen.
Figure 1.
The tissue-specific pathogen spreading in roots of infected banana (Musa spp. AAA) seedlings. (A,B) Visualization of Fusarium oxysporum f. sp. cubense hyphae by calcofluor white stain in the resistant cultivar “Yueyoukang 1” (A) and susceptible “Baxijiao” (B) 48 h after infection; (C) Quantification of hyphae in A and B. Bars represent 100 μm. CC, cortical cells; Ep, epidermis; En, endodermis; PE, periphery of epidermis; R, resistant cultivar “Yueyoukang 1”; S, susceptible cultivar “Baxijiao”. Quantitative data represent an average of three biological replicates. Comparison of groups was conducted using a paired t-test of variance. Values marked with * were considered significant at p < 0.05.
2.2. The Quality of Digital Gene Expression (DGE) Data
To identify genes important for banana resistance against Foc4, in total eight cDNA libraries were constructed consisting from two biological replications of 4 samples including controls (BXCK, YKCK) and inoculated plantlets (BXT and YKT), and subjected to sequencing in Illumina HiSeq 2000 platform.
As shown in Table S1, clean reads of all tested eight cDNA libraries were more than 10 M and the ratio of clean data to raw data was more than 97% for each library. The percentage of these clean reads that could be mapped to the reference database ranged from 87.59% to 94.29% in all eight libraries. The percentage of unique mapped reads to total clean reads was between 64.63% and 75.79% while it was 17.61–25.83% for multiple mapped reads. The GC content of each sample was more than 50%. The percentage of Q20 and Q30 was more than 98% and 95%, respectively. This evaluation indicated the high quality of DGE data and it could be used for further analysis.
2.3. Differential Expression of Genes in the Roots of Banana Cultivars Resistant or Susceptible to Foc4
In our study, we compared gene expression patterns between the cultivars before and after infection. Furthermore, we compared gene expression changes in the response to infection in both cultivars individually. Thus, we evaluated together four different comparison pairs (Table S2). Log2 (Fold Change) > 1 and p value < 0.05 were used as the threshold to select significantly differentially expressed genes (DEGs). In total, 955 DEGs were obtained from these four pairs of comparison. They comprised of 575 up-regulated and 380 down-regulated genes. The list of all DEGs including quantification details is presented as Tables S3–S6. Only 32 DEGs (18 up-regulated and 14 down-regulated) were found in the resistant cultivar after pathogen attack compared to the control. On the other hand, 193 DEGs were found in the susceptible cultivar after infection, 54 being up-regulated and 139 down-regulated. After pathogen infection, we found 228 genes with different expression levels between the two cultivars, out of which 159 DEGs showed higher expression levels in the resistant cultivar as compared with the susceptible one (Table S6).
We used a Venn diagram to evaluate genes up-regulated only in the resistant cultivar after infection (as compared to the control) or in the comparison of this cultivar to the susceptible one. As shown in Figure S1A, 12 identified genes fulfilled at least one of these criteria (Table 1), thus they represented genes determining the resistance of banana to Foc4. Out of them, four genes (UDP-glycosyltransferase 73C3-like, LOB domain-containing protein 41-like, transcription factor JUNGBRUNNEN 1-like, probable LRR receptor-like serine/threonine-protein kinase At1g74360) were showed higher expression levels in the resistant cultivar compared to the susceptible one after pathogen infection. The expression level of a phenylalanine ammonia-lyase-like gene in the resistant cultivar was significantly higher than that in the susceptible one before pathogen infection while probable WRKY transcription factor (TF) 50 was showed higher expression levels in the resistant cultivar both before and after pathoen infection. Another six genes (luminal-binding protein 2, α-carbonic anhydrase 7-like, curcumin synthase 3-like, proline-rich receptor-like protein kinase PERK3, uncharacterized LOC103972968 and uncharacterized LOC103984595) were transcriptionally induced exclusively in response to the pathogen in the resistant cultivar (Table 1). Forty eight genes were up-regulated only in the susceptible cultivar. There were 286 and 119 DEGs showing higher expression levels in the resistant cultivar in comparison to the susceptible, either before or after the infection, respectively. Together, 33 genes showed higher expression levels in the resistant cultivar when compared to the susceptible one, both before and after pathogen infection. These included some well-known defense related genes, such as PR-4-like, chitinase 1-like and cytochrome P450. They also included some genes related to plant cell wall, such as probable xyloglucan endotransglucosylase/hydrolase protein 23 and β-glucosidase 32-like (Table 2). The detailed functional classification of DEGs revealed by this study is elaborated in the next section.
Table 1.
Genes up-regulated by Fusarium oxysporum f. sp. cubense race 4 exclusively in the resistant banana (Musa spp. AAA) cultivar and genes showing higher expression levels in the resistant cultivar in comparison to the susceptible one after the infection.
| Gene ID | Gene Description |
|---|---|
| LOC103991268 | Probable WRKY transcription factor 50 |
| LOC103971627 | Phenylalanine ammonia-lyase-like |
| LOC104000780 | Probable LRR receptor-like serine/threonine-protein kinase At1g74360 |
| LOC103983205 | UDP-glycosyltransferase 73C3-like |
| LOC103980149 | LOB domain-containing protein 41-like |
| LOC103990974 | Transcription factor JUNGBRUNNEN 1-like |
| LOC103978674 | Luminal-binding protein 2 |
| LOC103984595 | Uncharacterized |
| LOC103984038 | α-carbonic anhydrase 7-like |
| LOC103968789 | Curcumin synthase 3-like |
| LOC103983427 | Proline-rich receptor-like protein kinase PERK3 |
| LOC103972968 | Uncharacterized |
LOB. lateral organ boundaries; LRR: leucine-rich repeat; UDP: uridine diphosphate.
Table 2.
Genes with higher expression levels in the resistant banana (Musa spp. AAA) cultivar before and after infection with Fusarium oxysporum f. sp. cubense race 4.
| Gene ID | Gene Description |
|---|---|
| LOC103993913 | Uncharacterized |
| LOC103978702 | Chitinase 1-like (PR-3) |
| LOC104000237 | Momilactone A synthase-like |
| LOC103978704 | Chitinase 1-like (PR-3) |
| LOC103989437 | Protein GOS9-like |
| LOC103975319 | Uncharacterized protein At1g15400-like |
| LOC103969557 | Transmembrane protein 45A-like |
| LOC103970573 | β-glucosidase 32-like |
| LOC103992490 | NAC domain-containing protein 68-like |
| LOC103977235 | Phenylpropanoylacetyl-CoA synthase-like |
| LOC103989217 | Uncharacterized |
| LOC103989972 | PR-4-like |
| LOC103992834 | Putative calcium-transporting ATPase 13, plasma membrane-type |
| LOC103988092 | Scarecrow-like protein 23 |
| LOC103980421 | Uncharacterized |
| LOC103993506 | Uncharacterized |
| LOC103974823 | α-carbonic anhydrase 7-like |
| LOC103987744 | Cytochrome P450 CYP72A219-like |
| LOC103979138 | Cytochrome P450 71A1-like |
| LOC104000899 | Uncharacterized |
| LOC104000297 | Leucine-rich repeat receptor protein kinase EXS |
| LOC103974226 | Uncharacterized |
| LOC103977900 | Uncharacterized |
| LOC103989489 | Miraculin-like |
| LOC103983075 | Uncharacterized |
| LOC103976880 | Probable xyloglucan endotransglucosylase/hydrolase protein 23 |
| LOC103979054 | Calcium-binding protein PBP1-like |
| LOC103974727 | Probable small nuclear ribonucleoprotein G |
| LOC103992513 | Probable WRKY transcription factor 70 |
| LOC103992023 | RING-H2 finger protein ATL2-like |
| LOC103989914 | Hexose carrier protein HEX6 |
| LOC103991268 | Probable WRKY transcription factor 50 |
| LOC104000052 | Putative UDP-glucuronate:xylan α-glucuronosyltransferase 3 |
HEX. hexose; NAC: NAM, ATAF1/2, CUC2; PR: pathogenesis-related; UDP:uridine diphosphate.
2.4. Gene Ontology (GO) Annotation Items Involved in the Resistance of Banana to Foc4
In order to identify protein functions associated with the resistance of banana against Foc4, we performed a GO annotation of DEGs. Only limited numbers of genes were assigned by GO annotations. Five genes (out of 32) differentially regulated after pathogen infection in the resistant cultivar were annotated by 17 GO items. We obtained 99 GO annotations assigned to 33 DEGs (from 193 in total) found after pathogen infection in the susceptible cultivar. Considering DEGs found between the two cultivars before pathogen infection (502 in total), we detected 223 GO annotations for 88 of them. After the infection, 122 GO categories were assigned to 44 genes (from 228 DEGs in total) which were differentially expressed between the two cultivars (Table S7, Figures S2–S5).
Similar to that for DEGs, a Venn diagram was constructed for GO annotation items, classified according to biological process (BP), molecular function (MF) and cellular compartment (CC), for all four pairs of comparison. GO categories common for resistant and susceptible cultivars after pathogen attack represent a general response of banana to Foc. They included four CC items (chloroplast, cytoplasmic membrane-bounded vesicle, cytoplasm and plasma membrane) and four MF items (DNA binding, zinc ion binding, chromatin binding and calmodulin binding), but no such items were assigned for BP. Together 26, 6 and 13 GO categories, assigned according to BP (Figure S6A), CC (Figure S6B) and MF (Figure S6C) respectively, appeared exclusively in DEGs found after the infection of the sensitive cultivar (BXCK-BXT). The detailed information of these GO categories is provided in Table S8. Most abundant annotations are related to anaerobic respiration, ammonia assimilation cycle, nitrate assimilation, “de novo” GDP-l-fucose biosynthetic process, glutamate metabolic process, glutamate decarboxylase activity, nonphotochemical quenching, development and photosynthesis.
However, for DEGs found exclusively after the infection of the resistant cultivar (YKCK-YKT), only two GO categories were found according to BP; cellular zinc ion homeostasis and one-carbon metabolic process) and two GO items were found according to MF (carbonate dehydratase activity and calcium-transporting ATPase activity). Five other GO categories belonging to BP (RNA splicing, calcium ion transport, pollen development, regulation of transcription, DNA-templated, response to nematode) were present in the comparison of YKCK-YKT and BXCK-YKCK or BXT-YKT or both (Figure S6).
Furthermore, 37 GO annotation items were found exclusively between the two cultivars both before and after pathogen infection. They include 18, 11 and 8 GO annotation items assigned to BP, CC and MF, respectively (Figure S6, detailed information is listed in Table 3). Most abundant annotations include plant cell wall biosynthesis and degradation and responses to stresses. Among these 37 GO annotation items, those related to plant cell wall biosynthesis and degradation belonged the top 10 abundant annotations, such as cellular polysaccharide metabolic process, chitinase activity, pectinesterase activity and xyloglucan:xyloglucosyl transferase activity.
Table 3.
Gene ontology (GO) terms detected in genes differentially expressed between the resistant and susceptible banana (Musa spp. AAA) cultivars before and after infection with Fusarium oxysporum f. sp. cubense race 4.
| GO ID | Term | Percentage of DEGs before Infection (%) | Percentage of DEGs after Infection (%) |
|---|---|---|---|
| GO:0015706 | Nitrate transport | 4.17 | 4.17 |
| GO:0006073 | Cellular glucan metabolic process | 7.69 | 7.69 |
| GO:0005975 | Carbohydrate metabolic process | 4.29 | 2.86 |
| GO:0010030 | Positive regulation of seed germination | 33.33 | 33.33 |
| GO:0080167 | Response to karrikin | 6.82 | 2.27 |
| GO:0009611 | Response to wounding | 7.04 | 1.41 |
| GO:0010167 | Response to nitrate | 3.57 | 3.57 |
| GO:0016998 | Cell wall macromolecule catabolic process | 16.67 | 16.67 |
| GO:0009408 | Response to heat | 1.82 | 3.64 |
| GO:0044264 | Cellular polysaccharide metabolic process | 25 | 25 |
| GO:0006508 | Proteolysis | 3.77 | 0.94 |
| GO:0009407 | Toxin catabolic process | 5.26 | 5.26 |
| GO:0050832 | Defense response to fungus | 5.13 | 5.13 |
| GO:0032259 | Methylation | 1.92 | 5.76 |
| GO:0010583 | Response to cyclopentenone | 10 | 10 |
| GO:0009409 | Response to cold | 1.08 | 1.08 |
| GO:0006032 | Chitin catabolic process | 16.67 | 16.67 |
| GO:0009736 | Cytokinin-activated signaling pathway | 20 | 20 |
| GO:0009505 | Plant-type cell wall | 5.56 | 4.17 |
| GO:0005618 | Cell wall | 1.34 | 0.67 |
| GO:0005887 | Integral component of plasma membrane | 11.11 | 11.11 |
| GO:0043231 | Intracellular membrane-bounded organelle | 4.83 | 1.61 |
| GO:0044444 | Cytoplasmic part | 3.13 | 3.13 |
| GO:0009506 | Plasmodesma | 0.47 | 0.94 |
| GO:0016020 | Membrane | 1.13 | 0.23 |
| GO:0005773 | Vacuole | 1.33 | 1.33 |
| GO:0003700 | Sequence-specific DNA binding transcription factor activity | 1.22 | 2.45 |
| GO:0008168 | Methyltransferase activity | 2.5 | 7.5 |
| GO:0016757 | Transferase activity, transferring glycosyl groups | 4.76 | 2.38 |
| GO:0004568 | Chitinase activity | 12.5 | 12.5 |
| GO:0004674 | Protein serine/threonine kinase activity | 0.63 | 0.63 |
| GO:0000166 | Nucleotide binding | 0.61 | 0.61 |
| GO:0004601 | Peroxidase activity | 4 | 4 |
| GO:0030599 | Pectinesterase activity | 10 | 10 |
| GO:0004722 | Protein serine/threonine phosphatase activity | 3.33 | 3.33 |
| GO:0016762 | Xyloglucan:xyloglucosyl transferase activity | 7.69 | 7.69 |
| GO:0004190 | Aspartic-type endopeptidase activity | 6.67 | 6.67 |
DEG: differentially expressed genes; GO: gene ontology; NAC: NAM, ATAF1/2, CUC2.
Table 4 shows 10 significantly accumulated GO categories and 18 genes included in these GO items, considering corrected p-value ≤ 0.05 as threshold. Pathogen infection caused significant accumulation in five (assigned to two genes) and four GO categories (assigned to eight genes) in the resistant and susceptible cultivars, respectively. In the resistant cultivar, the significantly accumulated GO categories include cellular zinc ion homeostasis, RNA splicing, one-carbon metabolic process, carbonate dehydratase activity and calcium-transporting ATPase activity. In the susceptible cultivar, significantly accumulated GO categories were anaerobic respiration, iron ion binding, heme binding and electron carrier activity. After pathogen infection, GO annotation named DNA binding was significantly accumulated in the susceptible but not in the resistant cultivar. There was only one GO annotation item which included nine genes. However, there were no significantly accumulated GO items between two cultivars before pathogen infection, although the number of GO categories was the highest.
Table 4.
GO items and relevant genes significantly accumulated in resistant and susceptible banana (Musa spp. AAA) cultivars after infection with Fusarium oxysporum f. sp. cubense race 4.
| Comparison Pair | GO Annotation | Gene ID | Gene Description |
|---|---|---|---|
| YKCK–YKT | RNA splicing | LOC103989140 | Uncharacterized |
| Cellular zinc ion homeostasis | LOC103984038 | α-carbonic anhydrase 7-like | |
| One-carbon metabolic process | |||
| Carbonate dehydratase activity | |||
| Calcium-transporting ATPase activity | |||
| BXCK–BXT | Anaerobic respiration | LOC103970727 | Kelch repeat-containing protein At3g27220-like |
| LOC103984439 | Uncharacterized | ||
| Iron ion binding | LOC103985201 | Cytochrome c-like | |
| LOC103996064 | Glutamate synthase 1 [NADH], chloroplastic-like | ||
| LOC103990398 | Abscisic acid 8′-hydroxylase 1-like | ||
| LOC103974663 | Trans-cinnamate 4-monooxygenase-like | ||
| LOC103972072 | Cytochrome P450 84A1-like | ||
| Heme binding | LOC103985201 | Cytochrome c-like | |
| LOC103990398 | Abscisic acid 8′-hydroxylase 1-like | ||
| LOC103974663 | Trans-cinnamate 4-monooxygenase-like | ||
| LOC103972072 | Cytochrome P450 84A1-like | ||
| Electron carrier activity | LOC103985201 | Cytochrome c-like | |
| LOC103990398 | Abscisic acid 8′-hydroxylase 1-like | ||
| LOC103974663 | Trans-cinnamate 4-monooxygenase-like | ||
| LOC103972072 | Cytochrome P450 84A1-like | ||
| BXT–YKT | DNA binding | LOC103972990 | Protein CUP-SHAPED COTYLEDON 3-like |
| LOC103984324 | Uncharacterized | ||
| LOC103982987 | Auxin-responsive protein IAA4-like | ||
| LOC103985820 | NAC domain-containing protein 21/22-like | ||
| LOC103990974 | TF JUNGBRUNNEN 1-like | ||
| LOC103975993 | Ethylene-responsive TF ERF107-like | ||
| LOC103993650 | Ethylene-responsive TF 11-like | ||
| LOC103992490 | NAC domain-containing protein 68-like | ||
| LOC103971736 | TF MYB6-like |
BX: “Baxijiao”, highly susceptible; YK: “Yueyoukang 1”, highly resistant. CK: control; T: treatment with pathogen. GO: gene ontology; TF: transcription factor.
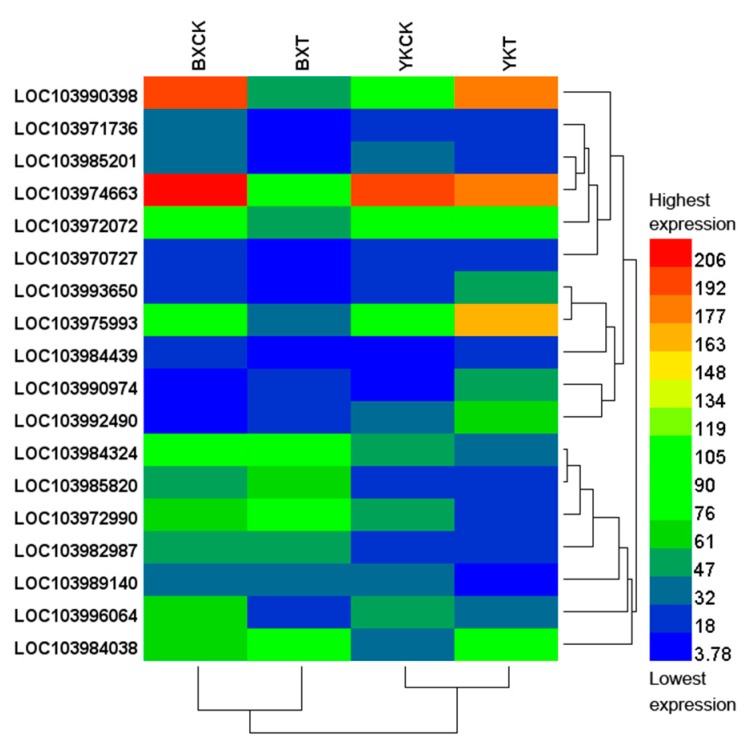
Next, we attempted to evaluate 18 genes classified in the top ten GO annotations (Table 4) using a heat map in terms of their reads per kilobase of transcript per million mapped reads (RPKM) values. As shown in Figure 2, these 18 genes could be grouped into the following gene clusters: (1) four genes (LOC10394439, LOC103993650, LOC103990398 and LOC103975993) accumulated in the resistant cultivar after pathogen infection, but it was opposite in the susceptible one; (2) Genes (LOC103992490, LOC103984038 and LOC103990974) with increased expression after pathogen infection in both cultivars but more intensive increase was observed in the resistant cultivar; (3) Genes with increased expression in the sensitive cultivar, but decreased or stable in the resistant cultivar (LOC103984324, LOC103972990 and LOC103985820); (4) Genes with decreased expression in the susceptible cultivar but less or nearly no decrease in the resistant one (LOC103971736, LOC103970727 and LOC103972072); (5) Genes with decreased expression in both cultivars but more intensive decrease was observed in the susceptible cultivar (LOC103985201, LOC103996064 and LOC103974663); (6) Genes which expression decreased significantly in the resistant cultivar, but with no change in the susceptible one (LOC103989140); (7) Genes not responding to the pathogen but showing significantly higher expression in the susceptible cultivar than in the resistant one both before and after pathogen infection (LOC103982987).
Figure 2.
Evaluation of the 18 genes classified in the top 10 GO annotations using heat map in terms of their RPKM values. Red represents the highest expression level while blue represents the lowest one. BX: “Baxijiao”, highly susceptible to Fusarium oxysporum f. sp. cubense race 4; YK: “Yueyoukang 1”, highly resistant to Fusarium oxysporum f. sp. cubense race 4. CK: control; T: treatment with pathogen. GO: gene ontology; RPKM: reads per kilobase of transcript per million mapped reads.
2.5. Kyoto Encyclopedia of Genes and Genomes (KEGG) Analysis
DEGs found between the two cultivars before and after pathogen infection were classified into 67 (corresponding to 113 genes) and 47 (corresponding to 45 genes) KEGG pathways, respectively. On the other hand, 14 (corresponding to 8 genes) and 51 (corresponding to 59 genes) KEGG pathways were found after the pathogen infection in the resistant and susceptible cultivar, respectively (Table S7, Figures S7–S10). Together we detected 86 different KEGG pathways and ten of them appeared in all four pairs of comparison. Thirteen KEGG pathways were found simultaneously in DEGs detected at least in three pairs of comparison including BXCK-BXT, YKCK-YKT and BXT-YKT or/and BXCK-YKCK. This suggests that they are generally involved in the responses of banana to Foc4. Six pathways (fructose and mannose metabolism, sphingolipid metabolism, butanoate metabolism, porphyrin and chlorophyll metabolism, carotenoid biosynthesis and ribosome) are exclusively present after the pathogen infection of the sensitive cultivar. On the other hand, there was only one pathway (protein export) exclusively present after the pathogen infection of the resistant cultivar. Numbers of accumulated KEGG pathways exclusively present in BXCK-YKCK and BXT-YKT comparisons were 15 and 9, respectively (Figure S11). Ten KEGG pathways, including steroid biosynthesis, carbon fixation in photosynthetic organisms and several pathways related to amino acid metabolism, were present in both of BXT-YKT and BXCK-YKCK comparisons (Figure S11 and Table 5).
Table 5.
KEGG pathways of genes differentially expressed between two banana (Musa spp. AAA) cultivars before and after infection with Fusarium oxysporum f. sp. cubense race 4.
| KEGG ID | Term |
|---|---|
| mus00020 | Citrate cycle |
| mus00100 | Steroid biosynthesis |
| mus00260 | Glycine, serine and threonine metabolism |
| mus00280 | Valine, leucine and isoleucine degradation |
| mus00310 | Lysine degradation |
| mus00450 | Selenocompound metabolism |
| mus00710 | Carbon fixation in photosynthetic organisms |
| mus00750 | Vitamin B6 metabolism |
| mus03040 | Spliceosome |
| mus04145 | Phagosome |
KEGG: Kyoto encyclopedia of genes and genomes.
When considering corrected p value ≤ 0.05 as the threshold, 21 KEGG pathways showed significant enrichment (Table 6 and Table S9). Ten significantly enriched items were found in DEGs after pathogen infection in the susceptible cultivar but only one in the resistant one (circadian rhythm—plant). Among DEGs found between the two cultivars before pathogen infection, ten significantly enriched KEGG pathways were identified; however, there was no significantly enriched KEGG pathway after the infection.
Table 6.
The top 21 Kyoto encyclopedia of genes and genomes (KEGG) pathways with corrected p value ≤ 0.05.
| Comparison Pair | Pathway ID | Pathway | No. of DEGs | Up-Regulated DEGs | Down-Regulated DEGs |
|---|---|---|---|---|---|
| BXCK–YKCK | mus01110 | Biosynthesis of secondary metabolites | 52 | 36 | 16 |
| mus00940 | Phenylpropanoid biosynthesis | 19 | 13 | 6 | |
| mus00941 | Flavonoid biosynthesis | 9 | 9 | 0 | |
| mus00360 | Phenylalanine metabolism | 14 | 5 | 9 | |
| mus01100 | Metabolic pathways | 68 | 39 | 29 | |
| mus00960 | Tropane, piperidine and pyridine alkaloid biosynthesis | 5 | 4 | 1 | |
| mus00950 | Isoquinoline alkaloid biosynthesis | 4 | 3 | 1 | |
| mus00500 | Starch and sucrose metabolism | 12 | 4 | 8 | |
| mus00480 | Glutathione metabolism | 6 | 5 | 1 | |
| mus00100 | Steroid biosynthesis | 4 | 0 | 4 | |
| YKCK–YKT | mus04712 | Circadian rhythm—plant | 2 | 1 | 1 |
| BXCK–BXT | mus01100 | Metabolic pathways | 44 | 13 | 31 |
| mus01110 | Biosynthesis of secondary metabolites | 27 | 11 | 16 | |
| mus00430 | Taurine and hypotaurine metabolism | 4 | 0 | 4 | |
| mus00940 | Phenylpropanoid biosynthesis | 9 | 5 | 4 | |
| mus00010 | Glycolysis/Gluconeogenesis | 6 | 0 | 6 | |
| mus00500 | Starch and sucrose metabolism | 7 | 0 | 7 | |
| mus00360 | Phenylalanine metabolism | 5 | 3 | 2 | |
| mus00941 | Flavonoid biosynthesis | 3 | 2 | 1 | |
| mus00250 | Alanine, aspartate and glutamate metabolism | 3 | 0 | 3 | |
| mus00592 | α-Linolenic acid metabolism | 3 | 1 | 2 |
BX: “Baxijiao”, a cultivar highly susceptible to Fusarium oxysporum f. sp. cubense race 4; YK: “Yueyoukang 1”, a cultivar highly resistant to Fusarium oxysporum f. sp. cubense race 4. CK: control; T: treatment with pathogen. DEG: differentially expressed genes; KEGG: Kyoto encyclopedia of genes and genomes.
2.6. Changes in Thespatio-Temporal Distribution of Xyloglucan in Foc-Infected Banana
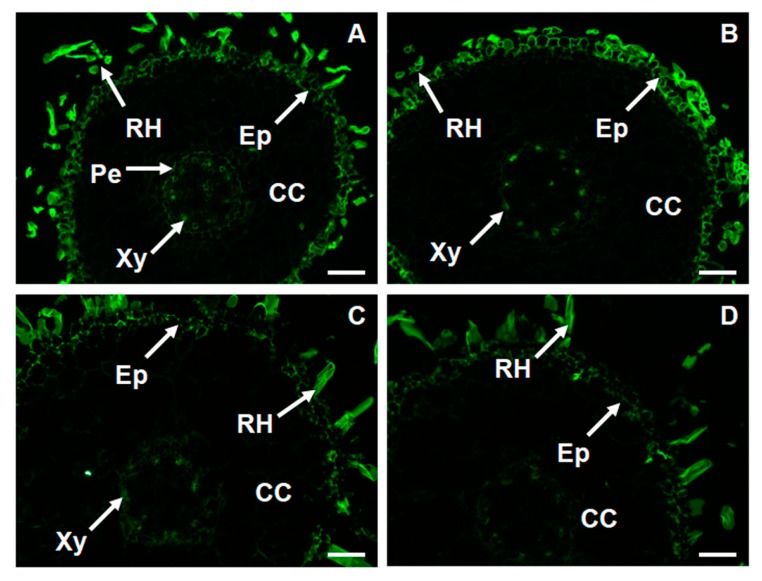
As mentioned above, we have found several genes and GO annotation items related to plant cell wall hemi-cellulose perhaps involved in banana resistance to Foc4. In order to validate these results, we conducted immunofluorescent histological observations of xyloglucan using LM15 monoclonal antibody in root sections of the two banana cultivars. We observed strong fluorescent signal in root hairs and epidermis while slightly weaker signal was detected in xylem and pericycle. Remarkably, fluorescent signal of lower intensity was observed in the susceptible cultivar. Pathogen infection caused obvious increase in the signal intensity in the epidermis and in the xylem of the resistant cultivar. Unlike in the resistant cultivar, Foc4 infection did not enhance the fluorescence signal in the susceptible cultivar (Figure 3).
Figure 3.
Immunolocalization of xyloglucan in banana (Musa spp. AAA) root cross sections by LM15 antibody. (A) Resistant cultivar before infection with Foc4; (B) Resistant cultivar after infection with Foc4; (C) Susceptible cultivar before infection with Foc4; (D) Susceptible cultivar after infection with Foc4; Bars represent 100 μm. CC, cortical cells; Ep, epidermis; Foc4, Fusarium oxysporum f. sp. cubense; Pe, pericycle; RH, root hairs; Xy, xylem.
2.7. Quantitative Real-Time PCR (qRT-PCR) Validation of DGE Data
To confirm the results of the Illumina Solexa sequencing, eight unigenes for quantitative real-time PCR (qRT-PCR) assays were selected from genes included in the significantly enriched GO annotations or KEGG pathways. These were: LOC103987502 (chalcone synthase 2-like), LOC103972276 (tropinone reductase homolog At1g07440-like), LOC103995575 (pathogenesis-related protein 1-like), LOC103978702 (chitinase 1-like), LOC103992490 (NAC domain-containing protein 68-like), LOC103990974 (TF JUNGBRUNNEN 1-like), LOC103993974 (probable 2-aminoethanethiol dioxygenase), LOC103974663 (trans-cinnamate 4-monooxygenase-like). As shown in Figure S12, six of them were consistent with the DGE analyses. These results approved Illumina Solexa sequencing data.
3. Discussion
We carried out a DGE analysis to screen which genes are specifically responsible for Fusarium resistance at the young seedling stage for better understanding of the mechanism underlying the resistance of banana to Foc4.
The studied two cultivars significantly differ in the resistance to Foc4, as shown by the high abundance of the pathogen hyphae in the inner root tissues of the susceptible cultivar. Resistant cultivar likely developed effective mechanism to minimize the spread of the pathogen, which remained predominantly at the root periphery. As proposed by our DGE analysis, resistant cultivar is equipped with unique set of genes involved in various functions to minimize colonization by the pathogen. These genes are discussed below.
We present a general view on differences in the gene expression patterns between two banana cultivars with Foc resistance or susceptibility using various bioinformatic tools. Enriching results reported by Bai et al. [19], more genes were found to be differently expressed in the susceptible cultivar as compared to the resistant one in the present study. This might be caused by higher susceptibility of tissue-cultured young banana plantlets [21] used in our study in comparison to older plants used by Bai et al. [19], and by in vitro conditions.
3.1. Receptor Like Kinases
Two genes acting as receptor-like kinases were identified as determinants of banana resistance against Foc4. These kinases are involved in the sensing and perception of the pathogen derived signal [22], therefore they might be important for the banana resistance against Foc4. One of them, probable LRR receptor-like serine/threonine-protein kinase At1g74360 was previously reported to be required for immunity against parasitic nematodes in Arabidopsis [23]. Proline-rich receptor-like protein kinase PERK3 belongs to the receptor kinases which share a putative extracellular domain related to cell wall proteins. They are suggested to sense changes induced by pathogens in the cell wall [24].
3.2. Trancription Factors
Plant TFs are involved in the regulation of defense-related gene expression against different types of microbial pathogens. For example, Jin et al. [25] suggested that, ethylene-responsive factor (ERF) CaPTI1 gene regulates defense response of pepper (Capsicum annuum L.) to Phytophthoracapsici. Liu et al. [26] found that SpMYB TF expression was significantly induced after Arabidopsis infection with Fusarium oxysporum or Botrytis cinerea. Furthermore, SpMYB overexpressing transgenic tobacco plants are more resistant to F. oxysporum and B. cinereaas compared to the wild-type plants, suggesting that SpMYB positively regulates plant disease resistance. In banana, up-regulated TFs including WRKY6, WRKY26, WRKY31, WRKY33, WRKY65 and WRKY72, ethylene responsive TFs, such as ethylene insensitive 3 (EIN3) and ethylene insensitive 3-like 1 (EIL1) were found in Foc-resistant cultivar “Nongke 1” [18]. Bai et al. [19] also reported that three TFs including WRKY22, WRKY33 and DREB showed different expression patterns between Foc-resistant and susceptible banana cultivars. The NAC (NAM, ATAF1/2, CUC2) gene family encodes a large family of plant-specific TFs that play diverse roles in plant development and stress regulation. Based on our study, NAC domain-containing protein 68-like determines the resistance of banana to Foc. On the other hand, the expression of another member of this TF family, NAC domain-containing protein 21/22-like, positively correlated with the increased susceptibility to Foc. The involvement of NAC21/22 in the susceptibility of plants to diseases was also reported in wheat (Triticum aestivum) [27]. Chen et al. [28] revealed that over-expression of SmNAC decreases resistance to bacterial wilt. These data suggest that plant NAC could play an important function in plant-pathogen interaction.
WRKY TFs are established regulators of defense genes. Products of these genes might be phosphorylated by mitogen activated protein kinases [29]. In the present study, probable MaWRKY50 was up-regulated by the pathogen exclusively in the resistant cultivar. Furthermore, this TF was more expressed in the resistant cultivar as compared to the susceptible one, both before and after pathogen infection. These results suggested that MaWRKY50 is an important TF determining both basal and induced resistance of banana to Foc4. WRKY50 is well-known TF participating in the salicylic acid signaling pathway in Arabidopsis [30]. Other promising TF candidates supporting the banana resistance against Fusarium might be JUNGBRUNNEN1 (JUB1) and LATERAL ORGAN BOUNDARIES (LOB) DOMAIN (LBD)-containing protein 41-like. In contrast to banana, JUB1 and LOB 20 have been shown rather to suppress the defense responses of Arabidopsis against Pseudomonas syringae and Fusarium oxysporum [31,32].
3.3. Canonical Defense Related and Cell Wall Associated Genes
Several canonical defense-related genes exert higher expression in the resistant cultivar before and after the infection (Table 2). For example, cytochrome P450 is the member of jasmonic acid pathway, a well-known signalling pathway associated with plant defense [33]. Many biochemical conversions in plant steroid hormone biosynthesis are catalyzed by cytochrome P450 enzymes (CYPs or P450s). Recently, Yang et al. [34] found that heterologous expression of wild eggplant (Solanummelongena) cytochrome P450 gene, StoCYP77A2, in tobacco confers enhanced disease resistance against Verticillium dahliae.
PR proteins play a very important role in plant innate immunity. PR-1 is one of the most abundant from this family of proteins. In the present study, PR-1 like (LOC103977651 and LOC103977653) was up-regulated by the pathogen exclusively in the susceptible cultivar. Another PR-1 member, LOC103975649, was down-regulated by the pathogen in the resistant cultivar. Differently, some other PRs, such as PR-3 and PR-4, belonging to chitinases, showed significantly higher expression levels in the resistant cultivar than in the susceptible one, both before and after pathogen infection. Chitinases are in the first line of plant defense response. They can disrupt fungal cell walls and produce chitin oligomers eliciting the plant defense signalling pathways [35]. They have been shown to be key players in the response to Foc infection in Cavendish banana [18]. The fact that not all PR genes (see PR-1) are induced in the resistant banana cultivar, might suggest that this cultivar activates different defense cascades including activation of PR-3 and PR-4 or accumulation of hemicelluloses to form different constitutive cell barriers.
Significant number of genes is involved in cell wall metabolism, indicating that cell wall, as a mechanical barrier preventing pathogen spreading, may be one of the crucial mechanisms of banana resistance. This is also reflected by enrichment of cell wall related genes after the infection observed by the KEGG pathway analysis. Specific remodeling of cell wall components such as extensins, arabinogalactan proteins and pectins substantially enhances the resistance of banana to Fusarium [7,8]. We identified enzymes metabolizing xylan and xyloglucans, which are important cell wall structural constituents [36]. These enzymes contribute to the banana resistance against Foc4. Immunofluorescence observation of xyloglucan validated these results and showed their profound accumulation in epidermis of the resistant cultivar. This is also in agreement with the accumulation of the pathogen in the epidermal periphery (Figure 3). Consistent outputs were obtained by comparative GO annotation. Several cell wall biosynthesis or degradation-related GO annotations were found to be preferentially enriched in the resistant cultivar (e.g., cellular glucan metabolic process, cellular polysaccharide metabolic process, pectinesterase activity, xyloglucan:xyloglucosyl transferase activity and transferase activity, transferring glycosyl groups). Our previous work revealed that the susceptible banana cultivar showed higher pectinesterase activities both before and after Foc4 infection, but pathogen infection lowered activity of this enzyme in both cultivars [7]. This was consistent with the expression levels of LOC103975191, the only gene assigned to GO: 0030599 (pectinesterase activity).
3.4. Secondary Metabolism
Our data also indicate that similarly to other species [37], secondary metabolites play very important roles in plant pathogen defense. Enzymes involved in phenyl-propanoid secondary metabolites or curcumin have been up-regulated preferentially in the resistant banana cultivar after the Foc4 infection.
3.5. New Genes with Putative Role in Foc4 Resistance
Several genes, which were preferentially up-regulated in the resistant cultivar, were not reported in plant disease resistance so far (e.g., α-carbonic anhydrase 7-like, phenylpropanoylacetyl-CoA synthase-like, probable small nuclear ribonucleoprotein G, hexose carrier protein HEX6, protein GOS9-like). They are promising candidates for regulators of banana resistance against Foc4. However, the precise mechanism of their function remains to be elucidated in the future.
3.6. Comparative GO and KEGG Analysis and Screening of Potential Resistant Genes
We provide here a comprehensive comparative elaboration of KEGG pathways important for resistance or sensitivity of banana against Foc. Taking corrected p value ≤ 0.05 as the threshold, there was only one KEGG pathway, circadian rhythm—plant, exclusively enriched in inoculated resistant cultivar. Circadian clock is the internal time-keeping machinery which helps plants to anticipate diurnal changes [38]. In the recent years, many lines of evidences, including our study, showed that the circadian rhythm modulate plant immune responses [39,40].
Further, ten KEGG pathways which were enriched between the two cultivars both before and after pathogen attack might be responsible for the basal resistance/susceptibility of banana against Foc. Most of these pathways were not reported in previous works [18,19], which might be caused by higher susceptibility of tissue-cultured young banana plantlets [21]. Three of them belong to amino acid metabolism (glycine, serine and threonine metabolism, valine, leucine and isoleucine degradation, lysine degradation). Recently, plant amino acid metabolism was also found to be involved in plant resistance to diseases. For example, Seifi et al. [41] found that infection of wild-type tomato (Solanum lycopersicum) leads to a strong transcriptional up-regulation of asparagine synthetase, followed by a severe depletion of asparagine titers. In contrast, resistant sitiens tomato plants displayed a strong induction of asparagine throughout the course of infection, and asparagine synthetase played an immune-regulatory role in Botrytis cinerea-tomato interaction. Over-expression of cytosolic aspartate amino transferase not only led to changes in the content of aspartate and aspartate-derived amino acids, but also affected defense responses against B. cinerea infection in Arabidopsis thaliana [42]. Thus, amino acid metabolism likely contributes to the defense strategy against Foc4 in banana. Citrate cycle, TCA cycle and Krebs cycleare important aerobic pathways for the final steps of the oxidation of carbohydrates and fatty acids. The DEGs assigned in this KEGG pathway showed higher expression levels in the susceptible cultivar than in the resistant one both before and after infection. In addition, GO annotation analysis also showed that GO annotation items, such as anaerobic respiration, aromatic amino acid family biosynthetic process, cysteine biosynthetic process, and glutamate metabolic process were detected exclusively in the susceptible cultivar (Table S8). These suggest higher demand for energy in the susceptible cultivar to cope with the negative effects of the pathogen.
4. Materials and Methods
4.1. Materials
Two banana cultivars, Musa spp. AAA cv. “Baxijiao” and “Yueyoukang 1” were used in this study. “Yueyoukang 1”, a cultivar from Cavendish selection GCTCV-218, is highly resistant to Foc4 (disease incidence in the field is under 5%) while “Baxijiao” is highly susceptible to this pathogen (with disease incidence of 44.4% in the field [43]).
Single-spore cultures of highly virulent Foc4 were used in the present study for banana inoculation. The pathogen cultivated on solid potato dextrose agar (PDA) medium (200 g/L patato + 20 g/L dextrose + 13 g/L agar) and kept at 4 °C for two cycles was activated on the fresh PDA medium and cultivated at 28 °C for 3 days in dark before transferring to the liquid potato lactose medium (200 g/L patato + 20 g/L lactose + 13 g/L agar) to produce spores. After 7-day-culture on a shaker rotating at 140 rpm, spore suspension was ready for use.
4.2. Inoculation of Banana Cultivars with Pathogen
Preparation of pathogen and the inoculation of two banana cultivars with this Foc4 were carried out according to the protocol described in our previous work [7]. In brief, tissue cultured banana seedlings without roots were cultivated two weeks in liquid rooting medium (MS [44] + 0.1 mg/L indole-3-butyric acid + 0.2 mg/L α-naphthaleneacetic acid). One newly emerged root (0.5–1.0 mm in diameter) of each plant was cut off to facilitate the penetration of the pathogen and it was transferred to a new rooting medium containing Foc4 at a final concentration of 5 × 102 conidia per mL (inoculation treatment). Control plants were cut-treated likewise, and they were transferred to a rooting medium without fungus.
Roots (devoid of excess liquid media by brief blotting on filter paper) were collected 24 h after treatments. One biological replicate of each individual sample consisted of whole roots from 21 seedlings. RNA extracted from the roots was subjected to DGE and qRT-PCR analysis. Values reported represent the average of three and two biological replicates for qRT-PCR and DGE, respectively. Individual roots were used for histological study.
4.3. Immunolabelling of Xyloglucan
Samples were collected 24 h after treatments. Fixation and embedding of samples were carried out as described in Xu et al. [45]. In detail, root tips of about 1 cm in length were fixed in 3.7% (v/v) formaldehyde in stabilizing buffer MTSB (50 mM piperazine-N,N′-bis(2-ethanesulfonic acid), 5 mM MgSO4·7H2O, 5 mM ethyleneglycol bis (2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), pH 6.9) for 1 h at room temperature, dehydrated in a successive ethanol series (30%, 50%, 70%, 90%, and 100%; v/v) and embedded in Steedman’s wax [46]. Thin sections (8–10 μm) were de-waxed and rehydrated in a successive ethanol series (100%, 90%, 70% and 50%; v/v), blocked in phosphate-buffered saline (PBS) supplemented with 50 mM glycine and 2% (w/v) bovine serum albumin (BSA). To detect the spatio-temporal distribution of xyloglucans, tissue sections were labelled with LM15, a major primary monoclonal antibody (diluted 1:20 in PBS containing 1% (w/v) BSA) recognizing XXXG motif of xyloglucan [47] at 4 °C overnight. After washing in PBS three times, sections were incubated in anti-rat IgG secondary antibody conjugated with FITC diluted 1:20 in PBS containing 1% (w/v) BSA for 1 h at room temperature. Afterwards, the sections were washed with PBS three times and they were stained with 0.01% of toluidine in PBS for 10 min to quenchtissue auto-fluorescence. Finally, the sections were rinsed with PBS (three times, each for 10 min) and mounted in anti-bleach medium before observation. Sections probed only with secondary antibodies were used as controls. Three biological replicates each consisting of 2 sections were prepared for individual treatment. Fluorescence was examined with an Imager D2 (ZEISS, Oberkochen, Germany). Exposure time was 30 ms.
4.4. Observation of Pathogen Diffusion in Root Tissues of Banana
The protocols for fixation and embedding of samples (collected 48 h after treatments) were carried out as described above. To observe the differences in pathogen diffusion between the resistant and susceptible cultivars, de-waxed cross sections of roots were stained with Calcofluor White Stain (Sigma-Aldrich, Saint Louis, MO, USA) for 10 min followed by three times of rinse with PBS buffer. Images were taken by using UV light for excitation of with a Olympus BH-2-FRCA microscope (Olympus, Tokyo, Japan).
The quantification of the hyphae represented an average of three biological replicates (one biological replicate was calculated as an average hypha number counted from 10 banana cell area of 0.01 mm2) ± standard deviation. A comparison of groups was conducted using a paired t-test of variance.
4.5. DGE Analysis
RNA preparation, library preparation for DGE sequencing and data analysis were carried out as described in our former paper [8].
4.6. GO and KEGG Enrichment Analysis of Differentially Expressed Genes
The GO enrichment analysis of DEGs was carried out by Blast2Go software [48]. GO terms with corrected p value less than 0.05 were considered as significantly enriched by DEGs. Application of Blast2GO algorithm for GO function classification enabled to get three categories of all the sequences in GO, respectively: MF, CC, BP. KEGG is a database resource for understanding high-level functions and utilities of the biological system, from molecular-level information, especially large-scale molecular datasets generated by genome sequencing and other high through-put experimental technologies (http://www.genome.jp/kegg/). We used KOBAS software to test the statistical enrichment of DEGs in KEGG pathways.
4.7. qRT-PCR Validation Result
The total RNA was extracted as described previously [8]. RNA was reverse transcribed in a 10 uL reaction system using the PrimeScriptTM RT Master Mix Kit (TaKaRa, Otsu, Japan). Gene-specific primers were designed base on the gene sequences using Primer 3.0 software and the primer sequences are listed in Table S10. The 18S rRNAgene of banana was used as a reference gene. The qRT-PCR was performed using the SYBR Premix Ex Taq Kit (TaKaRa, Otsu, Japan) according to the manufacturer’s protocol. And all qRT-PCR reactions were carried out using Thermal Cycler Dice (TaKaRa, Otsu, Japan). Individual reactions were run with each primer pair with annealing temperatures ranging from 55 to 60 °C. The conditions were as follows: initial holding at 95 °C for 3 min, followed by a two-step program of 95 °C for 10 s and annealing temperature for 30 s for 40 cycles. Each sample was analyzed in three technical replicates. The relative changes in gene expression levels were calculated using the2−ΔΔCt method [49].
4.8. Statistical Analysis
The DEGs of the resistant and susceptible banana cultivars before and after infection with Foc4 were compared with the method described by Audic et al. [50]. The false discovery rate (FDR) was used to determine the threshold of p value in multiple test and analysis. A threshold of FDR < 0.001 was used to judge the significance of gene expression difference. p ≤ 0.01, FDR ≤ 0.1, and the absolute value of log2 ratio ≥ 1 were used as threshold to assess the significance of gene expression difference.
5. Conclusions
Thank to comparative bioinformatic analyses and exploitation of tissue-cultured plants, we were able to provide a unique set of genes which are important for banana resistance to Foc. The results showed that in addition to canonical PR genes, increased expression of TFs, receptor-like kinases, cell wall regulatory enzymes as well as metabolic genes play important roles in the resistance of banana to Foc. The resistant banana cultivar developed structural barriers for restricting the pathogen spread. The results obtained in the present study will help to better understand banana resistance mechanism against Foc. Precise function and the regulation of the most important genes related to the resistance of banana to Foc4 should be of main interest for future studies, and for generation of resistant banana germplasm using genetic modification [51].
Acknowledgments
This work was supported by National Natural Science Foundation of China (31272117), Natural Science Foundation of Guangdong Province (2015A030313421), Key Technologies Research and Development Program of Guangdong Province (2014A020208111), the earmarked fund for Modern Agro-industry Technology Research System (nycytx-33), and by National Program for Sustainability I (grant No. LO1204) provided by the Czech Ministry of Education.
Abbreviations
| BP | Biological process |
| BSA | Bovine serum albumin |
| CC | Cellular compartment |
| DEG | Differentially expressed genes |
| DGE | Digital gene expression |
| FDR | False discovery rate |
| Foc | Fusarium oxysporum f. sp. cubense |
| Foc4 | Race 4 of Foc |
| GO | Gene ontology |
| KEGG | Kyoto encyclopedia of genes and genomes |
| LOB | Lateral organ boundaries |
| LRR | Leucine-rich repeat |
| MF | Molecular function |
| NAC | NAM, ATAF1/2, CUC2 |
| PBS | Phosphate-buffered saline |
| PDA | Potato dextrose agar |
| PR | Pathogenesis-related |
| qRT-PCR | Quantitative real time PCR |
| RPKM | Reads per kilobase of transcript per million mapped reads |
| TFs | Transcription factors |
| UDP | Uridine diphosphate |
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/19/2/350/s1.
Author Contributions
Chunxiang Xu planned experiments, Yuqing Niu and Bei Hu performed experiments, Chunxiang Xu, Xiaoquan Li, Tomáš Takáč, Jozef Šamaj and Houbin Chen analyzed data and prepared data presentation, Chunxiang Xu, Tomáš Takáč and Jozef Šamaj wrote the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.FAOSTAT. [(accessed on 20 January 2018)]; Available online: http://www.fao.org/faostat/en/#data/QC/visualize.
- 2.Hwang S.C., Ko W.H. Cavendish banana cultivars resistant to Fusarium wilt acquired through somaclonal variation in Taiwan. Plant Dis. 2004;88:580–588. doi: 10.1094/PDIS.2004.88.6.580. [DOI] [PubMed] [Google Scholar]
- 3.Ploetz R.C. Fusarium wilt of banana is caused by several pathogens referred to as Fusarium oxysporum f. sp. cubense. Phytopathology. 2006;96:653–656. doi: 10.1094/PHYTO-96-0653. [DOI] [PubMed] [Google Scholar]
- 4.Pegg K.G., Moore N.Y., Bentiey S. Fusarium wilt of banana in Australia: A review. Aust. J. Agric. Res. 1996;47:637–650. doi: 10.1071/AR9960637. [DOI] [Google Scholar]
- 5.Swarupa V., Ravishankar K.V., Rekha A. Plant defense response against Fusarium oxysporum and strategies to develop tolerant genotypes in banana. Planta. 2014;239:735–751. doi: 10.1007/s00425-013-2024-8. [DOI] [PubMed] [Google Scholar]
- 6.Li C.Y., Chen S., Zuo C.W., Sun Q.M., Ye Q., Yi G.J., Huang B.H. The use of GFP-transformed isolates to study infection of banana with Fusarium oxysporum f. sp. cubense race 4. Eur. J. Plant Pathol. 2011;131:327–340. doi: 10.1007/s10658-011-9811-5. [DOI] [Google Scholar]
- 7.Ma L., Jiang S., Lin G., Cai J., Ye X., Chen H., Li M., Li H., Takác T., Samaj J., et al. Wound-induced pectin methylesterases enhance banana (Musa spp. AAA) susceptibility to Fusarium oxysporum f. sp. cubense. J. Exp. Bot. 2013;64:2219–2229. doi: 10.1093/jxb/ert088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Y.L., Fan W., Li X., Chen H.B., Takac T., Samajova O., Fabrice M.R., Xie L., Ma J., Samaj J., et al. Expression and distribution of extensins and AGPs in susceptible and resistant banana cultivars in response to wounding and Fusarium oxysporum. Sci. Rep. 2017;7:42400. doi: 10.1038/srep42400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Y., Yi G., Peng X., Huang B., Liu E., Zhang J. Systemic acquired resistance in Cavendish banana induced by infection with an incompatible strain of Fusarium oxysporum f. sp. cubense. J. Plant Physiol. 2013;170:1039–1046. doi: 10.1016/j.jplph.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Mahdavi F., Sariah M., Maziah M. Expression of rice thaumatin-like protein gene in transgenic banana plants enhances resistance to Fusarium wilt. Appl. Biochem. Biotechnol. 2012;166:1008–1019. doi: 10.1007/s12010-011-9489-3. [DOI] [PubMed] [Google Scholar]
- 11.Endah R., Beyene G., Kiggundu A., van den Berg N., Schlüter U., Kunert K., Chikwamba R. Elicitor and Fusarium-induced expression of NPR1-like genes in banana. Plant Physiol. Biochem. 2008;46:1007–1014. doi: 10.1016/j.plaphy.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Luo J.Y., Pan X.L., Peng T.C., Chen Y.Y., Zhao H., Mu L., Peng Y., He R., Tang H. DNA methylation patterns of banana leaves in response to Fusarium oxysporum f. sp. cubense tropical race 4. J. Integr. Agric. 2016;15:2736–2744. doi: 10.1016/S2095-3119(16)61495-8. [DOI] [Google Scholar]
- 13.Guo Y., Qiu C.S., Long S.H., Chen P., Hao D.M., Preisner M., Wang H., Wang Y.F. Digital gene expression profiling of flax (Linumusitatissimum L.) stem peel identifies genes enriched in fiber-bearing phloem tissue. Gene. 2017;626:32–40. doi: 10.1016/j.gene.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Li Q.Q., Niu Z.B., Bao Y.G., Tian Q.J., Wang H.G., Kong L.R., Feng D.S. Transcriptome analysis of genes related to resistance against powdery mildew in wheat-Thinopyrum alien addition disomic line germplasm SN6306. Gene. 2016;590:5–17. doi: 10.1016/j.gene.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Cao K., Li H.Y., Wang Q., Zhao P., Zhu G.Y., Fang W.C., Chen C.W., Wang X.W., Wang L. Comparative transcriptome analysis of genes involved in the response of resistant and susceptible peach cultivars to nematode infection. Sci. Hortic. 2017;215:20–27. doi: 10.1016/j.scienta.2016.11.054. [DOI] [Google Scholar]
- 16.Wang Z., Zhang J.B., Jia C.H., Liu J.H., Li Y.Q., Yin X.M., Xu B.Y., Jin Z.Q. De novo characterization of the banana root transcriptome and analysis of gene expression under Fusarium oxysporum f. sp. cubense tropical race 4 infection. BMC Genom. 2012;13:65. doi: 10.1186/1471-2164-13-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li C., Shao J., Wang Y., Li W., Guo D., Yan B., Xia Y., Peng M. Analysis of banana transcriptome and global gene expression profiles in banana roots in response to infection by race 1 and tropical race 4 of Fusarium oxysporum f. sp. cubense. BMC Genom. 2013;14:851. doi: 10.1186/1471-2164-14-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li C.Y., Deng G.M., Yang J., Viljoen A., Jin Y., Kuang R.B., Zuo C.W., Lv Z.C., Yang Q.S., Sheng O., et al. Transcriptome profiling of resistant and susceptible Cavendish banana roots following inoculation with Fusarium oxysporum f. sp. cubense tropical race 4. BMC Genom. 2012;13:374. doi: 10.1186/1471-2164-13-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bai T.T., Xie W.B., Zhou P.P., Wu Z.L., Xiao W.C., Zhou L., Sun J., Ruan X.L., Li H.P., Zhang Z.G. Transcriptome and expression profile analysis of highly resistant and susceptible banana roots challenged with Fusarium oxysporum f. sp. cubense tropical race 4. PLoS ONE. 2013;8:e73945. doi: 10.1371/journal.pone.0073945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Hont A., Denoeud F., Aury J.M., Baurens F.C., Carreel F., Garsmeur O., Noei B., Bocs S., Droc G., Rouard M., et al. The banana (Musa acuminata) genome and the evolution of monocotyledonous plants. Nature. 2012;488:213–217. doi: 10.1038/nature11241. [DOI] [PubMed] [Google Scholar]
- 21.Smith M.K., Whiley A.W., Searle C., Langdon P.W., Schaffer B., Pegg K.G. Micropropagated bananas are more susceptible to Fusarium wilt than plants grown from conventional material. Aust. J. Agric. Res. 1998;49:1133–1139. [Google Scholar]
- 22.Afzal A.Z., Wood A.J., Lightfoot D.A. Plant receptor-like serine threonine kinases: Roles in signaling and plant defense. Mol. Plant Microbe Interact. 2008;21:507–517. doi: 10.1094/MPMI-21-5-0507. [DOI] [PubMed] [Google Scholar]
- 23.Mendy B., Wang’ombe M.W., Radakovic Z.S., Holbein J., Ilyas M., Chopra D., Holton N., Zipfel C., Grundler F.M., Siddique S. Arabidopsis leucine-rich repeat receptor-like kinase NILR1 is required for induction of innate immunity to parasitic nematodes. PLoS Pathog. 2017;13:e1006284. doi: 10.1371/journal.ppat.1006284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borassi C., Sede A.R., Mecchia M.A., Salgado Salter J.D., Marzol E., Muschietti J.P., Estevez J.M. An update on cell surface proteins containing extensin-motifs. J. Exp. Bot. 2016;67:477–487. doi: 10.1093/jxb/erv455. [DOI] [PubMed] [Google Scholar]
- 25.Jin J.H., Zhang H.X., Tan J.Y., Yan M.J., Li D.W., Khan A., Dong Z.H. A new ethylene-responsive factor CAPTI1 gene of pepper (Capsicum annuum L.) involved in the regulation of defense response to Phytophthora capsici. Front. Plant Sci. 2016;6:1217. doi: 10.3389/fpls.2015.01217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu L.J., Sonbol F.M., Huot B., Gu Y.N., Withers J., Mwimba M., Yao J., He S.Y., Dong X. Salicylic acid receptors activate jasmonic acid signalling through a non-canonical pathways to promote effector-triggered immunity. Nat. Commun. 2016;7:13099. doi: 10.1038/ncomms13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng H., Duan X., Zhang Q., Li X., Wang B., Huang L., Wang X., Kang Z. The target gene of tae-miR164, a novel NAC transcription factor from the NAM subfamily, negatively regulates resistance of wheat to stripe rust. Mol. Plant Pathol. 2014;15:284–296. doi: 10.1111/mpp.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen N., Wu S., Fu J., Cao B., Lei J., Chen C., Jiang J. Overexpression of the eggplant (Solanummelongena) NAC family transcription factor SmNAC suppresses resistance to bacterial wilt. Sci. Rep. 2016;6:31568. doi: 10.1038/srep31568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pandey S.P., Somssich I.E. The role of WRKY transcription factors in plant immunity. Plant Physiol. 2009;150:1648–1655. doi: 10.1104/pp.109.138990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Q.M., Venugopal S., Navarre D., Kachroo A. Low oleic acid-derived repression of jasmonic acid-inducible defense responses requires the WRKY50 and WRKY51 proteins. Plant Physiol. 2011;155:464–476. doi: 10.1104/pp.110.166876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thatcher L.F., Powell J.J., Aieken E.A., Kazan K., Manners J.M. The lateral organ boundaries domain transcription factor LBD20 functions in Fusarium wilt susceptibility and jasmonate signaling in Arabidopsis. Plant Physiol. 2012;160:407–418. doi: 10.1104/pp.112.199067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shahnejat-Bushehri S., Nobmann B., Devi Allu A., Balazadeh S. JUB1 suppresses Pseudomonas syringae-induced defense responses through accumulation of DELLA proteins. Plant Signal. Behav. 2016;11:e1181245. doi: 10.1080/15592324.2016.1181245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di X.T., Gomila J., Takken F.L.W. Involvement of salicylic acid, ethylene and jasmonic acid signalling pathways in the susceptibility of tomato to Fusarium oxysporum. Mol. Plant Pathol. 2017;18:1024–1035. doi: 10.1111/mpp.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang L., Shi C., Mu X.Y., Liu C., Shi K., Zhu W.J., Yang Q. Cloning and expression of a wild eggplant cytochrome P450 gene, StoCYP77A2, involved in plant resistance to Verticillium dahliae. Plant Biotechnol. Rep. 2015;9:167–177. doi: 10.1007/s11816-015-0355-6. [DOI] [Google Scholar]
- 35.Sels J., Mathys J., DeConinck B.M., Cammue B.P., DeBolle M.F. Plant pathogenesis-related (PR) proteins: A focus on PR peptides. Plant Physiol. Biochem. 2008;46:941–950. doi: 10.1016/j.plaphy.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Farrokhi N., Burton R.A., Brownfield L., Hrmova M., Wilson S.M., Bacic A., Fincher G.B. Plant cell wall biosynthesis: Genetic, biochemical and functional genomics approaches to the identification of key genes. Plant Biotechnol. J. 2006;4:145–167. doi: 10.1111/j.1467-7652.2005.00169.x. [DOI] [PubMed] [Google Scholar]
- 37.Pusztahelyi T., Holb I.J., Pócsi I. Secondary metabolites in fungus-plant interactions. Front. Plant Sci. 2015;6:573. doi: 10.3389/fpls.2015.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhattacharya A., Khanale V., Char B. Plant circadian rhythm in stress signaling. Ind. J. Plant Physiol. 2017;22:147–155. doi: 10.1007/s40502-017-0299-7. [DOI] [Google Scholar]
- 39.Wang W., Barnaby J.Y., Tada Y., Li H., Tör M., Caldelari D., Lee D.U., Fu X.D., Dong X. Timing of plant immune responses by a central circadian regulator. Nature. 2011;470:110–114. doi: 10.1038/nature09766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan G., Dong Y., Deng M., Zhao Z., Niu S., Xu E. Plant-pathogen interaction, circadian rhythm, and hormone-related gene expression provide indicators of phytoplasma infection in Paulownia fortunei. Int. J. Mol. Sci. 2014;15:23141–23162. doi: 10.3390/ijms151223141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seifi H., De Vleesschauwer D., Aziz A., Höfte M. Modulating plant primary amino acid metabolism as a necrotrophic virulence strategy: The immune-regulatory role of asparagine synthetase in Botrytis cinerea-tomato interaction. Plant Signal. Behav. 2014;9:e27995. doi: 10.4161/psb.27995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brauc S., De Vooght E., Claeys M., Höfte M., Angenon G. Influence of over-expression of cytosolic aspartate amino transferase on amino acid metabolism and defence responses against Botrytis cinerea infection in Arabidopsis thaliana. J. Plant Physiol. 2011;168:1813–1819. doi: 10.1016/j.jplph.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Chen H.B., Feng Q.R., Xu C.X., He R.X., Li J.G., Wang Z.H. Screening of banana clones for resistance to Fusarium wilt (Fusarium oxysporum f. sp. cubense) J. South China Agric. Univ. 2006;27:9–12. [Google Scholar]
- 44.Murashige T., Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 45.Xu C.X., Takáč T., Burbach C., Menzel D., Šamaj J. Developmental localization and the role of hydroxyproline rich glycoproteins during somatic embryogenesis of banana (Musa spp. AAA) BMC Plant Biol. 2011;11:38. doi: 10.1186/1471-2229-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vitha S., Baluška F., Braun M., Šamaj J., Volkmann D., Barlow P.W. Comparison of cryofixation and aldehyde fixation for plant actin immunocytochemistry: Aldehydes do not destroy F-actin. Histochem. J. 2000;32:457–466. doi: 10.1023/A:1004171431449. [DOI] [PubMed] [Google Scholar]
- 47.Marcus S.E., Verhertbruggen Y., Hervé C., Ordaz-Ortiz J.J., Farkas V., Pedersen H.L., Willats W.G.T., Knox J.P. Pectic homogalacturonan masks abundant sets of xyloglucan epitopes in plant cell walls. BMC Plant Biol. 2008;8:60. doi: 10.1186/1471-2229-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Götz S., García-Gómez J.M., Terol J., Williams T.D., Nagaraj S.H., Nueda M.J., Robles M., Talón M., Dopazo J., Conesa A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008;36:3420–3435. doi: 10.1093/nar/gkn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 [Delta] [Delta] CT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 50.Audic S., Claverie J.M. The significance of digital gene expression profiles. Genome Res. 1997;7:986–995. doi: 10.1101/gr.7.10.986. [DOI] [PubMed] [Google Scholar]
- 51.Dale J., James A., Paul J.Y., Khanna H., Smith M., Peraza-Echeverria S., Garcia-Bastidas F., Kema G., Waterhouse P., Mengersen K., et al. Transgenic Cavendish bananas with resistance to Fusarium wilt tropical race 4. Nat. Commun. 2017;8:1496. doi: 10.1038/s41467-017-01670-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.