Abstract
Background
Human lung cancer is still the leading cause of cancer-related mortality around the world, although a variety of new therapies have been used in the treatment of this disease. Antibody-drug conjugate (ADC) has revolutionized the field of cancer therapy in recent decades. Unlike traditional chemotherapy that damages the healthy cells, ADC first utilizes monoclonal antibodies to bind tumor-specific antigen targets and then deliver a highly potent cytotoxic agent to kill tumor cells. Thus, ADC can benefit cancer patients because this drug has less severe adverse effects.
Material/Methods
One type of ADC for non-small cell lung cancer (NSCLC) was designed in this study: Erbitux-vc-PAB-MMAE. It is a mouse/human chimeric monoclonal antibody, Erbitux, conjugating to the tubulin inhibitor auristatin. The efficacy of ADC was investigated through in vitro and in vivo studies.
Results
Our in vitro study demonstrated that Erbitux-vc-PAB-MMAE could effectively inhibit proliferation of human lung cancer A549 cells, and arrested cell cycle at G2/M phase. In a mouse xenograft model, the results indicated that Erbitux-vc-PAB-MMAE could be exactly delivered to tumor tissues, and effectively inhibited tumor growth via promoting apoptosis of cancer cells.
Conclusions
The antibody portion of an ADC drug (Erbitux) was used as a vector to bring the effector molecule (tubulin inhibitor MMAE) to the targeted tumor tissue. This antibody-drug conjugate can exert a strong anti-tumor effect.
MeSH Keywords: Antibodies, Monoclonal; Carcinoma, Non-Small-Cell Lung; Immunotoxins
Background
Lung cancer is the leading cause of cancer-related death in the world, and more than 1 million new cases are diagnosed per year. The economic burden increases year by year. Lung cancer is classified into 2 categories: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). The NSCLC patients account for 80% of all lung cancer patients. Because of the difficulty in early detection, most NSCLC patients have already reached advanced stage at diagnosis. The traditional chemotherapy has been used as primary therapy for treating advanced-stage NSCLC patients, but it has nonspecific toxicity and a narrow therapeutic window [1,2]. As more studies unveil the cancer cell biology, alternative targeted therapies (e.g., EGFR-TKI) can provide safer and more effective treatments compared with traditional chemotherapy. However, drug resistance is the new challenge to targeted therapies. Therefore, it is imperative to explore new therapy strategies for the treatment of NSCLC.
Antibody-drug conjugate (ADC) emerges as a promising class of anti-cancer drug, because it can specifically deliver drug to antigen-expressing tumor cells and kill tumor cells [3,4]. ADC has 3 components: a monoclonal antibody that specifically targets antigens expressed on the surface of tumor cells, a highly cytotoxic drug, and a stable cleavable or noncleanable linker. Monoclonal antibody can recognize and combine the specific antigens on the surface of tumor cells, and then ADC enters the tumor cells through cell phagocytosis. The highly cytotoxic drug is released to kill tumor cells after the linker is cleaved. This antibody-mediated drug delivery approach can specifically kill tumor cells, and also significantly expand the therapeutic window compared with the conventional chemotherapy. There are 2 types of ADCs are available in the market [5,6]: Kadcyla and Adcetris. Kadcyla was designed by conjugating a CD30-tageted antibody to a tubulin polymerization inhibitor monomethyl auristatin E. This ADC was approved for the treatment of relapsed or refractory Hodgkin’s lymphoma and systemic anaplastic large cell lymphoma. Adcetris was approved for treating HER2-positive breast cancer [7]. It is consisted of an antibody that targets human epidermal growth factor receptor 2 (HER2) and a tubulin polymerization inhibitor DM1. In addition, more than 40 types of ADCs are in the clinical trials stage now.
In this study, a new ADC (Erbitux-vc-PAB-MMAE) was designed. The monoclonal antibody part is Erbitux (Cetuximab). Cetuximab is a human/mouse chimeric monoclonal antibody that targets to epidermal growth factor receptor (EGFR). Thus, ADC can specifically target to NSCLC with EGFR gene mutation. The highly cytotoxic part is a tubulin inhibitor, monomethyl auristatin E (MMAE). The main mechanism of MMAE is to interfere with microtubules dissociation and polymerization of the tumor cells, so tumor cells cannot be normal stage for mitosis to proliferate. In this study, in vitro and in vivo studies were used to investigate the effectiveness of ADC (Erbitux-vc-PAB-MMAE) in inhibiting proliferation and promoting apoptosis of human lung cancer A549 cells.
Material and Methods
Cell lines and reagents
Human adenocarcinomic alveolar epithelial cell line A549 cells were from BOSTER (Hubei, China). Human lung cancer A549 cell line was grown in cell culture medium with DEME high-sugar medium (mixture of 10% fetal bovine serum and 1% streptomycin) (Biological Industries, Israel), and the control cell line SK-LU-1 (HTB-57) was from ATCC (USA), and cultured in a constant-temperature incubator (5% CO2, 37°C).
Generation of Erbitux-vc-PAB-MMAE
Erbitux (Lilly, USA) was conjugated to MC-vc-PAB-MMAE (Concortis Biosystem, Inc, USA) at 4.28: 1 drug-antibody ratio. Erbitux-vc-PAB-MMAE was verified by Hydrophobic Interaction Chromatography (HIC) using an Agilent 1100 HPLC system (Agilent Technologies, USA).
Analysis of cell proliferation
Cell proliferation was determined by using the Cell Counting Kit-8, CCK-8 (Transgen biotech, Beijing, China). Briefly, 5×103 A549 cells/well were seeded in a 96-well plate for 24 h, and then treated with 3 concentrations of epidermal growth factor receptor (EGFR) monoclonal antibody conjugate tubulin inhibitor (ADC), Cetuximab (Lilly, USA), and Cisplatin (Qilu Pharma, China) (Table 1). CCK-8 assay was performed according to the manufacturer’s instructions. All experiments were performed at least 3 times. The cell inhibition rate was calculated according to the OD value of each well. The inhibition rate formula is (average D450 of control group5D450 of experimental group)/D450 of control group×100%.
Table 1.
The inhibition rates of different concentrations of Cetuximab-conjugated tubulin inhibitor (ADC), Cetuximab, and Cisplatin on human lung cancer A549 cells (mean ±SD).
| Drug | Cell line | Concentration | Inhibition rate (24 hours) | Inhibition rate (48 hours) |
|---|---|---|---|---|
| ADC | A549 | 0.0089 mmol/L | 79.73±2.0** | 84.50±1.56**,# |
| 0.0178 mmol/L | 83.96±0.82* | 89.14±3.48* | ||
| 0.0267 mmol/L | 89.64±3.72* | 92.78±1.46 | ||
| SK-LU-1 | 0.0267 mmol/L | 8.68±3.98 | 10.18±1.78 | |
| Cetuximab | A549 | 0.0045 mmol/L | 15.63±0.93 | 25.74±0.66 |
| 0.0089 mmol/L | 23.51±1.05* | 27.92±1.13* | ||
| 0.0134 mmol/L | 27.16±2.60* | 28.13±1.16 | ||
| SK-LU-1 | 0.0134 mmol/L | 12.78±1.06 | 15.98±1.87 | |
| Cisplatin | A549 | 0.8867 mmol/L | 75.94±1.68 | 81.32±2.05 |
| 1.3300 mmol/L | 84.96±1.66# | 87.65±1.42# | ||
| 1.7734 mmol/L | 86.11±1.54 | 88.71±1.2 | ||
| SK-LU-1 | 1.7734 mmol/L | 81.78±1.51 | 87.54±1.09 |
P values compared among different concentrations of ADC or Cetuximab:
P<0.05;
P<0.01.
P values compared among different concentrations of ADC or Cisplatin:
P<0.05.
Analysis of cell cycle
Propidium iodide (PI) staining was applied to the analysis of cell cycle distribution through flow cytometry as previously described. Briefly, cells were cultured in a 25 cm2 flask for 24 h, then exposed to various concentrations of Erbitux-vc-PAB-MMAE (0.12, 0.24 mg/ml), Cetuximab (0.06, 0.12 mg/ml), Cisplatin (0.0125, 0.025 mg/ml), and negative control for 24 h. Cells were subsequently harvested with trypsin, collected by centrifugation (1000 rpm for 5 min), and washed once with cold PBS. The pellet was incubated with 1.8 ml (1 mg/ml) propidium iodide (PI, Solarbio, Beijing, China) in the dark at 4°C. Subsequently, cells were centrifuged and washed again, and then samples were analyzed using a BD Accuri C6 device (Becton Dickinson, CA, USA). Data were analyzed with the ModFit DNA analysis program.
Mouse xenograft tumor model and ADC treatment
All experiments using mice were approved by The First Affiliated Hospital of Nanchang University’s Institutional Review Board. Twenty female athymic nude mice (ages 4–6 weeks) (SJA Lab animal, Hunan, China) were raised in a specific pathogen-free IVC animal house. Mice were injected with 2×106 A549 cells in the subcutaneous space of the left flank. The long diameter and short diameter of tumors were evaluated using a vernier caliper. Tumor volume size was calculated according to the formula: volume=ab2/2 (a=long diameter, b=short diameter). Treatment began when tumors reaches a mean tumor volume of 300~500 mm3. Mice were randomized into 4 groups (5 mice per group): ADC group, Cetuximab group, Cisplatin group, and control group. Each treatment was based on the body surface area. The nude mouse body surface area formula is 0.0913×(body weight)2/3, and average body weight of nude mice is about 20 g. According to the concentration of the required dose of the corresponding drug, a disposable sterile insulin needle was used to extract the appropriate dose and inject it into the nude mice tail vein. The mice were injected with 300 μl of ADC (250 mg/m2), 400 ul of Cetuximab (250 mg/m2), 200 ul Cisplatin (120mg/m2), and 300 ul of 0.9% sodium chloride solution (control group), individually. Nude mice in each group were distinguished by a mark on the ear. After injection of the corresponding intervention drug into the tail vein, long and short diameters of the nude mouse subcutaneous tumors were measured every 3 days (a total of 8 times) using vernier caliper, and were used to calculate the corresponding tumor size. On the 21st day, the tumor tissue was removed from nude mice using spinal cord dislocation method, and fixed with 10% formalin before proceeding to the next step.
Apoptosis assay
Tumor cells apoptosis was investigated by Terminal deoxynucleotidyl transferase (TdT) dUTP Nick-End Labeling (TUNEL) assay. Briefly, tumor tissues were collected and prepared as paraffin sections. Then, TUNEL assay was carried out following the TUNEL Assay Kit (Keygenbio, Nanjing, China) manufacturer’s instructions. Images of tissues were taken using an Olympus IX71 microscope at a magnification of 200×. The ratios of apoptosis were examined by counting positively stained cells in 4 different randomly selected visual fields. The formula for apoptotic rate was positive number/total number×100%).
ELISA analysis
Mice were treated for 1 week. Serum and tumor tissues were collected for MMAE detection using ELISA. The procedure was performed according to the ELISA kit protocol (Epitope Diagnostics inc, San Diego, CA, USA). MMAE concentration in the samples was determined through the use of standard dose-response curves.
Statistical analysis
All data were analyzed with SPSS 22.0 statistics software (Chicago, USA). The data are shown as the means ± standard error of the mean (SEM). Significant differences between more than 2 groups were determined using one-way analysis of variance (ANOVA). Comparisons between 2 groups were made using the 2-tailed unpaired t test (* P<0.05; ** P<0.01).
Results
The cell growth inhibition rate results
The inhibition rates of different concentrations of Cetuximab-conjugated tubulin inhibitor (ADC), Cetuximab, and Cisplatin on human lung cancer A549 cells are shown in Table 1. ADC had an obvious inhibitory effect on the A549 cells at all doses. Cetuximab had some degree of inhibitory effect on A549 cells. For ADC and Cetuximab, the cell inhibition rate increased with increased concentration at 24 h, and the difference was statistically significant (P<0.05) (e.g., 0.0178 mmol/L ADC vs. 0.0089 mmol/L ADC, and 0.0267 mmol/L ADC vs. 0.0178 mmol/L ADC). At 48 h, the cell inhibition rate increased along with increased dose in low and medium volume (P<0.05) (e.g., 0.0178 mmol/L ADC vs. 0.0089 mmol/L ADC, and 0.0089 mmol/L Cetuximab vs. 0.0045 mmol/L Cetuximab). The inhibition rates with 0.0089 mmol/L ADC vs. 0.0089 mmol/L Cetuximab were significantly different (P<0.01) at 24 h and 48 h, individually. The result show that the effect of ADC is clearly stronger than that of Cetuximab at the same molar concentrations.
Cisplatin also had a significant inhibitory effect on the A549 cells at all concentrations. The cell inhibition rate significantly increased with increased concentration from low to medium (e.g., 1.3300 mmol/L Cisplatin vs. 0.8867 mmol/L Cisplatin) at 24 h and 48 h (P<0.05), individually. There was no significant difference between the inhibition rates in the 0.0089 mmol/L ADC group and 0.8867 mmol/L Cisplatin group (ADC molarity is 1% that of Cisplatin) at 24 h (P>0.05). However, a significant difference was observed between these 2 groups at 48 h. The results indicate that the effect of ADC is significantly better than that of Cisplatin at the same molar concentration.
Effects of ADC, Cetuximab, and Cisplatin on A549 cells were compared with the control cell line (SK-LU-1), a well-defined lung cancer cell line with non-alveolar epithelial origin. Cisplatin showed the highest inhibition rate, and ADC had the lowest inhibition rate compared with Cetuximab and Cisplatin (Table 1). Further, ADC, Cetuximab, and Cisplatin showed no inhibition effects on the linker of MC-vc-PAB-MMAE (data not shown). The effect of ADC was better than that of Cetuximab. ADC was better than Cisplatin at the same molarity.
Cell cycle results
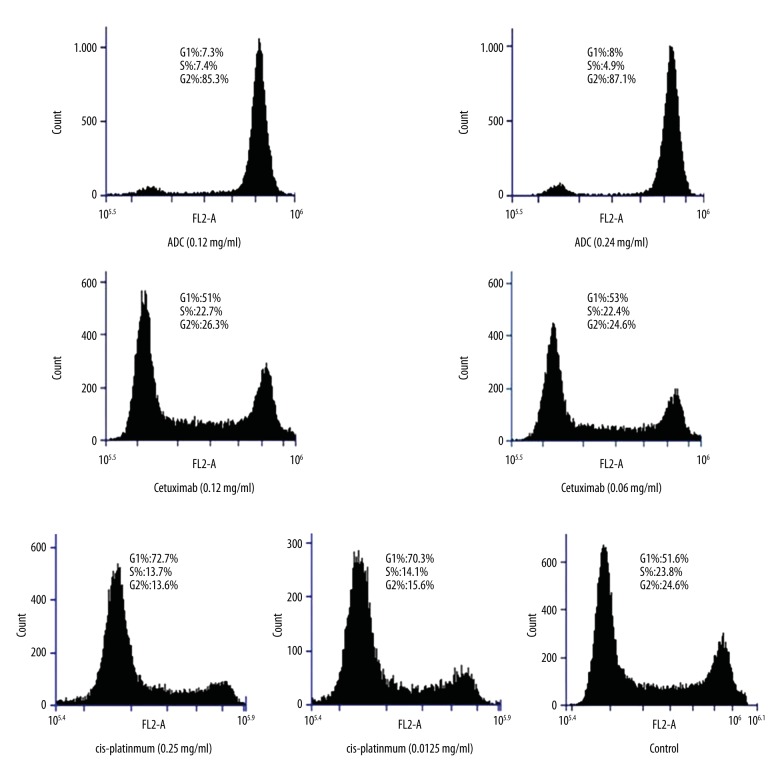
The results of cell cycle experiments of ADC, Cetuximab, and Cisplatin in all concentration groups are shown in Table 2 and Figure 1. Under the effect of ADC, G2/M phase significantly increased and the G1 and S phase cell was reduced compared to the control group; significant differences were observed (P<0.01), suggesting the retardation of the ADC in G2/M phase. G1 phase cell was dominant under different concentrations of Cetuximab, and G1, S, and G2 had no obvious difference compared with control groups (P>0.05). The Cisplatin group was compared with control groups with different concentration cell periods, showing that G1-phase cells increased obviously, but S-phase and G2-phase cells decreased (P<0.05), suggesting the retardation of G1 phase. There were no significant differences observed between different concentrations of the same drug, suggesting that the concentration has no effect on retardation of cell cycle. The dominant cells were different between the same concentrations of ADC and Cetuximab (0.0134 mmol/L) (P<0.01), suggesting that the retardation cycles of ADC and Cetuximab are different. Significant differences were observed (P<0.01) between 0.0134 mmol/L ADC and 1.33 mmol/L Cisplatin (ADC molarity about 0.01 of Cisplatin), suggesting that ADC and Cisplatin block the cell cycle in different stages. In the control cell line of SK-LU-1, the effects of ADC, Cetuximab, and Cisplatin indicated similar patterns.
Table 2.
The results of cell cycle experiments of ADC, Cetuximab and Cisplatin in each concentration group (mean ±SD).
| Drug | Cell line | Cell cycle | ||
|---|---|---|---|---|
| G1 | S | G2/M | ||
| ADC (0.0134 mmol/L) | A549 | 7.3±0.51** | 7.4±0.65** | 85.3±1.02** |
| ADC (0.0267 mmol/L) | 8.0±0.56** | 4.9±0.86* | 87.1±1.41** | |
| ADC (0.0267 mmol/L) | SK-LU-1 | 2.0±0.98 | 3.9±0.35 | 94.1±1.88 |
| Cetuximab (0.0067 mmol/L) | A549 | 53.0±0.78 | 22.4±0.50 | 24.6±0.67 |
| Cetuximab (0.0134 mmol/L) | 51.0±0.57 | 22.7±0.08 | 26.3±0.51 | |
| Cetuximab (0.0134 mmol/L) | SK-LU-1 | 41.2±0.96 | 20.3±0.80 | 38.5±1.40 |
| Cisplatin (1.33 mmol/L) | A549 | 70.3±0.88** | 14.1±0.37** | 15.6±0.73* |
| Cisplatin (2.66 mmol/L) | 72.7±1.12** | 13.7±0.41** | 13.6±0.73* | |
| Cisplatin (2.66 mmol/L) | SK-LU-1 | 71.6±0.98 | 12.9±0.96 | 15.5±1.01 |
| Control | A549 | 51.6±0.86 | 23.8±0.50 | 24.6±0.80 |
P values compared with control group.
P<0.05;
P<0.01.
Figure 1.
The cell cycle results with different concentrations of ADC, Cetuximab, and Cisplatin. Most cells in the control group are in G1 phase. In ADC groups (0.0134 mmol/L and 0.0267 mmol/L), most cells are in G2/M phase. In Cetuximab groups (0.0067 mmol/L and 0.0134 mmol/L) and Cisplatin groups (1.33 mmol/L and 2.66 mmol/L), most cells are in G1 phase.
The growth of lung cancer xenografts
As shown in Table 3, before administration and 3 and 6 days after the administration of drugs, the pairwise comparisons of tumor sizes showed that the difference was not statistically significant compared with the control group (P>0.05) (Supplementary Figure 1). After 9 days after drug administration, the differences between the ADC group and Cisplatin group, and between the ADC group and control group, were statistically significant (P<0.05), while the difference between the Cetuximab group and control group were still not statistically significant (P>0.05). From 12 days after drug administration, the ADC group and Cisplatin group showed significant differences when compared to the control group (P<0.05). At 9, 12, and 15 days of after drug administration, ADC group and Cisplatin group showed statistically significant difference (P<0.05). Statistical differences were not observed at the remaining days (18 and 21 days) between these 2 groups.
Table 3.
The average tumor sizes (mm3, n=5) of each group before and after administration of drugs (mean ±SD).
| Day | ADC group | Cetuximab group | Cisplatin group | Control group |
|---|---|---|---|---|
| 0 | 427.60±21.85 | 400.67±23.15 | 423.76±29.33 | 410.29±26.08 |
| 3 | 446.83±64.67 | 423.87±50.38 | 408.45±87.66 | 437.53±67.43 |
| 6 | 409.14±71.51 | 424.36±27.58 | 417.23±56.17 | 473.36±84.66 |
| 9 | 373.32±69.09*,# | 516.54±33.14 | 461.55±81.54 | 525.60±54.76 |
| 12 | 298.20±90.07*,# | 580.78±32.50 | 474.26±58.93* | 589.26±83.62 |
| 15 | 330.50±102.54*,# | 607.22±43.83 | 511.90±78.59* | 621.38±74.44 |
| 18 | 427.34±97.07* | 670.82±70.69 | 513.89±75.26* | 708.39±76.93 |
| 21 | 467.24±97.71* | 740.97±59.70 | 541.42±104.06* | 796.48±56.69 |
Means the P values of between each group and control group are less than 0.05, statistical differences were observed.
Means the P values of between each group and Cisplatin group are less than 0.05, statistical differences were observed.
As shown in Figure 2, with the increasing number of days after drug administration, the tumor sizes in the ADC group and Cisplatin group were significantly smaller than in the Cetuximab group and the control group. Especially, the tumor size change of ADC group was more obvious than in the Cisplatin group, while the tumor sizes in the Cetuximab group and the control group increased significantly, and there were no significant differences between these 2 groups.
Figure 2.
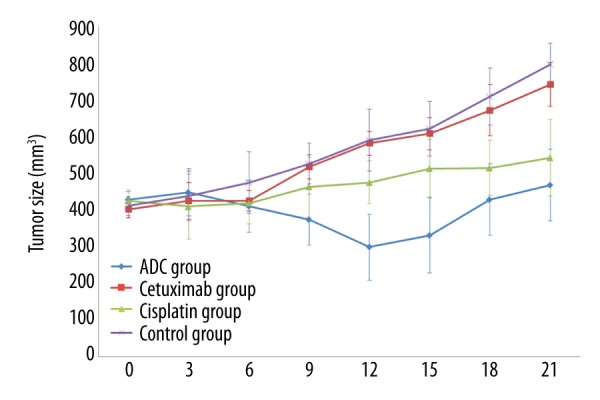
The average tumor sizes (mean ±SD) of each group before and after administration of drugs (mm3, n=5).
Apoptosis assay
As shown in Table 4, the difference was statistically significant when ADC group or Cisplatin group were compared with Cetuximab group and control group (P<0.05). However, the P values between the Cetuximab group and control group, and between the ADC group and Cisplatin group, were higher than 0.05, indicating no statistically significant differences.
Table 4.
The average cell apoptosis rates of fours group (n=5, mean ±SD).
| Group | Apoptosis rate (%) |
|---|---|
| ADC | 8.35±1.43*,# |
| Cetuximab | 0.99±0.76 |
| Cisplatin | 8.04±1.77*,# |
| Control | 1.11±0.81 |
Means the P values of between each group and control group are less than 0.05, statistical differences were observed.
Means the P values of between each group and Cetuximab group are less than 0.05, statistical differences were observed.
ELISA analysis
According to the standard concentration curve, the concentrations of all samples were calculated and listed in Table 5. The MMAE concentrations of the tissue samples were around 1 ug/ml, while the MMAE concentrations of serum samples were negative or significantly lower than the corresponding tissue sample concentrations. This supports that the ADC drugs can go through the blood circulation to exert an anti-tumor effect and stay stable in the plasma, further confirming the ADC drug targeting effect.
Table 5.
The average MMAE concentrations in various tissue samples (n=2).
| Sample | OD value | Concentration (ug/ml) |
|---|---|---|
| Tissue1 | 2.176 | 0.729 |
| Tissue2 | 2.159 | 0.759 |
| Tissue3 | 1.880 | 1.260 |
| Serum1 | 2.909 | −0.586 |
| Serum2 | 2.836 | −0.445 |
| Serum3 | 2.477 | 0.189 |
Discussion
Antibody-drug conjugate (ADC) utilizes monoclonal antibodies (MAb) that specifically binds to tumor-associated antigen. ADC conjugates highly potent cytotoxic agents through a stable linker, and form an extremely effective anti-cancer drug. ADC’s linker has important influence on the safety and efficacy of ADC. The linker should be stable in plasma and around tumor cells to avoid the early release of cytotoxins and rapid release of cytotoxic after endocytosis to the target cells. Unlike traditional therapeutic treatments [8–10], ADC binds to target antigens and mediates endocytosis through receptors, which lead to release of cytotoxin substances and cancer cell apoptosis. ADC does not kill normal cells because the monoclonal antibody of ADC cannot bind to normal cells without epidermal growth factor receptor (EGFR) expression. In our experiment, the monoclonal antibody of ADC is Cetuximab, which is highly specific to EGFR and is the most clinically advanced human/mouse chimeric IgG1 monoclonal antibody. Cetuximab has stronger affinity with EGFR than EGF or transforming growth factor alpha (TGFa), blocking its binding with EGFR, and thus cannot activate a series of downstream signaling pathways to avoid the tumor. In this experiment, the effector molecule of ADC is MMAE. MMAE is a depolymerization inhibitor of microtubules, the mechanism is that MMAE inhibits its polymerization by binding with tubulin and interferes with cell mitotic process, resulting in cell cycle arrest and apoptosis induction. MMAE mainly interferes with cell mitosis in M phase, so MMAE inhibits cells in G2/M phase [11]. Cytotoxic drugs have great value in cancer chemotherapy due to their high tumor cell killing efficiency. However, the nonspecific toxicity of cytotoxic drugs also results in no differential killing of normal tissue cells. ADC can delivery cytotoxic drugs to target cells through specific antibodies and then release cytotoxic drugs that reduces the adverse effects. The efficacy of ADC was compared with Cetuximab and Cisplatin in in vitro and in vivo studies. Cetuximab is a chimeric (mouse/human) monoclonal antibody that binds to and inhibits EGFR. Cisplatin is used to treat a number of cancers, and the mechanism is to interfere with DNA replication, which kills the fastest-proliferating cells.
Some in vitro and in vivo studies have already been conducted to investigate the effectiveness of ADC [9,12–14]. In the present study, the in vivo and in vitro experiments confirmed that the antibody portion of an ADC drug (Cetuximab) served as a vector for the effector molecule (tubulin inhibitor MMAE) to bring MMAE to the targeted tumor tissue. The small molecule cytotoxic cytokines play use high cytotoxicity to kill local cells, while monoclonal antibodies can also maintain their own antibody-dependent cytotoxicity (ADCC) or Fc-mediated complement-dependent cytotoxicity (CDC) to exert their own effects [15]. This method combines their strengths and complements their weaknesses, and they can exert a strong anti-tumor effect, reduce damage to normal tissues and cells, and also the antibody resistance problem [16].
Conclusions
The epidermal growth factor receptor (EGFR) monoclonal antibody conjugate tubulin has an obvious inhibitory effect on human lung cancer A549 cells in vitro, and the effect is better than with Cetuximab and Cisplatin. The (EGFR) monoclonal antibody conjugate tubulin is in G2/M phase retardation, and the retardation cell cycle is different from that of Cetuximab and Cisplatin. Cetuximab-conjugated tubulin inhibitor can significantly inhibit the growth of lung cancer xenografts compared with the control group and Cetuximab group, and also has better inhibition compared with the Cisplatin group. The apoptosis rate in the Cetuximab-conjugated tubulin inhibitor group and the Cisplatin group were higher than in the other 2 groups. Cetuximab-conjugated tubulin inhibition can be targeted into the lung cancer tissue after intravenous injection. Cetuximab-conjugated tubulin inhibition can be targeted into A549 human lung cancer xenografts and has obvious cytotoxicity-related inhibition of lung cancer.
The findings in this study support that ADC can be used to treat NSCLC in the future. However, there are still some questions that need to be addressed. First, more studies are needed to detect cell cycle changes and to explore the apoptosis mechanism. Second, the efficacy and drug resistance of Cetuximab-conjugated tubulin inhibitor after repeated administration need to be investigated. Furthermore, the maximum dosage and adverse effects of Cetuximab-conjugated tubulin inhibitor also should be determined before its application in NSCLS treatment.
Statement
The opinions, findings, and conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect those of the Jiangxi Provincial Education Department.
Supplementary Figure
The tumor size of nude mice before and after drug administration. (A) The tumor size of ADC group before drug administration; (B) The tumor size of ADC group after 21 days drug administration; (C) The tumor size of Cisplatin group before drug administration; (D) The tumor size of Cisplatin group after 21 days drug administration; (E) The tumor size of Cetuximab group before drug administration; (F) The tumor size of Cetuximab group after 21 days drug administration; (G) The tumor size of Control group before drug administration; (H) The tumor size of Control group after 21 days drug administration.
Footnotes
Source of support: This work was supported by award no. 2016-GJJ160244, awarded by the Jiangxi Provincial Education Department Youth Project, Jiangxi, China and Science an technology plan of Jiangxi Provincial Health Department 20165184
References
- 1.Cheng H, Perez-Soler R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol. 2018;19:e43–e55. doi: 10.1016/S1470-2045(17)30689-7. [DOI] [PubMed] [Google Scholar]
- 2.Cho JH. Immunotherapy for non-small-cell lung cancer: Current status and future obstacles. Immune Netw. 2017;17:378–91. doi: 10.4110/in.2017.17.6.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moek KL, de Groot DJA, de Vries EGE, Fehrmann RSN. The antibody-drug conjugate target landscape across a broad range of tumour types. Ann Oncol. 2017;28:3083–91. doi: 10.1093/annonc/mdx541. [DOI] [PubMed] [Google Scholar]
- 4.Willuda J, Linden L, Lerchen HG, et al. Preclinical antitumor efficacy of BAY 1129980-a novel auristatin-based anti-C4.4A (LYPD3) antibody-drug conjugate for the treatment of non-small cell lung cancer. Mol Cancer Ther. 2017;16:893–904. doi: 10.1158/1535-7163.MCT-16-0474. [DOI] [PubMed] [Google Scholar]
- 5.Kaplon H, Reichert JM. Antibodies to watch in 2018. MAbs; 2018. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heist RS, Guarino MJ, Masters G, et al. Therapy of advanced non-small-cell lung cancer with an SN-38-anti-trop-2 drug conjugate, sacituzumab govitecan. J Clin Oncol. 2017;35:2790–97. doi: 10.1200/JCO.2016.72.1894. [DOI] [PubMed] [Google Scholar]
- 7.Hotta K, Aoe K, Kozuki T, et al. A phase II study of trastuzumab emtansine in HER2-positive non-small-cell lung cancer. J Thorac Oncol. 2018;13(2):273–79. doi: 10.1016/j.jtho.2017.10.032. [DOI] [PubMed] [Google Scholar]
- 8.Nasiri H, Valedkarimi Z, Aghebati-Maleki L, Majidi J. Antibody-drug conjugates: Promising and efficient tools for targeted cancer therapy. J Cell Physiol. 2018 doi: 10.1002/jcp.26435. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Wang R, Li L, Zhang S, et al. A novel enediyne-integrated antibody-drug conjugate shows promising anti-tumor efficacy against CD30+ lymphomas. Mol Oncol. 2018 doi: 10.1002/1878-0261.12166. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katoh M. Antibody-drug conjugate targeting protein tyrosine kinase 7, a receptor tyrosine kinase-like molecule involved in WNT and vascular endothelial growth factor signaling: effects on cancer stem cells, tumor microenvironment and whole-body homeostasis. Ann Transl Med. 2017;5:462. doi: 10.21037/atm.2017.09.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin K, Rubinfeld B, Zhang C, et al. Preclinical development of an anti-NaPi2b (SLC34A2) antibody-drug conjugate as a therapeutic for non-small cell lung and ovarian cancers. Clin Cancer Res. 2015;21:5139–50. doi: 10.1158/1078-0432.CCR-14-3383. [DOI] [PubMed] [Google Scholar]
- 12.Zhao H, Atkinson J, Gulesserian S, et al. Modulation of macropinocytosis-mediated internalization decreases ocular toxicity of antibody-drug conjugates. Cancer Res. 2018 doi: 10.1158/0008-5472.CAN-17-3202. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Sussman D, Westendorf L, Meyer DW, et al. Engineered cysteine antibodies: An improved antibody-drug conjugate platform with a novel mechanism of drug-linker stability. Protein Eng Des Sel. 2018;31(2):47–54. doi: 10.1093/protein/gzx067. [DOI] [PubMed] [Google Scholar]
- 14.Adumeau P, Vivier D, Sharma SK, et al. Site-specifically labeled antibody-drug conjugate for simultaneous therapy and immunoPET. Mol Pharm. 2018 doi: 10.1021/acs.molpharmaceut.7b00802. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trotta AM, Ottaiano A, Romano C, et al. Prospective evaluation of cetuximab-mediated antibody-dependent cell cytotoxicity in metastatic colorectal cancer patients predicts treatment efficacy. Cancer Immunol Res. 2016;4:366–74. doi: 10.1158/2326-6066.CIR-15-0184. [DOI] [PubMed] [Google Scholar]
- 16.Weiss E, Ford JC, Olsen KM, et al. Apparent diffusion coefficient (ADC) change on repeated diffusion-weighted magnetic resonance imaging during radiochemotherapy for non-small cell lung cancer: A pilot study. Lung Cancer. 2016;96:113–19. doi: 10.1016/j.lungcan.2016.04.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The tumor size of nude mice before and after drug administration. (A) The tumor size of ADC group before drug administration; (B) The tumor size of ADC group after 21 days drug administration; (C) The tumor size of Cisplatin group before drug administration; (D) The tumor size of Cisplatin group after 21 days drug administration; (E) The tumor size of Cetuximab group before drug administration; (F) The tumor size of Cetuximab group after 21 days drug administration; (G) The tumor size of Control group before drug administration; (H) The tumor size of Control group after 21 days drug administration.