Abstract
Mycotoxins are the toxic secondary metabolites produced by fungi and they are a worldwide public health concern. A VHH antibody (or nanobody) is the smallest antigen binding entity and is produced by heavy chain only antibodies. Compared with conventional antibodies, VHH antibodies overcome many pitfalls typically encountered in clinical therapeutics and immunodiagnostics. Likewise, VHH antibodies are particularly useful for monitoring mycotoxins in food and feedstuffs, as they are easily genetic engineered and have superior stability. In this review, we summarize the efforts to produce anti-mycotoxins VHH antibodies and associated assays, presenting VHH as a potential tool in mycotoxin analysis.
Keywords: mycotoxin, VHH antibody, immunoassay
1. Introduction
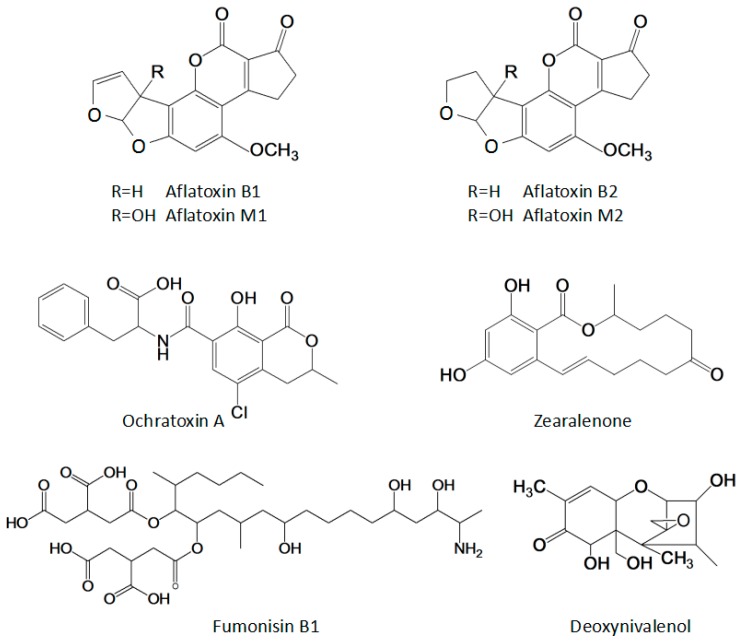
Mycotoxins are the secondary metabolites naturally produced by fungi that colonize crops. Among more than 400 mycotoxins are discovered so far, Aflatoxins (AFTs), ochratoxins (OTs), fumonisins (FBs), deoxynivalenol (DON) and zearalenone (ZEN) are gaining attention due to their frequent occurrence and high toxicity in animals and humans (Figure 1) [1]. The maximum allowable limits for these mycotoxins have been set by national and international institutions and organizations [2,3]. The European Commission set maximum limits in unprocessed cereal for AFB1 at 2 µg/kg, OTA at 5 µg/kg, FB at 4000 µg/kg, DON at 1250 µg/kg and ZEN at 100 µg/kg [3]. Thus, sensitive analytical methods are used to identify and quantify them, including high-performance liquid chromatography (HPLC) with mass spectrometryc (MS) [4,5,6,7,8] and some nano-sensor technologies [9,10,11]. Among them, immunoassays based on specific interactions between antibodies and antigens are the most useful tools for rapidly monitoring of mycotoxins [12]. These assays have a number of advantages, including high selectivity toward the target analytes, greater than or comparable sensitivity to the instrumental methods and ease of operation and high-throughput screening ability [13] for mycotoxins in agricultural foodstuffs.
Figure 1.
Molecular structures of the main mycotoxins.
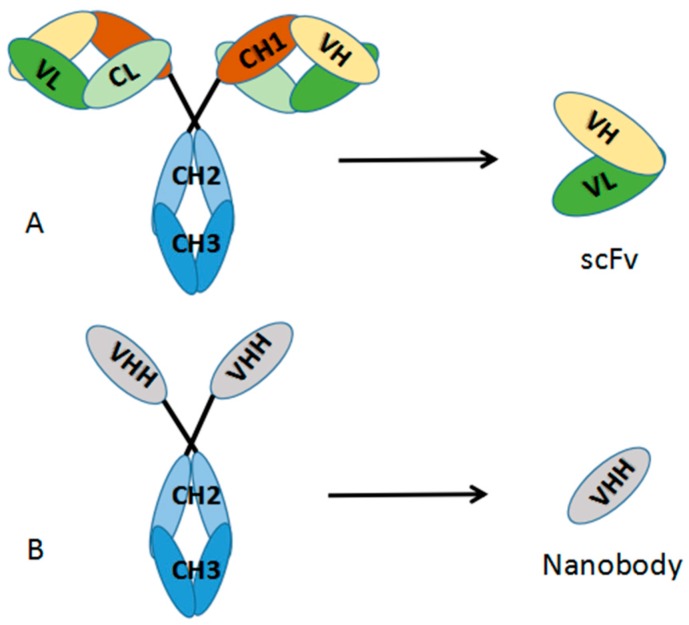
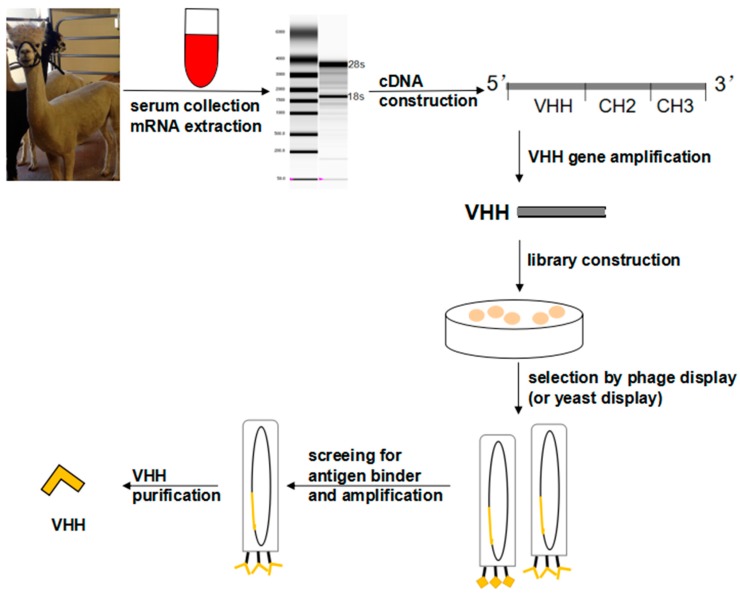
Antibodies are the core reagents for immunoassays. In the serum of camelidae family and cartilaginous fish, heavy chain only antibodies (HcAbs) are existed devoid of light chains and lacking the CH1 domains (Figure 2) [14,15] The variable domain of HcAbs, which binds to specific antigens, is referred to as VHH. These unique antibody fragments are the smallest entities of the antigen-antibody interaction, so they are also called nanobodies [16]. VHH production is summarized in Figure 3 [17]. Compared with conventional antibodies (polyclonal antibodies [pAbs] and monoclonal antibodies [mAbs]), the VHHs are able to recognize the active sites of the antigen, which are inaccessible or cryptic for pAbs and mAbs [18] and they can be isolated and produced in expression systems (e.g., phage, yeast & ribosome selection systems) [19,20] with higher antibody yields. Compared with single-chain variable fragments antibodies (scFvs), the VHHs have greater or comparable sensitivity; can be produced in large quantities; are easily manipulated genetically; and have superior physicochemical stability and high refolding capacity [21,22], such as excellent solubility and resilience to organic solvents and high temperature [23,24,25]. The lower molecular weight (15 kD, 1/10 of conventional antibody), the single domain nature of VHHs and the evolutionary replacement of the light chain (VL) for antigen binding are compensated for by sequence heterogeneity in CDR regions and greater topological diversity in paratopes [26,27]. An naturally occurring disulfide bond between cysteine residues exits in VHHs and contributes largely to their conformational and thermal stabilities, without affecting antigen-antibody binding abilities [28]. Although VHH antibodies are also promising reagents for detecting small chemicals (MW < 1500 Da) in environmental monitoring [25] (e.g., picloram [29], triclocarban [30], 3-phenoxybenzoic acid [23], brominated diphenyl ether-47 [31] and tetrabromobisphenol A [24]), in the current review, we will focus only on the development and optimization of immunoassays for monitoring mycotoxins. In these cases, VHHs are used as antigen-binding entities, anti-idiotypic antibodies and recombinant proteins.
Figure 2.
Schematic presentation of a (A) conventional antibody (IgG) and (B) heavy chain antibody, with their antigen-binding fragments (A) scFv and (B) VHH.
Figure 3.
Overview of VHH generation from camelid.
2. Anti-Hapten VHH-Based Immunoassay
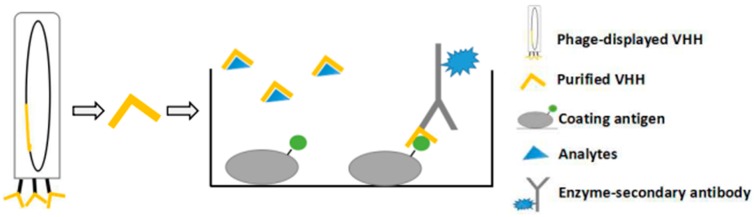
Generally, immunoassays that detect mycotoxins are based on a competitive assay, typically in direct or indirect format. Take the indirect format as an example, the solid phase is sensitized by coating antigen, followed by incubating with test analytes and anti-hapten antibodies. The antibodies bound to the coating antigen are measured and this determines the amount of analyte present [32] (Figure 4). The VHHs, function as binding with analytes preferred to be isolated mostly from an antigen-immunized VHH library. The anti-hapten VHHs are selected from phage displayed VHH fragment libraries, with a sequential competition strategy [33]. The immunogen is synthesized chemically, attached to a carrier protein and injected subcutaneously; three to five injections are used to boost the immune reaction. RNA is isolated from the blood after the last immunization. The first strand of cDNA was synthesized and the VHH domain is amplified and ligated into the expression system to construct the library [34]. Phage display technology is a preferable expression system, with the foreign nucleotide sequence encoded by the virion and foreign peptides or proteins expressed on its surface [35]. VHHs can be easily isolated, enriched, expressed and purified from phage display VHH library (Figure 4) [36]. The VHHs can be expressed and purified in columns. With phage display technology, the displayed VHH and phage DNA can be used directly as the detection antibody and DNA template in phage display-mediated immune-polymerase chain reaction (PD-IPCR), a technique with ultra-sensitivity. In PD-IPCR, the SYBR premix is added into each reaction and the fluorescent signal is detected during PCR amplification. The procedure is the same as the indirect immunoassay, except the VHH phage supernatant is used in place of the primary antibody.
Figure 4.
VHH production and anti-hapten VHH-based immunoassay. Coating antigen and analytes competitively bind to primary antibody, followed by the enzyme-secondary antibody and enzyme substrates. A weaker signal indicates a higher analyte concentration.
A phage displayed OTA-specific VHH, obtained from a phage displayed VHH library with OTA protein as immunogen in alpaca, was employed to develop a competitive indirect ELISA [37,38]. Then, a competitive real-time PD-IPCR for OTA was developed. The detection level of this method was 3.7 pg/L, with a linear range of 0.01–1000 pg/mL [39]. A His-tag can help anti-OTA VHH be purified on a nickel sepharose column with high purity. With the competing antigen OTA-BSA on a polyvinylidene fluoride membrane, a VHH-based dot ELISA was developed and the color intensity of the test squares was determined either visually or by scanning and quantitative analysis with densitometry. The cut-off level of this method was 5 µg/kg visually for OTA, with weak cross-reactivity with OTB and no cross-reactivity with DON, AFB1 and ZEN [40].
Anti-AFB1 VHH was also displayed in phage and purified by nickel sepharose column [41]. The magnetic-bead-carrying poly (acrylic acid) brushes (MB@PAA) were fabricated with anti-AFB1 VHH for improving AFB1 adsorption capacity. The MB@PAA@VHH exhibited good AFB1 adsorption (0.23 mg/g) and reusability with AFB1-spiked corn samples [42]. VHHs show much better tolerance to high temperature and organic solvents than mAbs. During exposure to 95 °C for 5 min, mAbs lost their antigen-binding abilities, while VHHs did not [41,43]. After incubation at 85 °C for 1 h, the anti-AFB1 VHHs Nb26 and Nb28 still retained about 70% and 40% of their binding activities, respectively [41]. VHHs are resistant to acetone, acetonitrile and methanol, which are the preferred solvents of highly lipophilic analytes in sample extraction. Both Nb26 and Nb28 retained 100% of their binding abilities in 80% of methanol, whereas a representative mAb lost 50% of its binding ability under the same conditions. Moreover, the mAb lost all of its AFB1-binding ability in the presence of 60% acetonitrile [41].
3. Anti-Idiotypic VHH Immunoassay
Anti-idiotypic antibodies, induced by paratopes of a primary antibody, are used as the secondary antibody against “idiotypic” or idiotopes [44]. This antibody competes with target analytes to bind with the same region of a primary antibody. Anti-idiotypic antibody is an exciting reagent because (1) it represents the “internal image” of the original antigen [45]; (2) it serves as a surrogate for the original antigen [46]; and (3) it competes with the original antigen for the primary antibody. In contrast to the VHH selection, anti-idiotypic VHHs are usually isolated from the library constructed by primary antibody immunization and eluted with the free antigen. Combined with the VHHs, anti- idiotypic VHH is a promising alternative to using the highly toxic mycotoxins in immunoassay.
3.1. Anti-Idiotypic VHH as a Substituent for Analytes
Due to the toxicity of mycotoxins and their hazardous effects to operator and environment, surrogate analytes were introduced to generate a standard calibration curve and assist in calculating the analyte concentration, based on the equation from the surrogate analyte [47]. In competitive immunoassays, anti-idiotypic antibodies (non-toxic reagents) were able to bind to the primary antibody and mimic the configuration with analytes; thus, they can be used as a surrogate analytes to develop an immunoassay without mycotoxins [48]. Anti-idiotypic VHH 2-5 against AFB1 was developed by immunizing an alpaca with anti-AFB1 mAb 1C11. VHH 2-5 showed almost the same binding abilities to mAb 1C11 as the homologous scFv 2G7 from 1C11, which indicated that VHH2-5 (by some key amino acid residues) might bind to AFB1 in the same way with that scFv 2G7 bound to the AFB1 [46]. With AFB1-BSA coated on the microplate and series dilution of VHH 2-5 incubation, the calibration curve of surrogate VHH also fit a four-parameter logistic equation (R2 = 0.9969). The same percentage of inhibition by VHH 2-5 and AFB1 were compared and it reflected the high correlation between their concentrations (R2 = 0.9985). Therefore, VHH 2-5 can be used as a surrogate analyte for AFB1 to develop a toxin-free ELISA. After a simple extraction with methanol/H2O (3:1, v/v), the VHH 2-5 concentration was determined first and then converted to AFB1 concentration with a linear equation. The assay had a comparable limit of detection and correlated well (R2 = 0.988) with the HPLC method in 20 contaminated peanut samples [49].
Because the anti-idiotypic antibody can compete with analytes for binding to the primary antibody, it can be used as a substitute for analytes (Figure 5A). Thus, an indirect competitive phage ELISA was developed for citrinin, a secondary metabolite found in food and feeds and a by-product of fermentation by the red yeast Monascus purpureus [50]. Compared with conventional anti-idiotypic antibodies, the anti-idiotypic VHHs can serve as both the detection antigens and DNA templates, therefore they can be used for immune-PCR (IPCR) with high sensitivity. A non-toxin quantitative IPCR assay was then developed for detecting OTA in agricultural products, with a limit of detection (LOD) of 4.17 pg/mL [51]. The M13 phage containing AFB1 anti-idiotypic VHH was used as a reagent for accurately quantifying AFB1 in corn, rice, peanut and feedstuffs. The real-time IPCR method brought higher sensitivity (4-fold increase over phage ELISA) and a wider linear range [52].
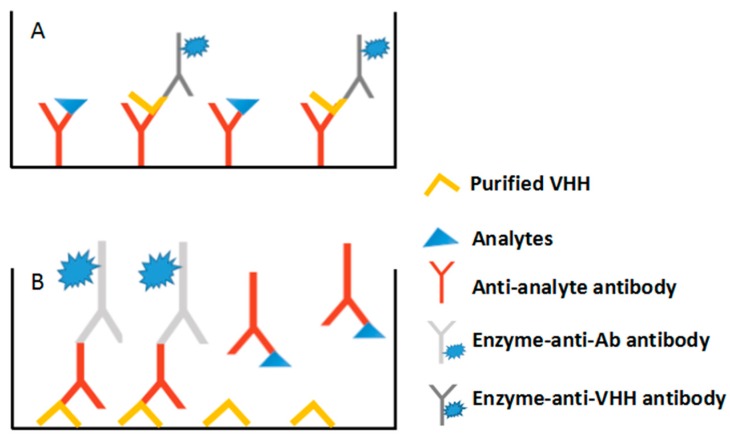
Figure 5.
Anti-idiotypic VHH-based Immunoassay. (A) Anti-idiotypic VHH functions as substitute for analytes. (B) Anti-idiotypic VHH functions as a mimic competing antigen.
Anti-idiotypic VHH has also been used as a non-toxic surrogate for ZEN for a “green” immunoassay. One DNA sequence was obtained after four rounds of biopanning. Coating with anti-ZEN mAb, the phage ELISA for ZEN was developed; it gives an IC50 of 0.25 ng/mL and a linear range of 0.11–0.55 ng/mL. The PD-IPCR for ZEN gave a detection limit of 6.5 pg/mL, a 12-fold improvement over phage ELISA [53].
Anti-idiotypic VHHs also represent the benefits of VHHs, showing high tolerance to methanol and thermostability [46]. Polyclonal anti-idiotype AFM1 antibody lost all of its primary antibody-binding ability after 5 min incubation at 80°C; whereas, VHH retained 50% of its activity after 20 min incubation [49].
3.2. Anti-Idiotypic VHH as a Mimic Competing Antigen in Immunoassay
Competitive immunoassays is the typical method used to detect mycotoxins in contaminated food products [54]. The coating antigens, usually obtained by coupling the mycotoxin with a carrier protein via chemical synthesis, are commonly used as the competing compound against primary antibodies [54]. Heterologous site systems, coupling carrier proteins with haptens as the immunogen and coating antigen from different positions [55] or different length in spacer-arm [56], are effective tools for improving the sensitivity and specificity of immunoassay. Unfortunately, the chemical synthesis of coating antigen inevitably exposes operators to these toxic analytes. Besides, the extensive modification and bridge group interference may make the conjugates less efficient or produce some unwanted cross-reactions [57]. Phage displayed peptide, isolated from the phage-displayed peptide library, is also a reagent to replace the chemical conjugated coating antigen. Although the peptide recognizes the primary antibody binding site and shows competition with the analytes for binding, it is rarely used as the coating antigen directly. Conjugating with carrier protein and fusion with binding protein [58] are the two options for using peptides as coating antigens. However, the molecular ratio of the peptide and carrier protein to the expression system in vivo may decrease the binding abilities of peptides to some extent. Due to the monovalence and mimicry of the toxins binding to primary antibodies, VHH can serve as the coating antigen [59] and potentially replace the conventional coating antigens (Figure 5B).
As a model, VHH was used to develop an immunoassay for DON. VHHs (N-28 and N-31) were isolated from a naïve phage display library through coating with anti-DON mAb and were used as coating antigen mimics in a heterologous immunoassay. Compared with chemically synthesized coating antigen (DON-BSA), the N-28 and N-31–based assays enhanced the assay sensitivity by 18-fold and 8-fold, respectively. Molecular modeling and alanine-scanning mutagenesis revealed that the residues Thr 102-Ser 106 of N-28 contributed to the binding with mAbs [60]. To improve the sensitivity of the assay further and to understand the relationship between anti-idiotypic VHH and mAb, site-saturation mutagenesis was applied to study the key residue Thr 102 [61]. An immunoassay with the mutant N28-T102Y gave an IC50 of 24.49 ng/mL, with sensitivity 3.2 times lower than N-28. Compared with the homology modeling of N-28, the mutant N28-T102Y had a larger side chain at position 102, shifting the binding site to the edge of the binding pocket, decreasing binding activity with anti-DON scFv [62].
As with FB1 and citrinin analysis, anti-idiotypic VHHs are an excellent alternative to chemosynthetic coating antigen. The anti-idiotypic VHH Ab2β Nb anti-FB1 was obtained from a naïve alpaca nanobody library. The Ab2β Nb was used as an alternative to chemosynthetic FB1-BSA conjugates, increasing the assay sensitivity by ~20-fold. The equilibrium dissociation constant (KD) measured for Ab2β Nb: anti-FB1 mAb was 164.6nM, which was 270 times higher than the KD of FB1-BSA: anti-FB1 mAb (KD = 0.62 nM) [63]. The weaker affinity indicates that less coating antigen participated in the competitive immunoassay [64,65]. As a result, the sensitivity of the assay was improved. Similarly, one β-type VHH was selected as a coating antigen from a naïve phage displayed VHH library and used to develop an immunoassay for citrinin detection; it produced an IC50 that was twice as good as that of citrinin-ovalbumin conjugates-based ELISA [66].
The anti-idiotypic VHH can be used as a signal-amplification carrier for an indirect competitive phage ELISA, showing comparable sensitivity with conventional chemosynthetic citrinin conjugate-based immunoassays [67,68]. Anti-idiotypic nanobodies can also be conjugated with the Eu/Tu (III) nanosphere as a probe to establish the competitive time-resolved strip methods for AFB1 and ZEN detection simultaneously. In this case, the probes were applied to a nitrocellulose membrane as capture antigens, with anti-AFB1 mAb and anti-ZEN mAb as detectors, providing a quantitative relationship ranging from 0.13 to 4.54 ng/mL for AFB1 and 0.20 to 2.77 ng/mL for ZEN [69].
4. VHH-Based Recombinant Proteins
VHHs can be chemically modified for site-specific immobilization to a transducer surface [70,71] or branched to nanoparticles and magnetic nanoparticles [42]. VHHs have great potential as bio recognition elements in the construction of immunosensors for mycotoxins [36]. Although, mAb- and pAb-based biosensors are traditionally used, recombinant antibody (rAb)-based biosensors have been reported to detect sub-regulatory levels of fungal (mycotoxins), marine (phycotoxins) and aquatic bio toxins in a wide range of food and environmental matrices. Due to this great potential for rAb technology, we can predict the inherent advantages of engineered rAb to provide the next generation of ultra-high performance binding reagents for rapid and specific toxin detection [53]. VHHs, the smallest specific-binding entities, can be genetically manipulated for a variety of applications [72]. To simplify immunoassay protocols, fluorescent proteins [72] or enzymes, such as alkaline phosphatase (AP), can be fused with VHHs for one-step immunoassay [73]. Besides, avoiding random chemical conjugation and the use of secondary antibodies, VHHs with fused proteins enlarge the paratopes and thus improve the affinities of VHHs due to the homodimeric nature of AP [74]. Anti-idiotypic nanobodies can also be fused with AP and expressed in E. coli, as demonstrated by the one-step detection of FB1 [75]. The anti-idiotypic VHH-AP retained both the enzymatic activity of AP and the ability to bind with primary antibody. The KD was 65.5 nM for Ab2b-Nb-AP: anti-FB1 mAb, which was half as large as that of Ab2b-Nb: anti-FB1 mAb (164.6 nM). In other words, the affinity of Ab2b-Nb-AP for anti-FB1 mAb was twice as large as for VHH, showing that the fused VHH-AP can enhance the binding affinity [76]. The colorimetric ELISA and chemiluminescence ELISA were developed with the “clonable” anti-idiotypic VHH-AP homogeneous probe for FB1, with an IC50 of 2.69 ng/mL and 0.12 ng/mL, respectively [75]. A direct competitive fluorescence enzyme immunoassay was performed for OTA detection, with IC50 and detection limit of 0.13 and 0.04 ng/mL, respectively [77]. Finally, 15-acetyl-deoxynivalenol (15-AcDON) was one of the mostly commonly DON acetylated metabolites in contaminant maize and small-grain cereals [78]. A monomer VHH was isolated from an antigen-immunized library and a VHH pentamer was formed by fusing the monomer DNA to the verotoxin B subunit in the pVT2 expression vector. The pentamer fragment showed structural flexibility for binding to antigen and pentamerization increased the affinity of the VHH for 15-AcDON [79]. Both the monomer and pentamer fragments can bind to 15-AcDON, with IC50 values of 1.24 and 0.5 µM respectively (determined with a competitive fluorescence polarization assay) [80].
5. Conclusions
Antigen-specific antibodies (also called recognition elements) are the most important and challenging components for mycotoxins immunoassays. VHHs, as an emerging reagent for monitoring hazardous mycotoxins in food (Table 1), have many advantages, including high solubility, ease of genetic manipulation and resistance to high temperature and organic solvents, despite the fact that they can be expensive to produce and their efficacy can be affected by the varied response of the animals and primer bias. VHHs are also very useful in crystallizing conformationally and the interaction between VHHs and the target [81]. However, to our knowledge, no crystal structure of an anti-mycotoxin VHHs against mycotoxin has been reported. Once we identify the VHH structures, it would be useful to identify the amino acid that influence binding affinity and carry out a mutagenesis study to enhance the assay sensitivity. In summary, VHHs are promising tools for detecting mycotoxins in food products.
Table 1.
VHH generation and applications for mycotoxin detection.
| Anti-Hapten VHH | ||||
| Analyte | VHH Library | Immunoassays | Sensitivity, IC50 | Linear Range (LOD) |
| OTA | Immunogen OTA-KLH | Indirect competitive ELISA [37] | 12 ng/mL | 8–20 ng/mL (6 ng/mL) |
| Immunogen OTA-KLH | Indirect competitive ELISA [38] | 0.64 ng/mL | 0.27–1.47 ng/mL | |
| Immunogen OTA-KLH | Competitive real time PD-IPCR [39] | 0.01–1000 pg/mL (3.7 pg/L) | ||
| Immunogen OTA-KLH | VHH-based dot ELISA [40] | (5 µg/kg) | ||
| AFB1 | Immunogen AFB1-BSA | Indirect competitive ELISA [41] | 0.754 ng/mL | 0.117–5.676 ng/mL (0.05 ng/mL) |
| Anti-Idiotypic VHH | ||||
| AFB1 | Immunogen: anti -AFB1 mAb 1C11 | Indirect competitive ELISA [49] | 0.018–0.079 ng/mL (0.015 ng/mL) | |
| Immunogen: anti -AFB1 mAb 1C11 | VHH ELISA [46] | 0.16 ng/mL | ||
| Immunogen: anti -AFB1 mAb 1C11 | Real-Time Immuno-PCR [52] | (0.02 ng/mL) | ||
| OTA | Naïve VHH phage display library | Immuno-PCR [51] | 300 pg/mL | (4.19 pg/mL) |
| ZEN | Naïve VHH phage display library | Indirect competitive phage ELISA [53] | 0.257 ng/mL | 0.11–0.55 ng/mL (0.08 ng/mL) |
| Naïve VHH phage display library | Phage display mediated IPCR [53] | 0.01–100 ng/mL (6.5 pg/mL) | ||
| DON | Naïve VHH phage display library | Indirect competitive immunoassay [60] | 8.77 ng/mL | 2.18–62.25 ng mL (1.16 ng/mL) |
| Naïve VHH phage display library | Competitive phage ELISA [62] | 24.49 ng/mL | 9.51–180.15 ng/mL | |
| citrinin | Naïve VHH phage display library | Phage ELISA [50] | 10.9 µg/kg | 2.5–100.0 µg/kg |
| Naïve VHH phage display library | VHH ELISA [66] | 44.6 ng/mL | 5.0–300.0 ng/mL | |
| FB | Naïve VHH phage display library | Competitive ELISA [63] | 0.95 ng/mL | 0.27–5.92 ng/mL (0.15 ng/mL) |
| AFB1 ZEN | Immunized alpaca library | Time-resolved fluorescence immunochromatographic assay [69] | 0.46 and 0.86 ng/mL for AFB1 and ZEN | AFB1: 0.13–4.54 ng/mL (0.05 ng/mL) ZEN: 0.20–2.77 ng/mL (0.07 ng/mL) |
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 31601539, 31772054), the Natural Science Foundation of Hubei Province (No. 2016CFB143), the Fundamental Research Funds for the Central Universities (No. 2662015QD027).
Author Contributions
All authors contributed to the literature collections. W.J. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Lee H.J., Ryu D. Advances in mycotoxin research: Public health perspectives. J. Food Sci. 2015;80:T2970–T2983. doi: 10.1111/1750-3841.13156. [DOI] [PubMed] [Google Scholar]
- 2.Van Egmond H.P., Jonker M.A. Worldwide Regulation for Mycotoxins in Food and Feed in 2003. Food and Agriculture Organization of the United Nations; Rome, Italy: 2005. pp. 22661–23667. FAO Food and Nutrition paper. [Google Scholar]
- 3.Commission E. Commission Regulation (EC) No. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union. 2006;364:5–24. [Google Scholar]
- 4.Saqerm H. Determination of aflatoxins in eggs, milk, meat and meat products using HPLC fluorescent and UV detectors. Food. Chem. 2009;114:1141–1146. [Google Scholar]
- 5.Cappiello A., Famiglini G., Tirillini B. Determination of aflatoxins in peanut meal by LC/MS with a particle beam interface. Chromatographia. 1995;40:411–416. doi: 10.1007/BF02269904. [DOI] [Google Scholar]
- 6.Li P., Zhaowei Z., Hu X., Zhang Q. Advanced hyphenated chromatographic–mass spectrometry in mycotoxin determination: Current status and prospects. Mass Spectrom. Rev. 2013;32:420–452. doi: 10.1002/mas.21377. [DOI] [PubMed] [Google Scholar]
- 7.Buiarelli F., Filippo P.D., Riccardi C., Pomata D., Rumolo E., Giannetti L., Neri B. Analytical method for the determination of mycotoxins in indoor/outdoor airborne particulate matter by HPLC-MS-MS. Int. J. Environ. Anal. Chem. 2015;95:713–729. doi: 10.1080/03067319.2015.1055468. [DOI] [Google Scholar]
- 8.Gullino M.L., Stack J.P., Fletcher J., Mumford J.D. Practical Tools for Plant and Food Biosecurity. Volume 8. Springer International Publishing; Cham, Switzerland: 2017. pp. 143–162. Plant Pathology in the 21st Century. [Google Scholar]
- 9.Appell M., Mueller A. Mycotoxin analysis using imprinted materials technology: Recent developments. J. AOAC Int. 2016;99:861–864. doi: 10.5740/jaoacint.16-0113. [DOI] [PubMed] [Google Scholar]
- 10.Berthiller F., Brera C., Iha M.H., Krska R., Lattanzio V.M.T., MacDonald S., Malone R.J., Maragos C., Solfrizzo M., Stranska-Zachariasova M., et al. Developments in mycotoxin analysis: An update for 2015–2016. World Mycotoxin J. 2017;10:5–29. doi: 10.3920/WMJ2016.2138. [DOI] [Google Scholar]
- 11.Vidal J.C., Bonel L., Ezquerra A., Hernández S., Bertolín J.R., Cubel C., Castillo J.R. Electrochemical affinity biosensors for detection of mycotoxins: A review. Biosens. Bioelectron. 2013;49:146–158. doi: 10.1016/j.bios.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Zheng M.Z., Richard J.L., Binder J. A review of rapid methods for the analysis of mycotoxins. Mycopathologia. 2006;161:261–273. doi: 10.1007/s11046-006-0215-6. [DOI] [PubMed] [Google Scholar]
- 13.Vasylieva N., Barnych B., Rand A.A., Inceoglu B., Gee S.J., Hammock B.D. Sensitive immunoassay for detection and quantification of the neurotoxin, tetramethylenedisulfotetramine (TETS) Anal. Chem. 2017;89:5612–5619. doi: 10.1021/acs.analchem.7b00846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamers-Casterman C., Atarhouch T., Muyldermans S., Robinson G., Hamers C., Songa E.B., Bendahman N., Hamers R. Naturally occurring antibodies devoid of light chains. Nature. 1993;363:446–448. doi: 10.1038/363446a0. [DOI] [PubMed] [Google Scholar]
- 15.Barelle C., Porter A. VNARs: An ancient and unique repertoire of molecules that deliver small, soluble, stable and high affinity binders of proteins. Antibodies. 2015;4:240–258. doi: 10.3390/antib4030240. [DOI] [Google Scholar]
- 16.Muyldermans S., Baral T.N., Retamozzo V.C., De B.P., De G.E., Kinne J., Leonhardt H., Magez S., Nguyen V.K., Revets H., et al. Camelid immunoglobulins and nanobody technology. Vet. Immunol. Immunopathol. 2009;128:178–183. doi: 10.1016/j.vetimm.2008.10.299. [DOI] [PubMed] [Google Scholar]
- 17.Pardon E., Laeremans T., Triest S., Rasmussen S.G.F., Wohlkonig A., Ruf A., Muyldermans S., Hol W.G.J., Kobilka B.K., Steyaert J. A general protocol for the generation of Nanobodies for structural biology. Nat. Protoc. 2014;9:674–693. doi: 10.1038/nprot.2014.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stijlemans B., Conrath K., Cortez-Retamozo V., Xong H.V., Wyns L., Senter P., Revets H., De Baetselier P., Muyldermans S., Magez S. Efficient targeting of conserved cryptic epitopes of infectious agents by single domain antibodies. African trypanosomes as paradigm. J. Biol. Chem. 2004;279:1256–1261. doi: 10.1074/jbc.M307341200. [DOI] [PubMed] [Google Scholar]
- 19.Tereshko V., Uysal S., Koide A., Margalef K., Koide S., Kossiakoff A.A. Toward chaperone-assisted crystallography: Protein engineering enhancement of crystal packing and X-ray phasing capabilities of a camelid single-domain antibody (VHH) scaffold. Protein Sci. 2008;17:1175–1187. doi: 10.1110/ps.034892.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norman H.L.C., Christopoulos T.K. Advances in Immunoassay Technology. InTech; Rijeka, Croatia: 2012. [Google Scholar]
- 21.Muyldermans S. Nanobodies: Natural single-domain antibodies. Annu. Rev. Biochem. 2013;82:775–797. doi: 10.1146/annurev-biochem-063011-092449. [DOI] [PubMed] [Google Scholar]
- 22.Marco A.D. Biotechnological applications of recombinant single-domain antibody fragments. Microb. Cell Fact. 2011;10:44–58. doi: 10.1186/1475-2859-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim H.J., Mccoy M.R., Majkova Z., Dechant J.E., Gee S.J., Tabaresda R.S., Gonzalez-Sapienza G.G., Hammock B.D. Isolation of alpaca anti-hapten heavy chain single domain antibodies for development of sensitive immunoassay. Anal. Chem. 2012;84:1165–1171. doi: 10.1021/ac2030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J., Bever C.R., Majkova Z., Dechant J.E., Yang J., Gee S.J., Xu T., Hammock B.D. Heterologous antigen selection of camelid heavy chain single domain antibodies against tetrabromobisphenol A. Anal. Chem. 2014;86:8296–8302. doi: 10.1021/ac5017437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bever C.S., Dong J.X., Vasylieva N., Barnych B., Cui Y., Xu Z.L., Hammock B.D., Gee S.J. VHH antibodies: Emerging reagents for the analysis of environmental chemicals. Anal. Bioanal. Chem. 2016;408:1–18. doi: 10.1007/s00216-016-9585-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saerens D., Muyldermans S. Single Domain Antibodies. Humana Press; Totowa, NJ, USA: 2012. [Google Scholar]
- 27.Kavanagh O., Elliott C.T., Campbell K. Progress in the development of immunoanalytical methods incorporating recombinant antibodies to small molecular weight bio toxins. Anal. Bioanal. Chem. 2015;407:2749–2770. doi: 10.1007/s00216-015-8502-z. [DOI] [PubMed] [Google Scholar]
- 28.Saerens D., Conrath K., Govaert J., Muyldermans S. Disulfide bond introduction for general stabilization of immunoglobulin heavy-chain variable domains. J. Mol. Biol. 2008;377:478–488. doi: 10.1016/j.jmb.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 29.Yau K.Y., Groves M.A., Li S., Sheedy C., Lee H., Tanha J., Mackenzie C.R., Jermutus L., Hall J.C. Selection of hapten-specific single-domain antibodies from a non-immunized llama ribosome display library. J. Immunol. Methods. 2003;281:161–175. doi: 10.1016/j.jim.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 30.Tabaresda R.S., Rossotti M., Carleiza C., Carrión F., Pritsch O., Ahn K.C., Last J.A., Hammock B.D., Gonzalez-Sapienze G. Competitive selection from single domain antibody libraries allows isolation of high-affinity antihapten antibodies that are not favored in the llama immune response. Anal. Chem. 2011;83:7213–7220. doi: 10.1021/ac201824z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bever C.R., Majkova Z., Radhakrishnan R., Suni I., Mccoy M., Wang Y., Dechant J., Gee S., Hammock B.D. Development and utilization of camelid VHH antibodies from alpaca for 2,2′,4,4′-tetrabrominated diphenyl ether detection. Anal. Chem. 2014;86:7875–7882. doi: 10.1021/ac501807j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodrow M.H., Harrison R.O., Hammock B.D. Hapten synthesis, antibody development and competitive inhibition enzyme immunoassay for s-triazine herbicides. J. Agric. Food Chem. 1990;38:990–996. doi: 10.1021/jf00094a016. [DOI] [Google Scholar]
- 33.Pírezschirmer M., Rossotti M.A., Badagian N., Leizagoyen C., Brena B.M., Gonzalezsapienza G.G. Comparison of three anti-hapten VHH selection strategies for the development of highly sensitive immunoassays for microcystins. Anal. Chem. 2017;89:6800–6806. doi: 10.1021/acs.analchem.7b01221. [DOI] [PubMed] [Google Scholar]
- 34.Spinelli S., Frenken L.G.J., Hermans P., Verrips T., Brown K., Tegoni M., Cambillau C. Camelid heavy-chain variable domains provide efficient combining sites to haptens. Biochemistry. 2000;39:1217–1222. doi: 10.1021/bi991830w. [DOI] [PubMed] [Google Scholar]
- 35.Smith G.P. Filamentous fusion phage: Novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228:1315–1317. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- 36.Bazin I., Tria S.A., Hayat A., Marty J.L. New bio recognition molecules in biosensors for the detection of toxins. Biosens. Bioelectron. 2016;87:285–298. doi: 10.1016/j.bios.2016.06.083. [DOI] [PubMed] [Google Scholar]
- 37.Houwelingen A.V., Saeger S.D., Waalwijk C., Beekwilder J. Generation of recombinant alpaca VHH antibody fragments for the detection of the mycotoxin ochratoxin A. World Mycotoxin J. 2008;1:407–417. doi: 10.3920/WMJ2008.1070. [DOI] [Google Scholar]
- 38.Liu X., Tang Z., Duan Z., He Z., Shu M., Wang X., Gee S.J., Hammock B.D., Xu Y. Nanobody-based enzyme immunoassay for ochratoxin A in cereal with high resistance to matrix interference. Talanta. 2017;164:154–158. doi: 10.1016/j.talanta.2016.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu X., Xu Y., Xiong Y., Tu Z., Li Y., He Z., Qiu Y., Fu J., Gee S.J., Hammock B.D. VHH phage-based competitive real-time immune-polymerase chain reaction for ultrasensitive detection of ochratoxin A in Cereal. Anal. Chem. 2014;86:7471–7477. doi: 10.1021/ac501202d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun Z., Duan Z., Liu X., Deng X., Tang Z. Development of a nanobody–based competitive dot ELISA for visual screening of ochratoxin A in cereals. Food. Anal. Methods. 2017;10:3558–3564. doi: 10.1007/s12161-017-0915-1. [DOI] [Google Scholar]
- 41.He T., Wang Y., Li P., Zhang Q., Lei J., Zhang Z., Ding X., Zhou H., Zhang W. Nanobody-based enzyme immunoassay for aflatoxin in agro-products with high tolerance to cosolvent methanol. Anal. Chem. 2014;86:8873–8880. doi: 10.1021/ac502390c. [DOI] [PubMed] [Google Scholar]
- 42.Xiong Y., Tu Z., Huang X., Xie B., Xiong Y.H., Xu Y. Magnetic beads carrying poly(acrylic acid) brushes as “nanobody containers” for immunoaffinity purification of aflatoxin B1 from corn samples. RSC Adv. 2015;5:77380–77387. doi: 10.1039/C5RA15843E. [DOI] [Google Scholar]
- 43.Ladenson R.C., Crimmins D.L., Landt Y., Ladenson J.H. Isolation and characterization of a thermally stable recombinant anti-caffeine heavy-chain antibody fragment. Anal. Chem. 2006;78:4501–4508. doi: 10.1021/ac058044j. [DOI] [PubMed] [Google Scholar]
- 44.Oudin J., Michel M. A new allotype form of rabbit serum gamma-globulins, apparently associated with antibody function and specificity. Des. Sci. 1963;257:805–808. [PubMed] [Google Scholar]
- 45.Jerne N.K., Roland J., Cazenave P.A. Recurrent idiotopes and internal images. EMBO J. 1982;1:243–247. doi: 10.1002/j.1460-2075.1982.tb01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y., Li P., Majkova Z., Bever C.R.S., Kim H.J., Zhang Q., Dechant J.E., Gee S.J., Hammock B.D. Isolation of alpaca anti-idiotypic heavy-chain single-domain antibody for the aflatoxin immunoassay. Anal. Chem. 2013;85:8298–8303. doi: 10.1021/ac4015885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li W., Cohen L.H. Quantitation of endogenous analytes in biofluid without a true blank matrix. Anal. Chem. 2003;75:5854–5859. doi: 10.1021/ac034505u. [DOI] [PubMed] [Google Scholar]
- 48.Guan D., Li P., Cui Y., Zhang Q., Zhang W. A competitive immunoassay with a surrogate calibrator curve for aflatoxin M1 in milk. Anal. Chim. Acta. 2011;703:64–69. doi: 10.1016/j.aca.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y., Li P., Qi Z., Hu X., Wen Z. A toxin-free enzyme-linked immunosorbent assay for the analysis of aflatoxins based on a VHH surrogate standard. Anal. Bioanal. Chem. 2016;408:1–8. doi: 10.1007/s00216-016-9370-x. [DOI] [PubMed] [Google Scholar]
- 50.Xu Y., Xiong L., Li Y., Xiong Y., Tu Z., Fu J., Tang X. Citrinin detection using phage-displayed anti-idiotypic single-domain antibody for antigen mimicry. Food Chem. 2015;177:97–101. doi: 10.1016/j.foodchem.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 51.Ji Y., He Q., Xu Y., Tu Z., Yang H., Qiu Y., Wang X., Liu Y. Phage displayed anti-idiotypic nanobody mediated immune-PCR for sensitive and environmentally friendly detection of mycotoxin ochratoxin A. Anal. Methods. 2016;8:7824–7831. doi: 10.1039/C6AY01264G. [DOI] [Google Scholar]
- 52.Lei J., Li P., Zhang Q., Wang Y., Zhang Z., Ding X., Zang W. Anti-idiotypic nanobody-phage based real-time immune-PCR for detection of hepatocarcinogen aflatoxin in grains and feedstuffs. Anal. Chem. 2014;86:10841–10846. doi: 10.1021/ac5029424. [DOI] [PubMed] [Google Scholar]
- 53.Wang X., He Q., Yang X., Xing L., Mei S., Tu Z., Li Y., Wang W., Cao D. Anti-idiotypic VHH phage display-mediated immune-PCR for ultrasensitive determination of mycotoxin zearalenone in cereals. Talanta. 2016;147:410–415. doi: 10.1016/j.talanta.2015.09.072. [DOI] [PubMed] [Google Scholar]
- 54.Goryacheva I.Y., Rusanova T.Y., Burmistrova N.A., Saeger S.D. Immunochemical methods for the determination of mycotoxins. J. Anal. Chem. 2009;64:768–785. doi: 10.1134/S1061934809080024. [DOI] [Google Scholar]
- 55.Wie S.I., Hammock B.D. Comparison of coating and immunizing antigen structure on the sensitivity and specificity of immunoassays for benzoylphenylurea insecticides [Penfluron] J. Agric. Food. Chem. 1984;32:1294–1301. doi: 10.1021/jf00126a021. [DOI] [Google Scholar]
- 56.Zhang Q., Wang L., Ahn K.C., Sun Q., Hu B., Wang J., Liu F. Hapten heterology for a specific and sensitive indirect enzyme-linked immunosorbent assay for organophosphorus insecticide fenthion. Anal. Chim. Acta. 2007;596:303–311. doi: 10.1016/j.aca.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 57.Xiao H., Clarke J.R., Marquardt R.R., Frohlich A.A. Improved methods for conjugating selected mycotoxins to carrier proteins and dextran for immunoassays. J. Agric. Food. Chem. 1995;43:2092–2097. doi: 10.1021/jf00056a025. [DOI] [Google Scholar]
- 58.Xu Y., Chen B., He Q., Qiu Y., Liu X., He Z., Xiong Z. New approach for development of sensitive and environmentally friendly immunoassay for mycotoxin fumonisin B1 based on using peptide-MBP fusion protein as substitute for coating antigen. Anal. Chem. 2014;86:8433–8440. doi: 10.1021/ac502037w. [DOI] [PubMed] [Google Scholar]
- 59.Yuan Q., Pestka J.J., Hespenheide B.M., Kuhn L.A., Linz J.E., Hart L.P. Identification of mimotope peptides which bind to the mycotoxin deoxynivalenol-specific monoclonal antibody. Appl. Environ. Microbiol. 1999;65:3279–3286. doi: 10.1128/aem.65.8.3279-3286.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qiu Y., He Q., Xu Y., Bhunia A.K., Tu Z., Chen B., Liu Y. Deoxynivalenol-mimic nanobody isolated from a naïve phage display nanobody library and its application in immunoassay. Anal. Chim. Acta. 2015;887:201–208. doi: 10.1016/j.aca.2015.06.033. [DOI] [PubMed] [Google Scholar]
- 61.Siloto R.M.P., Weselake R.J. Site saturation mutagenesis: Methods and applications in protein engineering. Biocatal. Agric. Biotechnol. 2012;1:181–189. doi: 10.1016/j.bcab.2012.03.010. [DOI] [Google Scholar]
- 62.Qiu Y., He Q., Xu Y., Wang W., Liu Y. Modification of a deoxynivalenol-antigen-mimicking nanobody to improve immunoassay sensitivity by site-saturation mutagenesis. Anal. Bioanal. Chem. 2016;408:895–903. doi: 10.1007/s00216-015-9181-5. [DOI] [PubMed] [Google Scholar]
- 63.Shu M., Xu Y., Wang D., Liu X., Li Y., He Q., Tu Z., Qiu Y., Ji Y., Wang X. Anti-idiotypic nanobody: A strategy for development of sensitive and green immunoassay for Fumonisin B₁. Talanta. 2015;143:388–393. doi: 10.1016/j.talanta.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 64.Emon J.M.V. Immunoassay and Other Bioanalytical Techniques. CRC Press; Boca Raton, FL, USA: 2007. [Google Scholar]
- 65.Wild D. The Immunoassay Handbook. 4th ed. Elsevier; Amsterdam, The Netherlands: 2013. [Google Scholar]
- 66.Yang X., Liang X., Li Y., Xiong Y., Tu Z., Fu J., Chen B. Anti-idiotypic nanobody as citrinin mimotope from a naive alpaca heavy chain single domain antibody library. Anal. Bioanal. Chem. 2015;407:5333–5341. doi: 10.1007/s00216-015-8693-3. [DOI] [PubMed] [Google Scholar]
- 67.Abramson D. Determination of citrinin in barley by indirect and direct enzyme immunoassay. J. AOAC Int. 1996;79:1325–1329. [PubMed] [Google Scholar]
- 68.Li Y., Wu H., Guo L., Zheng Y., Guo Y. Microsphere-based flow cytometric immunoassay for the determination of citrinin in red yeast rice. Food. Chem. 2012;134:2540–2545. doi: 10.1016/j.foodchem.2012.04.072. [DOI] [PubMed] [Google Scholar]
- 69.Tang X., Li P., Zhang Q., Zhang Z., Zhang W., Jiang J. Time-resolved fluorescence immunochromatographic assay developed using two idiotypic nanobodies for rapid, quantitative and simultaneous detection of aflatoxin and zearalenone in maize and its products. Anal. Chem. 2017;89:11520–11528. doi: 10.1021/acs.analchem.7b02794. [DOI] [PubMed] [Google Scholar]
- 70.Saerens D., Frederix F., Reekmans G., Conrath K., Jans K., Brys L., Huang L., Bosmans E., Maes G., Borghs G., et al. Engineering camel single-domain antibodies and immobilization chemistry for human prostate-specific antigen sensing. Anal. Chem. 2005;77:7547–7555. doi: 10.1021/ac051092j. [DOI] [PubMed] [Google Scholar]
- 71.Saerens D., Huang L., Bonroy K., Muyldermans S. Antibody fragments as probe in biosensor development. Sensors. 2008;8:4669–4686. doi: 10.3390/s8084669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rothbauer U., Zolghadr K., Tillib S., Nowak D., Schermelleh L., Gahl A., Backmann N., Conrath K., Muylermans S., Cardoso M.C., et al. Targeting and tracing antigens in live cells with fluorescent nanobodies. Nat. Methods. 2006;3:887–889. doi: 10.1038/nmeth953. [DOI] [PubMed] [Google Scholar]
- 73.Seo H.D., Lee J.J., Yu J.K., Hantschel O., Lee S.G., Kim H.S. Alkaline phosphatase-fused repebody as a new format of immune-reagent for an immunoassay. Anal. Chim. Acta. 2016;950:184–191. doi: 10.1016/j.aca.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 74.Wang J., Majkova Z., Bever C.R.S., Yang J., Gee S.J., Li J., Xu T., Hammock B.D. One-step immunoassay for tetrabromobisphenol A using a camelid single domain antibody-alkaline phosphatase fusion protein. Anal. Chem. 2015;87:4741–4748. doi: 10.1021/ac504735p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shu M., Xu Y., Liu X., Li Y., He Q., Tu Z., Fu J., Gee S.J., Hammock B.D. Anti-idiotypic nanobody-alkaline phosphatase fusion proteins: Development of a one-step competitive enzyme immunoassay for fumonisin B1 detection in cereal. Anal. Chim. Acta. 2016;924:53–59. doi: 10.1016/j.aca.2016.03.053. [DOI] [PubMed] [Google Scholar]
- 76.Liu J., Zabetakis D., Lee A.B., Goldman E.R., Anderson G.P. Single domain antibody-alkaline phosphatase fusion proteins for antigen detection—Analysis of affinity and thermal stability of single domain antibody. J. Immunol. Methods. 2013;393:1–7. doi: 10.1016/j.jim.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 77.Liu X., Xu Y., Wan D., Xiong Y., He Z., Wang X., Gee S.J., Ryu D., Hammock B.D. Development of a nanobody-alkaline phosphatase fusion protein and its application in a highly sensitive direct competitive fluorescence enzyme immunoassay for detection of ochratoxin A in cereal. Anal. Chem. 2015;87:1387–1394. doi: 10.1021/ac504305z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abramson D., Clear R.M., Gaba D., Smith D.M., Patrick S.K., Saydak D. Trichothecene and moniliformin production by Fusarium species from western Canadian wheat. J. Food. Prot. 2001;64:1220–1225. doi: 10.4315/0362-028X-64.8.1220. [DOI] [PubMed] [Google Scholar]
- 79.Zhang J., Tanha J., Hirama T., Khieu N.H., To R., Tong-Sevinc H., Stone E., Brission J.R., Mackenzie C.R. Pentamerization of single-domain antibodies from phage libraries: A novel strategy for the rapid generation of high-avidity antibody reagents. J. Mol. Biol. 2004;335:49–56. doi: 10.1016/j.jmb.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 80.Doyle P.J., Arbabi-Ghahroudi M., Gaudette N., Furzer G., Savard M.E., Gleddie S., Malean M.D., Mackenzie C.R., Hall J.C. Cloning, expression and characterization of a single-domain antibody fragment with affinity for 15-acetyl-deoxynivalenol. Mol. Immunol. 2008;45:3703–3713. doi: 10.1016/j.molimm.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 81.Desmyter A., Spinelli S., Roussel A., Cambillau C. Camelid nanobodies: Killing two birds with one stone. Curr. Opin. Struct. Biol. 2015;32:1–8. doi: 10.1016/j.sbi.2015.01.001. [DOI] [PubMed] [Google Scholar]