Summary
Multiple myeloma (MM) is a disease with known immune dysregulation. Natural killer (NK) cells have shown preclinical activity in MM. We conducted a first-in-human study of umbilical cord blood-derived (CB) NK cells for MM patients undergoing high dose chemotherapy and autologous haematopoietic stem cell transplantation (auto-HCT). Patients received lenalidomide (10 mg) on days −8 to −2, melphalan 200 mg/m2 on day −7, CB-NK cells on day −5 and auto-HCT on day 0. Twelve patients were enrolled, 3 on each of four CB-NK cell dose levels: 5×106, 1×107, 5×107 and 1×108 CB-NK cells/kg. Ten patients had either high-risk chromosomal changes or a history of relapsed/progressed disease. There were no infusional toxicities and no graft-versus-host disease. One patient failed to engraft due to poor autologous graft quality and was rescued with a back-up autologous graft. Overall, 10 patients achieved at least a very good partial response as their best response, including 8 with near complete response or better. With a median follow-up of 21 months, 4 patients have progressed or relapsed, 2 of whom have died. CB-NK cells were detected in vivo in 6 patients, with an activated phenotype (NKG2D+/NKp30+). These data warrant further development of this novel cellular therapy.
Keywords: Myeloma, natural killer, cord blood, ex vivo expansion, autologous transplant
Introduction
Multiple myeloma (MM) is the second most common haematological malignancy in adults and, despite many recent advances in therapy, (Alexanian, et al 2014) remains incurable (Laubach, et al 2015). MM is a disease characterized by immune dysregulation, whereby a suppressed immune system can allow for unchecked plasma cell proliferation (Rossi, et al 2013) and the malignant plasma cells can themselves further suppress the immune system (Brown, et al 2001, Frassanito, et al 2015, Ratta, et al 2002). Long-term remission with allogeneic haematopoietic stem cell transplantation (HCT) for some patients suggests the possibility of an allogeneic graft-versus-myeloma effect (Cavo, et al 2000, Giaccone, et al 2015). However, the morbidity and mortality associated with allotransplantation for MM has limited its application.
Natural killer (NK) cells are part of the innate immune system and have been implicated in tumour immunity and defence(Guillerey and Smyth 2015). Importantly, NK cells do not require prior exposure or sensitization to kill a specific target. While the exact mechanism of NK cells anti-tumour immunity is not known, a complex interplay between activating and inhibitory receptors probably determines cytotoxicity against a specific target (Long, et al 2013). This includes possible dis-inhibition of the killer immunoglobulin like receptor (KIR) due to absence of human leucocyte antigen (HLA) class I molecules on target cells (“missing self hypothesis”) as well as death receptor-induced apoptosis via Fas ligand and tumour necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) (Zamai, et al 1998).
The anti-MM effect of NK cells was first described by Frohn, et al (2002). Since then, we and others have corroborated these findings, demonstrating that NK cells are cytotoxic against MM cells in vitro and in vivo (Frohn, et al 2002, Garg, et al 2012, Shah, et al 2013). Unfortunately, autologous NK cells from patients with MM appear to be dysfunctional. These NK cells can have an unfavourable balance between activating and inhibitory receptors (Costello, et al 2013, Fauriat, et al 2006) and can be inhibited by the products of plasma cells (Gherman, et al 1987) and the hypoxic bone marrow microenvironment itself (Sarkar, et al 2013). Furthermore possible protection by class I expression (Carbone, et al 2005, Gao, et al 2014) in some patients suggests that any successful activity against MM requires highly active NK cells, ideally from an allogeneic source. While immunomodulatory drugs, such as lenalidomide, may augment NK cell function (Lagrue, et al 2015, Zhu, et al 2008) clinical experience suggests that this may not be sufficient to thwart disease progression. Because of these limitations of autologous NK cell function, we have been interested in the application of allogeneic NK cells as an adoptive cellular therapy to treat MM.
The clinical safety of peripheral blood-derived allogeneic NK cell infusion in MM patients has been demonstrated (Shi, et al 2008, Szmania, et al 2015). This requires collection of peripheral blood from a normal donor, which can be logistically cumbersome. To minimize these obstacles we have been interested in NK cells derived from cryopreserved umbilical cord blood (CB), a known source of haematopoietic progenitor cells (Robin, et al 2015). Our group has previously published a Good Manufacturing Practice (GMP)-compliant method of NK cell expansion from thawed CB mononuclear cells. This method yields a >1000-fold expansion of NK cells which demonstrate anti-MM activity in vitro and in vivo (Shah, et al 2013). Based on these findings we launched a first-in-human study of CB-NK cells for patients with MM who are receiving high-dose chemotherapy and autologous HCT.
Our data demonstrate that CB-NK cells in doses up to 1×108 cells/kg can be reliably produced for clinical use. Furthermore, these CB-NK cells are well tolerated in the setting of high dose chemotherapy and autologous-HCT. In addition, our correlative analyses suggest that CB-NK cells maintain an active phenotype in vivo, after adoptive transfer. These results provide a platform for further development of this novel cellular therapy.
Methods
Patients
All research was approved by the M.D. Anderson Cancer Center Institutional Review Board and all subjects gave written informed consent. This study was registered at clinicaltrials.gov (NCT01729091). Patients with symptomatic MM, aged 18–75 years with adequate cardiac, hepatic, renal and pulmonary function were eligible (Figure S1). An available umbilical cord blood unit matched at 4/6 HLA loci (HLA-A, -B, -DR) was also required. When possible, CB units with potential NK alloreactivity were chosen (Ruggeri, et al 1999). Specifically, for patients whose HLA typing included two HLA-C alleles belonging to the same group (C1 or C2 homozygous) we attempted to choose a CB unit with the opposite C allele group or a unit that was heterozygous for the C1 or C2 alleles. For patients who had two HLA-B alleles that did not belong to the Bw4 group, we attempted to select a Bw4 positive CB unit. As an allogeneic NK cell infusion was planned in the setting of autologous peripheral blood progenitor cell (PBPC) transplantation after myeloablative chemotherapy, patients were required to have at least 6×106 CD34+ cells/kg available for haematopoietic rescue. This would allow for a PBPC dose of 4×106 CD34+ cells/kg for the primary autograft and a back-up PBPC dose of 2×106 CD34+ cells/kg in the case of NK cell-mediated graft failure. Due to the inclusion of lenalidomide in the preparative regimen, patients were also registered in the accompanying REMS® program (Celgene Corp; Summit, NJ). Patients who were receiving other investigational agents or had ongoing illness (aside from MM), a history of hypersensitivity to lenalidomide or thalidomide or human immunodeficiency virus positivity were excluded from the study.
CB-NK cell production and release criteria
CB units were obtained from the MD Anderson Cord Blood Bank (Houston, TX) under an Institutional Review Board-approved protocol. The method for NK cell expansion from CB units has previously been published (Shah, et al 2013). Briefly, on day −19, the CB unit was thawed and mononuclear cells (MNCs) were isolated by Ficoll density gradient centrifugation. MNCs were cultured in a gas permeable bioreactor (G-Rex100, Wilson Wolf Corporation, Minneapolis, MN) with irradiated (100 Gy) K562-based artificial antigen presenting cells (aAPCs) expressing membrane bound interleukin (IL) 21 “Clone 9.mbIL21” (courtesy of Dr. Laurence Cooper), at a 2:1 aAPC:MNC ratio, and IL-2 (100 IU/mL, Novartis, Basel, Switzerland). On day 7, cells were CD3-depleted immunomagnetically (Clini MACS, Miltenyi, Bergisch Gladback, Germany) and the remaining cells were re-stimulated with the aAPC feeder cells and cultured for an additional 7 days.
On culture day 14, cells were harvested and NK cell purity was determined by flow cytometry (CD56+/CD3−). If the CD3+ cell dose was predicted to be >2 × 105 cells/kg, another CD3-depletion was performed. The cells were then washed on the Sepax System (Biosafe, Eysins, Switzerland) and resuspended in 100 ml PlasmaLyte supplemented with 0.5% human serum albumin (HSA). An aliquot of the NK cell product was removed prior to infusion to assess the functional status of the CB-NK cells using intracellular flow cytometry [interferon (IFN)-γ, TNF-α], and a standard chromium-51 (51Cr) assay (Shah, et al 2013) with K562 erythroleukaemia and MM cell lines RPMI 8226 and MM.1S (American Type Culture Collection, Manassas, VA) as targets. Final NK product release criteria included: negative Gram stain, ≤ 2×105 CD3+ cells/kg of patient weight, visual inspection negative for contamination, endotoxin assay <5 EU/kg patient weight and cell viability of ≥70%.
Treatment
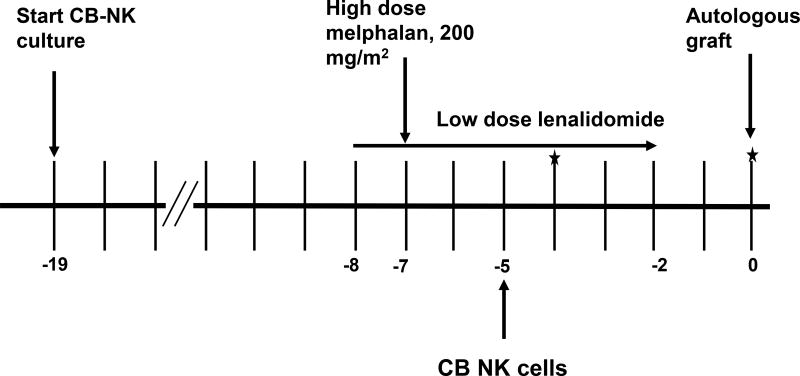
The treatment schema is outlined in Figure 1. Due to pre-clinical data demonstrating synergy between lenalidomide and NK cells (Acebes-Huerta, et al 2014, Lagrue, et al 2015) and safety of the combination of high dose melphalan with lenalidomide (Shah, et al 2015), patients received lenalidomide (10 mg orally daily) from days −8 to −2. Melphalan 200 mg/m2 was given intravenously on day −7. The freshly expanded CB-NK cells were infused on day −5. We hypothesized that this would allow for minimal interference of melphalan with the CB-NK cell product and allow for CB-NK cells to treat any remaining disease present after melphalan treatment. Autologous PBPC were infused on day 0. Correlative analyses were performed on day −4, day 0 and weekly thereafter for up to 4 weeks.
Figure 1. Trial schema.
Cord blood Natural Killer (CB-NK) cell culture was initiated at day −19. Patients received low dose lenalidomide from days −8 to −2 with high dose melphalan at day −7. CB-NK cells were infused on day −5 with infusion of the autologous haematopoietic stem cell graft on day (0). Stars indicate timing of correlative analyses, which continued weekly thereafter.
Trial design
This was a phase I, single-centre, dose escalation study of CB-NK cells for patients with MM. The primary objective was to determine the maximum tolerated dose (MTD) of CB-NK cells in combination with high dose melphalan and low dose lenalidomide. Four doses of CB-NK cells were tested: 5×106, 1×107, 5×107 and 1×108 CB-NK cells/kg. Patients were enrolled in cohorts of 3 per dose level. To ensure safety, a 42-day waiting period and full review by the Investigational New Drug (IND) office was required between the infusion of NK cells for the last patient on a dose level and enrolment on the next dose level. Dose-limiting toxicities (DLT) were defined as grade 4 CB-NK cell infusion-related toxicity, failure to engraft (defined as absolute neutrophil count of 0.5×109/l for 3 consecutive days) by day (+28), grade 3–5 allergic reaction related to CB-NK cell infusion, grade 3–5 organ toxicity within 30 days of CB-NK infusion, grade 3–4 graft-versus-host disease (GVHD) or any treatment-related death within 8 weeks of CB-NK cell infusion. Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 (http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf) was used to grade these toxicities. At the highest dose level, cohort expansion and subsequent phase II enrolment would be permitted if ≤ 1 of 3 patients experienced a DLT. Clinical response was determined according to the International Myeloma Working Group (IMWG) criteria and updated criteria for light chain disease (Durie, et al 2006, Kyle and Rajkumar 2009). Analysis for minimal residual disease (MRD) in the bone marrow was done by flow cytometry, examining the following markers: CD38, CD138, CD45, CD19, CD27, CD56, CD81 and CD117.
Correlative analyses
The secondary objective was to determine persistence of donor-derived CB-NK cells in the recipient. To do this, we collected serial PB samples at day −4, day 0 (before infusion of PBPC), day 7 and weekly thereafter until negative results were obtained. We applied our institution’s standard DNA microsatellite chimerism assay (De Lima, et al 2002) to detect donor CB-NK cells. Briefly, polymerase chain reaction (PCR)-based microsatellite polymorphism analysis was performed using capillary electrophoresis (CE-PCR) on DNA from the pre-transplant, CB donor and post-transplant PB samples. DNA from sorted T-lymphocytes (T-cells), myeloid cell (M-cells) and/or NK-cells was also used in addition to the total DNA as applicable. A total of 8 microsatellite regions (D6S264, D3S1282, D18S62, D3S1300, DM1, AR, D11S987 and D9S171) were assessed in each specimen to identify the most distinct or discriminating (informative) marker/s between CB donor and recipient DNA. The area under the curve for the informative marker was used to calculate the per cent engraftment using the formula: % engraftment = 100 × [Donor/(Donor+Recipient)] DNA in post-transplant sample. The lower limit of detection for this assay was established to be 1%.
In parallel, we performed multiparameter flow cytometry to analyze the source (patient or CB) and the phenotype of circulating NK cells. Briefly, PB MNCs were subjected to Ficoll-separation. Thereafter, cells were stained for the following: CD56, CD3, CD16, PAN KIR, NKG2D, NKG2A, NKp30, NK p44, NKp46 and NKG2C. When possible, a “flow chimerism” assay was performed: fluorochrome-conjugated antibodies against HLA groups Bw4 or Bw6 were applied to determine origin (donor CB unit versus recipient) of NK cells.
Results
Patients
A total of 12 patients were enrolled, with 3 patients per dose cohort. Table I summarizes the patients’ disease characteristics and risk factors. Three patients had abnormal cytogenetics or fluorescence in situ hybridization (FISH) changes generally accepted as high risk (Avet-Loiseau, et al 2012). Seven patients had a history of relapsed or progressive disease and 3 patients had received a prior autologous HCT. Patients had received a median of 2 lines of prior therapy (range 1–6).
Table I.
Patient Characteristics
| NK dose/kg | Patient | Age at HCT (years) |
Response at time of HCT |
ISS stage |
Durie Salmon stage |
Prior lines of therapy |
Non-standard risk feature |
|---|---|---|---|---|---|---|---|
| 5×106 | 1 | 66 | VGPR | 2 | IIIA | 1 |
|
| 2 | 51 | PR | NA | IIA | 4 |
|
|
| 3 | 46 | nCR | 3 | IIIA | 1 | ||
| 1×107 | 4 | 72 | PR | 3 | IIA | 1 | |
| 5 | 48 | VGPR | 2 | IIIA | 1 |
|
|
| 6 | 70 | nCR | 1 | IIIA | 3 |
|
|
| 5×107 | 7 | 52 | PR | 3 | IIIA | 2 |
|
| 8 | 52 | CR | 1 | IIIA | 2 |
|
|
| 9 | 66 | PR | 2 | IIIA | 1 | ||
| 1×108 | 10 | 67 | PR | NA | NA | 6* |
|
| 11 | 62 | VGPR | 1 | IIIA | 4* |
|
|
| 12 | 57 | PR | 1 | IA | 3* |
|
Indicates previous autologous HSCT
CG: cytogenetics; CR: complete response; FISH: Fluorescence in situ hybridization; HCT: haematopoietic stem cell transplantation; ISS: International staging system; NA: Not available; nCR: near complete response; NK: natural killer cell; PCL: plasma cell leukaemia; PR: partial response; sCR: stringent complete response; VGPR: very good partial response.
CB-NK cell product
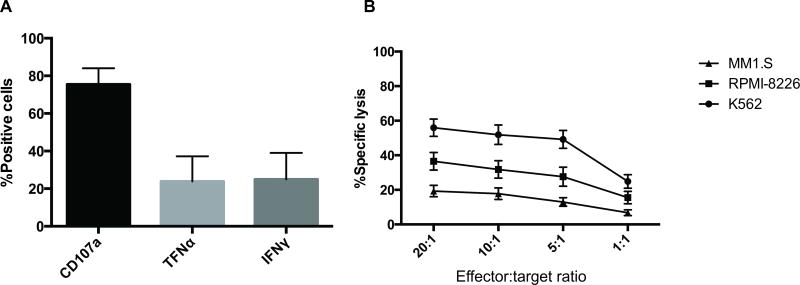
Expansion of CB-NK cells to target dose was reached in all 12 patients with a mean NK cell purity of 98.9% (96.8–99.7%) and were able to assess the functionality of these cells in a subset of patients (6/12). Consistent with our pre-clinical data, the NK cells were highly functional with CD107a degranulation in response to the K562 cell line as well as in vitro cytotoxicity against K562 and MM cell lines (Figure 2A–B). All CB-NK cell products met the release criteria mentioned above.
Figure 2. Expanded CB-NK cells demonstrate an active, unexhausted phenotype before infusion.
A. CD107a degranulation and production of tumour necrosis factor-α (TNF-α) and γ-interferon (IFN-γ) of expanded cord blood Natural Killer (CB-NK) cells against K562 targets (effector:target ratio =1:1, n=6). B. Expanded CB-NK cells demonstrate dose-dependent cytotoxicity against K562 and the MM cell lines RPMI 8226 and MM1.S (n=6).
Toxicities
There were no toxicities with infusion of CB-NK cells. There were no cases of GVHD. Eleven of 12 patients engrafted at a median time of 10 days (9–12). One patient at Dose Level 4 (1×108 CB-NK cells/kg) failed to engraft. Further examination of reference vials from this patient’s PBPC product (collected several years prior at an outside institution) revealed poor viability (16–34% by 7-Aminoactinomycin D staining) of the cells. This patient received a back-up autologous PBPC product (2.37×106 CD34+ cells/kg) that had been collected on a different day and exhibited greater CD34 viability. The patient then engrafted 10 days later.
Clinical outcomes
Overall, 10 patients achieved a very good partial response (VGPR) or better as a best response. Eight patients achieved a near complete response (CR) or better (Table II). Six patients achieved MRD negativity by bone marrow flow cytometry. In comparison to responses going into the autologous HCT, 7 patients were able to upgrade their response by day 90. All patients went on to receive lenalidomide- or bortezomib-based maintenance therapy. With a median follow-up of 21 months, 4 patients have progressed or relapsed with 2 of those 4 patients expiring due to progression.
Table II.
Clinical outcomes
| Patient | Response at time of HCT |
Best response post- HCT |
Time to best response post- HCT (days) |
Maintenance details | Relapse/progression? | Time to relapse or progression (days) |
|---|---|---|---|---|---|---|
| 1 | VGPR | VGPR | Stable VGPR | Len+ixazomib* | Progression | 321 |
| 2 | PR | nCR | 648 | Len | No | NA |
| 3 | nCR | nCR | 30 | Len | Relapse | 568 |
| 4 | PR | sCR | 30 | Len | No | NA |
| 5 | VGPR | CR | 30 | Len | Relapse | 230 |
| 6 | nCR | sCR | 101 | Len | No | NA |
| 7 | PR | sCR | 344 | Len + ixazomib* | No | NA |
| 8 | CR | CR | 35 | Len | Relapse | 345 |
| 9 | PR | PR | Stable PR | Len + ixazomib* | No | NA |
| 10 | PR | VGPR | 368 | Bortezomib | No | NA |
| 11 | VGPR | sCR | 22 | Bortezomib+ len | No | NA |
| 12 | PR | PR | Stable PR | Bortezomib+ len | No | NA |
as part of a clinical trial
HCT: haematopoietic stem cell transplantation; Len: lenalidomide; nCR: near complete response; PR: partial response; sCR: stringent complete response; VGPR: very good partial response.
Correlative analyses
To determine the persistence of the CB-derived NK cells, chimerism studies were performed. By microsatellite chimerism assay, donor-derived CB-NK cells were detected in 2/3 patients treated at Dose Level 3 (5×107 CB-NK cells/kg) and all 3 patients treated at Dose Level 4 (1×108 CB-NK cells/kg). The duration of CB-NK cell detection in these groups ranged from 5–12 days after CB-NK infusion (Table III). Of note, while at least 2 patients seemed to increase their donor NK chimerism from day −1 to day 0, the corresponding decrease in peripheral blood leucocyte count suggests that this increase was most likely a relative versus an absolute in vivo expansion.
Table III.
PCR-based microsatellite chimerism results from patients who demonstrated evidence of donor cord blood DNA in the peripheral blood. Per cent values indicate % contribution of donor DNA
| Day −4 | Day 0 | Day 7 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient | NK dose/kg | % of total DNA | % of NK DNA | WBC count (× 109/l) |
% of total DNA |
% of NK DNA |
WBC count (× 109/l) |
% of total DNA | % of NK DNA |
WBC count (× 109/l) |
| 7 | 5×107 | 0 | 0 | 2.8 | 24 | 39 | 0.7 | Not reported | 4 | 0.1 |
| 8 | 5×107 | 1 | 23 | 3.0 | 2 | 23 | 0.8 | 0 | 0 | 0.2 |
| 10 | 1×108 | 1 | 3 | 0.9 | 92 | 95 | 0.4 | 0 | 0 | 0.3 |
| 11 | 1×108 | 0 | 0 | 0.6 | 0.6 | 71 | 100 | 0.1 | ||
| 12 | 1×108 | 3* | 0* | 1.0 | 28 | 83 | 0.3 | 3** | Sub-optimal analysis | 0.3 |
Collected on Day (−1)
Collected on Day (+1)
NK: natural killer cell; PCR: polymerase chain reaction; WBC: white blood cell.
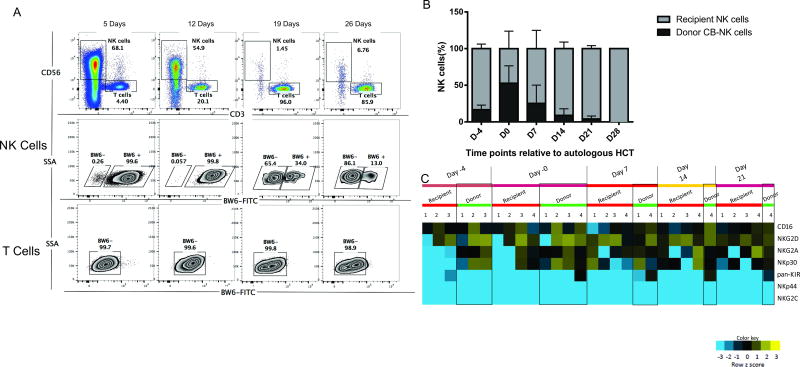
In 4 of 12 patients the donor CB unit differed from the patient with regards to alleles in the Bw4 or Bw6 group. The flow chimerism assay was thus feasible in those patients, as shown in a representative sample presented in Figure 3. In each of these cases donor CB-NK cells were detected and persisted for up to 5 days (2 patients), 12 days (1 patient) or 26 days (1 patient) after infusion. One of these patients (whose donor CB-NK persisted 12 days from infusion) had been treated on Dose Level 2 with 1×107 CB-NK cells/kg. This patient did not demonstrate CB-NK detection by our traditional DNA microsatellite chimerism assay, suggesting that the flow chimerism assay is more sensitive.
Figure 3. Correlative analyses by flow cytometry.
A. Representative sample of flow chimerism analysis during patient course. Using fluorochome-tagged flow cytometry antibodies against human leucocyte antigens (HLAs), differences in HLA types were exploited to identify the source of Natural Killer (NK) cells in vivo. In this case, the donor cord blood (CB) unit was Bw6+ while the recipient was Bw6−. Serial analyses of CD56+ population demonstrate persistence of CB-NK up to 26 days after infusion (day 21 after haematopoietic stem cell transplantation [HCT]). The T cell population (CD3+) remains recipient-derived throughout the patient’s course. B. Summary graph of relative frequencies of recipient versus donor CB-NK cells at 6 time points (n=4 patients). Data shown is mean +/− SEM. C. In vivo phenotype of infused CB-NK cells (n=4). Relative receptor expression frequencies on donor-derived and recipient NK cells were studied at 5 different time points following NK cell infusion. Each column represents a peripheral blood sample; each row represents a NK cell receptor. At any given time point, the same number under “Recipient’ and “Donor” correspond to the same sample, where both sources of NK cells were detected. The scaled expression value, denoted as Z-score, is plotted in yellow-blue colour scale, with blue colour denoting low expression and yellow indicating high expression.
To further characterize the in vivo phenotype of donor CB-NK cells, multicolour flow cytometry was performed. These data demonstrated that the adoptively transferred CB-NK cells expressed CD16, PAN KIR, NKG2D, NKG2A and NKp30. As seen in Figure 3C, donor CB-NK cells maintained expression of NKG2D in vivo. Additionally, the expression of activating receptors CD16 and NKp30 was generally higher in donor CB-NK cells versus endogenous NK cells. However, donor CB-NK cells appeared to express more NKG2A versus recipient NK cells. Expression of activating receptors NKp46 and NKG2D appeared to be similar between the two NK populations.
Discussion
While there have recently been a number of innovative strategies for the treatment of MM, the cure for this malignancy remains elusive. Our experience with allogeneic HCT, immunomodulatory agents and, more recently, antibody therapy (Lokhorst, et al 2015, Lonial, et al 2015), suggests that immunotherapy is probably a necessary weapon in the fight against this disease. Furthermore, the interaction between the permissive immune system and the aggressive plasma cell indicates that exogenous immunotherapy is probably needed.
In this study we demonstrate, for the first time, the safety and feasibility of allogeneic NK cells derived from umbilical cord blood and their potential role in myeloma immunotherapy. For all 12 patients enrolled on this trial we were able to reliably expand NK cells from CB units in our GMP laboratory to reach our planned maximal dose of 1×108 CB-NK cells/kg. Of note, the product at time of infusion mirrored the activated phenotype demonstrated in our pre-clinical data.(Shah, et al 2013) While there was no dose-related toxicity associated with the CB-NK cell infusions, cost and logistics dictated that we cap the dose as above. Though we did not expand the entire CB unit maximally, we estimate that, with expansion of the full CB unit, we could have produced another log-fold of CB-NK cells for each patient, even those at the highest dose level (up to 1×109 CB-NK cells/kg).
From a clinical perspective, the CB-NK cells were well tolerated and there were no cases of GVHD. Though one patient did not initially engraft, this was attributed to the poor viability in the primary PBPC autograft and not the CB-NK cells. This was a relatively high-risk group of MM patients and clinical outcomes were similar to a comparable patient population at our centre (Kazmi, et al 2015, Shah, et al 2015). However, we hesitate to make any conclusions about the clinical efficacy of these CB-NK cells, as we are limited by the small number of patients in this study and the subsequent administration of maintenance therapy (Table II).
Our correlative data demonstrate that the adoptively transferred CB-NK cells are detectable in recipients for up to 26 days. While a DNA microsatellite chimerism assay was able to detect these cells at the 5×107/kg dose, the flow chimerism assay increased our sensitivity and allowed for detection at one dose level below. Analysis of this data revealed that the CB-NK cells do maintain an active phenotype in vivo, with strong expression of NKG2D. Of note, this receptor has been implicated in the successful killing of MM by NK cells (El-Sherbiny, et al 2007, Martin-Antonio, et al 2015). Though small patient numbers prevented quantitative comparisons between donor and recipient-derived NK cells, some qualitative suggestions could be made. We noted that there was expression of the inhibitory receptor NKG2A on the adoptively transferred CB-NK cells; this may suggest a role for combination therapy with recently developed NKG2A blocking antibodies.
The phenotype data in our study are similar to those of a previous study with adoptively transferred PB-NK cells (Szmania, et al 2015). We were not, however, able to demonstrate significant in vivo expansion or prolonged persistence, probably due to the absence of interleukin 2 (IL2) administration and subsequent rejection by autologous lymphocytes. The use of systemic IL2 to enhance donor NK cell persistence, as practiced by several groups (Miller, et al 2005, Szmania, et al 2015), was omitted from our protocol to eliminate the possible induction of a regulatory T cell response, also reported with systemic IL2 (Kennedy-Nasser, et al 2014, Liu, et al 2015).
There remain several unanswered questions: while we were able to choose CB units with optimal KIR-HLA I alloreactivity in 7 of the 12 patients, we did not have such units in our CB bank for all patients. Thus we cannot yet determine what role, if any, KIR-HLA mismatch plays in this therapy. In addition, the lack of CB-NK cell persistence remains an issue. While donor CB-NK cells were detected after infusion, there was no long-term persistence (in the peripheral blood) and it is unclear whether the duration of persistence would correlate with clinical outcome. Furthermore, the short duration of the donor CB-NK cells makes it difficult to draw conclusions on the potential for GVHD of more persistent allogeneic NK cells. Finally, while lenalidomide has been shown in the pre-clinical setting to increase NK cell proliferation and function (Hernandez-Ilizaliturri, et al 2005) it is not clear if this has been successfully translated to our in vivo setting.
Based on our findings a phase II study of this therapy is currently in progress (NCT01729091). This trial will employ the same schema, at the dose of 1×108 CB-NK cells/kg, and will be powered to determine efficacy of this regimen. Based on our experience with our correlative studies, we will also apply a more comprehensive flow cytometry panel to examine additional markers of in vivo activation and exhaustion of adoptively transferred CB-NK cells. Future directions include administering multiple doses of CB-NK cells, genetic modification of the NK cells to improve specificity and persistence and combining CB-NK cells with antibody therapy. This last option is particularly intriguing, as the CB-NK cells strongly expressed CD16 in vivo and thus would be ideally poised to be effector cells for a MM-specific antibody. Ultimately a multimodal approach with chemotherapy, immunomodulation, antibody and cellular therapy may finally level the complicated and unbalanced immunological playing field in favour of the patient.
Supplementary Material
Acknowledgments
Funding
This work was supported by Celgene Corporation, the M.D. Anderson Cancer Center High Risk Multiple Myeloma Moon Shot, the Stading-Younger Cancer Research Foundation, the M.D. Anderson Molecular Evaluation and/or Biopsy Related Support Program (MEBRS) and by the M.D. Anderson Cancer Center Support Grant (P30 CA016672). In addition, R.Z.O., the Florence Maude Thomas Cancer Research Professor, would also like to acknowledge support from the National Cancer Institute (P50 CA142509, R01 CA184464 and CA194264, and U10 CA032102).
Conflicts of interest
NS, QB, RZO and YN receive research support from Celgene. YN receives research support from Novartis. On May 7, 2015, LC was appointed as the Chief Executive Officer at ZIOPHARM and is now a Visiting Scientist at MD Anderson Cancer Center. QB receives research support from Takeda. NS, RZO and QB have served on advisory boards for Takeda. RZO has participated in advisory boards with Array BioPharma, Bristol-Myers Squibb, Celgene, FORMA Therapeutics, Janssen and Onyx/Amgen. RZO receives grant/research support from Bristol-Myers Squibb, Millennium/Takeda, Onyx/Amgen and Spectrum Pharma
Footnotes
Author contributions
NS, MQ, RC and EJS designed the clinical trial. NS, JM, RZO, SP, KC, CH, SA, YN, QB, KP, MQ, RC, KR and EJS collected and analysed patient data. NS, IK, EY, LC, DL, SP, CB, KR, and EJS developed and implemented the NK cell expansion procedure. NS, LL, HS, MM, EL, CS, KR and EJS performed and/or analysed the correlative data. All authors participated in manuscript preparation and review.
References
- Acebes-Huerta A, Huergo-Zapico L, Gonzalez-Rodriguez AP, Fernandez-Guizan A, Payer AR, Lopez-Soto A, Gonzalez S. Lenalidomide induces immunomodulation in chronic lymphocytic leukemia and enhances antitumor immune responses mediated by NK and CD4 T cells. Biomed Res Int. 2014;2014:265840. doi: 10.1155/2014/265840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexanian R, Wang M, Delasalle K, Wang S, Qazilbash M, Handy B, Weber D. Value of novel agents and intensive therapy for patients with multiple myeloma. Bone Marrow Transplant. 2014;49:422–425. doi: 10.1038/bmt.2013.189. [DOI] [PubMed] [Google Scholar]
- Avet-Loiseau H, Attal M, Campion L, Caillot D, Hulin C, Marit G, Stoppa AM, Voillat L, Wetterwald M, Pegourie B, Voog E, Tiab M, Banos A, Jaubert J, Bouscary D, Macro M, Kolb B, Traulle C, Mathiot C, Magrangeas F, Minvielle S, Facon T, Moreau P. Long-term analysis of the IFM 99 trials for myeloma: cytogenetic abnormalities [t(4;14), del(17p), 1q gains] play a major role in defining long-term survival. J Clin Oncol. 2012;30:1949–1952. doi: 10.1200/JCO.2011.36.5726. [DOI] [PubMed] [Google Scholar]
- Brown RD, Pope B, Murray A, Esdale W, Sze DM, Gibson J, Ho PJ, Hart D, Joshua D. Dendritic cells from patients with myeloma are numerically normal but functionally defective as they fail to up-regulate CD80 (B7-1) expression after huCD40LT stimulation because of inhibition by transforming growth factor-beta1 and interleukin-10. Blood. 2001;98:2992–2998. doi: 10.1182/blood.v98.10.2992. [DOI] [PubMed] [Google Scholar]
- Carbone E, Neri P, Mesuraca M, Fulciniti MT, Otsuki T, Pende D, Groh V, Spies T, Pollio G, Cosman D, Catalano L, Tassone P, Rotoli B, Venuta S. HLA class I, NKG2D, and natural cytotoxicity receptors regulate multiple myeloma cell recognition by natural killer cells. Blood. 2005;105:251–258. doi: 10.1182/blood-2004-04-1422. [DOI] [PubMed] [Google Scholar]
- Cavo M, Terragna C, Martinelli G, Ronconi S, Zamagni E, Tosi P, Lemoli RM, Benni M, Pagliani G, Bandini G, Tura S. Molecular monitoring of minimal residual disease in patients in long-term complete remission after allogeneic stem cell transplantation for multiple myeloma. Blood. 2000;96:355–357. [PubMed] [Google Scholar]
- Costello RT, Boehrer A, Sanchez C, Mercier D, Baier C, Le Treut T, Sebahoun G. Differential expression of natural killer cell activating receptors in blood versus bone marrow in patients with monoclonal gammopathy. Immunology. 2013;139:338–341. doi: 10.1111/imm.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lima M, St John LS, Wieder ED, Lee MS, McMannis J, Karandish S, Giralt S, Beran M, Couriel D, Korbling M, Bibawi S, Champlin R, Komanduri KV. Double-chimaerism after transplantation of two human leucocyte antigen mismatched, unrelated cord blood units. Br J Haematol. 2002;119:773–776. doi: 10.1046/j.1365-2141.2002.03893.x. [DOI] [PubMed] [Google Scholar]
- Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, Gertz M, Dimopoulos M, Westin J, Sonneveld P, Ludwig H, Gahrton G, Beksac M, Crowley J, Belch A, Boccadaro M, Cavo M, Turesson I, Joshua D, Vesole D, Kyle R, Alexanian R, Tricot G, Attal M, Merlini G, Powles R, Richardson P, Shimizu K, Tosi P, Morgan G, Rajkumar SV. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- El-Sherbiny YM, Meade JL, Holmes TD, McGonagle D, Mackie SL, Morgan AW, Cook G, Feyler S, Richards SJ, Davies FE, Morgan GJ, Cook GP. The requirement for DNAM-1, NKG2D, and NKp46 in the natural killer cell-mediated killing of myeloma cells. Cancer Res. 2007;67:8444–8449. doi: 10.1158/0008-5472.CAN-06-4230. [DOI] [PubMed] [Google Scholar]
- Fauriat C, Mallet F, Olive D, Costello RT. Impaired activating receptor expression pattern in natural killer cells from patients with multiple myeloma. Leukemia. 2006;20:732–733. doi: 10.1038/sj.leu.2404096. [DOI] [PubMed] [Google Scholar]
- Frassanito MA, Ruggieri S, Desantis V, Di Marzo L, Leone P, Racanelli V, Fumarulo R, Dammacco F, Vacca A. Myeloma cells act as tolerogenic antigen-presenting cells and induce regulatory T cells in vitro. Eur J Haematol. 2015;95:65–74. doi: 10.1111/ejh.12481. [DOI] [PubMed] [Google Scholar]
- Frohn C, Höppner M, Schlenke P, Kirchner H, Koritke P, Luhm J. Anti-myeloma activity of natural killer lymphocytes. British Journal of Haematology. 2002;119:660–664. doi: 10.1046/j.1365-2141.2002.03879.x. [DOI] [PubMed] [Google Scholar]
- Gao M, Gao L, Yang G, Tao Y, Hou J, Xu H, Hu X, Han Y, Zhang Q, Zhan F, Wu X, Shi J. Myeloma cells resistance to NK cell lysis mainly involves an HLA class I-dependent mechanism. Acta Biochim Biophys Sin (Shanghai) 2014;46:597–604. doi: 10.1093/abbs/gmu041. [DOI] [PubMed] [Google Scholar]
- Garg TK, Szmania SM, Khan JA, Hoering A, Malbrough PA, Moreno-Bost A, Greenway AD, Lingo JD, Li X, Yaccoby S, Suva LJ, Storrie B, Tricot G, Campana D, Shaughnessy JD, Jr, Nair BP, Bellamy WT, Epstein J, Barlogie B, van Rhee F. Highly activated and expanded natural killer cells for multiple myeloma immunotherapy. Haematologica. 2012;97:1348–1356. doi: 10.3324/haematol.2011.056747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherman M, Manciulea M, Bancu AC, Sulica A, Stanworth DR, Herberman RB. Regulation of human natural cytotoxicity by IgG--I. Characterization of the structural site on monomeric IgG responsible for inhibiting natural killer cell activity. Mol Immunol. 1987;24:743–750. doi: 10.1016/0161-5890(87)90057-5. [DOI] [PubMed] [Google Scholar]
- Giaccone L, Brunello L, Festuccia M, Gilestro M, Maffini E, Ferrando F, Talamo E, Passera R, Boccadoro M, Omede P, Bruno B. Clinical impact of immunophenotypic remission after allogeneic hematopoietic cell transplantation in multiple myeloma. Bone Marrow Transplant. 2015;50:511–516. doi: 10.1038/bmt.2014.319. [DOI] [PubMed] [Google Scholar]
- Guillerey C, Smyth MJ. NK Cells and Cancer Immunoediting. Curr Top Microbiol Immunol. 2015;395:115–145. doi: 10.1007/82_2015_446. [DOI] [PubMed] [Google Scholar]
- Hernandez-Ilizaliturri FJ, Reddy N, Holkova B, Ottman E, Czuczman MS. Immunomodulatory drug CC-5013 or CC-4047 and rituximab enhance antitumor activity in a severe combined immunodeficient mouse lymphoma model. Clin Cancer Res. 2005;11:5984–5992. doi: 10.1158/1078-0432.CCR-05-0577. [DOI] [PubMed] [Google Scholar]
- Kazmi SM, Nusrat M, Gunaydin H, Cornelison AM, Shah N, Kebriaei P, Nieto Y, Parmar S, Popat UR, Oran B, Shah JJ, Orlowski RZ, Champlin RE, Qazilbash MH, Bashir Q. Outcomes Among High-Risk and Standard-Risk Multiple Myeloma Patients Treated With High-Dose Chemotherapy and Autologous Hematopoietic Stem-Cell Transplantation. Clin Lymphoma Myeloma Leuk. 2015;15:687–693. doi: 10.1016/j.clml.2015.07.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy-Nasser AA, Ku S, Castillo-Caro P, Hazrat Y, Wu MF, Liu H, Melenhorst J, Barrett AJ, Ito S, Foster A, Savoldo B, Yvon E, Carrum G, Ramos CA, Krance RA, Leung K, Heslop HE, Brenner MK, Bollard CM. Ultra low-dose IL-2 for GVHD prophylaxis after allogeneic hematopoietic stem cell transplantation mediates expansion of regulatory T cells without diminishing antiviral and antileukemic activity. Clin Cancer Res. 2014;20:2215–2225. doi: 10.1158/1078-0432.CCR-13-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrue K, Carisey A, Morgan DJ, Chopra R, Davis DM. Lenalidomide augments actin remodeling and lowers NK-cell activation thresholds. Blood. 2015;126:50–60. doi: 10.1182/blood-2015-01-625004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laubach J, Garderet L, Mahindra A, Gahrton G, Caers J, Sezer O, Voorhees P, Leleu X, Johnsen HE, Streetly M, Ludwig H, Mellqvist UH, Chng WJ, Pilarski L, Einsele H, Hou J, Turesson I, Zamagni E, Chim J, Mazumder A, Westin J, Lu J, Reiman T, Kristinsson S, Joshua D, Roussel M, O'Gorman P, Terpos E, Dimopoulos M, Moreau P, Anderson K, Palumbo A, Kumar S, Rajkumar V, Durie B, Richardson P. Management of relapsed multiple myeloma: Recommendations of the international myeloma working group. Leukemia. 2015 doi: 10.1038/leu.2015.356. [DOI] [PubMed] [Google Scholar]
- Liu Z, Gerner MY, Van Panhuys N, Levine AG, Rudensky AY, Germain RN. Immune homeostasis enforced by co-localized effector and regulatory T cells. Nature. 2015;528:225–230. doi: 10.1038/nature16169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M, Minnema MC, Lassen U, Krejcik J, Palumbo A, van de Donk NW, Ahmadi T, Khan I, Uhlar CM, Wang J, Sasser AK, Losic N, Lisby S, Basse L, Brun N, Richardson PG. Targeting CD38 with Daratumumab Monotherapy in Multiple Myeloma. N Engl J Med. 2015;373:1207–1219. doi: 10.1056/NEJMoa1506348. [DOI] [PubMed] [Google Scholar]
- Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol. 2013;31:227–258. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I, Walter-Croneck A, Moreau P, Mateos MV, Magen H, Belch A, Reece D, Beksac M, Spencer A, Oakervee H, Orlowski RZ, Taniwaki M, Rollig C, Einsele H, Wu KL, Singhal A, San-Miguel J, Matsumoto M, Katz J, Bleickardt E, Poulart V, Anderson KC, Richardson P. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N Engl J Med. 2015;373:621–631. doi: 10.1056/NEJMoa1505654. [DOI] [PubMed] [Google Scholar]
- Martin-Antonio B, Najjar A, Robinson SN, Chew C, Li S, Yvon E, Thomas MW, Mc Niece I, Orlowski R, Munoz-Pinedo C, Bueno C, Menendez P, Fernandez de Larrea C, Urbano-Ispizua A, Shpall EJ, Shah N. Transmissible cytotoxicity of multiple myeloma cells by cord blood-derived NK cells is mediated by vesicle trafficking. Cell Death Differ. 2015;22:96–107. doi: 10.1038/cdd.2014.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, Orchard PJ, Blazar BR, Wagner JE, Slungaard A, Weisdorf DJ, Okazaki IJ, McGlave PB. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- Ratta M, Fagnoni F, Curti A, Vescovini R, Sansoni P, Oliviero B, Fogli M, Ferri E, Della Cuna GR, Tura S, Baccarani M, Lemoli RM. Dendritic cells are functionally defective in multiple myeloma: the role of interleukin-6. Blood. 2002;100:230–237. doi: 10.1182/blood.v100.1.230. [DOI] [PubMed] [Google Scholar]
- Robin M, Ruggeri A, Labopin M, Niederwieser D, Tabrizi R, Sanz G, Bourhis JH, van Biezen A, Koenecke C, Blaise D, Tischer J, Craddock C, Maillard N, Mohty M, Russel N, Schetelig J, Finke J, Gluckman E, de Witte TM, Rocha V, Kroger N. Comparison of unrelated cord blood and peripheral blood stem cell transplantation in adults with myelodysplastic syndrome after reduced-intensity conditioning regimen: a collaborative study from Eurocord (Cord blood Committee of Cellular Therapy & Immunobiology Working Party of EBMT) and Chronic Malignancies Working Party. Biol Blood Marrow Transplant. 2015;21:489–495. doi: 10.1016/j.bbmt.2014.11.675. [DOI] [PubMed] [Google Scholar]
- Rossi M, Botta C, Correale P, Tassone P, Tagliaferri P. Immunologic microenvironment and personalized treatment in multiple myeloma. Expert Opin Biol Ther. 2013;13(Suppl 1):S83–93. doi: 10.1517/14712598.2013.799130. [DOI] [PubMed] [Google Scholar]
- Ruggeri L, Capanni M, Casucci M, Volpi I, Tosti A, Perruccio K, Urbani E, Negrin RS, Martelli MF, Velardi A. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94:333–339. [PubMed] [Google Scholar]
- Sarkar S, Germeraad WT, Rouschop KM, Steeghs EM, van Gelder M, Bos GM, Wieten L. Hypoxia induced impairment of NK cell cytotoxicity against multiple myeloma can be overcome by IL-2 activation of the NK cells. PLoS One. 2013;8:e64835. doi: 10.1371/journal.pone.0064835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah N, Martin-Antonio B, Yang H, Ku S, Lee DA, Cooper LJ, Decker WK, Li S, Robinson SN, Sekine T, Parmar S, Gribben J, Wang M, Rezvani K, Yvon E, Najjar A, Burks J, Kaur I, Champlin RE, Bollard CM, Shpall EJ. Antigen presenting cell-mediated expansion of human umbilical cord blood yields log-scale expansion of natural killer cells with anti-myeloma activity. PLoS One. 2013;8:e76781. doi: 10.1371/journal.pone.0076781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah N, Thall PF, Fox PS, Bashir Q, Shah JJ, Parmar S, Lin P, Kebriaei P, Nieto Y, Popat UR, Hosing CM, Cornelison A, Shpall EJ, Orlowski RZ, Champlin RE, Qazilbash MH. Phase I/II trial of lenalidomide and high-dose melphalan with autologous stem cell transplantation for relapsed myeloma. Leukemia. 2015;29:1945–1948. doi: 10.1038/leu.2015.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Tricot G, Szmania S, Rosen N, Garg TK, Malaviarachchi PA, Moreno A, Dupont B, Hsu KC, Baxter-Lowe LA, Cottler-Fox M, Shaughnessy JD, Jr, Barlogie B, van Rhee F. Infusion of haplo-identical killer immunoglobulin-like receptor ligand mismatched NK cells for relapsed myeloma in the setting of autologous stem cell transplantation. Br J Haematol. 2008;143:641–653. doi: 10.1111/j.1365-2141.2008.07340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmania S, Lapteva N, Garg T, Greenway A, Lingo J, Nair B, Stone K, Woods E, Khan J, Stivers J, Panozzo S, Campana D, Bellamy WT, Robbins M, Epstein J, Yaccoby S, Waheed S, Gee A, Cottler-Fox M, Rooney C, Barlogie B, van Rhee F. Ex vivo-expanded natural killer cells demonstrate robust proliferation in vivo in high-risk relapsed multiple myeloma patients. J Immunother. 2015;38:24–36. doi: 10.1097/CJI.0000000000000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamai L, Ahmad M, Bennett IM, Azzoni L, Alnemri ES, Perussia B. Natural killer (NK) cell-mediated cytotoxicity: differential use of TRAIL and Fas ligand by immature and mature primary human NK cells. J Exp Med. 1998;188:2375–2380. doi: 10.1084/jem.188.12.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Corral LG, Fleming YW, Stein B. Immunomodulatory drugs Revlimid (lenalidomide) and CC-4047 induce apoptosis of both hematological and solid tumor cells through NK cell activation. Cancer Immunol Immunother. 2008;57:1849–1859. doi: 10.1007/s00262-008-0512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.