Abstract
Background
Disturbances in sexual function are common among dialysis patients. Normal erections require a complex balance of physiological, psychological, emotional, hormonal, neurological and vascular factors. This study examined a possible association of overhydration (OH) with male sexual dysfunction and depression in hemodialysis (HD) patients.
Patients and methods
This cross-sectional study assessed hydration status by whole-body bioimpedance spectroscopy in patients on maintenance HD for more than 12 months. Patients were categorized according to OH to extracellular water (ECW) ratio: OH/ECW ratio >0.15 and OH/ECW ratio ≤0.15. Sexual function was assessed using the International Index of Erectile Function (IIEF) score. Psychological status was evaluated using the Beck Depression Inventory (BDI) score. Serum sex hormones were determined.
Results
Of 39 stable participants on HD, 53.8% were overhydrated (OH/ECW ratio >0.15) and 46.2% not overhydrated (OH/ECW ratio ≤0.15). Of participants with OH/ECW ratio >0.15, 85.7% had mild to severe ED, and 71.4% had abnormal BDI scores, ranging from mild mood disturbance to severe depression. Compared to patients with OH/ECW ratio ≤0.15, BDI scores, serum estradiol and plasma hsCRP were higher (18.48±8.34 vs 10.61±5.46, p<0.001; 140.10±44.51 vs 126.10±32.26, p=0.034; and, 17.70±12.14 vs 9.76±8.79, p=0.013; respectively) in those with OH/ECW ratio >0.15, while their IIEF score, serum total testosterone and dehydroepiandrosterone (DHEA) were lower (12.81±7.31 vs 41.44±23.79, p<0.001; 8.97±5.43 vs 14.10±8.30, p=0.013; and 85.31±55.14 vs 133.3±95.48, p=0.029; respectively). The OH/ECW ratio correlated inversely with the IIEF score (r=−0.69, p<0.001) and positively with BDI scores (r=0.64, p<0.001). IIEF scores were inversely correlated with BDI scores (r=−0.54, p<0.001).
Conclusion
OH in HD patients was found to be associated with a higher prevalence of sexual dysfunction and depression, lower serum levels of total testosterone and DHEA, and higher levels of serum estradiol.
Keywords: hemodialysis, overhydration, erectile dysfunction, depression, sex hormones, International Index of Erectile Function score, Beck Depression Inventory score
Introduction
Disturbances in sexual function are common among chronic kidney disease and dialysis patients.1,2 A substantial proportion of uremic patients complain of symptoms that include erectile dysfunction (ED) and decreased libido.1,2 Reported prevalences of sexual dysfunction in dialysis patients have ranged widely, from 41 to 93%.3–7
Normal erections require a complex balance of physiological, psychological, emotional, social, hormonal, neurological and vascular factors.1–4 Any disturbance in one or more of these components can compromise erection.1–4 ED among dialysis patients is thus a multifactorial problem that usually leads to a negative impact on patients’ quality of life.8,9 Various conditions may contribute to ED in this population, including uremic milieu, medications, diabetes mellitus, hypertension, liver disease, sleep apnea, tobacco smoking, alcohol consumption, zinc deficiency, pelvic radiation or surgery, and changes in hormone levels: reduced testosterone and elevated levels of prolactin, follicle-stimulating, luteinizing and parathyroid hormones.9–11
Overhydration (OH) is one of the most common problems of the hemodialysis (HD) population.12,13 However, to the best of our knowledge, data are lacking regarding the impact of hydration status on sexual function and depression in male patients treated with chronic HD. Specifically, we are unaware of studies that evaluated the direct impact of OH on ED in HD patients. This study aimed to evaluate the relationship between OH and ED in this setting.
Methods
Thirty-nine stable male patients on maintenance HD for more than 12 months participated in this cross-sectional study. Psychiatric disorders, heart failure (New York Heart Association class III or IV), acute or recent infection in the past 3 months, immunosuppressive therapy, liver cirrhosis, malignancy, amputations of limbs, implantation of prostheses, pelvic radiation, and surgery for prostate, bladder, colorectal cancer and pelvic arteries were considered exclusion criteria. The study was approved by the Helsinki Ethics Committee of the Galilee Medical Center, Nahariya, Israel. All participants signed a written informed consent form before participating in this study. All followed their usual recommended diet and continued their regular medications and dialysis regimen.
After undergoing full medical history and physical examination, all participants were assessed for hydration status, sexual function, psychological status and sex hormonal profile. Hydration status was assessed by a safe, simple, reproducible and noninvasive whole-body bioimpedance spectroscopy technique (BIS), using a Fresenius Medical Care Body Composition Monitor (BCM) device (Fresenius Medical Care, Bad Homburg, Germany).14,15 The OH to extracellular water (ECW) ratio (OH/ECW ratio) was used as an independent and comparable indicator of hydration status. Assessment of hydration status was performed just before starting the midweek HD session. Sexual function was assessed using the International Index of Erectile Function (IIEF) score, with a range of 1–25. A score of 1–7 is considered as severe ED, 8–11 as moderate ED, 12–16 as mild-moderate ED, 17–21 as mild ED and 22–25 as normal function.16 Psychological status was evaluated using the Beck Depression Inventory (BDI) score, with a range of 1–63. A score of 1–10 is considered as normal, 11–16 as mild mood disturbance, 17–20 as borderline depression, 21–30 as moderate depression, 31–40 as severe depression and >40 as extreme depression.17 The IIEF and BDI scores were validated as screening tools for the detection of ED and depression in dialysis patients.18–22 Blood samples were drawn for the determination of sex hormonal profile including serum levels of total testosterone, dehydroepiandrosterone (DHEA), estradiol and luteinizing hormone (LH). Patients were categorized into two groups according to their hydration status obtained by BIS, using the BCM device: patients with OH/ECW ratio >0.15 were considered overhydrated and those with OH/ECW ratio ≤0.15 not overhydrated.
Statistical analyses
Data were compared between participants with OH/ECW ratio >0.15 and those with OH/ECW ratio ≤0.15. Statistical analysis was carried out using SPSS (IBM SPSS Statistics version 21, Armonk, NY, USA) software. p<0.05 was considered to be significant. Quantitative (continuous) variables were described as means ± SD. Qualitative (categorical) variables were described as frequencies and percentages. Unpaired t-test was used to compare differences between the study groups, including age, body mass index (BMI), PD vintage, Kt/V (a dimensionless ratio, representing fractional urea clearance [liter per hour] [K], dialysis session length [hours] [t] and V is the distribution volume of urea [liter]), daily urinary output (DUO), biochemical and hormonal parameters and OH, as well as IIEF and BDI scores. Fisher’s exact test was used to compare frequencies between the study groups, including the presence of diabetes, primary renal disease and antihypertensive agents. Pearson’s correlation coefficient test was used to describe associations of OH (OH/ECW ratio) with IIEF scores and BDI scores, as well as the association of IIEF scores with BDI scores.
Results
Thirty-nine hemodialysis patients, aged 18–75 years (mean age: 62.7±12.2 years), participated in this study. Of them, 21 (53.8%) were overhydrated (OH/ECW ratio >0.15) and 18 not overhydrated (OH/ECW ratio >0.15). Patients who were overhydrated and those who were not overhydrated were comparable in age distribution, the presence of diabetes mellitus, BMI, hemodialysis vintage, Kt/V, DUO, hemoglobin, primary renal disease and antihypertensive medications (Table 1).
Table 1.
Demographic and clinical variables of hemodialysis patients according to OH/ECW ratio (>0.15 and ≤0.15)
| Variables | OH/ECW ratio >0.15; n (%) =21 (53.8%) | OH/ECW ratio ≤0.15; n (%) =18 (46.2%) | p-value |
|---|---|---|---|
| Age (years) | 64.67±12.20 | 60.39±12.21 | 0.141* |
| DM | 10 (47.6) | 8 (44.4) | 0.549§ |
| BMI (kg/m2) | 28.09±3.21 | 29.62±3.28 | 0.074* |
| HD vintage (months) | 30.2±29.2 | 34.67±25.24 | 0.312* |
| Kt/V | 1.15±0.18 | 1.24±0.19 | 0.083* |
| DUO (mL/day) | 457.1±700.4 | 583.3±712.3 | 0.291* |
|
| |||
| OH/ECW ratio | 0.179±0.019 | 0.132±0.011 | <0.001* |
| IIEF score | 12.81±7.31 | 41.44±23.79 | <0.001* |
| BDI score | 18.48±8.34 | 10.61±5.46 | <0.001* |
|
| |||
| Total testosterone (nmol/L) | 8.97±5.43 | 14.10±8.30 | 0.013* |
| DHEA (mcg/dL) | 85.31±55.14 | 133.3±95.48 | 0.029* |
| Estradiol (pmol/L) | 140.10±44.51 | 126.10±32.26 | 0.034* |
| LH (mIU/mL) | 13.07±8.1 | 12.37±13.65 | 0.422* |
|
| |||
| Serum albumin (g/dL) | 3.50±0.41 | 3.96±0.21 | <0.001* |
| hsCRP (mg/dL) | 17.70±12.14 | 9.76±8.79 | 0.013* |
| Hemoglobin (g/dL) | 10.64±1.33 | 11.05±1.04 | 0.094* |
|
| |||
| Primary renal disease | |||
| DM | 10 (47.6) | 8 (44.4) | 0.549§ |
| Hypertension | 6 (28.6) | 6 (33.3) | 0.509§ |
| CGN | 3 (14.3) | 2 (11.1) | 0.576§ |
| Unknown | 2 (9.5) | 2 (11.1) | 0.636§ |
|
| |||
| Medications | |||
| ACEIs/ARBs | 7 (33.3) | 5 (27.8) | 0.491§ |
| Beta blockers | 4 (19.0) | 4 (22.2) | 0.558§ |
| CCB | 6 (28.6) | 5 (27.8) | 0.620§ |
Notes:
Unpaired t-test;
Fisher’s exact test; data are presented as number (%) or as means and standard deviations. Kt/V, a dimensionless index that measures the fractional urea clearance during dialysis: K = blood urea clearance (L per hour), t = dialysis length (hour), V = distribution volume of urea (L).
Abbreviations: OH/ECW ratio, overhydration to extracellular water ratio; DM, diabetes mellitus; BMI, body mass index; HD, hemodialysis; DUO, daily urinary output; IIEF, International Index of Erectile Function; BDI, Beck Depression Inventory; DHEA, dehydroepiandrosterone; LH, luteinizing hormone; hsCRP, high-sensitivity C-reactive protein; CGN, chronic glomerulonephritis; ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; CCB, calcium channel blockers.
Mean values of OH/ECW ratio, IIEF score and BDI score were 0.179±0.019, 12.81±7.31 and 18.48±8.34, respectively, in those with OH/ECW ratio >0.15 compared to 0.132±0.011, 41.44±23.79 and 10.61±5.46, respectively, for those with OH/ECW ratio ≤0.15 (p<0.001, p<0.001, p<0.001, respectively) (Table 1).
Of participants with OH/ECW ratio >0.15, 85.7% (18/21) had mild to severe ED (Table 2) and 71.4% (15/21) had abnormal BDI scores, ranging from mild mood disturbance to severe depression (Table 3).
Table 2.
IIEF scores in hemodialysis patients according to OH/ECW ratio (>0.15 and ≤0.15)
| IIEF score | Patients with OH/ECW ratio >0.15; (n=21) | Patients with OH/ECW ratio ≤0.15; (n=18) | p-value | |
|---|---|---|---|---|
| 22–25 | Normal, n (%) | 3 (14.3) | 10 (55.6) | |
| 17–21 | Mild dysfunction, n (%) | 2 (9.5) | 5 (27.8) | |
| 12–16 | Mild-moderate dysfunction, n (%) | 4 (19.1) | 1 (5.5) | |
| 8–11 | Moderate dysfunction, n (%) | 7 (33.3) | 1 (5.5) | |
| 1–7 | Severe dysfunction, n (%) | 5 (23.8) | 1 (5.5) | |
| Total patients with abnormal IIEF scores, n (%) | 18 (85.7) | 8 (44.4) | 0.008* |
Note:
Fisher’s exact test.
Abbreviations: IIEF, International Index of Erectile Function; OH/ECW, overhydration to extracellular water.
Table 3.
BDI scores in hemodialysis patients according to OH/ECW ratio (>0.15 and ≤0.15)
| BDI score | Patients with OH/ECW ratio >0.15 (n=21) | Patients with OH/ECW ratio ≤0.15 (n=18) | p-value | |
|---|---|---|---|---|
| 1–10 | Normal, n (%) | 6 (28.6) | 11 (72.2) | |
| 11–16 | Mild mood disturbance, n (%) | 2 (9.5) | 4 (11.1) | |
| 17–20 | Borderline depression, n (%) | 5 (23.8) | 2 (11.1) | |
| 21–30 | Moderate depression, n (%) | 6 (28.6) | 1 (5.6) | |
| 31–40 | Severe depression, n (%) | 2 (9.5) | 0 | |
| >41 | Extreme depression, n (%) | 0 | 0 | |
| Total patients with abnormal BDI scores, n (%) | 15 (71.4) | 7 (38.9) | 0.042* |
Note:
Fisher’s exact test.
Abbreviations: BDI, Beck Depression Inventory; OH/ECW, overhydration to extracellular water.
Compared to patients with OH/ECW ratio ≤0.15, in those with OH/ECW ratio >0.15, mean BDI scores, serum estradiol and plasma hsCRP were significantly higher ([18.48±8.34 vs 10.61±5.46, p<0.001], [140.10±44.51 vs 126.10±32.26, p=0.034], [17.70±12.14 vs 9.76±8.79, p=0.013], respectively), while mean IIEF scores, serum total testosterone and DHEA were significantly lower ([12.81±7.31 vs 41.44±23.79, p<0.001], [8.97±5.43 vs 14.10±8.30, p=0.013], [85.31±55.14 vs 133.3±95.48, p=0.029], respectively) (Table 1).
The OH/ECW ratio significantly and inversely correlated with IIEF scores (r=−0.69, p<0.001) and significantly and positively with BDI scores (r=0.64, p<0.001) (Figures 1 and 2). IIEF scores were significantly and inversely correlated with BDI scores (r=−0.54, p<0.001) (Figure 3).
Figure 1.
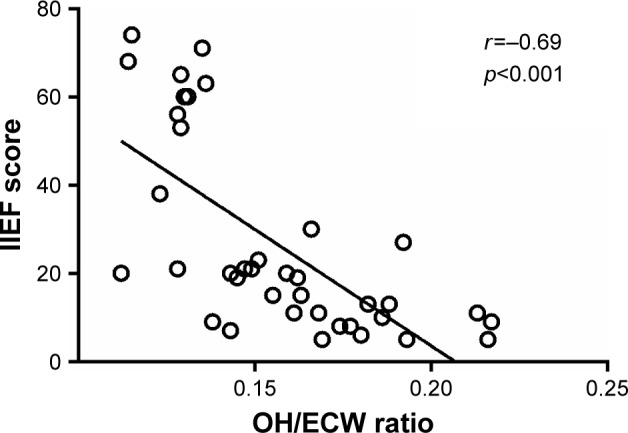
Correlation of hydration status index overhydration to extracellular water (OH/ECW) ratio with International Index of Erectile Function (IIEF) scores.
Figure 2.
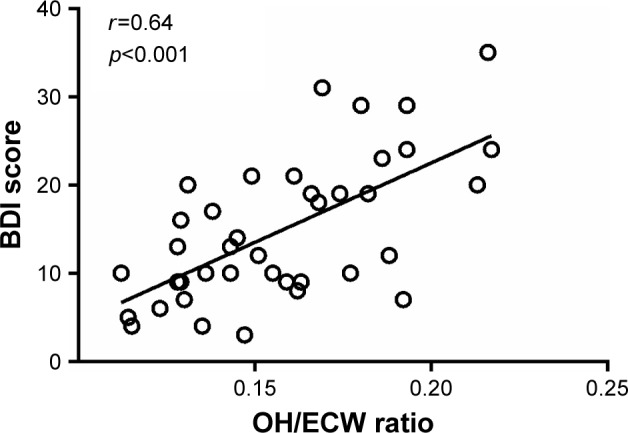
Correlation of hydration status index overhydration to extracellular water (OH/ECW) ratio with Beck Depression Inventory (BDI) scores.
Figure 3.
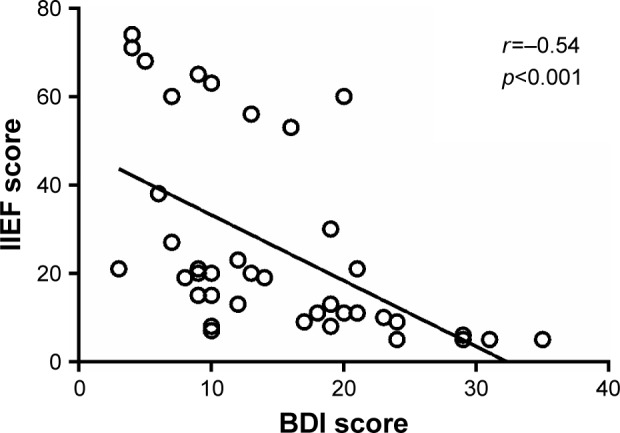
Correlation of Beck Depression Inventory (BDI) scores with International Index of Erectile Function (IIEF) scores.
No significant correlations were found in the OH/ECW ratio or sexual function (IIEF scores) with age, BMI, HD vintage, Kt/V, DUO, hemoglobin and serum LH levels.
Discussion
The main findings of this study were higher prevalence of sexual dysfunction and depression, lower serum levels of total testosterone and DHEA, and higher levels of serum estradiol in overhydrated HD patients, as well as the inverse association of OH with sexual function and the direct association of OH with depression.
Among dialysis patients, reported prevalences of ED and depression were 40%–90%3–5 and 20%–50%, respectively.18–20 In the present study, 85.7% (18/21) of overhydrated patients, with OH/ECW ratio >0.15, had mild to severe ED, and 71.4% (15/21) had abnormal BDI scores, ranging from mild mood disturbances to severe depression.
Overhydrated patients (OH/ECW ratio >0.15) were similar to those who were not overhydrated (OH/ECW ratio ≤0.15) in demographic and clinical characteristics, primary renal disease and medications. Yet they had higher serum estradiol, plasma hsCRP and BDI scores, and lower serum total testosterone, DHEA and IIEF scores. In addition, we report an inverse correlation of the OH/ECW ratio with sexual function and a direct correlation with BDI scores. Moreover, sexual function was inversely correlated with depression. Although previous studies showed a relationship between depression and ED in dialysis patients,22 only one study reported an indirect association between OH and depression.23
Plasma hsCRP, a marker of systemic inflammation, was found to be significantly higher among overhydrated patients. In HD patients, uremic toxins, oxidative stress, increased susceptibility to infections, HD catheter-related infections, prolonged exposure to the dialyzer membrane and OH have been reported to contribute to the development of inflammation.24–27 Systemic inflammation may adversely affect sexual function through interfering with sex hormones.28
OH is considered as an important cause for elevated blood pressure and plasma brain natriuretic peptide (BNP) in dialysis patients;12,13,29 and this may contribute to the development of vascular complications.12,13,29–33 Thus, OH can adversely affect sexual function also through its adverse effects on blood pressure and plasma BNP levels.
The present study showed lower IIEF scores, total serum testosterone and DHEA levels, and higher BDI scores and serum estradiol in overhydrated patients. For normal erectile function, combined activity of nerves, vessels and hormones is necessary to produce penile structural changes and erection.34 In dialysis patients, all the above-mentioned components may be seriously affected. Both low serum testosterone and elevated estradiol levels independently increase the incidence of ED.35 ED related to increased estradiol exposure is independent of testosterone levels.35 In animal models, elevated estradiol has demonstrated an unfavorable effect on erectile function through increasing venous leakage.36 Moreover, administration of exogenous estradiol produces ED in humans through an inhibitory effect on testosterone production; this reduces serum testosterone levels and impairs the structural integrity of the corpus cavernosum.37 Furthermore, estradiol administration reduces spontaneous and nocturnal erections by reducing testosterone levels.38 Additionally, in men with low serum testosterone levels, ED, as assessed using IIEF scores, was more severe in those with higher estradiol levels, suggesting an additive effect.39 In rats, when ED was related to high estradiol levels, testosterone therapy was not useful in restoring erections.40 Considering the described associations of OH with total testosterone, DHEA and estradiol, together with the associations observed of sexual function with hydration status and these hormones, the results of the present study suggest that OH may influence sexual function also through unknown mechanisms that affect sex hormones.
In summary, OH may contribute to sexual dysfunction in several ways. First, testosterone plays a crucial role in regulating male sexual function and has a primary function in controlling and synchronizing male sexual desire and arousal.41 Low serum testosterone levels usually contribute to ED.35 The present study showed lower total testosterone levels in overhydrated HD patients compared to those not overhydrated. Second, low DHEA levels have been related to a higher risk for ED also in people younger than 60 years.42 DHEA supplementation has been related to improvements in ED, desire, sexual interest, sexual activity and arousal.43 The present study showed lower DHEA levels in overhydrated HD patients compared to those without OH. Third, lower IIEF scores were found in overhydrated HD patients compared to those not overhydrated; a significant inverse and previously unreported correlation was demonstrated between hydration status and IIEF scores (Figure 1). Fourth, depression is the most common psychiatric illness in patients with end-stage renal disease and is one of the main disabling diseases contributing to ED.18,44 Higher BDI scores were found in overhydrated HD patients compared to those not overhydrated. Moreover, a significant direct and not previously described correlation was found between hydration status and BDI scores (Figure 2) as well as a significant and inverse correlation between IIEF and BDI scores (r=−0.54, p<0.001) (Figure 3). Fifth, higher levels of estradiol were found in overhydrated HD patients compared to those not overhydrated. In this regard, elevated serum estradiol levels reduce nocturnal erections and can contribute to ED, independent of testosterone levels.35–38 Sixth, inflammation is known to be common among HD patients;24–26 OH has been shown to aggravate systemic inflammation in this population.45 Systemic inflammation may adversely affect sexual function by interfering with sex hormones.28 The present study showed higher hsCRP levels in overhydrated HD patients compared to those not overhydrated. These findings suggest that OH may contribute to the development of ED through worsening inflammatory status. Seventh, since OH is usually accompanied by elevated blood pressure and plasma BNP, it can contribute to the development of vascular complications;12,13,29–33 and therefore adversely affect sexual function.
Finally, this study showed novel and statistically significant differences between HD patients who were overhydrated and were not overhydrated. Taken together, the findings of the present study suggest that OH may have a detrimental effect on sexual function in HD patients.
Conclusion
OH in HD patients was found to be associated with a higher prevalence of sexual dysfunction and depression, lower serum levels of total testosterone and DHEA, and higher levels of serum estradiol.
Although ED itself is a multifactorial problem and treatment strategies should be directed to improve all issues that may contribute to sexual dysfunction, improving treatable causes such as OH should be considered in this setting.
This study was conducted in one center and comprised a relatively small number of patients. Nonetheless, the findings may prompt multi-center studies to verify and confirm the role of OH on ED in HD patients.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Palmer B. Sexual dysfunction in men and women. Semin Dial. 2003;10:48–60. doi: 10.1053/jarr.2003.50003. [DOI] [PubMed] [Google Scholar]
- 2.Navaneethan SD, Vecchio M, Johnson DW, et al. Prevalence and correlates of self-reported sexual dysfunction in CKD: a meta-analysis of observational studies. Am J Kidney Dis. 2010;56(4):670–685. doi: 10.1053/j.ajkd.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 3.Palmer BF. Sexual dysfunction in uremia. J Am Soc Nephrol. 1999;10(6):1381–1388. doi: 10.1681/ASN.V1061381. [DOI] [PubMed] [Google Scholar]
- 4.Finkelstein SH, Finkelstein FO. Evaluation of sexual dysfunction. In: Nissenson AR, Fine RN, editors. Dialysis Therapy. 2nd ed. Philadelphia, PA: Hanley & Belfus; 1993. pp. 270–273. [Google Scholar]
- 5.Lew-Starowicz M, Gellert R. The sexuality and quality of life of hemodialyzed patients – ASED multicenter study. J Sex Med. 2009;6(4):1062–1071. doi: 10.1111/j.1743-6109.2008.01040.x. [DOI] [PubMed] [Google Scholar]
- 6.Weisbord SD. Sexual dysfunction and quality of life in patients on maintenance dialysis. Semin Dial. 2013;26(3):278–280. doi: 10.1111/sdi.12068. [DOI] [PubMed] [Google Scholar]
- 7.Fadem SZ, Walker DR, Abbott G, et al. Satisfaction with renal replacement therapy and education: the American Association of Kidney Patients survey. Clin J Am Soc Nephrol. 2011;6(3):605–612. doi: 10.2215/CJN.06970810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foulks CJ, Cushner HM. Sexual dysfunction in the male dialysis patient: pathogenesis, evaluation, and therapy. Am J Kidney Dis. 1986;8(4):211–222. doi: 10.1016/s0272-6386(86)80029-4. [DOI] [PubMed] [Google Scholar]
- 9.Rosas SE, Joffe M, Franklin E, et al. Prevalence and determinants of erectile dysfunction in hemodialysis patients. Kidney Int. 2001;59(6):2259–2266. doi: 10.1046/j.1523-1755.2001.00742.x. [DOI] [PubMed] [Google Scholar]
- 10.Hochstetler LA, Flanigan MJ, Lim VS. Abnormal endocrine tests in a hemodialysis patient. J Am Soc Nephrol. 1994;4(10):1754–1759. doi: 10.1681/ASN.V4101754. [DOI] [PubMed] [Google Scholar]
- 11.Leavey SF, Weitzel WF. Endocrine abnormalities in chronic renal failure. Endocrinol Metab Clin North Am. 2002;31(1):107–119. doi: 10.1016/s0889-8529(01)00006-8. [DOI] [PubMed] [Google Scholar]
- 12.Kim YJ, Jeon HJ, Kim YH, et al. Overhydration measured by bioimpedance analysis and the survival of patients on maintenance hemodialysis: a single-center study. Kidney Res Clin Pract. 2015;34(4):212–218. doi: 10.1016/j.krcp.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merhametsiz O, Oguz EG, Yayar O, Bektan B, Canbakan B, Ayli D. Bioimpedance spectroscopy method to determine hypervolemia in maintenance hemodialysis patients. Hippokratia. 2015;19(4):324–331. [PMC free article] [PubMed] [Google Scholar]
- 14.Chamney PW, Krämer M, Rode C, Kleinekofort W, Wizemann V. A new technique for establishing dry weight in hemodialysis patients via whole body bioimpedance. Kidney Int. 2002;61(6):2250–2258. doi: 10.1046/j.1523-1755.2002.00377.x. [DOI] [PubMed] [Google Scholar]
- 15.Chamney PW, Wabel P, Moissl UM, et al. A whole-body model to distinguish excess fluid from the hydration of major body tissues. Am J Clin Nutr. 2007;85(1):80–89. doi: 10.1093/ajcn/85.1.80. [DOI] [PubMed] [Google Scholar]
- 16.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822–830. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 17.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8(1):77–100. [Google Scholar]
- 18.King-Wing Ma T, Kam-Tao Li P. Depression in dialysis patients. Nephrology (Carlton) 2016;21(8):639–646. doi: 10.1111/nep.12742. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Yang X, Yao L, et al. Prevalence and related factors of depressive symptoms in hemodialysis patients in northern China. BMC Psychiatry. 2017;17(1):128. doi: 10.1186/s12888-017-1294-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murillo-Zamora E, Macías-de la Torre AA, Higareda-Almaraz MA. Depression prevalence among end stage renal disease patients in maintenance hemodialysis. Rev Med Inst Mex Seguro Soc. 2016;54(4):429–433. Spanish [with English abstract] [PubMed] [Google Scholar]
- 21.Chan LK, Yu EC, Li SY. Depression in patients receiving peritoneal dialysis. East Asian Arch Psychiatry. 2011;21(3):99–107. [PubMed] [Google Scholar]
- 22.Soykan A, Boztaş H, Idilman R, et al. Sexual dysfunctions in HCV patients and its correlations with psychological and biological variables. Int J Impot Res. 2005;17(2):175–179. doi: 10.1038/sj.ijir.3901267. [DOI] [PubMed] [Google Scholar]
- 23.Kursat S, Colak HB, Toraman A, Ekmekci C, Tekce H, Alici T. The relationship between depression-malnutrition and echocardiographic-blood pressure parameters in chronic hemodialysis patients. Int Urol Nephrol. 2008;40(3):793–799. doi: 10.1007/s11255-008-9342-y. [DOI] [PubMed] [Google Scholar]
- 24.Kalantar-Zadeh K, Stenvinkel P, Pillon L, Kopple JD. Inflammation and nutrition in renal insufficiency. Adv Ren Replace Ther. 2003;10(3):155–169. doi: 10.1053/j.arrt.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Avila-Díaz M, Ventura MD, Valle D, et al. Inflammation and extracellular volume expansion are related to sodium and water removal in patients on peritoneal dialysis. Perit Dial Int. 2006;26(5):574–580. [PubMed] [Google Scholar]
- 26.van der Walt C, Malan L, Uys AS, Malan NT. Low grade inflammation and ECG left ventricular hypertrophy in urban African males: the SABPA study. Heart Lung Circ. 2013;22(11):924–929. doi: 10.1016/j.hlc.2013.03.075. [DOI] [PubMed] [Google Scholar]
- 27.Monfared A, Salari A, Kazemnezhad E, et al. Association of left ventricular hypertrophy with high-sensitive C-reactive protein in hemodialysis patients. Int Urol Nephrol. 2013;45(6):1679–1686. doi: 10.1007/s11255-012-0375-x. [DOI] [PubMed] [Google Scholar]
- 28.Carrero JJ, Qureshi AR, Parini P, et al. Low serum testosterone increases mortality risk among male dialysis patients. J Am Soc Nephrol. 2009;20(3):613–620. doi: 10.1681/ASN.2008060664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wizemann V, Wabel P, Chamney P, et al. The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transplant. 2009;24(5):1574–1579. doi: 10.1093/ndt/gfn707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voroneanu L, Cusai C, Hogas S, et al. The relationship between chronic volume overload and elevated blood pressure in hemodialysis patients: use of bioimpedance provides a different perspective from echocardiography and biomarker methodologies. Int Urol Nephrol. 2010;42(3):789–797. doi: 10.1007/s11255-010-9767-y. [DOI] [PubMed] [Google Scholar]
- 31.Chan CT, Greene T, Chertow GM, et al. Frequent Hemodialysis Network (FHN) Trial Group Determinants of left ventricular mass in patients on hemodialysis: frequent Hemodialysis Network (FHN) Trials. Circ Cardiovasc Imaging. 2012;5(2):251–261. doi: 10.1161/CIRCIMAGING.111.969923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nwafor CE, Adebiyi AA, Ogah OS, Falase AO. Relationship between 24-hour blood pressure pattern and left ventricular structure and function in hypertensive Nigerians. Ethn Dis. 2013;23(4):474–479. [PubMed] [Google Scholar]
- 33.Ducloux D, Bresson-Vautrin C, Kribs M, Abdelfatah A, Chalopin JM. C-reactive protein and cardiovascular disease in peritoneal dialysis patients. Kidney Int. 2002;62(4):1417–1422. doi: 10.1111/j.1523-1755.2002.kid562.x. [DOI] [PubMed] [Google Scholar]
- 34.Kasapoglu B, Turkay C, Bayram Y, Koca C. Role of GGT in diagnosis of metabolic syndrome: a clinic-based cross-sectional survey. Indian J Med Res. 2010;132:56–61. [PubMed] [Google Scholar]
- 35.Schulster M, Bernie AM, Ramasamy R. The role of estradiol in male reproductive function. Asian J Androl. 2016;18(3):435–440. doi: 10.4103/1008-682X.173932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mancini A, Milardi D, Bianchi A, Summaria V, De Marinis L. Increased estradiol levels in venous occlusive disorder: a possible functional mechanism of venous leakage. Int J Impot Res. 2005;17(3):239–242. doi: 10.1038/sj.ijir.3901287. [DOI] [PubMed] [Google Scholar]
- 37.Srilatha B, Adaikan PG. Estrogen and phytoestrogen predispose to erectile dysfunction: do ER-alpha and ER-beta in the cavernosum play a role? Urology. 2004;63(2):382–386. doi: 10.1016/j.urology.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 38.Kwan M, VanMaasdam J, Davidson JM. Effects of estrogen treatment on sexual behavior in male-to-female transsexuals: experimental and clinical observations. Arch Sex Behav. 1985;14(1):29–40. doi: 10.1007/BF01541350. [DOI] [PubMed] [Google Scholar]
- 39.El-Sakka AI. Impact of the association between elevated oestradiol and low testosterone levels on erectile dysfunction severity. Asian J Androl. 2013;15(4):492–496. doi: 10.1038/aja.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kataoka T, Hotta Y, Ohno M, Maeda Y, Kimura K. Limited effect of testosterone treatment for erectile dysfunction caused by high-estrogen levels in rats. Int J Impot Res. 2013;25(6):201–205. doi: 10.1038/ijir.2013.21. [DOI] [PubMed] [Google Scholar]
- 41.Corona G, Isidori AM, Aversa A, Burnett AL, Maggi M. Endocrinologic control of men’s sexual desire and arousal/erection. J Sex Med. 2016;13(3):317–337. doi: 10.1016/j.jsxm.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Reiter WJ, Pycha A, Schatzl G, et al. Serum dehydroepiandrosterone sulfate concentrations in men with erectile dysfunction. Urology. 2000;55(5):755–758. doi: 10.1016/s0090-4295(99)00567-1. [DOI] [PubMed] [Google Scholar]
- 43.Reiter WJ, Pycha A, Schatzl G, et al. Dehydroepiandrosterone in the treatment of erectile dysfunction: a prospective, double-blind, randomized, placebo-controlled study. Urology. 1999;53(3):590–594. doi: 10.1016/s0090-4295(98)00571-8. discussion 594–595. [DOI] [PubMed] [Google Scholar]
- 44.Soterio-Pires JH, Hirotsu C, Kim LJ, Bittencourt L, Tufik S, Andersen ML. The interaction between erectile dysfunction complaints and depression in men: a cross-sectional study about sleep, hormones and quality of life. Int J Impot Res. 2017;29(2):70–75. doi: 10.1038/ijir.2016.49. [DOI] [PubMed] [Google Scholar]
- 45.Hassan K, Hassan F, Edgem R, Moshe S, Hassan S. The impact of the peritoneal glucose load index on hydration status and inflammation in peritoneal dialysis patients. J Int Med Res. 2015;43(1):42–53. doi: 10.1177/0300060514550013. [DOI] [PubMed] [Google Scholar]


