Abstract
Objectives
Ovarian cancer is one of the most lethal malignant tumors in women. Secreted phosphoprotein 1 (SPP1) plays an important role in some cancer types. Therefore, the role of SPP1 in ovarian cancer was determined and the potential mechanism was elucidated.
Materials and methods
The expression of SPP1 in ovarian cancer was determined by immunohistochemistry in ovarian cancer tissues and normal ovarian tissues. Cellular proliferation, migration, and invasion were determined by cell counting kit-8 assay, wound healing assay, and Matrigel invasion assay in SKOV3 and A2780 cells. The protein expression of SPP1, integrin subunit β1 (Integrin β1), focal adhesion kinase (FAK), and phosphorylation protein kinase B (p-AKT) was detected by Western blotting in SKOV3 cells after silencing SPP1. The expression of SPP1 was determined in SKOV3 cells after transfecting with miR-181a mimics or inhibitors. The growth of SKOV3 cells in vivo was determined in a nude mouse model of ovarian cancer after silencing SPP1.
Results
The expression of SPP1 was higher in epithelial ovarian cancer tissues than in normal ovarian tissues. Silencing SPP1 decreased the cell proliferation, migration, and invasion. Ectopic expression of SPP1 increased the cell proliferation, migration, and invasion. Silencing SPP1 prevented ovarian cancer growth in mice. Silencing SPP1 inhibited Integrin β1/FAK/AKT pathway. In agreement, ectopically expressed SPP1 activated Integrin β1/FAK/AKT pathway. Also, SPP1 was regulated by miR-181a.
Conclusion
SPP1 is a biomarker for the prognosis of ovarian cancer. It is also oncogenic and a potential target for ovarian cancer therapy.
Keywords: ovarian cancer, SPP1, Integrin β1, FAK, AKT, miR-181a
Introduction
Ovarian carcinoma is one of the most common gynecologic malignant tumors with a high mortality rate.1 Despite rapid progress in the chemotherapy and radiotherapy of ovarian carcinoma, patients with recurrent ovarian cancer are essentially incurable.2 The 5-year survival rate after diagnosis of ovarian cancer has not improved significantly.3 The pathogenesis of ovarian cancer remains unclear.
Secreted phosphoprotein 1 (SPP1) is a secreted arginine glycine aspartic acid containing phosphorylated glycoprotein. It has a molecular weight of about 325,000.4 The human SPP1 gene is located on chromosome 4 (4q13) with seven exons and six introns.5 The protein can be secreted by various cells, such as osteoclasts, macrophages, epithelial cells, and endothelial cells.6 The expression of SPP1 is strongly related to tumor metastasis in gastric cancer and esophageal adenocarcinoma.7–9 Previous studies reported that the expression of SPP1 is high in numerous tumors, including lung cancer, colon cancer, breast cancer, and prostate cancer.10–12 SPP1 promotes the progress of cancer through modulation of vascular endothelial growth factor expression and regulation of extracellular matrix protein.13,14 However, the localization and function of SPP1 in ovarian cancer are not known.
We hypothesized that SPP1 plays an important role in the development of ovarian cancer. Therefore, we intended to detect the expression of SPP1 in ovarian cancer tissue. Then, we studied the effect of SPP1 on ovarian cancer in vitro. Finally, we studied the growth of SKOV3 cells after silencing SPP1 in vivo. In this research, we found an aberrant localization and expression of SPP1 in ovarian cancer tissues. Additionally, we found that SPP1 was involved in the regulation of cellular migration and invasion, and cell proliferation in ovarian cancer. We also found that SPP1 can modulate Integrin β1 (ITGB1)/focal adhesion kinase (FAK)/AKT pathway. Further, we identified SPP1 to be an authentic target of miR-181a.
Materials and methods
The tissue microarray slides including ovarian cancer tissues and normal ovarian tissues (n=55) were purchased from US Biomax Inc. (Rockville, MD, USA). The use of human samples was approved by the Ethics Committee of Chongqing Medical University.
Immunohistochemistry
Immunohistochemistry was performed according to the instructions given in SP kit (ab80436, Abcam Company). The procedure of immunohistochemical staining was in accordance with our previous studies.15,16 All the sections were observed by three pathologists using a light microscope.17 The intensity of SPP1-positive cells was as follows: 0, 1, 2, and 3 for negative, weak, moderate, and strong intensity. The percentage of SPP1-positive cells was scored as follows: 0 for no cytoplasm expression, 1 for 1%–25% positive tumor cytoplasm, 2 for 26%–50% positive tumor cytoplasm, 3 for 51%–75% positive tumor cytoplasm, and 4 for 76%–100% positive tumor cytoplasm.18 The multiplication of the intensity and percentage scores led to the final SPP1 staining score and was defined as follows: staining score <6 was considered as low expression, while staining score of 7 or more was considered as high expression. All values were shown as the mean ± standard error of the mean.
Cell culture, transfection procedure, and reagents
Human ovarian cancer cells lines SKOV3 and A2780 were purchased from Jiangsu KeyGEN BioTECH Corp., Ltd. The cells were cultured in RPM1 1640 medium. The medium was supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin in an atmosphere of 5% carbon dioxide at 37°C. The siRNA sequences were obtained from Guangzhou RiboBio Co., Ltd (Guangzhou, China). The following sequences were targeted for human SPP1, ITGB1, FAK, and AKT siRNA, respectively. SPP1-1: 5′-GGAUGAUAUGGAUGAUGAAGA-3′; SPP1-2: 5′-GAACGACUCUGAUGAUGUAGA-3′; ITGB1: 5′-GGUUACUCUUGUCAGCUAAGG-3′; FAK: 5′-CGAUUAUAUGUUAGAGAUAGC-3′; AKT: 5′-UGUGUAUUAUGUUGUUCAAAU-3′; and negative control (NC) siRNA: 5′-UUCUUCGAAGGUGUCACGUTT-3′. Lentiviral vector expressing shRNA targeting SPP1 (named LV3-shPP1-1 and LV3-shPP1-2) and SPP1-lentiviral expression vector (named LV5-SPP1) were provided by Guangzhou RiboBio Co., Ltd. miR-181a mimics were synthesized at Guangzhou RiboBio Co., Ltd.
Quantitative real-time polymerase chain reaction (PCR)
Total RNAs were extracted as described previously.19 The reverse transcriptase and quantitative PCR reactions were conducted as previously described.17 PCR amplifications were started with a 10 min denaturation step at 95°C, followed by 40 amplification cycles (10 sec at 95°C, 20 sec at 60°C, 10 sec at 72°C). Relative quantification of mRNA was performed using the comparative threshold cycles method. The value was used to plot the gene expression employing the formula 2−ΔΔCT. The primers used for amplifying SPP1, ITGB1, FAK, and AKT and GAPDH were synthesized by Guangzhou Funeng Co., Ltd.
Detection of protein expression by Western blotting
Western blot analysis was performed as previously described.20 Three independent experiments in a certain condition were subjected to Western blot analysis. The SPP1 (ab33046), ITGB1 (ab52971), FAK (ab40794), AKT1 (ab81283), and GAPDH (ab8245) antibodies were purchased from Abcam Inc. The antibodies were diluted 1:1,000. The bands were detected with enhanced chemiluminescence reagent (Beyotime Institute of Biotechnology, Jiangsu, China) and quantified by ImageQuant 3.3 software (Molecular Dynamics).
Cell proliferation assay
Cellular proliferation was determined by cell counting kit-8 assay following the manufacturer’s instructions (Beyotime).21,22 Cells were seeded into 96-well plates and cultured for an additional 24 h. After treatment with lentiviral vector, 10 μL of the kit reagent was added and then incubated for another 2 h. The OD value was read at 450 nM to obtain the final results.
Wound healing assay and Matrigel invasion assays
Cell migration and invasion assay was performed in accordance with our previous studies.23,24 Briefly, ovarian cancer cells were cultured in six-well plates to about 80% confluence. The medium was replaced with serum-free medium. After wounding was achieved, the distance between two wounds was measured at 0 and 72 h. Invasion assay was performed as follows. The upper side was coated using Matrigel basement membrane matrix for 2 h at 37°C. The cells were added into the top chamber and then incubated for 48 h. Six percent paraformaldehyde was used to fix the invasive cells. Then, they were stained with 0.5% crystal violet (Beyotime) and counted.
Dual-luciferase reporter gene assay
Luciferase reporter gene assay was performed using the dual-luciferase reporter assay system (Promega, Madison, WI, USA) according to the instructions provided by the manufacturer. For SPP1 3′ untranslated region (UTR) luciferase reporter assay, wild-type or mutant reporter constructs (purchased from Genepharma Co., Ltd., Shanghai, China) were co-transfected into SKOV3 cells in 24-well plates with 100 nM miR-181a or 100 nM miR-NC and Renilla plasmid by using Endofectin™-Plus (GeneCopoeia). Reporter gene assay was performed 48 h post-transfection using the dual-luciferase assay system (Promega). Firefly luciferase activity was normalized for transfection efficiency using the corresponding Renilla luciferase activity. All experiments were performed at least three times.
Animal experiment
All animal care and handling procedures followed the guidelines approved by the Institutional Animal Use and Care Committee of Chongqing Medical University. SKOV3 cells were infected with LV3-NC or LV3-shSPP1-1. Six-week-old BALB/c nude mice were grouped into LV3-NC infected group (n=5) and LV3-shSPP1-1 infected group (n=5). The infected SKOV3 cells were injected subcutaneously into the left armpit. Twenty-eight days later, the weight and volume of tumors were calculated.
Statistical analysis
All statistical analyses were performed using SPSS 19.0 software. Each experiment was performed in triplicate. Student’s t-test or analysis of variance was used for statistical analysis. The differences were considered significant when p<0.05.
Results
Increased expression of SPP1 in epithelial ovarian carcinoma tissues
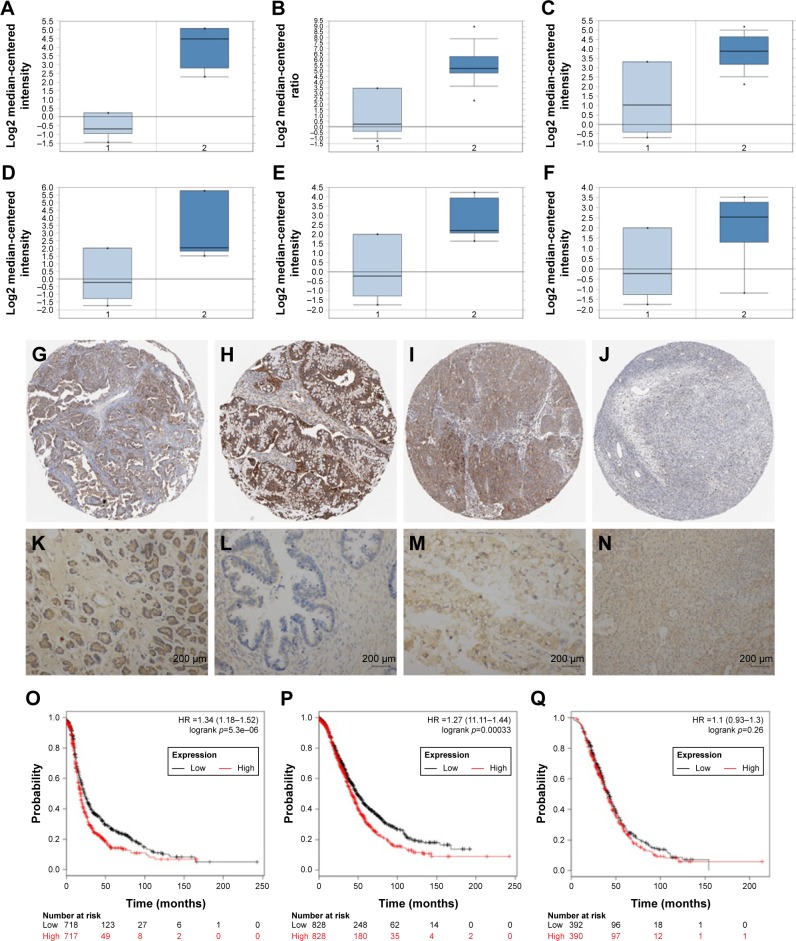
Using Oncomine analysis, we investigated the mRNA levels of SPP1 genes in ovarian cancers. Three independent data (Adib Ovarian, Yoshihara Ovarian, and Lu Ovarian; https://www.oncomine.org/resource/login.html) showed that the mRNA level of SPP1 in ovarian serous adenocarcinoma was higher than in normal ovarian tissues (fold changes were 25.623, 31.013, and 6.844, respectively; p=3.12E–5, 6.61E–7, and 0.008, respectively; Figure 1A–C). Then we analyzed the expression of SPP1 in other epithelial cancer histologic types (data derived from Lu Ovarian). The mRNA level of SPP1 in ovarian clear cell adenocarcinoma, ovarian endometrioid adenocarcinoma, and ovarian mucinous adenocarcinoma was higher than in normal ovarian tissues (fold changes were 9.504, 7.185, and 4.496, respectively; p=0.003, 0.004, and 0.015, respectively; Figure 1D–F).
Figure 1.
Immunohistochemical analysis of SPP1 expression in epithelial ovarian cancer.
Notes: The mRNA expression of SPP1 in epithelial cancer was investigated. Data were derived from Oncomine database. (A–C) Three independent data (Adib Ovarian, Yoshihara Ovarian, and Lu Ovarian) showed that the mRNA level of SPP1 in ovarian serous adenocarcinoma was higher than in normal ovarian tissues. (D–F; data derived from Lu Ovarian) The mRNA level of SPP1 in ovarian clear cell adenocarcinoma, ovarian endometrioid adenocarcinoma, and ovarian mucinous adenocarcinoma was higher than in normal ovarian tissues. (G–I) The location and expression of SPP1 protein in ovarian cancer tissues was analyzed. The data were derived from the human protein atlas. The expression of SPP1 in ovarian serous adenocarcinoma, ovarian mucinous adenocarcinoma, and ovarian endometrioid adenocarcinoma showed moderate staining. (J) SPP1 in the follicle cells showed medium staining. SPP1 was not detected in ovarian stroma cells. (K–N) The expression pattern of SPP1 was determined in normal ovarian tissues and epithelial ovarian cancer tissue samples using IHC. Original magnification, 200×. SPP1 expression reversely correlated with survival time. Kaplan–Meier survival analysis for the relationship between survival time and SPP1 signature in ovarian cancer was performed by using the online tool (http://kmplot.com/analysis/). (O) PFS curves are plotted for ovarian cancer patients (n=1,436). (P) OS curves are plotted for ovarian cancer patients (n=1,657). (Q) PPS curves are plotted for ovarian cancer patients (n=782). p<0.05 was considered to be a statistically significant difference.
Abbreviations: HR, hazard ratio; OS, overall survival; PFS, progression-free survival; PPS, post-progression survival; SPP1, secreted phosphoprotein 1.
We next analyzed the location of SPP1 protein in ovarian cancer tissues derived from the human protein atlas. SPP1 was mainly localized in the plasma membrane and cytoplasm (Figure 1G–I). The expression of SPP1 in ovarian serous adenocarcinoma, ovarian mucinous adenocarcinoma, and ovarian endometrioid adenocarcinoma showed moderate staining (Figure 1G–I). The SPP1 in follicle cells was medium staining. However, SPP1 was not detected in ovarian stroma cells (Figure 1J).
We then determined the expression of SPP1 in normal ovarian tissues and ovarian cancer tissues. The clinicopathologic data were obtained from US Biomax Inc. The expression of SPP1 was increased in epithelial ovarian cancer than in normal ovary tissues (p<0.05; Figure 1K–N; Table 1). Mann–Whitney U test was used to compare the associations between SPP1 overexpression and clinicopathologic variables of 45 epithelial ovarian cancer samples (Table 1). The associations between SPP1 expression and age were not significant (p>0.05; Table 1). SPP1 expression was significantly higher in cancer with advanced stages (stage III/IV) as compared with those in the early stages (stage I/II; p<0.05). The expression of SPP1 was correlated with tumor grade (grades 2–3 versus 1, p<0.05). Evaluation of the SPP1 genes with progression-free survival, overall survival, and post-progression survival was performed using the KM Plotter Online Tool (http://www.kmplot.com). Low expression of SPP1 mRNA was significantly correlated with favorite PFS and OS, but PPS for all ovarian cancer patients was not significant (Figure 1O–Q).
Table 1.
Association of SPP1 expression with clinicopathologic characteristics in 55 patients of EOC
| Characteristics | No of patients (N=55) | SPP1 expression
|
p-value | |
|---|---|---|---|---|
| Low no (%) | High no (%) | |||
| Age (years) | ||||
| <50 | 24 | 9 (37.50) | 15 (62.50) | >0.05 |
| ≥50 | 31 | 11 (35.48) | 20 (64.52) | |
| Normal ovarian | 10 | 8 (80.00) | 2 (20.00) | <0.05 |
| Cancer tissues | 45 | 12 (36.67) | 33 (73.33) | |
| FIGO stage | ||||
| I/II | 23 | 10 (43.48) | 13 (56.52) | <0.05 |
| III/IV | 22 | 2 (9.09) | 20 (90.91) | |
| Grade | ||||
| 1 | 12 | 5 (41.67) | 7 (58.33) | <0.05 |
| 2 | 14 | 4 (28.57) | 10 (71.43) | |
| 3 | 19 | 3 (15.79) | 16 (84.21) | |
| Grade 2–3 versus 1 | ||||
Abbreviations: SPP1, secreted phosphoprotein 1; EOC, epithelial ovarian cancer; FIGO, International Federation of Gynecology and Obstetrics.
SPP1 regulates cellular proliferation, migration, and invasion
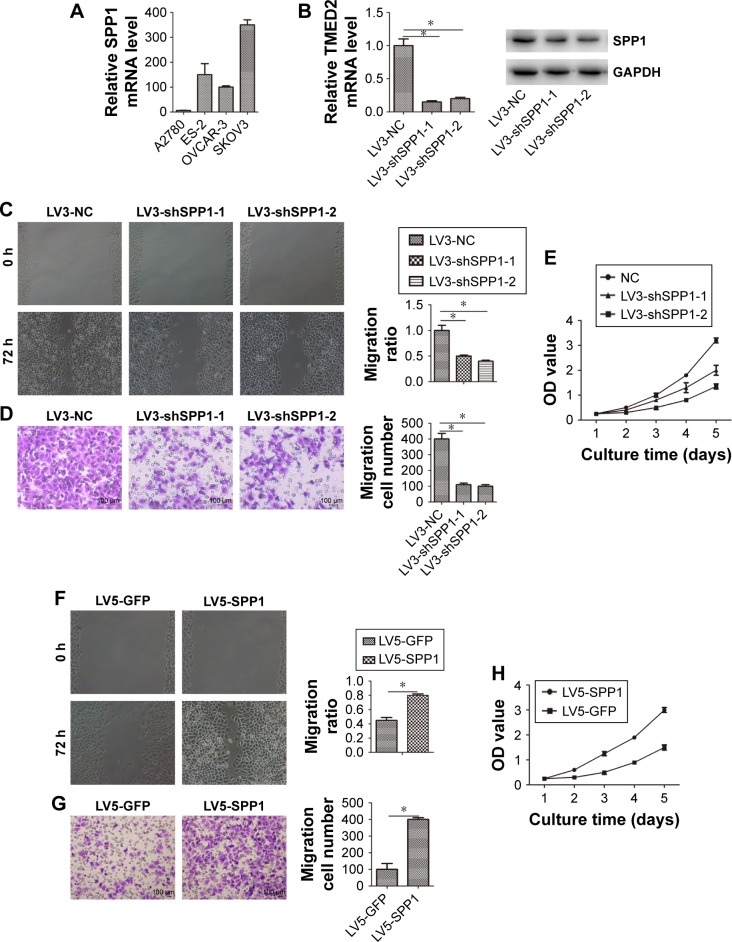
Cellular proliferation, migration, and invasion is an important cause of ovarian cancer progression and recurrence. So, we determined the role of SPP1 in cellular proliferation, migration, and invasion. We firstly determined the expression of SPP1 in four cell lines. Of these, SPP1 expression was higher in the SKOV3 cell line in comparison with the A2780 cell line (Figure 2A). The mRNA and protein levels of SPP1 were reduced in LV3-shSPP1-1- and LV3-shSPP1-2-infected SKOV3 cells compared to LV3-NC-infected SKOV3 cells (Figure 2B). Our data showed that the ability for cell proliferation, migration, and invasion was decreased in SKOV3 cells infected with LV3-shSPP1-1 and LV3-shSPP1-2 compared to that in SKOV3 cells infected with LV3-NC (p<0.05; Figure 2C–E). We also determined the ectopic expression of SPP1 in A2780 cells infected with LV5-SPP1. We found that the ectopic expression of SPP1 in A2780 cells increased the ability of cell proliferation, migration, and invasion, compared to LV-5-GFP-infected cells (p<0.05; Figure 2F–H).
Figure 2.
SPP1 regulates cellular proliferation, migration, and invasion.
Notes: (A) The relative expression of SPP1 mRNA in ovarian cancer cell lines. (B) SPP1 mRNA and protein levels were downregulated on infecting with LV3-shSPP1-1 or LV3-shSPP1-2. (C) Ovarian cancer SKOV3 cells’ migration ability was detected by the wound healing assay. The migration of LV3-shSPP1-1- and LV3-shSPP1-2-infected SKOV3 cells was lower as compared to that of LV3-NC-infected cells. (D) Ovarian cancer SKOV3 cells’ invasion ability was detected by Matrigel invasion assay. The invasion ability of LV3-shSPP1-1- and LV3-shSPP1-2-infected SKOV3 cells was decreased compared to that of LV3-NC-infected cells. (E) Cell proliferation was determined by CCK-8 assay. (F) Ovarian cancer A2780 cells’ migration ability was detected by wound healing assay. The migration ability of LV5-SPP1-infected A2780 cells was increased compared to that of LV3-NC-infected cells. (G) Ovarian cancer A2780 cells’ invasion ability was detected by Matrigel invasion assay. The invasion ability of LV5-SPP1-infected A2780 cells was increased compared to that of LV5-GFP-infected cells. (H) Cell proliferation was determined by CCK-8 assay. Error bars represent standard error. *p<0.05 (C, D, F, G) Magnification 200×.
Abbreviations: CCK-8, cell counting kit-8; NC, negative control; SPP1, secreted phosphoprotein 1.
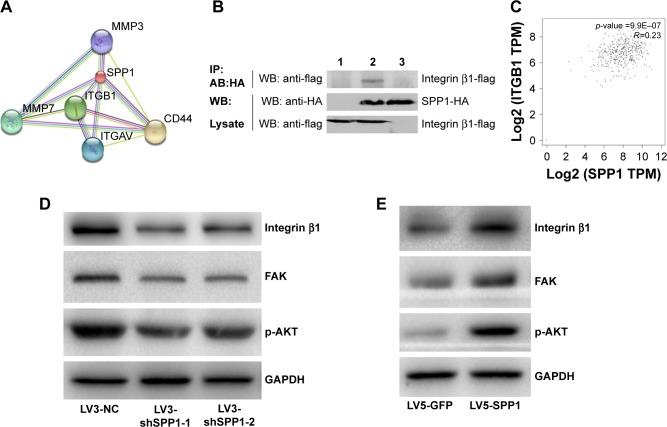
SPP1 regulated ITGB1/FAK/AKT pathway
Bioinformatics analyses (https://string-db.org/) showed that SPP1 may interact with ITGB1 (Figure 3A). In order to identify the interaction between SPP1 and ITGB1, we designed a co-immunoprecipitation experiment. Cells were co-transfected with Flag-ITGB1 and HA-SPP1. Control group was established simultaneously, and then cells were harvested 24 h later. Anti-HA antibodies were used to pull the interaction protein. Then, they were detected by anti-Flag antibodies using Western blot. Our results indicated that Flag bands could not be detected in cells infected with Flag-ITGB1 or HA-SPP1 only. However, they could be detected in cells that were co-transfected with Flag-ITGB1 and HA-SPP1 (Figure 3B). The Cancer Genome Atlas data (http://gepia.cancer-pku.cn/detail.php) indicated that the expression of SPP1 in ovarian cancer was positively correlated with ITGB1 (R=0.23; p=9.9E–07; Figure 3C).25
Figure 3.
SPP1-promoted A2780 cellular proliferation, migration, and invasion involves ITGB1/FAK/AKT pathway.
Notes: (A) Bioinformatics analyses showed that SPP1 may interact with Integrin β1 (https://string-db.org/). (B) The interaction between SPP1 and Integrin β1 was identified by co-immunoprecipitation assay. Cells were co-transfected with Flag-Integrin β1 and HA-SPP1, and a control group was established simultaneously. Cells were then harvested 24 h later. Anti-HA antibodies were used to pull the interaction protein. Then, they were detected by anti-Flag antibodies. Results showed that Flag bands could not be detected in the cells transfected with Flag-Integrin β1 (lane 3) or HA-SPP1 (lane 1) only. However, they could be detected in cells co-transfected with both Flag-Integrin β1 and HA-SPP1 (lane 2), which indicated that there existed an interaction between SPP1 and Integrin β1 in vivo. (C) TCGA data indicated that the expression of SPP1 in ovarian cancer was positively correlated with Integrin β1 (http://gepia.cancer-pku.cn/detail.php). (D, E) The expression of Integrin β1, FAK, and p-AKT was determined by Western blotting.
Abbreviations: FAK, focal adhesion kinase; TCGA, The Cancer Genome Atlas.
Previous studies revealed that ITGB1 is involved in the development of ovarian cancer via FAK/AKT pathway.26–29 We guessed that SPP1 may regulate the ITGB1/FAK/AKT pathway. ITGB1, FAK, and p-AKT protein levels were decreased in SKOV3 cells when SPP1 was silenced (Figure 3D). However, ectopic SPP1 gene expression in A2780 cells increased the protein level of ITGB1, FAK, and p-AKT (Figure 3E). These data indicated that SPP1 may activate ITGB1/FAK/AKT signaling axis in human epithelial ovarian cancer.
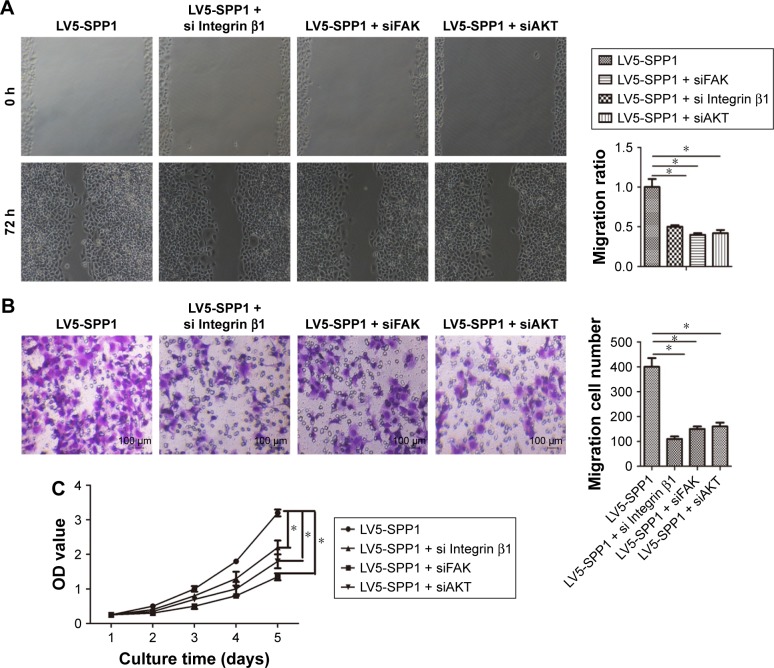
SPP1-promoted A2780 cellular proliferation, migration, and invasion involves ITGB1/FAK/AKT pathway
We have demonstrated that SPP1 can regulate the ITGB1/FAK/AKT signaling axis. Hence, we presumed that SPP1 regulation of epithelial ovarian cancer proliferation, migration, and invasion involves ITGB1/FAK/AKT pathway. We found that silencing of ITGB1, FAK, or AKT partially inhibited the cellular proliferation, migration, and invasion ability of LV5-SPP1-infected A2780 cells (Figure 4A–C). These results suggest that SPP1 promotes epithelia ovarian cancer cell proliferation, which is mediated by ITGB1/FAK/AKT pathway.
Figure 4.
SPP1-promoted A2780 cellular proliferation, migration, and invasion involves Integrin β1/FAK/AKT pathway.
Notes: A2780 cells were infected with LV5-GFP and LV5-SPP1. After 48 h, puromycin was added at a concentration of 2.5 μg/mL. The cells were transfected with Integrin β1 siRNA, FAK siRNA, or AKT siRNA for 48 h. Then, the cellular migration ability (A), invasion ability (B), and proliferation ability (C) were detected. (A and B) Original magnification, ×200. *p<0.05.
Abbreviations: FAK, focal adhesion kinase; SPP1, secreted phosphoprotein 1.
miR-181a regulates SPP1 expression in ovarian cancers
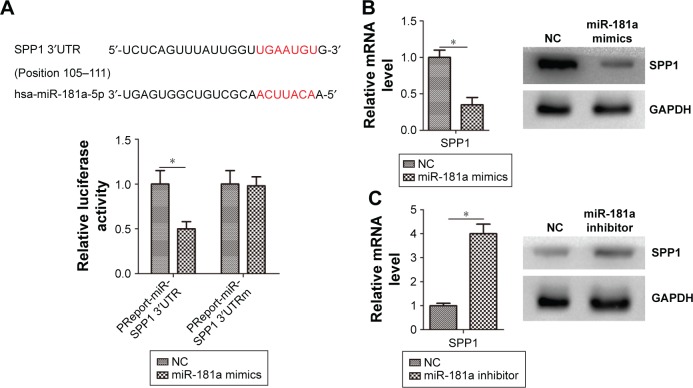
Bioinformatics analyses predicted that SPP1 is a potential target of miR-181a (http://www.targetscan.org/). We also predicted that miR-181a-specific binding site was located within the 3′UTR of SPP1 mRNA (Figure 5A). We observed that miR-181a significantly reduced luciferase activity when SPP1 3′UTR was inserted downstream of luciferase cDNA in the reporter vector (pMIR-SPP1-3′UTR) (Figure 5A). We also observed that miR-181a mimics reduced the expression of SPP1 (Figure 5B). miR-181a inhibitors increased the expression of SPP1 (Figure 5C).
Figure 5.
miR-181a regulates SPP1 expression in ovarian cancers.
Notes: (A) Putative binding sites targeted by miR-181a were predicted to be located in the 3′UTR of SPP1 mRNA. SKOV3 cells were co-transfected with miR-181a mimics or control RNA (NC) with luciferase reporter plasmids containing either wild-type (pMIR-SPP1-3′UTR) or mutant 3′UTR (pMIR-SPP1-3′UTRm) SPP1 genes. Luciferase expression was measured. (B) Ovarian cancer SKOV3 cells were transfected with miR-181a mimics or control RNA (NC). (C) Ovarian cancer SKOV3 cells were transfected with miR-181a inhibitor or control RNA (NC). Error bars represent standard error. *p<0.05.
Abbreviations: NC, negative control; SPP1, secreted phosphoprotein 1; UTR, untranslated region.
Silencing SPP1 inhibited the growth of SKOV3 cells in vivo
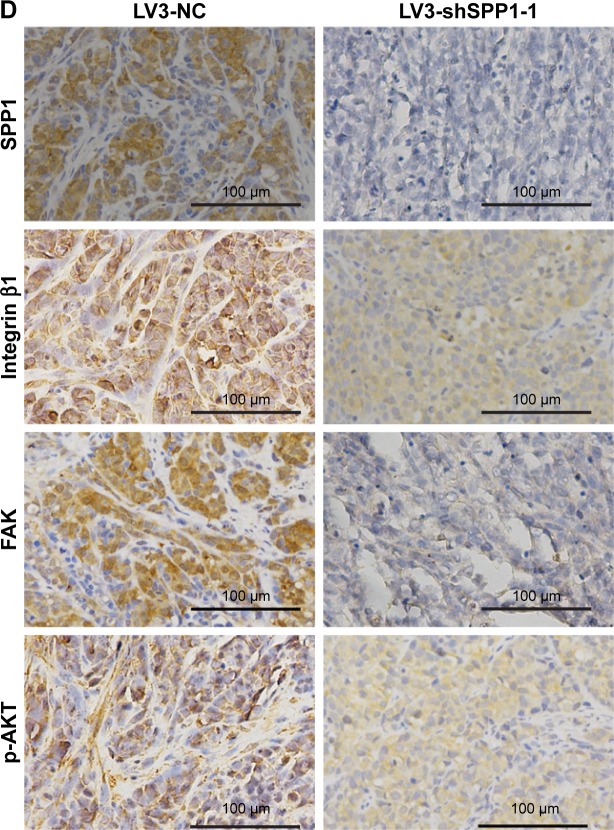
To determine the effect of SPP1 on the growth of SKOV3 cells in vivo, an animal model was constructed. SKOV3 cells were infected with LV3-NC and LV3-shSPP1-1, which formed tumors in all nude mice. The average volume of tumors was decreased in LV3-shSPP1-1 group compared to that in LV3-NC group (p<0.05; Figure 6A and B). The average weight of tumors was decreased in LV3-shSPP1-1 group compared to that in LV3-NC group (Figure 6C). The expression of ITGB1, FAK, or p-AKT was decreased in tumors derived from LV3-shSPP1-1-infected group compared to that in the LV3-NC group (Figure 6D).
Figure 6.
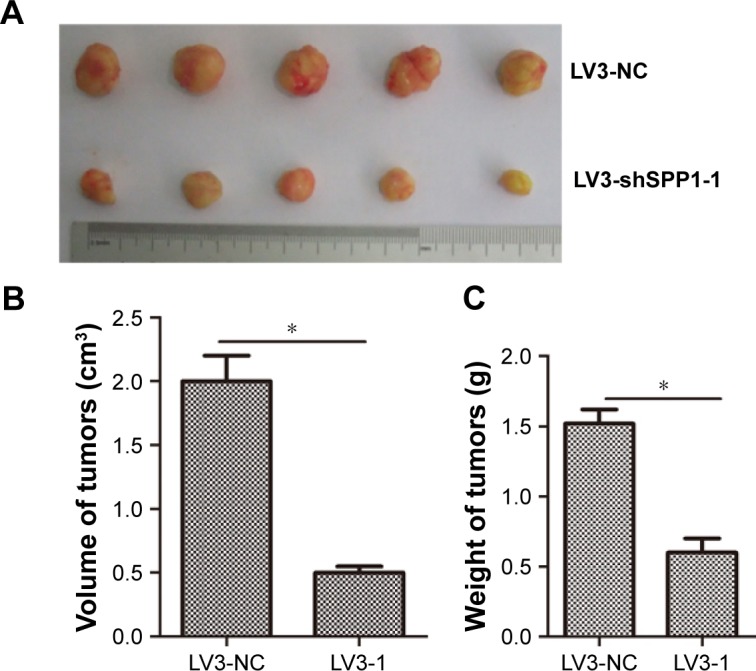
SPP1 regulated tumorigenesis in nude mice model.
Notes: (A–C) Mean tumor volume and weight on day 28 after tumor cell injection. LV3-shSPP1-1- or LV3-NC-infected SKOV3 cells were implanted s.c. into the left armpit. (D) Immunohistochemical analysis of Integrin β1, FAK, and p-AKT expression was performed on tumor xenografts. Representative images are shown (original magnification, ×200). Error bars represent standard error. *p<0.05.
Abbreviations: FAK, focal adhesion kinase; NC, negative control; s.c., subcutaneously; SPP1, secreted phosphoprotein 1.
Discussion
In this research, we observed that the expression of SPP1 was increased in epithelial ovarian cancer, compared to normal ovarian tissues. We also found that the expression of SPP1 in ovarian cancer was correlated with tumor stage and histologic grades. Our data revealed that high expression of SPP1 in ovarian cancer promoted cellular migration, invasion, and cell proliferation. These results indicated that SPP1 promoted the progress of ovarian cancer. Our data also demonstrate that SPP1 can regulate ITGB1/FAK/AKT pathway and that miR-181a controls SPP1 by targeting SPP1 3′UTR. Based on the above mentioned results, SPP1 is a potential therapeutic target and a biologic marker of ovarian carcinoma.
The expression of SPP1 is related to tumorigenesis and tumor metastasis. It has been reported that the expression of SPP1 is high in numerous tumors, including lung cancer, colon cancer, breast cancer, and prostate cancer.10–12 SPP1 levels were positively correlated with the number of extrapulmonary metastases, indicating that patients with high levels of SPP1 expression had higher risk of extrapulmonary metastasis.30 In colorectal cancer, SPP1 promotes metastasis by activating the epithelial-mesenchymal transition pathway.31 In this study, we confirmed that the expression of SPP1 was increased in ovarian cancer. Silencing SPP1 inhibited the malignant behaviors of ovarian cancer. Ectopic expression of SPP1 promoted the malignant behaviors of ovarian cancer. Our data indicated that SPP1 accelerated ovarian cancer progression.
ITGB1 is frequently upregulated in ovarian cancer and promotes ovarian tumorigenesis and cancer progression. ITGB1 regulates many functional activities such as cellular proliferation, adhesion, and invasion. Previous studies have suggested its implication in therapeutic resistance in numerous solid cancer models.32–34 FAK is increased in various malignancies including breast, colon, prostate, neck, and ovarian cancer.35–40 FAK is an important regulator of survival, proliferation, migration, and invasion, processes that are all involved in tumorigenesis and metastasis.41,42 Blocking the ITGB1/FAK/AKT pathway inhibits the metastatic ability of head and neck cancer cells.43 Activating the ITGB1/FAK/AKT pathway promoted the lymph node metastasis of gastric cancer.29 In this study, we observed that silencing SPP1 inhibited ITGB1/FAK/AKT pathway. Ectopic expression of SPP1 activated this pathway. This data indicated that SPP1 promoted the progression of ovarian cancer through ITGB1/FAK/AKT pathway.
Several studies report mixed indications of miR-181 as a tumor suppressor. miR-181 inhibited metastasis and proliferative ability of cancer.44–46 Concomitant analysis of P-Smad2 and miR-181a-5p in surgical samples may be capable of identifying those ovarian cancer patients with poor outcome and little chance of response to Pt-based neoadjuvant chemotherapy.47 Our results showed that miR-181 mimics inhibited the expression of SPP1 in SKOV3 cells. We further confirmed miR-181a reduced SPP1 expression by targeting its mRNA 3′UTR. This data indicated that SPP1 is a tumor-promoting gene in ovarian cancer. miR-181a is a potential oncotarget of ovarian cancer.
Conclusion
This study confirms that SPP1 is a tumor-promoting gene in ovarian cancer, which is involved in the ITGB1/FAK/AKT pathway. These results suggest that SPP1 may be a potential therapeutic target in epithelial ovarian cancer.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Hızlı D, Boran N, Yılmaz S, et al. Best predictors of survival outcome after tertiary cytoreduction in patients with recurrent platinum-sensitive epithelial ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 2012;163(1):71–75. doi: 10.1016/j.ejogrb.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 3.Lenhard SM, Bufe A, Kümper C, et al. Relapse and survival in early-stage ovarian cancer. Arch Gynecol Obstet. 2009;280(1):71–77. doi: 10.1007/s00404-008-0877-z. [DOI] [PubMed] [Google Scholar]
- 4.Oldberg A, Franzén A, Heinegård D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci U S A. 1986;83(23):8819–8823. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saitoh Y, Kuratsu J, Takeshima H, Yamamoto S, Ushio Y. Expression of osteopontin in human glioma. Its correlation with the malignancy. Lab Invest. 1995;72(1):55–63. [PubMed] [Google Scholar]
- 6.Ahmed M, Behera R, Chakraborty G, et al. Osteopontin: a potentially important therapeutic target in cancer. Expert Opin Ther Targets. 2011;15(9):1113–1126. doi: 10.1517/14728222.2011.594438. [DOI] [PubMed] [Google Scholar]
- 7.Zhuo C, Li X, Zhuang H, et al. Elevated THBS2, COL1A2, and SPP1 expression levels as predictors of gastric cancer prognosis. Cell Physiol Biochem. 2016;40(6):1316–1324. doi: 10.1159/000453184. [DOI] [PubMed] [Google Scholar]
- 8.Lin J, Myers AL, Wang Z, et al. Osteopontin (OPN/SPP1) isoforms collectively enhance tumor cell invasion and dissemination in esophageal adenocarcinoma. Oncotarget. 2015;6(26):22239–22257. doi: 10.18632/oncotarget.4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weng S, Zhou L, Han L, Yuan Y. Expression and purification of non-tagged recombinant mouse SPP1 in E. coli and its biological significance. Bioengineered. 2014;5(6):405–408. doi: 10.4161/bioe.34424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ue T, Yokozaki H, Kitadai Y, et al. Co-expression of osteopontin and CD44v9 in gastric cancer. Int J Cancer. 1998;79(2):127–132. doi: 10.1002/(sici)1097-0215(19980417)79:2<127::aid-ijc5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 11.Fedarko NS, Jain A, Karadag A, Van Eman MR, Fisher LW. Elevated serum bone sialoprotein and osteopontin in colon, breast, prostate, and lung cancer. Clin Cancer Res. 2001;7(12):4060–4066. [PubMed] [Google Scholar]
- 12.Chuang CY, Chang H, Lin P, et al. Up-regulation of osteopontin expression by aryl hydrocarbon receptor via both ligand-dependent and ligand-independent pathways in lung cancer. Gene. 2012;492(1):262–269. doi: 10.1016/j.gene.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Cui R, Takahashi F, Ohashi R, et al. Osteopontin is involved in the formation of malignant pleural effusion in lung cancer. Lung Cancer. 2009;63(3):368–374. doi: 10.1016/j.lungcan.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 14.Hu Z, Lin D, Yuan J, et al. Overexpression of osteopontin is associated with more aggressive phenotypes in human non-small cell lung cancer. Clin Cancer Res. 2005;11(13):4646–4652. doi: 10.1158/1078-0432.CCR-04-2013. [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Jiang B, Li H, et al. Wip1 is associated with tumorigenity and metastasis through MMP-2 in human intrahepatic cholangiocarcinoma. Oncotarget. 2017;8(34):56672–56683. doi: 10.18632/oncotarget.18074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costa F, Toscani D, Chillemi A, et al. Expression of CD38 in myeloma bone niche: a rational basis for the use of anti-CD38 immunotherapy to inhibit osteoclast formation. Oncotarget. 2017;8(34):56598–56611. doi: 10.18632/oncotarget.17896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu J, Meng Y, Yu T, et al. Ubiquitin E3 ligase MARCH7 promotes ovarian tumor growth. Oncotarget. 2015;6:12174–12187. doi: 10.18632/oncotarget.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin Q, Huang F, Wang X, et al. High Eg5 expression predicts poor prognosis in breast cancer. Oncotarget. 2017;8(37):62208–62216. doi: 10.18632/oncotarget.19215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poillet-Perez L, Jacquet M, Hervouet E, et al. GABARAPL1 tumor suppressive function is independent of its conjugation to autophagosomes in MCF-7 breast cancer cells. Oncotarget. 2017;8(34):55998–56020. doi: 10.18632/oncotarget.19639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Jin L, Ye F, et al. Isoform expression patterns of EPHA10 protein mediate breast cancer progression by regulating the E-Cadherin and β-catenin complex. Oncotarget. 2017;8(18):30344–30356. doi: 10.18632/oncotarget.15910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu H, Fu S, Chen Q, et al. The function of oxytocin: a potential biomarker for prostate cancer diagnosis and promoter of prostate cancer. Oncotarget. 2017;8(19):31215–31226. doi: 10.18632/oncotarget.16107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.JingSong H, Hong G, Yang J, et al. siRNA-mediated suppression of collagen type iv alpha 2 (COL4A2) mRNA inhibits triple-negative breast cancer cell proliferation and migration. Oncotarget. 2017;8(2):2585–2593. doi: 10.18632/oncotarget.13716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu H, Bi J, Peng Y, et al. Nuclear receptor NR4A1 is a tumor suppressor down-regulated in triple-negative breast cancer. Oncotarget. 2017;8(33):54364–54377. doi: 10.18632/oncotarget.17532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xue MQ, Liu J, Sang JF, Su L, Yao YZ. Expression characteristic of CXCR1 in different breast tissues and the relevance between its expression and efficacy of neo-adjuvant chemotherapy in breast cancer. Oncotarget. 2017;8(30):48930–48937. doi: 10.18632/oncotarget.16893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017 Apr 12; doi: 10.1093/nar/gkx247. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang GS, Brouwer-Visser J, Ramirez MJ, et al. Insulin-like growth factor 2 expression modulates Taxol resistance and is a candidate biomarker for reduced disease-free survival in ovarian cancer. Clin Cancer Res. 2010;16(11):2999–3010. doi: 10.1158/1078-0432.CCR-09-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang CR, Lee CT, Chang KY, et al. Down-regulation of ARNT promotes cancer metastasis by activating the fibronectin/integrin β1/FAK axis. Oncotarget. 2015;6(13):11530–11546. doi: 10.18632/oncotarget.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin J, Tang J, Jiao L, et al. A diterpenoid compound, excisanin A, inhibits the invasive behavior of breast cancer cells by modulating the integrin β1/FAK/PI3K/AKT/β-catenin signaling. Life Sci. 2013;93(18–19):655–663. doi: 10.1016/j.lfs.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, Wang Z, Li G, et al. CXCL1 from tumor-associated lymphatic endothelial cells drives gastric cancer cell into lymphatic system via activating integrin β1/FAK/AKT signaling. Cancer Lett. 2017;385:28–38. doi: 10.1016/j.canlet.2016.10.043. [DOI] [PubMed] [Google Scholar]
- 30.Zhang H, Liu HB, Yuan DM, Wang ZF, Wang YF, Song Y. Prognostic value of secreted phosphoprotein-1 in pleural effusion associated with non-small cell lung cancer. BMC Cancer. 2014;14:280. doi: 10.1186/1471-2407-14-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu C, Sun L, Jiang C, et al. SPP1, analyzed by bioinformatics methods, promotes the metastasis in colorectal cancer by activating EMT pathway. Biomed Pharmacother. 2017;91:1167–1177. doi: 10.1016/j.biopha.2017.05.056. [DOI] [PubMed] [Google Scholar]
- 32.Cordes N, Park CC. beta1 integrin as a molecular therapeutic target. Int J Radiat Biol. 2007;83(11–12):753–760. doi: 10.1080/09553000701639694. [DOI] [PubMed] [Google Scholar]
- 33.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10(1):9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer. 2009;9(9):665–674. doi: 10.1038/nrc2714. [DOI] [PubMed] [Google Scholar]
- 35.Lark AL, Livasy CA, Dressler L, et al. High focal adhesion kinase expression in invasive breast carcinomas is associated with an aggressive phenotype. Mod Pathol. 2005;18(10):1289–1294. doi: 10.1038/modpathol.3800424. [DOI] [PubMed] [Google Scholar]
- 36.Genna A, Lapetina S, Lukic N, et al. Pyk2 and FAK differentially regulate invadopodia formation and function in breast cancer cells. J Cell Biol. 2017;217(1):375–395. doi: 10.1083/jcb.201702184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li C, Gao S, Li X, Li C, Ma L. Procaine inhibits proliferation and migration of colon cancer cells through inactivation of the ERK/MAPK/FAK pathways by regulation of RhoA. Oncol Res. 2017 May 11; doi: 10.3727/096504017X14944585873622. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Song G, Xu S, Zhang H, et al. TIMP1 is a prognostic marker for the progression and metastasis of colon cancer through FAK-PI3K/AKT and MAPK pathway. J Exp Clin Cancer Res. 2016;35(1):148. doi: 10.1186/s13046-016-0427-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benelli R, Monteghirfo S, Venè R, Tosetti F, Ferrari N. The chemopreventive retinoid 4HPR impairs prostate cancer cell migration and invasion by interfering with FAK/AKT/GSK3beta pathway and beta-catenin stability. Mol Cancer. 2010;9:142. doi: 10.1186/1476-4598-9-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El HCP, Sharma PK, Singh R, et al. PI3Kp110-, Src-, FAK-dependent and DOCK2-independent migration and invasion of CXCL13-stimulated prostate cancer cells. Mol Cancer. 2010;9:85. doi: 10.1186/1476-4598-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ucar DA, Hochwald SN. FAK and interacting proteins as therapeutic targets in pancreatic cancer. Anticancer Agents Med Chem. 2010;10(10):742–746. doi: 10.2174/187152010794728675. [DOI] [PubMed] [Google Scholar]
- 42.Lechertier T, Hodivala-Dilke K. Focal adhesion kinase and tumour angiogenesis. J Pathol. 2012;226(2):404–412. doi: 10.1002/path.3018. [DOI] [PubMed] [Google Scholar]
- 43.Kim SA, Kwon SM, Kim JA, Kang KW, Yoon JH, Ahn SG. 5′-Nitro-indirubinoxime, an indirubin derivative, suppresses metastatic ability of human head and neck cancer cells through the inhibition of Integrin β1/FAK/Akt signaling. Cancer Lett. 2011;306(2):197–204. doi: 10.1016/j.canlet.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 44.Shin KH, Bae SD, Hong HS, Kim RH, Kang MK, Park NH. miR-181a shows tumor suppressive effect against oral squamous cell carcinoma cells by downregulating K-ras. Biochem Biophys Res Commun. 2011;404(4):896–902. doi: 10.1016/j.bbrc.2010.12.055. [DOI] [PubMed] [Google Scholar]
- 45.Roth E, Cao J. miR-181 suppresses metastasis via MMP-14. Aging (Albany NY) 2015;7(10):740–741. doi: 10.18632/aging.100824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen G, Zhu W, Shi D, et al. MicroRNA-181a sensitizes human malignant glioma U87MG cells to radiation by targeting Bcl-2. Oncol Rep. 2010;23(4):997–1003. doi: 10.3892/or_00000725. [DOI] [PubMed] [Google Scholar]
- 47.Petrillo M, Zannoni GF, Beltrame L, et al. Identification of high-grade serous ovarian cancer miRNA species associated with survival and drug response in patients receiving neoadjuvant chemotherapy: a retrospective longitudinal analysis using matched tumor biopsies. Ann Oncol. 2016;27(4):625–634. doi: 10.1093/annonc/mdw007. [DOI] [PubMed] [Google Scholar]