ABSTRACT
Amyloid-β (Aβ) peptides, as well as a variety of other protein fragments, are derived from proteolytical cleavage of the amyloid precursor protein (APP) and have been demonstrated to play a key role in the pathological changes underlying Alzheimer disease (AD). In AD mouse models, altered neurogenesis has been repeatedly reported to be associated with further AD-typical pathological hallmarks such as extracellular plaque deposition, behavioral deficits or neuroinflammation. While a toxic role of Aβ in neurodegeneration and impaired neuronal progenitor proliferation is likely and well-accepted, recent findings also suggest an important influence of APP-derived proteolitical fragments like the APP intracellular domain (AICD), as well as of APP itself.
KEYWORDS: Alzheimer, amyloid precursor protein, neurogenesis, proliferation, transgenic mice
Introduction
Alzheimer disease (AD) is the most prevalent form of dementia, with the number of patients constantly rising as a function of the demographic trend. Predictions and extrapolations from data from 2005 expect, assuming that there won't be a major breakthrough in prevention or therapy, a number of ∼42.3 million dementia patients worldwide in 2020, with about 4.6 million new cases every year.1 The prevalence rate rises steeply with age, with ∼1% affected in the group of people being 60 to 65 y old, but 30% affected in the group of people aged 90 and older.
During the last 25 years, a variety of transgenic mouse lines has been developed that partially reproduce the major hallmark lesions of AD. On a neuropathological level these comprise extracellular deposition of amyloid-β (Aβ) peptides in the form of plaques, as well as intracellular accumulation and hyperphosphorylation of tau protein (reviewed in e.g. refs. 2, 3). While a myriad of experimental therapeutic interventions has been reported that describe an ambiguous situation with regard to an amelioration of these pathological alterations, literature on non-pharmacological treatment and prevention by increased physical activity is more consistent.4
Numerous studies in the literature report positive effects of physical exercise on various brain functions and favorable influence on brain plasticity by facilitating neurogenerative, neuroadaptive, as well as neuroprotective processes. These include enhanced executive functions of cognition and some types of learning, including motor learning in the spinal cord among others.5 It has been demonstrated by neuroimaging approaches that aerobic physical exercise represents a sufficient instrument to increase hippocampal volume,6 resulting in reduced hippocampal atrophy in individuals at genetic risk for AD.7 This has been mainly attributed to an elevated release of neurotrophic factors and boosted angiogenesis, both facilitating increased neuro- and synaptogenesis (reviewed in ref. 8). Transgenic AD mouse models have been extensively studied with respect to exercise-mediated effects on deposition of amyloid-β (Aβ) peptides and the impact on hippocampal neurogenesis and cognitive function has been repeatedly described (reviewed in ref. 9).
It is widely accepted that hippocampal neurogenesis plays a necessary role in the maintenance of learning and memory abilities depending on proper function of the hippocampal circuitry. New-born neurons in the subgranular zone (SGZ) of the dentate gyrus (DG) become incorporated into the functional local network and can be identified using a variety of markers or labeling techniques (e.g., bromodeoxyuridine (BrdU)) (reviewed in ref. 10). While only few and conflicting studies about the extent of adult hippocampal neurogenesis in human AD patients are available (reviewed in ref. 11), numerous reports on the involvement of neurogenesis in transgenic AD mouse models have been published in recent years.
Amyloid precursor protein (APP) processing
The human APP gene is located on chromosome 21 and alternative splicing yields 8 isoforms which are expressed in a cell-type-specific manner, with APP695 being the most abundant transcript in neuronal cells (reviewed in ref. 12). APP can undergo a variety of different cleavage steps executed by certain secretases, which can be roughly subdivided into amyloidogenic and non-amyloidogenic processing (reviewed in ref. 13). In the amyloidogenic processing pathway, APP is initially cleaved by β-secretase, leading to the liberation of a long soluble extracellular fragment (sAPPβ) and a membrane-bound C-terminal stub (β-CTF or C99), containing the Aβ sequence. Subsequent intramembrane cleavage by γ-secretase leads to the liberation of the Aβ domain, as well as of an APP intracellular domain termed AICD (Fig. 1B). Alternatively, initial cleavage by α-secretase within the Aβ domain precludes Aβ peptide generation by releasing a slightly shorter soluble APP fragment (sAPPα). Following γ-secretase cleavage again releases the AICD fragment, as well as another small peptide named p3 with so far unknown function13 (Fig. 1A).
Figure 1.
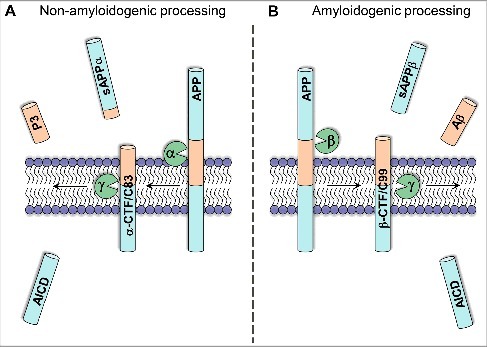
Simplified overview of APP processing. The amyloidogenic APP processing pathway (right) initially generates β-C-terminal fragments (β-CTF) by β-secretase cleavage. Further cleavage by γ-secretase leads to the release of Aβ peptides and the generation of the AICD fragment (B). Alternatively, initial cleavage by α-secretase precludes Aβ generation, thereby releasing sAPPα and producing α-CTFs. Subsequent γ-secretase cleavage releases the P3 fragment and also liberates the intracellular AICD fragment (A).
Impaired neurogenesis is a common feature of AD transgenic mouse models
Most of the reported transgenic AD mouse models make use of overexpression of familial AD-associated mutant genes (amyloid precursor protein (APP), presenilin 1/2 (PSEN1/2)) and differ by expressing either single or multiple transgenes, as well as in the employment of different mutations and promotor constructs used to drive transgene expression.3 In general, an increased age-dependent accumulation of Aβ peptides is associated with an overall decrease in neurogenesis in the predominant number of studies. In one of the earliest reports, 12–14-month-old mutant APP transgenic and age-matched control mice were injected daily with the thymidine analog BrdU for 5 consecutive days and were killed either one or 12 d after the last injection. Neuropathological analysis revealed the presence of numerous hippocampal amyloid deposits and a stereological quantification indicated a significant reduction in the number of BrdU-positive cells in transgenic compared with control mice. Interestingly, no such difference could be detected in 3-month-old mice which had not yet developed extracellular amyloid deposits.14 Related observations have been subsequently published in aged APP/PS1ΔEx9 mice which also presented reduced numbers of BrdU- or Doublecortin (DCX)-positive cells at 9 months of age, while no differences compared with wildtype (WT) mice could be detected at the age of 5 months in the absence of overt hippocampal amyloid pathology.15 The widely-used Tg2576 mouse model of AD develops an age-dependent amyloidosis in hippocampus and cortex at 9–12 months of age. A quantification of adult neurogenesis revealed a significantly increased number of proliferating BrdU-positive cells in 3-month-old Tg2576 mice in comparison to age-matched WT mice, while 5- and 12-month-old mice showed comparable numbers. The number of surviving BrdU-positive cells was however significantly reduced in 3-month-old Tg2576 mice, while again no differences could be detected at later time points, suggesting that neurogenesis is altered already at time points preceding considerable extracellular amyloid deposition.16
Rodriguez and colleagues used a triple-transgenic model expressing both mutant APP and mutant Tau on a homozygous PSEN1 knock-in background (3xTg). Interestingly, female 3xTg mice showed a significant reduction in neurogenesis starting at the age of 4 months compared with controls, which was directly associated with the presence of intraneuronal Aβ accumulation in the CA1 region of the hippocampus, while extracellular amyloid deposition started much later at 12 months of age.17 Another model with an early degenerative and behavioral phenotype expresses human mutant APP on a homozygous mutant PSEN1 knock-in background (APP/PS1KI). These mice show a significant reduction in the number of mitotic Ki67-immunoreactive cells in the dentate gyrus, accompanied by an almost complete absence of DCX-positive cells already at the age of 6 months. While numbers of Ki67- and DCX-positive cells showed a positive correlation in PS1KI control animals, no such correlation could be established in APP/PS1KI mice suggesting that the exhaustive loss of DCX-positive cells only partly reflects alterations in multipotent progenitor cell proliferation.18 Interestingly, a stereological quantification of granule cell layer (GCL) neurons in the dentate gyrus revealed a significant reduction in the number of GCLs in aged (12-month-old) APP/PS1KI mice in comparison to age-matched PS1KI control animals (-44%).19 This might suggest that defective neurogenesis indeed plays a role; however, it is questionable whether merely reduced neurogenesis accounts for this dramatic cell loss at this age, as it is known that DG neurogenesis in rodents in general strongly decreases with aging.20
To rescue impaired neurogenesis, environmental enrichment (EE) and enhanced physical activity have been demonstrated to result in significantly increased neurogenesis in the adult brains of rodents.21,22 Numerous studies reported such effects in various transgenic AD mouse models, however with conflicting results (e.g., refs. 23-26; Table 1).
Table 1.
Selection of different AD and DS mouse models in which impaired neurogenesis has been reported.
| Transgenic mouse model | Mutation APP | Mutation PS1 | Promoter | Plaque onset | Neuron loss | Neuro-genesis | Effect of PA/EE on NG | Reference |
|---|---|---|---|---|---|---|---|---|
| Tg2576 | Swedish | – | Hamster Prion Protein | 12m | no | ↓ | ↑ | 23,16 |
| 3xTg-AD | Swedish | M146V | Thy1 (APP, Tau) | 6m | n.a. | ↓ | ↑ | 17,24 |
| PS1 knock-in | ||||||||
| APP751SL/PS1KI | Swedish, London | M233T, L235P | Thy1 (APP) | 2m | ✓(6m) | ↓ | ⇆ | 18,25,32,33 |
| PS1 knock-in | ||||||||
| Tg4–42 | – | – | Thy1 (Aβ4–42) | – | ✓(5m) | n.a. | ↑ | 26,35 |
| TTA/APP | Swedish, Indiana | – | CaMKIIα-TTA | < 6m | n.a. | ⇆ | n.a. | 27 |
| APP-wt | – | – | PDGF | – | n.a | ↓ | ↑ | 37 |
| Ts65Dn | trisomic | – | – | – | n.a. | ↓ | ↑ | 42,41 |
Is Aβ the pivotal factor involved in impaired neurogenesis?
Taken together, most of the studies suggest that hippocampal accumulation of Aβ peptides in the form of extracellular deposits has a strong impact on neural progenitor cells. This view might reflect the observation that disturbance of neurogenesis in most models only becomes obvious upon substantial amyloid plaque deposition, with the consequence that potential confounding effects of APP overexpression have been largely neglected. To investigate the role of Aβ in more detail, a transgenic mouse model has been recently established, which expresses human mutant APP selectively in mature forebrain neurons under the control of an inducible CAMKIIα promotor construct. This allows discrimination between effects caused by extracellular release of Aβ or APP fragments on the one hand and the impact of human APP expression within dividing neuronal precursor cells on the other.27 Abundant amyloid plaques could be detected in both forebrain and dentate gyrus after gene activation for a period of 6 months. A detailed analysis using BrdU injections and subsequent quantification after 7 or 30 d to assess recently born and survival of immature neurons respectively, revealed no differences between transgenic and control mice. The unaltered proliferation and survival of adult-born hippocampal neurons despite of the proximity to amyloid pathology and APP-overexpressing neighboring neurons in this model suggests that APP expression within neural progenitor cells might have a crucial influence.27 In support of this hypothesis, substantial human mutant APP expression has been reported in dentate gyrus granule cells of e.g., APP/PS1KI mice already at 2 months of age19 or in neurospheres isolated from TgCRND8 transgenic mice.28
Another recent study addressed the question whether Aβ or APP is accountable for neurogenesis disturbance. The authors found impaired neurogenesis in the dentate gyrus of hAPP-I5 mice, a model overexpressing wildtype human APP under the control of the PDGF-β promotor. This impairment was more distinct than in hAPP-J20 mice, a model expressing mutant APP with the Swedish and Indiana mutations at comparable mRNA levels, which in addition to hAPP-I5 mice harbours strongly increased levels of Aβ peptides. Deletion of Cystatin C in hAPP-J20 mice led to a significant reduction in Aβ levels, however, no influence on the number of DCX-positive neurons in the dentate gyrus could be detected suggesting that Aβ was not the major factor accounting for impaired hippocampal neurogenesis in this model.29
Neuron loss in transgenic AD mouse models
While most of the available transgenic AD models, at least to a certain degree, reflect major pathological hallmarks of AD such as abundant extracellular amyloid-β peptide deposition or neuroinflammation, a convincing neurodegenerative phenotype with quantifiable neuron loss is mostly lacking.30 Extensive neuron loss in the CA1 region of the hippocampus has been described in the APP/PS1KI model,31 leading to a loss of ∼30% neurons already at the age of 6 months compared with PS1KI control mice and a significant reduction of the volume of the CA1 pyramidal cell layer to a comparable extent.32 This cell loss is intimately linked to the intraneuronal accumulation of Aβ peptides. Analyses in other brain regions like frontal cortex revealed comparable results. While APP/PS1KI mice show a comparable extracellular plaque load in frontal cortex and thalamus at an age of 12 months, neuron loss was only evident in the cortex where neurons overexpress human mutant APP and accumulate significant amounts of intraneuronal Aβ peptides. No such neuron loss could be detected in the thalamus, a brain region also harbouring massive extracellular amyloid pathology, however, in the absence of APP expression and intraneuronal Aβ accumulation.33 To assess whether cognitive stimulation and enhanced physical activity might have an influence on behavioral alterations and neuron loss, APP/PS1KI mice were maintained under EE or standard conditions starting at 2 months of age. Surprisingly, no beneficial effects on working memory, extracellular Aβ plaque load, neuron loss, as well as hippocampal neurogenesis could be detected.25
A direct link between intraneuronal Aβ accumulation and subsequent neuron loss in distinct brain regions has been also established in mouse models like TBA2.134 or Tg4–42,35 engineered to directly overexpress mutant Aβ peptides in the absence of mutant APP overexpression. These models use the neuron-specific Thy1-promotor to overexpress the N-terminal truncated Aβ species Aβ3–42 or Aβ4–42 and are characterized by extensive CA1 neuron loss and associated behavioral deficits.34-36 In the Tg4–42 model, enhanced physical activity using enriched housing in cages equipped with running wheels resulted in an amelioration of CA1 neuron loss, accompanied by a significantly increased overall dentate gyrus granule cell number and a concomitant increase in the number of DCX-positive cells in the DG.26
So far, no accumulation of Aβ peptides within dentate gyrus neurons has been reported in any of the mouse models that have been studied. The fact that many of the models that show alterations in neurogenesis also express substantial levels of mutant APP within this brain region (Fig. 2), might at least render such a mechanism possible. In support of this hypothesis, both Aβ40 and Aβ42 peptide secretion has been demonstrated in neural progenitor cells isolated from TgCRND8 mice, which show higher cytotoxicity in comparison to control cells derived from non-transgenic control animals.28
Figure 2.
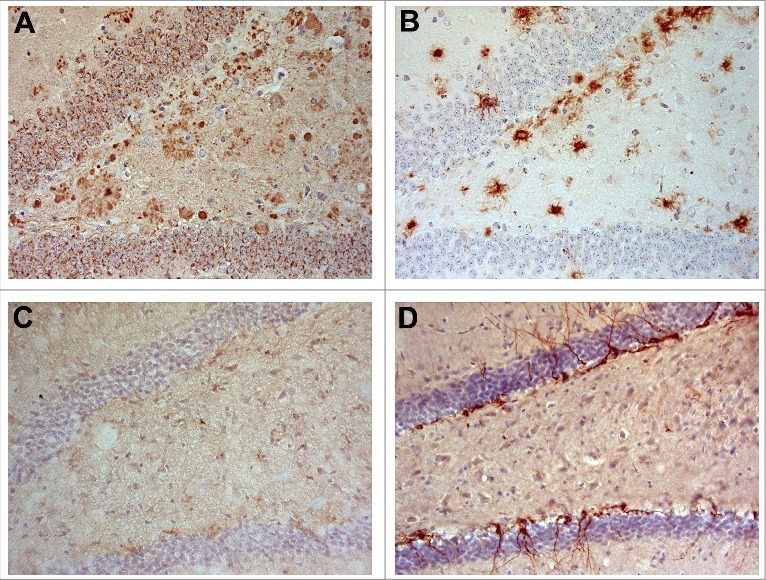
APP and Aβ might impair neurogenesis in APP/PS1KI mice. Abundant APP immunoreactivity in granule cells of the dentate gyrus and plaque-associated dystrophic neurites in 6-month-old APP/PS1KI mice (A). Aβ staining is mainly restricted to extracellular deposits in an adjacent section (B). Doublecortin (DCX)-immunoreactivity is almost lacking in APP/PS1KI mice (C), but clearly detectable in an age-matched wildtype animal (D).
APP expression and its impact on neurogenesis
In addition to overexpression of mutant human APP as mentioned before, transgenic overexpression of human wildtype APP also has an impact on hippocampal neurogenesis. In comparison to WT mice, young mice overexpressing APP under the control of the platelet-derived growth factor (PDGF) promotor showed a significantly reduced number of BrdU-labeled cells in the dentate gyrus, when housed under standard conditions. Enriched housing in larger cages equipped with running wheels, tubes and nesting material restored neurogenesis to normal levels.37 Retroviral expression of β-CTF, an APP fragment generated by amyloidogenic processing through β-secretase, resulted in significantly reduced glutamatergic connectivity of dentate gyrus granule cells at 21 d post infection, as well as a decreased dendritic length. Interestingly, overexpression of α-CTF resulted in similar defects, indicating that human APP overexpression can compromise dendritic development via amyloidogenic and non-amyloidogenic processing.38 Genetic loss-of-function studies provide further support to the notion that APP is capable of regulating neuronal progenitor proliferation. Wang and colleagues investigated whether APP deficiency affects neurogenesis by administering BrdU in 4–6-month-old APP knockout (APP−/−) mice. Interestingly, APP−/− mice showed enhanced proliferation of dentate gyrus progenitor cells, but an impaired maintenance of newborn neurons.39
No detrimental effect on neurogenesis could be established in mice carrying human APP with the Swedish double mutation, which has been inserted in a homozygous fashion into the endogenous gene locus (“knock-in”). These mice do not develop detectable extracellular amyloid pathology up to an age of 20 months. Only a “double knock-in,” combining mutant APP with an introduction of the FAD-linked P264L mutation in the endogenous mouse PSEN1 gene, resulted in a long-lasting impairment in neurogenesis with concomitant amyloid deposition.40 This suggests that either APP overexpression and/or Aβ accumulation in the hippocampus is needed to induce disturbances in neuronal progenitor proliferation.
In good agreement, impaired proliferation of neuronal precursors has been also reported in the Ts65Dn mouse model of Down syndrome (DS), as well as in the DG of DS fetuses, when compared with controls.41 DS is caused by a triplication of chromosome 21, which involves an elevation of the APP gene dose. Ts65Dn mice contain 3 copies of most of the genes of murine chromosome 16 that are homologues for human chromosome 21 genes. A combination of environmental enrichment and enhanced physical exercise starting in young mice resulted in a strongly increased neurogenesis rate in the dentate gyrus comparable to that of euploid mice.42 In addition to an increased gene dose of full-length APP, levels of fragments derived from proteolytical APP processing are also elevated in Ts65Dn mice43 and likely also in mouse models with APP overexpression. A normalization of the triplicated APP expression using APP shRNA lentiviral particles led to a restoration of neuronal maturation and differentiation and increased neurite-outgrowth to normal levels in a dose-dependent manner.44
On the contrary, there is also accumulating evidence that at least soluble secreted sAPP possesses neutrophic properties and exerts growth factor-like functions that also impact neurogenesis. Caille and colleagues have demonstrated that sAPP binds to EGF receptor expressing cells in the subventricular zone (SVZ) of the lateral ventricle, while sAPP infusion increases the number of EGF-responsive progenitors via raising their proliferation. This effect could be reversed by decreasing APP expression or blocking sAPP secretion.45 In support of this observation, Lopez-Toledano and Shelanski reported increased proliferation and differentiation of neuronal precursor cells in the dentate gyrus of 3-month-old APP-overexpressing J20 mice. This effect was reverted in an age-dependent manner, coinciding with increasing Aβ peptide levels.46
Role of the AICD fragment in altered neurogenesis
The APP-derived AICD fragment has been demonstrated to form a transcriptionally active complex with the nuclear adaptor protein Fe65 and the histone acetyltransferase Tip60.47 Studies using chromatin immunoprecipitation (ChIP) detected AICD in transcriptional complexes on promotors of target genes such as neprilysin (NEP) or the Sonic Hedgehog (Shh) receptor Patched (PTCH1) (reviewed in ref. 48). A functional ligand of APP named TAG1 has been identified that promotes the release of AICD in a γ-secretase-dependent manner. The TAG1-APP signaling pathway negatively modulates neurogenesis via Fe65, while an increase in neurogenesis could be demonstrated in TAG1-null mice that could be reverted by AICD expression. This confirms that AICD is able to act as a negative modulator of neurogenesis as one of its potential physiologic functions.49
Ts65Dn mice show a defective responsiveness to Shh, representing an important mitogen responsible for controlling cell division during neurodevelopment. Trisomic neural precursor cells derived from Ts65Dn mice have been demonstrated to exhibit increased expression levels of the Shh receptor Ptch1, resulting in suppression of Smoothend (Smo), a second receptor involved in this signaling pathway.43 Elevated AICD levels, and therefore increased AICD binding to the Ptch1 promotor, resulted in Ptch1 overexpression while Ptch1 silencing using antisense oligonucleotides lead to a restoration of cell proliferation in trisomic neural precursor cells.43 In support of this observation, a recent study has been demonstrated that γ-secretase inhibitor treatment normalized AICD levels and restored impaired neurogenesis and Sonic Hedgehog signaling in Ts65Dn-derived neurospheres.50 The crucial role of AICD is underscored by an age-dependent decrease in BrdU incorporation and DCX-immunoreactive cells in the dentate gyrus of AICD transgenic mice. While neuronal differentiation was unaffected, proliferation and survival of progenitor cells was strongly reduced. Long-term treatment using anti-inflammatory drugs like ibuprofen or naproxen rescued impaired neurogenesis, leading to the assumption that neuroinflammation is also a critical contributor.51
Conclusion
Impaired neurogenesis is a common feature of transgenic AD mouse models that are based on overexpression of mutant APP. While neurotrophic and growth-promoting functions are ascribed to the large secreted N-terminal portion of APP, other APP-derived proteolytic fragments like AICD and Aβ are believed to exert suppressing properties with regard to hippocampal neurogenesis. While the presence of extracellular Aβ deposits coincides with impaired neurogenesis in a variety of transgenic AD mouse models, the potential role of intraneuronal Aβ peptides or soluble Aβ species is currently less clear and warrants further studies.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work has been supported by the Alzheimer Forschung Initiative (grant #16013 to O.W.)
References
- [1].Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, et al.. Global prevalence of dementia: a Delphi consensus study. Lancet 2005; 366(9503):2112-7; https://doi.org/ 10.1016/S0140-6736(05)67889-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].LaFerla FM, Green KN. Animal Models of Alzheimer Disease. Cold Spring Harb Perspect Med 2012; 2(11). pii: a006320; PMID:23002015; https://doi.org/ 10.1101/cshperspect.a006320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Duyckaerts C, Potier MC, Delatour B. Alzheimer disease models and human neuropathology: similarities and differences. Acta Neuropathol 2008; 115(1):5-38; PMID:18038275; https://doi.org/ 10.1007/s00401-007-0312-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Intlekofer KA, Cotman CW. Exercise counteracts declining hippocampal function in aging and Alzheimer's disease. Neurobiol Dis 2013; 57:47-55; PMID:22750524; https://doi.org/ 10.1016/j.nbd.2012.06.011 [DOI] [PubMed] [Google Scholar]
- [5].Dishman RK, Berthoud H-R, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, Gandevia SC, Gomez-Pinilla F, Greenwood BN, Hillman CH, et al.. Neurobiology of Exercise. Obesity 2006; 14(3):345-56; PMID:16648603; https://doi.org/ 10.1038/oby.2006.46 [DOI] [PubMed] [Google Scholar]
- [6].Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, et al.. Exercise training increases size of hippocampus and improves memory. Proc Nat Acad Sci 2011; 108(7):3017-22; https://doi.org/ 10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Smith JC, Nielson KA, Woodard JL, Seidenberg M, Durgerian S, Hazlett KE, Figueroa CM, Kandah CC, Kay CD, Matthews MA, et al.. Physical activity reduces hippocampal atrophy in elders at genetic risk for Alzheimer's disease. Front Aging Neurosci 2014; 6:61; https://doi.org/ 10.3389/fnagi.2014.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Paillard T, Rolland Y, de Souto Barreto P. Protective Effects of Physical Exercise in Alzheimer's Disease and Parkinson's Disease: A Narrative Review. J Clin Neurology (Seoul, Korea) 2015; 11(3):212-9; https://doi.org/ 10.3988/jcn.2015.11.3.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ryan SM, Kelly ÁM. Exercise as a pro-cognitive, pro-neurogenic and anti-inflammatory intervention in transgenic mouse models of Alzheimer's disease. Ageing Res Rev 2016; 27:77-92; PMID:27039886; https://doi.org/ 10.1016/j.arr.2016.03.007 [DOI] [PubMed] [Google Scholar]
- [10].Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci 2010; 11(5):339-50; PMID:20354534; https://doi.org/ 10.1038/nrn2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Winner B, Winkler J. Adult Neurogenesis in Neurodegenerative Diseases. Cold Spring Harb Perspect Biol 2015; 7(4)a021287; PMID:25833845; https://doi.org/ 10.1101/cshperspect.a021287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bayer TA, Wirths O, Majtenyi K, Hartmann T, Multhaup G, Beyreuther K, Czech C. Key Factors in Alzheimer's Disease: ß-amyloid Precursor Protein Processing, Metabolism and Intraneuronal Transport. Brain Pathology 2001; 11:1-11; PMID:11145195; https://doi.org/ 10.1111/j.1750-3639.2001.tb00376.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nhan HS, Chiang K, Koo EH. The multifaceted nature of amyloid precursor protein and its proteolytic fragments: friends and foes. Acta Neuropathol 2015; 129(1):1-19; PMID:25287911; https://doi.org/ 10.1007/s00401-014-1347-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Haughey NJ, Nath A, Chan SL, Borchard AC, Rao MS, Mattson MP. Disruption of neurogenesis by amyloid β-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer's disease. J Neurochem 2002; 83(6):1509-24; PMID:12472904; https://doi.org/ 10.1046/j.1471-4159.2002.01267.x [DOI] [PubMed] [Google Scholar]
- [15].Taniuchi N, Niidome T, Goto Y, Akaike A, Kihara T, Sugimoto H. Decreased proliferation of hippocampal progenitor cells in APPswe/PS1dE9 transgenic mice. Neuroreport 2007; 18(17):1801-1805; PMID:18090315; https://doi.org/ 10.1097/WNR.0b013e3282f1c9e9 [DOI] [PubMed] [Google Scholar]
- [16].Krezymon A, Richetin K, Halley H, Roybon L, Lassalle J-M, Francès B, Verret L, Rampon C. Modifications of Hippocampal Circuits and Early Disruption of Adult Neurogenesis in the Tg2576 Mouse Model of Alzheimer's Disease. PLOS ONE 2013; 8(9):e76497; PMID:24086745; https://doi.org/ 10.1371/journal.pone.0076497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rodriguez JJ, Jones VC, Tabuchi M, Allan SM, Knight EM, LaFerla FM, Oddo S, Verkhratsky A. Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer's disease. PLoS ONE 2008; 3(8):e2935; PMID:18698410; https://doi.org/ 10.1371/journal.pone.0002935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Faure A, Verret L, Bozon B, El Tannir El Tayara N, Ly M, Kober F, Dhenain M, Rampon C, Delatour B. Impaired neurogenesis, neuronal loss, and brain functional deficits in the APPxPS1-Ki mouse model of Alzheimer's disease. Neurobiol Aging 2011; 95(1):92-101; https://doi.org/ 10.1016/j.neurobiolaging.2009.03.009 [DOI] [PubMed] [Google Scholar]
- [19].Cotel MC, Bayer TA, Wirths O. Age-dependent loss of dentate gyrus granule cells in APP/PS1KI mice. Brain Res 2008; 1222:207-13; PMID:18585693; https://doi.org/ 10.1016/j.brainres.2008.05.052 [DOI] [PubMed] [Google Scholar]
- [20].Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci 1996; 16(6):2027-33; PMID:8604047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci 1998; 18(9):3206-12; PMID:9547229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 1999; 2(3):266-70; PMID:10195220; https://doi.org/ 10.1038/6368 [DOI] [PubMed] [Google Scholar]
- [23].Jeong YH, Kim JM, Yoo J, Lee SH, Kim H-S, Suh Y-H. Environmental enrichment compensates for the effects of stress on disease progression in Tg2576 mice, an Alzheimer's disease model. J Neurochem 2011; 119(6):1282-93; PMID:21967036; https://doi.org/ 10.1111/j.1471-4159.2011.07514.x [DOI] [PubMed] [Google Scholar]
- [24].Marlatt MW, Potter MC, Bayer TA, van Praag H, Lucassen PJ. Prolonged running, not fluoxetine treatment, increases neurogenesis, but does not alter neuropathology, in the 3xTg mouse model of Alzheimer's disease. Curr Top Behav Neurosci 2013; 15:313-40; PMID:23670818; https://doi.org/ 10.1007/7854_2012_237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cotel MC, Jawhar S, Christensen DZ, Bayer TA, Wirths O. Environmental enrichment fails to rescue working memory deficits, neuron loss, and neurogenesis in APP/PS1KI mice. Neurobiol Aging 2012; 33(1):96-107; PMID:20359774; https://doi.org/ 10.1016/j.neurobiolaging.2010.02.012 [DOI] [PubMed] [Google Scholar]
- [26].Huttenrauch M, Brauss A, Kurdakova A, Borgers H, Klinker F, Liebetanz D, Salinas-Riester G, Wiltfang J, Klafki HW, Wirths O. Physical activity delays hippocampal neurodegeneration and rescues memory deficits in an Alzheimer disease mouse model. Translational Psychiatry 2016; 6:e800; PMID:27138799; https://doi.org/ 10.1038/tp.2016.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yetman MJ, Jankowsky JL. Wild-Type Neural Progenitors Divide and Differentiate Normally in an Amyloid-Rich Environment. J Neurosci 2013; 33(44):17335-41; PMID:24174666; https://doi.org/ 10.1523/JNEUROSCI.1917-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kanemoto S, Griffin J, Markham-Coultes K, Aubert I, Tandon A, George-Hyslop PS, Fraser PE. Proliferation, differentiation and amyloid-β production in neural progenitor cells isolated from TgCRND8 mice. Neuroscience 2014; 261:52-9; PMID:24361736; https://doi.org/ 10.1016/j.neuroscience.2013.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pan H, Wang D, Zhang X, Zhou D, Zhang H, Qian Q, He X, Liu Z, Liu Y, Zheng T, et al.. Amyloid β Is Not the Major Factor Accounting for Impaired Adult Hippocampal Neurogenesis in Mice Overexpressing Amyloid Precursor Protein. Stem Cell Reports 2016; 7(4):707-18; PMID:27693425; https://doi.org/ 10.1016/j.stemcr.2016.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wirths O, Bayer TA. Intraneuronal Abeta accumulation and neurodegeneration: Lessons from transgenic models. Life Sci 2012; 91(23-24):1148-52; PMID:22401905; https://doi.org/ 10.1016/j.lfs.2012.02.001 [DOI] [PubMed] [Google Scholar]
- [31].Casas C, Sergeant N, Itier JM, Blanchard V, Wirths O, van der Kolk N, Vingtdeux V, van de Steeg E, Ret G, Canton T, et al.. Massive CA1/2 neuronal loss with intraneuronal and N-terminal truncated Abeta42 accumulation in a novel Alzheimer transgenic model. Am J Pathol 2004; 165(4):1289-300; PMID:15466394; https://doi.org/ 10.1016/S0002-9440(10)63388-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Breyhan H, Wirths O, Duan K, Marcello A, Rettig J, Bayer TA. APP/PS1KI bigenic mice develop early synaptic deficits and hippocampus atrophy. Acta Neuropathol 2009; 117(6):677-85; PMID:19387667; https://doi.org/ 10.1007/s00401-009-0539-7 [DOI] [PubMed] [Google Scholar]
- [33].Christensen DZ, Kraus SL, Flohr A, Cotel MC, Wirths O, Bayer TA. Transient intraneuronal Abeta rather than extracellular plaque pathology correlates with neuron loss in the frontal cortex of APP/PS1KI mice. Acta Neuropathol 2008; 116(6):647-55; PMID:18974993; https://doi.org/ 10.1007/s00401-008-0451-6 [DOI] [PubMed] [Google Scholar]
- [34].Alexandru A, Jagla W, Graubner S, Becker A, Bauscher C, Kohlmann S, Sedlmeier R, Raber KA, Cynis H, Ronicke R, et al.. Selective Hippocampal Neurodegeneration in Transgenic Mice Expressing Small Amounts of Truncated A{beta} Is Induced by Pyroglutamate-A{beta} Formation. J Neurosci 2011; 31(36):12790-801; PMID:21900558; https://doi.org/ 10.1523/JNEUROSCI.1794-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bouter Y, Dietrich K, Wittnam JL, Rezaei-Ghaleh N, Pillot T, Papot-Couturier S, Lefebvre T, Sprenger F, Wirths O, Zweckstetter M, et al.. N-truncated amyloid beta (Abeta) 4–42 forms stable aggregates and induces acute and long-lasting behavioral deficits. Acta Neuropathol 2013; 126(2):189-205; PMID:23685882; https://doi.org/ 10.1007/s00401-013-1129-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Meissner JN, Bouter Y, Bayer TA. Neuron Loss and Behavioral Deficits in the TBA42 Mouse Model Expressing N-Truncated Pyroglutamate Amyloid-beta3-42. J Alzheimers Dis 2015; 45(2):471-82; PMID:25547635; https://doi.org/ 10.3233/jad-142868 [DOI] [PubMed] [Google Scholar]
- [37].Naumann N, Alpár A, Ueberham U, Arendt T, Gärtner U. Transgenic expression of human wild-type amyloid precursor protein decreases neurogenesis in the adult hippocampus. Hippocampus 2010; 20(8):971-9; PMID:19714567; https://doi.org/ 10.1002/hipo.20693 [DOI] [PubMed] [Google Scholar]
- [38].Morgenstern NA, Giacomini D, Lombardi G, Castaño EM, Schinder AF. Delayed dendritic development in newly generated dentate granule cells by cell-autonomous expression of the amyloid precursor protein. Mol Cell Neurosci 2013; 56:298-306; PMID:23851186; https://doi.org/ 10.1016/j.mcn.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wang B, Wang Z, Sun L, Yang L, Li H, Cole AL, Rodriguez-Rivera J, Lu H-C, Zheng H. The Amyloid Precursor Protein Controls Adult Hippocampal Neurogenesis through GABAergic Interneurons. J Neurosci 2014; 34(40):13314-25; PMID:25274811; https://doi.org/ 10.1523/JNEUROSCI.2848-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhang C, McNeil E, Dressler L, Siman R. Long-lasting impairment in hippocampal neurogenesis associated with amyloid deposition in a knock-in mouse model of familial Alzheimer's disease. Exp Neurology 2007; 204(1):77-87; PMID:17070803; https://doi.org/ 10.1016/j.expneurol.2006.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Contestabile A, Fila T, Ceccarelli C, Bonasoni P, Bonapace L, Santini D, Bartesaghi R, Ciani E. Cell cycle alteration and decreased cell proliferation in the hippocampal dentate gyrus and in the neocortical germinal matrix of fetuses with down syndrome and in Ts65Dn mice. Hippocampus 2007; 17(8):665-78; PMID:17546680; https://doi.org/ 10.1002/hipo.20308 [DOI] [PubMed] [Google Scholar]
- [42].Chakrabarti L, Scafidi J, Gallo V, Haydar TF. Environmental Enrichment Rescues Postnatal Neurogenesis Defect in the Male and Female Ts65Dn Mouse Model of Down Syndrome. Dev Neurosci 2011; 33(5):428-41; PMID:21865665; https://doi.org/ 10.1159/000329423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Trazzi S, Mitrugno VM, Valli E, Fuchs C, Rizzi S, Guidi S, Perini G, Bartesaghi R, Ciani E. APP-dependent up-regulation of Ptch1 underlies proliferation impairment of neural precursors in Down syndrome. Human Mol Genet 2011; 20(8):1560-73; PMID:21266456; https://doi.org/ 10.1093/hmg/ddr033 [DOI] [PubMed] [Google Scholar]
- [44].Trazzi S, Fuchs C, Valli E, Perini G, Bartesaghi R, Ciani E. The Amyloid Precursor Protein (APP) Triplicated Gene Impairs Neuronal Precursor Differentiation and Neurite Development through Two Different Domains in the Ts65Dn Mouse Model for Down Syndrome. J Biol Chem 2013; 288(29):20817-29; PMID:23740250; https://doi.org/ 10.1074/jbc.M113.451088 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [45].Caillé I, Allinquant B, Dupont E, Bouillot C, Langer A, Müller U, Prochiantz A. Soluble form of amyloid precursor protein regulates proliferation of progenitors in the adult subventricular zone. Development 2004; 131(9):2173-81; PMID:15073156; https://doi.org/ 10.1242/dev.01103 [DOI] [PubMed] [Google Scholar]
- [46].Lopez-Toledano MA, Shelanski ML. Increased neurogenesis in young transgenic mice overexpressing human APP(Sw, Ind). J Alzheimers Dis 2007; 12(3):229-40; PMID:18057556; https://doi.org/ 10.3233/JAD-2007-12304 [DOI] [PubMed] [Google Scholar]
- [47].Cao X, Südhof TC. A Transcriptively Active Complex of APP with Fe65 and Histone Acetyltransferase Tip60. Science 2001; 293(5527):115-20; PMID:11441186; https://doi.org/ 10.1126/science.1058783 [DOI] [PubMed] [Google Scholar]
- [48].Multhaup G, Huber O, Buée L, Galas M-C. Amyloid Precursor Protein (APP) Metabolites APP Intracellular Fragment (AICD), Aβ42, and Tau in Nuclear Roles. J Biol Chem 2015; 290(39):23515-22; PMID:26296890; https://doi.org/ 10.1074/jbc.R115.677211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ma Q-H, Futagawa T, Yang W-L, Jiang X-D, Zeng L, Takeda Y, Xu R-X, Bagnard D, Schachner M, Furley AJ, et al.. A TAG1-APP signalling pathway through Fe65 negatively modulates neurogenesis. Nat Cell Biol 2008; 10(3):283-94; PMID:18278038; https://doi.org/ 10.1038/ncb1690 [DOI] [PubMed] [Google Scholar]
- [50].Giacomini A, Stagni F, Trazzi S, Guidi S, Emili M, Brigham E, Ciani E, Bartesaghi R. Inhibition of APP gamma-secretase restores Sonic Hedgehog signaling and neurogenesis in the Ts65Dn mouse model of Down syndrome. Neurobiol Dis 2015; 82:385-396; PMID:26254735; https://doi.org/ 10.1016/j.nbd.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ghosal K, Stathopoulos A, Pimplikar SW. APP Intracellular Domain Impairs Adult Neurogenesis in Transgenic Mice by Inducing Neuroinflammation. PLOS ONE 2010; 5(7):e11866; PMID:20689579; https://doi.org/ 10.1371/journal.pone.0011866 [DOI] [PMC free article] [PubMed] [Google Scholar]


