On April 12, 2009, Mexico responded to a request for verification by the World Health Organization (WHO) of an outbreak of acute respiratory illness in the small community of La Gloria, Veracruz. During April 15–17, the Mexico Ministry of Health received informal notification of clusters of rapidly progressive severe pneumonia occurring mostly in Distrito Federal (metropolitan Mexico City) and San Luis Potosi. In response, on April 17, Mexico intensified national surveillance for acute respiratory illness and pneumonia. During April 22–24, novel influenza A (H1N1) virus infection, previously identified in two children in the United States (1), was confirmed in several patients. This report updates a previous report (2) on the outbreak in Mexico and summarizes public health actions taken to date by Mexico to monitor and control the outbreak. During March 1–May 29, national surveillance identified 41,998 persons with acute respiratory illness; specimens from 25,127 (59.8%) patients were tested, of which 5,337 (21.2%) were positive for novel influenza A (H1N1) virus infection by real-time reverse transcription–polymerase chain reaction (rRT-PCR). As of May 29, 97 patients with laboratory-confirmed infection had died. Epidemiologic evidence to date suggests that the outbreak likely peaked nationally in late April, although localized cases continue to be identified.
Enhanced Surveillance
The outbreak of acute respiratory illness in La Gloria, Veracruz (population 2,155), was characterized by a large number of cases (616 or 28.5% of the population) reported during March 5–April 10. This outbreak was likely of mixed cause; later testing of respiratory specimens collected during this period identified two patients as positive for seasonal influenza A (H3N2), one for seasonal influenza B, and one patient for novel influenza A (H1N1) virus with an adenovirus coinfection. Most of the respiratory illnesses from this outbreak remain undiagnosed; no severe cases or deaths were observed.
During March and April, 47 cases of rapidly progressive severe pneumonia were identified from clusters in Mexico City, San Luis Potosi, and other cities. Twelve deaths were reported; in four of the deaths, specimens were positive for novel influenza A (H1N1) infection. In response to the La Gloria outbreak and the pneumonia clusters, the National Committees for Epidemiological Surveillance and Emerging Infectious Disease released an epidemiologic alert on April 17 to enhance national surveillance for acute respiratory illness and severe pneumonia. This enhanced surveillance was implemented through active case finding in hospitals throughout the country, including daily zero-reporting (requiring facilities and jurisdictions to report even if no suspected cases had been identified) and monitoring of news media and other sources. The Mexico Ministry of Health also initiated investigations of outbreaks throughout the country, with the assistance of the WHO Global Outbreak and Alert Response Network, coordinated by the Pan American Health Organization.
During April 18–19, a survey conducted in 23 hospitals in Mexico City indicated increased pneumonia-related hospital admissions since April 10, particularly among young adults. On April 21, respiratory specimens collected as a result of these enhanced surveillance activities were sent to the National Microbiology Laboratory of the Public Health Agency of Canada and to the Influenza Division at CDC. During April 22–24, both laboratories identified novel influenza A (H1N1) virus in specimens from Mexican patients. The Directorate General of Epidemiology (DGE) established an Internet-based reporting platform to collect case-based epidemiologic information and a daily epidemiologic bulletin to disseminate results of ongoing investigations and recommendations from DGE. The first release of this bulletin occurred on April 26.
After identification of novel influenza A (H1N1) virus infection in Mexico, a case definition was developed. The initial definition of suspected novel influenza A (H1N1) virus infection included any hospitalized patient with severe acute respiratory illness. On May 1, this definition was expanded to include any person with acute respiratory illness defined as fever and either sore throat or cough. On May 11, the definition of suspected case was changed again to include any person with fever, cough, and headache, plus at least one of the following: rhinorrhea, coryza, arthralgia, myalgia, prostration, sore throat, chest pain, abdominal pain, or nasal congestion. In children aged <5 years, irritability replaced headache. A laboratory-confirmed case of novel influenza A (H1N1) virus infection was defined as illness in any person who had a respiratory specimen that tested positive for novel influenza A (H1N1) by rRT-PCR.
During 2008, to comply with new international health regulations, Mexico increased its number of influenza sentinel sites from 380 to 520 and expanded influenza testing capacity to four additional states. Enhanced surveillance for novel influenza A (H1N1) cases in mid-April 2009 generated an increase in the number of clinical specimens collected from patients with acute respiratory illness and a surge in testing at the National Laboratory from approximately 30 specimens to 900 daily. Enhancement of surveillance also included expansion of influenza testing capacity with rRT-PCR to eight states and with immunofluorescence to 30 of 31 Mexico states. As of May 29, a total of 25,127 specimens had been tested using rRT-PCR and, of those tested, 5,337 (21.2%) had been confirmed as positive for novel influenza A (H1N1) virus.
Of the 5,337 laboratory-confirmed cases of novel influenza A (H1N1) virus infection, 41.9% of patients were aged <15 years, 32.3% were aged 15–29 years, 23.7% were aged 30–59 years, and 2.1% were aged ≥60 years. Among patients with novel influenza A (H1N1) virus infection, 55.7% of deaths occurred among those aged 30–59 years (Table). Forty-nine percent of patients with confirmed infection were female.
TABLE.
Number of suspected,* tested, and laboratory-confirmed novel influenza A (H1N1) cases and deaths — Mexico, March–May 2009
| Age group (yrs) | No. suspected cases | No. tested | No. laboratory-confirmed positive† | (%) | Rate per 100,000 population | Deaths among laboratory-confirmed cases (% of confirmed deaths) | (%) | 2009 population§ |
|---|---|---|---|---|---|---|---|---|
| 0–4 | 6,428 | 3,520 | 695 | (13.2) | 7.26 | 5 | (5.2) | 9,578,579 |
| 5–14 | 7,742 | 4,229 | 1,517 | (28.7) | 7.11 | 7 | (7.2) | 21,327,734 |
| 15–29 | 11,568 | 7,591 | 1,704 | (32.3) | 5.83 | 26 | (26.8) | 29,221,168 |
| 30–59 | 12,687 | 8,507 | 1,251 | (23.7) | 3.26 | 54 | (55.7) | 38,330,279 |
| ≥60 | 2,249 | 1,016 | 112 | (2.1) | 1.23 | 5 | (5.2) | 9,092,937 |
| Age missing | 1,324 | 264 | 58 | — | — | — | — | — |
| Total | 41,998 | 25,127 | 5,337 | (100.0) | 4.96 | 97 | (100.0) | 107,550,697 |
The initial definition of suspected novel influenza A (H1N1) virus infection included any hospitalized patient with severe acute respiratory illness. On May 1, this definition was expanded to include any person with acute respiratory illness defined as fever and either sore throat or cough. On May 11, the definition of suspected case was changed again to include any person with fever, cough, and headache, plus at least one of the following: rhinorrhea, coryza, arthralgia, myalgia, prostration, sore throat, chest pain, abdominal pain, or nasal congestion. In children aged <5 years, irritability replaced headache.
Reported as of May 29, 2009.
From Consejo Nacional de Población. Available at http://www.conapo.gob.mx/index.php?option=com_content&view=article&id=36&Itemid=234.
As of May 29, Distrito Federal (Mexico City) had the highest number of laboratory-confirmed novel influenza A (H1N1) cases (1,804) and deaths (38); Mexico State reported 21 deaths (Figure 1). The peak number of confirmed cases (375) had onset of April 27 (Figure 2). As of May 29, all states in Mexico had reported laboratory-confirmed cases of novel influenza A (H1N1) virus.
FIGURE 1. Number of laboratory-confirmed cases of novel influenza A (H1N1) virus infection (N = 5,337)* and deaths (N = 97),† by state and Distrito Federal — Mexico, March–May 2009.
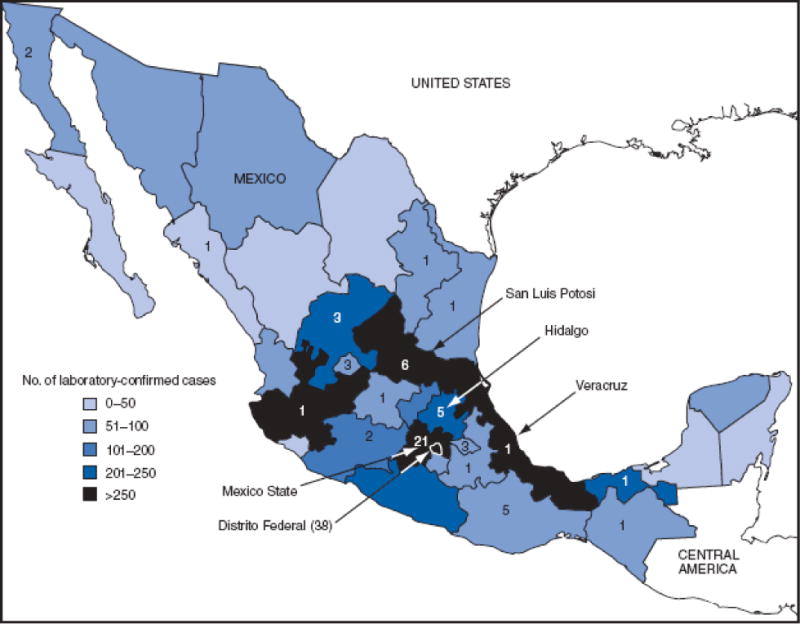
* Reported as of May 29, 2009.
† Indicated by numerals.
Alternative Text: The figure above shows the number of laboratory-confirmed cases of novel influenza A (H1N1) virus infection (N = 5,337) and deaths (N = 97) by state and Distrito Federal in Mexico from March through May 2009. As of May 29, Distrito Federal (Mexico City) had the highest number of laboratory-confirmed novel influenza A (H1N1) cases (1,804) and deaths (38); Mexico State reported 21 deaths.
FIGURE 2. Number (N = 5,305) of laboratory-confirmed cases of novel influenza A (H1N1) virus infection,* by date of illness onset — Mexico, March–May 2009.
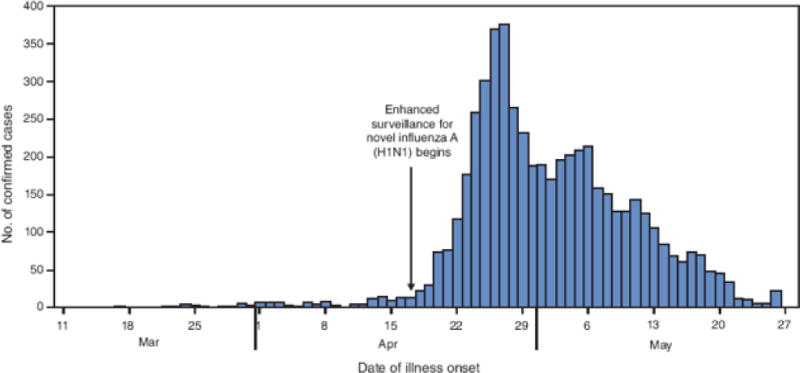
* Includes all confirmed cases with onset data reported as of May 29, 2009. Does not reflect all cases because of a backlog of untested specimens.
Alternative Text: The figure above shows the number (N = 5,305) of laboratory-confirmed cases of novel influenza A (H1N1) virus infection, by date of illness onset in Mexico from March through May 2009. The peak number of confirmed cases (375) had onset of April 27. As of May 29, all states in Mexico had reported laboratory-confirmed cases of novel influenza A (H1N1) virus.
Control Measures
On April 24, the federal government of Mexico activated the National Pandemic Preparedness and Response Plan and announced closure of schools in metropolitan Mexico City. Concurrently, the Ministry of Health launched a mass media campaign to promote respiratory hygiene and to alert the public about transmission of influenza. Additional social distancing measures included closure of restaurants and entertainment venues and cancellation of large public gatherings nationwide. To date, Mexico continues enhanced national surveillance and early antiviral treatment to decrease transmission of novel influenza A (H1N1) virus. Respiratory hygiene and hand washing are promoted through television and print media. On May 11, as schools reopened, parents were reminded to keep their children home if they had symptoms of influenza. In addition, a team of teachers and parents screened children at school entrances to determine whether they had fever or respiratory symptoms. The Ministries of Education and Health recommended closure of classrooms where two or more children had respiratory symptoms and closure of schools with ill children in two or more classrooms. On the first day of using this strategy, 91,357 children were determined symptomatic. This screening at schools was suspended on May 23. Screening for respiratory illness is ongoing at airports, where passengers complete a brief questionnaire about respiratory symptoms. Symptomatic travelers are asked to delay their journeys and referred for medical evaluation. At Mexico City’s International Airport, thermal scanners of body temperatures also are in use.*
Footnotes
Editorial Note:
Trends in case counts in Mexico suggest that novel influenza A (H1N1) activity is now decreasing, although localized transmission continues to occur. The epidemic curve of laboratory-confirmed cases remains incomplete because of a backlog of untested specimens. However, data regarding suspected cases (3) also indicate a peak in late April, and delays from case identification to reporting have decreased to a median of <2 days (Mexico Office of the Secretary of Health, unpublished data, 2009). Taken together, these data suggest that the outbreak likely has moved beyond its peak nationally, although a pattern of heterogeneous transmission and focal outbreak activity remains.
Several features of the outbreak in Mexico are consistent with outbreaks of the same novel influenza virus strain circulating in the United States and other countries. These features include person-to-person transmission during a period that is typically the low season for circulation of influenza viruses (4) and an age distribution of laboratory-confirmed cases that includes severe disease and deaths among children and adults in Mexico aged <60 years (4). Some deaths have occurred among previously healthy persons (4), and several patients have experienced an aggressive clinical course with severe pneumonia requiring ventilator support and progression to acute respiratory distress syndrome (2,5,6).
A recently reported serologic study suggested that children and younger adults have no or low levels of serum antibody, respectively, that are cross-reactive for the novel influenza A (H1N1) virus. Approximately one third of U.S. adults aged >60 years who were tested had cross-reactive neutralizing antibodies; however, the extent to which such antibody might be protective remains unknown (7). The serologic data, along with the age distribution of illness and clinical severity from the outbreak in Mexico, suggest age <60 years as a risk for infection and serious illness from novel A (H1N1) infection.
The current pattern of novel influenza A (H1N1) transmission in the northern hemisphere includes many localized outbreaks, including several among school children (8). This pattern is consistent with influenza outbreaks occasionally reported outside of the usual influenza season (9). However an unprecedented number of such off-season outbreaks are occurring currently. These outbreaks also involve extension into the community, as demonstrated by confirmed illness among travelers with no known epidemiologic link to focal outbreaks. Similar patterns of off-season outbreaks have been observed previously with the emergence and sustained transmission of other novel influenza A virus strains among humans (10).
The recent introduction of novel influenza A (H1N1) into several countries in the southern hemisphere at the beginning of its influenza season and the presumed susceptibility among much of the population to this new virus suggest that this strain might become a dominant circulating virus in the southern hemisphere during the coming months. The government of Mexico continues to coordinate a national response, engage partners, increase surge capacity, and implement mitigation measures to slow the spread of novel influenza A (H1N1). Investigations are ongoing to monitor virus circulation and evaluate mitigation strategies that might help guide prevention and control strategies in Mexico and worldwide.
Guidance from the Mexico government regarding screening, prevention, and control of novel influenza A (H1N1) virus infection is available at http://portal.salud.gob.mx/contenidos/noticias/influenza/lineamientos.html.
Contributor Information
JA Cordova, Office of the Secretary of Health.
M Hernandez, Office of the Secretary of Health.
H Lopez-Gatell, Directorate General of Epidemiology.
I Bojorquez, Directorate General of Epidemiology.
E Palacios, Directorate General of Epidemiology.
G Rodriguez, Directorate General of Epidemiology.
B de la Rosa, Directorate General of Epidemiology.
R Ocampo, Directorate General of Epidemiology.
C Alpuche, National Institute for Epidemiologic Reference and Diagnostics.
R Flores, National Institute for Epidemiologic Reference and Diagnostics.
JE Hernandez, National Institute of Public Health, Mexico. Pan American Health Organization, World Health Organization.
J Tustin, Public Health Agency of Canada.
K Watkins, Public Health Agency of Canada.
TL Stuart, Public Health Agency of Canada.
T Kuschak, Public Health Agency of Canada.
U Ströher, Public Health Agency of Canada.
G Soule, Public Health Agency of Canada.
B Balcewich, Public Health Agency of Canada.
E Azziz-Baumgartner, National Center for Immunization and Respiratory Diseases.
K Lafond, National Center for Immunization and Respiratory Diseases.
J Mott, National Center for Immunization and Respiratory Diseases.
F Mahoney, National Center for Immunization and Respiratory Diseases.
T Uyeki, National Center for Immunization and Respiratory Diseases.
M McCarron, National Center for Immunization and Respiratory Diseases.
A Mounts, National Center for Immunization and Respiratory Diseases.
MA Widdowson, National Center for Immunization and Respiratory Diseases.
X Xu, National Center for Immunization and Respiratory Diseases.
B Shu, National Center for Immunization and Respiratory Diseases.
S Lindstrom, National Center for Immunization and Respiratory Diseases.
A Klimov, National Center for Immunization and Respiratory Diseases.
J Katz, National Center for Immunization and Respiratory Diseases.
J Winchell, National Center for Immunization and Respiratory Diseases.
S Penaranda, National Center for Immunization and Respiratory Diseases.
N Dybdahl-Sissoko, National Center for Immunization and Respiratory Diseases.
K Ching, National Center for Immunization and Respiratory Diseases.
A Warner, National Center for Immunization and Respiratory Diseases.
K Etienne, National Center for Immunization and Respiratory Diseases.
S Waterman, National Center for Preparedness, Detection, and Control of Infectious Diseases.
J McAuliffe, Coordinating Office for Global Health.
S Dowell, Coordinating Office for Global Health.
PR Chavez, EIS Officer, CDC.
References
- 1.CDC. Update: swine influenza A (H1N1) infections—California and Texas, April 2009. MMWR. 2009;58:435–7. [PubMed] [Google Scholar]
- 2.CDC. Outbreak of swine-origin influenza A (H1N1) virus infection—Mexico, March–April 2009. MMWR. 2009;58:467–70. [PubMed] [Google Scholar]
- 3.Dirección General Adjunta de Epidemiología. Brote de influenza A H1N1 México. Boletín Diario. 2009 May 18;22 [Google Scholar]
- 4.Viboud C, Alonso WJ, Simonsen L. Influenza in tropical regions. PLoS Med. 2006;3:e89. doi: 10.1371/journal.pmed.0030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Human infection with new influenza A (H1N1) virus: clinical observations from Mexico and other affected countries, May 2009. Wkly Epidemiol Rec. 2009;84:185–9. [PubMed] [Google Scholar]
- 6.CDC. Hospitalized patients with novel influenza A (H1N1) virus infection—California, April–May, 2009. MMWR. 2009;58:536–41. [PubMed] [Google Scholar]
- 7.CDC. Serum cross-reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccine. MMWR. 2009;58:521–4. [PubMed] [Google Scholar]
- 8.CDC. Swine-origin influenza A (H1N1) virus infections in a school—New York City, April 2009. MMWR. 2009;58:470–2. [PubMed] [Google Scholar]
- 9.Kohn MA, Farley TA, Sundin D, Tapia R, McFarland LM, Arden NH. Three summertime outbreaks of influenza type A. J Infect Dis. 1995;172:246–9. doi: 10.1093/infdis/172.1.246. [DOI] [PubMed] [Google Scholar]
- 10.Taubenberger JK, Morens DM. 1918 influenza: the mother of all pandemics. Emerg Infect Dis. 2006;12:15–22. doi: 10.3201/eid1201.050979. [DOI] [PMC free article] [PubMed] [Google Scholar]


