Abstract
OBJECTIVES
Endoscopic surveillance of patients with Barrett’s Esophagus (BE) is recommended to detect esophageal adenocarcinoma (EAC) and its dysplasia precursors, but survival benefits are unclear. Using Surveillance, Epidemiology, and End Results (SEER) and linked Medicare data, we sought to determine the impact of a prior BE diagnosis on survival in patients with EAC.
METHODS
Our analysis focused on patients over age 65 with primary EAC diagnosed in a SEER region from 2000–2011 and enrolled in Medicare. We identified patients with preexisting BE prior to EAC diagnosis and compared this group to EAC patients without a prior BE diagnosis. A Cox Proportional Hazards model compared survival and included variables such as age, sex, cancer stage, treatment, and medical comorbidities.
RESULTS
Among 4,978 SEER-Medicare patients identified with EAC, 577 (12%) had preexisting BE; 4,401 (88%) did not. BE patients had overall lower stage (28.5% stage I vs. 12.8% stage IV) than those without preexisting BE (16.4% stage I vs. 30.6% stage IV). Overall survival was better among patients in the BE group (hazard ratio (HR), 0.56; 95% confidence interval (CI), 0.50–0.61); this benefit persisted in the adjusted model (HR, 0.72; 95%, 0.65–0.80). After adjusting for lead-time bias, the HRs attenuated to the null, with an unadjusted HR of 0.96 (95% CI: 0.86–1.05, P =0.39) and adjusted HR of 0.99 (CI: 0.89–1.10, P =0.92).
CONCLUSIONS
Survival outcomes in patients with a BE diagnosis prior to EAC were statistically better in both the unadjusted and adjusted Cox proportional hazards model. However, this benefit appears to be predominantly lead-time and length-time bias.
INTRODUCTION
Esophageal cancer is a highly lethal disease, with a five-year survival rate of only 20% (1). The incidence of esophageal adenocarcinoma (EAC), the most common form of this cancer in the United States, has dramatically risen in recent years (2–4). In particular, the proportion of patients with early-stage EAC has increased, due in part to increased surveillance of individuals with Barrett’s Esophagus (BE), the pre-malignant condition associated with the highest identified risk of developing EAC (5,6). To date, screening programs have focused on performing upper endoscopy with diagnostic biopsies to diagnose BE. Those patients found to have BE are then recommended to undergo endoscopic surveillance at set intervals to detect EAC at its earliest stages.
However, there are conflicting data on whether this surveillance strategy provides survival benefit, as potentially underpowered studies have suggested there is a down staging of EAC at diagnosis in patients enrolled in BE surveillance programs but no corresponding benefit (7–9). In one cohort, receipt of endoscopy at least 1 year before EAC diagnosis, which is indicative of prediagnosis screening, was found to be associated with a significantly reduced risk of death (10). Another study performed in the Netherlands demonstrated a reduced mortality among BE patients who underwent endoscopy surveillance (11). Alternatively, in a study of cases who died of EAC matched to controls living with EAC, surveillance within 3 years of cancer diagnosis was not associated with a decreased risk of death, and fatal cases were nearly as likely to have received surveillance as controls (9). A study of EAC among US veterans concluded that a prior endoscopy improved stage but did not change survival outcomes (12). Furthermore, survival estimates in these patients are subject to both lead-time and length-time bias. Lead-time bias can occur when surveillance detects a cancer at an earlier time point, which then contributes to the observed survival benefit. Length-time bias occurs when slower growing, less aggressive tumors are more likely detected through surveillance, with survival benefit among these cancers largely a result of more indolent tumor biology. Although there is conflicting evidence, BE screening and surveillance programs continue in many medical settings.
In this study, we used the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) and SEER-Medicare linked data to analyze the impact of a prior diagnosis of BE on EAC patients. Th e prior diagnosis of BE was used as a proxy to estimate the potential benefit of BE screening and surveillance.
METHODS
Cohort inclusion/exclusion criteria
We identified EAC patients diagnosed between 2000 and 2011 from the 2015 release of the SEER-Medicare data. The SEER-Medicare database is the result of a collaborative effort between the SEER registries, the Center for Medicare and Medicaid Services, and the National Cancer. The SEER database includes cancer incidence and survival data collected from cancer registries covering about 28% of the United States population. The Medicare database includes information for the vast majority, or ~97% of patients aged 65 and older, who receive Medicare benefits and is maintained by the Center for Medicare and Medicaid Services in a master Enrollment Database file (13). The SEER-Medicare data links SEER to Medicare enrollment and claims files, including Parts A and B claims for covered health care services. Patients were included if they had EAC diagnosed between 1 January 2000 and 31 December 2011 using International Classification of Diseases for Oncology (ICD-O-3) codes as outlined in Table 1. Patients with Tis tumors and those whose T, N, and M stages were all unknown were excluded. Only patients with EAC as their primary cancer pathologically confirmed, continuous enrollment in Medicare Parts A and B, and who were not enrolled in a Health Maintenance Organization (HMO), were included in our analysis. Endoscopies were identified using Current Procedural Terminology (CPT) codes (see Table 1).
Table 1.
SEER-Medicare claims codes
| Variable | Source | Codes |
|---|---|---|
| EAC diagnosis | ICD-0-3 codes | 8050, 8140–8147, 8160–8162, 8180–8221, 8250–8507, 8514, 8520–8551, 8560, 8570–8574, 8576, and 8940–8941 |
| Endoscopy | CPT codes | 43200, 43202, 43220, 43226, 43231, 43235, 43237, 43238, 43239, 43242, 43245, 43248, and 43249. |
| BE diagnosis | IDC-9 codes | 530.85, 530.22 (prior to 2003) |
BE, Barrett’s Esophagus; EAC, esophageal adenocarcinoma; ICD, International Classification of Diseases for Oncology.
Patients were identified as having BE if they had at least one claim with an ICD-9 code that occurred more than 6 months prior to their cancer diagnosis. The 6-month window for BE was chosen for this group to exclude patients who may have had their BE diagnosis occur concurrently with their EAC diagnosis on a single endoscopy (9, 10). Although Medicare benefits begin at age 65, patients were excluded if they were 65 years old (or <66) to ensure we had claims data at least 6 months prior to diagnosis for all patients. EAC patients who did not have a prior BE claim at least 6 months before diagnosis were categorized as the control comparison group; those who had an endoscopy at least 6 months before diagnosis were excluded from this group as these patients may represent either BE patients that were not identified or patients in a screening program; they were used as an additional control in a sensitivity analysis. The final cohort for the main analysis included 4,978 EAC patients.
Statistical analysis
Our primary outcomes of interest were overall and cancer-specific survival among EAC patients in patients with a prior diagnosis of BE compared to those with no prior diagnosis and no prior endoscopies. Th e broader aim was to estimate the impact of screening and surveillance on EAC survival. Overall survival of the two groups was plotted using a Kaplan–Meier curve. A Cox Proportional Hazard model was constructed to examine whether patients with a prior BE diagnosis had improved overall survival compared to those with no prior BE diagnosis. The first model estimated the unadjusted hazard ratio and the second model estimated the hazard ratio after adjustment for a select number of potential confounders: age at diagnosis (66–69, 70–74, 75–79, 80–84, 85+); sex; race, and ethnicity; year of diagnosis (2000–2007, 2008–2011); SEER region; marital status; median income (census tract quintile); college education (census tract quintile); comorbidity score; and treatment. We analyzed the adjusted model without stage and treatment and again with stage (T, N, and M tumor stages) and treatment (surgery/endoscopic therapy, radiation, or chemotherapy) included. Since earlier stage and more effective treatment are the only known biologic explanation for the association between screening and outcome, adjusting for stage and treatment should result in attenuation of the observed association fully to the null. The comorbidity score was estimated by applying the Deyo et al. adaptation (13–15) of the Charlson comorbidity index (16), which allows for index scores from ICD-9 diagnosis and procedure codes to Medicare inpatient, outpatient, and physician claims during the 13-month period prior to cancer diagnosis and classified into the groups 0, 1, and 2+. T, N, and M stage were mapped to the American Joint Committee on Cancer (AJCC) 7th edition according to the Collaborative Stage Data Collection System Version 02.05 using SEER variables for extension, lymph nodes, and metastasis, respectively. Cause of death was determined from SEER data.
We evaluated differences in the distribution of baseline characteristics between the two groups using χ 2 t-tests. To examine whether there was a difference in survival between patients who had a prior diagnosis of BE and those who did not, we fit Cox proportional hazards models for overall survival using all above independent variables as covariates. Survival was defined as time from date of diagnosis to date of death or 31 December 2013, whichever came first.
Sensitivity analyses were conducted to assess differences when: (1) the cohort was restricted to those aged 68 or older; (2) the BE patient population was restricted to only those with two or more endoscopies; and (3) when compared to the patient group with no prior BE and at least one endoscopy more than 6 months before EAC diagnosis.
We attempted to adjust for lead-time and length-time biases using the approaches published by Duffy et al. (17). Lead-time bias occurs when the survival time appears longer due to an earlier detection of disease, rather than improved outcomes. For lead-time bias, an estimate of additional follow-up time observed as a result of the BE patient’s lead time was calculated using an estimated sojourn time from incidence of cancer to clinical presentation with cancer and an estimate of each patient’s follow-up time. We used an estimated sojourn time of 3 years (18) for the analysis and assumed an exponential distribution (1). In length-time bias, screen-detected cancers appear to have a longer survival due to the presence of more slowly growing tumors, which are more likely to be detected, but less likely to be fatal. The adjustment for length time uses estimates of the proportion of BE patients with less aggressive tumors compared to those with more aggressive tumors. To calculate this, we examined a range of possible values for these estimates and conducted sensitivity analyses as suggested by Duffy et al. (17) and compared to the unadjusted relative risk (RR) of cancer death at 3 years.
Statistical significance was determined with a P <0.05 in a two-sided test. All statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Inc).
RESULTS
Patient characteristics
A total of 4,978 patients with EAC were identified in SEER-Medicare and the detailed results of patient characteristics are presented in Table 2. Of these patients, 577 (12%) had a prior diagnosis of BE, while the control group of 4,401 (88%) did not. Th e summary AJCC stage distribution for preexisting BE patients was 28.5, 9.5, 5.1, and 13.1% for stages I, II, III, and IV among preexisting BE patients, and 16.4, 18.4, 20.8, and 31.5% for stages I, II, II, and IV, among patients without preexisting BE. 13% of patients had unknown AJCC stage. At time of cancer diagnosis, patients in the group with a prior diagnosis of BE were more likely to have: an earlier T, N, or M stage of EAC, older age, higher Charlson comorbidity score, unknown tumor grade, and to undergo surgical treatment. Patients without a prior BE diagnosis were more likely to have a poor/undifferentiated tumor grade, undergo radiation, or chemotherapy and to have died of their cancer. There were no statistically significant differences in sex, race/ethnicity, diagnosis year, socioeconomic status, college education level, or SEER region between the two groups (P >0.05).
Table 2.
EAC patient characteristics: SEER-Medicare, 2000–2011
| Characteristic | No prior BE diagnosis (N=4401) |
Prior BE diagnosis (N=577) |
P value |
|---|---|---|---|
| Age | |||
| 66–69 | 931 (21.2%) | 72 (12.5%) | <0.0001 |
| 70–74 | 1105 (25.1%) | 124 (21.5%) | |
| 75–79 | 1055 (24.0%) | 154 (26.7%) | |
| 80–84 | 769 (17.5%) | 123 (21.3%) | |
| 85+ | 541 (12.3%) | 104 (18.0%) | |
| Age (mean, s.d.) | 75.9 (6.8) | 77.9 (6.8) | <0.0001 |
| Sex | |||
| Male | 3585 (81.5%) | 467 (81.0%) | 0.76 |
| Female | 816 (18.5%) | 110 (19.0%) | |
| Race/ethnicity | |||
| White | 4186 (95.1%) | 557 (96.5%) | 0.36 |
| Black | 74 (1.7%) | a | |
| Asian | 43 (1.7%) | a | |
| Hispanic/Latino | 39 (0.9%) | a | |
| Other/unknown | 59 (1.3%) | a | |
| Year of diagnosis | |||
| 2000–2003 | 1186 (27.0%) | 160 (27.7%) | 0.62 |
| 2004–2007 | 1601 (36.4%) | 198 (34.3%) | |
| 2008–2011 | 1614 (36.7%) | 219 (38.0%) | |
| SEER region | |||
| Northeast | 947 (21.5%) | 142 (24.6%) | 0.41 |
| South | 926 (21.0%) | 118 (20.5%) | |
| Midwest | 660 (15.0%) | 82 (14.2%) | |
| West/Hawaii | 1868 (42.4%) | 235 (40.7%) | |
| T stage | |||
| T1a | 281 (6.4%) | 167 (28.9%) | <0.0001 |
| T1b | 165 (3.8%) | 57 (9.9%) | |
| T1NOS | 774 (17.6%) | 116 (20.1%) | |
| T2 | 394 (8.9%) | 53 (9.2%) | |
| T3 | 1159 (26.3%) | 62 (10.8%) | |
| T4 | 843 (19.2%) | 48 (8.3%) | |
| Unknown | 785 (17.8%) | 74 (12.8%) | |
| N stage | |||
| N0 | 1806 (41.0%) | 382 (66.2%) | <0.0001 |
| N1 | 1782 (40.5%) | 112 (19.2%) | |
| Unknown | 813 (18.5%) | 83 (14.4%) | |
| M stage | |||
| M0 | 3053 (69.4%) | 503 (87.2%) | <0.0001 |
| M1 | 1348 (30.6%) | 74 (12.8%) | |
| Summary AJCC stage | |||
| I | 721 (16.4%) | 287 (28.5%) | <0.0001 |
| II | 809 (18.4%) | 85 (9.5%) | |
| III | 953 (21.7%) | 45 (4.5%) | |
| IV | 1348 (30.6%) | 74 (12.8%) | |
| Unknown | 570 (13.0%) | 86 (14.9%) | |
| Charlson score | |||
| 0 | 2494 (56.7%) | 267 (46.3%) | <0.0001 |
| 1 | 1138 (25.9%) | 169 (29.3%) | |
| 2+ | 769 (17.5%) | 141 (24.4%) | |
| Tumor grade | |||
| Low/lntermediate | 1573 (35.7%) | 224 (38.8%) | <0.0001 |
| Poor/undifferentiated | 2147 (48.8%) | 192 (33.3%) | |
| Unknown | 681 (15.5%) | 161 (27.9%) | |
| SES | |||
| 0 (lowest) | 846 (19.2%) | 101 (17.5%) | 0.20 |
| 1 | 870 (19.8%) | 108 (18.7%) | |
| 2 | 873 (19.8%) | 103 (17.9%) | |
| 3 | 878 (20.0%) | 137 (23.7%) | |
| 4 (highest) | 934 (21.2%) | 128 (22.2%) | |
| College | |||
| 0 (lowest) | 848 (19.3%) | 98 (17.0%) | 0.30 |
| 1 | 884 (20.1%) | 118 (20.5%) | |
| 2 | 894 (20.3%) | 106 (18.4%) | |
| 3 | 886 (20.1%) | 121 (21.0%) | |
| 4 (highest) | 889 (20.2%) | 134 (23.2%) | |
| Surgery | |||
| No | 3172 (72.1%) | 300 (52.0%) | <0.0001 |
| Yes | 1229 (27.9%) | 277 (48.0%) | |
| Radiation | |||
| No | 1789 (40.6%) | 337 (58.4%) | <0.0001 |
| Yes | 2612 (59.4%) | 240 (41.6%) | |
| Chemotherapy | |||
| No | 2416 (54.9%) | 408 (70.7%) | <0.0001 |
| Yes | 1985 (45.1%) | 169 (29.3%) |
BE, Barrett’s Esophagus; EAC, esophageal adenocarcinoma; SEER, Surveillance, Epidemiology, and End Results; NOS, not otherwise specified; SES, ecological socioeconomic status quintiles, based on median income by census tract ZIP code.
Data masked to comply with SEER-Medicare policy for groups <11.
Survival outcomes
The mean overall survival for the entire cohort was 21.4 months (s.d.=28.5) and the median was 10.1 months (range 0.5–168.9). Among BE patients, the mean was 35.3 months (s.d.=37.6), compared to 9.6 months (s.d.=6.5) for the control group. The median (range) survival was 21.4 months (0.6–168.0) and 9.5 months (0.5–168.9) for patients with and without a prior diagnosis of BE, respectively. Among patients who died, 75.5% (3,000/3,975) of patients without a prior diagnosis of BE died due to their cancer and 64.4% (273/424) of patients with a prior diagnosis of BE died due their cancer.
Overall and cancer-specific survival rates at one, three and five years are presented for both groups in Table 3. At 5 years, the overall survival rate was 31% for the BE group and 11% for the control group; the corresponding Kaplan–Meier survival curve is shown in Figure 1. When we examined cancer-specific survival between the two groups, 5-year survival rates were 47 and 21% for patients with and without a prior BE diagnosis, respectively. When stratified by AJCC 7th edition stage, a statistically significant increase in overall survival is seen in stages I and II; however, there are no survival differences between the two groups in stages III and IV. The Kaplan–Meier survival curves are shown in Figure 2.
Table 3.
1, 3 and 5-year survival rate (95% CI) for overall and cancer-specific survival
| No prior BE diagnosis (N=4401) |
Prior BE diagnosis (N=577) |
|
|---|---|---|
| Overall survival | ||
| 1 year | 43% (41–44%) | 62% (57–65%) |
| 3 years | 17% (16–18%) | 39% (35–43%) |
| 5 years | 11% (10–12%) | 31% (27–35%) |
| Cancer-specific survival | ||
| 1 year | 50% (49–52%) | 70% (66–74%) |
| 3 years | 26% (24–27%) | 52% (48–57%) |
| 5 years | 21% (20–22%) | 47% (42–51%) |
BE, Barrett’s Esophagus; CI, confidence interval; SEER, Surveillance, Epidemiology, and End Results.
Figure 1.

Kaplan–Meier survival curve for overall survival in patients with and without a prior Barrett’s Esophagus diagnosis.
Figure 2.
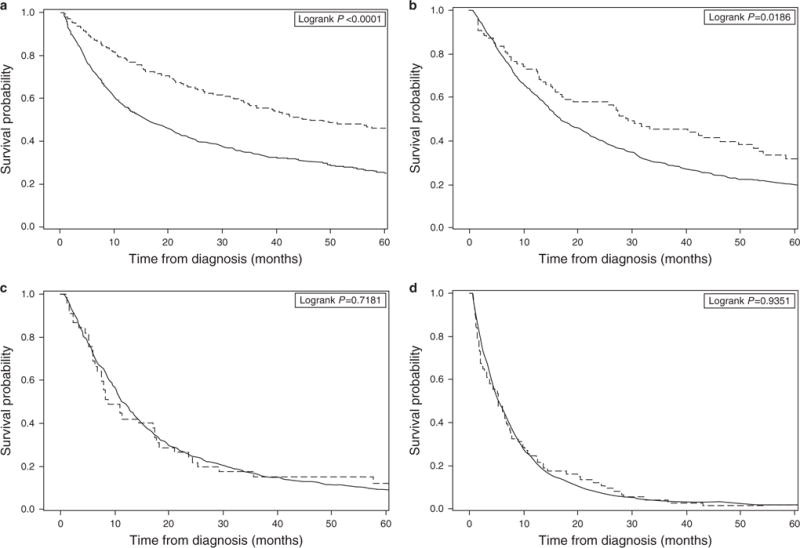
Kaplan–Meier survival curve by AJCC stage for overall survival in patients with and without a prior Barrett’s Esophagus diagnosis. Stage I is presented in (a), Stage II in (b), Stage III in (c), and Stage IV in (d).
When comparing overall survival in Cox proportional hazard models, the unadjusted model demonstrated a statistically significant difference among the two patient groups, with a survival benefit in the BE group (hazard ratio (HR), 0.56; 95% confidence interval (CI), 0.50–0.61; Table 4). Adjusting for potential confounders resulted in a HR of 0.53 (95% CI: 0.48–0.59). This benefit remained even aft er including the additional variables of stage and treatment adjustment (HR, 0.72; 95%, 0.65–0.80). In the fully adjusted model (with stage and treatment), patients who received any treatment had significantly better survival outcomes than those who did not (HR 0.47, 95% CI 0.43–0.51 for surgery/ endoscopic therapy, P <0.0001; HR 0.77, 95% CI 0.72–0.82 for radiation, P <0.0001; HR 0.71, 95% CI 0.66–0.75 for chemotherapy, P <0.0001). Those with a higher Charlson comorbidity score had significantly worse survival outcomes than those with a lower score (HR 1.14, 95% CI 1.06–1.23 for Charlson score 1, P =0.0003; HR 1.38, 95% CI 1.27–1.49 for Charlson score 2, P <0.0001). Lower stage patients (either T, N, or M stage) had better survival compared to higher stage patients. Th e multivariate hazard ratios for all covariates included in the Cox Proportional Hazard model are shown in Table 4. Similar results were found in the unadjusted and adjusted models for cancer-specific survival.
Table 4.
Cox Proportional hazard ratios for overall and EAC-specific mortality after adjustment for patient characteristicsa
| Characteristic | Overall | EAC specific | ||
|---|---|---|---|---|
| All | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value |
| Unadjusted model | 0.56 (0.50–0.61) | <0.0001 | 0.50 (0.44–0.56) | <0.0001 |
| Adjusted model (without stage or treatment) | 0.53 (0.48–0.59) | <0.0001 | 0.49 (0.43–0.55) | <0.0001 |
| Adjusted model (with stage and treatment) | 0.72 (0.65–0.80) | <0.0001 | 0.72 (0.63–0.82) | <0.0001 |
| Age at diagnosis | ||||
| 65–69 | 1.0 (ref) | 1.0 (ref) | ||
| 70–74 | 1.20 (1.08–1.31) | 0.0001 | 1.20 (1.08–1.34) | 0.0008 |
| 75–79 | 1.29 (1.17–1.41) | <0.0001 | 1.25 (1.12–1.40) | <0.0001 |
| 80–84 | 1.47 (1.33–1.63) | <0.0001 | 1.36 (1.21–1.53) | <0.0001 |
| 85+ | 1.89 (1.68–2.12) | <0.0001 | 1.75 (1.53–1.99) | <0.0001 |
| Sex | ||||
| Male | 1.0 (ref) | 1.0 (ref) | ||
| Female | 1.04 (0.97–1.13) | 0.27 | 1.11 (1.02–1.22) | 0.02 |
| Race/ethnicity | ||||
| White | 1.0 (ref) | 1.0 (ref) | ||
| Non-white | 0.86 (0.75–0.997) | 0.046 | 0.81 (0.69–0.96) | 0.01 |
| Year of diagnosis | ||||
| 2000–2003 | 1.0 (ref) | 1.0 (ref) | ||
| 2004–2007 | 0.96 (0.89–1.04) | 0.35 | 0.96 (0.88–1.06) | 0.42 |
| 2008–2011 | 0.87 (0.80–0.95) | 0.001 | 0.80 (0.73–0.89) | <0.0001 |
| SEER region | ||||
| Northeast | 1.0 (ref) | 1.0 (ref) | ||
| South | 1.16 (1.06–1.28) | 0.001 | 1.18 (1.06–1.32) | 0.003 |
| Midwest | 1.03 (0.94–1.14) | 0.51 | 1.09 (0.97–1.23) | 0.13 |
| West/Hawaii | 1.003 (0.93–1.09) | 0.95 | 1.05 (0.96–1.15) | 0.29 |
| T stage | ||||
| 1a | 1.0 (ref) | 1.0 (ref) | ||
| 1b | 1.04 (0.85–1.27) | 0.71 | 1.22 (0.93–1.60) | 0.16 |
| 1NOS | 1.77 (1.54–2.05) | <0.0001 | 2.22 (1.84–2.68) | <0.0001 |
| 2 | 1.46 (1.24–1.71) | <0.0001 | 1.82 (1.48–2.24) | <0.0001 |
| 3 | 1.87 (1.62–2.16) | <0.0001 | 2.36 (1.96–2.85) | <0.0001 |
| 4 | 2.14 (1.84–2.50) | <0.0001 | 2.70 (2.21–3.28) | <0.0001 |
| Unknown | 1.88 (1.62–2.19) | <0.0001 | 2.30 (1.89–2.80) | <0.0001 |
| N stage | ||||
| 0 | 1.0 (ref) | <0.0001 | 1.0 (ref) | |
| 1 | 1.16 (1.08–1.25) | <0.0001 | 1.25 (1.15–1.36) | <0.0001 |
| Unknown | 1.16 (1.05–1.27) | 0.0002 | 1.17 (1.05–1.30) | 0.0005 |
| M stage | ||||
| 0 | 1.0 (ref) | 1.0 (ref) | ||
| 1 | 1.68 (1.56–1.82) | <0.0001 | 1.74 (1.60–1.90) | <0.0001 |
| Charlson score | ||||
| 0 | 1.0 (ref) | 1.0 (ref) | ||
| 1 | 1.14 (1.06–1.23) | 0.0003 | 1.11 (1.02–1.20) | 0.02 |
| 2+ | 1.38 (1.27–1.49) | <0.0001 | 1.22 (1.11–1.34) | <0.0001 |
| Tumor grade | ||||
| Low/intermediate | 1.0 (ref) | 1.0 (ref) | ||
| Poor/undifferentiated | 1.31 (1.23–1.40) | <0.0001 | 1.39 (1.29–1.51) | <0.0001 |
| Unknown | 0.95 (0.87–1.04) | 0.26 | 0.94 (0.85–1.05) | 0.39 |
| SES | ||||
| 0 (lowest) | 1.0 (ref) | 1.0 (ref) | ||
| 1 | 0.98 (0.89–1.08) | 0.72 | 1.02 (0.92–1.15) | 0.70 |
| 2 | 0.94 (0.85–1.04) | 0.21 | 0.92 (0.81–1.03) | 0.16 |
| 3 | 0.97 (0.87–1.08) | 0.57 | 0.98 (0.87–1.11) | 0.77 |
| 4 (highest) | 0.94 (0.83–1.06) | 0.31 | 0.97 (0.84–1.12) | 0.68 |
| College | ||||
| 0 (lowest) | 1.0 (ref) | 1.0 (ref) | ||
| 1 | 0.98 (0.89–1.08) | 0.73 | 0.97 (0.84–1.11) | 0.56 |
| 2 | 0.92 (0.83–1.02) | 0.11 | 0.97 (0.86–1.08) | 0.41 |
| 3 | 0.94 (0.84–1.05) | 0.26 | 0.94 (0.83–1.07) | 0.37 |
| 4 (highest) | 0.93 (0.83–1.06) | 0.27 | 0.95 (0.83–1.07) | 0.50 |
| Surgery | ||||
| No | 1.0 (ref) | 1.0 (ref) | ||
| Yes | 0.47 (0.43–0.51) | <0.0001 | 0.39 (0.35–0.43) | <0.0001 |
| Radiation | ||||
| No | 1.0 (ref) | 1.0 (ref) | ||
| Yes | 0.77 (0.72–0.82) | <0.0001 | 0.75 (0.69–0.81) | <0.0001 |
| Chemotherapy | ||||
| No | 1.0 (ref) | 1.0 (ref) | ||
| Yes | 0.71 (0.66–0.75) | <0.0001 | 0.67 (0.62–0.73) | <0.0001 |
BE, Barrett’s Esophagus; CI, confidence interval; EAC, esophageal adenocarcinoma; SEER, Surveillance, Epidemiology, and End Results.
Adjusted models include all characteristics listed in the table, either with or without stage and treatment.
Sensitivity analyses
In order to reduce the potential of mislabeling any patients with a prior BE diagnosis as having no BE due to a lack of enough Medicare claim years, we restricted the cohort to patients who were at least 68 years old, creating a cohort of patients who all had at least three years of Medicare enrollment. Th e unadjusted HR for overall survival was 0.54 (95% CI: 0.49–0.69, P <0.0001), the adjusted HR (without stage and treatment variables) was 0.53 (95% CI: 0.48–0.59, P <0.0001) and the adjusted HR (including stage and treatment variables) was 0.71 (95% CI: 0.63–0.79, P <0.0001).
When we examined the number of endoscopies performed in the BE group, 36% (208/577), had at least two endoscopies more than 6 months prior to their EAC diagnosis. We compared this group to those patients without BE and found a statistically significant increase in overall survival (HR: 0.53; 95% CI: 0.45–0.62, P <0.0001). Th is persisted aft er adjustment for all variables (HR: 0.62; 95% CI: 0.53–0.74, P <0.0001).
We found 292 additional patients who had no BE claims but had at least one endoscopy more than 6 months prior to their EAC diagnosis. It is possible that this group could be patients who had BE that was either missed or not coded correctly. Another possibility is that patients were not diagnosed with BE, but underwent an upper endoscopy for other indications. We compared the BE patients to this group to determine whether the diagnosis of BE still had an effect on overall survival. We found the unadjusted HR for overall survival was 0.51 (95% CI: 0.44–0.60, P <0.0001) and the adjusted HR was 0.74 (95% CI: 0.60–0.92, P =0.007), showing a survival benefit for those patients in the BE group.
Lead-time and length-time bias
There is concern for lead-time bias impacting the results. To try to correct for this, we used the statistical method provided by Duffy et al. and an estimated sojourn time of 3 years (18). This resulted in an unadjusted HR for overall survival of 0.96 (95% CI: 0.86–1.05, P =0.39). Th e adjusted HR (without stage and treatment) was 0.99 (CI: 0.89–1.10, P =0.92). As a sensitivity analysis, we also used and analyzed results with sojourn times of 2 years and 4 years. Decreasing the sojourn time to 2 years attenuated the results, but they remained statistically significant (unadjusted HR: 0.85 (95% CI: 0.77–0.94, P =0.001); adjusted HR: 0.87 (CI: 0.79–0.87, P =0.009)). Increasing to 4 years resulted in an unadjusted HR of 1.06 (95% CI: 0.95–1.17, P =0.30) and an adjusted HR of 1.11 (CI: 1.004–1.23, P =0.042). To understand the impact of lead-time bias on EAC-specific mortality, we ran the models using the 3-year correction. We found an unadjusted HR of 0.83 (95% CI: 0.73–0.94, P =0.004). Th e adjusted model HR was 0.89 (95% CI: 0.89, 0.78–1.01, P =0.06). The potential for length-time bias is also a concern in this cohort. In an attempt to adjust for this, we used a suggested range of proportions of patients with length-time bias (slower, less aggressive) tumors, ranging from 50 to 90% and a range of relative fatality rates, from 0.50 to 0.90 (Supplementary Table 1) (17, 19) We based the sensitivity analyses on a RR of cancer death at 3 years for patients with a prior diagnosis of BE vs. those without (unadjusted RR=0.85). Th e RR aft er adjustment for length bias ranged from 0.85 to 0.97 (Supplementary Table 1), with a median of 0.87. If we define the patients in our cohort with low or intermediate grade tumors as those affected by length-time bias (19) we would categorize 36% of our patients as affected. This results in an adjusted 3-year RR of 0.86 compared to our initial or base case RR of 0.85.
DISCUSSION
We analyzed the linked SEER-Medicare database to test our hypothesis that a prior diagnosis of BE would impact EAC survival. Our analysis finds that EAC patients with a prior diagnosis of BE have better survival outcomes compared to the control group, even aft er adjusting for numerous variables. Patients within the BE group had earlier cancer stages, and were more likely to undergo surgery, demonstrating that patients with a prior BE diagnosis were typically downstaged. However, much of the observed effects is most likely related to selection bias, such as lead-time and length-time bias. When we adjusted for these two biases, we saw an attenuation in our results for both, suggesting that this is what is driving the survival outcomes. Lead-time in particular had a large effect on survival outcomes, with length-time having a lesser effect. When we included stage and treatment in the Cox proportional hazard models, survival outcomes among BE patients worsened.
A recently published 2015 VA study demonstrated that BE patients were more likely to be diagnosed at an earlier stage and have improved survival outcomes if they were in a surveillance program compared to those who were not (HR: 0.47) (18). We found comparable HRs in our study in both the unadjusted and adjusted models when we compared patients with BE to those without and when we compared number of endoscopes among BE patients.
In contrast, a 2013 study of patients at Kaiser Permanente of Northern California compared 51 BE patients who died from EAC (assumed to be surveillance eligible) with 101 matched controls comprised of BE patients who did not die of EAC and found no improved survival among the case group (9). However, this study had a small number of EAC patients and was focused on one region of the US.
Our results are consistent with an earlier and smaller 2002 SEER-Medicare analysis by Cooper et al. (10), which demonstrated that having at least one upper endoscopy prior to EAC diagnosis was associated with an earlier stage at diagnosis and improved survival (HR: 0.73, P =0.01). They also found that having a BE diagnosis was associated with improved survival among EAC patients (HR: 0.61, P =0.004). Our analyses used the most recent available data in contrast to data used by the Cooper study (mid 1990’s) and was considerably larger encompassing 11 years of data compared to four years. In addition, we were able to identify additional patients with a BE-specific ICD-9 code, rather than the more general diagnosis code used prior to 2003, which was less specific and could include patients with esophageal ulcers, for example.
As with any study that relies on administrative claims data, our study is subject to limitations. Because we used SEER-Medicare data and excluded 65 year olds, our cohort was limited to patients aged 66 or older, so we were not able to study how prior BE diagnosis may affect EAC survival in younger patients. However, EAC is associated with older age, with 59% of patients greater than age 65 (20). Furthermore, we potentially misclassified any patients who were diagnosed with BE prior to enrolling in Medicare as patients without BE because their diagnoses occurred when they were younger than 65. We attempted to control for this in a sensitivity analysis of patients aged 68 and older to ensure all had Medicare claims for at least 3 years.
With the acknowledged limitations of our analysis, a corollary of findings is that aggressive screening and surveillance for BE and endoscopic therapy for BE may not result in significant cancer survival benefit for those patients who progress to EAC. Additional methods to risk stratify and more accurately predict an individual patient’s or “personalized” risk could improve the net benefit of BE management strategies. Risk factors to incorporate may include demographic, clinical, and molecular biomarkers to successfully identify higher risk patients who may benefit from targeted screening and treatment. In conclusion, our analysis found that the survival in patients over age 65 with a diagnosis of BE prior to an EAC were statistically better in both the unadjusted and adjusted Cox proportional hazards model. However, the benefits found in our analysis appear to be mainly a result of lead-time and length-time bias. In this setting of imperfect data, from a conservative patient cancer prevention perspective, we would endorse endoscopic surveillance in patients with Barrett’s esophagus until more definitive data are available.
Supplementary Material
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
-
✓
Endoscopic surveillance of patients with Barrett’s Esophagus (BE) is recommended to detect dysplasia and early esophageal adenocarcinoma (EAC).
-
✓
Survival benefits are unclear.
WHAT IS NEW HERE
-
✓
Patients aged 65+ with BE and EAC appear to have an earlier stage than EAC patients without BE.
-
✓
Overall survival was better in EAC patients with BE.
-
✓
Survival differences can be explained by lead-time and length-time biases in the BE patient group.
Acknowledgments
Financial Support: NIH grant R01 CA140574, the study sponsor had no role in this manuscript.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/ajg
CONFLICT OF INTEREST
Guarantor of the article: Chin Hur, MD, MPH.
Author contributions: Angela C. Tramontano: statistical analysis, interpretation of data, study design, drafting of the manuscript, critical revision of the manuscript and approval of the final version. Deirdre F. Sheehan, Jennifer M. Yeh, Julian A. Abrams, Joel H. Rubenstein, John M. Inadomi, Deborah Schrag: interpretation of data, critical revision of the manuscript and approval of the final version. Chung Yin Kong and Emily C. Dowling: critical revision of the manuscript and approval of the final version. Chin Hur: study conception and design, interpretation of data, drafting of the manuscript, critical revision of the manuscript and approval of the final version.
Potential Competing Interests: None.
References
- 1.Thrift AP, Whiteman DC. The incidence of esophageal adenocarcinoma continues to rise: analysis of period and birth cohort effects on recent trends. Ann Oncol. 2012;23:3155–62. doi: 10.1093/annonc/mds181. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Kong CY, Kroep S, Curtius K, et al. Exploring the recent trend in esophageal adenocarcinoma incidence and mortality using comparative simulation modeling. Cancer Epidemiol Biomarkers Prev. 2014;23:997–1006. doi: 10.1158/1055-9965.EPI-13-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hur C, Miller M, Kong CY, et al. Trends in esophageal adenocarcinoma incidence and mortality. Cancer. 2013;119:1149–58. doi: 10.1002/cncr.27834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rice TW, Blackstone EH, Goldblum JR, et al. Superficial adenocarcinoma of the esophagus. J Thorac Cardiovasc Surg. 2001;122:1077–90. doi: 10.1067/mtc.2001.113749. [DOI] [PubMed] [Google Scholar]
- 6.Eloubeidi MA, Mason AC, Desmond RA, et al. Temporal trends (1973–1997) in survival of patients with esophageal adenocarcinoma in the United States: a glimmer of hope? Am J Gastroenterol. 2003;98:1627–33. doi: 10.1111/j.1572-0241.2003.07454.x. [DOI] [PubMed] [Google Scholar]
- 7.Corley DA, Levin TR, Habel LA, et al. Surveillance and survival in Barrett’s adenocarcinomas: a population-based study. Gastroenterology. 2002;122:633–40. doi: 10.1053/gast.2002.31879. [DOI] [PubMed] [Google Scholar]
- 8.Streitz JM, Jr, Andrews CW, Jr, Ellis FH., Jr Endoscopic surveillance of Barrett’s esophagus. Does it help? J Thorac Cardiovasc Surg. 1993;105:383–7. discussion 7–8. [PubMed] [Google Scholar]
- 9.Corley DA, Mehtani K, Quesenberry C, et al. Impact of endoscopic surveillance on mortality from Barrett’s esophagus-associated esophageal adenocarcinomas. Gastroenterology. 2013;145:312–9.e1. doi: 10.1053/j.gastro.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper GS, Yuan Z, Chak A, et al. Association of prediagnosis endoscopy with stage and survival in adenocarcinoma of the esophagus and gastric cardia. Cancer. 2002;95:32–8. doi: 10.1002/cncr.10646. [DOI] [PubMed] [Google Scholar]
- 11.Verbeek RE, Leenders M, Ten Kate FJ, et al. Surveillance of Barrett’s esophagus and mortality from esophageal adenocarcinoma: a population-based cohort study. Am J Gastroenterol. 2014;109:1215–22. doi: 10.1038/ajg.2014.156. [DOI] [PubMed] [Google Scholar]
- 12.Rubenstein JH, Sonnenberg A, Davis J, et al. Effect of a prior endoscopy on outcomes of esophageal adenocarcinoma among United States veterans. Gastrointest Endosc. 2008;68:849–55. doi: 10.1016/j.gie.2008.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV, 3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 14.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 15.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46:1075–9. doi: 10.1016/0895-4356(93)90103-8. discussion 81–90. [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Duffy SW, Nagtegaal ID, Wallis M, et al. Correcting for lead time and length bias in estimating the effect of screen detection on cancer survival. Am J Epidemiol. 2008;168:98–104. doi: 10.1093/aje/kwn120. [DOI] [PubMed] [Google Scholar]
- 18.El-Serag HB, Naik AD, Duan Z, et al. Surveillance endoscopy is associated with improved outcomes of oesophageal adenocarcinoma detected in patients with Barrett’s oesophagus. Gut. 2015;65:1252–60. doi: 10.1136/gutjnl-2014-308865. [DOI] [PubMed] [Google Scholar]
- 19.Bhat SK, McManus DT, Coleman HG, et al. Oesophageal adenocarcinoma and prior diagnosis of Barrett’s oesophagus: a population-based study. Gut. 2015;64:20–25. doi: 10.1136/gutjnl-2013-305506. [DOI] [PubMed] [Google Scholar]
- 20.Surveillance Research Program, National Cancer Institute. Fast Stats (Esophageal Cancer): an interactive tool for access to SEER cancer statistics. Available at http://seer.cancer.gov/statfacts/html/esoph.html. Accessed on 1 July 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


