Abstract
Background
Filanesib (ARRY-520) is a highly selective inhibitor of kinesin spindle protein, which has shown preclinical anti-myeloma activity.
Methods
This open-label Phase 1/2 study determined the maximum tolerated dose (MTD) of filanesib administered on Days 1 and 2 of 14-day cycles in patients with multiple myeloma (MM) and included expansion cohorts with/without dexamethasone (40 mg/week). Patients in the dose escalation (N=31) and Phase 2 single-agent cohorts (N=32) received prior bortezomib as well as prior thalidomide and/or lenalidomide. Patients in the Phase 2 filanesib + dexamethasone cohort (N=55) had prior alkylator therapy and disease refractory to lenalidomide, bortezomib and dexamethasone. Prophylactic filgrastim was incorporated during the dose escalation and used throughout Phase 2.
Results
Patients in each cohort had a median of ≥6 prior therapies. The most common dose-limiting toxicities were febrile neutropenia and mucosal inflammation. In Phase 2, grade 3/4 cytopenias were reported in approximately 50% of patients. Nonhematologic toxicities were infrequent. Phase 2 response rates (≥ partial response) were 16% (single agent) and 15% (filanesib + dexamethasone). All responding patients had low baseline levels of alpha 1-acid glycoprotein, a potential selective biomarker.
Conclusions
Filanesib 1.50 mg/m2/day administered with prophylactic filgrastim has a manageable safety profile and encouraging activity in heavily pretreated patients. This study is registered at www.clinicaltrials.gov as NCT00821249.
Keywords: Multiple Myeloma, Kinesin, Spindle Poles, Maximum Tolerated Dose, Pharmacokinetics, Dexamethasone, Filanesib
INTRODUCTION
Multiple myeloma (MM) is a cancer of antibody-producing plasma cells, typically characterized by uncontrolled proliferation of malignant plasma cells at multiple sites in the bone marrow and by the secretion of immunoglobulins (Ig). The development of novel active agents such as proteasome inhibitors (PIs) and immunomodulatory agents (IMiDs) represents significant advancement in the treatment of MM; however, the disease remains incurable and fatal in almost all patients. A need therefore remains for therapeutic options with novel mechanisms of action to treat patients with MM whose disease has relapsed after treatment with, or is refractory to, existing agents.
Targeting cellular mitosis by inhibiting kinesin spindle protein (KSP, also known as Eg5 or kinesin-5) represents a unique approach to treat MM. KSP is critical to the separation of spindle poles and generation of bipolar spindles early in mitosis.1 Inhibition results in the formation of a monopolar spindle, then mitotic arrest and ultimately, activation of apoptotic pathways and cell death.2,3 In certain cell types, KSP inhibition results in rapid onset of cell death due to depletion of the survival protein myeloid cell leukemia sequence 1 (Mcl-1), a member of the Bcl-2 family of anti-apoptotic regulators,4 during sustained mitotic arrest. As proliferating hematopoietic cells (unlike most nonhematologic cells) show Mcl-1 dependence for their survival, KSP inhibition represents a novel mechanism of action in the treatment of patients with MM. In addition, because KSP inhibition is not expected to share resistance mechanisms with conventional MM therapies, it is likely patients refractory to PI and/or IMiD therapies may retain sensitivity to a KSP inhibitor.
Filanesib (also known as ARRY-520) is a highly selective, targeted small-molecule inhibitor of KSP that has demonstrated significant tumor growth inhibition, including durable regressions, in nonclinical mouse xenograft models and superior efficacy compared with microtubule-targeted agents (paclitaxel or vincristine) in several of these models.5,6 Filanesib has also shown activity in several taxane-resistant models.5,6
This paper reports the results of a Phase 1/2 study (clinicaltrials.gov: NCT00821249) designed to establish the maximum tolerated dose (MTD) of single-agent filanesib in patients with relapsed/refractory MM (RRMM) and to evaluate filanesib as a single agent and in combination with dexamethasone in Phase 2 expansion cohorts. Based on preclinical data, the same dose delivered by a divided dose schedule was better tolerated and demonstrated superior efficacy than when administered as a single dose. The hypothesis was that it is necessary to maintain drug exposure such that cells in mitotic arrest would become apoptotic. The more dose intense Day 1 and Day 2 q 2 weeks schedule was the first step taken to try to prolong drug exposure. A retrospective exploratory assessment was also made to determine whether elevated baseline levels of alpha 1-acid glycoprotein (AAG) were associated with diminished filanesib activity.
METHODS
Study Design
The primary objective of the Phase 1 dose escalation was to determine the MTD of filanesib as a single agent. Secondary objectives included assessment of pharmacokinetics (PK), preliminary estimates of filanesib activity, and identification of potential markers for patient selection. The study included two Phase 2 cohorts evaluating filanesib administered at the Phase 1 MTD as a single agent (“Phase 2-Filanesib”) and with low-dose dexamethasone (“Phase 2-Filanesib/Dex”). The primary objective of Phase 2 was determination of filanesib activity by overall response rate (ORR). Secondary objectives included other measures of clinical activity, safety and biomarker assessments.
All patients received filanesib as a 1-hour intravenous (IV) infusion on Day 1 and Day 2 in 14-day cycles until unacceptable toxicity or disease progression (PD). Patients in the Phase 2-Filanesib/Dex cohort also received 40 mg dexamethasone orally (PO) once per week starting on Day 2. Due to the incidence of neutropenia in a concurrent study of filanesib7, prophylactic filgrastim was added to the treatment regimen during the Phase 1 dose escalation. All patients received filgrastim (dose determined per institutional standards) as a single daily subcutaneous (SC) bolus injection for a total of 5 to 7 days, beginning on Day 3 or Day 4 of each cycle; filgrastim was also administered in this manner in both Phase 2 cohorts.
Due to the relatively late onset of responses observed in this study and the fluctuations in laboratory values preceding those responses, the protocol was amended prior to enrollment of the Phase 2-Filanesib/Dex cohort to allow study treatment for up to 3 months despite PD, after which treatment was to be discontinued if progression continued. The study was also amended to allow patients in the Phase 1 and Phase 2-Filanesib cohorts with long-term stable disease (SD) and those with PD following partial response (PR) or minimal response (MR) to add low-dose dexamethasone to the treatment regimen.
Upon treatment discontinuation, patients not demonstrating PD were followed every 4 weeks until documented progression or initiation of subsequent therapy. For patients in the Phase 2 cohorts, follow-up continued every 2 months thereafter to document subsequent therapy and survival.
Patients
Phase 1 and Phase 2-Filanesib enrolled patients ≥18 years of age with RRMM or plasma cell leukemia (PCL). Patients had measurable disease, defined as any of the following: measurable serum M-protein (≥ 1.0 g/dL for IgG, ≥ 0.5 g/dL for IgA, ≥ 0.1 g/dL for IgD), urine M-protein ≥ 200 mg/24 hours, serum free light chain (FLC) ≥ 10 mg/dL with abnormal ratio, or oligo- or nonsecretory disease with bone marrow involvement with ≥ 30% plasmacytosis. Patients had received at least 2 prior regimens including bortezomib and an IMiD (thalidomide and/or lenalidomide), and had PD during or after the last prior regimen.
Patients enrolled in the Phase 2 Filanesib/Dex cohort met slightly different criteria regarding measurable disease (serum M-protein ≥ 0.5 g/dL for IgG). In addition to the prior regimens described above, patients were required to have received ≥ 2 consecutive cycles of prior treatment that included lenalidomide and bortezomib, and to have disease that was refractory to lenalidomide, bortezomib and dexamethasone (i.e., progressed on treatment or within 60 days of cessation) and refractory to the regimen immediately prior to study participation. These patients were also required to have prior alkylator therapy (i.e., autologous stem cell or bone marrow transplant with melphalan, or 2 cycles of either melphalan or cyclophosphamide).
All patients were required to have Eastern Cooperative Oncology Group performance status of 0 or 1, adequate liver and renal function (transaminases ≤ 2.5 × upper limit of normal, bilirubin < 1.5 mg/dL, and serum creatinine ≤ 2.5 mg/dL or calculated creatinine clearance ≥ 50 mL/min) and adequate hematology values without transfusion support within 2 weeks of screening (hemoglobin ≥ 8 g/dL, neutrophils ≥ 1.5 × 109/L, platelets ≥ 75 × 109/L or ≥ 50 × 109/L if marrow contained ≥ 50% plasma cells). Key exclusion criteria included primary amyloidosis and any stem cell or bone marrow transplant within 3 months prior to initiating study drug.
This study was conducted in adherence with International Conference on Harmonisation Good Clinical Practice guidelines and all applicable regulatory requirements. The study was approved by the institutional review boards of all participating centers, and patients provided written informed consent.
Determination of MTD in Phase 1
In Phase 1, a standard 3+3 design was used to determine the MTD, defined as the dose below which ≥ 33% of patients experienced a dose-limiting toxicity (DLT). A DLT was defined as an adverse event (AE) in Cycle 1 or Cycle 2 that met any of the following criteria: Grade 4 neutropenia > 7 days; febrile neutropenia; Grade 4 thrombocytopenia > 7 days and not responding to platelet transfusions; any thrombocytopenia associated with ≥ Grade 3 bleeding attributed to filanesib; any Grade 3 or 4 nonhematologic AE except nausea, vomiting or diarrhea in the absence of prophylaxis; or any treatment-related AE that delayed the start of Cycle 2 or Cycle 3 by > 2 weeks. Patients who did not complete Cycle 1 and Cycle 2 for reasons other than toxicity were considered unevaluable for assessment of DLT and were replaced.
At the investigator’s discretion, patients experiencing a DLT were permitted to be rechallenged with filanesib at a lower dose, and patients in Phase 1 were permitted to escalate to higher tolerated doses.
Assessments
AEs were assessed for severity using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.8
Disease response was categorized by the investigators according to the International Myeloma Working Group (IMWG) uniform response criteria for MM9 with the addition of minimal response (MR) per European Group for Blood and Marrow Transplantation (EBMT) criteria.10
Blood and 24-hour urine samples were collected at baseline and every 4 weeks until documented PD for the following assessments: serum protein electrophoresis, serum immunofixation electrophoresis, serum FLC, urine protein electrophoresis and urine immunofixation electrophoresis.
The primary efficacy analysis was objective response rate (ORR), defined as ≥ PR. Secondary efficacy analyses were time to response (TTR) and duration of response (DOR). Time to next treatment (TNT), treatment-free interval (TFI), progression-free survival (PFS) and overall survival (OS) were calculated for patients in the Phase 2 cohorts. A post hoc analysis was performed to calculate clinical benefit rate (CBR), defined as the proportion of response-evaluable patients whose best overall response was ≥ MR.
AAG, a biomarker hypothesized to correlate with filanesib activity, was measured in baseline peripheral blood samples using a validated immunoturbidimetric assay (Randox Laboratories, Crumlin, UK). An exploratory analysis of ORR by baseline AAG level was performed for response-evaluable patients in the Phase 2 cohorts.
PK evaluations
Blood samples for PK were collected in Phase 1 during Cycle 1 Day 1 (1 and 8 hours after beginning of infusion [BOI]) and Day 2 (before and at 1 and 8, 24, 48 and 144 hours after Day 2 BOI). Samples were analyzed using a validated liquid chromatography tandem mass spectrometry (LC-MS/MS) method (lower limit of detection 1 ng/mL filanesib). Noncompartmental analysis was done in Phoenix® WinNonlin®, Version 6.3 (Certara, St. Louis, MO, USA) as described by LoRusso et al.7
Statistical analysis
Up to 25 patients were anticipated to enroll in Phase 1. The Phase 2-Filanesib cohort used a 2-stage Simon minimax design in which a response in ≥ 1 of 18 evaluable patients was required to enroll an additional 14 evaluable patients. The Phase 2-Filanesib/Dex cohort utilized a 2-stage Simon optimal design in which a response in ≥ 3 of 18 evaluable patients was required to enroll an additional 30 evaluable patients.
The response-evaluable population included patients who received ≥ 1 dose of filanesib and had a post-Baseline disease assessment or who discontinued from the study due to PD, intolerable toxicity or death prior to that assessment. ORR was summarized by cohort. Survival and time to-event analyses were estimated using the Kaplan-Meier method. No formal comparisons were planned or performed.
A cutpoint for optimal prediction of ORR for the presumptive predictive biomarker AAG was determined retrospectively using the Youden method11–13 by comparing best clinical response with baseline AAG values from 79 patients in the Phase 2 cohorts of the current study, then adjusted upward to the maximum value to yield identical sensitivity and specificity.
Safety data were summarized using descriptive statistics. The safety population included all patients who received ≥ 1 dose of filanesib.
RESULTS
Patient characteristics
Patients were enrolled between March 2009 and July 2013 at 5 centers in the United States. A total of 31 patients (30 MM, 1 PCL) were enrolled in six dose cohorts in Phase 1, and 87 patients with MM were enrolled in Phase 2 (32 Phase 2-Filanesib, 55 Phase 2-Filanesib/Dex).
This paper reports data collected through 16 March 2016. At the time of data cutoff, all patients were no longer receiving study treatment and all patients had completed the follow-up study phase.
Patient characteristics and prior therapies are summarized in Table 1.
Table 1.
Demographic and baseline disease characteristics and prior therapies
| Phase 1 dose escalation (all doses) (N=31) |
Phase 2- Filanesib (N=32) |
Phase 2- Filanesib/Dex (N=55) |
|
|---|---|---|---|
| Male, n(%) | 20 (65) | 18 (56) | 27 (49) |
| White, n(%) | 24 (77) | 25 (78) | 39 (71) |
| Median age, years (range) | 60 (43, 79) | 65 (51, 82) | 63 (33, 82) |
| Heavy chain at diagnosis, n(%) | |||
| IgA | 7 (23) | 11 (34) | 14 (25) |
| IgD | 2 (6) | 0 (0) | 1 (2) |
| IgG | 17 (55) | 18 (56) | 28 (51) |
| IgM | 0 (0) | 1 (3) | 0 (0) |
| None | 5 (16) | 2 (6) | 12 (22) |
| Light chain at diagnosis, n(%) | |||
| Kappa | 22 (71) | 22 (69) | 38 (69) |
| Lambda | 8 (26) | 10 (31) | 17 (31) |
| Both or none | 1 (3) | 0 (0) | 0 (0) |
| Oligo- or non-secretory disease at diagnosis, n(%) | 1 (3) | 0 (0) | 0 (0) |
| ECOG status, n(%) | |||
| 0 | 6 (19) | 5 (16) | 6 (11) |
| 1 | 25 (81) | 26 (81) | 49 (89) |
| 2 | 0 (0) | 1 (3) | 0 (0) |
| ISS stage at study baseline, n(%) | |||
| I | 17 (55) | 10 (31) | 16 (29) |
| II | 12 (39) | 13 (41) | 22 (40) |
| III | 1 (3) | 9 (28) | 16 (29) |
| Missing | 1 (3) | 0 (0) | 1 (2) |
| High-risk cytogenetics at baseline, n(%)* | |||
| Yes | 0 (0) | 3 (9) | 17 (31) |
| No | 21 (68) | 25 (78) | 36 (65) |
| Missing | 10 (32) | 4 (12) | 2 (4) |
| Prior therapies, median (range) | 6 (2, 16) | 6 (2, 19) | 8 (2, 22) |
| Prior stem cell transplant, n(%) | 24 (77) | 26 (81) | 49 (89) |
| Prior carfilzomib, n(%) | 4 (13) | 2 (6) | 14 (25) |
| Prior pomalidomide, n(%) | 1 (3) | 1 (3) | 7 (13) |
| Prior bortezomib | |||
| Refractory† | 22 (71) | 18 (56) | 54 (98) |
| Relapsed | 8 (26) | 11 (34) | 1 (2) |
| Not applicable | 1 (3) | 3 (9) | 0 (0) |
| Prior dexamethasone | |||
| Refractory†,‡ | 23 (74) | 22 (69) | 54 (98) |
| Relapsed | 7 (23) | 10 (31) | 0 (0) |
| Not applicable | 1 (3) | 0 (0) | 1 (2) |
| Prior lenalidomide | |||
| Refractory† | 21 (68) | 25 (78) | 55 (100) |
| Relapsed | 8 (26) | 6 (19) | 0 (0) |
| Not applicable | 2 (6) | 1 (3) | 0 (0) |
Defined as one or more of the following: del(17p), t(4;14), t(14;16), 1q21 gain.
Documented progressive disease on therapy or within 60 days of completing treatment.
The required dexamethasone dose was ≥ 40 mg per week on treatment weeks.
Abbreviations: Dex = dexamethasone; IgA = immunoglobulin A; IgD = immunoglobulin D; IgG = immunoglobulin G; IgM = immunoglobulin M; ECOG = Eastern Cooperative Oncology Group; ISS = International Staging System; n = number
Determination of MTD and recommended Phase 2 dose
Dose levels of 1.0 and 1.25 mg/m2/day were uneventful. Dose levels ≥ 1.50 mg/m2/day included prophylactic filgrastim. At 1.50 mg/m2/day + filgrastim, 1 of 6 evaluable patients had a DLT (febrile neutropenia). The next dose level of 2.0 mg/m2/day (n=3 evaluable) was without DLT. At 2.25 mg/m2/day + filgrastim, however, both treated patients experienced DLTs of febrile neutropenia and mucosal inflammation, one of whom also experienced toxic epidermal necrolysis and fatal pneumonia. The dose of 2.00 mg/m2/day + filgrastim was revisited and expanded to 6 evaluable patients, and 2 patients experienced DLTs (febrile neutropenia and mucosal inflammation in both patients, one of whom also had corneal epitheliopathy). Further dose reduction was undertaken to a previously unevaluated dose (1.75 mg/m2/day), which was not tolerated due to DLTs in 2 patients (febrile neutropenia in both, one of whom also had mucosal inflammation). Therefore, 1.50 mg/m2/day administered with prophylactic filgrastim was declared the MTD/recommended Phase 2 dose.
Onset of all DLTs occurred within 9 days of treatment initiation in Cycle 1 (Table 2). Two patients (one each in the 1.25 mg/m2/day and 1.5 mg/m2/day cohorts) were not evaluable for assessment of DLTs due to PD prior to completing Cycle 2.
Table 2.
Dose Limiting Toxicities in Phase 1
| Cohort | Patients with DLTs/ evaluable patients |
DLT (grade) |
|---|---|---|
| 1.00 mg/m2/day | 0/3 | |
| 1.25 mg/m2/day | 1/6 | neutropenia (G4) |
| 1.50 mg/m2/day + filgrastim | 1/6 | febrile neutropenia (G4) |
| 1.75 mg/m2/day + filgrastim | 2/6 | febrile neutropenia (G3, G3) |
| mucosal inflammation (G3) | ||
| 2.00 mg/m2/day + filgrastim | 2/6 | febrile neutropenia (G3, G3) |
| mucosal inflammation (G3, G3) | ||
| corneal epitheliopathy (G3) | ||
| 2.25 mg/m2/day + filgrastim | 2/2 | febrile neutropenia (G3, G4) |
| mucosal inflammation (G3, G3) | ||
| pneumonia (G5) | ||
| toxic epidermal necrolysis (G4) |
All events occurred in Cycle 1. Filgrastim was administered prophylactically.
Abbreviations: DLT = dose limiting toxicity; G3/G4/G5 = Grade 3/4/5; mg = milligrams; m2 = meters squared
Treatment exposure and safety
Patients in Phase 1 (all cohorts combined) received a median of 7 cycles of filanesib (range, 1 to 81 cycles), equating to a median exposure of 105 days (range, 14 to 1603 days), with 35% of patients receiving treatment beyond 6 months. PD was the primary reason for treatment discontinuation in the overall Phase 1 population (61%) and in each cohort except the highest dose evaluated, in which both treated patients discontinued due to toxicity.
Patients in the Phase 2-Filanesib cohort received filanesib for a median of 5 cycles (range, 1 to 92 cycles), equating to a median exposure of 74 days (range, 14 to 1497 days), with 28% of patients receiving filanesib beyond 6 months. Patients in the Phase 2-Filanesib/Dex cohort received filanesib for a median of 6 cycles (range, 1 to 84 cycles), equating to a median exposure of 84 days (range, 14 to 1310 days), with 29% of patients receiving filanesib beyond 6 months. PD was the primary reason for treatment discontinuation in each cohort (78% and 85%, respectively).
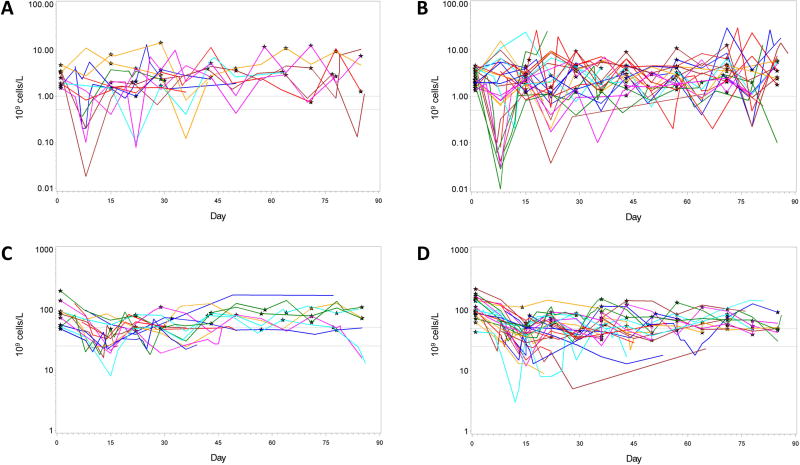
Grade 3/4 neutropenia was reported in approximately 40% of patients in each of the Phase 2 cohorts (Table 3). As expected, the incidence was higher in the dose escalation, attributable primarily to the evaluation of nontolerated doses. Grade 3/4 thrombocytopenia and anemia also were observed in approximately 50% of patients in each Phase 2 cohort. Cytopenias generally were reversible and not cumulative (Figure 1).
Table 3.
Incidence and severity of treatment-emergent AEs (≥ 20%) and SAEs (≥ 5%)
| AE, n (%) | Phase 1 dose escalation (all doses) (N=31) |
Phase 2-Filanesib (N=32) |
Phase 2-Filanesib/Dex (N=55) |
||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Grade | Grade | Grade | |||||||
|
| |||||||||
| 1/2 | 3 | 4 | 1/2 | 3 | 4 | 1/2 | 3 | 4 | |
| Hematologic abnormalities | |||||||||
| Neutropenia | 3 (10) | 7 (23) | 18 (58) | 8 (25) | 5 (16) | 10 (31) | 8 (15) | 10 (18) | 18 (33) |
| Thrombocytopenia | 10 (32) | 6 (19) | 9 (29) | 12 (38) | 7 (22) | 8 (25) | 24 (44) | 17 (31) | 11 (20) |
| Anemia | 11 (35) | 7 (23) | 1 (3) | 12 (38) | 10 (31) | 2 (6) | 12 (22) | 23 (42) | 3 (5) |
| Nonhematologic AEs | |||||||||
| Nausea | 12 (39) | 1 (3) | 0 (0) | 10 (31) | 0 (0) | 0 (0) | 18 (33) | 0 (0) | 0 (0) |
| Diarrhea | 10 (32) | 0 (0) | 0 (0) | 8 (25) | 0 (0) | 0 (0) | 16 (29) | 1 (2) | 0 (0) |
| Constipation | 4 (13) | 0 (0) | 0 (0) | 8 (25) | 0 (0) | 0 (0) | 9 (16) | 0 (0) | 0 (0) |
| Fatigue | 9 (29) | 4 (13) | 0 (0) | 11 (34) | 4 (12) | 1 (3) | 13 (24) | 4 (7) | 0 (0) |
| Pyrexia | 9 (29) | 1 (3) | 0 (0) | 5 (16) | 1 (3) | 0 (0) | 12 (22) | 1 (2) | 0 (0) |
| Hypokalemia | 5 (16) | 0 (0) | 1 (3) | 3 (9) | 1 (3) | 1 (3) | 11 (20) | 2 (4) | 0 (0) |
| Arthralgia | 4 (13) | 0 (0) | 0 (0) | 9 (28) | 0 (0) | 0 (0) | 11 (20) | 1 (2) | 0 (0) |
| Back pain | 5 (16) | 0 (0) | 0 (0) | 5 (16) | 2 (6) | 0 (0) | 9 (16) | 0 (0) | 0 (0) |
| Upper respiratory tract infection | 8 (26) | 0 (0) | 0 (0) | 5 (16) | 0 (0) | 0 (0) | 15 (27) | 2 (4) | 0 (0) |
| Cough | 10 (32) | 0 (0) | 0 (0) | 4 (13) | 0 (0) | 0 (0) | 14 (25) | 0 (0) | 0 (0) |
| Insomnia | 3 (10) | 0 (0) | 0 (0) | 3 (9) | 0 (0) | 0 (0) | 13 (24) | 1 (2) | 0 (0) |
| Febrile neutropenia | 0 (0) | 6 (19) | 2 (6) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 1 (2) | 2 (4) |
| Mucosal inflammation | 4 (13) | 5 (16) | 0 (0) | 5 (16) | 0 (0) | 0 (0) | 6 (11) | 0 (0) | 0 (0) |
| Edema peripheral | 6 (19) | 1 (3) | 0 (0) | 3 (9) | 0 (0) | 0 (0) | 7 (13) | 0 (0) | 0 (0) |
| Headache | 7 (23) | 0 (0) | 0 (0) | 6 (19) | 0 (0) | 0 (0) | 5 (9) | 0 (0) | 0 (0) |
| Anorexia | 7 (23) | 0 (0) | 0 (0) | 4 (12) | 0 (0) | 0 (0) | 8 (15) | 2 (4) | 0 (0) |
| Other AEs of Interest | |||||||||
| Peripheral neuropathy* | 3 (10) | 0 (0) | 0 (0) | 1 (3) | 1 (3) | 0 | 4 (7) | 0 (0) | 0 (0) |
| Alopecia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 0 (0) |
| SAEs | |||||||||
| Pneumonia | 0 (0) | 3 (10) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 1 (2) | 4 (7) | 0 (0) |
| Febrile neutropenia | 0 (0) | 6 (19) | 2 (6) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 1 (2) | 1 (2) |
| Pyrexia | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 3 (5) | 1 (2) | 0 (0) |
| Mucosal inflammation | 1 (3) | 4 (13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
This table is based on the safety population. Hematologic abnormalities are based on CTCAE grade rather than reported AEs. In addition, a total of 13 Grade 5 AEs were observed, 9 of which were disease progression; the 4 others were treatment-related pneumonia, treatment-related septic shock, and 2 deaths of unknown cause (1 of which was treatment-related).
Includes all events of peripheral neuropathy and peripheral sensory neuropathy.
Abbreviations: AE = adverse event(s); Dex = dexamethasone; N or n = number; SAE = serious adverse event(s)
Figure 1.
Neutrophil and platelet values over time for Phase 2 patients with post-baseline abnormalities ≥ Grade 3. (A and B) Absolute neutrophil values in Phase 2-Filanesib and Phase 2 Filanesib/Dex, respectively. (C and D) Platelet values in Phase 2-Filanesib and Phase 2 Filanesib/Dex, respectively. Black stars indicate Day 1 dosing of each cycle. Gray lines represent thresholds for Grade 3 and Grade 4 values.
Fatigue and pneumonia were the nonhematologic ≥ Grade 3 events reported with the highest incidence. Grade 3/4 events of febrile neutropenia and mucosal inflammation were observed at a relatively high incidence in the dose escalation phase (26% and 16%, respectively), occurred primarily at nontolerated doses, and these events were reported infrequently in the Phase 2 cohorts.
Of note, peripheral neuropathy was reported in 10% of patients in the dose escalation and 6–7% of patients in each Phase 2 cohort. These events were almost exclusively exacerbations of baseline symptoms; all were Grade 1/2 except a single Grade 3 event in the Phase 2-Filanesib group in a patient with Grade 1 neuropathy at baseline that worsened during Cycle 9.
AEs resulting in dose reduction were reported in 23% of patients in Phase 1 (all receiving doses above the MTD), 34% of patients in Phase 2-Filanesib and 11% of patients in Phase 2-Filanesib/Dex (Table 4). AEs resulting in treatment discontinuation were reported in 13% of patients in Phase 1 (incidence not dose dependent), 16% of patients in Phase 2-Filanesib and 20% of patients in Phase 2-Filanesib/Dex, respectively (Table 5).
Table 4.
AEs Leading to dose reduction
| AE, n (%) | Phase 1 dose escalation (all doses) (N=31) |
Phase 2- Filanesib (N=32) |
Phase 2- Filanesib/Dex (N=55) |
|---|---|---|---|
| AEs leading to dose reduction | |||
| Thrombocytopenia | 1 (3) | 3 (9) | 3 (5) |
| Febrile neutropenia | 5 (16) | 1 (3) | 0 (0) |
| Mucosal inflammation | 5 (16) | 1 (3) | 0 (0) |
| Neutropenia | 2 (6) | 1 (3) | 1 (2) |
| Vomiting | 0 (0) | 3 (9) | 0 (0) |
| Diarrhea | 0 (0) | 2 (6) | 0 (0) |
| Pyrexia | 0 (0) | 2 (6) | 0 (0) |
| Lipase increased | 0 (0) | 1 (3) | 1 (2) |
| Pneumonia | 1 (3) | 1 (3) | 0 (0) |
| Leukopenia | 1 (3) | 0 (0) | 0 (0) |
| Corneal disorder | 1 (3) | 0 (0) | 0 (0) |
| Vision blurred | 1 (3) | 0 (0) | 0 (0) |
| Nausea | 0 (0) | 1 (3) | 0 (0) |
| Blood amylase increased | 0 (0) | 0 (0) | 1 (2) |
| Bacteremia | 0 (0) | 1 (3) | 0 (0) |
| Punctate keratitis | 0 (0) | 1 (3) | 0 (0) |
This table is based on the safety population.
Abbreviations: AE = adverse event(s); Dex = dexamethasone; N or n = number
Table 5.
AEs leading to treatment discontinuation
| AE, n (%) | Phase 1 dose escalation (all doses) (N=31) |
Phase 2- Filanesib (N=32) |
Phase 2- Filanesib/Dex (N=55) |
|---|---|---|---|
| Death | 0 (0) | 1 (3) | 1 (2) |
| Fatigue | 0 (0) | 2 (6) | 0 (0) |
| Arthritis bacterial | 0 (0) | 0 (0) | 1 (2) |
| Pneumonia | 0 (0) | 0 (0) | 1 (2) |
| Sepsis | 1 (3) | 0 (0) | 1 (2) |
| Septic shock | 0 (0) | 0 (0) | 1 (2) |
| Thrombocytopenia | 1 (3) | 0 (0) | 2 (4) |
| Febrile neutropenia | 0 (0) | 0 (0) | 1 (2) |
| Hypercalcemia | 0 (0) | 0 (0) | 2 (4) |
| Dehydration | 0 (0) | 0 (0) | 1 (2) |
| Fluid overload | 0 (0) | 1 (3) | 0 (0) |
| Acute respiratory distress syndrome | 0 (0) | 0 (0) | 1 (2) |
| Dyspnea | 0 (0) | 0 (0) | 1 (2) |
| Cardiac failure congestive | 0 (0) | 0 (0) | 1 (2) |
| Renal failure acute | 0 (0) | 0 (0) | 1 (2) |
| Blister | 0 (0) | 1 (3) | 0 (0) |
| Leukopenia | 1 (3) | 0 (0) | 0 (0) |
| Neutropenia | 1 (3) | 0 (0) | 0 (0) |
| Atrial fibrillation | 1 (3) | 0 (0) | 0 (0) |
| Vision blurred | 1 (3) | 0 (0) | 0 (0) |
This table is based on the safety population.
Abbreviations: AE = adverse event(s); Dex = dexamethasone; N or n = number
Deaths on study or within 30 days of last filanesib dose were reported in 6% of patients in Phase 1, 9% of patients in Phase 2-Filanesib and 15% of patients in Phase 2-Filanesib/Dex. Nine of 13 patient deaths were attributed to PD; other causes were pneumonia (treatment related), septic shock (treatment related) and unknown (2 patients; 1 event treatment related).
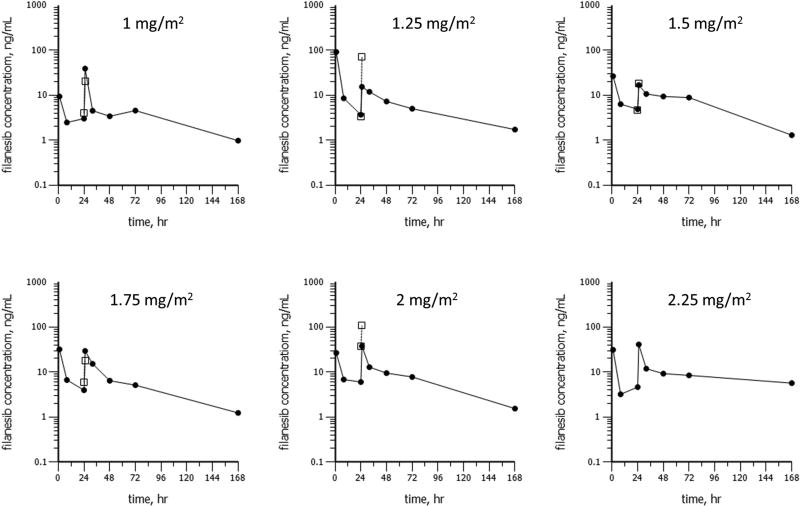
Pharmacokinetics
Filanesib had a prolonged terminal elimination phase (Figure 2). Geometric mean Cmax values were <50 ng/mL for all doses in Cycle 1. Median concentrations of filanesib were >1 ng/mL for 7 days of Cycle 1 at all doses. Exposure tended to be higher on Day 2 compared with Day 1 as the geometric mean accumulation ratio (based on AUC) was 1.58 (60.4% CV). The geometric mean systemic clearance (CL) was 5.39 L/hr and the steady-state volume of distribution (Vss) was 235 L.
Figure 2.
Geometric mean plasma concentrations for filanesib as a function of time following filanesib administration as a 1-hour infusion on Day 1 and Day 2 (vertical gray lines) of Cycle 1 (closed circles) and Cycle 2 (open boxes). Plots are shown for doses of 1, 1.25, 1.5, 1.75, 2, or 2.25 mg/m2/day. Error bars represent ± one geometric standard deviation.
Efficacy
Patients in Phase 1 had an ORR of 10% and a CBR of 14%, with a majority of responses observed at the 2.00 mg/m2/day dose (Table 6). The Phase 2-Filanesib cohort of patients with prior bortezomib and IMiD therapy had an ORR of 16% and a CBR of 23% (Table 7). The Phase 2-Filanesib/Dex cohort of patients with disease refractory to bortezomib, lenalidomide and dexamethasone had an ORR of 15% and a CBR of 20% (Table 7).
Table 6.
Clinical response in Phase 1
| 1.00 mg/m2 (N=3) |
1.25 mg/m2 (N=6) |
1.50 mg/m2 + GCSF (N=7) |
1.75 mg/m2 + GCSF (N=5) |
2.00 mg/m2 + GCSF (N=6) |
2.25 mg/m2 + GCSF (N=2) |
Total (N=29) |
|
|---|---|---|---|---|---|---|---|
| Best response, n (%) | |||||||
| Partial response | 1 (33) | 0 (0) | 0 (0) | 0 (0) | 2 (33) | 0 (0) | 3 (10) |
| Minimal response | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (17) | 0 (0) | 1 (3) |
| Stable disease | 2 (67) | 3 (50) | 4 (57) | 3 (60) | 1 (17) | 1 (50) | 14 (48) |
| Progressive disease* | 0 (0) | 3 (50) | 3 (43) | 2 (40) | 2 (33) | 0 (0) | 10 (34) |
| Not evaluable | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (50) | 1 (3) |
| Overall response rate (≥ PR) | 1 (33) | 0 (0) | 0 (0) | 0 (0) | 2 (33) | 0 (0) | 3 (10) |
| Clinical benefit rate (≥ MR) | 1 (33) | 0 (0) | 0 (0) | 0 (0) | 3 (50) | 0 (0) | 4 (14) |
This table is based on the Phase 1 response-evaluable population.
Progressive disease may not have been confirmed.
Abbreviations: GCSF = Granulocyte Colony Stimulating Factor; m2 = meter squared; mg = milligram; MR = minimal response; N or n = number; PR = partial response
Table 7.
Clinical response in Phase 2
| Phase 2-Filanesib | Phase 2-Filanesib/Dex | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| All Patients (N=31) |
High AAG (N=6) |
Low AAG (N=22) |
All Patients (N=54) |
High AAG (N=15) |
Low AAG (N=35) |
|
| Best response, n (%) | ||||||
| Very good partial response | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 0 (0) |
| Partial response | 5 (16) | 0 (0) | 5 (23) | 7 (13) | 0 (0) | 7 (20) |
| Minimal response | 2 (6) | 0 (0) | 2 (9) | 3 (6) | 0 (0) | 3 (9) |
| Stable disease | 12 (39) | 4 (67) | 7 (32) | 22 (41) | 7 (47) | 12 (34) |
| Progressive disease* | 12 (39) | 2 (33) | 8 (36) | 18 (33) | 6 (40) | 12 (34) |
| Not evaluable | 0 (0) | 0 (0) | 0 (0) | 3 (6) | 2 (13) | 1 (3) |
| Overall response rate (≥ PR) | 5 (16) | 0 (0) | 5 (23) | 8 (15) | 0 (0) | 7 (20) |
| Clinical benefit rate (≥ MR) | 7 (23) | 0 (0) | 7 (32) | 11 (20) | 0 (0) | 10 (29) |
The “All Patients” columns use the response-evaluable population for each Phase 2 cohort; other columns use response-evaluable patients who had a baseline AAG value
Progressive disease may not have been confirmed.
High AAG = Baseline value > 110 mg/dL; Low AAG = Baseline value ≤ 110 mg/dL
Abbreviations: AAG = alpha 1-acid glycoprotein at baseline; Dex = dexamethasone; MR = minimal response; N or n = number; PR = partial response
Responses were observed in patients with high-risk cytogenetics, 1/3 patients (33%) in the Phase 2-Filanesib cohort and 1/17 patients (6%) in the Phase 2-Filanesix/Dex cohort.
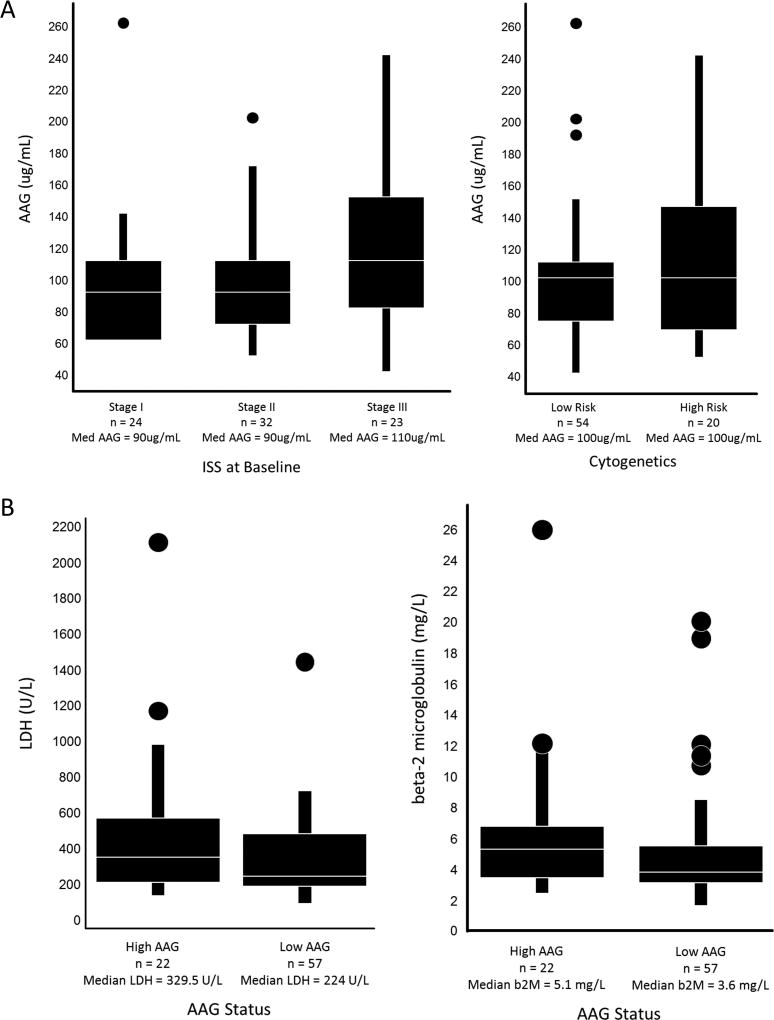
A posthoc exploratory analysis identified a baseline AAG value (110 mg/dL) above which no patient demonstrated a response of ≥ MR. In patients with low AAG at baseline in the Phase 2-Filanesib cohort, the ORR was 23% and the CBR 32%, and in the Phase 2-Filanesib/Dex cohort, the ORR was 20% and the CBR 29%. Baseline AAG concentration for 79 patients comprising the Phase 2 portion of the study was compared to Baseline ISS or cytogenetics status (Figure 3A), and Baseline levels of LDH or beta-2 microglobulin (Figure 3B). Statistical significance was not achieved for any of these prognostic variables.
Figure 3.
(A) AAG vs Baseline ISS or Cytogenetics Status Box plots display median (Med) AAG levels. ANOVA was performed to test the differences in mean AAG in the 3 levels of ISS (p=0.062) or high/low risk cytogenetics (p=0.20). (B) Baseline LDH or beta-2 microglobulin (b2M) vs AAG Status. Box plots display median LDH or b2M levels. ANOVA was performed to test the differences in mean LDH (p=0.066) or mean b2M (p=0.15) by high/low risk patient AAG status.
Dexamethasone was added to the treatment regimen for 3 patients in the Phase 2-Filanesib cohort who either had SD for at least 9 months (N=1) or had PD after a response of MR or better (N=2). None achieved a clinical response after the addition of dexamethasone.
Median TTR values for responding patients were 4.4 and 2.9 months, respectively, for the Phase 2-Filanesib and Phase 2-Filanesib/Dex cohorts, with corresponding DOR values of 8.6 and 4.4 months, respectively (Table 8).
Table 8.
Secondary efficacy endpoints
| Phase 2 Filanesib (N=32) |
Phase 2 Filanesib/Dex (N=55) |
|
|---|---|---|
| Time to response, months | ||
| N | 5 | 8 |
| Median (min, max) | 4.4 (2.6, 15.9) | 2.9 (0.8, 3.7) |
| 95% CI | 2.6, 15.9 | 0.8, 3.5 |
| Duration of response, months | ||
| N | 5 | 8 |
| Median (min, max) | 8.6 (1.4, 36.9*) | 4.4 (2.4, 21.4) |
| 95% CI | 1.4, NR | 2.4, 8.1 |
| PFS, months | ||
| N | 32 | 55 |
| Median (min, max) | 1.6 (0.0*, 39.4*) | 2.8 (0.3, 24.6) |
| 95% CI | 1.0, 3.3 | 1.0, 4.2 |
| TFI, months | ||
| N | 32 | 55 |
| Median (min, max) | 0.9 (0.0*, 16.1*) | 1.0 (0.0, 2.8*) |
| 95% CI | 0.8, 1.5 | 0.8, 1.4 |
| TNT, months | ||
| N | 32 | 55 |
| Median (min, max) | 4.1 (0.6, 39.9*) | 6.1 (0.4*, 26.9*) |
| 95% CI | 2.3, 12.0 | 3.8, 7.4 |
| OS, months | ||
| N | 32 | 55 |
| Median (min, max) | 19.0 (0.6*, 39.9*) | 10.7 (0.4, 28.3*) |
| 95% CI | 7.8, 23.3 | 5.6, 13.4 |
Time to response and duration of response were calculated using responders only. Other parameters were calculated using the safety population.
Patient ongoing.
Abbreviations: CI = confidence interval; Dex = dexamethasone; max = maximum; min = minimum; N or n = number; NR = not reached; OS = overall survival; PFS = progression-free survival; TFI = treatment-free interval; TNT = time to next treatment
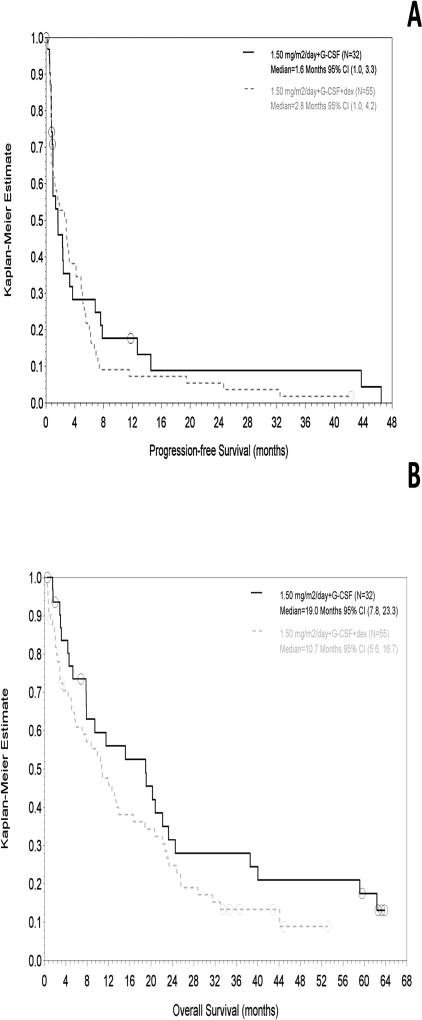
Median PFS values were 1.6 months (Phase 2-Filanesib) and 2.8 months (Phase 2-Filanesib/Dex), with OS values of 19.0 and 10.7 months, respectively (Figure 4). For the subset of patients with low AAG at baseline, median PFS values were 1.7 months (Phase 2-Filanesib) and 4.2 months (Phase 2-Filanesib/Dex), with OS values of 19.1 and 10.8 months, respectively. Patients alive at the time of analysis had been followed for survival for a median of 34 months (Phase 2-Filanesib) and 12 months (Phase 2-Filanesib/Dex).
Figure 4.
(A) PFS and (B) OS in Phase 2. PFS is based on investigator assessment.
DISCUSSION
Filanesib, a highly selective KSP inhibitor, induces apoptosis preferentially in cells that depend on short-lived survival proteins during mitosis.4 MM, which typically demonstrates an over-reliance on the survival protein Mcl-114,15 may thus be an appropriate target for filanesib. This study evaluated the safety and efficacy of filanesib in RRMM and evaluated AAG as a potential selection biomarker to identify patients most likely to respond to treatment.
The Phase 1 component of the study established the MTD of single-agent filanesib as 1.50 mg/m2/day administered as a 1-hour IV infusion on Days 1 and 2 of each 14-day cycle with prophylactic filgrastim.16 At doses exceeding the MTD, DLTs of febrile neutropenia, mucosal inflammation, corneal epitheliopathy and pneumonia were reported. At the MTD, a single DLT of febrile neutropenia was reported, and substantial proportions of patients experienced Grade 3/4 neutropenia (86%), thrombocytopenia (71%) and anemia (43%).
As dexamethasone has been reported to alter expression of certain apoptosis signaling proteins, such as Mcl-117, we hypothesized that dexamethasone may affect the anti-tumor activity of filanesib. Phase 2 evaluated filanesib at the Phase 1 MTD (including prophylactic filgrastim) with and without dexamethasone (40 mg weekly).
Toxicities in both Phase 2 cohorts were generally manageable and reversible. The most frequently reported nonhematologic adverse events ≥ Grade 3 were fatigue and pneumonia, with the former occurring at a higher incidence in the Phase 2-Filanesib cohort (16% vs. 7%) and the latter occurring more frequently in the Phase 2-Filanesib/Dex cohort (13% vs. 3%). Mucosal inflammation ≥ Grade 3 was reported in 36% of Phase 1 patients receiving doses ultimately determined to be above the MTD, but were limited to Grade 1 and infrequent Grade 2 events at the MTD in Phase 1 and in the Phase 2 cohorts. As in Phase 1, hematologic toxicities including Grade 3/4 thrombocytopenia, anemia and neutropenia were observed in about 50% of patients, but the rate of febrile neutropenia was low (5%). Cumulative toxicity was not observed with long-term administration. In both Phase 2 cohorts, the primary reason for treatment discontinuation was PD.
Unlike other currently available myeloma agents, filanesib is not expected to be active in terminally differentiated cells. Peripheral neuropathy was reported at a low incidence (< 10%) and in nearly all cases represented exacerbations of baseline conditions.
In addition to at least 2 prior therapies including an IMiD and PI, patients in the Phase 2-Filanesib/Dex cohort were required to have prior alkylator therapy and disease refractory to lenalidomide, bortezomib and dexamethasone. As the patient characteristics were significantly different in Phase 2 between the 2 cohorts, no comparisons can be made. High-risk cytogenetic characteristics were identified in 9% of Phase 2-Filanesib patients and 31% of Phase 2-Filanesib/Dex patients, with responses observed in both cohorts.
The goal of the Phase 2 single agent filanesib cohort was to evaluate for a signal of clinical activity. Importantly, filanesib has single-agent activity in RRMM with an ORR of 16% and a clinically meaningful CBR of 23%, comparable with single-agent activity observed with recently approved pomalidomide (ORR < 10% in RRMM) and carfilzomib (ORR 16–23%). In addition to the response rates, the activity of filanesib is durable with prolonged SD reported, and approximately 30% of patients continuing therapy beyond 6 months. OS was 19.0 months in the Phase 2-Filanesib cohort. Establishing that filanesib has single-agent activity is important as this provides an opportunity for combination therapy and future development with IMiDs and PIs.
The response rates in the distinct and refractory patient population with Phase 2-Filanesib/Dex (ORR 15%; CBR 20%) were also clinically relevant. These responses were also durable with an OS of 10.7 months. These findings are comparable to those reported for pomalidomide/dexamethasone and carfilzomib regimens approved in the United States for the treatment of similar, if not less refractory, MM patient populations.21,22
The TTR in the Phase 2 cohorts (median 3 to 4 months) was longer than that reported for currently used PI and IMiD treatment regimens.18–21 Although no sCRs or CRs were observed in this refractory patient population, the responses observed in this study suggest interesting activity in heavily pretreated and triple-refractory patient populations.
AAG is an acute-phase reactant protein that may be chronically elevated in individuals with cancer, including MM.23–25 Increased endogenous AAG levels have been shown to correlate with decreased unbound fraction of filanesib, potentially altering the PK and limiting the clinical activity of filanesib.26 In this study, a retrospective analysis of data from the Phase 2 cohorts indicated that all patients demonstrating objective responses had baseline AAG values ≤ 110 mg/dL. Patients with AAG levels > 110 mg/dL may therefore be unlikely to derive clinical benefit from filanesib. AAG levels vary considerably among patients with MM23–25,27 and can fluctuate over time. It is unclear whether this biomarker is predictive for response to filanesib or instead represents a prognostic biomarker. The clinical utility of baseline AAG as a predictor of response to filanesib administered as a single agent and in combination with carfilzomib is undergoing prospective evaluation in ongoing Phase 2 studies.
In conclusion, filanesib is a highly-selective KSP inhibitor with a novel mechanism of action undergoing evaluation in the treatment of MM. This Phase 1/2 study established a dose and schedule of filanesib treatment that has a manageable safety profile with a low incidence of nonhematologic toxicity and manageable hematologic toxicity. Encouraging clinical activity was observed, including durable responses in patients with triple-refractory disease previously treated with alkylating agents. AAG represents a potential biomarker that requires further prospective evaluation to determine its utility as a predictive or prognostic marker.
Based on the strength of data from this study and other ongoing studies, a single-agent Phase 2 study of filanesib in patients with refractory MM who have received prior bortezomib and lenalidomide (AfFIRM) and a Phase 2 study of carfilzomib ± filanesib in carfilzomib-naïve patients with refractory MM were initiated (clinicaltrials.gov:NCT02092922 and NCT01989325). Additional Phase 1 and 2 studies in combination with bortezomib, carfilzomib and pomalidomide, respectively, are also ongoing in RRMM (clinicaltrials.gov: NCT01248923, NCT01372540, and NCT02384083).28
Acknowledgments
Funding Sources:
Array Biopharma, Inc.
Grant : NIH/NCI P30 CA16672.
BH, SR, DW, BT, KL, and MP are current or former employees of and had stock options in Array BioPharma, Inc. JJS has received research funding from Array BioPharma, Celgene, Millennium/Takeda, Novartis, and Onyx/Amgen. JLK has consulted for Onyx/Amgen and Takeda, served on Data Safety Monitoring Committee of Incyte, and received research support from Celgene, Merck, Novartis, and Onyx/Amgen. JZ has served on the advisory boards for Bristol-Myers Squibb, Celgene, Janssen, Prothena, Seattle Genetics, Takeda and on Data Safety Monitoring Committee of Pharmacyclics, and has received research funding from Celgene. ADC has served on the advisory board for Bristol-Myers Squibb, Janssen and on the Independent Response Adjudication Committee for Celgene, and has received research support from Bristol-Myers Squibb. WIB has received research support from Acetylon, Amgen, Bristol-Myers Squibb, Celgene, Novartis, Sanofi, and Takeda, has seved on the advisory boards for Amgen, Bristol-Myers Squibb, Celgene, Sanofi and on speakers bureaus of Amgen and Celgene. R.Z.O. has served on advisory boards for and has research funding support from Celgene and Onyx/Amgen. SL has consulted for Onyx/Amgen, Bristol-Myers Squibb, Novartis, Janssen, Celgene, and Millennium/Takeda.
The authors thank our patients and their families. This study was funded by Array BioPharma Inc. Biostatistics support was provided by Roger Aitchison (Array BioPharma Inc.). Statistical programming was performed by Jennifer Regensburger (Array BioPharma Inc.). Catriona Byrne, Christopher Tennant and Anthony Maine, medical writers supported by funding from Array BioPharma Inc., provided drafts and editorial assistance to the authors during the preparation of this manuscript.
Footnotes
Author Contributions:
JJS, RZO, SL, BH, SR, DW, BT, KL, and MP conceived and designed the study. JJS, RZO, SL, JLK, JAZ, ADC, and WIB performed research. JJS, RZO, SL, JLK, JAZ, ADC, WIB, BH, SR, DW, BT, KL, and MP collected and interpreted data. JJS, BH, SR, DW, BT, KL, and MP performed statistical analysis. JJS, RZO, SL, JLK, JAZ, ADC, WIB, BH, SR, DW, BT, KL, and MP wrote the manuscript. All authors approved the final manuscript.
References
- 1.Blangy A, Lane HA, d'Hérin P, et al. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell. 1995;83(7):1159–1169. doi: 10.1016/0092-8674(95)90142-6. [DOI] [PubMed] [Google Scholar]
- 2.Stern BM, Murray AW. Lack of tension at kinetochores activates the spindle checkpoint in budding yeast. Curr Biol. 2001;11(18):1462–1467. doi: 10.1016/s0960-9822(01)00451-1. [DOI] [PubMed] [Google Scholar]
- 3.Milross CG, Mason KA, Hunter NR, Chung WK, Peters LJ, Milas L. Relationship of mitotic arrest and apoptosis to antitumor effect of paclitaxel. J Natl Cancer Inst. 1996;88(18):1308–1314. doi: 10.1093/jnci/88.18.1308. [DOI] [PubMed] [Google Scholar]
- 4.Tunquist BJ, Woessner RD, Walker DH. Mcl-1 stability determines mitotic cell fate of human multiple myeloma tumor cells treated with the kinesin spindle protein inhibitor ARRY-520. Mol Cancer Ther. 2010;9(7):2046–2056. doi: 10.1158/1535-7163.MCT-10-0033. [DOI] [PubMed] [Google Scholar]
- 5.Woessner RD, Corrette C, Allen S, et al. ARRY-520, a KSP inhibitor with efficacy and pharmacodynamic activity in animal models of solid tumors. Cancer Res. 2007;67:1433. [Google Scholar]
- 6.Woessner R, Tunquist B, Lemieux C, et al. ARRY-520, a novel KSP inhibitor with potent activity in hematological and taxane-resistant tumor models. Anticancer Res. 2009;29(11):4373–4380. [PubMed] [Google Scholar]
- 7.LoRusso P, Goncalves PH, Casetta L, et al. First-in-human phase 1 study of filanesib (ARRY520), a kinesin spindle protein inhibitor, in patients with advanced solid tumors. Invest New Drugs. 2015;33(2):440–449. doi: 10.1007/s10637-015-0211-0. [DOI] [PubMed] [Google Scholar]
- 8.Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events v3.0 (CTCAE) http://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf.
- 9.Durie BGM, Harousseau J-L, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467–1473. doi: 10.1038/sj.leu.2404284. (Comment in Leukemia 2007;21(4):818–820) [DOI] [PubMed] [Google Scholar]
- 10.Bladé J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haematopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102(5):1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 11.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 12.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39(4):561–577. [PubMed] [Google Scholar]
- 13.Schisterman EF, Perkins NJ, Liu A, Bondell H. Optimal cut-point and its corresponding Youden Index to discriminate individuals using pooled blood samples. Epidemiology. 2005;16:73–81. doi: 10.1097/01.ede.0000147512.81966.ba. [DOI] [PubMed] [Google Scholar]
- 14.Le Gouill S, Podar K, Harousseau JL, Anderson KC. Mcl-1 regulation and its role in multiple myeloma. Cell Cycle. 2004;3(10):1259–1262. doi: 10.4161/cc.3.10.1196. [DOI] [PubMed] [Google Scholar]
- 15.Derenne S, Monia B, Dean NM, et al. Antisense strategy shows that Mcl-1 rather than Bcl-2 or Bcl-x(L) is an essential survival protein of human myeloma cells. Blood. 2002;100(1):194–199. doi: 10.1182/blood.v100.1.194. [DOI] [PubMed] [Google Scholar]
- 16.Shah JJ, Zonder JA, Cohen AD, et al. A phase 1/2 trial of the KSP inhibitor ARRY-520 in relapsed/refractory multiple myeloma. Blood. 2010;116(21) Abstract 1959. [Google Scholar]
- 17.Lynch JT, Rajendran R, Xenaki G, et al. The role of glucocorticoid receptor phosphorylation in Mcl-1 and NOXA gene expression. Mol Cancer. 2010;9:38–53. doi: 10.1186/1476-4598-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richardson PG, Sonneveld P, Schuster M, et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of theAPEX trial. Blood. 2007;110(10):3557–3560. doi: 10.1182/blood-2006-08-036947. [DOI] [PubMed] [Google Scholar]
- 19.Richardson PG, Siegel DS, Vij R, et al. Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: a randomized Phase 2 study. Blood. 2014;123(12):1826–1832. doi: 10.1182/blood-2013-11-538835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richardson PG, Xie W, Jagannath S, et al. A phase 2 trial of lenalidomide, bortezomib, and dexamethasone in patients with relapsed and relapsed/refractory myeloma. Blood. 2014;123(10):1461–1469. doi: 10.1182/blood-2013-07-517276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siegel D, Martin T, Wang M, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120(14):2817–2825. doi: 10.1182/blood-2012-05-425934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lacy MQ, Hayman SR, Gertz MA, et al. Pomalidomide (CC4047) plus low-dose dexamethasone as therapy for relapsed multiple myeloma. J Clin Oncol. 2009;27(30):5008–5014. doi: 10.1200/JCO.2009.23.6802. [DOI] [PubMed] [Google Scholar]
- 23.Kremer JM, Wilting J, Janssen LH. Drug binding to human alpha-1 acid glycoprotein in health and disease. Pharmacol Rev. 1988;40(1):1–47. [PubMed] [Google Scholar]
- 24.Merlini G, Perfetti V, Gobbi PG, et al. Acute phase proteins and prognosis in multiple myeloma. Br J Haematol. 1993;83(4):595–601. doi: 10.1111/j.1365-2141.1993.tb04696.x. [DOI] [PubMed] [Google Scholar]
- 25.Pelliniemi TT, Irjala K, Mattila K, et al. Immunoreactive interleukin-6 and acute phase proteins as prognostic factors in multiple myeloma. Finnish Leukemia Group. Blood. 1995;85(3):765–771. [PubMed] [Google Scholar]
- 26.Tunquist B, Brown K, Hingorani G, et al. Identification of alpha 1-acid glycoprotein (AAG) as a potential patient selection biomarker for improved clinical activity of the novel KSP inhibitor ARRY-520 in relapsed and refractory multiple myeloma (MM) Blood. 2012;120(21) Abstract 1868. [Google Scholar]
- 27.Bruno R, Olivares R, Berille J, et al. Alpha-1-acid glycoprotein as an independent predictor for treatment effects and a prognostic factor of survival in patients with non- small cell lung cancer treated with docetaxel. Clin Cancer Res. 2003;9(3):1077–1082. [PubMed] [Google Scholar]
- 28.Chari A, Htut M, Zonder JA, et al. A phase 1 dose-escalation study of filanesib plus bortezomib and dexamethasone in patients with recurrent/refractory multiple myeloma. Cancer. 2016;122(21):3327–3335. doi: 10.1002/cncr.30174. [DOI] [PMC free article] [PubMed] [Google Scholar]