Abstract
Background
Serum (extracellular) histone levels are increased in inflammatory states and in the presence of coagulation dysfunction, eg, trauma, chemical/ischemic injury, infection. There is increasing evidence of a systemic inflammatory response associated with the presence of a pig xenograft in a nonhuman primate. We evaluated extracellular histone levels in baboons with various pig xenografts.
Methods
We measured serum histones in baboons with pig heterotopic heart (n=8), life-supporting kidney (n=5), orthotopic liver (n=4), and artery patch (n=9) grafts by ELISA. C-reactive protein (CRP), free triiodothyronine (fT3), serum amyloid A (SAA), and platelet counts were also measured, all of which may provide an indication of an inflammatory state. We investigated the effect of histones on platelet aggregation and on cytotoxicity of pig cells in vitro.
Results
Serum histones increased when baboons developed consumptive coagulopathy (CC) (eg, thrombocytopenia) or infection. CRP levels tended to be higher and fT3 levels lower when CC developed. Measurement of SAA correlated fairly well with CRP and indicated the state of inflammation. Treatment of the recipient with tocilizumab reduced the level of serum histones, CRP, and SAA, and increased the level of fT3 and platelet counts. In vitro, histone-induced platelet aggregation and endothelial cell apoptosis were both significantly reduced by the NF-kB pathway inhibitor, parthenolide.
Conclusions
These noninvasive assays may be useful for monitoring the health status of nonhuman primate recipients of pig organ grafts and may help in management after xenotransplantation. Tocilizumab and NF-κB inhibitors might prove valuable in reducing the inflammatory response to a pig xenograft.
INTRODUCTION
Histones are highly-conserved, alkaline, positively-charged proteins that include 5 main types - linker histone H1 and core histones H2A, H2B, H3, and H4.1,2 They are basic structural components of chromatin, namely nucleosomes that are responsible for DNA organization. Histones are found in 4 locations – (i) the cell nucleus, (ii) the cytosol, (iii) the cell surface, and (iv) in the extracellular space.3 Histones are released into the extracellular space from damaged and activated cells (eg, neutrophils4 and mast cells5 in situations such as trauma, chemical or ischemic injury,6,7 or infection, through the formation of neutrophil extracellular traps (NETs).3 When histones are released into the extracellular space, they cause cell and tissue damage, and act as danger/damage-associated molecular patterns (DAMPs) that mediate inflammation,6,8 coagulation disorders,8–10 an immune response,11 and/or cytotoxicity.12
Extracellular histones induce inflammation by binding to Toll-like receptors (TLRs), particularly to TLR2 and TLR4, of various cells, followed by cytokine secretion (eg, TNF-α, IL-1β, IL-6, IL-8, or IL-10).13 These pro-inflammatory cytokines also stimulate NETosis (an unique form of cell death associated with the release of chromatin and the granular contents of the cells into the extracellular space, with the involvement of other immune cells in the process), which increases histone release and amplifies inflammation.14 When extracellular histones bind to TLR2 and TLR4 on platelets, they induce platelet activation and aggregation15,16 that in turn induces NETosis.17 Extracellular histones enhance thrombin generation by (i) inducing tissue factor expression on endothelial cells potentially through the NF-κB pathway,18 (ii) releasing von Willebrand Factor (vWF),19,20 and (iii) reducing expression of coagulation-regulatory proteins (eg, thrombomodulin).9
There have been numerous investigations that have explored a correlation between extracellular histones and various disease processes.21–24 However, to our knowledge, there has been very limited study of extracellular histones and transplantation, especially in xenotransplantation.
With the increasing availability of genetically-engineered pigs and the introduction of novel immunosuppressive agents, markedly improved pig xenograft survival in nonhuman primates has been reported.25 There is increasing evidence that a systemic inflammatory response is associated with the presence of a pig xenograft.26,27 Furthermore, this response precedes the onset of features of coagulation dysfunction.28,29 Inflammation, coagulation, and the immune response have a complex inter-relationship.6,11 These observations suggested that prevention of an inflammatory response will impact the survival of pig xenografts in nonhuman primates.
Considering that extracellular histones are increased in inflammation and abnormal coagulation, histones may play an important role in xenotransplantation. The aims of present retrospective study were (i) to measure serum histone levels in baboons that had received various pig xenografts, (ii) to investigate the relationship between histones and other markers of inflammation and coagulation dysregulation (eg, C-reactive protein [CRP], free triiodothyronine [fT3], serum amyloid A [SAA], and platelet count), and (iii) to examine the effect of histones on pig cell cytotoxicity and human platelet aggregation in vitro.
MATERIALS AND METHODS
Animals
Baboons (n=26) (Oklahoma University Health Sciences Center, Oklahoma City, OK) received either a heterotopic heart graft (n=8),30 a life-supporting kidney graft (n=5),31 an orthotopic liver graft (n=4),32 or an artery patch graft (n=9)33,34 from genetically-engineered pigs (Revivicor, Blacksburg, VA) (Table 1).
Table 1.
Immunosuppressive and anti-inflammatory therapy in baboon recipients of pig organs or artery patches, and recipient survival
| Organ | Donor pig type | Baboon | Basis of maintenance immunosuppressive regimen | IL-6R blockade* (+/-) | Clinical outcome | Follow up (days) |
|---|---|---|---|---|---|---|
| Heart (n=8) |
GTKO/CD46 |
B17913 |
Anti-CD40mAb + CTLA4-Ig |
+ |
rejection# |
124 |
| GTKO/CD46 | B18013 | Anti-CD40mAb + CTLA4-Ig | + | rejection# | 118 | |
| GTKO/CD46/TBM | B5512 | Anti-CD40mAb + CTLA4-Ig | − | infectiona | 99 | |
| GTKO/CD46/TBM | B5712 | Anti-CD40mAb + CTLA4-Ig | − | rejection# | 130 | |
| GTKO/CD46 | B19010 | Anti-CD154mAb | − | CC | 18 | |
| GTKO/CD46 | B19110 | Anti-CD154mAb | − | CC | 33 | |
| GTKO/CD46 | B18910 | Anti-CD154mAb | − | CC/infectionb | 23 | |
| GTKO/CD46 | B19510 | Anti-CD154mAb | − | infectionc | 15 | |
|
| ||||||
| Kidney (n=5) |
GTKO/CD46/CD55/TBM/EPCR/CD39 |
B9313 |
Anti-CD40mAb |
+ |
infectiond |
136 |
| GTKO/CD46/CD55/EPCR/TFPI/CD47 | B17315 | Anti-CD40mAb | + | infectione | 237 | |
| GTKO/CD46/CD55/EPCR/TFPI/CD47 | B17615 | Anti-CD40mAb | + | infectionf | 260 | |
| GTKO/CD46/TBM | B17415 | Anti-CD40mAb | + | CC | 12 | |
| GTKO/CD46/TBM | B17515 | Anti-CD40mAb | + | CC | 12 | |
|
| ||||||
| Liver (n=4) |
GTKO/CD46 |
B7708 |
Tacrolimus |
− |
CC |
7 |
| GTKO/CD46 | B7808 | Tacrolimus | − | CC | 6 | |
| GTKO/CD46 | B18508 | Tacrolimus | − | CC | 5 | |
| GTKO/CD46 | B18908 | Tacrolimus | CC | 6 | ||
|
| ||||||
| Artery patch (n=9) |
GTKO/CD46/CIITA |
B6314 |
Anti-CD40mAb + CTLA4-Ig |
– |
** |
84 |
| GTKO/C D46/CIITA | B6514 | Anti-CD40mAb | − | ** | 84 | |
| GTKO/CD46 | B12912 | Anti-CD40mAb + CTLA4-Ig | − | ** | 48 | |
| GTKO/CD46 | B5412 | Anti-CD40mAb + CTLA4-Ig | − | ** | 28 | |
| GTKO/CD46 | B5912 | Anti-CD40mAb + CTLA4-Ig | − | ** | 28 | |
| GTKO/CD46/CIITA | B12612 | CTLA4-Ig | + | ** | 48 | |
| GTKO/CD46/CD55/ | B9512 | Anti-CD40mAb + CTLA4-Ig | + | ** | 48 | |
| CIITA/EPCR/TBM GTKO/CD46/CD55/ | B9212 | Anti-CD40mAb + CTLA4-Ig | ** | 48 | ||
| CIITA/EPCR/TBM GTKO/CD46 | B6414 | None | − | ** | 48 | |
Tocilizumab (10mg/kg, every 2 weeks)
Electively euthanized
focally extensive fibrosis with no significant inflammatory cell filtration
Cytomegalovirus pneumonia
Systemic Enterococcus faecium
Systemic Enterococcus faecium
Systemic Myroides spp
Systemic Methicillin-resistant staphylococcus aureus and enterococcus faecium.
Pneumocystis pneumonia
Immunosuppressive and anti-inflammatory therapy
For the heart, kidney or artery patch xenotransplantation experiments, immunosuppressive therapy was based on costimulation blockade using either (i) anti-CD154mAb (NIH NHP Reagent Resource, Boston, MA) or (ii) CTLA4-Ig (BMS, Princeton, NJ) +/- anti-CD40mAb (2C10R4; NIH NHP Reagent Resource) or (iii) anti-CD40mAb30–32,34,35 (Table 1). Nine immunosuppressed baboons with pig heart (n=2), kidney (n=5), or artery patch (n=2) grafts received interleukin-6 receptor (IL-6R) blockade with tocilizumab (10mg/kg; Actemra, Genentech, South San Francisco, CA). After liver xenotransplantation,32 immunosuppressive therapy was based on tacrolimus/mycophenolate mofetil (MMF) (Table 1).
Heart, kidney, liver, or artery patch transplantation
Anesthesia, intravascular catheter placement in baboons, and pig-to-baboon non-life-supporting heterotopic abdominal heart,30,36 life-supporting kidney,31 orthotopic liver,32 and artery patch33,34 xenotransplantation have been described previously.
All animal care was in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the National Research Council (8th edition, revised 2011), and was conducted in an AAALAC-accredited facility. Protocols were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Measurement of extracellular histones
Extracellular histone levels were measured using a commercially-available sandwich enzyme-linked immunosorbent assay (ELISA) (Cell Death Detection ELISAplus, Roche Diagnostics, Indianapolis, IN) according to the manufacturer’s instructions. Briefly, 20μL of serum was diluted 1:4 in the immunoreagent that was prepared by mixing of 5% volumes of anti-DNA-peroxidase and anti-histone-biotin with 90% volumes of incubation buffer, and added to streptavidin-coated microtiter plates. After incubation (3h) and washing, peroxidase activity of the retained immunocomplexes was developed by incubation with ABTS (2,2′-azino-di[3-ethylbenzthiazoline-sulfonate]) and read in a spectrophotometer at 405nm. The results are reported as optical density (OD).37
Measurement of C-reactive protein (CRP), free triiodothyronine (fT3), serum amyloid A (SAA), and platelet count
Whole blood and serum samples were obtained from recipients before and serially after transplantation. Serum CRP and platelet counts were measured by standard methods (Central Laboratory of Presbyterian Hospital, Pittsburgh, PA). The blood level of serum fT3 was measured by Antech Diagnostics (Southaven, MS). Serum amyloid A (SAA) was measured with a Rapid Test for Inflammation & Infection kit (Accuplex, Maynooth, Co Kildare, Ireland), as an indication of inflammation, per the manufacturer’s instructions (n=13). Briefly, serum was drawn up into an applicator and introduced into the port on the test strip; the result was indicated in the test window within 5-10min (Figure 1).
Figure 1.
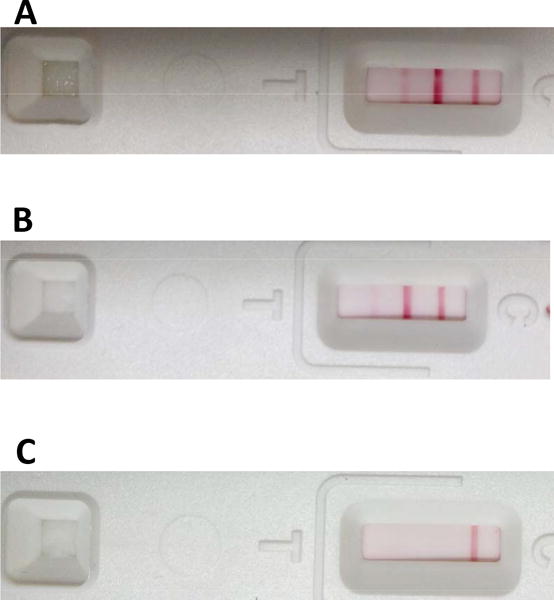
Representative results of assay for SAA showing (A) 3 lines (negative), indicating no active inflammation, (B) 2 lines, indicating mild-to-moderate inflammation, (C) 1 line, indicating significant inflammation.
Human platelet aggregation assay
Platelet aggregation assays were performed as previously described29. Whole blood samples were obtained from a healthy human volunteer and collected into a 3.2% trisodium citrate plastic tube. Human whole blood (500μL) was mixed with saline (500μL) in a plastic cuvette (Corning, Corning, NY), and platelet aggregation was measured by platelet aggregometry (2-sample, 4-channel, model 592 Whole Blood Aggregometer, Chrono-log, Harvertown, PA).29 By adding a small rotating magnet to each sample, shear stress was created to simulate intravascular flow conditions. The extent of platelet aggregation in 6min (based on the manufacturer’s instructions) was evaluated.
The effect of histones at various concentrations (40, 80, 160μg/mL) (Roche, Mannheim, Germany) on platelet aggregation was investigated in vitro. Inhibition of histone-induced platelet aggregation by the NF-κB inhibitor, parthenolide (at 2 and 8μM) (Sigma-Aldrich, St. Louis, MO) was investigated after co-incubation with whole blood for 30min at 37°C.
Porcine aortic endothelial cell (pAEC) culture
Wild-type pAECs were isolated from fresh pig aortas and cultured in collagen I-coated 6-well culture plates (BD Biosciences, San Jose, CA) in pAEC culture medium (10% heat-inactivated fetal bovine serum [FBS; Sigma-Aldrich], antibiotic-antimycotic [Invitrogen, Carlsband, CA] and endothelial growth factor [30μg/mL, BD]) at 37°C in a humidified atmosphere of 5% CO2, as previously described38,39
pAEC apoptosis assay
In order to determine the effect of histones on pAEC viability, apoptotic/necrotic cells were detected by flow cytometry using the Annexin V apoptosis detection kit I (BD, San Deigo, CA), as previously described.40 Confluent pAECs were incubated in serum-free medium (OPTI-MEM medium) (Invitrogen) in the presence of different concentrations of histone (Roche) (20, 40, 80, 160μg/mL) for 2h or 6h. The NF-κB inhibitor, parthenolide (2, 8 μM), was added with the histones and incubated for 6h. pAECs were harvested after incubation, and washed with PBS (Invitrogen), then resuspended with binding buffer (BD). Cells were stained with Annexin V for 20min and 7AAD for 15min, respectively, at room temperature. Annexin V-negative/7AAD-negative cells were identified as live cells. Annexin V-positive/7AAD-negative cells were identified as apoptotic, whereas Annexin V-negative/7AAD-positive cells were identified as necrotic or dead.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 7.0 software. Significance of the difference between 2 groups was determined by paired Student’s t-test or Mann–Whitney U-test. A P value of <0.05 was considered statistically significant.
RESULTS
In vivo studies
Serum histone levels in baboons with a pig xenograft (heart, kidney, liver, artery patch)
When graft function was stable, serum histone levels remained within the pretransplant range (which was generally <0.5). When a baboon developed consumptive coagulopathy (CC) or infection, serum histone levels increased.
For example, in one baboon with a pig heart transplant, histone levels increased to >3 when CC developed (B19010) (Figure 2A). There was also a significant increase in histone level (to >3) in one baboon that developed an early infection and required to be euthanized (B19510). In baboons that experienced no complications, the histone levels remained approximately <1.
Figure 2.
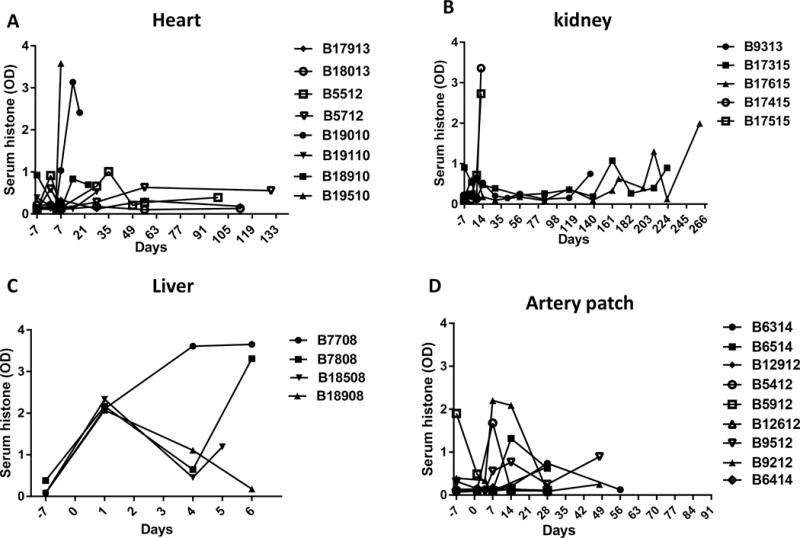
Changes in serum histone levels in baboons following pig (A) non-life-supporting heterotopic abdominal heart, (B) life-supporting kidney, (C) orthotopic liver, or (D) artery patch xenotransplantation.
(A) Following a pig heart transplant, in baboons that experienced no complications (eg, no infection or CC), serum histone levels remained approximately <1. Histone levels increased to >3 when CC (B19010) or infection (B19510) developed.
(B) Following a pig kidney transplant, serum histone levels increased when CC (B17415 and B17515) or infection (B9313, B17315, B17615) developed.
(C) Following a pig liver transplant, serum histone levels increased to >2 in all cases within 24h when CC developed (thrombocytopenia <50,000μl). This was followed by temporary recovery in 3 cases.
(D) Following a pig artery patch transplant, there was some fluctuation in serum histone levels during the first month (which is difficult to explain), but thereafter the levels stabilized at <1.
Similarly, serum histone levels increased in kidney recipients to >2 when early CC developed requiring euthanasia (B17415 and B17515) (Figure 2B). In the remaining baboons, when prolonged graft survival was obtained, histone levels increased terminally when infectious complications developed (eg, B17615).
After pig liver transplantation, histone levels increased to >2 in all cases within 24h, at a time when the platelet counts had fallen to <50,000/μl (Figure 2C). Some recovery occurred, but histones increased again in 3 cases when the state of CC became problematic.
In the presence of a pig artery patch graft, there was some fluctuation in serum histone level during the first month that is difficult to explain (B6514, B5412 and B9212), but in all baboons it recovered to the normal range (Figure 2D).
Tocilizumab was associated with a reduced level of serum histones and CRP, maintenance of a negative SAA (when the recipients were stable), and an increase in fT3 and platelet count
We divided the baboons into 2 groups based on whether they received treatment with tocilizumab (IL-6 receptor blockade n=9; heart [n=2], kidney [n=5], artery patch [n=2]) or not (n=17) (Table 1), and investigated the effect of this agent on serum histone, CRP, fT3, and SAA levels. (Because of insufficient serum, SAA was measured in only 8 baboons that received tocilizumab and in 5 that did not.)
Immediately posttransplantation, there was an increase in mean histone level (day 1) (Figure 3A), which was significantly higher in the baboons that did not receive tocilizumab (1.1 vs 0.3, p<0.05). The level also rose in 2 baboons with kidney grafts that rapidly developed CC, and required euthanasia on day 12.
Figure 3.
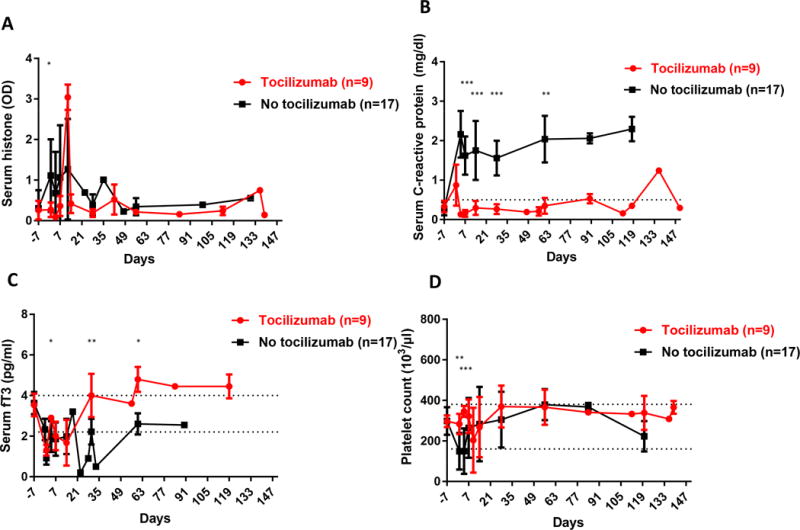
Changes in (A) serum histones, (B) C-reactive protein (CRP), (C) free triiodothyronine (fT3), and (D) platelet count after pig organ or artery patch transplantation in baboons receiving (n=9) in red or not receiving (n=17) in black tocilizumab.
(A) The mean serum histone level in baboons that did not receive tocilizumab was significantly higher on day 1 post-transplant than in baboons that received tocilizumab (day 1, 1.2 vs 0.3, p<0.05), except in 2 baboons that required euthanasia on day 12 for the early and rapid development of CC (indicated by the spikes in serum histone). (*p<0.05)
(B) In the tocilizumab-treated baboons, the mean CRP remained <0.5 from day 4, and on days 7, 14, 28, and 60 was significantly lower than in baboons that did not receive tocilizumab (day 7, 0.2 vs 1.6mg/dl, p<0.001; day 14, 0.3 vs 1.8mg/dl, p<0.001; day 28, 0.3 vs 1.6mg/dl, p<0.001; and day 60, 0.3 vs 2.0mg/dl, p<0.01, respectively). (**p<0.01; ***p<0.001)
(C) There was an immediate post-transplant significant decrease in fT3 in all baboons irrespective of tocilizumab treatment, but the fT3 level recovered more rapidly and to a higher level in those that received tocilizumab. The mean fT3 levels in baboons receiving tocilizumab on days 1, 30 and 60 were significantly higher than those in baboons not receiving tocilizumab (day1, 1.3 vs 0.9pg/ml, p<0.05; day 30, 4.0 vs 2.2pg/ml, p<0.01; day 60, 4.8 vs 2.6pg/ml, p<0.05, respectively). (*p<0.05; **p<0.01)
(D) The mean platelet count was significantly higher on day 1 and 4 in the tocilizumab-treated baboons than in those that did not receive tocilizumab (day 1, 283 ×103/μl vs 149 ×103/μl, p<0.01; day 4, 346 ×103/μl vs 149 ×103/μl, p<0.001; respectively). (**p<0.01; ***p<0.001)
Mean CRP rose by day 4 (the first day of measurement) in the baboons that did not receive tocilizumab, and remained elevated throughout the posttransplant course (Figure 3B). In contrast, tocilizumab administration largely prevented a rise in CRP (Figure 3B). In the baboons that received tocilizumab, the mean CRP levels on days 7, 14, 28 and 60 were significantly lower than those in the baboons that did not receive tocilizumab (0.2 vs 1.6mg/dl, p<0.001; 0.3 vs 1.8mg/dl, p<0.001; 0.3 vs 1.6mg/dl, p<0.001; and 0.3 vs 2.0mg/dl, p<0.01, respectively) (Figure 3B).
There was an immediate decrease in fT3 in all baboons irrespective of tocilizumab administration (Figure 3C), but fT3 levels recovered more rapidly and were sustained at a higher level in baboons that received tocilizumab (Figure 3C). The mean fT3 levels in tocilizumab-treated baboons on days 1, 30, and 60 were significantly higher than those in untreated baboons (1.3 vs 0.9pg/ml, p<0.05; 4.0 vs 2.2pg/ml, p<0.01; and 4.8 vs 2.6pg/ml, p<0.05, respectively).
The mean platelet count (Figure 3D) was significantly higher in tocilizumab-treated baboons on days 1 and 4 than in those that did not receive tocilizumab (283 ×103/μl vs 149 ×103/μl, p<0.01; 346 ×103/μl vs 149 ×103/μl, p<0.001; respectively). Thereafter there was no significant difference.
The SAA assay indicated no inflammation was present pretransplant (Table 2). However, when a baboon developed an infection (B19510, B9313) or CC (B17415, B17515), or was developing an immune response, eg, was becoming sensitized (B6414), the SAA assay became positive, indicating moderate or severe inflammation. In the presence of an artery patch graft, but in the absence of tocilizumab therapy, even when the baboon showed no features of CC, infection, or other complication, the SAA indicated some inflammation (eg, B12912 and B5412) (Table 2). In contrast, the baboons receiving tocilizumab showed a normal SAA when they were stable (no features of CC, infection, or other complication) indicating no inflammation (eg, B18013, B17315 [day 180], B17615 [day 180], B12612 and B9512) (Table 2).
Table 2.
Changes in serum amyloid A (SAA) in baboon recipients of pig organs or artery patches (n=13)*
| Organ | Baboon | SAA Pre-transplant Post-transplant | IL-6R blockade** (+/−) | Status on day of SAA measurement | Day of SAA measurement | |
|---|---|---|---|---|---|---|
| Heart (n=2) | ||||||
| B18013 | negative | negative | + | rejection# | 118 | |
| B19510 | negative | significant inflammation | − | infectiona | 15 | |
|
| ||||||
| Kidney (n=5) | ||||||
| B9313 | negative | significant inflammation | + | infectionb | 136 | |
| B17315 | negative | negative | + | stable | 180 | |
| B17615 | negative | negative | + | stable | 180 | |
| B17415 | negative | significant inflammation | + | CC | 12 | |
| B17515 | negative | significant inflammation | + | CC | 12 | |
|
| ||||||
| Liver (n=1) | ||||||
| B7708 | negative | moderate inflammation | − | CC | 7 | |
|
| ||||||
| Artery patch (n=5) | ||||||
| B12912 | negative | significant inflammation | stable | 48 | ||
| B5412 | negative | significant inflammation | − | stable | 28 | |
| B12612 | negative | negative | + | stable | 48 | |
| B9512 | negative | negative | + | stable | 48 | |
| B6414 | negative | moderate inflammation | − | sensitized | 48 | |
SAA could not be measured in all recipients because of a lack of serum samples.
Tocilizumab (10mg/kg, every 2 weeks)
focally extensive fibrosis with no significant inflammatory cell filtration
Systemic Enterococcus faecium
Systemic Myroides spp
Serum histone and CRP levels tended to increase, fT3 levels decreased, and SAA became positive when CC (thrombocytopenia) developed
As we have not seen CC develop in any baboon with a pig artery patch graft, we excluded these (n=9) from the analysis. Of the remaining 17 baboons with pig organ grafts, 9 developed CC (defined by a platelet count <50,000μl or <20% of pretransplant [Figure 4D]) following pig heart, kidney, or liver transplantation. Serum histone levels tended to rise to much higher levels in the early posttransplant period (first month) in those baboons that developed CC (Figure 4A). In these baboons, the mean serum histone level was higher on days 1, 4, and 7 than in those that did not develop CC, though these differences were not statistically significant (1.3 vs 0.5, ns; 1.0 vs 0.4, ns; 1.2 vs 0.9, ns; respectively). There was therefore a trend towards a correlation between high histone level and CC.
Figure 4.
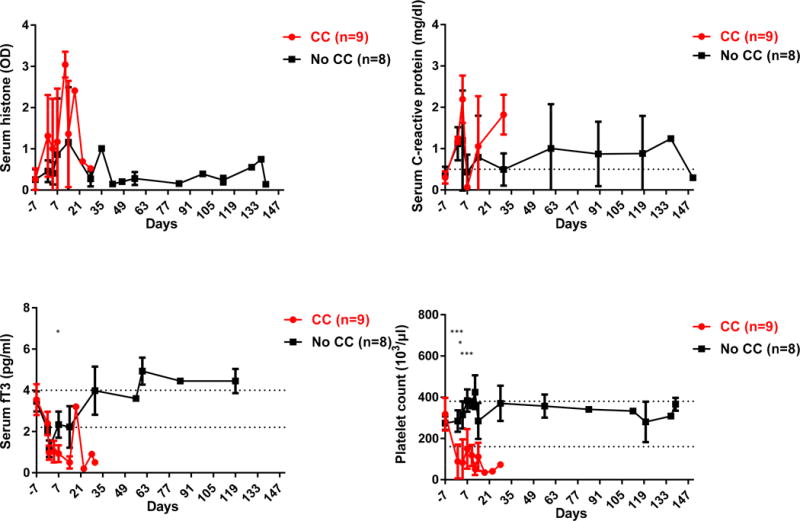
Changes in the levels of (A) serum histones, (B) CRP, (C) fT3, and (D) platelet count after pig organ transplantation in baboons that developed CC (n=9) or did not develop CC (n=8)
(A) Serum histone levels rose to higher levels in the first post-transplant month in baboons that developed CC. In the baboons that developed CC, mean serum histone level on day 1 was significantly higher than pre-transplant (1.3 vs 0.3, p<0.05). In these baboons, the mean serum histone level tended to be higher on days 1, 4, and 7 than in those that did not develop CC, though the differences were not statistically significant (day 1, 1.3 vs 0.5, ns; day 4, 1.0 vs 0.4, ns; day 7, 1.2 vs .09, ns; respectively).
(B) When CC developed, on days 4 and 30 the mean CRP level tended to be higher than in those that did not develop CC, though the differences were not statistically significant (day 4, 2.2 vs 1.2mg/dl, ns; day 30, 1.8 vs 0.5 mg/dl; ns respectively)
(C) In baboons that developed CC, the mean fT3 level on days 7 was significantly lower than in those that did not develop CC (0.9 vs 2.3 pg/ml, p<0.05). (*p<0.05)
(D) CC was associated with a significant fall in mean platelet count, which was lower than when CC did not develop. On days 1, 4, and 7, the mean platelet counts were lower in those baboons in which CC developed (day 1, 88 ×103/μl vs 284 ×103/μl, p<0.001; day 4, 82 ×103/μl vs 314 ×103/μl, p<0.05; and day 7, 150 ×103/μl vs 384 ×103/μl, p<0.001, respectively). (*p<0.05; ***p<0.001)
Similarly, mean CRP level tended to be higher (Figure 4B), and mean fT3 level lower (Figure 4C), when CC developed. On day 7, the mean fT3 in the baboons that developed CC was significantly lower than in those that did not develop CC (0.9 vs 2.3 pg/ml, p<0.05).
By definition, platelet counts fell dramatically when CC developed (Figure 4D).
The SAA assay indicated inflammation when CC developed, even when tocilizumab had been administered (B17415, B17515) (Table 2).
In vitro studies
Inhibition of histone-induced human platelet aggregation by the NF-κB inhibitor, parthenolide
In the absence of any platelet agonist (or exposure to pig cells), no platelet aggregation was observed in the absence of histone (Figure 5). Within 6min of adding a histone (160ug/mL) to the whole blood sample, 18% platelet aggregation was observed (after which aggregation slowly increased further to 21%). The addition of the NF-κB inhibitor, parthenolide (at 2 or 8μM), significantly reduced histone-induced aggregation at 6min to 5% (p<0.01). There was no significant difference in inhibitory effect between 2 and 8μM of the NF-κB inhibitor.
Figure 5.
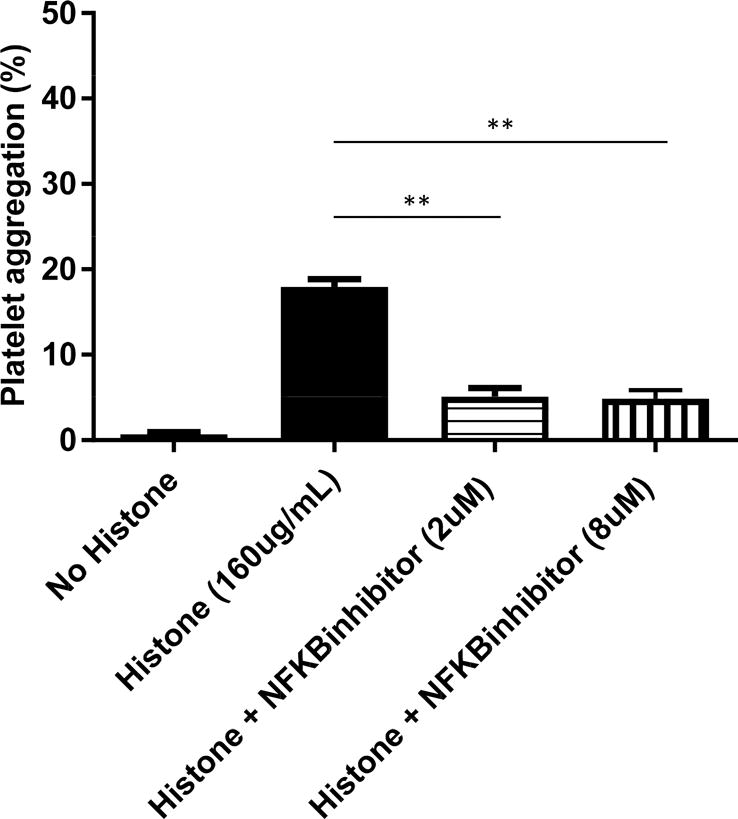
In vitro, in the absence of any platelet agonist or pig cells, the addition of histones (160μg/mL) to human platelets induced 18% platelet aggregation. This was significantly reduced (p<0.01) by the addition of the NF-κB inhibitor, parthenolide at 2 and 8μM, to 5% and 5%, respectively.
Histones induced cell apoptosis/death in vitro via an NF-κB-dependent pathway
With increasing concentrations of histone, and increasing periods of exposure, there was an increase in apoptosis/death of pAECs (Figure 6A, B). Histone (160μg/mL)-induced apoptosis/death was significantly greater than in the absence of histone. After 2h incubation with histone, mean percentage apoptosis/death was 67.3% vs 6.8% (Figure 6B), after 6h 94.6% vs 9.2% (Figure 6B), after 16h 94.0% vs 17.3% (Figure 6B), and after 24h 97.4% vs 17.2% (Figure 6B) (all p<0.05).
Figure 6.
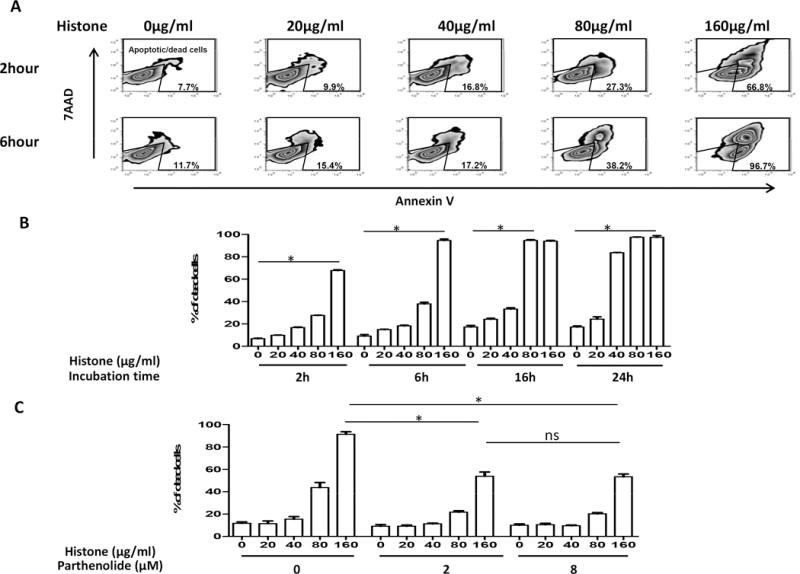
In vitro, incubation with histones induced apoptosis/death of pAECs in a concentration- and time-dependent manner (A, representative figure by flow cytometry; B, mean apoptosis/death percentage). (C) The NF-κB inhibitor, parthenolide (at 2 and 8μM), significantly reduced histone (160μg/ml)-induced cell apoptosis/death (mean apoptosis/death percentage 91.4% vs 53.8%, and 91.4% vs 53.6%, respectively) (both p<0.05). There was no significant difference in the protective effect of parthenolide between at 2μM and 8μM (mean apoptosis/death percentage 53.8% vs 53.6%).
Histone (160μg/ml)-induced cell apoptosis/death was significantly reduced by the NF-κB inhibitor, parthenolide, at 2μM (mean percentage apoptosis/death 91.4% vs 53.8%) and 8μM (mean percentage apoptosis/death 91.4% vs 53.6%) (both p<0.05) (Figure 6C). However, the higher concentration (8μM) of NF-κB inhibitor did not provide any further protective effect compared to the lower concentration (2μM) (mean percentage apoptosis/death 53.6% vs 53.8%).
DISCUSSION
Histones are one of the damage-associated molecular pattern molecules (DAMPs), that include high mobility group box-1 (HMGB-1).41,42 They are also basic unit structural components of chromatin (nucleosomes); lack of histone leads to disorganized and ineffectively structured human genomic DNA.3
Histones can be released in many conditions, eg, trauma, chemical or ischemic injury, infection.3 Under conditions of cellular stress or injury, once histones are released from both damaged cells and activated immune cells into the extracellular space and/or blood, they can induce systemic inflammation (eg, cytokine release),8,21 a thrombotic response (eg, platelet activation and aggregation),15,16,43 and/or endothelial cytotoxicity,6 which may lead to life-threatening complications, including acute organ injury44 or multiple organ failure.37,45 These findings imply an important role for extracellular histones in disease progression.
However, to our knowledge nothing is known of the extracellular histone levels in recipients of pig xenografts. The present study demonstrated that increased serum histone levels in xenograft recipients (of a heart, liver, kidney, or artery patch) were associated with the operative procedure, but more importantly with infection, tissue/cell injury (including rejection), and CC. In contrast, when the recipients were stable, serum histone levels also remained stable.
A systemic inflammatory response develops after pig organ transplantation in nonhuman primates.26,27 Therefore, anti-inflammatory agents have been administered. For example, a beneficial effect of tocilizumab (IL-6 receptor blockade) has been documented.26,31 In the present study, when baboons received tocilizumab, the levels of serum histone and CRP were reduced, and the level of fT3 and the platelet count were increased. The mechanisms by which tocilizumab results in a reduction in histone release is uncertain, but the prevention or suppression of an inflammatory response is likely to play a role. Furthermore, the decreased number of neutrophils (the main source of histones) associated with tocilizumab therapy might also result in a reduction of extracellular histone release.46,47
Emerging studies indicate that increased serum histone levels have been observed in patients with disseminated intravascular coagulation (DIC), which is characterized by widespread microvascular thrombosis in various organs with exhaustion of coagulation factors and platelets, contributing to multiple organ dysfunction syndrome.44 Kim et al, demonstrated that high circulating histone levels are related to mortality in patients with disseminated intravascular coagulation.48
In the present study, serum histone levels increased in baboons that developed CC when compared with those that did not develop CC. There was a correlation between high histone levels and CC although the mechanisms involved remain unclear. When CC develops, damaged cells/tissues may induce histone release. Sustained inflammation in the presence of a xenograft can also induce histone release by cell/tissue injury and activation, which can promote coagulation dysfunction. Exhaustion of coagulation factors and platelets may occur through thrombin generation by (i) inducing tissue factor (TF) expression,18 (ii) reducing expression of coagulation-regulatory proteins,9 or (iii) through direct platelet activation and aggregation.15,16,43
Serum amyloid A protein (SAA) is an acute phase inflammation-reactive protein that can be produced from the liver by induction of cytokines (interleukin-1 and interleukin-6),49,50 similar to the production of CRP.51,52 To our knowledge, the SAA assay has not been tested in a xenotransplantation model previously. Because it is a more rapid and simple test than other inflammatory markers (eg, CRP, histone), albeit currently only semi-quantitative, the SAA assay might prove valuable as a screening test for monitoring recipients with pig xenografts. Any indication that inflammation is developing could be followed by more intensive investigation, eg, to determine the presence of an infection, rejection, of CC.
We demonstrated that the SAA test indicated significant or moderate inflammation when baboons (i) developed an infection, or (ii) were developing CC, or (iii) when the pig graft was undergoing rejection, whereas the SAA indicated no inflammation when the baboons receiving tocilizumab were stable. However, the SAA assay indicated inflammation in some baboons not receiving tocilizumab even when they were stable, mimicking the CRP in this respect. Increased SAA levels have been observed in recipients of kidney or heart allografts when undergoing rejection or infection.53–56
In our in vitro study, we demonstrated that histones induced apoptosis of pAECs in a concentration- and time-dependent manner, as well as inducing human platelet aggregation through the NF-κB pathway, which is consistent with other reports.57,58 However, whether the same histone concentrations are achieved in vivo and with the same activity remains uncertain. Although the molecular mechanisms of protection from histone-mediated cytotoxicity and platelet activation and aggregation by NF-κB inhibitors remain unclear, NF-κB might be a potential therapeutic target in terms of anti-apoptosis and anti-platelet activation/aggregation in xenotransplantation. We demonstrated that NF-κB inhibitors reduce the histone-induced platelet aggregation in whole blood which is consistent with other reports59 although the mechanism remains uncertain. In our platelet aggregation assay, we used a whole blood sample where the NF-κB inhibitor may have reduced platelet aggregation indirectly through its effect on other immune cells.
The observations made in the in vivo studies are correlative, but there remains uncertainty of the exact role of histones in vivo in the pig-to-baboon model, eg, whether they are passive markers of inflammation or they actively increase inflammation (or both). However, it is well-known that when histones are released, they can cause tissue injury. It is therefore possible, if not probable, that in the present series of experiments the increase in histone level accelerated the pathologic state, eg, by accelerating the development of CC. An anti-histone antibody is available3,12,57,60,61 and so this could be administered to prevent an increase in histones or reverse it.
In summary, although the numbers in some of the groups were small, and there were several variables, we suggest that the following conclusions can reasonably be drawn. The present retrospective study demonstrated that significant inflammation could be identified by the measurement of serum histones when baboons were developing an infection or CC. Whether an increase in histones is simply a marker of inflammation or whether histones actively increase inflammation in the pig-to-baboon model requires further investigation. There was a correlation between high histone levels and CC. Similarly, CRP levels tended to be higher and fT3 levels lower when CC developed. Our limited monitoring of SAA showed similar results to those of CRP. Therefore, extracellular histones along with other inflammatory markers (eg, CRP, fT3, SAA) could serve as useful biomarkers, and might allow improved management of primates with pig organ grafts.
Acknowledgments
We thank Dr. Keith Reimann for providing anti-CD40mAb from the NHP Reagent Resource (contract HHSN2722001300031C). Work on xenotransplantation in the Thomas E. Starzl Transplantation Institute of the University of Pittsburgh is, or has been, supported in part by NIH grants #U19 AI090959, #U01 AI068642, and # R21 A1074844, and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Inc., Blacksburg, VA. The baboons used in the study were from the Oklahoma University Health Sciences Center, Division of Animal Resources, which is supported by NIH P40 sponsored grant RR012317-09.
ABBREVIATIONS
- CC
consumptive coagulopathy
- CRP
C-reactive protein
- DAMP
danger/damage-associated molecular pattern
- Gal
galactose-α1,3-galactose
- GTKO
α1,3-galactosyltransferase gene-knockout
- NET
neutrophil extracellular trap
- SAA
serum amyloid A
- fT3
free triiodothyronine
Footnotes
AUTHORS’ CONTRIBUTION:
The study was designed and initiated by TL, WL, YW, HI, CTE and DKCC. Genetically-engineered pigs were provided by DA. The transplants were carried out by TL, HH (Hidetaka Hara), ME, HI, and DKCC. The laboratory assays were conducted by TL, WL, HH (Hidetaka Hara), CL, HH (Hai Huang), and HI. Data were collected and analyzed by TL, WL, HH (Hidetaka Hara), ME, HH (Hai Huang), YW, HI, CTE and DKCC. The manuscript was prepared by TL, WL, HH (Hidetaka Hara), ME, YW, HI, CTE and DKCC, and revised and approved by all authors.
DISCLOSURE OF CONFLICT OF INTEREST
David Ayares is an employee of Revivicor, Inc. No other author has a conflict of interest.
References
- 1.Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 2.Campos EI, Reinberg D. Histones: annotating chromatin. Annu Rev Genet. 2009;43:559–599. doi: 10.1146/annurev.genet.032608.103928. [DOI] [PubMed] [Google Scholar]
- 3.Chen R, Kang R, Fan XG, Tang D. Release and activity of histone in diseases. Cell Death Dis. 2014;5:e1370. doi: 10.1038/cddis.2014.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 5.von Kockritz-Blickwede M, Goldmann O, Thulin P, et al. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood. 2008;111:3070–3080. doi: 10.1182/blood-2007-07-104018. [DOI] [PubMed] [Google Scholar]
- 6.Xu J, Lupu F, Esmon CT. Inflammation, innate immunity and blood coagulation. Hamostaseologie. 2010;30:5–6. 8–9. [PubMed] [Google Scholar]
- 7.Huang H, Chen HW, Evankovich J, et al. Histones activate the NLRP3 inflammasome in Kupffer cells during sterile inflammatory liver injury. J Immunol. 2013;191:2665–2679. doi: 10.4049/jimmunol.1202733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esmon CT. Molecular circuits in thrombosis and inflammation. Thromb Haemost. 2013;109:416–420. doi: 10.1160/TH12-08-0634. [DOI] [PubMed] [Google Scholar]
- 9.Ammollo CT, Semeraro F, Xu J, Esmon NL, Esmon CT. Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J Thromb Haemost. 2011;9:1795–1803. doi: 10.1111/j.1538-7836.2011.04422.x. [DOI] [PubMed] [Google Scholar]
- 10.Semeraro F, Ammollo CT, Esmon NL, Esmon CT. Histones induce phosphatidylserine exposure and a procoagulant phenotype in human red blood cells. J Thromb Haemost. 2014;12:1697–1702. doi: 10.1111/jth.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esmon CT, Xu J, Lupu F. Innate immunity and coagulation. J Thromb Haemost. 2011;9(Suppl 1):182–188. doi: 10.1111/j.1538-7836.2011.04323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu J, Zhang X, Pelayo R, et al. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu J, Zhang X, Monestier M, Esmon NL, Esmon CT. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J Immunol. 2011;187:2626–2631. doi: 10.4049/jimmunol.1003930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allam R, Kumar SV, Darisipudi MN, Anders HJ. Extracellular histones in tissue injury and inflammation. J Mol Med (Berl) 2014;92:465–472. doi: 10.1007/s00109-014-1148-z. [DOI] [PubMed] [Google Scholar]
- 15.Semeraro F, Ammollo CT, Morrissey JH, et al. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood. 2011;118:1952–1961. doi: 10.1182/blood-2011-03-343061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuchs TA, Bhandari AA, Wagner DD. Histones induce rapid and profound thrombocytopenia in mice. Blood. 2011;118:3708–3714. doi: 10.1182/blood-2011-01-332676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Etulain J, Martinod K, Wong SL, Cifuni SM, Schattner M, Wagner DD. P-selectin promotes neutrophil extracellular trap formation in mice. Blood. 2015;126:242–246. doi: 10.1182/blood-2015-01-624023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang X, Li L, Liu J, Lv B, Chen F. Extracellular histones induce tissue factor expression in vascular endothelial cells via TLR and activation of NF-kappaB and AP-1. Thromb Res. 2016;137:211–218. doi: 10.1016/j.thromres.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Ward CM, Tetaz TJ, Andrews RK, Berndt MC. Binding of the von Willebrand factor A1 domain to histone. Thromb Res. 1997;86:469–477. doi: 10.1016/s0049-3848(97)00096-0. [DOI] [PubMed] [Google Scholar]
- 20.Brill A, Fuchs TA, Savchenko AS, et al. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. 2012;10:136–144. doi: 10.1111/j.1538-7836.2011.04544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abrams ST, Zhang N, Manson J, et al. Circulating histones are mediators of trauma-associated lung injury. Am J Respir Crit Care Med. 2013;187:160–169. doi: 10.1164/rccm.201206-1037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang H, Evankovich J, Yan W, et al. Endogenous histones function as alarmins in sterile inflammatory liver injury through Toll-like receptor 9 in mice. Hepatology. 2011;54:999–1008. doi: 10.1002/hep.24501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Meyer SF, Suidan GL, Fuchs TA, Monestier M, Wagner DD. Extracellular chromatin is an important mediator of ischemic stroke in mice. Arterioscler Thromb Vasc Biol. 2012;32:1884–1891. doi: 10.1161/ATVBAHA.112.250993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang R, Zhang Q, Hou W, et al. Intracellular Hmgb1 inhibits inflammatory nucleosome release and limits acute pancreatitis in mice. Gastroenterology. 2014;146:1097–1107. doi: 10.1053/j.gastro.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper DK, Ekser B, Ramsoondar J, Phelps C, Ayares D. The role of genetically-engineered pigs in xenotransplantation research. J Pathol. 2016;238:288–299. doi: 10.1002/path.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwase H, Ekser B, Zhou H, et al. Further evidence for a sustained systemic inflammatory response in xenograft recipients (SIXR) Xenotransplantation. 2015;22:399–405. doi: 10.1111/xen.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ezzelarab MB, Cooper DK. Systemic inflammation in xenograft recipients (SIXR): A new paradigm in pig-to-primate xenotransplantation? Int J Surg. 2015;23:301–305. doi: 10.1016/j.ijsu.2015.07.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ezzelarab MB, Ekser B, Azimzadeh A, et al. Systemic inflammation in xenograft recipients precedes activation of coagulation. Xenotransplantation. 2015;22:32–47. doi: 10.1111/xen.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwase H, Ekser B, Zhou H, Dons EM, Cooper DK, Ezzelarab MB. Platelet aggregation in humans and nonhuman primates: relevance to xenotransplantation. Xenotransplantation. 2012;19:233–243. doi: 10.1111/j.1399-3089.2012.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwase H, Ekser B, Satyananda V, et al. Pig-to-baboon heterotopic heart transplantation--exploratory preliminary experience with pigs transgenic for human thrombomodulin and comparison of three costimulation blockade-based regimens. Xenotransplantation. 2015;22:211–220. doi: 10.1111/xen.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwase H, Liu H, Wijkstrom M, et al. Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation. 2015;22:302–309. doi: 10.1111/xen.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ekser B, Long C, Echeverri GJ, et al. Impact of thrombocytopenia on survival of baboons with genetically modified pig liver transplants: clinical relevance. Am J Transplant. 2010;10:273–285. doi: 10.1111/j.1600-6143.2009.02945.x. [DOI] [PubMed] [Google Scholar]
- 33.Ezzelarab MB, Ekser B, Echeverri G, et al. Costimulation blockade in pig artery patch xenotransplantation - a simple model to monitor the adaptive immune response in nonhuman primates. Xenotransplantation. 2012;19:221–232. doi: 10.1111/j.1399-3089.2012.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iwase H, Satyananda V, Zhou H, et al. Initial in vivo experience of pig artery patch transplantation in baboons using mutant MHC (CIITA-DN) pigs. Transpl Immunol. 2015;32:99–108. doi: 10.1016/j.trim.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lowe M, Badell IR, Thompson P, et al. A novel monoclonal antibody to CD40 prolongs islet allograft survival. Am J Transplant. 2012;12:2079–2087. doi: 10.1111/j.1600-6143.2012.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ezzelarab M, Garcia B, Azimzadeh A, et al. The innate immune response and activation of coagulation in alpha1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 2009;87:805–812. doi: 10.1097/TP.0b013e318199c34f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kutcher ME, Xu J, Vilardi RF, Ho C, Esmon CT, Cohen MJ. Extracellular histone release in response to traumatic injury: implications for a compensatory role of activated protein C. J Trauma Acute Care Surg. 2012;73:1389–1394. doi: 10.1097/TA.0b013e318270d595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hara H, Long C, Lin YJ, et al. In vitro investigation of pig cells for resistance to human antibody-mediated rejection. Transpl Int. 2008;21:1163–1174. doi: 10.1111/j.1432-2277.2008.00736.x. [DOI] [PubMed] [Google Scholar]
- 39.Iwase H, Ekser B, Hara H, et al. Regulation of human platelet aggregation by genetically modified pig endothelial cells and thrombin inhibition. Xenotransplantation. 2014;21:72–83. doi: 10.1111/xen.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujita M, Mehra R, Lee SE, et al. Comparison of proliferative capacity of genetically-engineered pig and human corneal endothelial cells. Ophthalmic Res. 2013;49:127–138. doi: 10.1159/000342978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liaw PC, Ito T, Iba T, Thachil J, Zeerleder S. DAMP and DIC: The role of extracellular DNA and DNA-binding proteins in the pathogenesis of DIC. Blood Rev. 2016;30:257–261. doi: 10.1016/j.blre.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev. 2012;249:158–175. doi: 10.1111/j.1600-065X.2012.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esmon CT. Extracellular histones zap platelets. Blood. 2011;118:3456–3457. doi: 10.1182/blood-2011-07-364380. [DOI] [PubMed] [Google Scholar]
- 44.Kawai C, Kotani H, Miyao M, et al. Circulating Extracellular Histones Are Clinically Relevant Mediators of Multiple Organ Injury. Am J Pathol. 2016;186:829–843. doi: 10.1016/j.ajpath.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 45.Zeerleder S, Stephan F, Emonts M, et al. Circulating nucleosomes and severity of illness in children suffering from meningococcal sepsis treated with protein C. Crit Care Med. 2012;40:3224–3229. doi: 10.1097/CCM.0b013e318265695f. [DOI] [PubMed] [Google Scholar]
- 46.Ogata A, Hirano T, Hishitani Y, Tanaka T. Safety and efficacy of tocilizumab for the treatment of rheumatoid arthritis. Clin Med Insights Arthritis Musculoskelet Disord. 2012;5:27–42. doi: 10.4137/CMAMD.S7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanaka T, Narazaki M, Kishimoto T. Anti-interleukin-6 receptor antibody, tocilizumab, for the treatment of autoimmune diseases. FEBS Lett. 2011;585:3699–3709. doi: 10.1016/j.febslet.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 48.Kim JE, Lee N, Gu JY, Yoo HJ, Kim HK. Circulating levels of DNA-histone complex and dsDNA are independent prognostic factors of disseminated intravascular coagulation. Thromb Res. 2015;135:1064–1069. doi: 10.1016/j.thromres.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 49.Jensen LE, Whitehead AS. Regulation of serum amyloid A protein expression during the acute-phase response. Biochem J. 1998;334(Pt 3):489–503. doi: 10.1042/bj3340489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uhlar CM, Whitehead AS. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem. 1999;265:501–523. doi: 10.1046/j.1432-1327.1999.00657.x. [DOI] [PubMed] [Google Scholar]
- 51.Cicarelli DD, Vieira JE, Bensenor FE. Comparison of C-reactive protein and serum amyloid a protein in septic shock patients. Mediators Inflamm. 2008;2008:631414. doi: 10.1155/2008/631414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Falsey AR, Walsh EE, Francis CW, et al. Response of C-reactive protein and serum amyloid A to influenza A infection in older adults. J Infect Dis. 2001;183:995–999. doi: 10.1086/319275. [DOI] [PubMed] [Google Scholar]
- 53.Hartmann A, Eide TC, Fauchald P, et al. Serum amyloid A protein is a clinically useful indicator of acute renal allograft rejection. Nephrol Dial Transplant. 1997;12:161–166. doi: 10.1093/ndt/12.1.161. [DOI] [PubMed] [Google Scholar]
- 54.Feussner G, Stech C, Dobmeyer J, Schaefer H, Otto G, Ziegler R. Serum amyloid A protein (SAA): a marker for liver allograft rejection in humans. Clin Investig. 1994;72:1007–1011. doi: 10.1007/BF00577745. [DOI] [PubMed] [Google Scholar]
- 55.Muller TF, Vogl M, Neumann MC, Lange H, Grimm M, Muller MM. Noninvasive monitoring using serum amyloid A and serum neopterin in cardiac transplantation. Clin Chim Acta. 1998;276:63–74. doi: 10.1016/s0009-8981(98)00100-4. [DOI] [PubMed] [Google Scholar]
- 56.Walker AH, Keevil BG, Yonan N. An investigation of the correlation between C-reactive protein, serum amyloid a concentration, and cardiac allograft rejection. Transplant Proc. 2002;34:1279–1280. doi: 10.1016/s0041-1345(02)02776-8. [DOI] [PubMed] [Google Scholar]
- 57.Allam R, Scherbaum CR, Darisipudi MN, et al. Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4. J Am Soc Nephrol. 2012;23:1375–1388. doi: 10.1681/ASN.2011111077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanz AB, Sanchez-Nino MD, Ramos AM, et al. NF-kappaB in renal inflammation. J Am Soc Nephrol. 2010;21:1254–1262. doi: 10.1681/ASN.2010020218. [DOI] [PubMed] [Google Scholar]
- 59.Malaver E, Romaniuk MA, D’Atri LP, et al. NF-kappaB inhibitors impair platelet activation responses. J Thromb Haemost. 2009;7:1333–1343. doi: 10.1111/j.1538-7836.2009.03492.x. [DOI] [PubMed] [Google Scholar]
- 60.Monestier M, Fasy TM, Losman MJ, Novick KE, Muller S. Structure and binding properties of monoclonal antibodies to core histones from autoimmune mice. Mol Immunol. 1993;30:1069–1075. doi: 10.1016/0161-5890(93)90153-3. [DOI] [PubMed] [Google Scholar]
- 61.Kusano T, Chiang KC, Inomata M, et al. A novel anti-histone H1 monoclonal antibody, SSV monoclonal antibody, improves lung injury and survival in a mouse model of lipopolysaccharide-induced sepsis-like syndrome. Biomed Res Int. 2015;2015:491649. doi: 10.1155/2015/491649. [DOI] [PMC free article] [PubMed] [Google Scholar]


