Abstract
There has been a longstanding interest in understanding whether the presence of inhomogeneity in myocardial sympathetic innervation can predict patients at risk of sudden cardiac arrest from lethal ventricular arrhythmias. The advent of radiolabeled norepinephrine analogs has allowed this to be imaged in patients with ischemic and non-ischemic cardiomyopathy using single, photon emission computed tomography (SPECT) and positron emission tomography (PET). Several observational studies have demonstrated that globally elevated myocardial sympathetic tone (as reflected by reduced myocardial norepinephrine analog uptake) can predict composite cardiac end-points including total cardiovascular mortality. More recent studies have indicated that quantifying the extent of regional denervation can predict the risk of lethal ventricular arrhythmias and sudden cardiac death. This review will summarize our current understanding of the prognostic significance of altered myocardial sympathetic innervation.
Keywords: Myocardial sympathetic innervation, 131I-meta-iodo-benzyguanidine, 11C-hydroxyephedrine, Sudden cardiac death, Regional denervation, Myocardial infarction, Hibernating myocardium
Introduction
Alterations in cardiac sympathetic activity have been linked to disease progression and increased mortality in cardiovascular disease [1••]. The clinical manifestations of cardiac sympathetic dysfunction are frequently subtle and may manifest as alterations in resting heart rate, heart rate variability, or impairment of cardiac autonomic reflexes such as blunting of the chronotropic response to changes in systemic hemodynamics. For the past several decades, compelling preclinical data has accumulated demonstrating a link between cardiac sympathetic nerve activity and ventricular arrhythmias leading to sudden cardiac death (SCD) [2]. This review will focus on imaging myocardial sympathetic innervation in patients with ischemic cardiomyopathy and review studies suggesting that it may help to better identify patients at risk for SCD.
Left Ventricular Dysfunction and Sudden Cardiac Death
Advances in coronary revascularization and the advent of highly potent pharmacological agents for the treatment of acute coronary syndromes have improved overall survival but increased the prevalence ischemic cardiomyopathy and heart failure as consequences of coronary artery disease. The development of ischemic cardiomyopathy is accompanied by dynamic myocyte and neuronal remodeling that exacerbates the risk for SCD from lethal ventricular arrhythmias. For example, the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II) found patients with ischemic cardiomyopathy and reduced left ventricular ejection fraction (LVEF; <30 %) to have an all-cause mortality approaching 20 % during an average follow-up period of 20 months [3]. Prophylactic insertion of an implantable cardiac defibrillator (ICD) reduced absolute risk by 5.6 %. Similar findings were demonstrated in the Sudden Cardiac Death in Heart Failure (SCD-HeFT) trial which also found no effect of prophylactic antiarrhythmic therapy with amiodarone on survival [4]. Although the ACC/AHA offers a class I recommendation for prophylactic ICD placement for the primary prevention of SCD in patients with ischemic cardiomyopathy and reduced EF, identification of those most likely to require the device remains challenging. Our current strategy continues to rely primarily on estimation of LVEF as obtained from angiography, echocardiography, nuclear imaging or cardiac MRI. Of patients who received a primary prevention ICD, appropriate ICD therapies occur in only 25 % of patients after 5 years [4]. Moreover, device therapies for ventricular tachycardia (VT) overestimate the incidence of aborted sudden cardiac arrest, with conventional programming strategies (e.g., short durations between detection and treatment, use of anti-tachycardia pacing, and treatment of a wide range of VT rates). Many therapies have subsequently been found to treat self-terminating ventricular arrhythmias that do not lead to SCD [5]. Thus, while prophylactic ICD therapy saves lives, there continues to be a need to develop better risk stratification approaches that can identify those within the primary prevention ICD population (i.e., with an EF ≤35 %) at highest risk of arrhythmic death. An equally important unmet need reflects the sobering fact that we currently have no way to prospectively identify those at risk of arrhythmic death with ejection fractions greater than 35 %. While the annual rate of SCD in this population is lower, they constitute the vast majority of patients that will ultimately succumb to arrhythmic death [6].
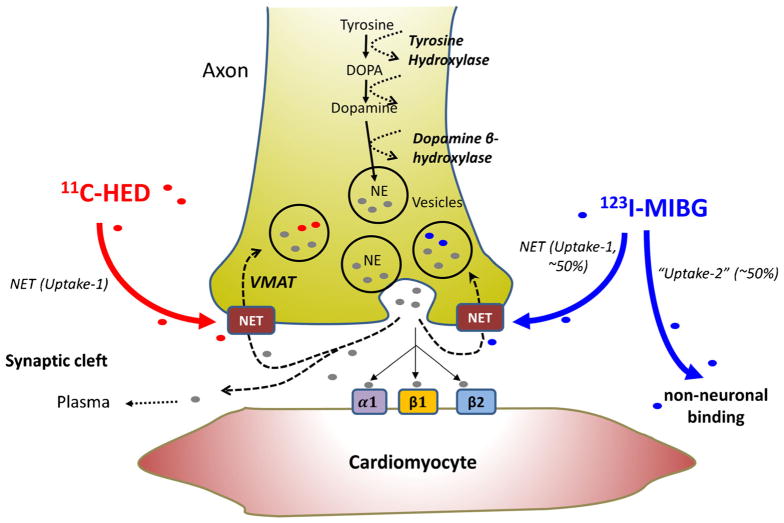
Radiotracer-Based Imaging of the Cardiac Sympathetic Nervous System—Factors Determining Alterations in Myocardial Tracer Activity
Advances in cardiac molecular imaging have paved the way for the emergence of radiotracers capable of characterizing myocardial sympathetic innervation in vivo. The utility and accurate interpretation of these radiotracers are based on the understanding of the biologic fate of the neurotransmitter, norepinephrine. Sympathetic projections from the central nervous system synapse primarily in the stellate ganglia, and post-ganglionic fibers travel in parallel with the epicardial coronary arteries to innervate the epicardium and subsequently, homogeneously distribute to the inner layers of the heart. Sympathetic activation results in the release of norepinephrine into the synaptic cleft to bind to β-adrenoreceptors located on the cardiomyocyte membrane. Sympathetic activity is terminated by the reuptake of norepinephrine from the synaptic cleft to the neuronal varicosities via norepinephrine transporter (NET) or ‘Uptake-1’ and undergoes degradation or repackaging. Although several other targets for sympathetic nerve imaging have been investigated, the bulk of the clinical data on radiotracer-based imaging is based on the evaluation of NET reuptake activity using norepinephrine analogs (Fig. 1). For conventional nuclear imaging, 123I-meta-iodobenzylguanidine (123I-MIBG) is the principal tracer. Using PET imaging, the most common norepinephrine analog used is 11C-meta-hydroxyephedrine (11C-HED). The myocardial retention of 11C-HED and 123I-MIBG are both dependent on the NET reuptake mechanism, as evidenced by reduced retention with NET blockade. Both radioligands compete with norepinephrine released from presynaptic sympathetic nerves as well as circulating norepinephrine levels in blood. Because of this, myocardial radioligand uptake is functionally reduced when there is elevated global myocardial sympathetic tone as well as elevated circulating norepinephrine levels as in heart failure. Retention of 123I-MIBG and 11C-HED in the myocardium can also be reduced by inhibiting the NET pharmacologically (e.g. using alpha-2 agonists), chemical sympathectomy using the topical application of phenol on the cardiac surface, or surgical denervation immediately following cardiac transplantation. Cardiac re-innervation after global denervation occurs slowly and remains relatively minor many years after cardiac transplantation [7].
Fig. 1.
Cardiac sympathetic nerve transmission and tracer uptake. Schematics of sympathetic transmission and neuronal uptake of 11C-HED and 123I-MIBG for imaging of sympathetic neuronal innervation. Norepinephrine (NE) is synthesized and packaged in the nerve terminals and released into the synaptic cleft. Released NE binds to target receptors or is recycled to the sympathetic nerve terminal via norepinephrine transporter (NET) for repackaging or degradation. 11C-HED and 123I-MIBG radiotracers are also incorporated into the nerve terminals using NET-dependent mechanism. VMAT vesicular monoamine transporter, DOPA 3,4-dihydroxyphenylalanine
While both MIBG and HED can assess myocardial sympathetic nerve function, there are differences that are important to consider. First, the target to background ratio can diminish due to increased competition from myocardial norepinephrine release as heart failure severity advances making it difficult to characterize regional 123I-MIBG uptake with conventional SPECT. Thus, most clinical studies employing 123I-MIBG are based on planar imaging of global myocardial uptake or washout compared with activity in a reference mediastinal region of interest. While the target to background activity of 11C-HED is more favorable and allows attenuation corrected images as well as kinetic analyses to be performed, it requires an on-site cyclotron for synthesis and is not available for widespread clinical distribution. Finally, animal studies suggest that nonneuronal uptake can vary between tracers [8, 9]. When compared with 123I-MIBG, 11C-HED appears to have significantly less extraneuronal uptake and it is resistant to degradation by monoamine oxidase and catecholamine-O-methyltransferase. These differences may favor the detection of regional variations in myocardial sympathetic innervation using 11C-HED.
Regional Myocardial Sympathetic Denervation from Reversible and Irreversible Ischemia
Early work in canine models demonstrated that sympathetic denervation occurred following transmural myocardial infarction. Denervation occurs in irreversibly injured myocardium, the entire myocardial region at risk of ischemia, and normal myocardium that is apical from an infarcted region [10]. Similar changes have been demonstrated in humans with myocardial infarction where the volume of denervated myocardium exceeds infarcted myocardium [11–15]. Viable but denervated myocardium, exhibits a ‘hypersensitivity response’ to norepinephrine and isoproterenol infusion with accentuated shortening of the effective refractory period [16]. In addition, norepinephrine infusion enhances induction of ventricular arrhythmias during programmed ventricular stimulation. This has led to the hypothesis that inhomogeneity in sympathetic innervation and/or denervation supersensitivity creates a substrate favorable to arrhythmogenesis by increasing spatial dispersion of action potential duration during sympathetic activation.
In addition to myocardial infarction, inhomogeneity in myocardial sympathetic innervation has also been demonstrated in chronic coronary artery disease and animal models of chronic reversible ischemia that results in hibernating myocardium [8, 9, 12, 13, 17–24]. These changes are accompanied by an increased risk of SCD from VT/VF in the absence of severely depressed global function as well as acute or chronic infarction [25–27]. Myocardial uptake of 123I-MIBG was reduced in swine with hibernating myocardium with the greatest reduction seen in the subendocardium [8], coinciding with the region with the most severe limitation in coronary flow reserve. PET imaging using 11C-HED demonstrated even larger reductions in 11C-HED uptake [9]. These imaging findings were supported by ex vivo western analyses of sympathetic markers and physiologic studies [28]. Thus, both reversible (acute and chronic ischemia) and irreversible ischemia (infarction) can lead to regional inhomogeneity in myocardial sympathetic innervation. These changes reflect the fact that myocardial sympathetic nerves are exquisitely sensitive to ischemia [29].
Sympathetic re-innervation and nerve sprouting may also contribute to inhomogeneity in myocardial sympathetic innervation and lethal ventricular arrhythmias [30]. Following infarction, there is increased cardiac expression of nerve growth factor which is critical for sympathetic nerve survival. Likewise, growth-associated protein-43 (GAP43), a marker of neuronal growth and nerve sprouting, increases at the border zone between normal and infarcted tissue [31]. Similar changes are seen in chronic reversible ischemia associated with hibernating myocardium [32]. These areas of neural remodeling and hyperinnervation may further contribute to inhomogeneity leading to lethal ventricular arrhythmias and SCD.
Clinical Evidence of Impaired Myocardial Sympathetic Innervation in Left Ventricular Dysfunction
Myocardial sympathetic denervation is prevalent among patients with ischemic heart disease. In a small study of patients with known coronary artery disease (CAD), 91 % of the patients had some degree of reduced myocardial uptake on 123I-MIBG SPECT, and this could occur in the absence of a prior history of a myocardial infarction (MI) [21]. The magnitude of reduced 123I-MIBG uptake was found to be related to stenosis severity. Patients with a prior myocardial infarction had greater reductions in 123I-MIBG uptake than those without infarction (16 vs. 7 % of LV mass). The benefit of cardiac neurohormonal modulation was demonstrated by large randomized trials studying the use of beta-blockers in heart failure [33–35]. Survival benefit was attributed to preventing heart failure progression as well as reducing sudden cardiac death and was hypothesized to be due to the suppression of myocardial sympathetic activity. This was confirmed by subsequent in vivo radionuclide studies evaluating the effects of beta-blockers on global 123I-MIBG uptake. Milliano et al. evaluated the effect of metoprolol therapy on cardiac sympathetic innervation in 59 patients with heart failure [36]. As compared with baseline imaging, myocardial 123I-MIBG uptake increased by approximately 22 % in patients receiving metoprolol for 6 months while it decreased by 7.8 % in placebo controls. The increase in 123I-MIBG uptake with metoprolol-paralleled reverse left ventricular (LV) remodeling with a reduction in LV end-diastolic diameter and an increase in the LVEF. Regional improvement in cardiac sympathetic innervation was reported in a randomized trial of carvedilol [37]. In this study, defect size on 123I-MIBG SPECT decreased among patients on carvedilol (20 to 15 %, p=0.03) with no change in placebo controls (22 vs. 21 %, p=NS). Thus, these imaging studies indicate that the myocardial uptake of norepinephrine analogs is favorably increased in response to blocking neurohormonal activation with beta-blockers in patients with heart failure. This most likely is an indirect action and secondary to reduced myocardial sympathetic nerve activity resulting from improvements in left ventricular function and functional class. Nevertheless, the studies indicate that the pharmacological treatment of heart failure can modulate the uptake of norepinephrine analogs.
Myocardial Sympathetic Denervation Predicts Clinical Outcomes
A logical extension of these data is determining whether sympathetic nerve imaging can predict cardiovascular events and impact prognosis. Merlet et al. followed 90 patients with cardiomyopathy (mixed ischemic and non-ischemic etiology) for up to 27 months and found that the heart to mediastinum ratio (HMR) of 123I-MIBG uptake was the most potent predictor of survival [38]. These findings were subsequently expanded to show that the 123I-MIBG HMR was the most important clinical predictor of cardiac mortality after 54 months of follow-up [39]. This predictive capacity was independent of the etiology of left ventricular dysfunction and was even prognostic among those with only mildly reduced EF (40–50 %) [39].
The largest prospective study evaluating global 123I-MIBG uptake and clinical outcomes was the AdreView Myocardial Imaging for Risk Evaluation in Heart Failure study (ADMIRE-HF). In this study, planar 123I-MIBG and SPECT perfusion imaging were performed in 961 patients with NYHA class II or III heart failure (HF) and LVEF ≤35 % [40]. The majority of patients (66 %) had ischemic cardiomyopathy. Over a median follow-up of 17 months, the primary composite end-point (heart failure progression, arrhythmic events and cardiac death) occurred more frequently among those with a global reduction in sympathetic innervation (prospectively defined as a late HMR <1.6). Although the frequency of arrhythmic events was significantly higher among those with a HMR <1.6, the vast majority were non-sustained VT. SCD, resuscitated sudden cardiac arrest, and appropriate ICD discharges (shock or anti-tachycardia pacing) were a small portion of the total composite end-points (21 %) [40]. Quantification of regional defects was attempted in a subgroup of patients but did not provide any additional value to global indices of 123I-MIBG uptake in predicting prognosis [40].
A sub-analysis of the ADMIRE-HF study explored whether 123I-MIBG HMR provided any improvement in risk stratification over LVEF. The ADMIRE-HF LVEF values reported by the core laboratory (some core LVEF measurements were >35 %) were stratified by a late HMR of 1.6, and the combined ADMIRE-HF end-points were estimated in each group [41]. A late HMR of <1.6 conferred, a greater risk of death and arrhythmic events across all LVEF subgroups. Interestingly, among subjects with an LVEF >40 %, a late HMR >1.6 was not associated with any risk of death or an arrhythmic event over the follow-up period. In contrast, individuals with an LVEF >40 % and a late HMR <1.6 had a 7.5 %/100 person-years risk of death and arrhythmic events. While this was a post-hoc analysis, the observations raise the possibility that assessing global cardiac sympathetic innervation may ultimately aid in identifying individuals at an increased risk of arrhythmic death who would otherwise be categorized as low risk based upon relatively preserved LV function.
In order to determine the most prognostic end-point associated with altered global 123I-MIBG uptake, Verschure et al. recently performed a patient level meta-analysis of 636 heart failure patients [42••]. Over a mean follow-up of 37 months, HMR was able to predict all categories of cardiac events, with the strongest predictive value for composite cardiac events, all-cause mortality, and cardiac mortality. Interestingly, the weakest univariate predictor of 123I-MIBG HMR was for arrhythmic events. In multivariate analysis, only NYHA heart failure class and age were independently associated with arrhythmias.
In contrast to these results, Tamaki et al. showed that an alternate assessment of 123I-MIBG can predict SCD [43]. Among 106 patients with an EF <40 % followed up for an average of 65 months, three measures of 123I-MIBG (early HMR (at 20 min), delayed HMR (at 200 min) and mIBG washout ratio (WR; between 20 and 200 min after injection)] predicted SCD. However, by multivariate analysis, only WR and EF remained independent predictors. In a separate multivariate analyses, WR was the only 123I-MIBG parameter that was an independent predictor of pump failure death and total cardiac mortality. It should be noted that there were several exclusion criteria for this study, most notably the use of beta-blockers at the time of imaging. Thus, the generalizability of these results to the contemporary management of patients on optimal medical therapy for heart failure at the time of imaging is uncertain.
Incremental Prognostic Value of Regional Sympathetic Denervation in Ischemic Cardiomyopathy
Bax et al. provided one of the first clinical studies exploring the relation between regional sympathetic denervation and arrhythmic events in a phase 2 study of cardiac 123I-MIBG imaging [44]. Fifty patients with ischemic cardiomyopathy and an LVEF ≤40 % referred for electrophysiological (EP) study for unexplained syncope or non-sustained VT underwent planar and SPECT 123I-MIBG imaging. Early and late HMR were determined from the planar images, while early and late 123I-MIBG summed scores (reflecting regional defect size) were obtained from the SPECT data. Thirty patients had a positive EP study and demonstrated significantly greater late 123I-MIBG SPECT summed score compared with patients with a negative EP study. There were no differences in the planar early or late HMR between those with and without a positive EP study. All patients had ischemic cardiomyopathy, but infarct sizes (Technetium-99 m SPECT) were not different between the two groups. The only independent predictor of a positive EP study (sustained VT) was the late 123I-MIBG SPECT summed score. This supports the potential of regional sympathetic denervation to predict arrhythmic events. Similar to reports from animal studies [45], the burden of sympathetic denervation on SPECT was much larger than the SPECT perfusion defect. Nevertheless, the size of the denervation-perfusion mismatch did not predict a positive EP study. A recently published reanalysis of the images from these patients focused on assessing 123I-MIBG uptake in the scar and border zone (40–60 % of normal perfusion) [46]. With this approach, 123I-MIBG uptake in the border zone was shown to predict inducibility of sustained VT at EP study.
These studies were subsequently expanded to determine whether the association of regional denervation and inducible ventricular arrhythmia among patients with ischemic and non-ischemic cardiomyopathy correlated with ICD therapies. One hundred and sixteen patients on optimal medical therapy after receiving any clinically indicated re-vascularization therapy underwent planar and SPECT 123I-MIBG imaging and SPECT Tc 99 m perfusion imaging [47]. Over an average follow-up period of 23 months, appropriate ICD therapy (shock or anti-tachycardia pacing) occurred in 21 % patients. ICD implantation for secondary prevention (vs. primary prevention) and late 123I-MIBG SPECT defect size were the only independent predictors of ICD therapy and cardiac death. The authors found that ICD therapies were more prevalent (40 vs. 3 %) among those with large 123I-MIBG defects (summed score >26) when compared with those with smaller defects. There was no relationship between the presence or size of denervation-perfusion mismatch and ventricular arrhythmias.
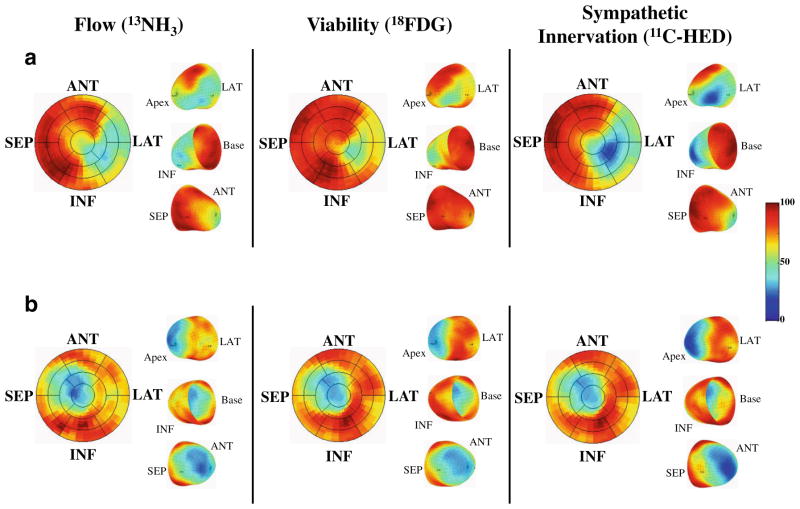
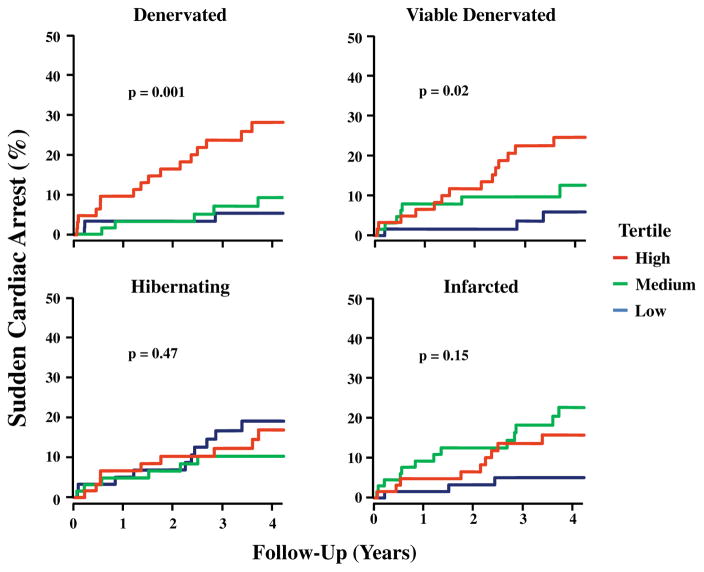
Recently, PET imaging with 11C-HED has been employed to quantify the extent of sympathetic denervation with cause specific mortality for sudden cardiac arrest [48]. The Prediction of Arrhythmic Events with Positron Emission Tomography (PAREPET) study specifically evaluated the occurrence of sudden cardiac arrest (SCA) among patients with ischemic cardiomyopathy (EF≤35 %) in relation to the volume of denervated myocardium using PET [49••]. The study enrolled 204 patients, who underwent PET assessment (Fig. 2) of myocardial perfusion (13NH3—ammonia retention), myocardial denervation (11C-meta-hydroxyephedrine (11C-HED)) and viability (18F-2-deoxyglucose (18FDG)). Thirty-three patients experienced SCA which included arrhythmic death (SCD) or ICD discharge for aborted arrhythmic death (ventricular fibrillation or VT >240 beats/min). Other device therapies including treatments for VT at rates <240 were not considered SCA surrogates. Patients developing SCA had a significantly greater volume of denervated myocardium and viable-denervated myocardium when compared with those without SCA, and Kaplan-Meier survival analysis demonstrated that both denervated and viable-denervated myocardium predicted time to SCA. Every 1 % increase in the volume of the denervated myocardium was associated with a 5.7 % increase in the risk of SCA and patients in the highest tertile of denervation had an SCA rate of 6.7 % per year (Fig. 3). In a multivariate analysis, the magnitude of denervated myocardium was the only independent predictor of SCA from PET imaging. Global 11C-HED retention fraction (analogous to the 123I-MIBG HMR) was not different among those with and without SCA. This study was the first to suggest a relationship between viable-denervated myocardium and lethal arrhythmic events. This parallels the preclinical observations in swine with hibernating myocardium [9, 26, 27]. Nevertheless, only the total volume of denervated myocardium remained a significant predictor in the multivariate analysis [44, 47]. Interestingly, scar volume assessed using 18FDG was not a multivariate predictor of arrhythmic death.
Fig. 2.
PAREPET imaging of myocardial flow, viability, and sympathetic innervation. a A subject who experienced SCA. There is a mismatch in infarct size (reduced 18F-2 deoxyglucose, 18FDG which was administered during a euglycemic-hyperinsulinemic clamp) which was smaller than the volume of sympathetic denervation (reduced 11C-HED). Within the region of viable but denervated myocardium (mismatch between reduced 11C-HED and preserved 18FDG), there was reduced perfusion (13N-ammonia (13NH3)) with preserved 18FDG indicating hibernating myocardium. In contrast, a subject with matched reductions in flow, infarct volume, and sympathetic denervation is shown in (b). (ANT anterior, INF inferior, LAT lateral, SEP septum). (Reprinted with permission from Elsevier [49••])
Fig. 3.
PAREPET imaging parameters and SCA. Kaplan-Meier curves show the incidence of SCA for tertiles of PET-defined myocardial substrates (median follow-up, 4.1 years). As continuous variables, the total volume of denervated myocardium as well as viable-denervated myocardium predicted SCA. Neither infarct volume nor hibernating myocardium was significant as continuous variables. (Reprinted with permission from Elsevier [49••])
Clinical Implications and Future Perspectives
The available data have consistently shown that imaging of cardiac sympathetic innervation provides independent prognostic information in patients with reduced LV systolic function. Recent meta-analyses have suggested that the strongest predictive potential of 123I-MIBG HMR is for all-cause cardiac mortality, as well as a composite including non-lethal clinical events [42••, 50••]. Our current medical approach to heart failure management recommends up-titration of medications as clinically tolerated and the clinical utility of incorporating 123I-MIBG imaging into managing heart failure patients remains unclear.
The potential clinical utility of imaging sympathetic innervation is much more obvious with regard to the end-point of preventing lethal arrhythmias and targeting ICDs for the primary prevention of sudden death. Unfortunately, the majority of studies have lumped all arrhythmic events and included those of dubious clinical impact as ‘appropriate ICD therapies.’ These not only over estimate SCA by nearly threefold [51, 52] but approaches to reduce ICD therapies for these nonmalignant ventricular arrhythmias actually improve survival [5]. Thus, although ‘appropriate ICD therapies’ is a convenient end-point, future studies would benefit from employing a more restrictive end-point that more closely approximates the observed benefit of ICD therapy as recently employed in the PAREPET trial [49••].
The available data also suggests that regional, rather than global, assessment of sympathetic innervation will be more accurate for predicting arrhythmic death and targeting ICD therapy. Although this assessment is possible with 123I-MIBG and conventional SPECT imaging (and potential improvements are likely with new imaging technologies), there will continue to be inherent limitations due to radionuclide imaging characteristics and photon attenuation. PET approaches therefore appear to hold more promise. Nevertheless, the preeminent PET sympathetic nerve tracer, HED, requires a local cyclotron and radiochemistry support. Thus, widespread clinical application will require a tracer incorporating a longer half-life isotope (e.g., 18F labeled LMI1195, Lantheus Medical Imaging) [53, 54], which could be regionally produced and then transported to individual PET centers for clinical use (as is currently used for oncologic imaging with 18F-2-deoxyglucose (FDG)).
Clinical assessment of the risk for SCA has two obvious clinical applications. The first would be to recommend additional therapy for those at elevated risk of SCA. At this point, the only specific therapy that could be realistically considered would be an ICD. Furthermore, expanding the clinical indication for ICDs would be challenging due to (a) the high cost of these devices and (b) the widespread recognition that ICDs are not efficiently used in those with current primary prevention indications (as previously noted, appropriate ICD therapies occur in only 25 % of patients after 5 years [4]). Alternatively, sympathetic imaging could be used to identify patients at low risk of SCA among those who are currently considered for a primary prevention ICD. For example, the PAREPET study enrolled subjects who were already eligible for an ICD. As illustrated in Fig. 4, the absence of all four independent risk factors was associated with a very low risk of SCA (<1 %/ year). This is a lower rate of SCA than in patients with coronary artery disease and mild LV dysfunction [55–57] who are not considered candidates for ICD therapy. Furthermore, this subgroup comprised over 44 % of the PAREPETcohort. Thus, with independent validation of these results and additional studies to determine the optimal time interval for retesting, it may become possible to safely delay implanting an ICD for low-risk patients who have a current clinical indication based solely on ejection fraction. A recent meta-analysis has also suggested that the addition of 123I-MIBG HMR was particularly helpful for the downward reclassification of a low-risk cohort [58••].
Fig. 4.
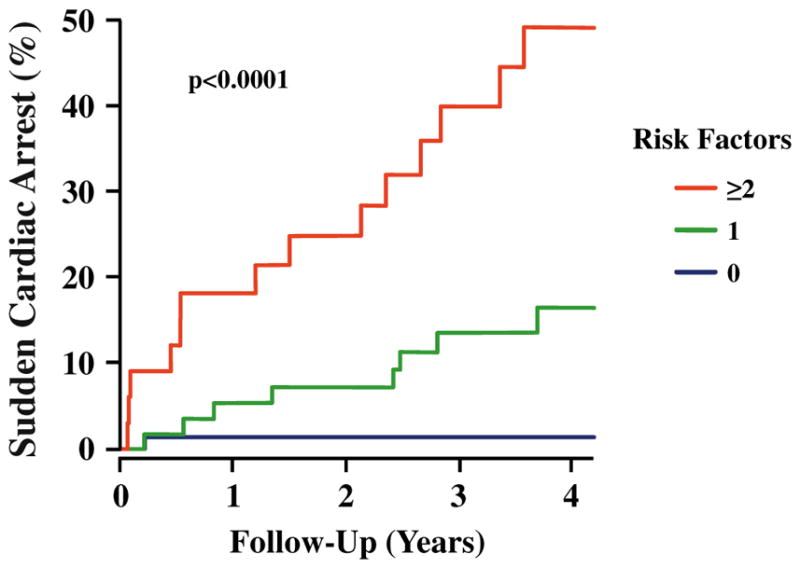
SCA risk factor model from PAREPET. Kaplan-Meier curves illustrating highly significant differences in the incidence of SCA in relation to the number of risk factors present (p<0.0001). Subjects with no risk factor (blue, 44 % of cohort) had an annual rate of SCA <1 %. With two or more risk factors (red, 20 % of cohort), the annual risk of SCA increased to ~12 %. Patients with one risk factor (green, 36 % of cohort) had an intermediate risk of SCA (~4 %/year). (Reprinted with permission from Elsevier [49••])
Conclusion
In conclusion, imaging cardiac sympathetic innervation provides prognostic information in patients with left ventricular dysfunction, and numerous studies have documented that this information is independent of routine clinical and demographic parameters. Nevertheless, the clinical translation of these findings to routine patient care remains unclear. Although there are clearly unanswered questions regarding optimal tracers and assessment methods, there appears to be sufficient preliminary data to move in the direction of pragmatic clinical trials which incorporate cardiac sympathetic imaging into algorithms with therapeutic implications. The design and funding of these studies will present considerable challenges to interested investigators, but they are critical if cardiac sympathetic imaging is going to enter the clinical armamentarium.
Acknowledgments
This study is supported by HL55324, HL61610, and the Albert and Elizabeth Rekate Fund. James A. Fallavollita has received grant support from NIH-NHLBI (HL-76252) and has pending grants from NHLBI (RO1) and Lantheus Medical Imaging. John M. Canty has received grant support from NHLBI and has pending grants from NHLBI and VA.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest Saurabh Malhotra and Stanley F. Fernandez declare that they have no conflict of interest. James A. Fallavollita has been a consultant for Lantheus Medical Imaging (written agreement but no compensation), and has been employed by Buffalo VA Medical Center and University of Buffalo. John M. Canty has received honoraria and travel expenses from Yale and University of Maryland for Grand Rounds.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
•• Of major importance
- 1••.Shen MJ, Zipes DP. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res. 2014;114:1004–21. doi: 10.1161/CIRCRESAHA.113.302549. This is a comprehensive review of the role of the autonomic system in the development of cardiac arrhythmias which covers basic as well as clinical pathophysiology. [DOI] [PubMed] [Google Scholar]
- 2.Cao JM, Chen LS, KenKnight BH, Ohara T, Lee MH, Tsai J, et al. Nerve sprouting and sudden cardiac death. Circ Res. 2000;86:816–21. doi: 10.1161/01.res.86.7.816. [DOI] [PubMed] [Google Scholar]
- 3.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 4.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 5.Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JP, et al. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med. 2012;367:2275–83. doi: 10.1056/NEJMoa1211107. [DOI] [PubMed] [Google Scholar]
- 6.Goldberger JJ, Basu A, Boineau R, Buxton AE, Cain ME, Canty JM, Jr, et al. Risk stratification for sudden cardiac death: a plan for the future. Circulation. 2014;129:516–26. doi: 10.1161/CIRCULATIONAHA.113.007149. [DOI] [PubMed] [Google Scholar]
- 7.Bengel FM, Permanetter B, Ungerer M, Nekolla SG, Schwaiger M. Alterations of the sympathetic nervous system and metabolic performance of the cardiomyopathic heart. Eur J Nucl Med Mol Imaging. 2002;29:198–202. doi: 10.1007/s00259-001-0694-0. [DOI] [PubMed] [Google Scholar]
- 8.Luisi AJ, Jr, Fallavollita JA, Suzuki G, Canty JM., Jr Spatial inhomogeneity of sympathetic nerve function in hibernating myocardium. Circulation. 2002;106:779–81. doi: 10.1161/01.cir.0000028604.23202.ac. [DOI] [PubMed] [Google Scholar]
- 9.Luisi AJ, Jr, Suzuki G, deKemp R, Haka MS, Toorongian SA, Canty JM, Jr, et al. Regional 11C-hydroxyephedrine retention in hibernating myocardium: chronic inhomogeneity of sympathetic innervation in the absence of infarction. J Nucl Med. 2005;46:1368–74. [PubMed] [Google Scholar]
- 10.Barber MJ, Mueller TM, Henry DP, Felten SY, Zipes DP. Transmural myocardial infarction in the dog produces sympathectomy in noninfarcted myocardium. Circulation. 1983;67:787–96. doi: 10.1161/01.cir.67.4.787. [DOI] [PubMed] [Google Scholar]
- 11.Simoes MV, Barthel P, Matsunari I, Nekolla SG, Schomig A, Schwaiger M, et al. Presence of sympathetically denervated but viable myocardium and its electrophysiologic correlates after early revascularised, acute myocardial infarction. Eur Heart J. 2004;25:551–7. doi: 10.1016/j.ehj.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Bulow HP, Stahl F, Lauer B, Nekolla SG, Schuler G, Schwaiger M, et al. Alterations of myocardial presynaptic sympathetic innervation in patients with multi-vessel coronary artery disease but without history of myocardial infarction. Nucl Med Commun. 2003;24:233–9. doi: 10.1097/00006231-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Bulow H, Stahl F, Lauer B, Nekolla SG, Schuler G, Schwaiger M, et al. Myocardial presynaptic sympathetic innervation evaluated with 11C-HED-PET in patients with severe coronary artery disease but no history of myocardial infarction. J Nucl Med. 2002;43(Suppl 5):184. doi: 10.1097/00006231-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Matsunari I, Schricke U, Bengel FM, Haase HU, Barthel P, Schmidt G, et al. Extent of cardiac sympathetic neuronal damage is determined by the area of ischemia in patients with acute coronary syndromes. Circulation. 2000;101:2579–85. doi: 10.1161/01.cir.101.22.2579. [DOI] [PubMed] [Google Scholar]
- 15.Bengel FM, Barthel P, Matsunari I, Schmidt G, Schwaiger M. Kinetics of 123I-MIBG after acute myocardial infarction and reperfusion therapy. J Nucl Med. 1999;40(6):904–10. [PubMed] [Google Scholar]
- 16.Inoue H, Zipes DP. Results of sympathetic denervation in the canine heart: supersensitivity that may be arrhythmogenic. Circulation. 1987;75:877–87. doi: 10.1161/01.cir.75.4.877. [DOI] [PubMed] [Google Scholar]
- 17.Sasano T, Abraham MR, Chang KC, Ashikaga H, Mills KJ, Holt DP, et al. Abnormal sympathetic innervation of viable myocardium and the substrate of ventricular tachycardia after myocardial infarction. J Am Coll Cardiol. 2008;51:2266–75. doi: 10.1016/j.jacc.2008.02.062. [DOI] [PubMed] [Google Scholar]
- 18.Fricke E, Fricke H, Eckert S, Zijlstra S, Weise R, Lindner O, et al. Myocardial sympathetic innervation in patients with chronic coronary artery disease: is reduction in coronary flow reserve correlated with sympathetic denervation? Eur J Nucl Med Mol Imaging. 2007;34:206–11. doi: 10.1007/s00259-006-0236-x. [DOI] [PubMed] [Google Scholar]
- 19.Sakata K, Yoshida H, Nawada R, Obayashi K, Tamekiyo H, Mochizuki M. Scintigraphic assessment of regional cardiac sympathetic nervous system in patients with single-vessel coronary artery disease. Ann Nucl Med. 2000;14:151–8. doi: 10.1007/BF02987853. [DOI] [PubMed] [Google Scholar]
- 20.Stevens MJ, Dayanikli F, Raffel DM, Allman KC, Sandford T, Feldman EL, et al. Scintigraphic assessment of regionalized defects in myocardial sympathetic innervation and blood flow regulation in diabetic patients with autonomic neuropathy. J Am Coll Cardiol. 1998;31:1575–84. doi: 10.1016/s0735-1097(98)00128-4. [DOI] [PubMed] [Google Scholar]
- 21.Hartikainen J, Mustonen J, Kuikka J, Vanninen E, Kettunen R. Cardiac sympathetic denervation in patients with coronary artery disease without previous myocardial infarction. Am J Cardiol. 1997;80:273–7. doi: 10.1016/s0002-9149(97)00345-7. [DOI] [PubMed] [Google Scholar]
- 22.Guertner C, Klepzig H, Jr, Maul FD, Hartmann A, Lelbach S, Hellmann A, et al. Noradrenaline depletion in patients with coronary artery disease before and after percutaneous transluminal coronary angioplasty with iodine-123 metaiodobenzylguanidine and single-photon emission tomography. Eur J Nucl Med. 1993;20:776–82. doi: 10.1007/BF00180908. [DOI] [PubMed] [Google Scholar]
- 23.Fallavollita JA, Banas MD, Suzuki G, deKemp RA, Sajjad M, Canty JM., Jr 11C-meta-hydroxyephedrine defects persist despite functional improvement in hibernating myocardium. J Nucl Cardiol. 2010;17:85–96. doi: 10.1007/s12350-009-9164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fallavollita JA, Canty JM., Jr Dysinnervated but viable myocardium in ischemic heart disease. J Nucl Cardiol. 2010;17:1107–15. doi: 10.1007/s12350-010-9292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fallavollita JA, Riegel BJ, Suzuki G, Valeti U, Canty JM., Jr Mechanism of sudden cardiac death in pigs with viable chronically dysfunctional myocardium and ischemic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2005;289:H2688–96. doi: 10.1152/ajpheart.00653.2005. [DOI] [PubMed] [Google Scholar]
- 26.Canty JM, Jr, Suzuki G, Banas MD, Verheyen F, Borgers M, Fallavollita JA. Hibernating myocardium: chronically adapted to ischemia but vulnerable to sudden death. Circ Res. 2004;94:1142–9. doi: 10.1161/01.RES.0000125628.57672.CF. [DOI] [PubMed] [Google Scholar]
- 27.Pizzuto MF, Suzuki G, Banas MD, Heavey B, Fallavollita JA, Canty JM., Jr Dissociation of hemodynamic and electrocardiographic indexes of myocardial ischemia in pigs with hibernating myocardium and sudden cardiac death. Am J Physiol Heart Circ Physiol. 2013;304:H1697–707. doi: 10.1152/ajpheart.00166.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ovchinnikov V, Suzuki G, Canty JM, Jr, Fallavollita JA. Blunted functional responses to pre- and postjunctional sympathetic stimulation in hibernating myocardium. Am J Physiol Heart Circ Physiol. 2005;289:H1719–28. doi: 10.1152/ajpheart.00273.2005. [DOI] [PubMed] [Google Scholar]
- 29.Gutterman DD, Morgan DA, Miller FJ. Effect of brief myocardial ischemia on sympathetic coronary vasoconstriction. Circ Res. 1992;71:960–9. doi: 10.1161/01.res.71.4.960. [DOI] [PubMed] [Google Scholar]
- 30.Chen PS, Chen LS, Cao JM, Sharifi B, Karagueuzian HS, Fishbein MC. Sympathetic nerve sprouting, electrical remodeling and the mechanisms of sudden cardiac death. Cardiovasc Res. 2001;50:409–16. doi: 10.1016/s0008-6363(00)00308-4. [DOI] [PubMed] [Google Scholar]
- 31.Zhou S, Chen LS, Miyauchi Y, Miyauchi M, Kar S, Kangavari S, et al. Mechanisms of cardiac nerve sprouting after myocardial infarction in dogs. Circ Res. 2004;95:76–83. doi: 10.1161/01.RES.0000133678.22968.e3. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez SF, Ovchinnikov V, Canty JM, Jr, Fallavollita JA. Hibernating myocardium results in partial sympathetic denervation and nerve sprouting. Am J Physiol Heart Circ Physiol. 2013;304:H318–27. doi: 10.1152/ajpheart.00810.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med. 1996;334:1349–55. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 34.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353(9146):9–13. [PubMed] [Google Scholar]
- 35.Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF) Lancet. 1999;353(9169):2001–7. [PubMed] [Google Scholar]
- 36.de Milliano PAR, de Groot AC, Tijssen JGP, van Eck-Smit BLF, Van Zwieten PA, Lie KI. Beneficial effects of metoprolol on myocardial sympathetic function: evidence from a randomized, placebo-controlled study in patients with congestive heart failure. Am Heart J. 2002;144:E3. doi: 10.1067/mhj.2002.121807. [DOI] [PubMed] [Google Scholar]
- 37.Cohen-Solal A, Rouzet F, Berdeaux A, Le Guludec D, Abergel E, Syrota A, et al. Effects of carvedilol on myocardial sympathetic innervation in patients with chronic heart failure. J Nucl Med. 2005;46:1796–803. [PubMed] [Google Scholar]
- 38.Merlet P, Valette H, Dubois-Rande JL, Moyse D, Duboc D, Dove P, et al. Prognostic value of cardiac metaiodobenzylguanidine imaging in patients with heart failure. J Nucl Med. 1992;33:471–7. [PubMed] [Google Scholar]
- 39.Wakabayashi T, Nakata T, Hashimoto A, Yuda S, Tsuchihashi K, Travin MI, et al. Assessment of underlying etiology and cardiac sympathetic innervation to identify patients at high risk of cardiac death. J Nucl Med. 2001;42:1757–67. [PubMed] [Google Scholar]
- 40.Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, et al. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. J Am Coll Cardiol. 2010;55:2212–21. doi: 10.1016/j.jacc.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 41.Shah AM, Bourgoun M, Narula J, Jacobson AF, Solomon SD. Influence of ejection fraction on the prognostic value of sympathetic innervation imaging with iodine-123 MIBG in heart failure. JACC Cardiovasc Imaging. 2012;5:1139–46. doi: 10.1016/j.jcmg.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 42••.Verschure DO, Veltman CE, Manrique A, Somsen GA, Koutelou M, Katsikis A, et al. For what endpoint does myocardial 123I-MIBG scintigraphy have the greatest prognostic value in patients with chronic heart failure? Results of a pooled individual patient data meta-analysis. Eur H J Cardiovasc Imaging. 2014;15(9):996–1003. doi: 10.1093/ehjci/jeu044. This is a comprehensive meta analysis of pooled individual patient data evaluating how various clincial end-points can be predicted using 123I-MIBG scintigraphy. [DOI] [PubMed] [Google Scholar]
- 43.Tamaki S, Yamada T, Okuyama Y, Morita T, Sanada S, Tsukamoto Y, et al. Cardiac iodine-123 metaiodobenzylguanidine imaging predicts sudden cardiac death independently of left ventricular ejection fraction in patients with chronic heart failure and left ventricular systolic dysfunction: results from a comparative study with signal-averaged electrocardiogram, heart rate variability, and QT dispersion. J Am Coll Cardiol. 2009;53:426–35. doi: 10.1016/j.jacc.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 44.Bax JJ, Kraft O, Buxton AE, Fjeld JG, Parizek P, Agostini D, et al. 123 I-mIBG scintigraphy to predict inducibility of ventricular arrhythmias on cardiac electrophysiology testing: a prospective multicenter pilot study. Circ Cardiovasc Imaging. 2008;1:131–40. doi: 10.1161/CIRCIMAGING.108.782433. [DOI] [PubMed] [Google Scholar]
- 45.Dae MW, Herre JM, O’Connell JW, Botvinick EH, Newman D, Munoz L. Scintigraphic assessment of sympathetic innervation after transmural versus nontransmural myocardial infarction. J Am Coll Cardiol. 1991;17:1416–23. doi: 10.1016/s0735-1097(10)80156-1. [DOI] [PubMed] [Google Scholar]
- 46.Zhou Y, Zhou W, Folks RD, Manatunga DN, Jacobson AF, Bax JJ, et al. I-123 mIBG and Tc-99m myocardial SPECT imaging to predict inducibility of ventricular arrhythmia on electrophysiology testing: a retrospective analysis. J Nucl Cardiol. 2014;21:913–20. doi: 10.1007/s12350-014-9911-7. [DOI] [PubMed] [Google Scholar]
- 47.Boogers MJ, Borleffs CJ, Henneman MM, van Bommel RJ, van Ramshorst J, Boersma E, et al. Cardiac sympathetic denervation assessed with 123-iodine metaiodobenzylguanidine imaging predicts ventricular arrhythmias in implantable cardioverter-defibrillator patients. J Am Coll Cardiol. 2010;55(24):2769–77. doi: 10.1016/j.jacc.2009.12.066. [DOI] [PubMed] [Google Scholar]
- 48.Fallavollita JA, Luisi AJ, Jr, Michalek SM, Valverde AM, deKemp RA, Haka MS, et al. Prediction of arrhythmic events with positron emission tomography: PAREPET study design and methods. Contemp Clin Trials. 2006;27:374–88. doi: 10.1016/j.cct.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 49••.Fallavollita JA, Heavey BM, Luisi AJ, Jr, Michalek SM, Baldwa S, Mashtare TL, Jr, et al. Regional myocardial sympathetic denervation predicts the risk of sudden cardiac arrest in ischemic cardiomyopathy. J Am Coll Cardiol. 2014;63:141–9. doi: 10.1016/j.jacc.2013.07.096. The PAREPET trial was the first to demonstrate that the volume of regionally denervated myocardium was a predictor of cause specific death from sudden cardiac arrest. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50••.Nakata T, Nakajima K, Yamashina S, Yamada T, Momose M, Kasama S, et al. A pooled analysis of multicenter cohort studies of (123)I-mIBG imaging of sympathetic innervation for assessment of long-term prognosis in heart failure. JACC Cardiovasc Imaging. 2013;6:772–84. doi: 10.1016/j.jcmg.2013.02.007. This is a pooled metaanalysis evaluating the use of MIBG to predict prognosis in patients with heart failure. [DOI] [PubMed] [Google Scholar]
- 51.Bocker D, Bansch D, Heinecke A, Weber M, Brunn J, Hammel D, et al. Potential benefit from implantable cardioverter-defibrillator therapy in patients with and without heart failure. Circulation. 1998;98:1636–43. doi: 10.1161/01.cir.98.16.1636. [DOI] [PubMed] [Google Scholar]
- 52.Moss AJ, Greenberg H, Case RB, Zareba W, Hall WJ, Brown MW, et al. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation. 2004;110:3760–5. doi: 10.1161/01.CIR.0000150390.04704.B7. [DOI] [PubMed] [Google Scholar]
- 53.Sinusas AJ, Lazewatsky J, Brunetti J, Heller G, Srivastava A, Liu YH, et al. Biodistribution and radiation dosimetry of LMI1195: first-in-human study of a novel 18F-labeled tracer for imaging myocardial innervation. J Nucl Med. 2014;55(9):1445–51. doi: 10.2967/jnumed.114.140137. [DOI] [PubMed] [Google Scholar]
- 54.Yu M, Bozek J, Lamoy M, Guaraldi M, Silva P, Kagan M, et al. Evaluation of LMI1195, a novel 18F-labeled cardiac neuronal PET imaging agent, in cells and animal models. Circ Cardiovasc Imaging. 2011;4:435–43. doi: 10.1161/CIRCIMAGING.110.962126. [DOI] [PubMed] [Google Scholar]
- 55.Solomon SD, Wang D, Finn P, Skali H, Zornoff L, McMurray JJ, et al. Effect of candesartan on cause-specific mortality in heart failure patients: the Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation. 2004;110:2180–3. doi: 10.1161/01.CIR.0000144474.65922.AA. [DOI] [PubMed] [Google Scholar]
- 56.Zile MR, Gaasch WH, Anand IS, Haass M, Little WC, Miller AB, et al. Mode of death in patients with heart failure and a preserved ejection fraction: results from the Irbesartan in heart failure with preserved ejection fraction study (I-Preserve) trial. Circulation. 2010;121(12):1393–405. doi: 10.1161/CIRCULATIONAHA.109.909614. [DOI] [PubMed] [Google Scholar]
- 57.Solomon SD, Zelenkofske S, McMurray JJ, Finn PV, Velazquez E, Ertl G, et al. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med. 2005;352:2581–8. doi: 10.1056/NEJMoa043938. [DOI] [PubMed] [Google Scholar]
- 58••.Nakajima K, Nakata T, Yamada T, Yamashina S, Momose M, Kasama S, et al. A prediction model for 5-year cardiac mortality in patients with chronic heart failure using 123I-metaiodobenzylguanidine imaging. Eur J Nucl Med Mol Imaging. 2014;41:1673–82. doi: 10.1007/s00259-014-2759-x. This study uses MIBG to develop a model to predict 5 year total cardiac mortality. [DOI] [PMC free article] [PubMed] [Google Scholar]