Abstract
Objective
To test the hypothesis that multitissue deficits in insulin sensitivity are greater among women than men with type 1 diabetes compared to respective controls.
Research Design and Methods
Three-stage hyperinsulinemic-euglycemic clamps (4, 8, 40mU/m2/min) were performed on 41 people with type 1 diabetes and 47 adults without diabetes (mean±SD age 46±8). Infusions of [1-13C]palmitate, [1,1,2,3,3-2H2]glycerol, and [6,6-2H2]glucose isotope tracers were used to determine free fatty acid (FFA), glycerol, and glucose kinetics in 52 of these participants (25 M and 27 W).
Results
There was no difference in age or BMI by type 1 diabetes status in either sex. Free fatty acid rate of appearance (FFA Ra) was higher in both sexes with type 1 diabetes compared to those without diabetes during stages 1 and 2. The same was seen with glycerol for stages 1 and 2. During stage 3 glucose rate of disappearance (Rd) was lower in those with type 1 diabetes among both sexes. All had sex by type 1 diabetes interactions with greater deficits in insulin sensitivity in women. While there was no sex by diabetes interaction in regards to glucose rate of appearance (Ra), those with type 1 diabetes had a higher glucose Ra than those without diabetes.
Conclusions
We found that type 1 diabetes affected adipose and skeletal muscle insulin sensitivity to a greater extent in women than in men, perhaps contributing to the greater relative increase in cardiovascular risk in women with type 1 diabetes.
Keywords: Diabetes, type 1; Insulin resistance; Gender differences
1.1 Introduction
The incidence of atherosclerotic disease in the general population is low in premenopausal women when compared to men of the same age, and rises abruptly after menopause. [1] In people with and without diabetes, men have higher cardiovascular disease (CVD) risk and mortality than women. [2] In the presence of type 1 diabetes the risk of developing and dying from coronary artery disease (CAD) is increased in both sexes, but the relative increase in risk is at least twice as high in women compared to men. [3]–[5] This loss of CV protection is particularly pronounced in young women, and some studies have reported equal risk of ischemic heart disease deaths in men and women in the presence of insulin dependent diabetes. [4] Thus, the CV protection of the premenopausal state seems to be lost when type 1 diabetes is present. [4] However, the mechanism for the loss of cardiovascular protection in women with diabetes remains poorly understood.
Evidence suggests that insulin resistance plays a role in CAD in type 1 diabetes. In the Coronary Artery Calcification in Type 1 Diabetes (CACTI) study cohort, insulin resistance was associated with higher levels of coronary artery calcification (CAC), [6] independent of other CVD risk factors (age, gender, LDL-C, HDL-C, smoking, and diabetes duration). In fact, study participants without diabetes had worse CVD risk profiles (higher LDL-C and triglycerides and lower HDL-C) yet had less CAC and higher estimated and measured insulin sensitivity than participants with diabetes. [6], [7] CAC has a high correlation with coronary atherosclerotic plaque burden, [8] and is often used as a surrogate measure of atherosclerosis. Among adults without diabetes, men had higher CAC scores than women; however, in the presence of type 1 diabetes this sex difference was mostly lost. [8]–[12] It is unknown why the sex difference in CAC scores is altered in the presence of type 1 diabetes.
Given that CAD risk is increased to a greater extent in women with type 1 diabetes than in men with type 1 diabetes and that insulin resistance correlates with CAC, we hypothesized that insulin sensitivity is reduced to a greater degree in women with type 1 diabetes than in men with type 1 diabetes. Thus, insulin resistance could account, at least partially, for the greater CAD risk in women with type 1 diabetes. We have previously reported results of skeletal muscle, adipose tissue, and liver insulin sensitivity by three-stage hyperinsulinemic euglycemic clamp study with tracers, performed in a subset of participants with and without type 1 diabetes from the CACTI cohort. [13] Here we report an analysis of sex-based differences in these parameters.
2.1 Research Design and Methods
The CACTI study enrolled 1416 adults between 19 and 56 years of age, 652 with type 1 diabetes and 764 without type 1 diabetes and completed a fasting examination including blood levels of lipid parameters, glucose, hemoglobin A1c, blood pressure, weight, height and waist/hip circumference as previously described. [7] All participants were invited to return at 3 and 6 year follow up points and the above measurements were repeated at each visit. All study participants had CAC measured by electron beam computed tomography at baseline, 3, and 6 year follow-ups. Inclusion criteria for initial enrollment of type 1 diabetes participants were: 19–56 years of age, no history of cardiovascular disease (CVD), on insulin therapy within 1 year of diagnosis of diabetes and current insulin therapy, diagnosed with diabetes before age 30 years, and/or with positive antibodies, and diabetes duration of at least 10 years. All participants provided informed consent, and the study was approved by the Colorado Combined Institutional Review Board.
For the hyperinsulinemic euglycemic clamp substudy, participants were recruited from the CACTI cohort at the time of their 6-year follow-up visit. [6] Inclusion criteria included HbA1c ≤ 9.5% (80 mmol/mol), albumin excretion rate < 200 μg/min, triglycerides < 400 mg/dL, blood pressure < 160/100 mmHg, and a CAC measurement present from the 6 year follow-up. A total of 41 subjects with type 1 diabetes (21 women and 20 men) and 47 subjects without diabetes (27 women and 20 men) were recruited. For the isotope tracer analysis 50 participants from the insulin clamp substudy were randomly selected (26 with and 26 without type 1 diabetes including 25 males and 27 females). Subjects underwent a standardized macronutrient composition diet (50% carbohydrate, 20% protein, 30% fat) and were asked to refrain from intense physical activity, smoking, and alcohol use for 3 days before the clamp study day. Daily energy requirements for diet design were estimated based on dual energy x-ray absorptiometry measurement of fat free mass using the equation: daily energy intake = 1.4 kcal/day × [372 + (23.9 × fat free mass)] premenopausal women were scheduled for their study visit during the follicular phase of their menstrual cycle (day 2–10).
Participants were admitted to the Clinical Translational Research Center inpatient unit the night before the clamp. Those with diabetes were asked to bolus their long acting insulin ≥12 hours before admission. Dinner was provided and subjects then fasted overnight and throughout the clamp protocol. Subjects with type 1 diabetes bolused for their dinner per their usual regimen. At 2200, insulin pumps were removed, if applicable, and all participants with type 1 diabetes were maintained overnight on intravenous (IV) regular insulin with adjustments to achieve and maintain euglycemia. The morning of the clamp study two antecubital catheters were placed in one arm for infusions of stable isotopes, insulin, potassium and dextrose as needed. A retrograde hand IV was placed in the contralateral hand for arterialized blood draws during the clamp using the heated hand vein technique. At 0800 hour, a primed, continuous infusion of [1,1,2,3,3-2H2]glycerol and [6,6-2H2]glucose were initiated at 0.011 umol/kg/min and 0.04 mg/kg/min, respectively, and a continuous infusion of [1-13C]palmitate was initiated with no prime at 0.04 μmol/kg/min and continued throughout a 2-h basal lead-in period and throughout the insulin clamp. For individuals with type 1 diabetes, the overnight insulin infusion was continued through the basal period until the initiation of the insulin clamp at 1000 hour. Resting metabolic rate measurement and blood samples for determination of baseline hormones and substrates were performed over the final 30 minutes before the clamp. At 1000 h, a three-stage hyperinsulinemic euglycemic clamp was initiated and continued for the next 4.5 hour using the method of DeFronzo et al. [14]. Briefly, a primed continuous infusion of insulin was administered at 4 mU/m2 · min for 1.5 h (stage 1), 8 mU/m2/min for 1.5 hours (stage 2), and then 40 mU/m2 · min for the final 1.5 h (stage 3). A variable infusion of [6,6-2H2]glucose spiked 20% dextrose was administered to maintain blood glucose at approximately 90 mg/dL. Arterialized blood was sampled every 5 minutes for bedside determination of glucose concentration (Analox Instruments USA, Inc., Lunenburg, MA), and the dextrose infusion was adjusted as necessary.
Glycerol, glucose, and palmitate isotope enrichment were measured by gas chromatography/mass spectrometry as previously described [13], [15] (GC model 6890 and MS model 5973A; Hewlett-Packard, Pal Alt, CA).
The isotope tracer data was used to determine glucose rate of appearance (glucose Ra), glucose rate of disappearance (glucose Rd), rates of appearance for free fatty acids (FFA Ra) and glycerol (glycerol Ra) and insulin concentration required to suppress glucose and glycerol Ra by 50% (IC50). The Steele equations for trace appearance rates were used to account for small changes in enrichment and concentration. Volumes of distribution used in the calculations were 180ml/kg for glycerol and 40ml/kg for palmitate. The FFA Ra was derived by dividing palmitate Ra by the percentage of total FFA accounted for by palmitate.
Least squares means (LSM) and 95% CIs for each outcome variable and insulin were calculated for each clamp stage, by sex and diabetes status. Mixed effects models with stage treated as a categorical variable (i.e., no assumptions about the trajectory by stage) were used to compare overall trajectories of each outcome variable by sex and diabetes, and to compare the least-squares means at each stage. The mixed effects models were adjusted for measured insulin at each stage. A p-value < 0.05 was considered statistically significant.
3.1 RESULTS
Characteristics of study participants by diabetes status and sex are shown in Table 1. There were no differences in age, blood pressure, BMI, total body fat percentage, or trunk fat percentage by diabetes status in either men or women. As expected, participants with diabetes had a higher HbA1c than those without. Levels of total and LDL-C and triglycerides were lower in participants with type 1 diabetes in both men and women including after accounting for statin use. Adiponectin was higher in men, but not women, with type 1 diabetes. There were no significant differences in HDL-C by type 1 diabetes status in women, though men with type 1 diabetes had a higher HDL-C than men without diabetes (p=0.03).
Table A.
Baseline characteristics by type 1 diabetes status and sex
| Men | Women | |||
|---|---|---|---|---|
| Type 1 Diabetes | Non-diabetes | Type 1 Diabetes | Non-diabetes | |
| Age (years) | 47 (10) | 47 (6) | 44 (9) | 45 (8) |
| Diabetes duration (years) | 29.2 (7.71) | - | 27.9 (8.32) | - |
| A1c (%) [mmol/mol] | 7.5 (0.8) [58] ** | 5.4 (0.3) [36] | 7.5 (0.9) [58]** | 5.5 (0.3) [37] |
| BMI | 28.3 (4.26) | 27.2 (3.58) | 25.8 (4.30) | 25.5 (4.26) |
| Total body Fat (%) | 24.4 (6.07) | 24.2 (3.25) | 32.4 (6.72) | 33.6 (6.57) |
| Trunk Fat (%) | 25.9 (7.28) | 27.4 (4.36) | 30.4 (8.60) | 31.2 (7.57) |
| Adiponectin | 11.5 (5.45)* | 7.30 (4.35) | 13.0 (6.13) | 11.3 (5.76) |
| Cholesterol (mg/dL) | 145 (32.10)* | 171 (25.35) | 135 (32.73)** | 171 (31.66) |
| Triglycerides (mg/dL) | 63 (11)* | 126 (73) | 69 (42)* | 94 (38) |
| LDL | 75 (30)* | 101 (25) | 66 (25)* | 93 (27) |
| HDL (mg/dL) | 60.8 (29.73)* | 44.5 (9.34) | 55.5 (13.13) | 59.9 (15.08) |
| SBP (mmHg) | 117 (11.93) | 118 (8.36) | 111 (8.99) | 109 (9.60) |
| DBP (mmHg) | 75.0 (6.74) | 80.4 (8.31) | 71.5 (7.30) | 72.8 (6.21) |
p< 0.05,
p<0.001: for comparison by diabetes status within sex; Mean ± SD
3.1.1 Skeletal muscle insulin sensitivity
M-value
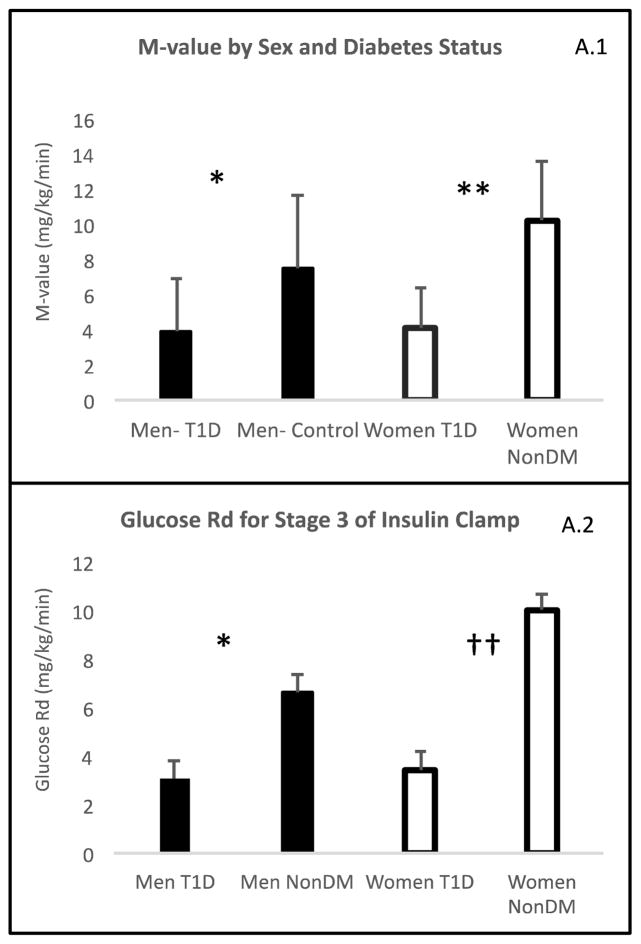
M-values during the final 30 minutes of stage 3 of the clamp are shown in Figure A.1. Participants without diabetes had higher M-values than those with diabetes among both men (7.52±4.14 vs. 4.14±2.24; p=0.006 non-diabetes vs. type 1 diabetes) and women (10.2±3.26 vs 4.14±2.96; p<0.001 non-diabetes vs. type 1 diabetes). A sex by diabetes interaction was tested and found (p=0.0141). Women without diabetes had a higher M-value than men without diabetes (p=0.022), whereas there was no difference by sex in M-value among participants with type 1 diabetes (p=0.998) indicating a greater deficit in whole body insulin sensitivity in women than in men with type 1 diabetes.
Figure A.1. Skeletal Muscle Insulin Sensitivity.
(A.1) M-value by sex and diabetes status, (A.2)) Glucose rate of disappearance. Results are shown by sexes based on diabetes status and expressed by least significant means (LSM).
*: P<0.05 men with type 1 diabetes vs men without type 1 diabetes
†: P=0.0001 men with type 1 diabetes vs men without type 1 diabetes
**: P<0.05 women with type 1 diabetes vs women without type 1 diabetes
††: P<0.0001 women with type 1 diabetes vs women without type 1 diabetes
Glucose rate of disappearance (Rd)
Glucose Rd, a measure of glucose uptake largely explained by skeletal muscle, was compared across clamp stages in regards to sex and diabetes status. There was a sex by diabetes by stage interaction (p<0.0001). There were no differences in glucose Rd by diabetes status in either sex at baseline (women p=0.1413 and men p=0.1758) or during stage 1 (women p=0.26 and men p=0.24). However, during stage 2 there was a difference in glucose Rd by diabetes status only among women (LSM difference for women with type 1 diabetes compared to controls was −0.85 ± 0.37, p=0.03). During stage 3 of the clamp, people with diabetes in both sexes had a decreased glucose Rd (Figure A.2), but the difference between women with versus without type 1 diabetes (LSM difference −6.59 ±1.00; p<0.0001) was greater than that between men with versus without type 1 diabetes (LSM difference −3.56 ±1.03; p=0.001).
3.1.2 Adipose tissue insulin sensitivity
Free Fatty Acid (FFA) concentration
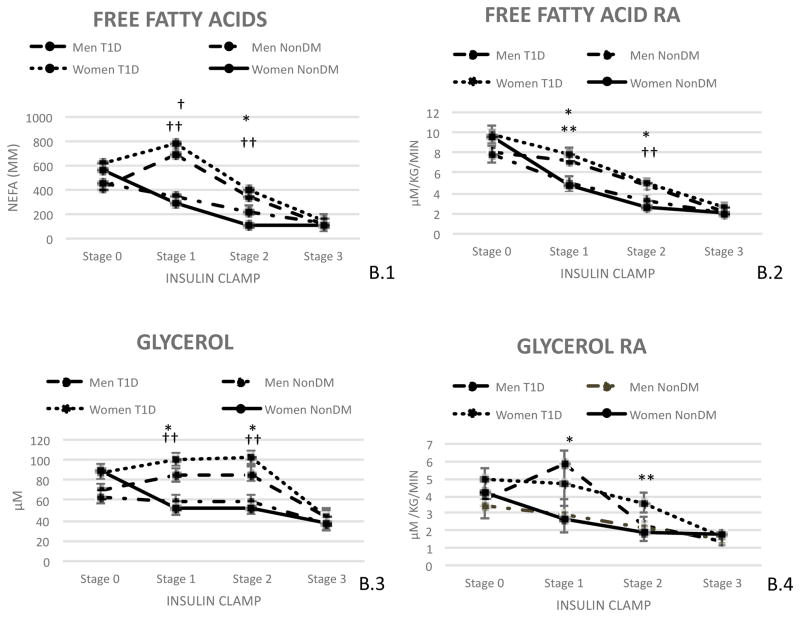
FFA concentration was compared across clamp stages by sex and diabetes status as shown in Figure B.1. There was a sex by diabetes by stage interaction (p<0.0001). At baseline, there was no difference in FFA concentration by diabetes status in either sex (women p=0.32 and men p=0.46). During stages 1 and 2 of the clamp, FFA concentrations were higher in people with type 1 diabetes than controls among both men (LSM stage 1: 357.20±53.97 p<0.0001; LSM stage 2: 112.71±54.073, p=0.04) and women (LSM stage 1: 496.15±49.47, p<0.0001; LSM stage 2: 289.83±49.55 p<0.0001), but the difference in FFA concentrations by type 1 diabetes status was greater in women than in men during both stages (Figure B.1). There were no differences in FFA concentrations by type 1 diabetes status during stage 3 of the clamp in either sex (women p=0.40 and men p=0.86).
Figure B. Adipose Tissue Insulin Sensitivity.
(B.1) Non Esterified Fatty Acid levels by time and diabetes status, (B.2) Free Fatty Acid rate of appearance, (B.3) Glycerol by time and diabetes status, (B.4) Glycerol rate of appearance. Results are shown by sexes based on diabetes status. Results are expressed by least significant means (LSM).
*: P<0.05 Men with type 1 diabetes vs men without type 1 diabetes
†: P=0.0001 Men with type 1 diabetes vs men without type 1 diabetes
**: P<0.05 Women with type 1 diabetes vs women without type 1 diabetes
††: P=0.0001 Women with type 1 diabetes vs women without type 1 diabetes
Free Fatty Acid rate of appearance (Ra)
FFA Ra, a marker of adipose tissue lipolysis, was compared across clamp stages by sex and diabetes status as shown in Figure B.2. During the clamp there was a sex by diabetes by stage interaction (p<0.0001). At baseline, there was no difference in FFA Ra by type 1 diabetes status in either sex (women p=0.73 and men p=0.82). During stages 1 and 2 of the clamp, FFA Ra was higher in people with type 1 diabetes than controls among both men and women, but the difference in FFA Ra was greater in women (LSM stage 1: 3.11±0.75 p=0.0002; LSM stage 2: 2.37±0.56 p=0.0001) than in men (LSM stage 1: 2.19±0.82 p=0.011; LSM stage 2: 1.41±0.61 p=0.03) during both stages. There were no differences in FFA Ra by type 1 diabetes status seen during stage 3 of the clamp in either sex (women p=0.10 and men p=0.88).
Glycerol Concentration
Glycerol concentration, as reflected in the FFA concentrations, was compared across clamp stages and by sex and diabetes status as seen in figure B.3. There was a sex by diabetes interaction present (p<0.0001). At baseline there was no difference in glycerol concentration in either sex based on diabetes status (men: p=0.62 and women: p=0.91). During stage 1 of the clamp those with diabetes were found to have higher glycerol concentrations than those without (Men: with type 1 diabetes: 85.16±6.87 vs without diabetes: 58.06±6.84; p=0.004; Women: with type 1 diabetes: 100.84±6.37 vs without diabetes: 51.81±6.00; p<0.0001). The same was seen during stage 2 of the insulin clamp (Men: with type 1 diabetes: 86.11±6.68 vs without diabetes: 58.70±6.66; p=0.003 and Women: with type 1 diabetes: 101.70±6.28 vs without diabetes: 52.57±5.76; p<0.0001). There was no difference seen in men (p=0.57) or women (p=0.45) in regards to diabetes status during stage 3 of the clamp.
Glycerol rate of appearance (Ra)
Glycerol Ra, a measure of adipose tissue lipolysis, was compared across clamp stages by sex and diabetes status as shown in Figure B.4. Across clamp stages, there was a sex by diabetes by stage interaction (p=0.05). At baseline, there was no significant difference in glycerol Ra by type 1 diabetes status in either sex (women p=0.41 and men p=0.68). During stage 1 of the clamp, men with type 1 diabetes had a greater glycerol Ra than men without type 1 diabetes (5.80±0.86 vs 2.88±0.99; p=0.03), but there was no significant difference in glycerol Ra among women with versus without type 1 diabetes (p=0.1). During stage 2 of the clamp women with type 1 diabetes had a higher glycerol Ra than women without type 1 diabetes (3.57±0.56 vs 1.89±0.48; p=0.03), but there was no significant difference in glycerol Ra by type 1 diabetes status among men (p=0.87). During stage 3 of the clamp there was no significant difference in glycerol Ra by type 1 diabetes status in either sex (women p=0.57 and men p=0.58). We also assessed glycerol IC 50 data. Men with type 1 diabetes were found to have a higher IC 50 of glycerol than men without diabetes (32.9±11.42 vs 15.8±8.86; p<0.001) however no difference was seen among women (p=0.12).
3.1.3 Hepatic insulin sensitivity
Glucose rate of appearance (Ra)
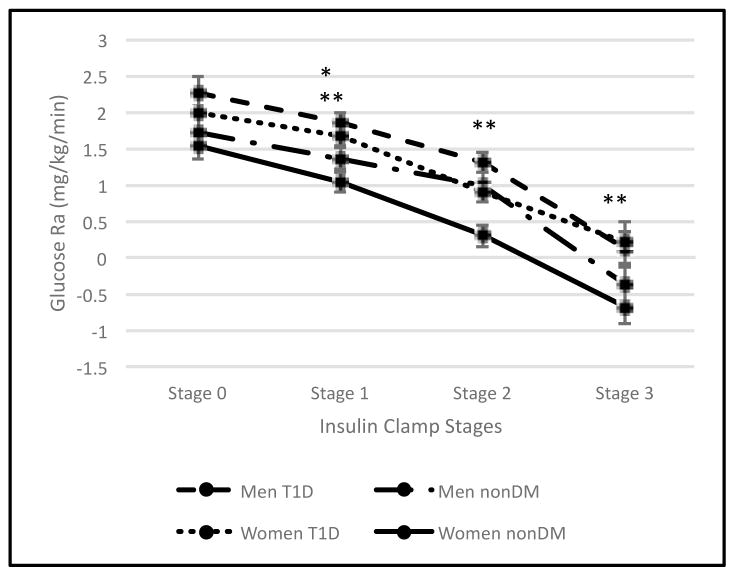
Glucose Ra, a measure of hepatic glucose production, was compared across clamp stages by sex and diabetes as shown in Figure C. There was no interaction of sex by diabetes (p=0.49) overall or by stage (p=0.23). Glucose Ra was not different by type 1 diabetes status in either men or women at baseline, but was higher in participants with type 1 diabetes during the first stage in both men (LSM 0.49±0.19; p=0.01) and women (LSM 0.62±0.19; p=0.002). During stages 2 and 3 of the clamp, glucose Ra was higher in women with type 1 diabetes than women without type 1 diabetes (LSM stage 2: 0.62±0.22, p=0.008; LSM stage 3: 0.88±0.29 p=0.004), but there was no difference between men with vs without type 1 diabetes (stage 2: p=0.15; stage 3: p=0.13). IC 50 for glucose was also assessed and while there were significant differences between men with and without type 1 diabetes (52.5±25.39 vs 32.8±17.89, p=0.045) and women with and without type 1 diabetes (69.0±46.02 vs 27.8±10.51, p=0.014), there was no sex by diabetes interaction (p=0.19).
Figure C.1. Hepatic Insulin Sensitivity.
(C) Glucose rate of appearance between sexes based on diabetes status. Results are expressed as least significant means (LSM).
*: P<0.05 men with type 1 diabetes vs men without type 1 diabetes
†: P=0.0001 men with type 1 diabetes vs men without type 1 diabetes
**: P< 0.05 women with type 1 diabetes vs women without type 1 diabetes
††: P=0.0001 women with type 1 diabetes vs women without type 1 diabetes
4.1 DISCUSSION
Previously adults with type 1 diabetes were shown to have increased whole body and adipocyte insulin resistance compared to non-diabetic controls matched for BMI and age, and similar in terms of habitual physical activity. [6], [15] In light of the known correlation of insulin resistance with CAD in other populations, our demonstration of a correlation with CAC in type 1 diabetes, and the loss of premenopausal protection from CAD in women with type 1 diabetes, we looked more closely at sex differences in insulin resistance in various tissues (skeletal muscle, adipose, and liver) among people with type 1 diabetes compared to controls without diabetes. Our data show that in the presence of type 1 diabetes women have a greater loss of skeletal muscle and adipose tissue insulin sensitivity compared to controls than men. While prior studies have reported on similar sex differences in cardiovascular risk and coronary artery calcification in people with type 1 diabetes, to our knowledge we are the first to present this novel look at insulin resistance differences by sex within type 1 diabetes.
First, we found a significant sex difference in skeletal muscle insulin resistance by diabetes status, such that the presence of type 1 diabetes impaired skeletal muscle insulin sensitivity in women to a greater extent than men. In fact, loss of skeletal muscle uptake of glucose in response to hyperinsulinemic conditions among adults with type 1 diabetes was twice as high in women as in men, such that the greater skeletal muscle insulin sensitivity in women without diabetes when compared to men is completely lost in the setting of type 1 diabetes. Adipose tissue insulin sensitivity is similarly decreased in type 1 diabetes as demonstrated when examining FFA suppression, FFA Ra, glycerol suppression, glycerol Ra, and glycerol IC50. Again, the decrement in adipose tissue insulin sensitivity in type 1 diabetes is markedly greater in women than in men. Of note, while glycerol concentrations do not follow the pattern of glycerol Ra exactly, they are overall consistent with the trends in FFA data. In contrast, while hepatic insulin sensitivity as measured by suppression of glucose Ra is also impaired in type 1 diabetes relative to subjects without diabetes, we find no clear sex-specific difference for this marker of hepatic insulin sensitivity. Both men and women with type 1 diabetes are roughly equally defective in hepatic insulin sensitivity when compared to same sex controls without diabetes. It is possible that one contributor to the insulin resistance in type 1 diabetes is the peripheral nature of insulin administration, which results in peripheral hyperinsulinemia but relative hepatic hypoinsulinemia, in contrast to the normal portal delivery of insulin. [16] However, it remains unclear why this would differentially affect adipose and skeletal muscle, but not hepatic, insulin sensitivity in women versus men.
Cardiovascular risk is increased in both sexes in the presence of type 1 diabetes but the relative increase in risk is greater in women than men. We have found that the decrease in adipose and skeletal muscle insulin sensitivity associated with type 1 diabetes follows the same pattern. Insulin resistance is a risk factor for cardiovascular disease in type 2 diabetes, [17] and we have previously reported that insulin resistance based on a surrogate measure of insulin sensitivity may explain the sex difference in cardiovascular risk in type 1 diabetes. [7] Here we support this hypothesis with direct gold-standard measurement of insulin sensitivity in a smaller subcohort of the CACTI cohort. The mechanisms responsible for these observed sex differences in both cardiovascular risk and insulin sensitivity among people with type 1 diabetes remain unclear. However, a possible mechanism is suggested by the apparent role of estrogen in both premenopausal cardiovascular protection and enhanced insulin sensitivity. Following menopause, women’s cardiovascular protection decreases and insulin resistance increases. [1], [18] Animal models have shown that estrogen deficiency is associated with insulin resistance. [19] With replacement of estrogen, insulin sensitivity improves in post-menopausal women. [20], [21] Martinez and colleagues found that type 1 diabetes was a significant factor that influenced estradiol activity in adolescents. They found lower levels of estradiol and estrogenic activity in women with type 1 diabetes than controls. [22] In women with type 1 diabetes and amenorrhea there is a picture of hypothalamic hypogonadism with low FSH, LH, and estradiol [23], [24] and in our cohort of premenopausal women with type 1 diabetes, we previously reported a high prevalence of irregular menses correlating to higher CAC levels. [8], [25] Some animal models have shown estrogen to protect against hyperglycemia via decreasing HGP and increasing skeletal muscle uptake of glucose. [11] From this evidence it seems reasonable to speculate that dysregulation of the hypothalamic-pituitary-gonadal (HPG) axis is partially responsible for the elevated insulin resistance seen in type 1 diabetes women compared to men.
One possible mechanism for this sex-specific effect of HPG axis changes can be found in known effects of HPG dysregulation and menopause on adipose tissue distribution. Following menopause, visceral adipose tissue (VAT), known to be associated with insulin resistance [26], increases. [27] In addition, pericardial adipose tissue (PAT) increases following menopause. [28], [29] PAT also correlates with CAC and is associated with CAD. [29] Within the CACTI clamp subcohort, pericardial to subcutaneous adipose tissue ratios were lower in men with type 1 diabetes and higher in women with type 1 diabetes than controls [30] and a higher ratio of PAT to SAT adipose tissue volume correlated with decreased insulin sensitivity. Unfortunately, in this study we were not able to examine subgroups by menopausal status, as there were only 6 postmenopausal women in this study. Thus, adipose tissue changes due to HPG axis disruption may partially explain differential insulin sensitivity changes seen between men and women with type 1 diabetes.
With estrogen replacement, insulin sensitivity improves even in those with diabetes. A meta analysis of 107 trials revealed a decrease in HOMA-IR by 35.8% with hormone replacement therapy (HRT) in those with diabetes compared to placebo/no treatment. This decrease in HOMA-IR was greater in women with diabetes than in women without diabetes. [31] The Heart and Estrogen/Progestin Replacement Study (HERS) revealed that hormone replacement did decrease type 2 diabetes risk in women with cardiovascular disease. The failure of HRT to decrease cardiovascular outcomes in the HERS study likely reflects differences in the mechanisms involved in secondary event prevention versus primary prevention of cardiovascular disease. [32], [33] It is also important to note that these studies were not in women with type 1 diabetes mellitus and that data in this area is very limited.
In summary, our present findings demonstrate that type 1 diabetes increases insulin resistance in skeletal muscle and adipose tissue, but not liver, to a greater extent in women than in men. These changes in skeletal muscle and adipose tissue insulin resistance may relate to disruptions of the HPG axis in type 1 diabetes and may explain why women with type 1 diabetes lose cardiovascular protection to a greater extent than men with type 1 diabetes. While abnormal menses seem to be common among women with type 1 diabetes, there is currently no data to suggest replacement of estrogen or regulation of the menstrual cycle with oral contraceptives would resolve the insulin resistance disparity between sexes with type 1 diabetes. A better understanding of the mechanism of altered insulin sensitivity is needed in order to evaluate potential interventions that could improve both insulin sensitivity and cardiovascular risk among women with type 1 diabetes.
Acknowledgments
The authors are grateful to the US Department of Veterans Affairs for fellowship funding and to the Eastern Colorado Geriatric Research, Education, and Clinical Center for research support for IES.
Grants: Support for this study was provided by NHLBI grants HL61753, HL79611, and HL113029, JDRF grant 17-2013-313, ADA grant 7-13-CD-10 (Snell-Bergeon) and DERC Clinical Investigation Core P30 DK57516. The study was performed at the Adult CTRC at UCD support by NIH-M01-RR00051, at the Barbara Davis Center for Childhood Diabetes and at Colorado Heart Imaging Center in Denver, CO.
Footnotes
Author contributions: R.J.M. wrote the manuscript. I.E.S. collected study data, and contributed to writing and reviewing the manuscript. L.L.P. conducted data analyses and contributed to writing and reviewing the manuscript. J.K.S. recruited participants, collected study data, and contributed to writing and reviewing the manuscript. D.M.M collected study data, and reviewed the manuscript. R.H.E contributed to designing the study, preliminary data interpretation, discussion, and review of the manuscript. M.J.R. designed the study and reviewed the manuscript. B.C.B. collected study data, performed tracer analyses and contributed to writing and reviewing the manuscript. J.K.S is the guarantor of this work.
Conflicts of Interest: There are no conflicts of interest.
References
- 1.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999 Jun;340(23):1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 2.Preis SR, et al. Trends in All-Cause and Cardiovascular Disease Mortality among Women and Men with and without Diabetes in the Framingham Heart Study, 1950–2005. Circulation. 2009 Apr;119(13):1728–1735. doi: 10.1161/CIRCULATIONAHA.108.829176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lloyd CE, Kuller LH, Ellis D, Becker DJ, Wing RR, Orchard TJ. Coronary artery disease in IDDM. Gender differences in risk factors but not risk. Arterioscler Thromb Vasc Biol. 1996 Jun;16(6):720–726. doi: 10.1161/01.atv.16.6.720. [DOI] [PubMed] [Google Scholar]
- 4.Laing SP, et al. Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia. 2003 Jun;46(6):760–765. doi: 10.1007/s00125-003-1116-6. [DOI] [PubMed] [Google Scholar]
- 5.de Ferranti SD, et al. Type 1 Diabetes Mellitus and Cardiovascular Disease. Circulation. 2014 Sep;130(13):1110–1130. doi: 10.1161/CIR.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 6.Schauer IE, et al. Insulin resistance, defective insulin-mediated fatty acid suppression, and coronary artery calcification in subjects with and without type 1 diabetes: The CACTI study. Diabetes. 2011 Jan;60(1):306–314. doi: 10.2337/db10-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dabelea D, et al. Effect of type 1 diabetes on the gender difference in coronary artery calcification: a role for insulin resistance? The Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Diabetes. 2003 Nov;52(11):2833–2839. doi: 10.2337/diabetes.52.11.2833. [DOI] [PubMed] [Google Scholar]
- 8.Colhoun HM, Rubens MB, Underwood SR, Fuller JH. The effect of type 1 diabetes mellitus on the gender difference in coronary artery calcification. J Am Coll Cardiol. 2000 Dec;36(7):2160–2167. doi: 10.1016/s0735-1097(00)00986-4. [DOI] [PubMed] [Google Scholar]
- 9.Sung K-C, et al. Relationship between Insulin Resistance and Coronary Artery Calcium in Young Men and Women. PLoS ONE. 2013 Jan;8(1) doi: 10.1371/journal.pone.0053316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Høeg LD, et al. Lipid-Induced Insulin Resistance Affects Women Less Than Men and Is Not Accompanied by Inflammation or Impaired Proximal Insulin Signaling. Diabetes. 2011 Jan;60(1):64–73. doi: 10.2337/db10-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geer EB, Shen W. Gender Differences in Insulin Resistance, Body Composition, and Energy Balance. Gend Med. 2009;6(Suppl 1):60–75. doi: 10.1016/j.genm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mauvais-Jarvis F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol Sex Differ. 2015 Sep;6 doi: 10.1186/s13293-015-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergman BC, et al. Features of Hepatic and Skeletal Muscle Insulin Resistance Unique to Type 1 Diabetes. J Clin Endocrinol Metab. 2012 May;97(5):1663–1672. doi: 10.1210/jc.2011-3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 15.Bergman BC, et al. The Importance of Palmitoleic Acid to Adipocyte Insulin Resistance and Whole-Body Insulin Sensitivity in Type 1 Diabetes. J Clin Endocrinol Metab. 2013 Jan;98(1):E40–E50. doi: 10.1210/jc.2012-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frystyk J, et al. Comparison of pancreas-transplanted type 1 diabetic patients with portal-venous versus systemic-venous graft drainage: impact on glucose regulatory hormones and the growth hormone/insulin-like growth factor-I axis. J Clin Endocrinol Metab. 2008 May;93(5):1758–1766. doi: 10.1210/jc.2007-2350. [DOI] [PubMed] [Google Scholar]
- 17.Patel TP, et al. Insulin resistance: an additional risk factor in the pathogenesis of cardiovascular disease in type 2 diabetes. Heart Fail Rev. 2016 Jan;21(1):11–23. doi: 10.1007/s10741-015-9515-6. [DOI] [PubMed] [Google Scholar]
- 18.Lovejoy J, Champagne C, de Jonge L, Xie H, Smith S. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes 2005. 2008 Jun;32(6):949–958. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louet JF, LeMay C, Mauvais-Jarvis F. Antidiabetic actions of estrogen: Insight from human and genetic mouse models. Curr Atheroscler Rep. 2004 May;6(3):180–185. doi: 10.1007/s11883-004-0030-9. [DOI] [PubMed] [Google Scholar]
- 20.Van Pelt RE, Gozansky WS, Schwartz RS, Kohrt WM. Intravenous estrogens increase insulin clearance and action in postmenopausal women. Am J Physiol Endocrinol Metab. 2003 Aug;285(2):E311. doi: 10.1152/ajpendo.00490.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Margolis KL, et al. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia. 2004 Jul;47(7):1175–1187. doi: 10.1007/s00125-004-1448-x. [DOI] [PubMed] [Google Scholar]
- 22.Martínez D, et al. Oestrogen activity of the serum in adolescents with Type 1 diabetes. Diabet Med J Br Diabet Assoc. 2016 Oct;33(10):1366–1373. doi: 10.1111/dme.13078. [DOI] [PubMed] [Google Scholar]
- 23.Djursing H, Hagen C, Nyholm HC, Carstensen L, Andersen AN. Gonadotropin Responses to Gonadotropin-Releasing Hormone and Prolactin Responses to Thyrotropin-Releasing Hormone and Metoclopramide in Women with Amenorrhea and Insulin-Treated Diabetes Mellitus. J Clin Endocrinol Metab. 1983 May;56(5):1016–1021. doi: 10.1210/jcem-56-5-1016. [DOI] [PubMed] [Google Scholar]
- 24.Djursing H, Hagen C, Andersen AN, Svenstrup B, Bennett P, Pedersen LM. Serum Sex Hormone Concentrations in Insulin Dependent Diabetic Women with and Without Amenorrhoea. Clin Endocrinol (Oxf) 1985 Aug;23(2):147–154. doi: 10.1111/j.1365-2265.1985.tb00209.x. [DOI] [PubMed] [Google Scholar]
- 25.Snell-Bergeon JK, et al. Reproductive History and Hormonal Birth Control Use Are Associated with Coronary Calcium Progression in Women with Type 1 Diabetes Mellitus. J Clin Endocrinol Metab. 2008 Jun;93(6):2142–2148. doi: 10.1210/jc.2007-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cefalu WT, et al. Contribution of visceral fat mass to the insulin resistance of aging. Metabolism. 1995 Jul;44(7):954–959. doi: 10.1016/0026-0495(95)90251-1. [DOI] [PubMed] [Google Scholar]
- 27.Franklin RM, Ploutz-Snyder L, Kanaley JA. Longitudinal changes in abdominal fat distribution with menopause. Metabolism. 2009 Mar;58(3):311–315. doi: 10.1016/j.metabol.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 28.Kim TH, et al. Pericardial fat amount is an independent risk factor of coronary artery stenosis assessed by multidetector-row computed tomography: the Korean Atherosclerosis Study 2. Obes Silver Spring Md. 2011 May;19(5):1028–1034. doi: 10.1038/oby.2010.246. [DOI] [PubMed] [Google Scholar]
- 29.El Khoudary SR, et al. Cardiovascular Fat, Menopause, and Sex Hormones in Women: The SWAN Cardiovascular Fat Ancillary Study. J Clin Endocrinol Metab. 2015 Sep;100(9):3304–3312. doi: 10.1210/JC.2015-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alman AC, et al. The ratio of pericardial to subcutaneous adipose tissues is associated with insulin resistance. Obes Silver Spring Md. 2017 May; doi: 10.1002/oby.21875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salpeter SR, Walsh JME, Ormiston TM, Greyber E, Buckley NS, Salpeter EE. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab. 2006 Sep;8(5):538–554. doi: 10.1111/j.1463-1326.2005.00545.x. [DOI] [PubMed] [Google Scholar]
- 32.Kanaya AM, et al. Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/progestin Replacement Study. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003 Jan;138(1):1–9. doi: 10.7326/0003-4819-138-1-200301070-00005. [DOI] [PubMed] [Google Scholar]
- 33.Grady D, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) JAMA. 2002 Jul;288(1):49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]