Abstract
The major factors determining myocardial perfusion and oxygen delivery have been elucidated over the past several decades, and this knowledge has been incorporated into the management of patients with ischemic heart disease (IHD). The basic understanding of the fluid mechanical behavior of coronary stenoses has also been translated to the cardiac catheterization laboratory where measurements of coronary pressure distal to a stenosis and coronary flow are routinely obtained. However, the role of perturbations in coronary microvascular structure and function, due to myocardial hypertrophy or coronary microvascular dysfunction, in IHD is becoming increasingly recognized. Future studies should therefore be aimed at further improving our understanding of the integrated coronary microvascular mechanisms that control coronary blood flow, and of the underlying causes and mechanisms of coronary microvascular dysfunction. This knowledge will be essential to further improve the treatment of patients with IHD.
Keywords: Coronary blood flow, Ischemic heart disease, Microvascular dysfunction, Hypertrophy, Coronary artery disease
The left ventricle (LV) is responsible for generating the arterial blood pressure, which is required to sustain blood flow in the systemic circulation. Among the regional vascular beds within the systemic circulation, the coronary circulation is unique in that its perfusion is impeded during the systolic phase of the cardiac cycle by the contracting muscle that surrounds it. Because the LV utilizes most of the oxygen (O2) delivered through the coronary vasculature to sustain its function, myocardial contraction is closely connected to coronary blood flow (CBF). As a result, O2 delivery, and the balance between O2 supply and demand are critical determinants of normal LV function. Knowledge of the regulation of CBF in health and disease is therefore essential for proper understanding of the pathophysiological basis and management of many cardiovascular (CV) disorders,1–3 including obstructive coronary artery disease (CAD),4–6 metabolic dysfunction,7–9 and cardiac hypertrophy.10–12 In this review we discuss the control of CBF in the normal healthy heart and in CV disease (CVD) states that are associated with chronic ischemic heart disease (IHD). We will briefly discuss the impact of these disease states on clinical measurements of flow capacity and stenosis severity in the setting of CAD.
Regulation of CBF in the healthy heart
CBF is characterized by marked phasic variations during the cardiac cycle with coronary arterial inflow out of phase with venous outflow.13 Systolic contraction increases LV wall tension and compresses the intramyocardial microvessels, thereby impeding coronary arterial inflow and increasing coronary venous outflow. Systolic compression is not uniformly distributed across the LV wall, resulting in a redistribution of blood flow from the subendocardial to the subepicardial layers of the LV.14 Conversely, during diastole, coronary arterial inflow increases with a transmural gradient that favors perfusion to the subendocardial layers, at which time the coronary venous outflow falls.13
Myocardial oxygen extraction in the LV at rest averages 60–80% of arterially delivered O2,15,16 implying that the ability to increase O2 extraction to increase O2 consumption is limited. Consequently, increases in myocardial O2 consumption, for example during physical activity, are principally met through proportional increases in CBF and hence O2 supply,15,16 that is also dependent on arterial O2 content, which is the product of hemoglobin concentration and arterial O2 saturation plus a small amount of O2 dissolved in plasma that is directly related to arterial O2 tension. This implies that for any given flow level, anemia results in proportional reductions in O2 delivery whereas hypoxia, due to the nonlinear O2 dissociation curve, results in relatively small reductions in O2 content until arterial O2 tension falls to the steep portion of the O2 dissociation curve, i.e. below 50 mm Hg.
Autoregulation of CBF
CBF remains relatively constant over a wide range of perfusion pressures, provided that the determinants of myocardial O2 consumption are kept constant (Fig 1A).17 This is accomplished by changes in diameter of the coronary microvasculature, which involves both myogenic and metabolic mechanisms.3 This autoregulation of blood flow is especially important to maintain myocardial perfusion when coronary pressure is reduced distal to a coronary artery stenosis. The pressure at which the resistance vessels become maximally dilated is the lowest pressure at which normal myocardial perfusion can be sustained and is referred to as the lower limit of autoregulation. Below this pressure, flow decreases in a pressure-dependent way, resulting in the onset of myocardial ischemia.
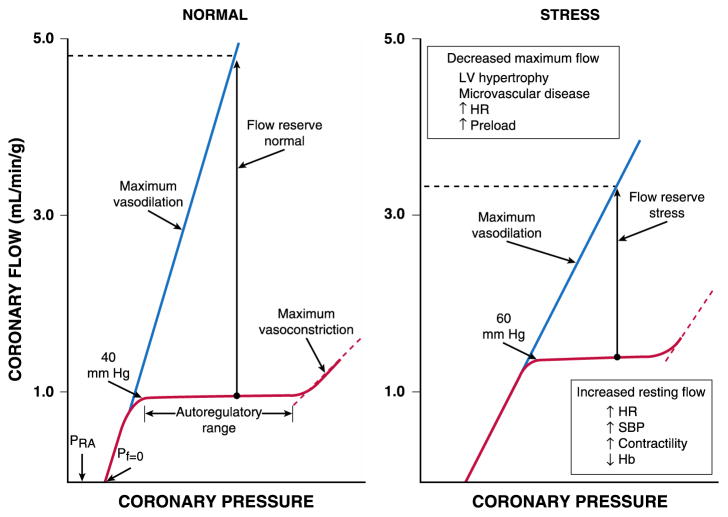
Fig. 1.
Autoregulatory relation under basal conditions and following metabolic stress (e.g., tachycardia). The normal heart maintains CBF constant (left panel) as regional coronary pressure is varied over a wide range when the global determinants of oxygen consumption are kept constant (red lines). Below the lower autoregulatory pressure limit (approximately 40 mm Hg), subendocardial vessels are maximally vasodilated and myocardial ischemia develops. During vasodilation (blue lines), flow increases four to five times above resting values at a normal arterial pressure. Coronary flow ceases at a pressure higher than right atrial pressure (PRA), called zero flow pressure (Pf = 0), which is the effective back pressure to flow in the absence of coronary collaterals. Following stress (right panel), tachycardia increases the compressive determinants of coronary resistance by decreasing the time available for diastolic perfusion and thus, reduces maximum vasodilated flow. Increases in myocardial oxygen demand or reductions in arterial oxygen content (e.g. from anemia or hypoxemia) increase resting flow. These changes reduce coronary flow reserve, the ratio between dilated and resting coronary flow, and cause ischemia to develop at higher coronary pressures. Abbreviations: LV, left ventricular; HR, heart rate; SBP, systolic blood pressure; Hb, hemoglobin. Reprinted from Canty and Duncker6 with permission.
Under normal hemodynamic conditions, resting LV-CBF averages 0.7–1.0 ml/min per g of myocardium and can increase 4–5 fold during vasodilation in the absence of microcirculatory dysfunction.15,16,18 The ability to increase CBF above resting values in response to pharmacological vasodilation is termed coronary flow reserve (CFR).6 Maximum perfusion and CFR are reduced when the diastolic time available for (subendocardial) perfusion is decreased (e.g. during an increase in heart rate) or the compressive determinants of diastolic perfusion (preload and hence, radial wall stress) are increased (Fig 1B).15,16,18 Coronary reserve is also reduced when resting flow is increased, for example in response to increases in the hemodynamic determinants of O2 consumption (systolic pressure, heart rate, and contractility) or with reductions in arterial O2 supply (anemia and hypoxia). Hence, conditions can develop that favor the development of subendocardial ischemia in the presence of normal coronary arteries.1 Studies in conscious dogs in the basal resting state have shown that autoregulation of coronary flow can maintain resting flow in the presence of mean coronary pressures as low as 40 mm Hg.17 These coronary pressure levels are similar to those recorded in humans without symptoms of ischemia during balloon occlusions, using pressure wire micromanometers.19 The lower pressure range of autoregulation increases to higher pressure values during tachycardia due to an increase in flow requirements in conjunction with a decrease in diastolic perfusion time (Fig 1B).20
Mechanical and hemodynamic determinants of CBF
CBF is dependent upon the effective perfusion pressure and the resistive properties of the coronary vascular bed. The effective perfusion pressure of the coronary bed is the pressure-drop (ΔP) across the coronary vascular bed, with the input pressure being arotic pressure. However, because extravascular forces are exerted on the compressible intramural coronary vasculature by the surrounding myocardium, the effective output- or backpressure, i.e. the pressure at which flow becomes zero (termed zero flow pressure or Pf = 0), cannot simply be equated to right atrial pressure (Fig 1A). In LV hypertrophy (LVH), compressive effects from elevated LV diastolic pressure also impede perfusion via passive compression of microcirculatory vessels by elevated extra-vascular tissue pressure during diastole.
Extravascular compressive forces
During systole, cardiac contraction raises myocardial tissue pressure to values equal to LV pressure at the subendocardium. This declines to values near pleural pressure at the subepicardium.15 The increased extravascular pressure is transmitted across the vessel wall and provides an effective backpressure to flow, which produces a time-varying reduction in the driving pressure for coronary flow that impedes perfusion particularly to the subendocardium. Although this paradigm can explain variations in systolic coronary inflow, it is not able to account for the increase in coronary venous systolic outflow. To explain both impaired inflow and accelerated venous outflow, some investigators have proposed the concept of the intramyocardial pump.14 In this model, micro-circulatory vessels are compressed during systole and produce a capacitive discharge of blood that accelerates flow from the microcirculation to the coronary venous system. At the same time, the upstream capacitive discharge impedes systolic coronary arterial inflow. Although this explains the phasic variations in coronary arterial inflow and venous outflow, as well as its transmural distribution in systole, vascular capacitance cannot explain compressive effects related to elevated tissue pressure during diastole. This concept is supported by observations that increases in preload effectively raise the normal backpressure to coronary flow above coronary venous pressure levels.14 Thus, intramyocardial capacitance, compressive forces changing effective coronary backpressure as well as coronary resistance, and a time-varying driving pressure all contribute to the mechanical compressive determinants of phasic CBF.
Coronary vascular resistance
Coronary vascular resistance (CVR) can be divided into several components.18 Under normal circumstances, there is no measurable pressure drop in the epicardial arteries, indicating negligible conduit resistance. With the development of hemodynamically significant epicardial artery narrowing (more than 50% diameter reduction), the fixed conduit artery resistance begins to contribute significantly to total CVR and, when the vessel is severely narrowed (more than 90% diameter reduction), may reduce basal resting flow. The major component of CVR under normal conditions primarily arises from small arteries and arterioles, so called resistance vessels.21 This resistance is dynamic and distributed throughout the myocardium across a broad range of vessel sizes (20–400 μm in diameter). Because the diameter of these vessels, and hence their resistance can be changed substantially both by passive and active mechanisms they play a pivotal role in the regulation of CBF. Interestingly, there is normally little resistance contributed by capillaries and coronary venules, and their resistance remains fairly constant during changes in vasomotor tone.21 Thus, minimal CVR of the microcirculation is principally determined by the size and density of arterial resistance vessels, while changes in vasomotor tone enable substantial variations in CBF in the healthy heart.
Transmural variations in minimum CVR and diastolic driving pressure
The vulnerability of the LV subendocardium to compressive forces1 is partially compensated for by a lower minimal vascular resistance in the innermost LV layer, which is the result of increased densities of arterioles and capillaries as compared to the subepicardium. Because of this vascular gradient, subendocardial flow during maximal pharmacological vasodilation of the non-beating heart is greater than subepicardial perfusion14; CVR in the maximally vasodilated heart is also pressure-dependent, reflecting passive distention of arterial resistance vessels.14 Thus, minimal CVR obtained at a normal coronary distending pressure will increase when coronary pressure is reduced.19 The precise determinants of the effective driving pressure for diastolic perfusion continue to be controversial.14 Most experimental studies demonstrate that the effective backpressure Pf = 0 to LV-CBF is higher than right atrial pressure, and its minimum value is approximately 10 mm Hg in the maximally vasodilated heart. This increases to values close to LV diastolic filling pressure when preload is elevated above 20 mm Hg.22 Elevated preload reduces coronary driving pressure and diminishes subendocardial perfusion. It is particularly important in determining flow when coronary pressure is reduced by a stenosis, as well as in the severely hypertrophied or failing heart.16,23
Transmural variations in autoregulation
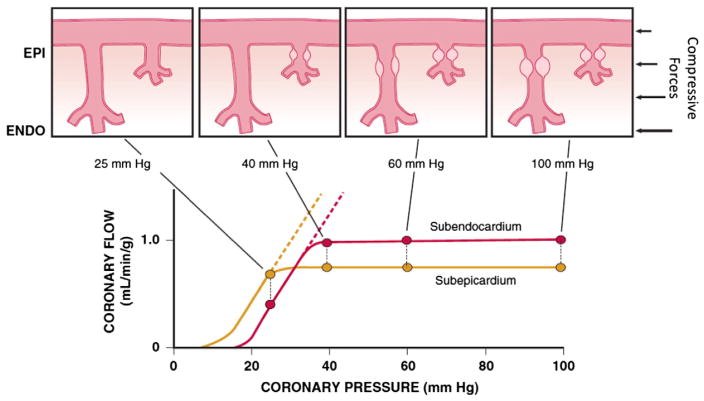
Figure 2 shows transmural variations in the lower autoregulatory pressure limit, which are responsible for the increased vulnerability of the subendocardium to ischemia.1 Subendocardial flow primarily occurs in diastole and resistance vessels are maximally vasodilated below a mean coronary pressure of 40 mm Hg.17 In contrast, subepicardial flow occurs throughout the cardiac cycle and is maintained until coronary pressure falls below 25 mm Hg. This difference arises from the more pronounced effects of systolic contraction on subendocardial vasodilator reserve,1 as well as the higher subendocardial oxygen consumption, requiring a higher resting flow level (Fig 2). The transmural difference in the minimal autoregulatory pressure translates into vulnerability of the subendocardium to ischemia in the presence of a coronary stenosis. Although there is no pharmacologically recruitable flow reserve during ischemia in the normal coronary circulation under resting conditions with low sympathetic activity,24 reductions in coronary flow below the lower limit of autoregulation can occur in the presence of pharmacologically recruitable coronary flow reserve during exercise.4,16
Fig. 2.
Transmural variations in coronary autoregulation and myocardial metabolism. Increased vulnerability of the subendocardium (ENDO; red) versus subepicardium (EPI; gold) to ischemia reflects the fact that autoregulation is exhausted at a higher coronary pressure (40 versus 25 mm Hg). This is the result of increased resting flow and oxygen consumption in the subendocardium and an increased sensitivity to systolic compressive effects because subendocardial flow only occurs during diastole. Subendocardial vessels become maximally vasodilated before those in the subepicardium as coronary artery pressure is reduced. These transmural differences can be increased further during tachycardia or during conditions with elevated preload, which reduce maximum subendocardial perfusion. Modified from Canty and Duncker6 with permission.
Structure and function of the coronary microcirculation
Individual coronary resistance arteries are a longitudinally distributed network and experimental studies of the coronary microcirculation have demonstrated considerable spatial heterogeneity of specific resistance vessel control mechanisms.4,25 To meet the metabolic requirements of the myocardium supplied by the downstream vascular bed, all resistance vessel segments need to dilate in an orchestrated manner, with a significant part of the resistance vessels being upstream of the site of metabolic control of coronary resistance. This is achieved independently of “metabolic signals” by sensing hemodynamic forces, such as intraluminal flow (shear stress–mediated control) or intraluminal pressure changes (myogenic control). Epicardial arteries (>400 μm in diameter) serve as conduit arteries that contribute little pressure drop (<5%) over a wide range of flows.21 Coronary arterial resistance vessels can be divided into small arteries (100–400 μm), which regulate their diameter in response to changes in wall shear stress and transmural pressure (myogenic response), and arterioles (<100 μm), which are sensitive to changes in local tissue metabolism and directly control perfusion of the low-resistance coronary capillary bed.26 Capillary density of the normal myocardium averages 3500/mm2, and is greater in the subendocardium than the subepicardium, enabling a higher O2 extraction in the subendocardium (83%) than in the subepicardium (70%).27
Most of the pressure drop in the microcirculation under basal conditions occurs across resistance arteries with a diameter of 50–200 μm, with little pressure drop occurring across small arteries of 200–400 μm.21 During pharmacological vasodilation with dipyridamole, vasodilation attenuates the pre-capillary drop in pressure across arterioles, while increasing the pressure drop across resistance arteries of 200–400 μm. Under these conditions, as much as 40% of the total CVR can reside in these small resistance arteries.28 There is also considerable heterogeneity in microcirculatory vasodilation to other stimuli. For example, as coronary pressure is reduced during autoregulation, vasodilation principally occurs in arterioles (<100 μm), whereas larger resistance arteries tend to decrease in diameter in response to the reduction in intraluminal distension pressure.29 In contrast, an increase in O2 consumption elicits a more uniform vasodilation of resistance vessels of all sizes.30 Similar heterogeneity in resistance vessel dilation is observed in response to endothelium-dependent agonists as well as pharmacological vasodilators.28 A special subset of coronary resistance vessels are the transmural penetrating arteries that course from the epicardium to the subendocardial plexus.4 These vessels are not only less sensitive to metabolic signals, but they are also beyond the reach of metabolic stimuli, when subendocardial ischemia occurs. Consequently, local vascular control mechanisms (i.e. flow and pressure) are critical determinants of diameter in this upstream resistance segment. This is particularly important since, even during maximal vasodilation, this segment harbors an additional longitudinal component of CVR that must be traversed before the subendocardial arteriolar microcirculation is reached. Due to this greater longitudinal pressure drop, pressures in coronary arterioles are lower in the subendocardium than in the subepicardium.28 This may contribute to vulnerability to subendocardial ischemia in pathophysiological states affecting the coronary microcirculation.
Hemodynamic forces regulating CVR
Because a significant part of CVR can be upstream from the segments that are under direct metabolic control, local vascular control mechanisms are critically important in orchestrating adequate regional tissue perfusion to the distal microcirculation. These mechanisms vary among different sizes and classes of coronary resistance vessels, which serves their diverse functions.
Resistance vessel responses to changes in shear stress
Small arteries and arterioles regulate their diameter in response to changes in local shear stress. This so called “flow-induced” dilation in isolated coronary arterioles was originally demonstrated by Kuo et al.25,31 They found this to be endothelium-dependent and mediated by nitric oxide (NO), as it could be abolished with NO synthase-inhibition. In contrast, in small arteries isolated from patients undergoing cardiac surgery flow-induced dilation is mediated by an endothelium-derived hyperpolarizing factor (EDHF).32,33 The disparity with animal studies may reflect age or species variability in the relative importance of EDHF versus NO in the coronary circulation and illustrates the presence of redundant mechanisms.34 Mechanisms also appear to vary as a function of vessel size, with studies in pigs demonstrating that EDHF is more important in epicardial conduit arteries,35 while NO dominates in the smaller arterial vessels.31 It also appears that EDHF represents a compensatory pathway that is normally inhibited by NO and becomes upregulated in disease states or exposure to risk factors, in which NO-mediated vasodilation is impaired.33,34 The exact identity of EDHF is subject to debate. Several studies have suggested that EDHF may be hydrogen peroxide (H2O2).34 Interestingly, a recent study by Gutterman and co-workers suggests that ceramide (a bioactive sphingolipid) is responsible for the shift from NO-mediated to H2O2-mediated flow-induced dilation.36 Notwithstanding the variability in isolated blood vessels, blocking NO synthase in the coronary circulation of humans reduces vasodilation to pharmacological endothelium-dependent agonists and blunt increases in CBF during metabolic vasodilation, indicating that NO-mediated vasodilation provides some contribution to overall CVR control in vivo.37
Resistance vessel responses to changes in wall stress
The myogenic response is the ability of vascular smooth muscle to respond to changes in vascular transmural pressure and, hence, wall stress changes.26 Thus, vessels decrease tone when pressure decreases and constrict when pressure increases. The myogenic response is a general property of vascular smooth muscle and is therefore present across a large range of coronary resistance artery sizes in animals as well as in humans,38 but in the coronary microcirculation in vivo it is particularly prominent in arterioles <100 μm. While the exact cellular mechanism remains incompletely understood, it is clear that the myogenic response depends on vascular smooth muscle calcium entry, possibly through stretch-activated L-type Ca2+- channels, leading to an increase in intracellular Ca2+-concentration that activates several downstream signaling pathways, ultimately eliciting cross-bridge activation.26 The changes in coronary resistance arising from the myogenic response restore local coronary flow back to the original level, supporting the concept that the myogenic response is a key mechanism in coronary autoregulation.29
Endothelium-dependent modulation of CVR
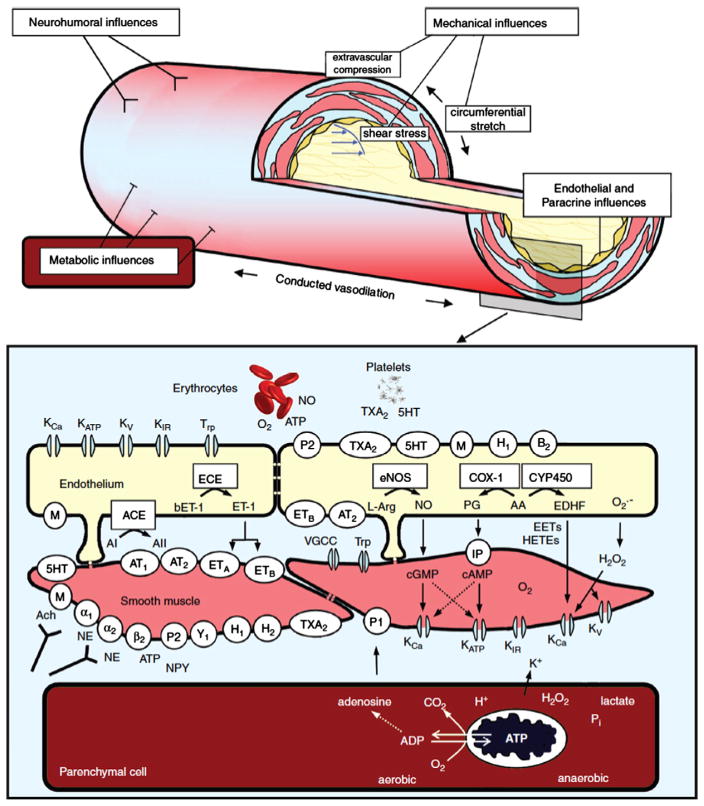
Coronary resistance vessels are modulated by a wide variety of paracrine factors that can be released from erythrocytes or platelets, circulating neurohormonal agonists, neural tone, and local control through vascular shear stress (Fig 3).3,4,6,39 The net effect of many of these factors depends critically on the presence of a functional endothelium. Furchgott and Zawadzki originally demonstrated that acetylcholine normally dilates arteries via an endothelium-dependent relaxing factor that was later identified to be NO.40 This binds to guanylyl cyclase and increases cyclic guanosine monophosphate (cGMP), resulting in vascular smooth muscle relaxation.41 When the endothelium is removed, the dilation to acetylcholine is converted to vasoconstriction, reflecting the effect of muscarinic receptor mediated vascular smooth muscle contraction. Subsequent studies have demonstrated that coronary resistance arteries also exhibit endothelial modulation of diameter and that the response to physical forces such as shear stress, as well as paracrine mediators, varies with resistance vessel size.25 The major endothelium-dependent biochemical pathways involved in regulating coronary resistance artery diameter include nitric oxide, prostaglandins, and EDHF (Fig 3). For an in-depth review of the role of these mediators in endothelium-dependent control of coronary resistance vessels the reader is referred to several recent reviews.3,6,34
Fig. 3.
Schematic drawing of an arteriole (top) and of endothelium, vascular smooth muscle and cardiomyocyte (at the bottom) illustrating mechanisms for control of vasomotor tone and diameter. Neurohumoral, endothelial, and metabolic influences are detailed in the bottom part of the figure. Abbreviations: KCa, calcium-activated K+ channel; KATP, ATP-sensitive K+channel; KV, voltage-gated K+ channel; KIR, inward rectifying K+ channel; Trp, transient receptor potential channels; O2, oxygen; ATP, adenosine triphosphate; NO, nitric oxide; TXA2, thromboxane A2 and receptor; 5HT, 5-hydroxytryptamine and receptor; P2, purinergic type 2 receptor; M, muscarinic receptor; H1 and H2, histamine type 1 and 2 receptors; B2, bradykinin type 2 receptor; ECE, endothelin-converting enzyme; bET-1, big endothelin-1; ET-1, endothelin-1; eNOS, endothelial nitric oxide synthase; L-arg, l-arginine; COX-1, cyclooxygenase 1; CYP450, cytochrome P450; ACE, angiotensin-converting enzyme; AI, angiotensin I; AII; angiotensin II; AT1, angiotensin type 1 receptor; AT2, angiotensin type 2 receptor; ETA, endothelin type A receptor; ETB, endothelin type B receptor; PG, prostaglandins; AA, arachidonic acid; EDHF, endothelium-derived hyperpolarizing factor; O2−, superoxide anion; VGCC, voltage-gated calcium channels; IP, prostacyclin receptor; EETs, epoxyeicosatrienoic acids; HETEs, hydroxyeicosatetraenoic acids; H2O2, hydrogen peroxide; α1, α1-adrenergic receptor; α2, α2-adrenergic receptor; β2, β2-adrenergic receptor; ACh, acetylcholine; NE, norepinephrine; NPY, neuropeptide Y; P1, purinergic type 1 receptor; and Y1, neuropeptide Y receptor. Reproduced from Laughlin et al.39 with permission.
Neural control of CVR
Sympathetic and parasympathetic (vagal) nerves innervate coronary resistance vessels and can affect tone through mechanisms directly on vascular smooth muscle cells as well as by stimulating the release of NO from the endothelium (Fig 3).3,39 The physiological role of vagal nerve control of CBF is uncertain, but coronary resistance arteries of humans and dogs are known to dilate to acetylcholine resulting in increases in CBF.3,39 In humans with atherosclerosis or risk factors for CAD the resistance vessel dilation to acetylcholine is attenuated.42
There is negligible sympathetic tone in the heart and coronary vessels under basal resting conditions. However, during sympathetic activation, coronary tone is modulated by norepinephrine released from sympathetic nerves innervating the myocardium and the coronary circulation, as well as by circulating norepinephrine and epinephrine.15,43 The effects of sympathetic activation on myocardial perfusion and coronary resistance vessel tone are complex and dependent on the net actions of β1-mediated increases in myocardial O2 consumption, direct β1 and β2-mediated coronary resistance vessel dilation, and α1-mediated constriction.3,39 Under normal healthy conditions, exercise-induced β-adrenergic receptor mediated “feed forward” dilation predominates, resulting in an increase in flow that is commensurate with the increase in myocardial O2 consumption.16 This neural control mechanism produces transient vasodilation, thereby preventing the development of subendocardial ischemia and buildup of local vasoactive metabolites during sudden increases in demand from exercise for example. In the presence of nonselective β-adrenoceptor blockade, sympathetic activation unmasks α1-mediated coronary artery constriction.16 Although flow is only mildly decreased, O2 delivery is maintained by increased O2 extraction resulting in a reduction in coronary venous O2 tension at similar levels of cardiac workload. Intense α1-adrenergic constriction can overcome intrinsic stimuli for metabolic vasodilation to result in ischemia in the presence of pharmacological vasodilator reserve.43 The role of pre- and postsynaptic α2 receptors in controlling CBF appears to be less significant, which may, at least in part be due to inhibition of norepinephrine release by presynaptic α2 receptor stimulation, as well as the presence of α2 receptors on the endothelium, leading to reduced vasoconstriction.16
Metabolic control of CVR
Despite increasing knowledge there is still no consensus regarding specific mediators of metabolic vasodilation, and CVR in any segment of the microcirculation is determined by the integration of local physical factors (e.g., pressure and flow), vasodilator metabolites (e.g., adenosine, PO2, and pH), autacoids, and neurohumoral modulation (Fig 3).3,39 Each of these mechanisms contributes to form the net coronary vascular smooth muscle tone, which may ultimately be controlled by opening and closing vascular smooth muscle ATP-sensitive K (KATP) channels,44 although other K+ channels are also likely involved.3 There is considerable redundancy in the available local control mechanisms.3,4 Thus, eliminating a single mechanism in experimental conditions by use of specific blockers or the early state of pathological conditions does not necessarily impair coronary autoregulation or metabolic flow regulation at normal coronary pressures.3,16 The lack of an important vasomotor mechanism can, however, be unmasked by stressing the heart and evaluating flow regulation at reduced pressures distal to a coronary stenosis at rest or during exercise.4 For an in depth review of this topic the reader is referred to elsewhere.2,3,39,44
Regulation of CBF in pathophysiological states
An epicardial artery stenosis arising from atherosclerosis increases total coronary resistance and thus reduces maximal CBF. In addition, abnormalities in coronary microcirculatory control can also contribute to causing myocardial ischemia in many patients with a coronary artery stenosis. For example, LVH is associated with lower CBF reserve due to failure of the coronary vascular tree to grow commensurate with the degree of LVH. Separating the role of an epicardial stenosis from coronary resistance vessels can be accomplished by simultaneously assessing coronary flow and distal coronary pressure using intracoronary transducers that are currently available for clinical care and reviewed elsewhere.19,45,46
Coronary artery stenosis
Stenosis pressure-flow relation
In the healthy heart, the angiographically visible epicardial coronary arteries are able to accommodate large increases in coronary flow without producing a significant drop in pressure and hence serve truly as conduit vessels to transport blood to the coronary resistance vasculature. This changes dramatically in CAD where the epicardial artery resistance (which increases with stenosis severity), becomes dominant and limits maximal myocardial perfusion.47
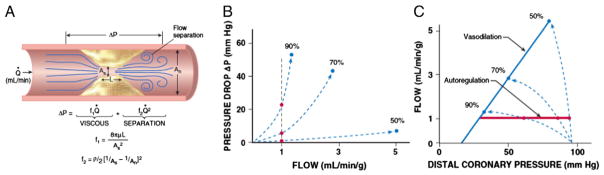
It is helpful to consider the idealized relation between stenosis severity, pressure drop, and flow, which has been validated in animals as well as humans studied in circumstances in which diffuse atherosclerosis and risk factors that can impair microcirculatory resistance vessel control are minimized.48 Fig 4A summarizes the major determinants of stenosis energy losses. The relation between pressure drop across a stenosis and coronary flow for stenoses between 30% and 90% diameter reduction can be described using the Bernoulli principle. The total pressure drop across a stenosis is governed by three hydrodynamic factors — viscous losses, separation losses, and turbulence. The single most important determinant of stenosis resistance for any given level of flow is the minimum lesional cross-sectional area within the stenosis.48 Because resistance is inversely proportional to the fourth power of radius, small dynamic changes in luminal area caused by thrombi or vasomotion in eccentric lesions (where vascular smooth muscle can relax or constrict in a portion of the stenosis) lead to major changes in the stenosis pressure-flow relation and impact on maximal perfusion. Separation losses determine the curvilinearity or “steepness” of the stenosis pressure-flow relation and become increasingly important as stenosis severity and/or flow rate increases.
Fig. 4.
A. Fluid mechanics of a stenosis. The pressure drop across a stenosis can be predicted by the Bernoulli equation. It is inversely related to the minimum stenosis cross-sectional area and varies with the square of the flow rate as stenosis severity increases. Abbreviations: ΔP, pressure drop; Q = flow; f1, viscous coefficient; f2, separation coefficient; As, area of the stenosis; An, area of the normal segment; L, stenosis length; μ, viscosity of blood; ρ, density of blood. Interrelation among the epicardial artery stenosis pressure flow relation (B), and the distal coronary pressure-flow relation (C). Red circles and lines depict resting flow and blue circles and lines maximal vasodilation for stenoses of 50, 70, and 90% diameter reduction. As shown in panel B, the stenosis pressure flow relation becomes extremely nonlinear as stenosis severity increases. As a result, there is very little pressure drop across a 50% stenosis, and distal coronary pressure and vasodilated flow remain near normal. However, a 90% stenosis critically impairs flow and, because of the steepness of the pressure flow relation, causes a marked reduction in distal coronary pressure. Modified from Canty and Duncker6 with permission.
Stenosis pressure drop and resistance increase exponentially as minimum cross-sectional area of the lesion decreases (Fig 4B). This reflects the fact that it becomes flow-dependent and varies with the square of the flow or flow velocity. As a result, the instantaneous stenosis resistance progressively increases during resistance vessel dilation. This becomes particularly important in determining the stenosis pressure-flow behavior for severely narrowed arteries and leads to a situation in which small reductions in luminal area result in large reductions in post-stenotic coronary pressure that limit maximum coronary perfusion.
Interrelation among distal coronary pressure, flow, and stenosis severity
Because maximum myocardial perfusion is ultimately determined by the coronary pressure distal to a stenosis, it is helpful to place the epicardial stenosis pressure-flow relation into the context of the coronary autoregulatory and vasodilated coronary pressure-flow relations, as depicted in Fig 4C. The effects of a stenosis on resting and vasodilated flows as a function of percentage diameter reduction, when diffuse intraluminal narrowing is absent and coronary microcirculatory resistance is normal, are summarized in Fig 4C. In the absence of microcirculatory dysfunction coronary flow can normally increase approximately five times from the resting flow values.47 As illustrated in Fig 4C, there is no significant pressure drop across a stenosis (ΔP) or stenosis-related alteration in maximal myocardial perfusion until stenosis severity exceeds a 50% diameter reduction (cross-sectional area reduction of 75%). As stenosis severity exceeds 50%, the curvilinear coronary stenosis pressure flow relation steepens and increases in stenosis resistance are accompanied by concomitant increases in ΔP across the stenosis (Fig 4B). This reduces distal coronary pressure, the major determinant of perfusion to the microcirculation, and maximum vasodilated flow (and CFR) decreases. A critical stenosis, one in which subendocardial flow reserve is completely exhausted at rest, usually develops when stenosis severity exceeds 90%. Under these circumstances, pharmacological vasodilation of subepicardial resistance vessels results in a reduction in distal coronary pressure that actually redistributes flow away from the subendocardium, leading to a “transmural steal” phenomenon.16
Concept of maximal perfusion and CFR
The concept of CFR was originally proposed by Gould.45 With technological advances, it has become possible to characterize this in humans using invasive catheter-based measurements of intracoronary pressure and flow as well as with noninvasive imaging of myocardial perfusion with positron emission tomography (PET), single-photon emission tomography (SPECT) and, more recently, cardiac magnetic resonance imaging (CMR). With these measurements, it has also become increasingly apparent that abnormalities in coronary microcirculatory control contribute to the functional significance of isolated epicardial artery stenosis in many patients with CAD, as well as lead to impaired CBF responses in the presence of normal coronary arteries. Because of these complexities, multiple complimentary approaches are frequently required to define limitations in myocardial perfusion that arise from stenosis severity vs. abnormalities of the coronary microcirculation6,45,49 The major indices currently used to quantify CFR are absolute flow reserve (maximal flow divided by basal flow), relative flow reserve (maximal myocardial perfusion in the stenosis region divided by maximal myocardial perfusion in the non-stenosis region) and fractional flow reserve (post-stenotic coronary pressure divided by aortic pressure during maximal vasodilation). For an in depth discussion of these indices the reader is referred to recent reviews published elsewhere.6,45
Impact of a chronic epicardial stenosis on the coronary microcirculation
As discussed above, an acute coronary artery inflow obstruction produces potent coronary resistance vessel dilation. In contrast, there is evidence that a chronic coronary artery stenosis, not only results in myocardial and interstitial,6,50 but also in structural and functional microvascular alterations distal to the stenotic artery. Thus, arteriolar inward remodeling5 and rarefaction51 have been reported, as well as increased vasoconstrictor responses to endothelin (due to a loss of ETB-mediated vasodilation) and blunting of myogenic responses.52 These findings support the concepts that endothelial dysfunction contributes to microvascular dysfunction distal to a stenosis, and does so via alterations in the control of vascular tone as well as through structural changes in microvessel diameter5 and densities.51 These findings indicate that a chronic epicardial stenosis may not only limit flow reserve by increasing proximal artery resistance, but also by producing microvascular dysfunction. This explains, at least in part, the observation that many patients with a significant coronary artery stenosis show an exaggerated reduction in coronary flow reserve for the level of stenosis severity.46
Impact of a chronic coronary artery occlusion on the coronary microcirculation
Following a total occlusion of a coronary artery, residual perfusion to the affected myocardium can be provided by pre-existing coronary collateral channels that are recruited when an intercoronary pressure gradient between the source and recipient vessel develops. In most animal species, the collateral flow during occlusion is less than 10% of the resting flow levels and is insufficient to maintain tissue viability for longer than 20 minutes. Coronary pressure during balloon angioplasty occlusion in patients undergoing percutaneous coronary intervention (PCI) is indicative of coronary collateral flow and exhibits considerable variability. In humans in which the existing coronary collaterals are sparse, coronary pressure during balloon angioplasty occlusion falls to ~10 mm Hg, and ischemia develops. However in some patients, collaterals develop to the point where they are sufficient to not only maintain resting perfusion normal but also prevent stress-induced ischemia at submaximal cardiac workloads. The “collateral flow index” is calculated as coronary wedge pressure during PCI balloon occlusion minus venous pressure divided by the mean arterial blood pressure. If this index is greater than 0.25, collateral flow is sufficient and ischemia does not develop during PCI balloon occlusion at rest.19 These patients have a lower CVD event rate and improved survival in a large observational cross sectional study.53
Regulation of CBF in the collateralized heart
The control of CBF to collateral-dependent myocardium is governed by a series resistance arising from interarterial collateral anastomoses, largely epicardial, as well as the native downstream microcirculation. Collateral resistance is therefore the major determinant of perfusion and coronary pressure distal to a chronic occlusion which is already near the lower autoregulatory pressure limit. Because of this, subendocardial perfusion is critically dependent on mean aortic pressure and LV preload with ischemia easily provoked by systemic hypotension, increases in LV end-diastolic pressure, and tachycardia. Like the distal resistance vessels, collaterals constrict when NO synthesis is blocked, which aggravates myocardial ischemia and can be overcome by nitroglycerin.4,16 Experimental studies have demonstrated that coronary collaterals are under tonic dilation from vasodilator prostaglandins, and blocking cyclooxygenase with aspirin exacerbates myocardial ischemia in dogs.23 The role of prostanoids in human coronary collateral resistance regulation is unknown.
The distal microcirculatory resistance in collateral-dependent myocardium appears to be regulated by mechanisms similar to those present in the normal circulation but is characterized by attenuated endothelium-dependent vasodilation as compared to normal vessels,16 similar to what has been observed with a chronic coronary artery occlusion.52 Interestingly, the remote normally perfused zone in collateralized hearts also shows alterations in coronary resistance vessel control, suggesting that abnormalities are not restricted to the collateral-dependent region. The extent that these microcirculatory abnormalities alter the normal metabolic and coronary autoregulatory responses in collateral-dependent and remote myocardial regions is unknown.16
Impact of cardiac hypertrophy on the coronary microcirculation
The effects of cardiac hypertrophy on CBF and its regulation are complex (Fig 5) and need to be thought of in terms of the absolute flow level (e.g., measured with an intracoronary Doppler probe) as well as the flow per gram of myocardium (e.g. measured by positron emission tomography). With acquired LVH (such as in hypertension), resting flow per gram of myocardium remains constant, but the increase in LV mass necessitates an increase in the absolute level of resting flow (ml/min) through the coronary artery.10,54 In terms of maximal perfusion, pathological LVH does not result in appreciable vascular proliferation (as opposed to physiological LVH produced by exercise training) and coronary resistance vessel densities remain essentially unchanged. Thus, while maximum absolute flow (ml/min) during vasodilation remains unchanged, the increase in LV mass reduces the maximum perfusion per gram of myocardium. The net effect of LVH is that CFR at any given coronary arterial pressure is reduced in a fashion that is inversely related to the change in LV mass. For example, in the absence of a change in mean aortic pressure, a two-fold increase in LV mass, as is associated with severe LVH, can reduce CFR in a non-stenotic artery from 4 to 2. This will increase the functional severity of any anatomical degree of coronary artery narrowing and can even precipitate subendocardial ischemia with normal coronary arteries. Moreover, structural alterations in microvessels, most notably medial hypertrophy in arterioles in pressure-overload hypertrophy,2,11 will further contribute to a reduction in coronary reserve. Finally, it is likely that the increased diffusion distance due to the increased size of cardiac fibers and reduced capillary density also contribute to the development of tissue hypoxia.10
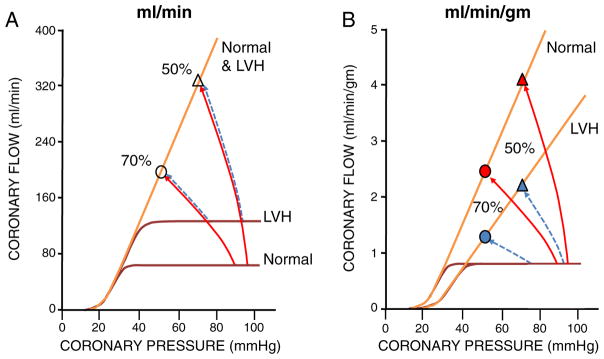
Fig. 5.
Effects of hypertrophy on absolute flow (ml/min) and flow per gm of tissue (ml/min/gm). A. With acquired hypertrophy, myocardial mass increases without proliferation of the microcirculatory resistance arteries. Absolute LV flow is shown in panel A. The increase in LV mass causes a proportional increase in absolute flow at rest although the maximum absolute flow per minute during vasodilation remains unchanged. B. When tissue perfusion is assessed using flow per gm of myocardium (as obtained using PET for example), the maximum flow per gram of tissue falls inversely with the increase in LV mass. In contrast, the resting flow per gram of myocardium remains constant since the increase in absolute resting flow is proportional to the increase in LV mass. Regardless of whether absolute flow or flow per gm is measured, the net effect of these opposing actions is to decrease coronary flow reserve at any coronary pressure in LVH. As a result of the reduction in microcirculatory reserve in the absence of a coronary stenosis, the functional significance of a 50% stenosis (triangles) in the hypertrophied heart could approach a more severe stenosis (in the example, 70%, circles) in normal myocardium. This can even result in ischemia with normal coronary arteries during stress. Modified from Canty and Duncker6 with permission.
In patients with coronary artery disease, some degree of LVH is common, as hypertension is an important risk factor for CAD. The presence of LVH will contribute to reductions in CFR, independently of stenosis severity.55 The actual CFR in LVH will be critically dependent on the underlying cause of LVH and its effects on coronary driving pressure. A similar degree of hypertrophy caused by untreated systemic hypertension will have a higher CFR than in aortic stenosis, in which mean arterial pressure remains normal.10 Similarly, when LVH is from systolic hypertension with increased pulse pressure caused by reduced aortic compliance, the accompanying reduction in diastolic pressure will result in a lower coronary flow reserve, as myocardial perfusion occurs primarily in diastole. Conversely, the increase in LV diastolic filling pressure that typically accompanies LVH results in elevated Pf = 0, thereby further contributing to a reduction in CFR.23 Finally, there is also evidence that alterations in the control of coronary microvascular tone may occur in hearts with pathological LVH. These include endothelial dysfunction, enhanced α-adrenergic vasoconstriction, downregulation of K+ channels and enhanced expression of smooth muscle Ca2+ channels.3,11 These observations suggest that not only structural but also functional abnormalities of the coronary microcirculation contribute to perturbations in the regulation of CBF in the hypertrophied heart.
Coronary microvascular dysfunction
Measurements of coronary flow reserve in humans with risk factors for atherosclerosis are systematically lower than in healthy individuals without CAD risk factors.54,56,57 Much of this may arise from abnormal local resistance vessel control via impaired endothelial-dependent vasodilation arising from NO inactivation associated with risk factors for CAD.3 Kuo and colleagues have demonstrated that experimental hypercholesterolemia markedly attenuates the dilation of coronary arterioles in response to shear stress as well as pharmacological agonists that stimulate NO synthase in the absence of epicardial stenosis.58 This was reversed with L-arginine, suggesting reduced substrate availability or overcoming the effect of endogenously produced methylated L-arginines, such as ADMA that are responsible for impaired NO synthesis or availability.59
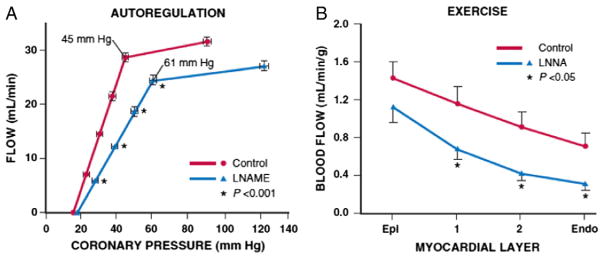
These in vitro abnormalities in NO-mediated vasodilation can be functionally significant and impair the ability of the cardiac muscle to regulate CBF. Fig 6A shows the effects of inhibiting NO on the regulation of CBF in normal healthy dogs.60 Although resting blood flow is not altered, there is a marked increase in the coronary pressure at which intrinsic autoregulatory adjustments become exhausted, with flow beginning to decrease at a distal coronary pressure of 60 versus 45 mm Hg, approximately similar to the shift occurring in response to a twofold increase in heart rate. In vivo microcirculatory studies have demonstrated that inhibiting NO production prevents resistance arteries from dilating maximally in response to shear stress.62 This likely reflects excess resistance in the transmural penetrating arteries, which are upstream of metabolic stimuli for vasodilation and extremely dependent on shear stress as a stimulus for local vasodilation. These functional abnormalities amplify the physiological effects of a coronary stenosis, resulting in the development of subendocardial ischemia at a lower workload (Fig 6B).61
Fig. 6.
Impaired microcirculatory control with abnormal NO-mediated endothelium-dependent resistance artery dilation. A. Effects of blocking nitric oxide synthase (NOS) with the L-arginine analog LNAME in chronically instrumented dogs. There is an increase in the lower autoregulatory pressure limit, resulting in the onset of ischemia at a coronary pressure of 61 mm Hg versus 45 mm Hg under normal conditions that occurred without a change in heart rate (modified from Smith and Canty60). B. Transmural perfusion before and after blocking NO-mediated dilation with LNNA in exercising dogs subjected to a coronary stenosis. Although coronary pressure and hemodynamics were similar, blood flow was lower in each layer of the heart after blocking NOS and was not overcome by metabolic dilator mechanisms during ischemia. Collectively, these experimental data support the notion that abnormalities in endothelium-dependent microvascular vasodilation can amplify the functional effects of a proximal coronary stenosis (modified from Duncker and Bache61). Abbreviations: Endo, endocardium; Epi, epicardium; LNAME, NG-nitro-L-arginine methyl ester; LNNA, NG-nitro-L-arginine. Reprinted from Canty and Duncker6 with permission.
The prognostic importance of abnormalities in coronary resistance vessel control is underscored by data in women evaluated for chest pain felt to be of ischemic origin.63,64 Abnormalities in CFR and endothelium-dependent vasodilation are common in women with insignificant epicardial CAD. These changes negatively affect prognosis and can produce metabolic evidence of myocardial ischemia as assessed by CMR spectroscopy.65 While these findings underline the importance of understanding gender differences in the regulation of CBF and development of CAD,64 a recent study indicates that coronary microvascular dysfunction is equally prevalent in both men and women and imparts a similarly poor prognosis.66
Impact of coronary microvascular dysfunction on physiological measures of stenosis severity
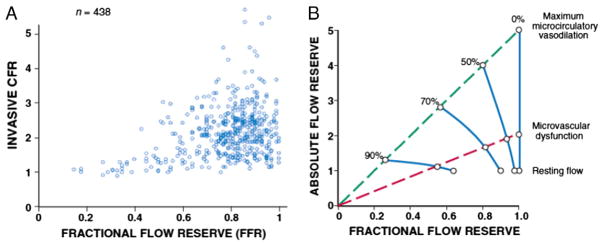
If microcirculatory function is normal, quantitative measures of stenosis severity during vasodilation that are derived using absolute flow reserve and fractional flow reserve should all be closely related. Unfortunately, this is the exception rather than the rule and microvascular dysfunction and/or variability in the microcirculatory response to pharmacological vasodilation dissociate the idealized relations between various indices of CFR for a given stenosis severity. Fig 7A summarizes the observed variability, showing the relation between paired invasive measurements of absolute flow reserve vs. distal coronary pressure derived FFR.46 These measurements demonstrate several points. As shown in Fig 7A, hemodynamically insignificant stenoses (i.e. FFR > 0.8) can have absolute flow reserves that vary between 1 and over 5. While this variability decreases when FFR is less than 0.8 it is still considerable until FFR falls below 0.5. There is also a wide variation between indices of absolute flow reserve and relative perfusion differences derived from quantitative PET perfusion measurements.46
Fig. 7.
A. There is a wide variation in paired measurements of functional stenosis severity using different indices of flow reserve in the same patient. Simultaneous intracoronary catheter based measurements of absolute flow reserve are compared to fractional flow reserve (FFR). This variability reflects differences in the contribution of the microcirculation and stenosis in individual patients (adapted from Johnson et al.46). B. Hypothetical effects of microvascular dysfunction on the stenosis pressure flow relation and measurements of fractional flow reserve. The upper blue dashed line shows the idealized linear relation between absolute flow reserve and FFR when the coronary microcirculation is normal and maximally vasodilated. The lower red dashed line indicates the relation between absolute flow reserve and FFR when there is microvascular dysfunction. Individual stenoses are illustrated by the solid blue lines. The presence of microvascular dysfunction will limit vasodilation. Thus, absolute flow reserve will be reduced and overestimate stenosis severity. In contrast, since distal coronary pressure is higher with submaximal vasodilation, fractional flow reserve, and relative flow reserve will underestimate stenosis severity. It is likely that these interactions contribute to the variability demonstrated in Fig 7A. Modified from Canty and Duncker6 with permission.
Experimental studies show that the variability in microvascular dysfunction and submaximal pharmacological vasodilator responses can have a significant impact on assessing the physiological significance of a coronary stenosis using FFR (or relative perfusion with imaging). This is schematized in Fig 7B. The two dashed lines show idealized relations between absolute flow reserve and FFR. Microvascular dysfunction in the presence of normal coronary arteries (0% stenosis) attenuates CFR despite an FFR of 1. Conversely, for any given stenosis, the FFR measured in the presence of microvascular disease will be higher than when vasodilator responses are normal. As the pressure-drop across the stenosis is flow-dependent, FFR will underestimate the physiological severity of the stenosis when maximum vasodilation is not achieved. All of these factors contribute to at least some of the discordance between FFR and CFR observed in clinical studies and underscore the importance of combining both pressure- and flow-derived indices to assess vasodilator reserve of the total coronary vascular bed. Indeed, the availability of high-fidelity pressure and flow measurements on a single wire has now facilitated the development of approaches to assess the stenosis pressure-flow relation as well as abnormalities in microcirculatory reserve by determining FFR and absolute CFR simultaneously.49,67 When assessed together, these measurements have the potential to identify circumstances in which mixed abnormalities from a stenosis and abnormal microcirculation contribute to the functional impact of a coronary stenosis.
Future perspectives
The major factors determining myocardial perfusion and O2 delivery that were established several decades ago have been incorporated into how we manage patients with angina and have stood the test of time. The basic understanding of the fluid mechanical behavior of coronary stenoses has also been translated to the cardiac catheterization laboratory where measurements of coronary pressure distal to a stenosis and CBF are routinely obtained. These physiological concepts now facilitate routine clinical decision making in a fashion that favorably impacts outcomes.
Despite progress in advancing our mechanistic understanding of the coronary circulation in health and CAD, important gaps remain in our basic knowledge as well as the translation of this to the clinical setting. For example, while basic research has identified the importance of physical factors such as shear stress and local coronary pressure in regulating isolated coronary resistance vessels, how these mechanisms interact in a complex vascular network to bring about the phenomenon of autoregulation and metabolic coronary vasodilation remains unanswered. Finally, although the role of microcirculatory dysfunction in symptoms as well as prognosis of patients with CAD is increasingly recognized, the mechanisms behind the cross-talk between the different cell-types in the heart and their impact on cardiac structure and function are a particularly important area of future investigation. For example, there is evidence that risk factors resulting in endothelial dysfunction in the coronary micro-circulation can adversely affect fibroblast and cardiomyocyte function through paracrine signaling,68,69 contributing to myocardial stiffening and possibly heart failure with preserved ejection fraction.70 These observations underscore the importance of the need for continued bench to bedside translational investigation in these and other areas in order to expand our fundamental knowledge and improve the care of patients with chronic IHD.
Acknowledgments
Financial support: DJD: European Commission (FP7-Health-2010; MEDIA-261409). AK: SMARTER, European Union’s Seventh Framework Programme grant 606998. Hungarian National Scientific Research Found (OTKA) K-108444. DM: European Commission (FP7-Health-2010; MEDIA-261409). JMC: National Heart Lung and Blood Institute (HL 55324; HL 61610) and the Albert and Elizabeth Rekate fund.
Abbreviations and Acronyms
- CAD
coronary artery disease
- CBF
coronary blood flow
- CFR
coronary flow reserve
- cGMP
cyclic guanylyl-monophosphate
- CMR
cardiac magnetic resonance
- CVD
cardiovascular disease
- CVR
coronary vascular resistance
- EDHF
endothelium-derived hyperpolarizing factor
- FFR
fractional flow reserve
- H2O2
hydrogen peroxide
- HF
heart failure
- IHD
ischemic heart disease
- KATP
ATP-sensitive potassium channel
- LV
left ventricle
- LVH
left ventricular hypertrophy
- NO
nitric oxide
- O2
oxygen
- PCI
percutaneous coronary intervention
- PET
positron emission tomography
- SPECT
single photon emission computed tomography
Footnotes
Statement of Conflict of Interest: There are no conflicts of interest to declare for any of the authors.
References
- 1.Hoffman JIE. Transmural myocardial perfusion. Prog Cardiovasc Dis. 1987;29:429–464. doi: 10.1016/0033-0620(87)90016-8. [DOI] [PubMed] [Google Scholar]
- 2.Tomanek RJ. Coronary Vasculature: Development, Structure-Function, and Adaptations. New York: Springer; 2013. [Google Scholar]
- 3.Tune JD. Morgan & Claypool Life Sciences. 2014. Coronary circulation. [Google Scholar]
- 4.Duncker DJ, Bache RJ. Regulation of coronary vasomotor tone under normal conditions and during acute myocardial hypoperfusion. Pharmacol Ther. 2000;86:87–110. doi: 10.1016/s0163-7258(99)00074-1. [DOI] [PubMed] [Google Scholar]
- 5.Canty JM, Jr, Suzuki G. Myocardial perfusion and contraction in acute ischemia and chronic ischemic heart disease. J Mol Cell Cardiol. 2012;52:822–831. doi: 10.1016/j.yjmcc.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canty JM, Jr, Duncker DJ. Coronary blood flow and myocardial ischemia. In: Bonow RO, Mann DL, Zipes DP, Libby P, editors. Braunwald’s Heart Disease. 10. Philadelphia: Elsevier; 2014. [Google Scholar]
- 7.Berwick ZC, Dick GM, Tune JD. Heart of the matter: coronary dysfunction in metabolic syndrome. J Mol Cell Cardiol. 2012;52:848–856. doi: 10.1016/j.yjmcc.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belin de Chantemele EJ, Stepp DW. Influence of obesity and metabolic dysfunction on the endothelial control in the coronary circulation. J Mol Cell Cardiol. 2012;52:840–847. doi: 10.1016/j.yjmcc.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J. 2014;35:1101–1111. doi: 10.1093/eurheartj/eht513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bache RJ. Effects of hypertrophy on the coronary circulation. Prog Cardiovasc Dis. 1988;31:403–440. doi: 10.1016/0033-0620(88)90005-9. [DOI] [PubMed] [Google Scholar]
- 11.Camici PG, Olivotto I, Rimoldi OE. The coronary circulation and blood flow in left ventricular hypertrophy. J Mol Cell Cardiol. 2012;52:857–864. doi: 10.1016/j.yjmcc.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 12.Merkus D, Duncker DJ. Coronary microvascular dysfunction in post-infarct remodelled myocardium. Eur Heart J. 2014;16:A74–A79. [Google Scholar]
- 13.Canty JM, Jr, Brooks A. Phasic volumetric coronary venous outflow patterns in conscious dogs. Am J Physiol. 1990;258:H1457–H1463. doi: 10.1152/ajpheart.1990.258.5.H1457. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman JIE, Spaan JAE. Pressure-flow relations in coronary circulation. Physiol Rev. 1990;70:331–390. doi: 10.1152/physrev.1990.70.2.331. [DOI] [PubMed] [Google Scholar]
- 15.Feigl EO. Coronary physiology. Physiol Rev. 1983;63:1–205. doi: 10.1152/physrev.1983.63.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev. 2008;88:1009–1086. doi: 10.1152/physrev.00045.2006. [DOI] [PubMed] [Google Scholar]
- 17.Canty JM., Jr Coronary pressure-function and steady-state pressure-flow relations during autoregulation in the unanesthetized dog. Circ Res. 1988;63:821–836. doi: 10.1161/01.res.63.4.821. [DOI] [PubMed] [Google Scholar]
- 18.Klocke FJ. Coronary blood flow in man. Prog Cardiovasc Dis. 1976;XIX:117–166. doi: 10.1016/0033-0620(76)90020-7. [DOI] [PubMed] [Google Scholar]
- 19.Kern MJ, Lerman A, Bech JW, et al. Physiological assessment of coronary artery disease in the cardiac catheterization laboratory. a scientific statement from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Circulation. 2006;114:1321–1341. doi: 10.1161/CIRCULATIONAHA.106.177276. [DOI] [PubMed] [Google Scholar]
- 20.Canty JM, Jr, Giglia J, Kandath D. Effect of tachycardia on regional function and transmural myocardial perfusion during graded coronary pressure reduction in conscious dogs. Circulation. 1990;82:1815–1825. doi: 10.1161/01.cir.82.5.1815. [DOI] [PubMed] [Google Scholar]
- 21.Chilian WM, Layne SM, Klausner EC, Eastham CL, Marcus ML. Redistribution of coronary microvascular resistance produced by dipyridamole. Am J Physiol. 1989;256:H383–H390. doi: 10.1152/ajpheart.1989.256.2.H383. [DOI] [PubMed] [Google Scholar]
- 22.Aversano T, Klocke FJ, Mates RE, Canty JM., Jr Preload-induced alterations in capacitance-free diastolic pressure-flow relations. Am J Physiol. 1984;246:H410–H417. doi: 10.1152/ajpheart.1984.246.3.H410. [DOI] [PubMed] [Google Scholar]
- 23.Duncker DJ, Zhang J, Bache RJ. Coronary pressure-flow relation in left ventricular hypertrophy: importance of changes in back pressure versus changes in minimum resistance. Circ Res. 1993;72:579–587. doi: 10.1161/01.res.72.3.579. [DOI] [PubMed] [Google Scholar]
- 24.Canty JM, Jr, Smith TP., Jr Adenosine-recruitable flow reserve is absent during myocardial ischemia in unanesthetized dogs studied in the basal state. Circ Res. 1995;76:1079–1087. doi: 10.1161/01.res.76.6.1079. [DOI] [PubMed] [Google Scholar]
- 25.Kuo L, Davis MJ, Chilian WM. Longitudinal gradients for endothelium-dependent and -independent vascular responses in the coronary microcirculation. Circulation. 1995;92:518–525. doi: 10.1161/01.cir.92.3.518. [DOI] [PubMed] [Google Scholar]
- 26.Davis MJ, Hill MA, Kuo L. Local regulation of microvascular perfusion. In: Tuma RF, Duran WN, Ley K, editors. Microcirculation. 2. Boston: Elsevier; 2008. pp. 161–284. [Google Scholar]
- 27.Weiss HR, Sinha AK. Regional oxygen saturation of small arteries and veins in the canine myocardium. Circ Res. 1978;42:119–126. doi: 10.1161/01.res.42.1.119. [DOI] [PubMed] [Google Scholar]
- 28.Zhang C, Rogers P, Merkus D, et al. Regulation of coronary microvascular resistance in health and disease. In: Tuma RF, Duran WN, Ley K, editors. Microcirculation. 2. Boston: Elsevier; 2008. pp. 521–549. [Google Scholar]
- 29.Kanatsuka H, Lamping KG, Eastham CL, Marcus ML. Heterogeneous changes in epimyocardial microvascular size during graded coronary stenosis. Evidence of the microvascular site for autoregulation. Circ Res. 1990;66:389–396. doi: 10.1161/01.res.66.2.389. [DOI] [PubMed] [Google Scholar]
- 30.Kanatsuka H, Lamping KG, Eastham CL, Dellsperger KC, Marcus ML. Comparison of the effects of increased myocardial oxygen consumption and adenosine on the coronary microvascular resistance. Circ Res. 1989;65:1296–1305. doi: 10.1161/01.res.65.5.1296. [DOI] [PubMed] [Google Scholar]
- 31.Kuo L, Davis MJ, Chilian WM. Endothelium-dependent, flow-induced dilation of isolated coronary arterioles. Am J Physiol. 1990;259:H1063–H1070. doi: 10.1152/ajpheart.1990.259.4.H1063. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Gutterman DD. Vascular control in humans: focus on the coronary microcirculation. Basic Res Cardiol. 2009;104:211–227. doi: 10.1007/s00395-009-0775-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miura H, Wachtel RE, Liu Y, et al. Flow-induced dilation of human coronary arterioles: important role of Ca2+-activated K+ channels. Circulation. 2001;103:1992–1998. doi: 10.1161/01.cir.103.15.1992. [DOI] [PubMed] [Google Scholar]
- 34.Beyer AM, Gutterman DD. Regulation of the human coronary microcirculation. J Mol Cell Cardiol. 2012;52:814–821. doi: 10.1016/j.yjmcc.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dube S, Canty JM., Jr Shear-stress induced vasodilation in porcine coronary conduit arteries is independent of nitric oxide release. Am J Physiol. 2001;280:H2581–H2590. doi: 10.1152/ajpheart.2001.280.6.H2581. [DOI] [PubMed] [Google Scholar]
- 36.Freed JK, Beyer AM, LoGiudice JA, Hockenberry JC, Gutterman DD. Ceramide changes the mediator of flow-induced vasodilation from nitric oxide to hydrogen peroxide in the human microcirculation. Circ Res. 2014;115:525–532. doi: 10.1161/CIRCRESAHA.115.303881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quyyumi AA, Dakak N, Andrews NP, Gilligan DM, Panza JA, Cannon RO., III Contribution of nitric oxide to metabolic coronary vasodilation in the human heart. Circulation. 1995;92:320–326. doi: 10.1161/01.cir.92.3.320. [DOI] [PubMed] [Google Scholar]
- 38.Miller FJ, Dellsperger KC, Gutterman DD. Myogenic constriction of human coronary arterioles. Am J Physiol Heart Circ Physiol. 1997;273:H257–H264. doi: 10.1152/ajpheart.1997.273.1.H257. [DOI] [PubMed] [Google Scholar]
- 39.Laughlin MH, Davis MJ, Secher NH, et al. Peripheral circulation. Compr Physiol. 2012;2:321–447. doi: 10.1002/cphy.c100048. [DOI] [PubMed] [Google Scholar]
- 40.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 41.Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 42.Ludmer PL, Selwyn AP, Shook TL, et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 43.Heusch G, Baumgart D, Camici P, et al. α-Adrenergic coronary vasoconstriction and myocardial ischemia in humans. Circulation. 2000;101:689–694. doi: 10.1161/01.cir.101.6.689. [DOI] [PubMed] [Google Scholar]
- 44.Duncker DJ, Bache RJ, Merkus D. Regulation of coronary resistance vessel tone in response to exercise. J Mol Cell Cardiol. 2012;52:802–813. doi: 10.1016/j.yjmcc.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 45.Gould KL. Does coronary flow trump coronary anatomy? J Am Coll Cardiol Img. 2009;2:1009–1023. doi: 10.1016/j.jcmg.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 46.Johnson NP, Kirkeeide RL, Gould KL. Is discordance of coronary flow reserve and fractional flow reserve due to methodology or clinically relevant coronary pathophysiology? JACC Cardiovasc Imaging. 2012;5:193–202. doi: 10.1016/j.jcmg.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 47.Wilson RF, Marcus ML, White CW. Prediction of the physiologic significance of coronary arterial lesions by quantitative lesion geometry in patients with limited coronary artery disease. Circulation. 1987;75:723–732. doi: 10.1161/01.cir.75.4.723. [DOI] [PubMed] [Google Scholar]
- 48.Klocke FJ. Measurements of coronary blood flow and degree of stenosis: current clinical implications and continuing uncertainties. J Am Coll Cardiol. 1983;1:31–41. doi: 10.1016/s0735-1097(83)80008-4. [DOI] [PubMed] [Google Scholar]
- 49.van de Hoef TP, Nolte F, Rolandi MC, et al. Coronary pressure-flow relations as basis fort he understanding of coronary physiology. J Mol Cell Biol. 2012;52:786–793. doi: 10.1016/j.yjmcc.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 50.Canty JM, Jr, Fallavollita JA. Pathophysiologic basis of hibernating myocardium. In: Zaret BL, Beller GA, editors. Clinical nuclear cardiology: state of the art and future direction. 4. Philadelphia: Elsiever Publishing; 2010. pp. 577–593. [Google Scholar]
- 51.Urbieta Caceres VH, Lin J, Zhu XY, et al. Early experimental hypertension preserves the myocardial microvasculature but aggravates cardiac injury distal to chronic coronary artery obstruction. Am J Physiol Heart Circ Physiol. 2011;300:H693–H701. doi: 10.1152/ajpheart.00516.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sorop O, Merkus D, de Beer VJ, et al. Functional and structural adaptations of coronary microvessels distal to a chronic coronary artery stenosis. Circ Res. 2008;102:795–803. doi: 10.1161/CIRCRESAHA.108.172528. [DOI] [PubMed] [Google Scholar]
- 53.Meier P, Gloekler S, Zbinden R, et al. Beneficial effect of recruitable collaterals: a 10-year follow-up study in patients with stable coronary artery disease undergoing quantitative collateral measurements. Circulation. 2007;116:975–983. doi: 10.1161/CIRCULATIONAHA.107.703959. [DOI] [PubMed] [Google Scholar]
- 54.Lanza GA, Crea F. Primary coronary microvascular dysfunction: clinical presentation, pathophysiology, and management. Circulation. 2010;121:2317–2325. doi: 10.1161/CIRCULATIONAHA.109.900191. [DOI] [PubMed] [Google Scholar]
- 55.Camici PG, d’Amati G, Rimoldi O. Coronary microvascular dysfunction: mechanisms and functional assessment. Nat Rev Cardiol. 2014 doi: 10.1038/nrcardio.2014.160. http://dx.doi.org/10.1038/nrcardio.2014.160. [DOI] [PubMed]
- 56.Marzilli M, Merz CN, Boden WE, et al. Obstructive coronary atherosclerosis and ischemic heart disease: an elusive link! J Am Coll Cardiol. 2012;60:951–956. doi: 10.1016/j.jacc.2012.02.082. [DOI] [PubMed] [Google Scholar]
- 57.Herrmann J, Kaski JC, Lerman A. Coronary microvascular dysfunction in the clinical setting: from mystery to reality. Eur Heart J. 2012;33:2771–2783. doi: 10.1093/eurheartj/ehs246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuo L, Davis MJ, Cannon MS, Chilian WM. Pathophysiological consequences of atherosclerosis extend into the coronary microcirculation: restoration of endothelium-dependent responses by l-arginine. Circ Res. 1992;70:465–476. doi: 10.1161/01.res.70.3.465. [DOI] [PubMed] [Google Scholar]
- 59.Chen JW, Hsu NW, Wu TC, Lin SJ, Chang MS. Long-term angiotensin-converting enzyme inhibition reduces plasma asymmetric dimethylarginine and improves endothelial nitric oxide bioavailability and coronary microvascular function in patients with syndrome X. Am J Cardiol. 2002;90:974–982. doi: 10.1016/s0002-9149(02)02664-4. [DOI] [PubMed] [Google Scholar]
- 60.Smith TP, Jr, Canty JM., Jr Modulation of coronary autoregulatory responses by nitric oxide: evidence for flow-dependent resistance adjustments in conscious dogs. Circ Res. 1993;73:232–240. doi: 10.1161/01.res.73.2.232. [DOI] [PubMed] [Google Scholar]
- 61.Duncker DJ, Bache RJ. Inhibition of nitric oxide production aggravates myocardial hypoperfusion during exercise in the presence of a coronary artery stenosis. Circ Res. 1994;74:629–640. doi: 10.1161/01.res.74.4.629. [DOI] [PubMed] [Google Scholar]
- 62.Jones CJ, Kuo L, Davis MJ, DeFily DV, Chilian WM. Role of nitric oxide in the coronary microvascular responses to adenosine and increased metabolic demand. Circulation. 1995;91:1807–1813. doi: 10.1161/01.cir.91.6.1807. [DOI] [PubMed] [Google Scholar]
- 63.Maas AH, van der Schouw YT, Regitz-Zagrosek V, et al. Red alert for women’s heart: the urgent need for more research and knowledge on cardiovascular disease in women: proceedings of the workshop held in Brussels on gender differences in cardiovascular disease, 29 September 2010. Eur Heart J. 2011;32:1362–1368. doi: 10.1093/eurheartj/ehr048. [DOI] [PubMed] [Google Scholar]
- 64.Vaccarino V, Badimon L, Corti R, et al. Presentation, management, and outcomes of ischaemic heart disease in women. Nat Rev Cardiol. 2013;10:508–518. doi: 10.1038/nrcardio.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buchthal SD, den Hollander JA, Merz CN, et al. Abnormal myocardial phosphorus-31 nuclear magnetic resonance spectroscopy in women with chest pain but normal coronary angiograms. N Engl J Med. 2000;342:829–835. doi: 10.1056/NEJM200003233421201. [DOI] [PubMed] [Google Scholar]
- 66.Murthy VL, Naya M, Taqueti VR, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129:2518–2527. doi: 10.1161/CIRCULATIONAHA.113.008507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gould KL, Johnson NP, Bateman TM, et al. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol. 2013;62:1639–1653. doi: 10.1016/j.jacc.2013.07.076. [DOI] [PubMed] [Google Scholar]
- 68.Shah AM. Paracrine modulation of heart cell function by endothelial cells. Cardiovasc Res. 1996;31:847–867. [PubMed] [Google Scholar]
- 69.Zhang M, Shah AM. ROS signalling between endothelial cells and cardiac cells. Cardiovasc Res. 2014;102:249–257. doi: 10.1093/cvr/cvu050. [DOI] [PubMed] [Google Scholar]
- 70.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]