Abstract
Background
Positron emission tomography (PET) with insulin-stimulated 18F-2-deoxyglucose (FDG) uptake is the gold standard for myocardial viability. However, insulin stimulation is infrequently performed due to time and inconvenience. We therefore assessed the clinical applicability of an abbreviated hyperinsulinemic-euglycemic clamp.
Methods and Results
Dynamic FDG PET was performed in 50 patients with ischemic cardiomyopathy (ejection fraction: .30 ± .10) using an abbreviated hyperinsulinemic-euglycemic clamp with separate Non-Diabetic (n = 26) and Diabetic (n = 24) protocols (American Society of Nuclear Cardiology guidelines), and supplemental potassium. In regions with normal resting perfusion (13N-ammonia uptake ≥80% maximal segment), there were no differences in either maximal (Non-Diabetic: .60 ± .20 vs Diabetic: .60 ± .17 μmol/min/g, P = .93) or mean rates of myocardial glucose uptake (MGU) (Non-Diabetic: .52 ± .18 vs Diabetic: .52 ± .14 μmol/min/g, P = .63) between the protocols. Multivariate analysis showed that diastolic blood pressure alone (maximal MGU, r2 = .20, P = .001) or with NYHA Heart Failure Class (mean MGU, r2 = .25, P = .003) could account for some of the variability in normal-region MGU. Potassium supplementation safely attenuated the decline in plasma levels.
Conclusions
This abbreviated hyperinsulinemic-euglycemic clamp produced similar MGU values in normal resting myocardium in non-diabetic and diabetic subjects, which are no different than published rates with a standard insulin clamp. Thus, this abbreviated approach is sufficient to overcome myocardial insulin resistance.
Keywords: Myocardial viability, positron emission tomography, insulin, FDG
INTRODUCTION
The assessment of 18F-2-deoxyglucose (FDG) uptake during hyperinsulinemic-euglycemic clamp (HEC) is widely accepted as the preferred approach to not only maximize myocardial glucose uptake but also to reduce the heterogeneity of FDG accumulation in viable myocardium.1 The enhanced myocardial uptake of FDG not only improves image quality and regional assessment but would also improve the predictive accuracy for the assessment of viability. Despite these advantages, widespread adoption of FDG imaging with HEC has been hampered by the associated increase in study time and inconvenience.
The American Society for Nuclear Cardiology (ASNC) has developed guidelines for the performance of FDG positron emission tomography (PET) viability imaging to promote standardization of this imaging modality.1 These recommendations included a modified HEC that uses intravenous dextrose and insulin in the fasting state to promote myocardial FDG uptake, with protocols for both diabetic and non-diabetic subjects. While the abbreviated ASNC clamp is much less time consuming than the formal method (a process that would take up to 150 min before FDG administration),2 and therefore more amenable to clinical application, an objective evaluation in patients most likely to require clinical FDG imaging has not been reported.
Therefore, the primary goal of this investigation was to determine the clinical applicability of the abbreviated HEC.1 We were particularly concerned that the abbreviated HEC might be inadequate to completely overcome insulin resistance in diabetic subjects, and thus would result in lower values for myocardial glucose utilization (MGU) as compared to non-diabetic subjects3 or those receiving a more prolonged, standard HEC.4,5 If this hypothesis was refuted, and there no differences between MGU values in diabetic and non-diabetic subjects, then a secondary aim was to attempt to explain the inter-patient variability in “normal” MGU values. We hypothesized that at least some of the variability in MGU could be accounted for by routine clinical parameters, as has been shown in subjects with normal left ventricular systolic function.6
METHODS
The study population consisted of 50 subjects with ischemic cardiomyopathy who are enrolled in the Prediction of ARrhythmic Events with Positron Emission Tomography (PAREPET) Trial.7 This NIH-sponsored trial is investigating the role of myocardial viability in the subsequent risk of sudden cardiac death (SCD). All patients had to have documented coronary artery disease, NYHA Class I–III heart failure symptoms, an ejection fraction (EF) of ≤.35, and be eligible to receive an implantable cardiac defibrillator for the primary prevention of SCD. Patients with recent MI or revascularization were excluded, as were those who had indications for the secondary prevention of SCD (i.e., unexplained syncope, sustain ventricular arrhythmias), or were being considered for coronary revascularization.7 The study protocol was approved by the Institutional Review Board at the University at Buffalo, and all subjects signed informed consent prior to enrollment. For the purposes of this study, subjects with ventricular pacing or a left bundle branch block pattern on their resting electrocardiogram were excluded due to potential alterations in septal FDG uptake independent of resting perfusion.8,9
PET Imaging Using an Abbreviated HEC
PET imaging of myocardial perfusion (with 13N-ammonia, NH3) and metabolism (with 18F-2-fluoro-2-deoxyglucose, FDG) was performed on an ECAT Exact HR+ (Siemens Medical Solutions, Malvern, PA). After a 15-min transmission scan to correct for attenuation, 10 mCi (370 MBq) NH3 was injected as a bolus and imaging performed over 24 min.7 After marking the chest with indelible ink for proper repositioning, subjects were removed from the scanner for the initiation of the abbreviated HEC as proposed by the ASNC.1 Non-diabetic patients received priming doses of insulin (5 units) and dextrose (10 g) intravenously, followed by a dextrose-insulin infusion (15 units insulin in 500 mL of 20% dextrose initially at 1.5 milliunits insulin/kg/min and 10.0 mg glucose/kg/min). Diabetic patients received priming doses of insulin (6–10 units IV), followed by separate insulin (100 units insulin in 500 mL of normal saline initially at 4.0 milliunits insulin/kg/min) and glucose (20% dextrose initially at 6.0 mg glucose/kg/min) infusions. In both groups, blood samples were drawn at 5–15 min intervals for serial measurements of plasma glucose and potassium levels (i-Stat, Abbott Laboratories, Chicago, IL) from a peripheral venous catheter located on the opposite extremity from the infusion site. After the establishment of euglycemia (~20–30 min after initiation of the infusions), subjects were returned to the scanner, repositioned, and 6.5 mCi (241 MBq) FDG was injected as a bolus.7 Dynamic imaging was performed with the following frame sequence: 8 × 15, 2 × 30, 2 × 120, 1 × 180, 4 × 300, and 3 × 600 s, resulting in 20 frames over 60 min. A repeat transmission scan was obtained for attenuation correction of the FDG emission data. Subjects were removed from the scanner and given a snack to prevent late hypoglycemia. All patients tolerated the imaging session without complication. The effective dose equivalent for this protocol (including transmission scans) was ~8.0 mSv.
As currently proposed, the administration of potassium is not included in the ASNC protocol.1 However, after the first five subjects showed significant declines in their potassium levels, we elected to preemptively administer potassium chloride to prevent HEC-induced hypokalemia. Accordingly, subjects were given potassium chloride (10 mEq) intravenously if their initial plasma potassium levels were <5 mmol/L, and oral supplementation of 40–80 mEq if initial plasma potassium was ≤4 and ≤3.5 mmol/L.
Determination of Myocardial Perfusion and Glucose Uptake
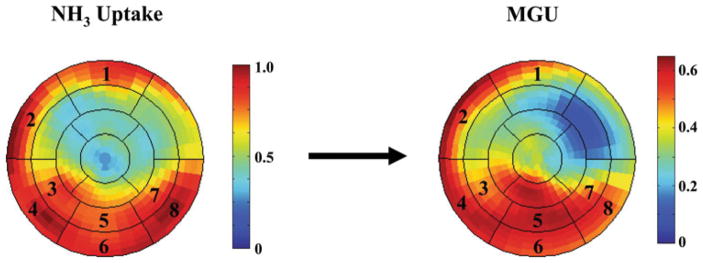
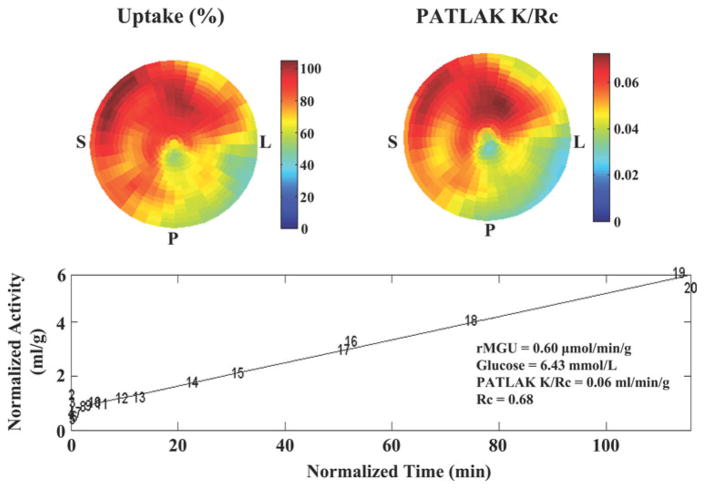
Regional myocardial perfusion was determined using NH3 uptake (MyoPC®, University of Ottawa Heart Institute, Ontario, Canada).7 The left ventricle was divided into 17 segments, and segments with NH3 uptake ≥80% of the maximal segment were considered “normal” for that individual subject (minimum 2 segments per subject, Figure 1). Next, MGU (in μmol/min/g) was determined for these “normal” segments by the graphical method of Patlak and Blasberg,10 assuming irreversible FDG uptake from 3 to 60 min. Briefly, this model defines the relation between myocardial activity divided by the instantaneous arterial blood activity (Cm(t)/Cb(t)) versus the integral of Cb(t) divided by the blood activity at that time t (∫Cb(t)dt/Cb(t)). This relationship of “normalized activity” versus “normalized time” becomes linear after the transient response of tissue FDG, similar to a constant FDG infusion (Figure 2). The slope of the linear portion of this relation was fit using a robust estimation (Bartlett method), and is equal to the net influx constant (Ki), representing the rate of FDG uptake and phosphorylation. In all patients, linearity was confirmed visually in the region with maximal uptake by two observers to ensure no “bending” of the Patlak plots (Figure 2).11 The arterial blood-pool time-activity curve was averaged from a 20-mm3 region of interest automatically centered in the left ventricle and the left atrial cavities.7,12 The rate of MGU was calculated as: MGU = Ki · Pglu/L, where Pglu was the plasma glucose concentration during the insulin clamp determined from the average of at least three measurements made immediately prior to and during FDG imaging. The lumped constant (L), which accounts for differences in uptake and phosphorylation between FDG and glucose, was assumed to be .67.13 To exclude the potentially confounding effects from the averaging of multiple segments, values in the maximal segment of each subject were also determined.
Figure 1.
Representative polar maps of NH3 uptake and MGU using FDG imaging. A 17-segment model of the LV and computational software (MyoPC®) was used to determine segmental uptake of 13N-ammonia. Segments with uptake ≥80% of the maximal segment were identified (i.e., segments 1–8 in this example) and considered to have normal perfusion. Myocardial glucose uptake (MGU, μmol/min/g) was quantified for these segments by the method of Patlak and Blasberg. The average MGU values in these segments as well as the maximal segment were determined for each subject.
Figure 2.
MGU by Patlak analysis in a representative subject. The polar tomograms from FDG PET illustrate both regional uptake (upper left image) and kinetic modeling by Patlak analysis (upper right image). The lower graph confirms the linear relationship expected between the normalized myocardial activity and normalized blood-pool integral (Time). The net influx constant, Ki = K/Rc, which accounts for myocardial FDG uptake and phosphorylation, is determined from the slope (K) of the linear portion of this relationship, divided by a recovery coefficient (Rc) accounting for partial volume losses in the myocardium. Data points are labeled by frame number (1–20). S, Interventricular septum; P, posterior wall; L, lateral wall.
Transthoracic Echocardiography
All subjects underwent transthoracic echocardiography (Sonos 5500, Philips Healthcare, Andover, MA) for the quantification of various cardiac parameters7 as recommended by the American Society of Echocardiography.14 LV mass was calculated using the area-length method, and left atrial volume, LV end-systolic and end-diastolic volumes, and ejection fraction were determined using the modified Simpson’s approach.14 All measurements were confirmed by a board-certified cardiologist.
Statistical Analyses
All values are presented as mean ± standard deviation. Differences between the Diabetic and Non-Diabetic protocols were compared using unpaired t-tests for continuous data and the χ2 test for categorical values, using Sigma-Stat 3.2. P < .05 was considered statistically significant.
Various demographic, medication, echocardiographic, hemodynamic, metabolic, and laboratory parameters were tested for their association with normal-segment MGU in univariate models. Demographic variables included: HEC protocol (Diabetic vs Non-Diabetic), age, clinical diagnosis of diabetes mellitus, Canadian Heart Society Angina Class, New York Heart Association Heart Failure Class, QRS duration, sex, weight, body mass index, and body surface area (BSA). Medication variables included the use of: beta-blocker, amiodarone, antiplatelet agents (aspirin and/or clopidogrel), angiotensin converting enzyme inhibitor or angiotensin receptor antagonist, aldosterone antagonist, and digoxin. Echocardiographic parameters included: ejection fraction, LV end-diastolic volume (absolute and indexed for BSA), LV end-systolic volume (absolute and indexed for BSA), left atrial volume (absolute and indexed for BSA), and left ventricular mass (absolute and indexed for BSA). Hemodynamic parameters included: systolic and diastolic blood pressure, pulse pressure, heart rate, and rate pressure product (systolic blood pressure · heart rate). Metabolic parameters included: baseline plasma glucose, total infused insulin, final insulin infusion rate, total infused glucose, final glucose infusion rate, and total infused glucose/insulin (g/U, absolute and indexed for BSA). Baseline laboratory values included: potassium, sodium, hematocrit, and creatinine. Variables with univariate P ≤ .10 were then included in multivariate analyses using forward and stepwise inclusion criteria (SAS version 9.1).
RESULTS
Subject Demographics
The demographic and echocardiographic parameters for the 50 subjects are shown in Table 1. The average age was 64 ± 12 years (range: 32–85 years) and the majority were male (42 male, 8 female). Consistent with a history of ischemic cardiomyopathy, the average ejection fraction was .30 ± .10. With the exception of greater body mass index and higher fasting plasma glucose level in the Diabetic protocol group, there were no demographic or echocardiographic differences between the subjects in the two HEC protocols (Table 1).
Table 1.
Demographic and echocardiographic parameters
| All patients | Non-Diabetic protocol | Diabetic protocol | P value | |
|---|---|---|---|---|
| n | 50 | 26 | 24 | |
| Age (years) | 64 ± 12 | 65 ± 12 | 64 ± 12 | .97 |
| Sex, Male (%) | 84 | 85 | 83 | .61 |
| Body mass index (kg/m2) | 26.2 ± 6.3 | 22.9 ± 4.6 | 29.8 ± 5.9 | <.001 |
| Fasting plasma glucose (mg/dL) | 117 ± 44 | 98 ± 10 | 138 ± 55 | <.001 |
| NYHA Class | 2.0 ± .7 | 1.9 ± .7 | 2.0 ± .7 | .55 |
| CCS Class | 1.6 ± .7 | 1.5 ± .7 | 1.8 ± .7 | .29 |
| LV EDVI (mL/m2) | 81 ± 24 | 84 ± 24 | 77 ± 23 | .35 |
| LV ESVI (mL/m2) | 57 ± 20 | 61 ± 23 | 53 ± 17 | .14 |
| Ejection fraction | .30 ± .10 | .28 ± .11 | .32 ± .08 | .08 |
NYHA, New York Heart Association heart failure classification; CCS, Canadian Cardiovascular Society angina classification; LV EDVI, left ventricular end-diastolic volume index; LV ESVI, left ventricular end-systolic volume index.
Normal-Segment MGU in Patients with Ischemic Cardiomyopathy
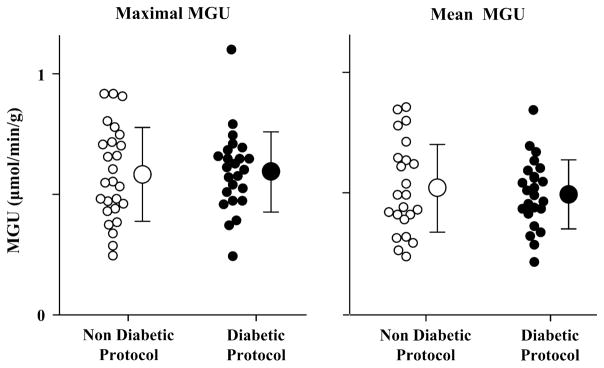
PET parameters for the Non-Diabetic and Diabetic protocol groups are shown in Table 2. Of the 850 segments in these patients with ischemic cardiomyopathy, 475 segments (56%, or 9.5 ± 3.1 segments per patient) had normal levels of resting NH3 uptake (≥80% of the maximal segment per patient). Maximal segment MGU averaged .60 ± .18 μmol/min/g, with no difference between those receiving the Diabetic vs Non-Diabetic protocols (Table 2 and Figure 3). Similarly, there were no differences in mean MGU in normally perfused segments which averaged .51 ± .16 μmol/min/g (Table 2 and Figure 3). Although there were slightly more “normal” segments among the patients treated with the Diabetic protocol (Table 2), the coefficient of variation (SD/mean) within the “normal” segments of each subject was similar in the two groups (Non-Diabetic: .12 ± .06 vs Diabetic: .14 ± .09, P = .24).
Table 2.
PET parameters
| HEC protocol | Segments with normal NH3 uptake | Metabolic glucose utilization (μmol/min/g) | |
|---|---|---|---|
|
| |||
| Maximal | Mean | ||
| Non-Diabetic | 8.3 ± 2.9 | .60 ± .20 | .52 ± .18 |
| Diabetic | 10.8 ± 2.8 | .60 ± .17 | .50 ± .14 |
| p value | <.01 | .93 | .63 |
Figure 3.
MGU values in normally perfused segments of patients with ischemic cardiomyopathy. The maximal (left graph; Non-Diabetic: .60 ± .20 μmol/min/g, Diabetic: .60 ± .17 μmol/min/g) and mean (right graph; Non-Diabetic: .52 ± .18 μmol/min/g, Diabetic: .50 ± .14 μmol/min/g) MGU values for each subject are plotted relative to the HEC protocol used during FDG imaging. For both analyses, there were no differences in the range, scatter, or average MGU values between those subjects that underwent the Non-Diabetic (white circles) or Diabetic (black circles) protocols.
Correlation of Normal-Segment MGU and Clinical Parameters
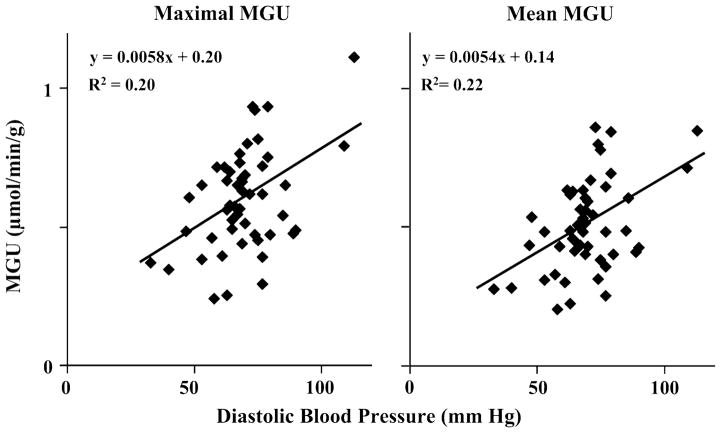
There was considerable variability in the maximal (Non-Diabetic: .60 ± .20 μmol/min/g, Diabetic: .60 ± .17 μmol/min/g) and mean (Non-Diabetic: .52 ± .18 μmol/min/g, Diabetic: .50 ± .14 μmol/min/g) normal-segment MGU values between subjects within each protocol group (Figure 3), which we thought might be due, at least in part, to differences in patient-specific variables. Table 3 lists the variables that were correlated (P < .10) with maximal and mean MGU values. For both maximal and mean MGU, diastolic blood pressure was the most significantly correlated variable by univariate analysis. As illustrated in Figure 4, diastolic blood pressure was positively correlated with MGU; however, it could only account for ~20–22% of the variability in normal-segment MGU. By multivariate analyses (both forward and stepwise approaches), diastolic blood pressure was the only independent predictor of maximal MGU. Both diastolic blood pressure and NYHA Heart Failure Class were independent predictors of mean normal-region MGU, accounting for ~25% of the variability among subjects (r2 = .25, P = .003).
Table 3.
Univariate and multivariate predictors of normal-region MGU
| Parameter estimate | P value | |
|---|---|---|
| Maximal normal-region MGU | ||
| Univariate predictors | ||
| Diastolic blood pressure | 5.72 × 10−3 | .001 |
| Rate pressure product | 3.26 × 10−5 | .008 |
| NYHA heart failure class | −7.14 × 10−2 | .059 |
| Systolic blood pressure | 2.27 × 10−3 | .060 |
| Multivariate predictor | ||
| Diastolic blood pressure | 5.72 × 10−3 | .001 |
| Mean normal-region MGU | ||
| Univariate predictors | ||
| Diastolic blood pressure | 5.35 × 10−3 | .0007 |
| Rate pressure product | 3.00 × 10−5 | .007 |
| NYHA heart failure class | −7.76 × 10−2 | .022 |
| Systolic blood pressure | 2.25 × 10−3 | .038 |
| Baseline hematocrit | 7.85 × 10−3 | .076 |
| Body mass index | −6.68 × 10−3 | .078 |
| Heart rate | 4.02 × 10−3 | .078 |
| Total infused glucose (g) | 2.94 × 10−3 | .086 |
| Multivariate predictors | ||
| Diastolic blood pressure | 4.25 × 10−3 | .016 |
| NYHA heart failure class | −6.90 × 10−2 | .035 |
Figure 4.
Relationship of MGU values with diastolic blood pressure. By univariate analysis, the maximal (left graph) and mean (right graph) MGU values from normally perfused segments were most highly correlated with diastolic blood pressure, and diastolic blood pressure remained an independent predictor by multivariate analysis (Table 3). However, diastolic blood pressure was only able to account for ~20–22% of the variability in MGU values among patients with ischemic cardiomyopathy.
Plasma Potassium Changes During the ASNC Clamp
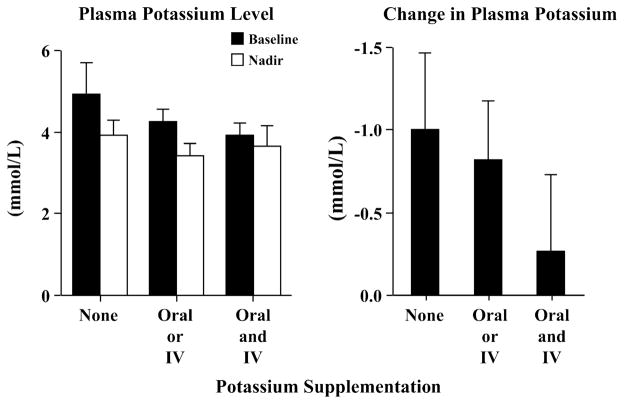
In subjects without potassium supplementation, there was a significant fall (>1 mmol/L) in plasma potassium due to the HEC (Figure 5). We subsequently modified our protocol to incorporate potassium supplementation in the majority of patients (those with plasma potassium <5 mmol/L), with an average supplementation of 25 ± 26 mmol/subject (range: 0–90 mmol). This standardized approach was successful at maintaining baseline potassium levels and circumventing the predictable decline in potassium during HEC (Figure 5). The final plasma potassium level before leaving the PET suite was 3.8 ± .4 mmol/L. Hyperkalemia (>5.5 mmol/L) was not observed.
Figure 5.
Changes in plasma potassium with an abbreviated HEC. Consistent with electrolyte shifts during insulin administration, the HEC protocols resulted in a fall in plasma potassium (left graph). After the first few subjects had dramatic falls in their plasma potassium without supplementation (>1 mmol/L), we modified our protocol to include potassium supplementation for the majority of subjects (those with baseline plasma potassium <5 mmol/L). As expected, this modification abated the decline in plasma potassium levels (right graph) without induction of hyperkalemia.
DISCUSSION
To our knowledge, this is the first study to examine quantitative myocardial glucose uptake using an abbreviated HEC as proposed by the ASNC, and the first study to compare MGU values in diabetic and non-diabetic subjects with ischemic cardiomyopathy. The results demonstrate that using only 20–30 min of HEC prior to FDG administration results in similar values for maximal and mean MGU using the Non-Diabetic and Diabetic protocols. Even more importantly, the resultant MGU values are comparable to those previously reported with the more prolonged HEC.4,5 Based upon these data, the routine use of a HEC to reduce the heterogeneity of FDG uptake would improve the discrimination between viable and non-viable myocardium.
Abbreviated HEC in Patients with Ischemic Cardiomyopathy
Inadequate insulin administration in diabetic subjects can result in poor cardiac FDG uptake and image quality. Since the quantification of infarction by PET is determined by the decrement in FDG uptake, this could confound assessment of myocardial viability in subjects with diabetes mellitus. Since MGU values in this study were similar in both Diabetic and Non-Diabetic protocols, insulin resistance in diabetics was successfully overcome using this abbreviated method. The prevalence of insulin resistance is particularly high in patients with ischemic cardiomyopathy (48% in our population), thus these results have significant implications not only for clinical trials such as the PAREPET study7 but also for clinical patient care since it is the patients with ischemic cardiomyopathy in which the assessment of myocardial viability is most crucial.15
To our knowledge, this is the first report of MGU values in diabetic and non-diabetic subjects with ischemic cardiomyopathy. A previous report suggested that mean MGU values in patients with diabetes (and no coronary disease or echocardiographic wall motion abnormalities) were lower than normal volunteers (.42 ± .12 vs .54 ± .11 μmol/min/g, P < .05).3 This discrepancy with our results was likely due to inadequate insulin infusion to overcome insulin resistance in the prior study. Our Diabetic protocol group had a mean MGU of .50 ± .14 μmol/min/g which compares favorably with their normal volunteers, and it is likely that our average insulin rate of 3.9 ± .4 mU/kg/min was higher than that achieved in the previous study (simply stated as “more than 1 mU/kg/min”).3
Our mean MGU estimates also compare very favorably to the results obtained in patients with ischemic heart disease using a more conventional and prolonged HEC.4,5 For example, Gerber et al reported MGU of .47 ± .18 μmol/min/g in remote regions of subjects with anterior infarction4 (as compared to .51 ± .16 μmol/min/g for all patients in this study). Furthermore, they obtained similar values when they restricted their analysis to subjects with an ejection fraction of ≤.35.4 Although they also found similar MGU values in control subjects,4 other investigators have found slightly higher values in normal volunteers,5,16 suggesting the presence of myocardial insulin resistance in the setting of ischemic cardiomyopathy.5
Inter-Patient Variability in Normal-Region MGU
In view of the large number of subjects treated with the Diabetic protocol, we anticipated a significant association between MGU and some metabolic variables. We were therefore surprised that only diastolic blood pressure and NYHA Heart Failure Class were the only independent variables by multivariate analysis. However, a previous study of subjects with normal LV systolic function found significant associations between HEC MGU and both systolic and diastolic blood pressures.6 The statistically significant variables in their multivariate analysis were systolic blood pressure and plasma-free fatty acid concentration, which accounted for ~55% of the variability in MGU (r = .74).6 Our overall results were less predictive for MGU (~20–25% explanation of variability by multivariate analysis) and may be in part related to the absence of free fatty acid concentration as a key metabolic variable. However, we also noted lower correlation coefficients for both systolic (r = .30 vs r = .506) and diastolic blood pressure (r = .46 vs r = .606) as compared to this previous study, suggesting that LV dysfunction in our subjects further increased MGU variability.
Some investigators have identified insulin resistance among subjects with cardiac hypertrophy17 or ischemic cardiomyopathy.5,18 We therefore anticipated significant correlations between MGU and ventricular volumes, ejection fraction, left ventricular mass, and/or left atrial volume. Although our data do not directly support these previous observations, this may be in part related to the higher insulin infusion rate and the ability to overcome insulin resistance among those subjects treated with the Diabetic protocol. We found little difference between maximal and mean MGU values suggesting that investigating the maximal myocardial segment could be sufficient in further studies.
Potassium Supplementation During HEC
Insulin administration induces an intracellular migration of potassium and a subsequent decline in plasma concentration. In view of the well-established association between electrolyte abnormalities (especially hypokalemia) and ventricular arrhythmias, we felt it was important to modify the protocol to include potassium supplementation to moderate this effect (Figure 5). Although we are unaware of any arrhythmic complications during HEC, and any induced hypokalemia should be temporary, we feel that it is prudent to avoid even transient hypokalemia in patients with ischemic cardiomyopathy. Furthermore, our approach was safe without any induction of hyperkalemia.
Methodological Limitations
In this study, the myocardial segments of interest were defined by the presence of 13N-ammonia uptake ≥80% of the maximal segment per subject. It is therefore possible that some of the included segments were in fact not normal, and viable dysfunctional myocardium may have been included. Although FDG accumulation in viable dysfunctional myocardium can be higher than normal regions in the fasting state16,19 or with sub-maximal stimulation,20 we and others have shown that MGU in these segments during insulin stimulation is either normal4,5,19 or slightly reduced.16 Thus, the inclusion of viable dysfunctional segments into the “normal” region is unlikely to have significantly influenced our results. The presence of normal wall motion on echocardiography to define normal segments would have been a more specific approach; however, it would have excluded the large proportion of subjects in whom there were no normal segments due to pathological remodeling, and would add the potential for segment misregistration. Furthermore, the point estimate of our average MGU is actually higher than Gerber et al’s in which normal regions were defined by normal wall motion.4
Acknowledgments
Supported by a Grant from the National Institutes of Health (RO1 HL-076252, JMC and JAF).
We would like to thank Brendan Heavey for his assistance with image analysis and statistical support, Sue Michalek and Marsha Barber for administration of the PAREPET trial, Paul Galantowicz for his assistance with the imaging protocol, and Anne Coe for her help with the preparation of this manuscript.
References
- 1.Bacharach SL, Bax JJ, Case J, Delbeke D, Kurdziel KA, Martin WH, et al. PET myocardial glucose metabolism and perfusion imaging: Part 1—Guidelines for data acquisition and patient preparation. J Nucl Cardiol. 2003;10:543–56. doi: 10.1016/s1071-3581(03)00648-2. [DOI] [PubMed] [Google Scholar]
- 2.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–23. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 3.Ohtake T, Yokoyama I, Watanabe T, Momose T, Serezawa T, Nishikawa J, et al. Myocardial glucose metabolism in noninsulin-dependent diabetes mellitus patients evaluated by FDG-PET. J Nucl Med. 1995;36:456–63. [PubMed] [Google Scholar]
- 4.Gerber BL, Vanoverschelde JL, Jr, Bol A, Michel C, Labar D, Wijns W, et al. Myocardial blood flow, glucose uptake, and recruitment of inotropic reserve in chronic left ventricular ischemic dysfunction: Implications for the pathophysiology of chronic myocardial hibernation. Circulation. 1996;94:651–9. doi: 10.1161/01.cir.94.4.651. [DOI] [PubMed] [Google Scholar]
- 5.Marinho NVS, Keogh BE, Costa DC, Lammertsma AA, Ell PJ, Camici PG. Pathophysiology of chronic left ventricular dysfunction: New insights from the measurement of absolute myocardial blood flow and glucose utilization. Circulation. 1996;93:737–44. doi: 10.1161/01.cir.93.4.737. [DOI] [PubMed] [Google Scholar]
- 6.Yokoyama I, Ohtake T, Momomura S, Yonekura K, Yamada N, Nishikawa J, et al. Organ-specific insulin resistance in patients with noninsulin-dependent diabetes mellitus and hypertension. J Nucl Med. 1998;39:884–9. [PubMed] [Google Scholar]
- 7.Fallavollita JA, Luisi AJ, Jr, Michalek SM, Valverde AM, deKemp RA, Haka MS, et al. Prediction of ARrhythmic Events with Positron Emission Tomography: PAREPET study design and methods. Contemp Clin Trials. 2006;27:374–88. doi: 10.1016/j.cct.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Thompson K, Saab G, Birnie D, Chow BJ, Ukkonen H, Ananthasubramaniam K, et al. Is septal glucose metabolism altered in patients with left bundle branch block and ischemic cardiomyopathy? J Nucl Med. 2006;47:1763–8. [PubMed] [Google Scholar]
- 9.Neri G, Zanco P, Zanon F, Buchberger R. Effect of biventricular pacing on metabolism and perfusion in patients affected by dilated cardiomyopathy and left bundle branch block: Evaluation by positron emission tomography. Europace. 2003;5:111–5. doi: 10.1053/eupc.2002.0272. [DOI] [PubMed] [Google Scholar]
- 10.Ha AC, Renaud JM, Dekemp RA, Thorn S, Dasilva J, Garrard L, et al. In vivo assessment of myocardial glucose uptake by positron emission tomography in adults with the PRKAG2 cardiac syndrome. Circ Cardiovasc Imaging. 2009;2:485–91. doi: 10.1161/CIRCIMAGING.109.853291. [DOI] [PubMed] [Google Scholar]
- 11.Herrero P, Dence CS, Sharp TL, Welch MJ, Gropler RJ. Impact of reversible trapping of tracer and the presence of blood metabolites on measurements of myocardial glucose utilization performed by PET and 18F-fluorodeoxyglucose using the Patlak method. Nucl Med Biol. 2004;31:883–92. doi: 10.1016/j.nucmedbio.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Lortie M, Beanlands RS, Yoshinaga K, Klein R, Dasilva JN, DeKemp RA. Quantification of myocardial blood flow with 82Rb dynamic PET imaging. Eur J Nucl Med Mol Imaging. 2007;34:1765–74. doi: 10.1007/s00259-007-0478-2. [DOI] [PubMed] [Google Scholar]
- 13.Ratib O, Phelps ME, Huang S-C, Henze E, Selin CE, Schelbert HR. Positron tomography with deoxyglucose for estimating local myocardial glucose metabolism. J Nucl Med. 1982;23:577–86. [PubMed] [Google Scholar]
- 14.Gottdiener JS, Bednarz J, Devereux R, Gardin J, Klein A, Manning WJ, et al. American Society of Echocardiography recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr. 2004;17:1086–119. doi: 10.1016/j.echo.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Beanlands RS, Nichol G, Huszti E, Humen D, Racine N, Freeman M, et al. F-18-fluorodeoxyglucose positron emission tomography imaging-assisted management of patients with severe left ventricular dysfunction and suspected coronary disease: A randomized, controlled trial (PARR-2) J Am Coll Cardiol. 2007;50:2002–12. doi: 10.1016/j.jacc.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Maki M, Luotolahti M, Nuutila P, Iida H, Voipio-Pulkki LM, Ruotsalainen U, et al. Glucose uptake in the chronically dysfunctional but viable myocardium. Circulation. 1996;93:1658–66. doi: 10.1161/01.cir.93.9.1658. [DOI] [PubMed] [Google Scholar]
- 17.Paternostro G, Pagano D, Gnecchi-Ruscone T, Bonser RS, Camici PG. Insulin resistance in patients with cardiac hypertrophy. Cardiovasc Res. 1999;42:246–53. doi: 10.1016/s0008-6363(98)00233-8. [DOI] [PubMed] [Google Scholar]
- 18.Paternostro G, Camici PG, Lammerstma AA, Marinho N, Baliga RR, Kooner JS, et al. Cardiac and skeletal muscle insulin resistance in patients with coronary heart disease. A study with positron emission tomography. J Clin Invest. 1996;98:2094–9. doi: 10.1172/JCI119015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fallavollita JA. Spatial heterogeneity in fasting and insulin-stimulated 18F-2-deoxyglucose uptake in pigs with hibernating myocardium. Circulation. 2000;102:908–14. doi: 10.1161/01.cir.102.8.908. [DOI] [PubMed] [Google Scholar]
- 20.Vanoverschelde JL, Jr, Wijns W, Depre C, Essamri B, Heyndrickx GR, Borgers M, et al. Mechanisms of chronic regional postischemic dysfunction in humans: New insights from the study of noninfarcted collateral-dependent myocardium. Circulation. 1993;87:1513–23. doi: 10.1161/01.cir.87.5.1513. [DOI] [PubMed] [Google Scholar]