Abstract
Purpose
Pharmaceutical companies paid at least $3.91 billion to prescribers in 2013, yet evidence indicating whether industry payments shift prescribing away from generics is limited. This study examined the association between amount of industry payments to prescribers and generic drug prescribing rates among Medicare Part D prescribers.
Methods
A cross-sectional analysis was conducted among 770,095 Medicare Part D prescribers after linking the 2013 national Open Payments data with 2013 Medicare Provider Utilization and Payment data. The exposure variable was categorized amount of total industry payments to prescribers (i.e., meals, travel, research, ownership). The outcome was prescriber’s annual generic drug prescribing rate. Multivariable generalized linear regression models were used to examine the association between amount of industry payments and prescriber’s annual generic drug prescribing rates, controlling for prescriber’s demographic and practice characteristics.
Results
In this sample, over one-third (38.0%) of Medicare Part D prescribers received industry payments in 2013. The mean annual generic drug prescribing rate was highest among prescribers receiving no payments and lowest among those receiving more than $500 of industry payments (77.5% vs. 71.3%, respectively; p<0.001). The receipt of industry payments was independently associated with prescribers’ generic drug prescribing rate; higher payments corresponded with lower generic drug prescribing rates. Other prescriber characteristics associated with higher annual generic drug prescribing rate included male sex, non-northeast region, specialty, and patient volume.
Conclusions
Receipt of industry payments was associated with a decreased rate of generic drug prescribing. How this affects patient care and total medical costs warrants further study.
Keywords: industry payments, generic drugs, prescribing, prescribers
INTRODUCTION
Generic drugs play an important role in controlling health care costs.1,2 Between 2005 and 2014, generic drug use realized savings of $1.68 trillion in health care expenditures.3 The generic drug market continues to grow, increasing from 19% of total drug spending in 2006 to 28% of total drug spending in 2014.4 Today, nearly 9 out of every 10 prescriptions dispensed in the United States are generic drugs.3 One way to combat rising prescription drug costs is to encourage use of generic prescription drugs.
Generic drug use is largely impacted by certain key groups including prescribers and industry manufacturers. A 2011 survey on physician’s perceptions of generic drugs found that nearly a quarter of physicians did not routinely use generics as first-line treatment.5 About 25% of the physicians expressed concerns about the efficacy of generics, and almost half reported concerns about quality.5 Additionally, pharmaceutical manufacturers may use direct-to-consumer (DTC) advertising to promote their products and this can lead to product switching or resistance to using a generic.6 The pharmaceutical industry also can have financial relationships with prescribers including providing money for research activities, gifts, speaking fees, meals, or travel.7
The Affordable Care Act requires the Centers for Medicare and Medicaid Services (CMS) to collect information from applicable manufacturers and group purchasing organizations (GPOs) in order to report information about their financial relationships with physicians and hospitals.7 The Open Payments data became available to the public in early 2015 and include consulting fees, research grants, travel reimbursements, and payments made from pharmaceutical industry to medical practitioners. From August to December 2013, a total of 4.46 million records of industry payments (summed to $3.91 billion) from 1,392 companies were made to 480,000 physicians and 1,025 teaching hospitals.8 These data provide a unique opportunity to examine the association between the amount of industry payments to prescribers and their generic drug prescribing rates. Several recently published studies linked the national Open Payments data with national and regional Medicare Part D prescribing data and found that industry payments were associated with an overall increase in prescribing rates of brand-name drugs.9–11 However, these studies only included general industry payments such as meals and travel, which were usually less costly than research and ownership payments.
By focusing on generic instead of brand-name drug prescribing rates, we took a further step and included not only general payments but also payments for research grants/consulting and ownership royalties (e.g., payments for royalty or license of sales of pharmaceutical products, or ownership or investment interests).12 We linked the 2013 Open Payments data and the Medicare Part D Prescriber public use file (PUF) to examine the association between industry payments to prescribers and generic drug prescribing rates. We focused on all types of payments (i.e., general, research, and ownership) paid by the pharmaceutical industry, and also conducted a series of sensitivity analyses to examine the associations by type of industry payments, by prescriber’s annual prescribing volume, and by prescriber’s specialty.
METHODS
Data Sources
This cross-sectional study was approved by Auburn University Institutional Review Board (IRB) and by the Research Involving Human Subjects Committee (RIHSC) at the U.S. Food and Drug Administration (FDA). The three data sources utilized for this study were the 2013 Open Payments data, the 2013 Medicare Part D Prescriber PUF, and the National Prescriber Identification (NPI) registry file. The 2013 Open Payments data include data on payments, other transfers of value, and ownership or investment interests made to physicians or teaching hospitals for the period from August 1 to December 31, 2013.13 The publicly available Open Payments data are self-reported by applicable manufacturers and GPOs. CMS published these data on June 30, 2015 after quality control and review, which included a dispute process and industry corrections from April 6 through June 6, 2015.14 The 2013 Medicare Part D Prescriber PUF includes the aggregated prescription drug information that individual physicians and other health care providers prescribed in 2013 under the Medicare Part D Prescription Drug Program.15 To protect the privacy of Medicare beneficiaries, any aggregated records which are derived from 10 or fewer claims have been excluded from the Part D Prescriber PUF. Therefore, the 2013 Medicare Part D Prescriber PUF retains information from 86.8% of claims and 78.1% of total costs from CMS’s Medicare Part D Prescription Drug Event claims data.16 We used the NPI registry file as the linkage between the 2013 Open Payments data and the 2013 Medicare Part D Prescriber PUF to merge industry payments and Part D prescribers’ prescription drug events.
Study Population
We included all prescribers with non-missing NPI number in the 2013 Medicare Part D Prescriber PUF dataset because we used NPI number to link prescribers with the 2013 Open Payments data. We further excluded prescribers with missing values in all three variables including total claims of brand-name drugs (i.e., prescription drugs approved from New Drug Application (NDA), NDA authorized generic, or Biologic License Application (BLA)), total claims of generic drugs (i.e., prescription drugs approved by Abbreviated New Drug Application (ANDA)), and total claims of other drugs. These variables were redacted by CMS and were used to calculate prescriber’s annual generic drug prescribing rate. The study population included a total of 770,095 Medicare Part D prescribers.
Measurements
The exposure variable was the receipt of industry payments to prescribers (i.e., general payments such as meals and travels, research grants/consulting, and ownership royalties) from August 1 to December 31, 2013, which were reported in the 2013 Open Payments data. We identified individual prescribers with non-missing first and last names and zip codes because we used this information to link individual prescribers with the NPI file to obtain their unique NPI numbers. We calculated total payments by summing all payment amounts – including general, research, and ownership payments – for each individual prescriber. Based on the distribution of payments, the total payments were further categorized as no payment ($0), $100 and less, $100-500, and more than $500.
The outcome was Medicare Part D prescriber’s annual generic drug prescribing rate in 2013. For each prescriber, the 2013 Medicare Part D Prescriber PUF includes the total number of Medicare Part D prescriptions (including brand-name, generic, and other prescriptions) that were dispensed through Medicare Advantage Prescription Drug (MAPD) plans and by stand-alone Prescription Drug Plans (PDP), which include original prescriptions and any refills.15 Each prescriber’s annual generic drug prescribing rate was calculated as the percentage of total claims of generic drugs divided by the total claims of Medicare Part D drugs dispensed in 2013.
We adjusted for covariates including each prescriber’s sex (male and female), region (Northeast, Midwest, South, West, and non-U.S.), specialty (primary care, medical specialty, surgical specialty, obstetrics/gynecology, hospital-based practice, psychiatry, and non-physician),17 and annual number of Medicare Part D beneficiaries served (0-50, 51-100, 101-250, and 251 and more).
Statistical analysis
First, we used Chi-square tests to compare the proportion of prescribers with and without industry payments between subcategories of Medicare Part D claims and covariates. We also used 2-sample t tests and ANOVA to compare the mean annual generic drug prescribing rate between subcategories of industry payments and covariates. Then we used multivariable generalized linear regression models with Poisson distributions to examine the association between amount of industry payments and prescriber’s annual generic drug prescribing rates, controlling for covariates. All analyses were performed using SAS 9.3 (SAS Institute, Inc., Cary, NC). Due to the large sample size and multiple comparisons, statistical significance was set at p<0.01 instead of p<0.05.
A series (n=11) of sensitivity analyses were performed to confirm the robustness of our results. First, we calculated industry payments by individual payment type. Instead of using the sum of general, research, and ownership payments as the exposure variable, we ran three separate multivariable generalized linear regression models to examine the associations between general, research, and ownership payments with generic drug prescribing rates, respectively. Second, in order to adjust for the impact of prescribing volume on prescriber’s generic drug prescribing rate, we ran separate multivariable generalized linear regression models within subgroups of prescribers who had 2,000 and more, 5,000 and more, and 10,000 and more claims of Medicare Part D prescriptions (based on the distribution of number of claims per prescriber), respectively. Finally, we performed subgroup multivariable analyses by limiting to different types of prescribers – physicians only, psychiatrists, cardiologists, family medicine physicians, and internal medicine physicians.
RESULTS
Industry payments and prescriber characteristics
The study population included 770,095 Medicare Part D prescribers. Of these, over one-third of Medicare Part D prescribers (38.0%) received industry payments in 2013. Since payment categories were not mutually exclusive, the most frequent payment was general payments (38.0%), followed by research payments (0.4%), and ownership payments (0.3%). The median of total industry payments was $146 (interquartile range (IQR)=$51-414).
Characteristics of the study population, divided between prescribers who did and did not receive industry payments of any kind, are shown in Table 1. Compared with prescribers receiving no payments, higher proportions of those receiving payments were men, practicing in South region, more likely to be primary care physicians, medical or surgical specialists, obstetrics/gynecology physicians, and psychiatrists, and more likely to treat higher numbers of Medicare Part D beneficiaries and prescribe higher numbers of Medicare Part D prescriptions (all p<0.01).
Table 1.
Characteristics of study population (n=770,095)
| Factors | Receipt of Industry Payments N (%) |
P value1 | |
|---|---|---|---|
| Yes N=292,763 (38.0) |
No N=477,332 (62.0) |
||
| Prescriber characteristics | |||
| Sex | <.0001 | ||
| Male | 216,798 (74.0) | 251,536 (52.7) | |
| Female | 75,965 (26.0) | 225,796 (47.3) | |
| Region | <.0001 | ||
| Northeast | 60,513 (20.7) | 108,699 (22.8) | |
| Midwest | 60,674 (20.7) | 107,089 (22.4) | |
| South | 116,745 (39.9) | 153,182 (32.1) | |
| West | 52,521 (17.9) | 105,483 (22.1) | |
| Non-US | 2,310 (0.8) | 2,879 (0.6) | |
| Prescriber practice factors | |||
| Specialty | <.0001 | ||
| Primary care | 99288 (33.9) | 131,968 (27.7) | |
| Medical specialty | 69,053 (23.6) | 33,662 (7.1) | |
| Surgical specialty | 45,631 (15.6) | 28,536 (6.0) | |
| Obstetrics/gynecology | 16,888 (5.8) | 11,640 (2.4) | |
| Hospital-based specialty | 15,460 (5.3) | 34,800 (7.3) | |
| Psychiatry | 15,786 (5.4) | 18,248 (3.8) | |
| Non-physician, unknown | 30,657 (10.5) | 218,478 (45.8) | |
| Annual number of distinct Medicare Part D beneficiaries served | <.0001 | ||
| 0-50 | 59,427 (20.3) | 190,733 (40.0) | |
| 51-100 | 46,474 (15.9) | 93,833 (16.7) | |
| 101-250 | 88,855 (30.4) | 127,402 (26.7) | |
| 251 and above | 98,007 (33.5) | 65,364 (13.7) | |
| Annual number of Medicare Part D claims | <.0001 | ||
| 0-100 | 35,213 (12.0) | 128,023 (26.8) | |
| 101-400 | 67,191 (23.0) | 157,545 (33.0) | |
| 401-2000 | 88,509 (30.2) | 118,972 (24.9) | |
| 2001 and above | 101,850 (34.8) | 72,792 (15.3) | |
Chi-square tests
Mean annual generic drug prescribing rates
Figure 1 shows the mean annual generic drug prescribing rates by amount of industry payments to prescribers. The mean annual generic drug prescribing rate was highest among prescribers receiving no payments and lowest among those receiving more than $500 of industry payments (77.5% vs. 71.3%, respectively; p<0.01).
Figure 1.
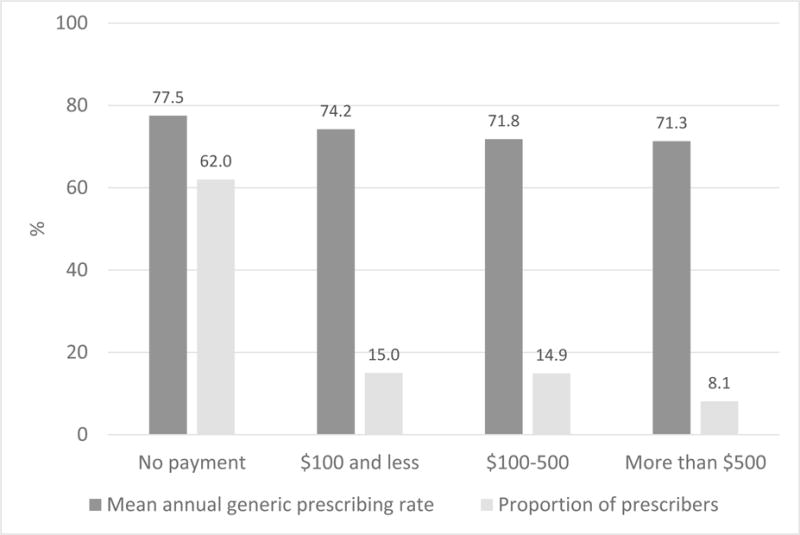
Mean annual generic drug prescribing rates by industry payments to prescribers (n=770,095)
Table 2 shows both unadjusted and adjusted results of predictors of annual generic drug prescribing rates. The receipt of industry payments was independently associated with prescriber’s generic drug prescribing rate. The higher the amount of the payments, the lower the generic drug prescribing rate. Adjusted results showed that prescribers who received payments of less than $100 had a 2% (rate ratio (RR)=0.98 with 99% confidence interval (CI)=0.97,0.98) reduction in annual generic drug prescribing rate compared to those without industry payments. While prescribers who received payments of more than $500 had a 5% (RR=0.95 with 99% CI=0.95,0.95) reduction in annual generic drug prescribing rate compared to those without industry payments. Other predictors of higher annual generic drug prescribing rate included male sex, non-northeast region, certain specialty (i.e., hospital-based prescribers and psychiatrist), and lower number of Medicare Part D beneficiaries (all p<0.01).
Table 2.
Unadjusted and adjusted results of predictors of annual generic drug prescribing rates (n=770,095)
| Factors | Mean Annual Generic Drug Prescribing Rate Rate Ratio (99% Confidence Interval)1 |
|
|---|---|---|
| Unadjusted Models2 | Adjusted Model3 | |
| Industry payments category | ||
| No payment | ref | ref |
| $100 and less | 0.96 (0.96, 0.96)** | 0.98 (0.97, 0.98)** |
| $100-500 | 0.93 (0.92, 0.93)** | 0.95 (0.95, 0.95)** |
| More than $500 | 0.92 (0.92, 0.92)** | 0.95 (0.95, 0.95)** |
| Prescriber characteristics | ||
| Sex | ||
| Male | ref | ref |
| Female | 0.99 (0.99, 0.99)** | 0.97 (0.97, 0.97)** |
| Region | ||
| Northeast | ref | ref |
| Midwest | 1.05 (1.04, 1.05)** | 1.05 (1.04, 1.05)** |
| South | 1.03 (1.03, 1.03)** | 1.03 (1.03, 1.03)** |
| West | 1.03 (1.02, 1.03)** | 1.02 (1.02, 1.02)** |
| Non-US | 1.06 (1.05, 1.07)** | 1.06 (1.05, 1.07)** |
| Prescriber practice factors | ||
| Specialty | ||
| Primary care | ref | ref |
| Medical specialty | 0.91 (0.91, 0.91)** | 0.92 (0.91, 0.92)** |
| Surgical specialty | 0.93 (0.92, 0.93)** | 0.93 (0.92, 0.93)** |
| Obstetrics/gynecology | 0.81 (0.80, 0.81)** | 0.82 (0.81, 0.82)** |
| Hospital-based specialty | 1.09 (1.09, 1.09)** | 1.07 (1.07, 1.08)** |
| Psychiatry | 1.05 (1.05, 1.05)** | 1.06 (1.06, 1.06)** |
| Non-physician, unknown | 1.02 (1.01, 1.02)** | 1.01 (1.01, 1.01)** |
| Annual number of distinct Medicare Part D beneficiaries served | ||
| 0-50 | ref | ref |
| 51-100 | 1.00 (1.00, 1.00)* | 1.01 (1.01, 1.01)** |
| 101-250 | 1.01 (1.01, 1.02)** | 1.02 (1.02, 1.03)** |
| 251 and above | 0.98 (0.98, 0.98)** | 1.00 (1.00, 1.01)** |
Generalized linear regression models with Poisson distribution
Each factor as the only exposure variable without controlling for any other factors
Industry payments as exposure variable, controlling for all covariates
P<0.01
P<0.001
Sensitivity analyses
The results of a total of 11 sensitivity analyses are summarized in Table 3. The association between higher amount of industry payments and lower prescriber’s annual generic drug prescribing rate was similar to the pooled analyses when we only accounted for general payments or research payments. However, our results showed that higher amounts of ownership payments were associated with higher generic drug prescribing, which was strongly modified by prescriber’s specialty (significant interaction term between payment category and specialty). Our findings were unchanged when we limited to prescribers with higher annual prescribing volumes and with certain specialties.
Table 3.
Results of sensitivity analyses of associations between amount of industry payments and annual generic drug prescribing rates
| Industry Payment Category | Mean Annual Generic Drug Prescribing Rate Rate Ratio (99% Confidence Interval)1 |
|---|---|
| Adjusted Model2 | |
| By type of industry payments | |
| General payments2 | |
| Exposure: No payment | ref |
| $100 and less | 0.98 (0.97, 0.98)* |
| $100-500 | 0.95 (0.95, 0.95)* |
| More than $500 | 0.95 (0.95, 0.95)* |
| Research payments3 | |
| Exposure: No payment | ref |
| $5,000 and less | 0.98 (0.96, 1.00) |
| $5,000-50,000 | 0.92 (0.90, 0.94)* |
| More than $50,000 | 0.89 (0.86, 0.91)* |
| Ownership payments4 | |
| Exposure: No payment | ref |
| $25,000 and less | 1.02 (1.00, 1.04) |
| $25,000-500,000 | 1.06 (1.05, 1.08)* |
| More than $500,000 | 1.06 (1.04, 1.09)* |
| By annual prescribing volume | |
| Limit to prescribers with annual claims ≥2,0005 | |
| Exposure: No payment | ref |
| $100 and less | 0.99 (0.98, 0.99)* |
| $100-500 | 0.97 (0.97, 0.97)* |
| More than $500 | 0.94 (0.93, 0.94)* |
| Limit to prescribers with annual claims ≥5,0006 | |
| Exposure: No payment | ref |
| $100 and less | 0.99 (0.99, 0.99)* |
| $100-500 | 0.98 (0.97, 0.98)* |
| More than $500 | 0.94 (0.93, 0.94)* |
| Limit to prescribers with annual claims ≥10,0007 | |
| Exposure: No payment | ref |
| $100 and less | 1.00 (0.99, 1.00) |
| $100-500 | 0.98 (0.98, 0.99)* |
| More than $500 | 0.95 (0.94, 0.95)* |
| By prescriber’s specialty | |
| Limit to physicians only8 | |
| Exposure: No payment | ref |
| $100 and less | 0.99 (0.98, 0.99)* |
| $100-500 | 0.96 (0.96, 0.97)* |
| More than $500 | 0.96 (0.96, 0.97)* |
| Limit to psychiatrists only9 | |
| Exposure: No payment | ref |
| $100 and less | 0.99 (0.99, 1.00)* |
| $100-500 | 0.98 (0.98, 0.99)* |
| More than $500 | 0.95 (0.94, 0.95)* |
| Limit to cardiologists only10 | |
| Exposure: No payment | ref |
| $100 and less | 1.00 (0.99, 1.00) |
| $100-500 | 0.98 (0.98, 0.99)* |
| More than $500 | 0.95 (0.95, 0.96)* |
| Limit to family medicine prescribers only11 | |
| Exposure: No payment | ref |
| $100 and less | 0.99 (0.99, 0.99)* |
| $100-500 | 0.97 (0.97, 0.97)* |
| More than $500 | 0.94 (0.94, 0.95)* |
| Limit to internal medicine prescribers only12 | |
| Exposure: No payment | ref |
| $100 and less | 0.98 (0.98, 0.99)* |
| $100-500 | 0.96 (0.96, 0.97)* |
| More than $500 | 0.92 (0.91, 0.92)* |
Generalized linear regression models with Poisson distribution, controlling for all covariates
Exposure variable is the sum of general payments calculated from the 2013 Open Payment data
Exposure variable is the sum of research payments calculated from the 2013 Open Payment data
Exposure variable is the sum of ownership payments calculated from the 2013 Open Payment data
Limiting to prescribers with annual Medicare Part D claims ≥2,000, n=174,700
Limiting to prescribers with annual Medicare Part D claims ≥5,000, n=78,131
Limiting to prescribers with annual Medicare Part D claims ≥10,000, n=26,567
Limiting to physicians only by removing non-physicians such as nurses and physician assistants, n=520,951
Limiting to psychiatrists only, n=34,034
Limiting to cardiologists only, n=21,903
Limiting to family medicine prescribers only, n=97,625
Limiting to internal medicine prescribers only, n=114,195
P<0.001
DISCUSSION
We linked 2 national datasets to examine the association between amount of industry payments to prescribers and Medicare Part D prescriber’s annual generic drug prescribing rate. We found that the receipt of industry payments was independently associated with prescriber’s generic drug prescribing rate. The higher amount payments, the lower generic drug prescribing rate. As compared with prescribers receiving no industry payments, we found that receipt of industry payments of less than $100 was associated with a 2% reduction in annual generic drug prescribing rate and receipt of payments of more than $500 was associated with a 5% reduction in annual generic drug prescribing rate, after controlling for prescriber’s demographic and practice characteristics. Our findings persisted after we further limited to individual types of industry payments and prescribers with higher prescribing volumes and certain specialties.
Our results are consistent with recent studies that linked the CMS Open Payments data and Medicare Part D prescribing data. These studies found that industry payments were associated with an overall increase in prescribing rates of brand-name drugs.9–11 Likewise, we found industry payments were associated with decreased prescribing rates of generic drugs. However, DeJong et al focus on payments specifically for industry-sponsored meals,11 while Ornstein et al and Perlis et al analyzed general industry payments (i.e., meals and travels) in the CMS Open Payments data.9,10 We took a further step by including not only general payments but also payments for research grants/consulting and ownership royalties. Although prescribers with any research and ownership payments were much less compared to prescribers with general payments, the amount of the payments was much higher than general payments. Results from our sensitivity analyses indicated that prescribers’ receipt of research payments of more than $50,000 was associated with even lower (11%) annual generic drug prescribing rates.
However, we found prescribers receiving ownership payments was associated with higher annual generic drug prescribing rates compared with those without ownership payments. The positive association between receiving ownership payments and generic drug prescribing rates was modified by prescriber’s specialty. Specifically, when we stratified prescribers based on their specialty, we found no association between receiving ownership payments and generic drug prescribing rates among primary care physicians, positive associations among medical and surgical specialists, and negative association among other specialties (data not shown but can be requested from authors). Since the majority (nearly 85%) of prescribers with ownership payments were medical or surgical specialists in this sample, the positive association between receiving ownership payments and generic drug prescribing rates shown in our sensitivity analysis can be explained by the oversampling of this group of prescribers. We did not observe the same effect modification of specialty on the main analysis of total payments. Our findings of ownership payments somewhat challenge the assumption discussed by Yeh et al,18 that larger industry payments to physicians are more likely to influence physicians’ prescribing behavior. We found higher industry payments were associated with lower generic drug prescribing rates; however, certain payment types may be more of a cause of concern than others.
In addition, we found noteworthy prescriber demographic characteristics that were associated with generic drug prescribing behaviors. Our findings indicate that female prescribers had lower annual generic drug prescribing rates than males. Additionally, prescribers’ practice region also made a difference – prescribers located in the Northeast region had the lowest annual generic drug prescribing rates compared to those in the Midwest, South, and West regions. The generic prescribing difference in geographic regions might also reflect individual prescriber’s perceptions towards generic drugs. For example, all U.S. states have adopted generic substitution laws to reduce medication costs.19 However, physicians may override the policy by prescribing brand-name drugs and requesting “dispense as written”. Using pharmacy claims from a large pharmacy benefits manager, Shrank et al found patients from Northeast region had the highest likelihood (adjusted odds ratio (OR)=1.77 with 95% CI=1.73,1.81 vs. reference=West region) of physician-assigned “dispense as written” compared with other regions.20 Therefore, policies such as financial penalties to reduce the rate of “dispense as written” may help reduce medication costs and improve medication adherence. To our knowledge, our study is the first study that demonstrates the associations between prescribers’ sex and geographic region with generic drug prescribing behaviors in the U.S. Targeted educational interventions regarding safety and effectiveness of generic drugs might improve prescribers’ perceptions about generic drugs and help reduce the difference in generic prescribing in their practice.
Last but not least, our results showed differences in generic drug prescribing rates between prescriber’s specialties. Specifically, medical/surgical specialists and obstetrics/gynecology physicians had lower generic drug prescribing rates compared to primary care physicians while hospital-based physicians, psychiatrists, and non-physician prescribers had higher generic drug prescribing rates. In addition, despite the differences in generic drug prescribing rates across specialty groups, results from sensitivity analyses confirmed that the relationship between higher industry payments and lower generic drug prescribing rates was quite consistent within physicians only as well as among individual specialty groups. Other than prescribers’ specialty, we found a moderate association between higher numbers of Medicare Part D patients served and higher generic drug prescribing rates. However, the relationship between higher industry payments and lower generic drug prescribing rates still held when we limited to prescribers with higher annual prescribing volumes.
Our findings should be interpreted in light of limitations. First, the cross-sectional analyses could only detect associations but not causality between industry payments to prescribers and their annual generic drug prescribing rates. Although we merged the same year’s CMS Open Payments data with the Medicare Part D Prescriber PUF, we cannot determine the temporal relationship between industry payments and generic drug prescribing. Second, our findings are limited by the completeness and accuracy of reporting of industry payments in the CMS Open Payment data. The 2013 Open Payments data only included payments for the period from August 1 to December 31, 2013, which means our estimates of the proportion of prescribers who received industry payments and their amounts of payments were underestimated. Our next step will be to analyze the complete 2014 and 2015 Open Payment data to confirm our findings. In addition, since the Medicare Part D Prescriber PUF does not contain specific prescription information such as drug name or dosage, we could not directly link industry payments from a specific pharmaceutical company to prescribing of a specific company’s products. We also excluded roughly 27% of Medicare Part D prescribers from the Medicare Part D Prescriber PUF due to their missing values in NPI number and brand/generic claim counts. Finally, our study population only included Medicare Part D prescribers, so our findings might not be generalizable to prescribers who did not prescribe for Medicare Part D beneficiaries.
Nonetheless, the first step towards overcoming barriers to generic drug use is to understand the key influential groups and the nature of the barriers that exist. Then, strategies can be developed and tailored to reduce or eliminate barriers for different influential groups. Our findings of an association between the pharmaceutical industry and prescribers highlight the potential influence of industry payments on prescribers’ decision-making about generic drug use. Future research could focus on whether the association between industry payments and generic prescribing changes over time or changes with different health insurance systems (i.e., public vs. private), and how this association impacts quality of prescribing and patient health outcomes.
In conclusion, we found that the amount of industry payments may influence generic prescribing. The amount and type of payments may influence generic drug prescribing. How this affects patient care and total medical costs warrants further study.
Key points.
Prescriber’s receipt of industry payments was independently associated with a decreased rate of generic drug prescribing. However, the association varied by type of payments.
Prescriber’s generic drug prescribing behaviors also varied by their sex, geographic location, specialty, and patient volume.
How this association between industry payments and generic drug prescribing affects patient care and total medical costs is important to safety and economics of health care delivery and warrants further study.
Acknowledgments
Funding source: This study was funded by the U.S. Food and Drug Administration through grant # U01FD005486. Views expressed in written materials or publications and by speakers do not necessarily reflect the official policies of the Department of Health and Human Services; nor does any mention of trade names, commercial practices, or organization imply endorsement by the United States Government.
Footnotes
Authors’ contributions: Dr. Qian had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Qian, Harris, Hansen. Acquisition, analysis, or interpretation of the data: all authors. Drafting of the manuscript: Qian. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Qian. Obtained funding: Qian, Harris. Study supervision: Qian, Hansen. Written permission has been obtained from all co-authors.
Conflict of interest statements: In the past 3 years, Richard Hansen has provided expert testimony for Boehringer Ingelheim. No other authors declare a potential conflict of interest.
Prior presentation: The results of this study have been presented at the 32nd International Conference on Pharmacoepidemiology & Therapeutic Risk Management, August 25-28, 2016 in Dublin, Ireland.
References
- 1.Fischer MA, Avorn J. Potential savings from increased use of generic drugs in the elderly: what the experience of Medicaid and other insurance programs means for a Medicare drug benefit. Pharmacoepidemiology and drug safety. 2004;13(4):207–214. doi: 10.1002/pds.872. [DOI] [PubMed] [Google Scholar]
- 2.Haas JS, Phillips KA, Gerstenberger EP, Seger AC. Potential savings from substituting generic drugs for brand-name drugs: medical expenditure panel survey, 1997-2000. Annals of internal medicine. 2005;142(11):891–897. doi: 10.7326/0003-4819-142-11-200506070-00006. [DOI] [PubMed] [Google Scholar]
- 3.Generic Pharmaceutical Association. Generic drug savings in the US Seventh annual edition. 2015 Available at http://www.gphaonline.org/media/wysiwyg/PDF/GPhA_Savings_Report_2015.pdf. (Accessed January 27th, 2016)
- 4.Generic Pharmaceutical Association. 2015 Generic Pharmaceutical Association Annual Report. 2015 http://www.gphaonline.org/media/wysiwyg/GPhA2015AnnualReport.pdf.
- 5.Shrank WH, Liberman JN, Fischer MA, Girdish C, Brennan TA, Choudhry NK. Physician perceptions about generic drugs. The Annals of pharmacotherapy. 2011;45(1):31–38. doi: 10.1345/aph.1P389. [DOI] [PubMed] [Google Scholar]
- 6.Hansen RA, Shaheen NJ, Schommer JC. Factors influencing the shift of patients from one proton pump inhibitor to another: the effect of direct-to-consumer advertising. Clinical therapeutics. 2005;27(9):1478–1487. doi: 10.1016/j.clinthera.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Medicare and Medicaid Services. Open Payments. Available at http://www.cms.gov/openpayments/ (Assessed May 1st, 2015)
- 8.Centers for Medicare and Medicaid Services. The facts about Open Payments data. Available at https://openpaymentsdata.cms.gov/summary (Assessed August 8th, 2016)
- 9.Ornstein C, Jones R, Tigas M. Now there’s proof: docs who get company cash tend to prescribe more brand-name meds. New York, NY: ProPublica; 2016. Available at https://www.propublica.org/article/doctors-who-take-company-cash-tend-to-prescribe-more-brand-name-drugs. Published March 17, 2016. [Google Scholar]
- 10.Perlis RH, Perlis CS. Physician Payments from Industry Are Associated with Greater Medicare Part D Prescribing Costs. PloS one. 2016;11(5):e0155474. doi: 10.1371/journal.pone.0155474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeJong C, Aguilar T, Tseng CW, Lin GA, Boscardin WJ, Dudley RA. Pharmaceutical Industry-Sponsored Meals and Physician Prescribing Patterns for Medicare Beneficiaries. JAMA internal medicine. 2016;176(8):1114–1110. doi: 10.1001/jamainternmed.2016.2765. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Medicare and Medicaid Services: Open Payments. Natures of Payment. Available at https://www.cms.gov/openpayments/about/natures-of-payment.html (Assessed September 5th, 2016)
- 13.Centers for Medicare and Medicaid Services. Open Payments Public Use Files: Methodology Overview & Data Dictionary. 2016 Jun; Available at https://www.cms.gov/OpenPayments/Downloads/OpenPaymentsDataDictionary.pdf (Assessed July 20th, 2016). 2016.
- 14.Centers for Medicare and Medicaid Services: Open Payments. Annual Report to Congress on the Open Payments Program. 2016 Apr; (Assessed August 25th, 2016) [Google Scholar]
- 15.Centers for Medicare and Medicaid Services. Medicare Provider Utilization and Payment Data: Part D Prescriber. Available at http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Provider-Charge-Data/Part-D-Prescriber.html (Assessed April 8th, 2015)
- 16.Centers for Medicare and Medicaid Services. Medicare Fee-For Service Provider Utilization & Payment Data Part D Prescriber Public Use File: A Methodological Overview. 2015 Apr 7; Available at https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Provider-Charge-Data/Downloads/Prescriber_Methods.pdf (Assessed May 6th, 2015). 2015.
- 17.Centers for Medicare and Medicaid Services. Medicare Data on Provider Practice and Speciality (MD-PPAS) User Documentation. 2016 Version 2.1. Available at https://www.resdac.org/sites/resdac.umn.edu/files/MD-PPAS%20User%20Documentation%20-%20Version%202.1.docx (Assessed March 15th, 2016)
- 18.Yeh JS, Franklin JM, Avorn J, Landon J, Kesselheim AS. Association of Industry Payments to Physicians With the Prescribing of Brand-name Statins in Massachusetts. JAMA internal medicine. 2016;176(6):763–768. doi: 10.1001/jamainternmed.2016.1709. [DOI] [PubMed] [Google Scholar]
- 19.Shrank WH, Choudhry NK, Agnew-Blais J, et al. State generic substitution laws can lower drug outlays under Medicaid. Health Aff (Millwood) 2010;29(7):1383–1390. doi: 10.1377/hlthaff.2009.0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shrank WH, Liberman JN, Fischer MA, et al. The consequences of requesting “dispense as written”. The American journal of medicine. 2011;124(4):309–317. doi: 10.1016/j.amjmed.2010.11.020. [DOI] [PubMed] [Google Scholar]


