Abstract
Here we describe two assays to measure dense core vesicle (DCV) exocytosis-mediated cargo secretion in neuroendocrine cells. To conduct siRNA screens for novel genes in regulated DCV exocytosis, we developed a plate reader-based secretion assay using DCV cargo, NPY-Venus, and an orthogonal 3H-serotonin secretion assay. The NPY-Venus secretion assay was successfully used for a high throughput siRNA screen, and the serotonin secretion assay was used to validate hits identified from the screen (Sorensen, 2017; Zhang et al., 2017 ).
Keywords: NPY, Serotonin, Exocytosis, Dense core vesicle (DCV), Neuroendocrine, High throughput screen, BON cells
Background
Dense core vesicle (DCV) exocytosis mediates the secretion of proteins, peptides and small molecules from endocrine and neuroendocrine cells. Protein and peptide hormones enter the secretory pathway while they are being synthesized by ribosomes on the endoplasmic reticulum and sorted into DCVs at the trans-Golgi network (TGN) ( Borgonovo et al., 2006 ; Bowman et al., 2009 ). Small molecules, such as serotonin, are directly taken up by vesicular monoamine transporters (VMATs) from the cytoplasm to the lumen of DCVs (Sudhof, 2004). Under non-stimulating conditions, cargo molecules are stored within DCVs in close proximity to the plasma membrane. When cells receive an external stimulation that increases cytoplasmic Ca2+, DCVs undergo regulated exocytosis and their contents are released to the outside of the cell (Klenchin and Martin, 2000). Released molecules control many critical biological processes, such as growth ( Guerineau et al., 1998 ), ‘fight or flight’ response ( Kishimoto et al., 2006 ), glucose metabolism (Lang, 1999), immune reactions ( Griffiths et al., 2010 ), fertilization ( Mayorga et al., 2007 ), etc. Due to the essential roles of regulated exocytosis, multiple electrophysiology- or microscopy-based methods have been developed to measure regulated exocytosis in live cells. Electrophysiological methods quantify regulated exocytosis by measuring either cell membrane capacitance or by amperometry to measure oxidizable secretory products (Martin, 2003; Zhang et al., 2011 ). Fluorescent microscopy-based methods measure regulated exocytosis by counting the number of fusion events at the plasma membrane by TIRF microscopy ( Kabachinski et al., 2016 ). However, the above methods are difficult to apply to high throughput screening. Here, we developed a plate reader-based assay relying on NPY-Venus fluorescence. The assay utilizes genetically engineered BON cell lines that stably express a DCV cargo, NPY-Venus. When cells are stimulated with ionomycin in the presence of external Ca2+, intracellular Ca2+ concentration is rapidly increased and NPY-Venus is quickly released from DCVs by regulated exocytosis. The percent secretion of NPY-Venus is used as a measurement of regulated exocytosis. We have successfully optimized the assay for high throughput screens ( Zhang et al., 2017 ). In addition, we described an orthogonal 3H-serotonin uptake and secretion assay that is similar to previous reports (Samuel Tran et al., 2004 ), which is also utilized to measure regulated DCV exocytosis ( Zhang et al., 2017 ).
Materials and Reagents
Pipette tips
1.7 ml microcentrifuge tubes (DOT Scientific, catalog number: 609-GMT)
TPP 15 ml conical centrifuge tube (TPP Techno Plastic Products, catalog number: 91015)
10 cm tissue culture dishes
96-well tissue culture plate (Corning, Costar®, catalog number: 3596)
96-well black bottom assay plate (Corning, catalog number: 3915)
0.2 μm syringe filter (Corning, catalog number: 431219)
NalgeneTM 0.45 μm cellulose acetate syringe filter (Thermo Fisher Scientific, catalog number: 190-2545)
Polycarbonate centrifuge tube (32 ml, Beckman Coulter, catalog number: 355631)
-
BON cells (a gift from C.M. Townsend, University of Texas Medical Branch, Galveston, TX)
Note: Maintain the BON cells in DMEM/F12 (1:1; Thermo Fisher Scientific, GibcoTM, catalog numbers: 11965092 and 11765062) supplemented with 10% FBS (PHENIX Research Products, catalog number: FBS-500US) at 37 °C with 5% CO2).
HEK293FT cells (Thermo Fisher Scientific, InvitrogenTM, catalog number: R70007)
DMEM (Thermo Fisher Scientific, GibcoTM, catalog number: 11965092)
F12 nutrients (Thermo Fisher Scientific, GibcoTM, catalog number: 11765062)
Fetal bovine serum (PHENIX Research Products, catalog number: FBS-500US; Thermo Fisher Scientific, catalog number: 16000044)
Metafectamine SI (Biontex Laboratories, catalog number: T100-1.0)
RNAiMAX (Thermo Fisher Scientific, catalog number: 13778030)
Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P3911)
Potassium phosphate monobasic (KH2PO4) (Sigma-Aldrich, catalog number: P0662)
Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S9888)
Sodium bicarbonate (NaHCO3) (Sigma-Aldrich, catalog number: 792519)
Sodium phosphate dibasic (Na2HPO4) (Sigma-Aldrich, catalog number: S9763)
Sodium phosphate dibasic heptahydrate (Na2HPO4·7H2O) (Sigma-Aldrich, catalog number: S9390)
Trypsin-EDTA 0.25% (Thermo Fisher Scientific, GibcoTM, catalog number: 25200056)
NPY-Venus lenti construct pTM14-NPY-Venus (request from the Martin Lab)
Opti-MEM medium (Thermo Fisher Scientific, catalog number: 31985070)
Calcium chloride (CaCl2) (Sigma-Aldrich, catalog number: 793639)
Magnesium chloride (MgCl2) (Sigma-Aldrich, catalog number: M9272)
HEPES (Sigma-Aldrich, catalog number: H3375)
Glucose (Sigma-Aldrich, catalog number: DX0145)
Ionomycin (Sigma-Aldrich, catalog number: I0634-5MG)
DMSO (Sigma-Aldrich, catalog number: D8418-50ML)
Hybri-MaxTM, cell culture grade DMSO (e.g., Sigma-Aldrich, catalog number: D2650)
Triton X-100 (Sigma-Aldrich, catalog number: T9284-1L)
3H-serotonin (PerkinElmer, catalog number: NET498001MC)
L-ascorbic acid (Sigma-Aldrich, catalog number: A5960-25G)
Envelop plasmid pMD2.G (Addgene, catalog number: 12259)
Packaging plasmid psPAX2 (Addgene, catalog number: 12260)
Protamine sulfate (Sigma-Aldrich, catalog number: P4020)
BON cell culture medium (see Recipes)
HANK’s buffer(see Recipes)
PSS-Na (see Recipes)
L-ascorbic acid solution (see Recipes)
HEK cell culture medium (see Recipes)
Viral production medium (see Recipes)
HBeS (2x) (see Recipes)
CaCl2 solution (see Recipes)
Protamine sulfate (see Recipes)
BON cell freezing medium (see Recipes)
Equipment
Tissue culture incubator
Multichannel pipettes
Automated liquid handling system (Biotek Microflo dispenser, Thermo Scientific Matrix Hydra DT 96-well liquid handler, optional)
Tecan plate reader (Infinite F500 with excitation filter 485/20 and emission filter 535/25 or Safire II with excitation 505/20 and emission 535/10) (Tecan Trading, model: Infinite® F500)
Beckman ultracentrifuge with a Ti70 rotor
Ti70 rotor (Beckman Coulter, model: Type 70 Ti)
Multichannel aspirator (Argos Technologies, catalog number: EV503)
FACS sorter (BD, model: FACSAriaTM II)
Fluorescent microscopy with FITC excitation and emission filters
Scintillator counter
Procedure
-
Maintenance of BON cells
BON cells are neuroendocrine tumor cells that are derived from a metastasized human pancreatic tumor ( Parekh et al., 1994 ). They contain numerous DCVs and secrete serotonin, neurotensin and pancreastatin under stimulatory conditions ( Parekh et al., 1994 ). Due to their relatively large DCVs and rapid growth, BON cells are good models to study DCV exocytosis ( Karatekin et al., 2008 ; Samuel Tran et al., 2004 ).
BON cells are cultured in 10 cm tissue culture dishes with 10 ml culture medium (see Recipes) in a 5% CO2 incubator at 37 °C.
-
BON cells are typically split every three days at a 1:5 or 1:6 ratio. To split cells from a confluent 10 cm dish:
Wash BON cells with 5 ml HANK’s (with 1 mM EDTA) buffer (see Recipes).
Add 1 ml 0.25% trypsin-EDTA and incubate at 37 °C for 3 min to detach cells.
Add 5 ml normal culture medium and mix well with cells to terminate trypsinization, transfer the cell suspension to a 15 ml conical centrifugation tube and pellet cells by a low-speed centrifugation (1,000 × g, 5 min).
Resuspend cells with 5 or 6 ml normal culture medium and add 1 ml to each 10 cm dish with 9 ml culture medium inside.
For siRNA transfection, we recommend using BON cells 48 h after splitting, when cells are healthy and growing at log phase.
-
NPY-Venus secretion assay
The NPY-Venus secretion assay uses BON cells that stably express NPY-Venus, which can be obtained from the Martin Lab or generated by following the steps described in Procedure D ‘Establishing NPY-Venus-expressing cell lines’. All the volumes in this section are for one well of a 96-well plate.-
siRNA transfection
-
Mix siRNA and transfection reagents in 96-well plates with either Metafectamine SI or RNAiMAX. For each siRNA treatment, we recommend having 8 replicates, with 4 of them used to determine Ca2+-stimulated secretion and the other 4 for background secretion (Figure 1A).
Step Metafectamine SI RNAiMAX 1 Dilute Metafectamine buffer with H2O to make 1x buffer Dilute RNAiMAX (0.3 μl) with 5 μl Opti-MEM medium 2 (see Note 1) Mix the following components:siRNA (5 μM stock): 1.2 μl1x Metafectamine SI buffer: 15 μlMetafectamine SI: 0.33 μlDilute siRNA (1.2 μl stock (5 μM)) with 5 μl Opti-MEM medium 3 Mix RNAiMAX and siRNA 4 Incubate for 15 min at room temperature Incubate for 5 min at room temperature - Prepare BON cell suspension with a concentration of 1.8 x 105 cells/ml in normal growth medium (see Procedure A).
- Add 100 μl cell suspension to siRNA mixture after the incubation step is complete. Leave the plate at room temperature for 10 min before transferring to tissue culture incubator at 37 °C and 5% CO2.
- Incubate cells for 48 h before conducting the secretion assays.
-
-
Prepare buffers
- Prewarm PSS-Na (see Recipes) to 37 °C.
- Prepare stimulation buffer by adding ionomycin (dissolved in DMSO, 5 mM stock) to PSS-Na at a final concentration of 1.25 μM. Each well designed for stimulation requires ~100 μl stimulation buffer.
- Prepare control buffer by adding DMSO to PSS and make sure DMSO concentration (1/4,000 dilution if ionomycin stock solution is 5 mM) is identical to that in the stimulation buffer. Each well designed as control requires ~100 μl control buffer.
- Prepare lysis buffer by adding Triton X-100 to PSS at a final concentration of 1%. Each well requires ~100 μl lysis buffer.
-
Stimulate cells for secretion (Figure 1B)
- Remove culture medium with a multichannel aspirator.
- Wash cells with 200 μl pre-warmed PSS-Na once and remove washing buffer.
- Add 100 μl stimulation buffer or control buffer to the cells. Incubate at 37 °C for 10 min.
- Transfer 95 μl supernatant to a 96-well assay plate. Avoid disrupting cells. Add plain buffers (stimulation and control buffer) to 3 empty wells for background normalization. Keep the plate in the dark.
- Add 95 μl lysis buffer to cells, and incubate at 37 °C for 10 min.
- Use a pipette to mix cells with lysis buffer and then transfer 95 μl to a 96-well assay plate. Add lysis buffer to 3 empty wells for background normalization.
-
Measure NPY-VenusDetermine NPY-Venus fluorescence in supernatant and lysates by a Tecan plate reader. We recommend using a monochromatic plate reader (excitation 505/20 nm, emission 535/10 nm), but we were also successful using the filter sets for GFP/FITC (excitation 485/20 nm, emission 535/25 nm).The NPY-Venus secretion assay can be adapted to high throughput screening with the help of automated liquid handling systems.
- For siRNA transfection, deliver 1.2 μl siRNA from siRNA library to 96-well plates with a multichannel pipette or an automated liquid handling system.
- For siRNA transfection, deliver 100 μl cell suspension to siRNA mixture by an automated liquid handling system (Biotek Microflo dispenser).
- For NPY-Venus secretion, conduct all the liquid handling steps with an automated liquid handling system (Thermo Scientific Matrix Hydra DT 96-well liquid handler).
-
-
3H-serotonin secretion assay
The serotonin secretion assay uses parental BON cells. The NPY-Venus cell lines should also work, although we haven’t tested them for this assay. Please be advised that the serotonin secretion assay deals with radioactivity and therefore the experiment has to be carried out according to local regulations in a radioactivity laboratory and waste has to be disposed of properly. All the volumes in this section are for one well of a 96-well plate.
-
siRNA transfection.
Follow the steps as described in Step B1 (see Note 2).
-
Load cells with 3H-serotonin 24 h after siRNA transfection by adding the following mixture to each well (do NOT replace culture medium).
3H-serotonin (1 mCi/ml): 0.2 μl
L-ascorbic acid (150 mM) (see Recipes): 0.33 μl
H2O: 1.47 μl
Total: 2 μl
-
Measure serotonin secretion after 18 h incubation with 3H-serotonin.
Follow the same steps as described in Step B3, except that:
For Step B3d: instead of transferring supernatant to assay plates, collect them into 1.7 ml microcentrifuge tubes and centrifuge to remove intact cells and cell debris.
For Step B3f: instead of transferring cell lysates to assay plates, simply transfer them to tubes for scintillation counting. No centrifugation is required.
After centrifugation, transfer secretion buffer and cell lysates into new scintillation counting tubes and quantify 3H-serotonin with a scintillation counter.
-
-
Establishing NPY-Venus-expressing cell lines
We have generated two stable BON cell lines, H8 and F6, that express DCV cargo, NPY-Venus (see Note 3 for differences), which can be obtained from the Martin Lab. Alternatively, one may generate NPY-Venus-expressing cell lines by the following steps. Please be advised that lentivirus packaging and transduction experiments should be conducted in a Biosafety Level 2 laboratory and wastes should be disposed of according to local regulations.
-
Lentivirus packaging
Split 2.5 x 106 HEK293FT cells to each 10 cm tissue culture plate, and incubate cells overnight in a 5% CO2 incubator at 37 °C.
-
The next morning, transfect HEK cells for lentivirus generation with the following protocol:
1 Mix the following components:NPY-Venus lenti construct: 20 μgEnvelop plasmid pMD2.G: 6 μgPackaging plasmid psPAX2: 15 μgCaCl2 (2.5 M) (see Recipes): 50 μlAdd H2O to bring total volume to 500 μl500 μl 2x HBeS (see Recipes) 2 Mix plasmid mixture dropwise with HBeS 3 Incubate at room temperature for 20 min 4 Add plasmid mixture (1 ml) dropwise to HEK cells 6-8 h after transfection, remove medium from cells and replace with viral production medium (DMEM with 30% FBS, see Recipes).
24 h after transfection, replace with fresh viral production medium again.
48-60 h after transfection, collect culture medium and properly dispose of cells.
Centrifuge culture medium at low-speed (1,000 × g, 5 min) to remove dead cells and cell debris, then filter supernatant through a 0.45 μm pore membrane and collect in a 32 ml sterile polycarbonate ultracentrifugation tube.
Pellet lentiviral virions by ultracentrifugation at 33,000 rpm (or 11,000 × g), 4 °C for 2 h in a Ti70 Beckman rotor.
Carefully take out the centrifugation tube and mark the position of the viral pellet at the outside wall of the tube near the bottom.
Carefully remove the supernatant and add 100 μl cold sterile PBS to cover the viral pellet. Incubate in cold room (4 °C) overnight.
The next morning, use a pipette to resuspend a few times and aliquot concentrated viral solution (20 μl each) into separate tubes. Store viral solution at -80 °C or use immediately. Avoid repeated freeze and thaw.
-
Lentiviral transduction
Seed 1.5 x 104 BON cells into each well of a 96-well plate (5 wells in total). Incubate overnight to allow cell attachment.
-
The next afternoon, transduce BON cells by replacing medium with the following lentiviral solution.
Well 1 2 3 4 5 Culture medium (μl) 100 100 100 100 100 Lentiviral solution (μl) 0 0.33 1 3 10 Protamine sulfate (μl) 1 1 1 1 1 18 h after transduction, remove lentiviral-containing medium and replace with fresh culture medium.
48-72 h after transduction, check NPY-Venus expression with a fluorescence microscope. Choose one well where the majority of cells are healthy and fluorescent for single cell screening (see Note 4).
-
Single cell screening
Trypsinize NPY-Venus expressing cells and make a cell solution.
Seed cells to 96-well plates with a FACS sorter (1 cell/well) or by serial dilution (0.5 cell/well). Place the plates in an incubator at 37 °C and 5% CO2.
After about a week, check the plates and mark wells that have a single clone. Discard empty wells or wells that have multiple clones.
Frequently check candidate wells and replace medium if necessary. When cells grow into aggregates with hundreds of cells, vigorously pipette with a 200 μl pipette tip to disperse into single cells.
Continue to culture the cells until they cover > 50% area of a well. Check cells by fluorescence microscopy and choose clones that express NPY-Venus for the next step.
Split candidate clones into three separate 96-well plates and incubate for 48 h.
Use two plates for screening. Follow the steps described in Procedure B to select clones that have high percent secretion with ionomycin stimulation and low percent secretion with DMSO control.
Keep the best clones (1~3) and discard the rest. Expand cells, freeze some vials in freezing medium (see Recipes) and store liquid nitrogen.
-
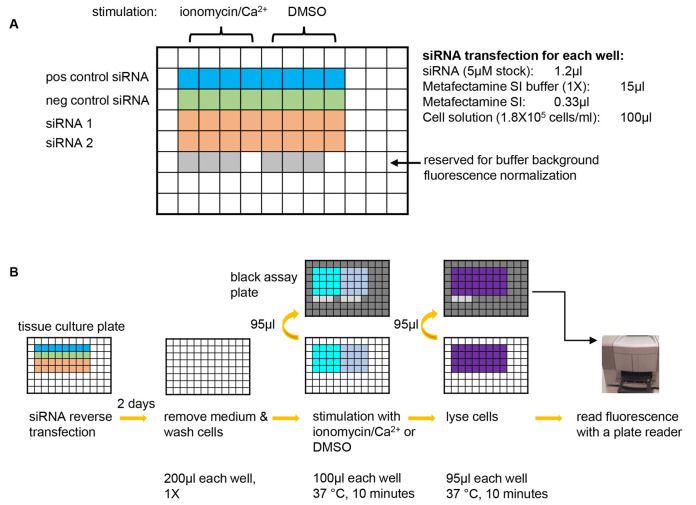
Figure 1. A graphic summary of the NPY-Venus secretion assay.
A. Plate design for an example experiment of testing two siRNAs of interest. A positive control siRNA that is known to inhibit NPY-Venus secretion (e.g., CADPS siRNA) and a negative control siRNA that is known not to affect NPY-venus secretion (e.g., Dharmacon non-targeting siRNA D-001210-03-20) should always be included in each experiment. 8 wells are used for each siRNA with 4 wells for stimulation with ionomycin/Ca2+ and the other 4 for background secretion. 6 wells are left blank so that the same wells on the black assay plates (see B) can be used for determining the background fluorescence of plain buffer. pos control siRNA: positive control siRNA; neg control siRNA: negative control siRNA. B. Steps of the NPY-Venus secretion assay. From left to right: conduct reverse transfection with siRNAs; after two days of incubation, remove culture medium and wash cells with pre-warmed PSS-Na; stimulate cells with PSS-Na containing either ionomycin or DMSO and then transfer the supernatant to a black assay plate; lyse cells with 1% Triton X-100 and then transfer to a black assay plate; add plain buffer to the reserved wells (see A) and then determine NPY-Venus fluorescence with a plate reader. Refer to texts for details.
Data analysis
-
NPY-Venus secretion assay
NPY-Venus percent secretion is measured by the percentage of NPY-Venus secreted into buffer divided by total NPY-Venus in both buffer and cell lysates.
Subtracting background fluorescence in empty buffer to calculate secreted NPY-Venus in supernatant and cell lysates.
-
Use the following equation to calculate the percent secretion of NPY-Venus.
NPY-Venus basal percent secretion under the treatment of control buffer should be low and does not significantly vary among different siRNA treatments. For simplicity, we simply used ionomycin-stimulated NPY-Venus percent secretion as a measurement of regulated exocytosis.
-
Serotonin secretion assay
3H-serotonin percent secretion is calculated in a similar way as NPY-Venus.
Basal secretion of serotonin is typically much larger than that in the NPY-Venus assay because the protocol does not wash cells very extensively and residue 3H-serotonin was carried over to the secretion buffer (see Note 5). Therefore, we always measure both stimulated and basal secretion and use the difference (referred to as ‘Ca2+-dependent secretion’) as a measurement of regulated exocytosis (see Figure 2).
Figure 2. Serotonin secretion assay in BON cells.
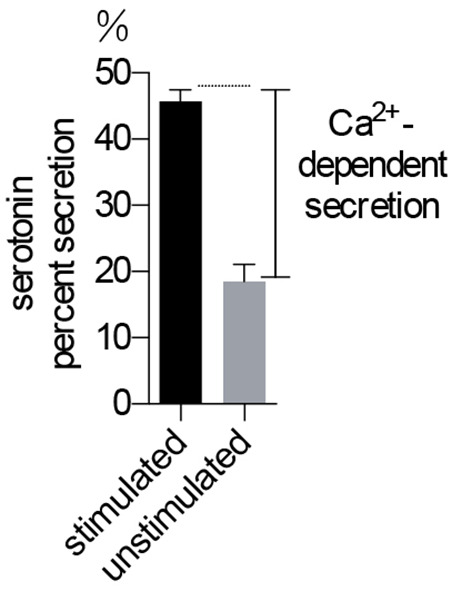
Ca2+-dependent secretion is used to indicate the level of exocytosis. n = 5 for each treatment.
Notes
The protocol uses siRNA at a final concentration of 50 nM. The optimal siRNA concentration is dependent on particular siRNAs and may need further optimization.
It is important to measure serotonin background secretion in the presence of DMSO because background secretion is high due to the retention of residual 3H-serotonin in the washing step.
BON cell Clone H8 (Clone H8) stably expresses NPY-Venus and a plasma membrane protein TfR-mcherry. TfR-mcherry only contains the membrane targeting domain of transferrin receptor (TfR) with the endocytosis signal mutated. BON cell Clone F6 only expresses NPY-Venus.
We chose Well 2, but it may differ based on the concentration of lentiviral particles.
Basal 3H-serotonin secretion can be minimized by washing cells multiple times before stimulation. However, more cells can be lost by multiple washes and the measurable 3H-serotonin signal may be attenuated.
Recipes
-
BON cell culture medium
Mix DMEM with the same volume of F12 nutrient
Add FBS (PHENIX Research, no heat inactivation) to make a final concentration of 10% and store at 4 °C
-
HANK’s buffer
Dissolve the following components in H2O
Component Weight (g) KCl 2 KH2PO4 0.3 NaCl 40 NaHCO3 1.75 Na2HPO4·7H2O 0.45 Adjust pH to 7.4~7.5 with HCl or NaOH solution, bring total volume to 5 L
Aliquot to 500 ml bottles and autoclave
Add sterile EDTA for a final concentration of 1 mM
Store at room temperature.
-
PSS-Na
Component Concentration (mM) NaCl 145 KCl 5.6 CaCl2 2.2 MgCl2 0.5 HEPES 15 Glucose 5.6 Adjust pH to 7.4 with NaOH solution and store at 4 °C
-
L-ascorbic acid solution
Dissolve L-ascorbic acid in H2O to a final concentration of 150 mM
Adjust pH to 5-6
Filter through a 0.2 μm membrane to sterilize
Store at -20 °C
-
HEK cell culture medium
Add FBS (Thermo Fisher Scientific, no heat inactivation) to DMEM for a final concentration of 10% and store at 4 °C
-
Viral production medium
Add FBS (Thermo Fisher Scientific, no heat inactivation) to DMEM for a final concentration of 30%
Prepare fresh solution right before use
-
HBeS (2x)
Component Concentration(mM) NaCl 280 Na2HPO4 1.5 HEPES 50 Adjust pH to 7.05 with NaOH solution, filter through a 0.2 μm membrane to sterilize and store at 4 °C
-
CaCl2 solution
Dissolve CaCl2 to make a 2.5 M solution in H2O
Filter through 0.2 μm membrane to sterilize and store at 4 °C
-
Protamine sulfate
Dissolve protamine sulfate to make a 1 mg/ml solution in H2O
Filter through a 0.2 μm membrane to sterilize and store at 4 °C
-
Freezing medium
Mix normal BON cell culture medium with cell culture grade DMSO (e.g., Sigma-Aldrich) at a 9:1 ratio
Prepare fresh solution right before use
Acknowledgments
The work was supported by National Institutes of Health grants DK040428, DK025861, and GM119158 (to Martin, T.F.J.). The NPY-Venus secretion assay was inspired by Nagai et al. (2002) and work by Bruinsma, S and Martin T.F.J. (unpublished). The serotonin secretion assay was inspired by Samuel Tran et al. (2004) and Berwin et al. (1998). We acknowledge current and previous members of the Martin Lab for their advice on developing the assays. The authors declare no competing financial interests.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Berwin B., Floor E. and Martin T. F.(1998). CAPS(mammalian UNC-31) protein localizes to membranes involved in dense-core vesicle exocytosis. Neuron 21(1): 137-145. [DOI] [PubMed] [Google Scholar]
- 2. Borgonovo B., Ouwendijk J. and Solimena M.(2006). Biogenesis of secretory granules. Curr Opin Cell Biol 18(4): 365-370. [DOI] [PubMed] [Google Scholar]
- 3. Bowman G.R., Cowan A.T. and Turkewitz A.P.(2009). Biogenesis of dense-core secretory granules. Trafficking Inside Cells 183-209. [Google Scholar]
- 4. Griffiths G. M., Tsun A. and Stinchcombe J. C.(2010). The immunological synapse: a focal point for endocytosis and exocytosis. J Cell Biol 189(3): 399-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guerineau N. C., Bonnefont X., Stoeckel L. and Mollard P.(1998). Synchronized spontaneous Ca2+ transients in acute anterior pituitary slices . J Biol Chem 273(17): 10389-10395. [DOI] [PubMed] [Google Scholar]
- 6. Kabachinski G., Kielar-Grevstad D. M., Zhang X., James D. J. and Martin T. F.(2016). Resident CAPS on dense-core vesicles docks and primes vesicles for fusion. Mol Biol Cell 27(4): 654-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karatekin E., Tran V. S., Huet S., Fanget I., Cribier S. and Henry J. P.(2008). A 20-nm step toward the cell membrane preceding exocytosis may correspond to docking of tethered granules. Biophys J 94(7): 2891-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kishimoto T., Kimura R., Liu T. T., Nemoto T., Takahashi N. and Kasai H.(2006). Vacuolar sequential exocytosis of large dense-core vesicles in adrenal medulla. EMBO J 25(4): 673-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klenchin V. A. and Martin T. F. J.(2000). Priming in exocytosis: Attaining fusion-competence after vesicle docking. Biochimie 82(5):399-407. [DOI] [PubMed] [Google Scholar]
- 10. Lang J.(1999). Molecular mechanisms and regulation of insulin exocytosis as a paradigm of endocrine secretion. Eur J Biochem 259(1-2): 3-17. [DOI] [PubMed] [Google Scholar]
- 11. Martin T. F.(2003). Tuning exocytosis for speed: fast and slow modes. Biochim Biophys Acta 1641(2-3): 157-165. [DOI] [PubMed] [Google Scholar]
- 12. Mayorga L. S., Tomes C. N. and Belmonte S. A.(2007). Acrosomal exocytosis, a special type of regulated secretion. IUBMB Life 59(4-5): 286-292. [DOI] [PubMed] [Google Scholar]
- 13. Nagai T., Ibata K., Park E. S., Kubota M., Mikoshiba K. and Miyawaki A.(2002). A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol 20(1): 87-90. [DOI] [PubMed] [Google Scholar]
- 14. Parekh D., Ishizuka J., Townsend C. M. Jr. Haber B., Beauchamp R. D., Karp G., Kim S. W., Rajaraman S., Greeley G. Jr.and Thompson J. C.(1994). Characterization of a human pancreatic carcinoid in vitro: morphology, amine and peptide storage, and secretion . Pancreas 9(1): 83-90. [DOI] [PubMed] [Google Scholar]
- 15. Sorensen J. B.(2017). Ride the wave: Retrograde trafficking becomes Ca2+ dependent with BAIAP 3. J Cell Biol 216(7): 1887-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sudhof T. C.(2004). The synaptic vesicle cycle. Annu Rev Neurosci 27: 509-547. [DOI] [PubMed] [Google Scholar]
- 17. Tran V. S., Marion-Audibert A. M., Karatekin E., Huet S., Cribier S., Guillaumie K., Chapuis C., Desnos C., Darchen F. and Henry J. P.(2004). Serotonin secretion by human carcinoid BON cells. Ann N Y Acad Sci 1014: 179-188. [DOI] [PubMed] [Google Scholar]
- 18. Zhang X., Jiang S., Mitok K. A., Li L., Attie A. D. and Martin T. F. J.(2017). BAIAP3, a C2 domain-containing Munc13 protein, controls the fate of dense-core vesicles in neuroendocrine cells. J Cell Biol 216(7): 2151-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Z., Wu Y., Wang Z., Dunning F. M., Rehfuss J., Ramanan D., Chapman E. R. and Jackson M. B.(2011). Release mode of large and small dense-core vesicles specified by different synaptotagmin isoforms in PC12 cells. Mol Biol Cell 22(13): 2324-2336. 21551071 [Google Scholar]