Abstract
The long-lived, hypoxic-tolerant naked mole-rat well-maintains cardiac function over its three-decade-long lifespan and exhibits many cardiac features atypical of similar-sized laboratory rodents. For example, they exhibit low heart rates and resting cardiac contractility, yet have a large cardiac reserve. These traits are considered ecophysiological adaptations to their dank subterranean atmosphere of low oxygen and high carbon dioxide levels and may also contribute to negligible declines in cardiac function during aging. We asked if naked mole-rats had a different myofilament protein signature to that of similar-sized mice that commonly show both high heart rates and high basal cardiac contractility. Adult mouse ventricles predominantly expressed α-myosin heavy chain (97.9 ± 0.4%). In contrast, and more in keeping with humans, β myosin heavy chain was the dominant isoform (79.0 ± 2.0%) in naked mole-rat ventricles. Naked mole-rat ventricles diverged from those of both humans and mice, as they expressed both cardiac and slow skeletal isoforms of troponin I. This myofilament protein profile is more commonly observed in mice in utero and during cardiomyopathies. There were no species differences in phosphorylation of cardiac myosin binding protein-C or troponin I. Phosphorylation of both ventricular myosin light chain 2 and cardiac troponin T in naked mole-rats was approximately half that observed in mice. Myofilament function was also compared between the two species using permeabilized cardiomyocytes. Together, these data suggest a cardiac myofilament protein signature that may contribute to the naked mole-rat’s suite of adaptations to its natural subterranean habitat.
Keywords: Naked mole-rat, Heart, Slow skeletal troponin I, β myosin heavy chain, Neoteny, Hypoxia
Introduction
The naked mole-rat (Heterocephalus glaber) has emerged as a rodent model of exceptional biomedical interest [22, 27, 51], best known for both its extraordinary longevity [7] and healthspan [8, 16] and concomitant resistance to cancer [28, 50] as well as anoxic conditions [34]. Previously, we have reported that naked mole-rats exhibit unusually low basal cardiac function for a mouse-sized (~ 45 g) rodent [16, 17]; however, this low level of function is maintained well into its third decade, ages thought to be equivalent to nonagenarian humans. It has an exceptionally low heart rate of 250 bpm for an unanesthetized small rodent (e.g., mice 700 bpm) and a diminished (28%) fractional shortening relative to similar-sized rodents [17]. Equivalent values in other laboratory rodents would be indicative of systolic dysfunction. However, the naked mole-rat heart clearly is not dysfunctional. Naked mole-rats have a very strong β-adrenergic response resulting in an almost twofold greater cardiac reserve in comparison to mice [17]. It is likely that this cardiac profile of low basal function yet high cardiac reserve reflects ecophysiological adaptations to an unforgiving and energetically constrained subterranean habitat that naked mole-rats have occupied since the early Miocene (23–16 million years ago) [6].
Naked mole-rats are strictly subterranean rodents native to the arid and semi-arid regions of the eastern horn of Africa. There, they rest in deep subterranean chambers 6–8 ft below ground. The nests are shared by numerous conspecifics as well as microorganisms, which together with plant roots, all respire, depleting the already limited underground supply of oxygen, since gas exchange with the air above ground occurs primarily by diffusion through soil, a slow process dependent upon soil porosity [5]. Naked mole-rats have a low basal metabolic rate—66 to 75% that of a mouse [9, 13]—reducing oxygen consumption and heat production and, thereby, the need for both gas exchange and evaporative cooling while at rest [5]. Naked mole-rats have, however, a very large metabolic scope and are able to increase their metabolic rate more than fivefold when actively excavating burrows in search of underground food sources [30]. This harsh, energetically constrained habitat of highly variable oxygen availability and high levels of carbon dioxide relative to that experienced above ground is, thus likely to have shaped both their metabolic physiology and their cardiovascular system. In many cardiovascular disease states, adult mammalian hearts, like those of healthy naked mole-rats, also have to contend with the stressful conditions of hypoxia and hypercapnia [23, 39, 45]. Such diseased states are also responsible for disrupting cardiac homeostasis and causing dysregulation of the contractile protein signature [39, 42, 43].
Cardiac function is fundamentally regulated by the composition of the contractile machinery, including the expression of different isoforms of cardiac myofilament proteins and post-translational modifications of these proteins. The different isoforms of myofilament proteins within the heart can cause differences in calcium sensitivity, contraction, and rates of cross-bridge cycling. For instance, myosin heavy chain (MHC) regulates contractility differently depending upon which isoform is predominantly expressed in the heart. Expression of α-MHC is associated with a high force of contraction, while β-MHC causes lower contractility [24]. Additionally, changes in troponin I isoforms can modulate the calcium sensitivity and cross-bridge cycling of cardiomyocytes [25].
Post-translational modifications of these proteins can also largely affect cardiac function, and such modifications often become dysregulated in the setting of homeostatic imbalance. Of these modifications, phosphorylation of myofilament proteins is the most well-studied [3, 25, 32]. Depending upon the myofilament protein, and even the specific site within the protein that is modified, the functional outcomes can differ widely [25, 48, 49]. Given their unusual cardiac function phenotype as well as their numerous ecophysiological adaptations to the challenges encountered living below ground, we hypothesized that the naked mole-rat may exhibit unique features in its cardiac myofilament profile.
Materials and methods
Animals
Male and female naked mole-rats (2–5 years) and C57BL6/J mice (3–6 months) housed at the University of Texas Health Science Center at San Antonio (UTHSCSA) were used in this study. The selected ages allowed for physiological age matching between species such that both were at approximately equivalent percentages of their maximum lifespan (6–15% of maximum lifespan for naked mole-rats and 6–13% of that for mice). Both species were maintained on a 12-h light-dark cycle. The captive-bred naked mole-rats were predominantly third generation descendants of animals captured in the wild in Africa in 1980. The naked mole-rats were housed in family groups in simulated burrow systems consisting of tubes and cages of varying sizes. Climatic conditions also approximated their native habitat (30 °C; 50% relative humidity), although atmospheric conditions were normoxic. Naked mole-rats met all their nutrient and water needs through an ad libitum supply of fruit and vegetables, supplemented with a protein-and vitamin-enriched cereal (Pronutro, Bokomo, South Africa).
We chose the extensively studied C57Bl/6 strain of mice as an “experimental control” with which to evaluate naked mole-rat data to assure that measurements made in our laboratory were comparable to those previously published and used these as the basis with which to evaluate our naked mole-rat data. C57Bl/6 mice were obtained from Envigo (formerly Harlan) and group housed (n = 5 per cage) at 25 °C and were given ad libitum access to mouse chow (Harlan Teklad 7912) and water.
Naked mole-rats are neither moles nor rats and are phylogenetically distant from both these organisms. Rather, they are a unique species, taxonomically isolated in a distinct family, the Heterocephalidae in the Hystricognath suborder of Rodentia and are evolutionarily equidistant from both mice and rats. Given that cardiac function scales allometrically with body size [40], we chose the more similarly sized mouse (25 vs. 45 g naked mole-rat) as controls rather than laboratory rats (250 g) or other species of African mole-rats (Bathyergidae 160–2000 g) that are phylogenetically more closely related to naked mole-rats, but are poorly characterized.
Both naked mole-rats and mice were sacrificed by isoflurane inhalation. Tissues were rapidly harvested and flash frozen in liquid nitrogen and then stored at −80 °C for protein and cardiomyocyte analyses or fixed for imaging. These studies were approved by the IACUC at UTHSCSA (protocol #13007x).
Electron microscopy
Several hearts were then sectioned through the mid-papillary region of the ventricles and placed in cold fixative buffer containing 4% formaldehyde and 1% glutaraldehyde. Tissue was embedded in polybed 812 resin and sections were cut at 90 nm and placed on copper grids. Sections were stained with uranyl acetate for 30 s followed by Reynolds lead citrate for 20 s. Samples were then visualized with a JEOL 1230 transmission electron microscope.
Protein isolation and analysis
Myofilament-enriched fractions and total homogenates were isolated in various buffers, all containing Halt Protease and Phosphatase Inhibitor (Life Technologies). Atrial and ventricular tissues were homogenized in ice-cold F60 buffer to isolate myofilament proteins. Protein content in these homogenates was analyzed using BCA assays (Life Technologies). Five micrograms of protein were loaded per well on large format Hoefer SDS-PAGE gels which were run at 30 mA for 26 h at 4 °C to separate MHC isoforms. The gels were then fixed and stained with SYPRO Ruby (Life Technologies) as described previously [1]. Aliquots of ventricular samples in F60 buffer were resuspended in 4 M urea buffer in order to solubilize myofilament proteins. Bradford assays (Bio-Rad) were used to determine protein quantity, and 4 μg of the urea samples were run at 90 V on 4–15% unstained Bio-Rad TGX mini gels. These gels were stained with Pro-Q Diamond and SYPRO Ruby (Life Technologies), to assess myofilament phosphorylation and total myofilament protein levels, respectively, according to previously described procedures [54]. Each sample in both the MHC and myofilament protein phosphorylation experiments were run in triplicate and the averages were then used for statistical analyses. All protein levels from gels were quantified with ImageJ (NIH).
Ventricular and quadriceps tissues were homogenized in RIPA buffer and total protein content was analyzed with BCA assays. Twenty microgram aliquots of these samples were run on 4–20% Bio-Rad TGX mini gels and then transferred to nitrocellulose membranes at 50 V for 2 h. Blots were probed with the following antibodies: internal and C-terminal regions of troponin I-SS (sc-20645 and sc-8119, respectively; Santa Cruz) both at 1:1000, cardiac troponin I (ab19615; Abcam) at 1:5000, and GAPDH (G8795; Sigma) at 1:20,000. The antibodies used in these Western blot analyses were chosen after confirmation with BLAST that each epitope had good homology with both the mouse and naked mole-rat proteins. LI-COR secondary antibodies for the respective primary antibody hosts were used (mouse 926–32,210, rabbit 926–68,021, and goat 925–68,024), each at a concentration of 1:10,000. The blots were then imaged using an Odyssey Infrared Imaging System (LI-COR Biosciences).
Mass spectrometry
Ventricular myofilament-enriched samples homogenized in F60 buffer were also used for mass spectrometry. Proteins were separated by SDS-PAGE (2-cm separation; Criterion XT Bis-Tris 12% gel; Bio-Rad). After staining with Coomassie blue, each gel lane was divided into seven slices and the proteins digested in situ with trypsin (sequencing grade; Promega). The digests were analyzed by capillary HPLC-electrospray ionization tandem mass spectrometry (HPLC-ESI-MS/MS) on a Thermo Fisher LTQ Orbitrap Velos mass spectrometer. On-line HPLC separation of the digests was accomplished with an Eksigent/AB Sciex NanoLC-Ultra 2-D HPLC system: column, PicoFrit™ (New Objective; 75 μm i.d.) packed to 15 cm with C18 adsorbent (Vydac; 218MS 5 μm, 300 Å). Precursor ions were acquired in the Orbitrap in centroid mode at 60,000 resolution (m/z 400); data-dependent collision-induced dissociation (CID) spectra of the six most intense ions in the precursor scan were acquired at the same time in the linear trap (30% normalized collision energy). Mascot (Matrix Science) was used to search the spectra against the rodent subset of the NCBInr database (v140522) concatenated with a database of common protein contaminants. Cysteine carbamidomethylation was set as a fixed modification and methionine oxidation and deamidation of glutamine and asparagine were considered as variable modifications; trypsin was specified as the proteolytic enzyme, with one missed cleavage allowed. Subset search of the identified proteins by X! Tandem, cross-correlation with the Mascot results and determination of protein and peptide identity probabilities were accomplished by Scaffold (Proteome Software). The thresholds for acceptance of peptide and protein assignments in Scaffold were 95 and 99%, respectively.
Steady-state force measurements
Cardiomyocytes were isolated from frozen ventricular tissue and chemically skinned for analysis of force as previously described [2]. The tissue was mechanically homogenized and then filtered through a 70 μm strainer. After centrifugation at 120×g for 1 min at 4 °C, the pellet was resuspended in 1% Triton X-100 in relaxing solution (97.92 μM KOH, 6.24 μM ATP, 10 μM EGTA, 10 μM Na2CrP, 47.58 μM Kprop, 100 μM BES, and 6.54 μM MgCl2) and rocked for 15 min at room temperature. Two subsequent centrifugations were carried out as above and the remaining pellet was resuspended in relaxing solution without Triton. This solution, now containing skinned cardiomyocytes, was examined using an inverted microscope (Leica DM IRB). Individual cardiomyocytes were singled out and attached to a force transducer and high-speed piezo translator (ThorLabs) using UV-sensitive glue. To measure force generation, cells were perfused with buffers containing different calcium concentrations (pCa 10–pCa 4.5). Force transduction was measured at two sarcomere lengths (SLs)—1.9 and 2.3 μm—in order to determine differences in length-dependency of calcium sensitivity of force development. Fitting of the force curves was done by using a modified Hill equation (P/PO = [Ca2+]n/(Kn + [Ca2+]n), where n is the Hill-slope and K is the pCa50). Data were acquired using custom-made LabView software (National Instruments).
Statistical analyses
Data from all experiments, other than the mass spectrometry analyses, were analyzed using Student’s t tests. F-tests for equality of variance were also conducted, and a Welch’s correction was made if there were unequal variances. MHC and phosphorylation measurements include both male and females of each species (with each sex representing approximately half of the sample size for each species) and were subsequently grouped together as there were no detected sex differences. The steady-state force measurements were conducted on only males of each species. The number of individuals in an experiment is noted in each figure legend. GraphPad Prism 5 software (GraphPad) was used for all statistical calculations. Data are expressed as mean ± standard error of the mean (SEM).
Results
Cardiac form and function of mice and naked mole-rats
We have previously reported on the differences in cardiac function in young, healthy mice and naked mole-rats [17], finding that naked mole-rats displayed much lower heart rates and fractional shortening compared to mice (Table 1). We also previously found that naked mole-rats have increased cardiomyocyte cross-sectional area (216 ± 10 vs. 178 ± 7 mm2) compared to mice, but this is accompanied by greater diastolic wall thicknesses (0.87 ± 0.02 vs. 0.77 ± 0.03 mm) and larger hearts overall (190 ± 10 vs. 130 ± 10 mg), as such it is unlikely that naked mole-rat cardiomyocytes are hypertrophied. Despite these differences in form and function of the two species’ hearts, electron micrographs taken of ventricles there showed no obvious differences in sarcomeric organization or striation patterns between both mice and naked mole-rat, despite the large differences in cardiac form and function (Fig. 1). Naked mole-rats had no overt changes in overall architecture, including sarcomere hallmarks such as Z-disks and M-lines when compared to mice.
Table 1.
Average cardiac morphological and functional parameters of naked mole-rats and mice
| Parameter | Mouse | Naked mole-rat |
|---|---|---|
| Average body size (g) | ~ 25 | ~ 45 |
| Heart weight (mg) | 130 ± 10 | 190 ± 10* |
| Heart rate (bpm) | 704 ± 11 | 256 ± 8* |
| Fractional shortening (%) | 39 ± 2 | 28 ± 2* |
| Diastolic ventricular wall thickness (mm) | 0.77 ± 0.03 | 0.87 ± 0.02* |
| Cardiomyocyte cross-sectional area (mm2) | 178 ± 7 | 216 ± 10* |
Data from [17]. n = 6–10 animals per species for each measurement
Denotes p < 0.05 versus mice
Fig. 1.
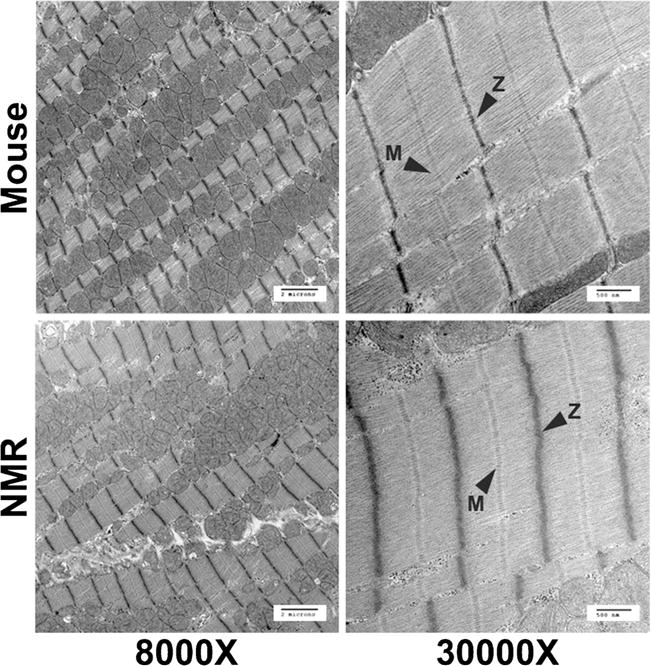
Electron micrographs of mouse and naked mole-rat ventricles displaying no overt differences in sarcomere organization or structure between species. M-lines and Z-disks are denoted in the images
Naked mole-rats have increased β-MHC in their hearts
We assessed if myosin heavy chain (MHC) isoform composition, known to regulate contractility, may partially explain the low basal fractional shortening previously observed in naked mole-rat hearts (Table 1). Like mice, naked mole-rats expressed mostly α-MHC in their atria (75.5 ± 4.0%). Strikingly, however, naked mole-rats predominantly expressed β-MHC (79.0 ± 2.0%) in their ventricles (Fig. 2). This MHC distribution was in stark contrast to mice which expressed almost no β-MHC in their hearts but, rather, had mainly α-MHC in both their atria (98.1 ± 0.4%) and ventricles (97.9 ± 0.4%). Of note, in both mice and naked mole-rats, the MHC isoform contributions were similar in the left and right ventricles, so the ventricular data reflect the isoform content in both ventricles, both here and in all following experiments using ventricular tissue.
Fig. 2.
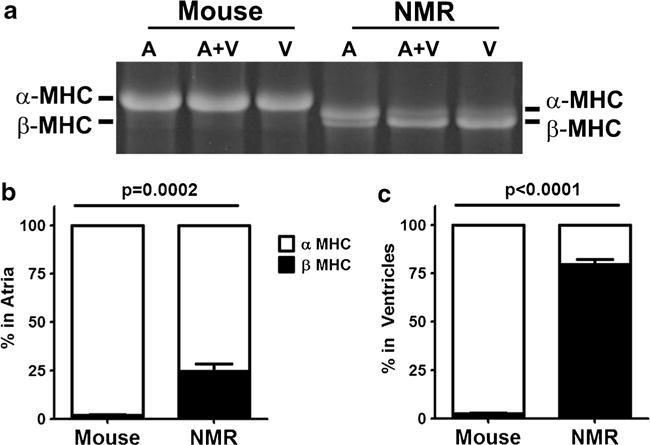
Composition of myosin heavy chain isoforms differs between mouse and naked mole-rat (NMR) in regions of the heart. a Large format SDS-PAGE electrophoresis showed α- and β-MHC in both the ventricles (V) and atria (A). Naked mole-rats had significantly more β-MHC than mice, but still expressed predominantly α-MHC in their atria. b Naked mole-rats had mostly β-MHC in their ventricles, making them more like humans (n = 11 ventricles and n = 6 atria/species)
Naked mole-rats have similar or lower phosphorylation of cardiac myofilament proteins to mice
We also questioned if there were species differences in levels of ventricular myofilament phosphorylation, as these modifications influence contractility (Fig. 3a, b). When corrected for total protein content, phosphorylation of both cardiac troponin T (TnT) and myosin light chain 2 (MLC-2) in naked mole-rats was half (p < 0.0001) that observed in mice (Fig. 3c). There were, however, no species differences in phosphorylation of cardiac myosin binding protein-C (cMyBP-C) or troponin I (TnI). Intriguingly, we observed pronounced interspecific differences in the molecular weights of the protein bands for TnT, TnI, and MLC-2 and questioned if these differences reflected variation in myofilament isoform expression.
Fig. 3.
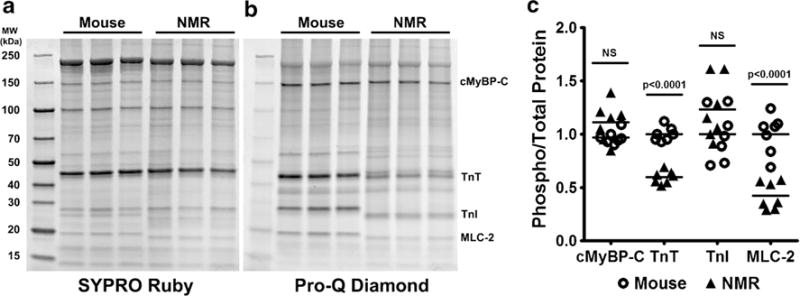
Phosphorylation of myofilament proteins is either lower or no different in naked mole-rat (NMR) ventricles when compared to those of mice. a SYPRO Ruby stain showed all myofilament fraction proteins. b Pro-Q Diamond stain showed total phosphorylation of myofilament proteins, with specific proteins highlighted: cardiac myosin binding protein C (cMyBP-C), troponin T (TnT), troponin I (TnI), and myosin light chain 2 (MLC-2). c Quantification of the highlighted proteins shows that naked mole-rats had significantly lower phosphorylation of TnT and MLC-2 than mice, but there were no species differences in cMyBP-C and TnI phosphorylation (n = 7/species)
Young, healthy naked mole-rats express slow skeletal troponin in their ventricles
Mass spectrometry analysis conducted on myofilament preparations from ventricular tissue detected the presence of all of the common cardiac isoforms of cTnT, cTnI, and MLC-2 in both species (Table 2). However, slow skeletal troponin I (ssTnI) was also detected in the naked mole-rat samples. Upon cluster analysis of the mass spectrometry data, it was found that naked mole-rat ssTnI shared no peptides with other protein hits (Supplementary Table S1), thus substantiating its presence in naked mole-rat ventricles. To confirm that ssTnI was present in naked mole-rat ventricles, Western blot analyses were undertaken (Fig. 4) using quadricep skeletal muscle as a positive control. Antibodies to both the internal and C-terminal regions of ssTnI showed, as expected, that both mouse and naked mole-rat quadriceps express ssTnI. Ventricles of both species expressed the cardiac isoform, cTnl. However, only the naked mole-rat ventricular tissue also expressed ssTnI, confirming the presence of both cardiac and skeletal troponin in the hearts of young, healthy adult naked mole-rats.
Table 2.
Mass spectrometry reveals the presence of slow skeletal troponin I in healthy adult naked mole-rat ventricles
| Species | Protein | Accession number | MW (kDa) | Total spectraa | Unique peptidesb | Sequence coverage (%) |
|---|---|---|---|---|---|---|
| Mouse | Troponin T2, cardiac isoform CRA_a (Mus musculus) | gi|148707615|gb|EDL39.562.1| (+15) | 32 | 64 | 3 | 47 |
| Troponin I, cardiac muscle (Mus musculus) | gi|6678393|ref|NP_033432.1| | 24 | 82 | 3 | 54 | |
| Myosin light chain 2 (Mus musculus domesticus) | gi|199985|gb|AAA39796.1| | 19 | 59 | 1 | 84 | |
| Naked mole-rat | Troponin T, cardiac muscle, partial (Heterocephalus glaber) | gi|351700845|gb|EHB03764.1| (+6) | 33 | 94 | 12 | 50 |
| Troponin I, cardiac muscle isoform X1 (Heterocephalus glaber) | gi|512907890|ref|XP_004901203.1| (+1) | 24 | 73 | 11 | 53 | |
| Troponin I, slow skeletal muscle (Heterocephalus glaber) | gi|351700843|gb|EHB03762.1| (+2) | 24 | 22 | 8 | 36 | |
| Myosin regulatory light chain 2, ventricular cardiac isoform X2 (Heterocephalus glaber) | gi|512809121|ref|XP_004876314.1| | 19 | 106 | 20 | 86 |
For a complete list, please see Table S1
Total number of spectra assigned with ≥95% confidence
Number of unique peptide sequences assigned with ≥95% confidence that are exclusive to the indicated protein
Fig. 4.
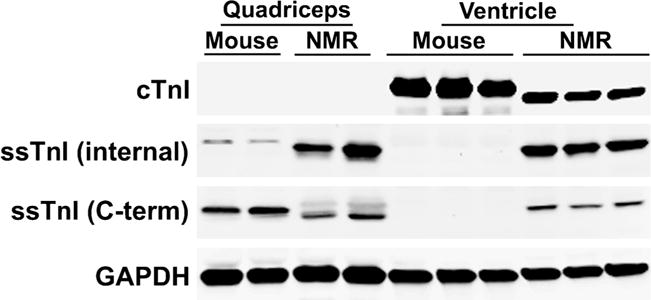
Western blot analysis of troponin I proteins confirms expression of ssTnI in naked mole-rat (NMR) ventricles. Quadriceps muscle from both species was loaded as a positive control for ssTnI. cTnI is expressed in both mouse and naked mole-rat ventricles but not quadriceps, as expected. Antibodies to both internal and C-terminal regions of ssTnI show that this protein was expressed in both mouse and naked mole-rat quadriceps, but only in the ventricles of naked mole-rats
Mechanical properties and force development in naked mole-rat and mouse ventricular cardiomyocytes
We questioned if species differences in contractile proteins affect the kinetics of cardiomyocyte contractility. Therefore, the steady-state force-calcium relationship was compared between species. These measurements were taken at two different sarcomere lengths (SLs) to approximate cardiomyocyte lengths at rest (short, 1.9 μM) and under maximal tension in vivo (long, 2.3 μM). At both short and long SLs, the naked mole-rat and mouse cardiomyocytes displayed comparable calcium sensitivity to force of contraction (EC50), maximal force of contraction (FMAX) and Hill coefficients (nHill), a measure of cooperativity of the contractile proteins (Table 3, Fig. 5). Importantly, both the mice and naked mole-rats exhibited increased force generation at the longer SL, compared to short sarcomere lengths. The naked mole-rat cardiomyocytes also exhibited a trend towards a higher length-dependent increase in force development than observed in mice (= 0.051).
Table 3.
Summary of steady-steady force results from mouse and naked mole-rat ventricular cardiomyocytes
| Group | n | SL = 1.9 μm
|
SL = 2.3 μm
|
SLΔFMAX (%) | SLΔEC50 (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| FMAX (mN/mm2) | nHill | EC50 (μM) | FMAX (mN/mm2) | nHill | EC50 (μM) | ||||
| Mouse | 4 (16) | 74.16 ± 0.73 | 2.06 ± 0.11 | 2.81 ± 0.13 | 85.10 ± 2.53a | 2.06 ± 0.09 | 2.38 ± 0.07a | 14.70 ± 2.67 | 14.84 ± 4.30 |
| NMR | 4 (11) | 74.85 ± 2.91 | 2.18 ± 0.15 | 3.28 ± 0.15 | 90.79 ± 3.73a | 2.09 ± 0.05 | 2.6 ± 0.11a | 21.26 ± 0.40 | 19.95 ± 3.44 |
For changes in FMAX and EC50, percent changes are shown
n represents number of samples, with the number in the parentheses representing number of cells analyzed (2–4 per sample)
Represents p < 0.05 SL 2.3 versus 1.9 μm. Data are shown as mean ± SEM
Fig. 5.
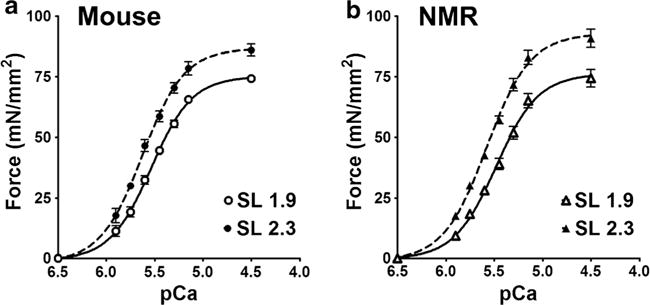
Force curves from steady-state force measurements on a mouse and b naked mole-rat cardiomyocytes at short (1.9 μm) and long (2.3 μm) sarcomere lengths (SLs). (n = 4 samples per species at each length, with n = 2–5 cardiomyocytes per sample)
Discussion
This study set out to determine if there were interspecific differences in the expression of protein isoforms and their post-translational modifications that may contribute to the low basal cardiac function and enhanced cardiac reserve (Table 1) observed in the naked mole-rat [17]. We found that the naked mole-rat has a 4-fold increased expression of β-MHC in the ventricles compared to mice. Additionally, the unexpected expression of ssTnl was identified, which was verified by mass spectrometry. Unique myofilament phosphorylation states were also identified in the naked mole-rat. In addition, we observed that naked mole-rat cardiomyocytes exhibit a trend towards higher length-dependent increase in force development compared to those of mice. These species differences could contribute to the disparate basal contractility and cardiac function that we have previously observed (Table 1). Although the cardiac myofilament signature and low levels of phosphorylation are more in keeping with end stage heart failure in other species, such as humans [4, 44], it is unlikely that this is the case for naked mole-rats. Rather, we postulate that this cardiac myofilament protein profile stems primarily from ecophysiological adaptations to the energetic constraints encountered below ground, where gas exchange is poor and oxygen availability is low.
The expression of ssTnI, like that of β-MHC, in adult naked mole-rat hearts concurs with several other reports of the retention of neotenous features into adulthood in this species. Adult naked mole-rats are extremely tolerant of low oxygen availability. Indeed, it was recently reported that naked mole-rats can survive 18 min without any oxygen, a trait attributed to the switching of carbohydrate fuels from glucose to fructose metabolism [34]. Moreover, like mammalian fetuses, naked mole-rats utilize a neonatal NMDA receptor and exhibit a blunted calcium response to hypoxic conditions [36, 37]. Both brain and lung tissues appear to be structurally malleable and retain morphological and molecular features commonly seen in utero (e.g., a double capillary system in lung [31] as well as the maintained expression of neuregulin-1, a neurotrophic growth factor, in the brain [12]). Recently, it was also shown that naked mole-rats have a very protracted period of brain development that is thought to allow for neuroplasticity and could be a major factor in their resilience to neurodegenerative diseases [35]. Although speculative, it is possible that the myofilament proteins of the naked mole-rat heart also reflect that cardiac developmental processes are not switched off postnatally and that neotenous features are retained, enabling the maintenance of proliferative capacity and/or a youthful phenotype well into adulthood [16]. Furthermore, our unexpected data support the idea that the naked mole-rat’s maintained expression of genes that are expressed in utero in other species may be indicative of evolved adaptations to atmospheres low in oxygen and high in carbon dioxide. For example, adult naked mole-rat expression of ssTnI in the heart could provide a mechanism for tolerating fluctuations in pH conditions that may occur in response to elevated levels of carbon dioxide and concomitant carbonic acid in deep burrows and the organism’s inability to expel excess carbon dioxide into this milieu. ssTnI has been previously shown to protect against the reduction in sarcomeric force production that occurs during acidosis [55].
Similarly, the predominant expression of ventricular β-MHC (Fig. 2) is consistent with limited oxygen availability and reduced energetic expenditure and the low basal cardiac function we previously observed in naked mole-rat [17]. The β-MHC isoform reduces actin gliding velocity in in vitro motility assays and exhibits a significantly lower ATPase activity compared to that of α-MHC [24, 38]. Similarly, β-MHC is associated with a more energetically economical contractility and has a lower tension cost compared to α-MHC [29], requiring less ATP, and as a result thereof, less oxygen, for force generation [33, 53]. Like other mammalian species [44], naked mole-rats express mostly α-MHC in their atria. It is interesting to note, however, that the naked mole-rat atria contained 25% β-MHC, five times more than that seen in mouse atria.
Fetal mouse and rat hearts express β-MHC, but switch to mostly α-MHC shortly after birth. In adulthood, expression of β-MHC in laboratory rodent hearts increases after induction of pathological conditions [43]. When rat cardiomyocytes were experimentally induced to express β-MHC, the cells exhibited a greater length-dependence of shortening velocity than those naturally expressing α-MHC [24]. This is similar to the trend towards higher length-dependent increases in force development observed in the β-MHC expressing naked mole-rat cardiomyocytes.
The expression of β-MHC in the naked mole-rat ventricle is similar to that of humans, who maintain high expression of ventricular β-MHC throughout development and adulthood [2]. Intriguingly, both humans and naked mole-rats have average heart rates that are approximately half that predicted allometrically on the basis of their body sizes (humans, 127 bpm predicted vs. 70 bpm observed; and naked mole-rats, 483 bpm predicted vs. 250 bpm observed) (Table 1) [15, 17]. In a multi-species comparison, larger mammals, which tend to have lower resting heart rates, also have ventricles that are mainly composed of the β-MHC isoform. Small mammals with resting heart rates above a threshold of 300 tend to express more ventricular α-MHC [18]. The low heart rate of the naked mole-rat heart thus correlates well with the predominance of β-MHC in the species’ ventricles.
Adult naked mole-rats, however, differ from humans, as well as rats and mice, by the presence of ssTnI in their ventricles (Fig. 4). This protein is usually expressed highly in the prenatal mammalian heart, but is rapidly downregulated after birth when hearts begin to express predominantly cTnI [19]. Transgenic mice overexpressing ssTnI in their hearts maintain ATP production during ischemia by upregulating glycolytic activity [39]. Expression of ssTnI in the adult mouse heart during heart failure can also help maintain systolic function during respiratory hypercapnia. This is thought to be due to the fact that the sequence of ssTnI allows it to be less modifiable by acidic pH. Acidosis reduces the affinity of cTnI for troponin C, thereby inhibiting actin and myosin interaction [52]. Evolution may have thus favored the presence of cardiac ssTnI into adulthood in this species so that naked mole-rats could maintain contractile function under the hypercapnic conditions commonly encountered in their poorly ventilated burrows.
Our investigation of myofilament protein phosphorylation further elucidated components of the naked mole-rat’s unique cardiac function, but also yielded new questions for future investigation such as how the naked mole-rat’s phospho-regulation of these proteins directly affects the species’ unique cardiac function. There were species differences in migration of MLC-2 on the Pro-Q and SYPRO gels (Fig. 3), which may be due to differences in protein sequences and post-translational modifications. Mouse and human sequences of MLC-2 differ in the N-terminus: where mice have two serines at positions 14 and 15, however human MLC-2 has only one phosphorylatable serine at position 15 and an asparagine at amino acid 14, resulting in different post-translational modifications[47]. Interestingly, the naked mole-rat sequence is more like that of humans, with asparagine-14 and serine-15. Consistent with the difference in sequences, naked mole-rat ventricles displayed a 50% reduction in phosphorylation of MLC-2 compared to mice. Previously, phosphorylation of MLC-2 had been shown to regulate contractility both under basal and β-adrenergic-stimulated conditions in the heart [48]. However, more recent data show that MLC-2 phosphorylation is not changed by either β-adrenergic stimulation or inhibition and is maintained at set levels in order to maintain cardiac function [10].
Unexpectedly, we noted that the calcium sensitivity of mice and naked mole-rats were similar, despite having quite different myofilament protein expression and post-translational modifications. Interestingly, it was shown that the expression of ssTnI in the mouse heart results in a leftward shift of the force-pCa relationship, increasing the myofilament calcium sensitivity [41, 55]. Conversely, reduced phosphorylation of MLC-2 results in a rightward shift of the force-pCa relationship [11,20]. The combination of these two factors in the naked mole-rat may at least partially explain why these two species exhibit similar myofilament calcium sensitivities despite disparate protein phosphorylation.
In contrast to MLC-2, naked mole-rats do not have lower levels of cMyBP-C phosphorylation compared to mice. The three main serines targeted for phosphorylation on cMyBP-C are all crucial for normal cardiac function. Moreover, these are highly phosphorylated under basal conditions in mice [46], and de-phosphorylated cMyBP-C is extremely susceptible to proteolytic cleavage [14]. It may be that high basal phospho-regulation of this protein is essential in naked mole-rats.
Phosphorylation of TnI was also not different between species. This protein has several sites which may become phosphorylated, and the differences in phosphorylation preference for these sites can cause opposite effects on inotropy, energy consumption, and calcium sensitivity [25]. To date, the specifics as to which phosphorylated region of cTnI is responsible for certain functional outcomes remain unresolved and controversial [32, 52]. It should also be noted that ssTnI lacks the N-terminal phosphorylation sites present on cTnI that are critical in β-adrenergic control of systolic function [25].
Many in vitro studies have shown that increased phosphorylation of cTnT, specifically at the critical threonine-206 site, reduces ATPase activity and force production [49]. Our results show that the naked mole-rat hearts have low phosphorylation of cTnT and low cardiac contractility compared to mice. Naked mole-rats do express a threonine-206 in their cTnT sequence; however, it has been recently proposed from mass spectrometry analyses that the only phosphorylation site on cTnT is serine-2, and it only controls the stability of the contractile apparatus and not contractility [21]. At present, given the incomplete annotation and sequencing of the naked mole-rat genome [26] and, in particular, the sequence of the cTnC N-terminal region (as seen in Tables 1 and S1), we currently cannot tell if the naked mole-rat cTnT protein contains this important serine-2.
Here, we have shown that adult naked mole-rats express several myofilament proteins that are only expressed in mice and rats in utero or during cardiomyopathies [10, 11]. This is the first report to study the composition and function of the naked mole-rat cardiac myofilament. As such, there remain questions as to the exact mechanism and functions of the altered myofilament composition. A possible explanation is that these myofilament proteins may safeguard against the detrimental effects of hypoxia and hypercapnia on cardiac contractility. How the regulation and expression of the myofilament proteins shown here contribute to the naked mole-rat’s large cardiovascular functional range in the face of many stressors merits further study to help better understand the species’ longevity and successful cardiovascular aging.
Supplementary Material
Acknowledgments
This work was supported in part by the following grants: American Heart Association, 12GRNT12030299 and 15GRNT22420022 (R. Buffenstein); National Institutes of Health Training Grant, T32 AG021890 (K.M. Grimes); National Institutes of Health, R01 HL105826 and K02 HL114749 (S. Sadayappan) and the American Heart Association Grant-in-Aid, 14GRNT20490025 (S. Sadayappan); National Institutes of Health, P01 HL62426 and R01 HL75494 (P.P. de Tombe); American Heart Association Midwest Predoctoral Fellowship, 11PRE7240022 (D.Y. Barefield); and National Institutes of Health shared instrumentation grant S10RR025111 (S.T. Weintraub). Mass spectrometry analyses were conducted in the University of Texas Health Science Center at San Antonio Institutional Mass Spectrometry Laboratory; the expert technical assistance of Sammy Pardo is gratefully acknowledged.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00424-017-2046-3) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflicts of interest.
References
- 1.Barefield D, Kumar M, de Tombe PP, Sadayappan S. Contractile dysfunction in a mouse model expressing a heterozygous MYBPC3 mutation associated with hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2014;306:H807–H815. doi: 10.1152/ajpheart.00913.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barefield D, Kumar M, Gorham J, Seidman JG, Seidman CE, de Tombe PP, Sadayappan S. Haploinsufficiency of MYBPC3 exacerbates the development of hypertrophic cardiomyopathy in heterozygous mice. J Mol Cell Cardiol. 2014;79C:234–243. doi: 10.1016/j.yjmcc.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barefield D, Sadayappan S. Phosphorylation and function of cardiac myosin binding protein-C in health and disease. J Mol Cell Cardiol. 2010;48:866–875. doi: 10.1016/j.yjmcc.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodor GS, Oakeley AE, Allen PD, Crimmins DL, Ladenson JH, Anderson PA. Troponin I phosphorylation in the normal and failing adult human heart. Circulation. 1997;96:1495–1500. doi: 10.1161/01.cir.96.5.1495. [DOI] [PubMed] [Google Scholar]
- 5.Buffenstein R. Ecophysiological responses of subterranean rodents to underground habitats. In: Lacey EA, Patton JL, Cameron GN, editors. Life underground: the biology of subterranean rodents. University of Chicago Press; Chicago: 2000. pp. 183–226. [Google Scholar]
- 6.Buffenstein R. Ecophysiological responses to a subterranean habitat; A Bathyergid perspective. Mammalia. 1996;60:591–605. [Google Scholar]
- 7.Buffenstein R. The naked mole-rat: a new long-living model for human aging research. J Gerontol A Biol Sci Med Sci. 2005;60:1369–1377. doi: 10.1093/gerona/60.11.1369. [DOI] [PubMed] [Google Scholar]
- 8.Buffenstein R. Negligible senescence in the longest living rodent, the naked mole-rat: insights from a successfully aging species. J Comp Physiol B. 2008;178:439–445. doi: 10.1007/s00360-007-0237-5. [DOI] [PubMed] [Google Scholar]
- 9.Buffenstein R, Yahav S. Is the naked mole-rat Heterocephalus glaber an endothermic yet poikilothermic mammal. J Therm Biol. 1991;16:227–232. [Google Scholar]
- 10.Chang AN, Battiprolu PK, Cowley PM, Chen G, Gerard RD, Pinto JR, Hill JA, Baker AJ, Kamm KE, Stull JT. Constitutive phosphorylation of cardiac myosin regulatory light chain in vivo. J Biol Chem. 2015;290:10703–10716. doi: 10.1074/jbc.M115.642165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colson BA, Locher MR, Bekyarova T, Patel JR, Fitzsimons DP, Irving TC, Moss RL. Differential roles of regulatory light chain and myosin binding protein-C phosphorylations in the modulation of cardiac force development. J Physiol. 2010;588:981–993. doi: 10.1113/jphysiol.2009.183897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edrey YH, Casper D, Huchon D, Mele J, Gelfond JA, Kristan DM, Nevo E, Buffenstein R. Sustained high levels of neuregulin-1 in the longest-lived rodents; a key determinant of rodent longevity. Aging Cell. 2012;11:213–222. doi: 10.1111/j.1474-9726.2011.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldman BD, Goldman SL, Lanz T, Magaurin A, Maurice A. Factors influencing metabolic rate in naked mole-rats (Heterocephalus glaber) Physiol Behav. 1999;66:447–459. doi: 10.1016/s0031-9384(98)00306-0. [DOI] [PubMed] [Google Scholar]
- 14.Govindan S, McElligott A, Muthusamy S, Nair N, Barefield D, Martin JL, Gongora E, Greis KD, Luther PK, Winegrad S, Henderson KK, Sadayappan S. Cardiac myosin binding protein-C is a potential diagnostic biomarker for myocardial infarction. J Mol Cell Cardiol. 2012;52:154–164. doi: 10.1016/j.yjmcc.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grimes KM, Lindsey ML, Gelfond JA, Buffenstein R. Getting to the heart of the matter: age-related changes in diastolic heart function in the longest-lived rodent, the naked mole rat. J Gerontol A Biol Sci Med Sci. 2012;67:384–394. doi: 10.1093/gerona/glr222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grimes KM, Reddy AK, Lindsey ML, Buffenstein R. And the beat goes on: maintained cardiovascular function during aging in the longest-lived rodent, the naked mole-rat. Am J Physiol Heart Circ Physiol. 2014;307:H284–H291. doi: 10.1152/ajpheart.00305.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimes KM, Voorhees A, Chiao YA, Han HC, Lindsey ML, Buffenstein R. Cardiac function of the naked mole-rat: ecophysiological responses to working underground. Am J Physiol Heart Circ Physiol. 2014;306:H730–H737. doi: 10.1152/ajpheart.00831.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamilton N, Ianuzzo CD. Contractile and calcium regulating capacities of myocardia of different sized mammals scale with resting heart rate. Mol Cell Biochem. 1991;106:133–141. doi: 10.1007/BF00230179. [DOI] [PubMed] [Google Scholar]
- 19.Hunkeler NM, Kullman J, Murphy AM. Troponin I isoform expression in human heart. Circ Res. 1991;69:1409–1414. doi: 10.1161/01.res.69.5.1409. [DOI] [PubMed] [Google Scholar]
- 20.Kampourakis T, Sun YB, Irving M. Myosin light chain phosphorylation enhances contraction of heart muscle via structural changes in both thick and thin filaments. Proc Natl Acad Sci U S A. 2016;113:E3039–E3047. doi: 10.1073/pnas.1602776113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katrukha IA, Gusev NB. Enigmas of cardiac troponin T phosphorylation. J Mol Cell Cardiol. 2013;65:156–158. doi: 10.1016/j.yjmcc.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 22.Keane M, Craig T, Alfoldi J, Berlin AM, Johnson J, Seluanov A, Gorbunova V, Di Palma F, Lindblad-Toh K, Church GM, de Magalhaes JP. The naked mole rat genome resource: facilitating analyses of cancer and longevity-related adaptations. Bioinformatics. 2014;30:3558–3560. doi: 10.1093/bioinformatics/btu579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konishi M, Akiyama E, Iwahashi N, Maejima N, Tsukahara K, Hibi K, Kosuge M, Ebina T, Kimura K. Hypercapnia in patients with acute heart failure. Eur Heart J. 2015;36:155–155. doi: 10.1002/ehf2.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korte FS, McDonald KS. Sarcomere length dependence of rat skinned cardiac myocyte mechanical properties: dependence on myosin heavy chain. J Physiol. 2007;581:725–739. doi: 10.1113/jphysiol.2007.128199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Layland J, Solaro RJ, Shah AM. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc Res. 2005;66:12–21. doi: 10.1016/j.cardiores.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 26.Lewis KN, Soifer I, Melamud E, Roy M, McIsaac RS, Hibbs M, Buffenstein R. Unraveling the message: insights into comparative genomics of the naked mole-rat. Mamm Genome. 2016;27:259–278. doi: 10.1007/s00335-016-9648-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis KN, Wason E, Edrey YH, Kristan DM, Nevo E, Buffenstein R. Regulation of Nrf2 signaling and longevity in naturally long-lived rodents. Proc Natl Acad Sci U S A. 2015;112:3722–3727. doi: 10.1073/pnas.1417566112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang S, Mele J, Wu Y, Buffenstein R, Hornsby PJ. Resistance to experimental tumorigenesis in cells of a long-lived mammal, the naked mole-rat (Heterocephalus glaber) Aging Cell. 2010;9:626–635. doi: 10.1111/j.1474-9726.2010.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Locher MR, Razumova MV, Stelzer JE, Norman HS, Moss RL. Effects of low-level α-myosin heavy chain expression on contractile kinetics in porcine myocardium. Am J Physiol Heart Circ Physiol. 2011;300:H869–H878. doi: 10.1152/ajpheart.00452.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lovegrove BG. The cost of burrowing by the social mole rats (Bathyergidae) Cryptomys damarensis and Heterocephalus glaber: the role of soil moisture. Physiol Zool. 1989;62:449–469. [Google Scholar]
- 31.Maina JN, Maloiy GMO, Makanya AN. Morphology and Morphometry of the lungs of 2 east-African mole rats, Tachyoryctes-Splendens and Heterocephalus-Glaber (Mammalia, Rodentia) Zoomorphology. 1992;112:167–179. [Google Scholar]
- 32.Marston SB, de Tombe PP. Troponin phosphorylation and myofilament Ca2+−sensitivity in heart failure: increased or decreased? J Mol Cell Cardiol. 2008;45:603–607. doi: 10.1016/j.yjmcc.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narolska NA, van Loon RB, Boontje NM, Zaremba R, Penas SE, Russell J, Spiegelenberg SR, Huybregts MA, Visser FC, de Jong JW, van der Velden J, Stienen GJ. Myocardial contraction is 5-fold more economical in ventricular than in atrial human tissue. Cardiovasc Res. 2005;65:221–229. doi: 10.1016/j.cardiores.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 34.Park TJ, Reznick J, Peterson BL, Blass G, Omerbasic D, Bennett NC, Kuich PHJL, Zasada C, Browe BM, Hamann W, Applegate DT, Radke MH, Kosten T, Lutermann H, Gavaghan V, Eigenbrod O, Begay V, Amoroso VG, Govind V, Minshall RD, Smith ESJ, Larson J, Gotthardt M, Kempa S, Lewin GR. Fructose-driven glycolysis supports anoxia resistance in the naked mole-rat. Science. 2017;356:305–308. doi: 10.1126/science.aab3896. [DOI] [PubMed] [Google Scholar]
- 35.Penz OK, Fuzik J, Kurek AB, Romanov R, Larson J, Park TJ, Harkany T, Keimpema E. Protracted brain development in a rodent model of extreme longevity. Sci Rep. 2015;5:11592. doi: 10.1038/srep11592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peterson BL, Larson J, Buffenstein R, Park TJ, Fall CP. Blunted neuronal calcium response to hypoxia in naked mole-rat hippocampus. PLoS One. 2012;7:e31568. doi: 10.1371/journal.pone.0031568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peterson BL, Park TJ, Larson J. Adult naked mole-rat brain retains the NMDA receptor subunit GluN2D associated with hypoxia tolerance in neonatal mammals. Neurosci Lett. 2012;506:342–345. doi: 10.1016/j.neulet.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 38.Pope B, Hoh JF, Weeds A. The ATPase activities of rat cardiac myosin isoenzymes. FEBS Lett. 1980;118:205–208. doi: 10.1016/0014-5793(80)80219-5. [DOI] [PubMed] [Google Scholar]
- 39.Pound KM, Arteaga GM, Fasano M, Wilder T, Fischer SK, Warren CM, Wende AR, Farjah M, Abel ED, Solaro RJ, Lewandowski ED. Expression of slow skeletal TnI in adult mouse hearts confers metabolic protection to ischemia. J Mol Cell Cardiol. 2011;51:236–243. doi: 10.1016/j.yjmcc.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prothero J, Jurgens K. Scaling of maximal life span in mammals: a review. In: Woodhead A, Thompson K, editors. Evolution of longevity in animals: a comparative approach. Plenum Press; New York: 1987. pp. 49–74. [Google Scholar]
- 41.Puglisi JL, Goldspink PH, Gomes AV, Utter MS, Bers DM, Solaro RJ. Influence of a constitutive increase in myofilament Ca(2+)-sensitivity on Ca(2+)-fluxes and contraction of mouse heart ventricular myocytes. Arch Biochem Biophys. 2014;552–553:50–59. doi: 10.1016/j.abb.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajabi M, Kassiotis C, Razeghi P, Taegtmeyer H. Return to the fetal gene program protects the stressed heart: a strong hypothesis. Heart Fail Rev. 2007;12:331–343. doi: 10.1007/s10741-007-9034-1. [DOI] [PubMed] [Google Scholar]
- 43.Razeghi P, Essop MF, Huss JM, Abbasi S, Manga N, Taegtmeyer H. Hypoxia-induced switches of myosin heavy chain iso-gene expression in rat heart. Biochem Biophys Res Commun. 2003;303:1024–1027. doi: 10.1016/s0006-291x(03)00478-9. [DOI] [PubMed] [Google Scholar]
- 44.Reiser PJ, Kline WO. Electrophoretic separation and quantitation of cardiac myosin heavy chain isoforms in eight mammalian species. Am J Phys. 1998;274:H1048–H1053. doi: 10.1152/ajpheart.1998.274.3.H1048. [DOI] [PubMed] [Google Scholar]
- 45.Sabbah HN, Sharov VG, Goldstein S. Cell death, tissue hypoxia and the progression of heart failure. Heart Fail Rev. 2000;5:131–138. doi: 10.1023/A:1009880720032. [DOI] [PubMed] [Google Scholar]
- 46.Sadayappan S, Gulick J, Osinska H, Martin LA, Hahn HS, Dorn GW, 2nd, Klevitsky R, Seidman CE, Seidman JG, Robbins J. Cardiac myosin-binding protein-C phosphorylation and cardiac function. Circ Res. 2005;97:1156–1163. doi: 10.1161/01.RES.0000190605.79013.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scruggs SB, Reisdorph R, Armstrong ML, Warren CM, Reisdorph N, Solaro RJ, Buttrick PM. A novel, in-solution separation of endogenous cardiac sarcomeric proteins and identification of distinct charged variants of regulatory light chain. Mol Cell Proteomics. 2010;9:1804–1818. doi: 10.1074/mcp.M110.000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scruggs SB, Solaro RJ. The significance of regulatory light chain phosphorylation in cardiac physiology. Arch Biochem Biophys. 2011;510:129–134. doi: 10.1016/j.abb.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Streng AS, de Boer D, van der Velden J, van Dieijen-Visser MP, Wodzig WK. Posttranslational modifications of cardiac troponin T: an overview. J Mol Cell Cardiol. 2013;63:47–56. doi: 10.1016/j.yjmcc.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 50.Tian X, Azpurua J, Hine C, Vaidya A, Myakishev-Rempel M, Ablaeva J, Mao Z, Nevo E, Gorbunova V, Seluanov A. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature. 2013;499:346–349. doi: 10.1038/nature12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Triplett JC, Swomley A, Kirk J, Lewis K, Orr M, Rodriguez K, Cai J, Klein JB, Buffenstein R, Butterfield DA. Metabolic clues to salubrious longevity in the brain of the longest-lived rodent: the naked mole-rat. J Neurochem. 2015;134:538–550. doi: 10.1111/jnc.13149. [DOI] [PubMed] [Google Scholar]
- 52.Urboniene D, Dias FA, Pena JR, Walker LA, Solaro RJ, Wolska BM. Expression of slow skeletal troponin I in adult mouse heart helps to maintain the left ventricular systolic function during respiratory hypercapnia. Circ Res. 2005;97:70–77. doi: 10.1161/01.RES.0000173849.68636.1e. [DOI] [PubMed] [Google Scholar]
- 53.van der Velden J, Moorman AFM, Stienen GJM. Age-dependent changes in myosin composition correlate with enhanced economy of contraction in guinea-pig hearts. J Physiol-London. 1998;507:497–510. doi: 10.1111/j.1469-7793.1998.497bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang L, Muthu P, Szczesna-Cordary D, Kawai M. Characterizations of myosin essential light chain’s N-terminal truncation mutant Delta43 in transgenic mouse papillary muscles by using tension transients in response to sinusoidal length alterations. J Muscle Res Cell Motil. 2013;34:93–105. doi: 10.1007/s10974-013-9337-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolska BM, Vijayan K, Arteaga GM, Konhilas JP, Phillips RM, Kim R, Naya T, Leiden JM, Martin AF, de Tombe PP, Solaro RJ. Expression of slow skeletal troponin I in adult transgenic mouse heart muscle reduces the force decline observed during acidic conditions. J Physiol. 2001;536:863–870. doi: 10.1111/j.1469-7793.2001.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


