Abstract
The west Africa Ebola virus disease (EVD) epidemic was extraordinary in scale. Now that the epidemic has ended, it is a relevant time to examine published studies with direct relevance to clinical care and, more broadly, to examine the implications of the clinical research response mounted. Clinically relevant research includes literature detailing risk factors for and clinical manifestations of EVD, laboratory and other investigation findings in patients, experimental vaccine and therapeutic clinical trials, and analyses of survivor syndrome. In this Review, we discuss new insights from patient-oriented research completed during the west Africa epidemic, identify ongoing knowledge gaps, and suggest priorities for future research.
Introduction
The world’s largest ever epidemic of Ebola virus disease (EVD) probably commenced in December, 2013, following the infection of the presumed index case, a 2-year-old child living in rural Guinea.1 The subsequent outbreak soon crossed into Sierra Leone and Liberia and case numbers escalated rapidly. When WHO acknowledged in August, 2014, that the outbreak was a public health emergency of international concern, there were already 1711 reported cases and there had been 932 deaths.2 By the end of the epidemic, 28 646 cases and 11 323 deaths had been reported,3 but the true numbers are likely to be much higher. The epidemic had far-reaching effects in west Africa, including enormous economic costs and significant strains on already stretched health-care systems.4,5 A staggering 881 health-care workers were infected and 513 died.6
The focus of global response efforts was, quite rightly, to provide humanitarian assistance and medical care, and to interrupt chains of transmission.7 But there were also calls from WHO, funding bodies, and governments to urgently increase the scale of scientific research to respond to the rapidly growing EVD epidemic.8 Before 2014, outbreaks were short-lived, occurred in remote locations, and involved relatively small case numbers. Such factors, coupled with little research interest and funding, meant that the general understanding of EVD was limited. The west Africa epidemic provided an important opportunity to improve patient outcomes through clinical studies that would enhance knowledge and allow investigation of potential interventions. There were major hurdles to overcome, however, including logistical challenges,9,10 and ethical and societal considerations11,12 that could affect the ability to reach conclusions within the lifetime of the epidemic.
This Review summarises published findings from clinical research completed during the epidemic, and then discusses the implications for countries at risk of EVD outbreaks, ongoing clinical research gaps, and priorities moving forward. There was a broad range of research done during this period, so we have placed emphasis on patient-centred developments and progress made investigating Ebola virus vaccines (appendix).
Clinical features of EVD
In the west Africa epidemic, the greatest burden of EVD was in young adults (median age 32 years, IQR 21–42).13 It is unclear whether this burden represents an increased risk in young adults (perhaps because of increased exposure) or a case ascertainment bias (if children or the elderly were less likely to be in the official count). There was no marked gender difference in disease prevalence (48·8% of probable and confirmed infections were in men).14
We now know that young age is a predictor of death (odds ratio [OR] per year of life 0·91, 95% CI 0·85–0·97) and that children tend to deteriorate rapidly, with a median of 3 days from admission to an Ebola treatment centre (ETC) to death in a cohort of 300 children.15,16 Likewise, some data show that mortality is higher in patients older than 45 years and in men.13,14,17–20 The previously published case fatality rates (CFRs) for maternal (90%) and neonatal EVD (100%) might be an overestimation,21 since there have been subsequent case reports of maternal22,23 and, very rarely, neonatal survival.24 Without systematic data collection, however, the prognosis for pregnant women is uncertain.
Although first described as Ebola haemorrhagic fever, because of the frequency of bleeding observed during the initial outbreaks of 1976,25,26 a spectrum of illness was evident in the west Africa epidemic and haemorrhage, when present, was a late finding associated with fatal disease.27,28 The hallmark of advanced disease in this epidemic was severe gastrointestinal illness.13,18,29–33
The most frequent symptoms at presentation (table 1) were fever, fatigue, anorexia, vomiting, diarrhoea, headache, and abdominal pain.13,18,31–33 Anecdotal reports of large volume, cholera-like diarrhoea emerged from ETCs in west Africa, and volumes of up to 10 L of diarrhoea per day were observed in medically evacuated patients.29,30 Notably, fever was absent in at least 10% of patients,13,18,31–33 which has important implications for clinical triage and case definitions that include fever as a prerequisite symptom. Less common clinical manifestations, including confusion, conjunctivitis, and hiccups,20,33 had good discriminatory importance in identifying EVD cases in all patients presenting to ETCs and therefore remain helpful for presumptive clinical diagnosis in the context of a known outbreak.
Table 1. Clinical manifestations of investigational findings in Ebola virus disease, reported by studies done during the west Africa epidemic.
| Signs and symptoms13 reported in more than 10% of patients with acute Ebola virus disease | Investigational findings that have been reported during acute Ebola virus disease | Signs and symptoms during survivor syndrome from Ebola virus disease | |
|---|---|---|---|
| General | Fever, fatigue, hiccups | Raised pro-inflammatory markers, including CRP; elevated lactate34 | Fatigue,35–37 depression,36–39 anxiety,36,37,39 insomnia35,36,38 |
| Neurological and visual | Headache, confusion | Detectable Ebola virus RNA in the cerebrospinal fluid;40,41 diffuse swelling, microvascular occlusions as observed by MRI42 | Difficulty concentrating, mood changes, and memory loss;38,39 headaches;35,36,38,43 dizziness;38 difficulties hearing;35,37–39,44 visual disturbances;35–37,39,43,44 peripheral paraesthesia or dysaethesia37 |
| Cardiovascular | Chest pain | Bradycardia,45 arrhythmias as shown by electrocardiogram;46 myocarditis shown during MRI47 | Chest pain,36,38,43 palpitations35,37,38 |
| Pulmonary | Cough, dyspnoea, sore throat | Pulmonary oedema and pulmonary effusion as observed on x-ray and USS46 | Dyspnoea37 |
| Gastrointestinal | Anorexia, vomiting, diarrhoea, abdominal pain, odynophagia | Paralytic ileus and bowel wall oedema shown by USS29,48 | Anorexia,35,38 abdominal pain,35,36,38,43 constipation38 |
| Hepatobiliary | Jaundice | Transaminitis with high AST:ALT ratio34,49 | ·· |
| Renal, urological, and electrolytes | ·· | Acute kidney injury,34,50,51 raised creatine kinase,34 hypokalaemia34,49,51 or hyperkalaemia,34 hyponatraemia,34 hypocalcaemia,49 hypoglycaemia16 | Decreased libido; sexual dysfunction and testicular pain36,38 |
| Haematological | Clinically significant haemorrhage uncommon, likely to be more frequent in pregnant women. | Leucopenia, thrombocytopenia, raised INR, haematoconcentration34 | Anaemia35 |
| Skin and musculoskeletal | Myalgia, arthralgia, conjunctivitis | ·· | Arthralgia;35,36,39,43,44 myalgia;36 alopecia, skin peeling, and pruritus35,36,38,39,43 |
USS=ultrasound scan. ALT=alanine transaminase. AST=aspartate transaminase. CRP=C-reactive protein. INR=international normalised ratio.
A cross-sectional seroepidemiological study done in Sierra Leone found that 14 (7·5%) of 187 individuals who had not been diagnosed with EVD had detectable anti-Ebola glycoprotein antibodies.52 12 of the 14 denied any symptoms compatible with EVD. These results, when considered alongside related data from previous outbreaks,53,54 suggest that a proportion of Ebola virus infections are subclinical, although the contribution of such cases to transmission or herd immunity is unknown and the specificities of serological assays need to be considered.
WHO’s estimated CFR for the epidemic was 70% (95% CI 69–72).32,55 Overall, mortality was lower in patients admitted to hospital (CFR 61%, 95% CI 59–62) compared with patients not admitted to hospital (88%, 86–90).32 Small hospital series have reported substantially improved survival (eg, CFR 32% in a hospital in Sierra Leone56), but these data should be interpreted with caution, since there are many potential explanations for the variability in CFR. For example, although medical intervention might have conferred a survival benefit, the influence of case selection bias (arising from self-presenting patients who are not representative of patients with EVD in the community) or a survival bias (when the most unwell patients succumbed to disease before admission) has not been fully assessed. The 19% CFR seen in patients treated in Europe and the USA was much lower than that reported in west Africa;57 although not confirmed, possible explanations include fewer untreated comorbidities and lower levels of viraemia at admission, and access to advanced physiological support and experimental therapies that were not available routinely in the three most affected countries in west Africa.
Complications of acute illness
EVD can be a severe and complex multisystem disease, with inflammation, vascular leakage, hypovolaemic shock, electrolyte disturbance, and direct organ damage all contributing to illness. Most existing knowledge about the pathogenesis of EVD has come from in-vitro studies and animal models (reviewed elsewhere58–60), and limited histopathological data from previous human cases of EVD.60 Improved characterisation of the broad spectrum of organ involvement (table 1) is an important contribution to knowledge about EVD from this outbreak.
Gastrointestinal complications
The mechanism of severe diarrhoea in EVD is unclear. Although clinical descriptions of large volume, so-called rice water diarrhoea draws analogy with cholera and implies a secretory process, previous autopsy findings indicate that intestinal wall inflammation also occurs.60 There have been small gains in explaining why patients experience abdominal pain (including peritonism in a subset of cases),29,46,48 with case reports from resource-rich countries identifying paralytic ileus by ultrasonography.29,48 In one case, marked bowel wall oedema was observed; the treating clinicians speculated that both viral-mediated damage and iatrogenic hypoproteinaemia might have contributed to this finding.29 They also suggested that an inflamed gastrointestinal tract was likely the source of the bacteraemia observed in this patient, but there are few similar data to suggest whether this was a common phenomenon in west Africa.
Renal complications
Renal dysfunction is more common than previously thought. In one series of 150 patients, acute kidney injury (defined according to the Risk, Injury, and Failure; Loss; and End-stage kidney disease [RIFLE] criteria) occurred in 50% of patients and was an independent predictor of mortality (OR 5·84, 95% CI 1·15–29·58);34 a similar pattern has been seen in other cohorts.19,50,51 Importantly, these studies suggest that renal dysfunction occurs earlier in the disease trajectory than previously recognised and, at times, before the onset of severe vomiting and diarrhoea. For these patients, this early onset of disease manifestation indicates a mechanism partly independent of prerenal hypovolaemia because of gastrointestinal losses.34,61 There are probably various contributors, including renal hypoperfusion from septic shock or, in patients with disseminated intravascular coagulopathy, thrombus formation in the renal microvascular system, or rhabdomyolysis.34 In particular, the risk of acute kidney injury from rhabdomyolysis has yet to be fully elucidated. Although approximately half of patients with EVD experience myalgia32 and suggestive laboratory findings of raised creatine kinase34,57,62 and hyperkalaemia have been reported, identification of true rhabdomyolysis has been limited by insufficient urine myoglobin measurement. Furthermore, there have been few mechanistic studies of Ebola-virus-induced muscle damage.
Hyperkalaemia has been reported in 13% of patients with EVD in one series based in west Africa.34 This finding is plausible given the prevalence of acute kidney injury and the hypothesis of rhabdomyolysis, but hyperkalaemia has been reported infrequently in other series, both in west Africa51 and in medically evacuated patients (albeit confounded by frequent use of renal replacement therapy in this setting). Therefore, there is no certainty and caution is required when interpreting potassium findings obtained under field conditions, since erroneous readings of hyperkalaemia due to specimen haemolysis is possible. Hypokalaemia is common,34,49,51 and although this is not an unexpected finding, given the severity of gastrointestinal losses in EVD, the variability in reported blood potassium disturbances highlights the necessity of biochemical testing to inform clinical decision making. Although data are limited, other commonly observed metabolic abnormalities include hyponatraemia, hypocalcaemia, and hypomagnesemia.34,49,57 Additionally, severe and frequent hypoglycaemia has been described in children with EVD.16
Hepatic complications
There is little new knowledge regarding liver injury in EVD. Normal bilirubin concentrations were considered normal in patients with EVD in west Africa, but transaminitis was common, typically with a high aspartate transaminase to alanine transaminase ratio.19,34,49,57 It is not clear whether the increased ratio represents liver damage, muscle damage, or both.34,57,62 A high AST concentration during the first week of illness was shown to be associated with fatal outcome.63 In the same study, AST correlated with the Ebola virus cycle threshold value during PCR analysis, suggesting it could be used as a surrogate marker of viral load.63
Respiratory complications
Dyspnoea and tachypnoea were observed frequently in west African patients with EVD. Difficulty in breathing was reported in between 41%20 and 50% of patients.64 Tachypnoea was observed in all 35 patients in one cohort.19 Other groups have reported much lower rates of dyspnoea,10,33,65 but there is likely to be variability in reporting since the intensity of monitoring is varied and dyspnoea is a subjective symptom. Acute lung injury has been observed in patients with EVD who were medically evacuated and had access to more intensive monitoring. In this setting, hypoxaemia was observed in 14 (52%) of 27 patients and non-invasive or invasive mechanical ventilation was required in nine patients (33%).57 Tachypnoea could occur secondary to acidosis, which is common in EVD,19,34,51 but pulmonary oedema associated with vascular leakage or fluid overload might also contribute to this condition.30,48 Direct viral pneumonitis was suggested as the cause of acute respiratory failure in one case, as shown by interstitial pulmonary infiltrates and the detection of Ebola virus in bronchial aspirate fluid.66
Cardiovascular complications
Further reports of inappropriate bradycardia in patients with EVD surfaced during this epidemic.45 Because some patients in this report were also encephalopathic, the authors suggested a possible central neurological cause, as opposed to the previous hypothesis of toxin-mediated damage.45 Arrhythmias have been reported in medically evacuated patients46 and have been the presumed proximal cause of sudden death in some patients with EVD during acute illness or during early recovery67 in west Africa. Electrolyte disturbances could be possible precipitants, but there is also evidence that viral myocarditis can occur during acute illness and recovery.35,47 Additionally, a hypercoagulable state has been shown during early recovery;68 although this raises the possibility of venous thrombosis and pulmonary thromboembolism,35 evidence of these complications is incomplete. Additionally, higher haemoglobin concentration and haematocrit were associated with mortality in a west Africa cohort;34 this finding might have resulted from haemoconcentration, but whether the increase in blood viscosity has clinically important consequences is unknown.
Neurological complications
Neurological complications were common in patients in west Africa and included headache (61%), confusion (13%), and coma or unconsciousness (6%).32 A third of patients treated in Europe and the USA were encephalopathic at some point during their illness.57 Encephalitis during acute illness and early recovery has been described, with detection of Ebola virus RNA in cerebrospinal fluid.40,41 This association alone is insufficient to assume an infective mechanism, but is supported by isolation of virus from cerebrospinal fluid in a survivor with meningoencephalitis.69 Detailed radiological investigation is challenging even in resource-rich settings, but MRI brain imaging done at day 33 of illness has shown microvascular disease and ischaemia in a patient with meningoencephalitis.42
Inflammatory response
The association between high viral load in blood and increased mortality is now well established,18–20,34,64,70–72 and has been shown to follow a sigmoid (logistic) function.73 Severe EVD is associated with an intense inflammatory response, characterised by high concentrations of pro-inflammatory mediators.60,74,75 The kinetics of soluble immune mediators and biomarkers in serial blood samples obtained from seven patients with EVD treated in the USA showed an association between more severe disease and biomarkers suggestive of endothelial or coagulatory dysfunction. These patients also showed a comparative absence of biomarkers indicative of an immune response (compared with those found in patients with less severe EVD).76 Two case series reported high concentrations of C-reactive protein and lactate, especially in fatal cases.34,57
Co-infections and sepsis
There are new and unexpected findings describing how concurrent infections affect EVD prognosis. Analysis of blood samples from 1182 patients infected with Ebola virus found that patients with Plasmodium spp parasitaemia were 20% more likely to survive, even after accounting for the mortality risk factors of marked Ebola virus viraemia and increasing patient age.77 In the same study, the survival advantage was independent of treatment with antimalarial drugs, and administration of different antimalarial drugs failed to improve survival in mice infected with EVD. The authors hypothesised that concurrent Plasmodium sp infection might moderate the host immune response, perhaps by reducing the exuberant cytokine response observed in EVD.77 By contrast, a separate study did multivariate analysis on data from 1047 cases and found that malaria parasite co-infection was an independent determinant of fatal outcome, but only for children who were aged 5–14 years; all patients in this study received antimalarial therapy.78 The reasons for these discrepant findings are unclear.
A study of 49 patients with EVD found that co-infection with GB virus C was associated with improved survival.79 Similar to the hypothesis for the effect seen with concurrent Plasmodium sp infection, GB virus C might also have beneficial immunomodulatory functions in EVD infection.77 Studies showing the effect of HIV co-infection on survival in EVD have either not been done or have yet to report their findings.
Physiological and biochemical findings that would fulfil the commonly accepted criteria for septic shock have been described for patients with EVD treated in Europe and the USA. It seems likely that sepsis and septic shock also occurred in many patients with EVD treated in west Africa, but there are insufficient data to confirm this. Sepsis could be caused by Ebola virus infection alone, or by bacterial co-infections (these have not been investigated systematically).29
Supportive care
Supportive care remains the principal management strategy for patients with EVD. Several authorities advocate focusing efforts on correcting gastrointestinal fluid losses and electrolyte imbalances, and preventing hypovolaemic shock.80 Recommended components of care often included oral or intravenous fluids, analgesia, antiemetics and antidiarrhoeal medications alongside empirical antimicrobials and antimalarials.81,82
The lower case fatality rate in patients treated in the USA and Europe (19%) suggests that intensive supportive care strategies can contribute substantially to improved survival.57 Trials of supportive care were not completed during the west Africa EVD epidemic, however, and the evidence base for defining optimal supportive care for EVD remains insufficient.83 Intensive intravenous fluid resuscitation was shown previously to be harmful in severe paediatric infections in resource-limited settings, albeit in a different context to EVD; therefore, a universal fluid resuscitation protocol for ETCs could potentially cause harm in some patients.84 The complexities of detecting and correcting abnormalities in fluid distribution and organ perfusion have been shown by studies of patients treated in the USA and Europe.57,85 Some have questioned whether EVD-associated sepsis differs significantly from bacterial or fungal sepsis and, accordingly, whether applying general principles of sepsis management (or administering experimental sepsis treatments) to patients with EVD could improve survival.80,86
Several interventions have been used routinely in some ETCs but not in others, with little evidence of their benefit or risks. For example, the role of empirical vitamin K remains unclear,87 given the limited understanding of the frequency and mechanisms of coagulopathy in EVD. Non-steroidal anti-inflammatory drugs were prescribed in some centres,56 despite their potential to worsen gastrointestinal and renal complications. Loperamide is known not to confer benefit in patients with cholera, but it is uncertain whether a similar, secretory process causes the large volume diarrhoea described in EVD. Additionally, paralytic ileus is a known complication of EVD and a contraindication to loperamide use, but might go unrecognised in a typical ETC setting. The apparent variability of electrolyte disturbances also raises concerns about routine empirical electrolyte supplementation in the absence of blood electrolyte monitoring.
Any future trials of supportive care strategies in EVD will be challenging if new outbreaks are more typical (ie, smaller and of shorter duration), but high-quality supportive care is clearly a major factor influencing survival and it is important that recommended supportive care strategies are evidence-based. An expert consensus statement on the optimal package of supportive care for EVD in various settings would be a helpful interim measure, even more so if this identified the most important evidence gaps to guide the design of prospective clinical studies should a situation arise where such trials were possible.
Survivors
The enormity of the west Africa outbreak has led to an unprecedented number of EVD survivors. The most frequently reported post-EVD complications in this epidemic (table 1) are consistent with previous outbreaks.88,89 These include arthralgia, visual disturbances (including uveitis and loss of visual acuity), hearing impairments, myalgia, fatigue, abdominal pain, and sleep disturbances.35,36,38,43,44 Neurological deficits were reported infrequently before this outbreak, but now appear to be an important contributor to morbidity.39 Psychological distress in response to a life-threatening illness could also contribute to neurocognitive manifestations.90 Survivors report very poor social acceptance by their communities and are often stigmatised.36,38 Although this is known to affect survivor confidence and social engagement,38 long-term psychological needs are unknown.
The pathogenic mechanisms that underlie EVD sequelae remain poorly understood. There is a long-held assumption that autoimmune or post-infectious inflammatory processes play prominent roles, but an association between viral replication in immune privileged sites and late complications in some survivors is newly established.69,91
Ebola virus was isolated from the aqueous humour of a survivor with panuveitis, 14 weeks after diagnosis.91 The total duration of viral sequestration was unknown, but was less than 18 months.92 Additionally, infectious virus was detected in the cerebrospinal fluid of a survivor with meningoencephalitis, 9 months following acute illness.69 At this time, there was also a transient viraemia, thought to represent a so-called spillover of Ebola virus from its site of replication in the CNS.24,69 Both of these patients were medically evacuated to settings with advanced care and received experimental therapies. Therefore, it is unclear if the nature, timing, and severity of these complications are representative of sequelae seen in west Africa. Follow-up of 151 survivors in Sierra Leone showed that late recrudescence, defined as illness or death that could not be attributed to a non-EVD related cause after a period of full recovery from confirmed EVD, was rare (maximum estimate of 0·7%).93
Persistence of Ebola virus in body fluids had been shown before this outbreak94 but the long duration of persistence has been an unexpected finding.95 For example, viral RNA is detectable in semen up to 18 months following discharge from an ETC.96 There are few data available to estimate the proportion of male survivors affected. In one small convenience sample of survivors who were at varying durations into recovery, the overall prevalence of viral RNA positive semen was 49%.97 Determinants of viral persistence in semen require further study.
There is also new evidence that women who recover from EVD during pregnancy can harbour persistent virus in the amniotic fluid and placenta and deliver an infected, stillborn fetus.23,98,99 Additionally, there are reports of viral persistence in other body fluids that would not be considered to be immune privileged, albeit for a briefer timeframe. Case reports suggest shorter-lived persistence of viable virus (and viral RNA) in urine and viral RNA in sweat85,100 and contribute to existing knowledge of persistence in vaginal, rectal, and conjunctival swab specimens and in breast milk.94,101,102 Some caution is required when interpreting these small case studies; for example, the method used to collect a positive urine sample from a male patient was not described, raising the possibility of cross-contamination by virus present in semen.85 Nonetheless, the viral kinetics of persistence in these fluids require closer examination, particularly when there are implications for guidance on preventing sexual transmission or potential transmission by breastfeeding.
The phenomenon of viral persistence means that, in limited circumstances, survivors can act as a reservoir for ongoing disease transmission. Convincing evidence now exists to show that men can transmit Ebola virus to women during sexual intercourse.103,104 The prolonged duration of viral persistence in semen raises the possibility of sexual transmission occurring long after the resolution of acute illness. There is evidence that a flare of EVD in Guinea, which occurred months after the end of the Guinean outbreak, was caused by male-to-female sexual transmission (at approximately 470 days after initial illness in the male partner).103 There are no population-level data that predict the risk for sexual partners of EVD survivors, but the low incidence of new flares of disease provides some indication that transmission leading to disease is uncommon. There is no published, definitive evidence of female-to-male sexual transmission having occurred, or of mother-to-child transmission by breastfeeding.
There are several ongoing research priorities for survivors. Long-term studies are a priority because the longest survivor follow-up reported to date has been just over 2 years,88 with ongoing symptoms reported at that time. Of note, there are no descriptions of the effect of EVD on childhood development and outcomes, and although the small amount of evidence so far suggests that EVD survivors might be at greater risk of pregnancy-related complications including stillbirth, these data require comparison with age-matched controls.24,105 The risk of EVD recurrence and subsequent transmission by survivors is a key concern and so biological sampling in survivor cohorts is important to direct guidance on prevention strategies.106 Although a biological sampling approach (based on sequential negative samples) seems reasonable, we first need to know the natural history of persistence (ie, whether detection of Ebola virus in semen can follow non-detection in earlier samples). Clinical trials of experimental drugs to clear persistent virus have commenced (registration numbers NCT2818582 and NCT02739477). To date, many of the available viral persistence studies have relied on reverse transcriptase PCR to identify viral presence, but future studies should also focus on identifying live virus, which is more indicative of potential transmission risk.
Therapeutics
Experimental treatments
Before the west Africa epidemic, experimental therapeutics had not been studied in patients with EVD, although transfusion of blood from convalescent patients had been tried.107 The sheer scale of the west Africa epidemic demanded that effective, specific treatments should be identified and made available to patients as soon as possible. Accordingly, an expert panel was convened by WHO in September, 2014, to prioritise promising candidates for clinical trials.12
Disappointingly, no clinical trial of potential therapeutic agents has produced conclusive evidence of a beneficial effect (table 2). None of the trials have shown safety concerns for the respective agents, but safety and tolerability will need to be confirmed in subsequent studies. A phase 2 clinical trial of the antiviral favipiravir showed no survival benefit for patients with EVD and with a high viral load (cycle threshold <20), but suggested that further efficacy studies in patients with less advanced disease (cycle threshold ≥20) may be warranted.50 A trial of the antiviral brincidofovir in Liberia was stopped before a conclusion could be reached after the drug company withdrew involvement in Ebola trials, in the setting of falling case numbers.110 A phase 2, single-arm trial of the small interfering RNA lipid nanoparticle compound TKM-130803, done in Sierra Leone, showed no survival advantage in patients with severe EVD, compared with survival in historical (untreated) controls.109 The Ebola-Tx trial showed no survival benefit in patients who received convalescent plasma compared with historical controls.71 A separate report of the antibody titres in the transfused plasma found that concentrations of neutralising antibody were generally low and no significant association was found between antibody concentrations in the transfused units and patient survival.111 A multicentre, randomised trial of the ZMapp triple monoclonal antibody cocktail found that the CFR in patients receiving ZMapp in addition to standard care (22%) was lower than in patients receiving standard of care alone (37%). Although this finding did not meet the prespecified statistical threshold for efficacy, the posterior probability that the addition of ZMapp improved survival was 91%.108
Table 2. Patient-based clinical trials of experimental therapeutics registered on clinical trial databases during the west Africa Ebola virus disease outbreak.
| Trial design | Research question (PICO model) | Registration number (declared status as of November, 2016) | Result | |
|---|---|---|---|---|
| ZMapp | Open label RCT with adaptive trial design | Intervention: 50 mg/kg ZMapp, intravenous, every 3 days, total of three doses; comparison with optimised care alone (including favipiravir in Guinea); outcome measured as day 28 survival | Registered as PACTR201503001065306, NCT02363322 (completed) | No statistically conclusive benefit108 |
| TKM-130803 | Open label, single arm, Component of a multi-stage approach | Intervention: 0⋅3 mg/kg of TKM-130803, intravenous, once daily, total of seven doses; comparison with historical controls; outcome measured as day 14 survival | Registered as PACTR201501000997429 (completed) | No overall survival benefit109 |
| Favipiravir | Open label, single arm | Intervention: 6000 mg (day 0) and 2400 mg (days 1–9), oral, daily of favipiravir, total of ten doses; comparison with historical controls; outcome measured as day 14 survival | Registered as NCT02329054 (completed) | No overall survival benefit50 |
| Convalescent plasma | Open label, single arm | Intervention: 400–500 mL of convalescent plasma from two donors, administered as two consecutive (200–250 mL) transfusions; one treatment cycle in total; comparison with historical controls; outcome measured as day 14 survival | Registered as NCT02342171 (completed) | No overall survival benefit71 |
| Convalescent plasma | Open label, single arm | Intervention: 180–220 mL of convalescent plasma from two donors, administered as two consecutive (90–110 mL) infusions; up to three treatment cycles, at least 48 h apart; no comparison made; outcome measured as Ebola virus load | Registered as NCT02333578 (recruiting) | NA |
| Convalescent plasma | Open label, single arm | Intervention: INTERCEPT plasma; dose not defined; comparison not defined; outcome measured as 1 year survival | Registered as NCT02295501 (open to enrolment) | NA |
| Convalescent plasma | Open label, random allocation | Intervention: single transfusion of convalescent plasma; dose not defined; comparison with Ringer’s Lactate solution; outcome measured as all-cause mortality as 14 days after treatment | Registered as ISRCTN13990511 (ongoing; no longer recruiting) | NA |
| Brincidofovir | Open label, single arm trial, component of a multistage approach | Intervention: 200 mg brincidofovir oral, initial dose, then 100 mg, oral, twice weekly; total of five doses; comparison with historical controls; outcome measured as day 14 survival | Registered as PACTR201411000939962 (recruitment suspended) | No statistical conclusion110 |
| Azithromycin, Sunitinib, Erlonitib, Atorvastatin, Irbesartan | Multi-arm RCT with adaptive trial design | Intervention: azithromycin (1500 mg, oral, daily for 5 days) vs sunitinib (50 mg, oral, daily for 7 days) and erlonitib (150 mg, oral, daily for 7 days) vs atorvastatin (40 mg, oral, daily until discharge) and irbesartan (150 mg, oral, daily until discharge); comparison with intravenous fluids and laboratory testing alone; outcome measured as day 14 survival | Registered as NCT02380625 (not yet open to recruitment) | NA |
| Interferon β | Open label, single arm | Intervention: subcutaneous interferon β once daily for up to 10 days; comparison not defined (safety and effectiveness study); undefined outcome | Registered as ISRCTN17414946 (completed) | NA |
| Amiodarone | Open label, RCT | Intervention: amiodarone (20 mg/kg, intravenous, on days 1–3 then 200 mg, oral, three times daily, on days 4–10); comparison with supportive care alone; outcome measured as day 10 survival | Registered as NCT02307591 and PACTR201501001014425 (withdrawn) | NA |
Where a dose of an intervention has been stated, it refers to the stated adult dose. Refer to trial protocols for weight adjustment. PICO=participant, intervention, comparison, outcome. RCT=randomised controlled trial. NA=not available.
Other patients with EVD received experimental therapies on a compassionate basis, outside of clinical trials.30,57,112,113 Many of these patients were treated in resource-rich countries and received a combination of experimental agents alongside intensive care support and nursing care, so it is difficult to assess safety or efficacy. A small number of patients in west Africa received repurposed agents (including lamivudine, amiodarone, atorvastatin, irbesartan, clomifene, and favipiravir) without enrolment in a registered trial.114,115 Anecdotal reports of survival benefit have been reported for some of these agents,114,116 but it is impossible to draw any meaningful conclusions.
A retrospective study of patient outcome data from an ETC in Liberia found a temporal association between the use of antimalarial combination artesunate–amodiaquine and a period of reduced EVD mortality.117 Patients received this combination when there was a supply failure of the first line agent (artemether–lumefantrine), rather than for hypothesis-driven reasons. This supply failure, along with other limitations described by the authors, makes it difficult to interpret the findings from this study, but additional studies are warranted since in-vitro activity of amodiaquine against Ebola virus provides biological plausibility.118
Despite the largely negative outcomes from clinical trials, it must be recognised that the ability of researchers to overcome regulatory and operational barriers to complete trials to internationally accepted standards represents real progress, compared with previous outbreaks caused by high-hazard or emerging pathogens. Several ongoing challenges remain, however. For some drugs, the 100% survival rates seen in non-human primate models119,120 were not replicated in clinical trials. The reasons underlying these discrepancies should be explored, to maximise the use of the animal model in drug development. Explanations might include inherent biological differences between species, animal models that do not match human illness,109,121 differences in exposure route and infectious dose, or that some patients present late in the course of illness with complex end-organ manifestations that cannot be simulated completely in an animal model.
Vaccines
The epidemic also prompted accelerated efforts to take leading vaccine candidates to clinical trials, and to advance preclinical pipelines for less-developed candidates.122 Overall, four candidate vaccines met WHO criteria for fast-tracked clinical assessment: the replication competent recombinant vesicular stomatitis virus (rVSV) vaccine expressing Zaire Ebola virus gylcoprotein (ZEBOV), the replication-deficient chimpanzee adenovirus serotype 3 vector vaccine (ChAd3-ZEBOV), followed later by another adenoviral vectored vaccine (Ad26-ZEBOV) with a heterologous boost (modified vaccinia virus Ankara, MVA), and a nanoparticle vaccine (Novavax).123 The first clinical trial in a highly affected country commenced in February, 2015; with the exception of the nanoparticle vaccine, for which the phase 1 trial is ongoing, all of the candidates have been investigated in clinical trials in the region (table 3).
Table 3. Vaccine trials recruiting in the most affected countries during the Ebola virus disease outbreak in west Africa.
| Trial design | Research question (PICO model) | Registration number (declared status as of November, 2016) | |
|---|---|---|---|
| rVSV ZEBOV | |||
| Ebola ça suffit! | Open label, cluster randomised, ring vaccination | Participants include contacts of confirmed EVD patients; intervention with immediate vaccination with rVSV ZEBOV; comparison with delayed (day 21) vaccination; outcome measured as safety and efficacy | Registered as PACTR201503001057193 (interim results available124) |
| Ebola ça suffit! | Open label, single arm | Participants include adult front-line workers; intervention with immediate vaccination with rVSV ZEBOV; comparison with delayed (day 21) vaccination; outcome measured as safety and efficacy | Registered as PACTR201503001057193 (closed to recruitment, follow up complete125) |
| STRIVE | Open label, randomised, with two substudies | Participants include adult front-line workers; intervention with immediate vaccination with vVSV ΔG ZEBOV; comparison with delayed (18–24 weeks) vaccination; outcomes measured as safety, efficacy, and immunogenicity | Registered as NCT02378753, PACTR201502001037220 (ongoing but not recruiting) |
| Multiple | |||
| PREVAC | Double-blind RCT | Participants include children and adults; intervention with immediate vaccination with rVSV-ZEBOV (with or without rVSV boost) or Ad26.ZEBOV + MVA-BN-Filo boost; comparison with placebo; outcomes measured as safety and immunogenicity | Registered as NCT02876328 (not yet open for recruitment) |
| PREVAIL | Double-blind RCT | Participants include adults with Ebola virus infection; intervention with immediate vaccination with VSVG-ZEBOV or ChAd3-EBO Z; comparison with placebo; outcomes measured as safety and immunogenicity | Registered as NCT02344407 (ongoing, but not recruiting, no results available) |
| Ad5-EBOV | |||
| Ad5-EBOV | Double-blind RCT | Participants include healthy adults aged 18–50 years in Sierra Leone; intervention with high dose, or low dose immediate vaccination with Ad5-EBOV; comparison with placebo; outcome measured as safety and immunogenicity | Registered as NCT02575456, PACTR201509001259869 (completed, no results available) |
| Ad26. ZEBOV + MVA-BN-Filo | |||
| EBOVAC | Open label, single arm, followed by double-blind RCT | Participants include healthy adults and children in Sierra Leone; intervention with immediate vaccination with Ad26-ZEBOV and with MVA-BN-Filo boost; comparison with placebo (meningococcal vaccine during immediate vaccination) during the second stage of the RCT; outcome measured as safety, immunogenicity, and efficacy | Registered as NCT02509494, PACTR201506001147964 (recruiting) |
EVD=Ebola virus disease. PICO=participant, intervention, comparison, outcome. RCT=randomised controlled trial.
The phase 3 Ebola ça suffit rVSV-ZEBOV trial done in Guinea yielded remarkable interim findings.124 This study used a novel approach of ring vaccination, a method that was first used during smallpox eradication programmes and involves vaccination of high-risk contacts (defined geographically or socially) of known EVD cases, with the aim of interrupting transmission. Rings of contacts received either immediate or delayed (21 days postexposure) vaccination in a cluster randomised trial. This pragmatic approach aimed to balance the requirement for high-quality efficacy and safety data against ethical concerns about using placebo designs in highly susceptible populations in the midst of an EVD outbreak.126 Preliminary results suggest excellent efficacy (100%, 95% CI 75–100). There were no new infections after 6 days in participants that were immediately vaccinated (n=2014), compared with 16 infections in the delayed vaccination group (n=1930).124 In light of these findings, randomisation was stopped and all subsequent participants received immediate vaccination. Concerns have been raised about the reactogenecity of rVSV-ZEBOV following observed, transient fever (up to 30%), arthritis (3–22%), rash, and dermatitis in phase 1 trials in Africa and Europe.127 Whether these findings apply to other populations is unknown, as is the effect of potential side-effects on the acceptability of the vaccine among individuals at varying levels of risk of EVD. A substantial practical challenge to rolling out this vaccine in an outbreak would be differentiating those with transient vaccine-related fever from those who are developing symptomatic EVD. Additionally, the transient viraemia triggered by vaccination could also result in a false-positive PCR result with some tests.128
Adenovirus vector vaccines were the second type of vaccine to reach clinical trials in the affected countries. Phase 1/2a trials of ChAd3-ZEBOV showed safety.129–131 However, a trial with study groups in the USA and Mali showed that a single dose of vaccine elicited sufficient immunogenicity likely to be effective in postexposure prophylaxis scenarios, but that a heterologous prime and boost (with modified vaccinia Ankara expressing Zaire Ebola virus glycoprotein) would be more appropriate when an extended period of protection was required.129 The superior protective efficacy of a heterologous prime-boost regimen has been shown in other phase 1 trials of ChAd3132 and Ad26-ZEBOV133 and, in practical terms, might make it important for groups who have prolonged exposure periods—eg, health-care workers and burial teams.129 As we learn more about viral sequestration and sexual transmission, more durable vaccine-induced immunity might be required to provide longer-term protection of sexual partners or survivors of EVD. However, the inclusion of a boosting component will add to the logistical complexity of mass vaccination. The results of field trials of adenovirus vector-based vaccines are awaited (table 3).
Other ongoing vaccination trials in the region commenced too late to identify effectiveness. However, they should be able to provide important safety and immunogenicity data, including comparative data for different candidate vaccines. This presents a dilemma with respect to licensure of these vaccines. Although it is possible that promising Ebola vaccines could receive regulatory approval if human safety and immunogenicity data are supported by evidence of efficacy in non-human primate studies, the limitations of the present animal model and an imprecise understanding of immune correlates of protection mean there is little certainty in this process. The ongoing development and assessment of different vaccines are important, because it is unlikely that a single vaccine will meet all of the criteria in the WHO target therapeutic profile.123
Conclusions
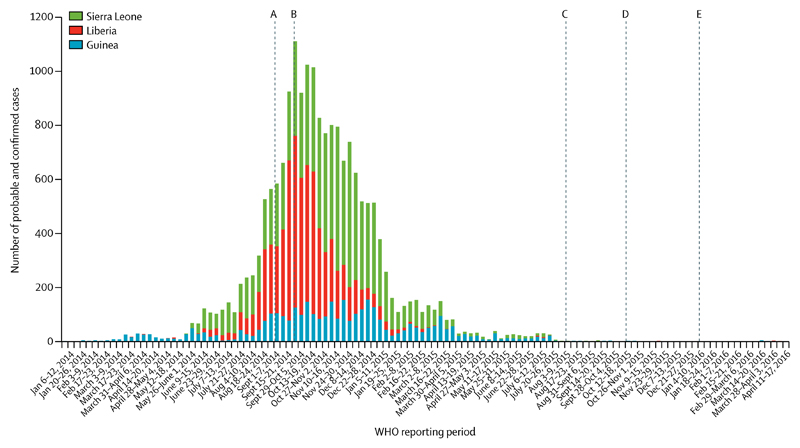
There have been several notable successes in the scientific response to this epidemic, including improved characterisation of EVD complications and the completion of clinical trials of experimental therapeutics (figure). Progress was slow in other areas. Despite the large number of patients, the reporting of clinical manifestations was fragmented and many published studies have described small cohorts or single cases. Data collection was frequently ad hoc or retrospective, highlighting the need to embed clinically relevant research in outbreak preparedness and response. Knowledge of how EVD affects susceptible populations, such as pregnant women and children, has not progressed substantially. We do not know the true benefits (or potential harms) of administering specific components of supportive care. Reporting on the outcomes of patients treated in resource-rich countries has been descriptive and repetitive, and only one medically evacuated patient was recruited to a clinical trial.
Figure. Significant research advances during west Africa Ebola virus disease epidemic.
Adapted from Bausch and Rojek (2016).134 (A) WHO holds consultation on potential Ebola therapeutics and vaccines.135 (B) WHO Response Team publishes first large observational patient data set.13 (C) Interim results of VSV-ZEBOV vaccine trial published.124 (D) Molecular evidence for sexual transmission published.104 (E) First clinical trial of experimental treatment (convalescent plasma) published.71
An important question is how to apply findings from studies that have generated new information, particularly when the results are inconclusive. For example, despite the absence of incontrovertible evidence of efficacy, it is possible that ZMapp will be included as standard of care in future EVD outbreaks; if this happens, it is likely that trials of any new agents will need to show superiority of the new agent given alongside ZMapp, compared with ZMapp alone. Such trials will also need to stratify by viral load on admission.73
Individual components of supportive care interventions have not been assessed in EVD-specific trials. The rationale of providing intravenous fluid replacement to patients with substantial gastrointestinal fluid losses is clear, but there is scope to compare different empirical fluid replacement regimens and investigate the optimal timing of fluid replacement. Although the observational studies of patients treated in Europe and the USA suggest that physiological support does contribute to survival, many of the advanced interventions used will be difficult to translate to the typical ETC environment and so a key component of assessment will be feasibility and practicality.
For pharmaceutical interventions that could alter the course of future outbreaks, the greatest hope comes from the Ebola ça suffit! ring vaccination trial. The final results from this trial136 were published after the literature search for this Review, and the results confirm the highly promising interim findings.124 It is likely that ring vaccination strategies will be adopted in future outbreaks caused by Zaire Ebola virus strains.
Additional findings from the west Africa epidemic are expected and it is hoped that new data will contribute to the knowledge base. The degree to which findings from this epidemic can be applied to future outbreaks, including those caused by different species of Ebola virus, is unknown; a comparison of key clinical findings from different outbreaks would be useful, but would rely on high-quality, comparable datasets being available. Future, smaller EVD outbreaks can be expected in at-risk countries and clinical studies will need to be rapid and efficient; greater yields may be obtained if research priorities are agreed in advance, with centralised coordination of studies.
In all of the fields reviewed, we have discussed areas of priority for future investigation. To achieve the most rigorous outcomes from future studies, there must be an improved commitment to producing protocol-directed, hypothesis-driven research whenever possible. When this is infeasible, recommendations should be based on careful, systematic data collection and use of shared platforms that facilitate data collation across different sites. This data collation will require not only a commitment from scientists but also funding and publishing mechanisms that facilitate and reward collaborative science.
Supplementary Material
Search strategy and selection criteria.
We searched PubMed for articles published from the beginning of the west Africa outbreak on Jan 1, 2014, to Nov 30, 2016, using search terms “Ebola” or “Ebola virus” or “Ebola Virus Disease” or “Ebola Haemorrhagic Fever” using British and American spelling variations. We reviewed the articles from the search that had an abstract available in English in addition to relevant references cited in those articles and conference and international meeting reports. We reviewed all publications that contained original research or patient data for quality and relevance. To identify ongoing unpublished clinical research, we searched clinical trial databases ClinicalTrials.gov, the Pan African Clinical Trials Registry, and the ISRCTN registry.
Two authors (AR, JD) categorised all papers according to predefined subject area, using publication review software (appendix). There were no discrepancies in individual categorisation that required mediation from the third author (PH). Papers were selected for inclusion on the basis of clinical relevance by joint review of two authors (AR, JD). A few papers that were published before the outbreak were included where comparison with existing knowledge was considered necessary; these were identified from the libraries of the authors.
Acknowledgments
AR is funded by a Rhodes Scholarship. The authors would like to thank Adrian Hill and Simon Mendelsohn at the University of Oxford for their advice on the review of vaccine progress. This work was supported by the Wellcome Trust of Great Britain (grant numbers 107834/Z/15/Z and106491/Z/14/Z), EU FP7 project PREPARE (602525), UK Medical Research Council (MC_PC_15001), and the Bill & Melinda Gates Foundation (OPP1116588).
Footnotes
Contributors
AR performed the literature search, according to the stated search strategy. AR, PH, and JD reviewed and selected articles to include in the Review, based on the stated selection criteria. AR produced the figures and tables. All authors contributed to writing the Review.
Declaration of interests
We declare no competing interests. The authors were investigators for two clinical trials described in this Review (TKM-130803 and brincidofovir trials).
References
- 1.Baize S, Pannetier D, Oestereich L, et al. Emergence of Zaire Ebola virus disease in Guinea. N Engl J Med. 2014;371:1418–25. doi: 10.1056/NEJMoa1404505. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Statement on the 1st meeting of the IHR Emergency Committee on the 2014 Ebola outbreak in west Africa. [accessed March 1, 2016];2014 http://www.who.int/mediacentre/news/statements/2014/ebola-20140808/en/
- 3.WHO. Ebola situation report—30 March 2016. [accessed March 30, 2016]; http://apps.who.int/ebola/current-situation/ebola-situation-report-30-march-2016.
- 4.UN Economic Commission for Africa. Socio-economic impacts of Ebola on Africa (Revised edition) [accessed Oct 11, 2016];2015 http://www.uneca.org/sites/default/files/PublicationFiles/eca_ebola_report_final_eng_0.pdf.
- 5.Parpia AS, Ndeffo-Mbah ML, Wenzel NS, Galvani AP. Effects of response to 2014–2015 Ebola outbreak on deaths from malaria, HIV/AIDS, and tuberculosis, west Africa. Emerg Infect Dis. 2016;22:433–41. doi: 10.3201/eid2203.150977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. Ebola situation reports, December 17, 2014. [accessed April 4, 2016];2016 http://apps.who.int/ebola/en/status-outbreak/situation-reports/ebola-situation-report-17-december-2014.
- 7.WHO. Ebola response roadmap. [accessed Sept 1, 2016];2014 http://www.who.int/csr/resources/publications/ebola/response-roadmap/en/
- 8.WHO. How can science inform our response to Ebola virus disease? [accessed Sept 1, 2016];2014 Oct 7; http://www.who.int/csr/resources/publications/ebola/science-ebola-response/en/2014.
- 9.Lang T. Ebola: embed research in outbreak response. Nature. 2015;524:29–31. doi: 10.1038/524029a. [DOI] [PubMed] [Google Scholar]
- 10.van Griensven J, De Weiggheleire A, Delamou A, et al. The use of Ebola convalescent plasma to treat Ebola virus disease in resource constrained settings: a perspective from the field. Clin Infect Dis. 2015;62:69–74. doi: 10.1093/cid/civ680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rid A, Emanuel EJ. Ethical considerations of experimental interventions in the Ebola outbreak. Lancet. 2014;384:1896–99. doi: 10.1016/S0140-6736(14)61315-5. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Ethical considerations for use of unregistered interventions for Ebola viral disease. [accessed March 1, 2016];2014 http://apps.who.int/iris/bitstream/10665/130997/1/WHO_HIS_KER_GHE_14.1_eng.pdf.
- 13.WHO Ebola Response Team. Ebola virus disease in west Africa— the first 9 months of the epidemic and forward projections. N Engl J Med. 2014;371:1481–95. doi: 10.1056/NEJMoa1411100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO Ebola Response Team. Ebola virus disease among male and female persons in west Africa. N Engl J Med. 2016;374:96–98. doi: 10.1056/NEJMc1510305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO Ebola Response Team. Agua-Agum J, Ariyarajah A, et al. Ebola virus disease among children in west Africa. N Engl J Med. 2015;372:1274–77. doi: 10.1056/NEJMc1415318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fitzgerald F, Naveed A, Wing K, et al. Ebola virus disease in children, Sierra Leone, 2014–2015. Emerg Infect Dis. 2016;22:1769–77. doi: 10.3201/eid2210.160579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bah EI, Lamah M-C, Fletcher T, et al. Clinical presentation of patients with Ebola virus disease in Conakry, Guinea. N Engl J Med. 2015;372:40–47. doi: 10.1056/NEJMoa1411249. [DOI] [PubMed] [Google Scholar]
- 18.Fitzpatrick G, Vogt F, Moi Gbabai OB, et al. The contribution of Ebola viral load at admission and other patient characteristics to mortality in a Medecins Sans Frontières Ebola case management centre, Kailahun, Sierra Leone, June–October, 2014. J Infect Dis. 2015;212:1752–58. doi: 10.1093/infdis/jiv304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schieffelin JS, Shaffer JG, Goba A, et al. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med. 2014;371:2092–100. doi: 10.1056/NEJMoa1411680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan T, Mu J, Qin E, et al. Clinical characteristics of 154 patients suspected of having Ebola virus disease in the Ebola holding center of Jui Government Hospital in Sierra Leone during the 2014 Ebola outbreak. Eur J Clin Microbiol Infect Dis. 2015;34:2089–95. doi: 10.1007/s10096-015-2457-z. [DOI] [PubMed] [Google Scholar]
- 21.Black BO, Caluwaerts S, Achar J. Ebola viral disease and pregnancy. Obstet Med. 2015;8:108–13. doi: 10.1177/1753495X15597354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oduyebo T, Pineda D, Lamin M, Leung A, Corbett C, Jamieson DJ. A pregnant patient with Ebola virus disease. Obstet Gynecol. 2015;126:1273–75. doi: 10.1097/AOG.0000000000001092. [DOI] [PubMed] [Google Scholar]
- 23.Caluwaerts S, Fautsch T, Lagrou D, et al. Dilemmas in managing pregnant women with Ebola: 2 case reports. Clin Infect Dis. 2016;62:903–05. doi: 10.1093/cid/civ1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO. Interim guidance: clinical care for survivors of Ebola virus disease. [accessed March 30, 2016];2016 http://apps.who.int/iris/bitstream/10665/204235/1/WHO_EVD_OHE_PED_16.1_eng.pdf.
- 25.Report of an International Commission. Ebola haemorrhagic fever in Zaire, 1976. Bull World Health Organ. 1978;56:271–93. [PMC free article] [PubMed] [Google Scholar]
- 26.Ebola haemorrhagic fever in Sudan, 1976. Report of a WHO/International study team. Bull World Health Organ. 1978;56:247–70. [PMC free article] [PubMed] [Google Scholar]
- 27.Barry MÉ, Touré A, Traoré FÉA, et al. Clinical predictors of mortality in patients with Ebola virus disease. Clin Infect Dis. 2015;60:1821–24. doi: 10.1093/cid/civ202. [DOI] [PubMed] [Google Scholar]
- 28.Barry M, Traoré FA, Sako FB, et al. Ebola outbreak in Conakry, Guinea: epidemiological, clinical, and outcome features. Med Mal Infect. 2014;44:491–94. doi: 10.1016/j.medmal.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Kreuels B, Wichmann D, Emmerich P, et al. A case of severe Ebola virus infection complicated by Gram-negative septicemia. N Engl J Med. 2014;271:2394–401. doi: 10.1056/NEJMoa1411677. [DOI] [PubMed] [Google Scholar]
- 30.Liddell AM, Davey RT, Jr, Mehta AK, et al. Characteristics and clinical management of a cluster of 3 patients with Ebola virus disease, including the first domestically acquired cases in the United States. Ann Intern Med. 2015;163:81–90. doi: 10.7326/M15-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dietz PM, Jambai A, Paweska JT, Yoti Z, Ksiazek TG. Epidemiology and risk factors for Ebola virus disease in Sierra Leone—23 May 2014 to 31 January 2015. Clin Infect Dis. 2015;61:1648–54. doi: 10.1093/cid/civ568. [DOI] [PubMed] [Google Scholar]
- 32.WHO Ebola Response Team. Agua-Agum J, Ariyarajah A, et al. West African Ebola epidemic after one year—slowing but not yet under control. N Engl J Med. 2015;372:584–87. doi: 10.1056/NEJMc1414992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lado M, Walker NF, Baker P, et al. Clinical features of patients isolated for suspected Ebola virus disease at Connaught Hospital, Freetown, Sierra Leone: a retrospective cohort study. Lancet Infect Dis. 2015;15:1024–33. doi: 10.1016/S1473-3099(15)00137-1. [DOI] [PubMed] [Google Scholar]
- 34.Hunt L, Gupta-Wright A, Simms V, et al. Clinical presentation, biochemical, and haematological parameters and their association with outcome in patients with Ebola virus disease: an observational cohort study. Lancet Infect Dis. 2015;15:1292–99. doi: 10.1016/S1473-3099(15)00144-9. [DOI] [PubMed] [Google Scholar]
- 35.Tiffany A, Vetter P, Mattia J, et al. Ebola virus disease complications as experienced by survivors in Sierra Leone. Clin Infect Dis. 2016;62:1360–66. doi: 10.1093/cid/ciw158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nanyonga M, Saidu J, Ramsay A, Shindo N, Bausch DG. Sequelae of Ebola virus disease, Kenema District, Sierra Leone. Clin Infect Dis. 2016;62:125–26. doi: 10.1093/cid/civ795. [DOI] [PubMed] [Google Scholar]
- 37.Epstein L, Wong KK, Kallen AJ, Uyeki TM. Post-Ebola signs and symptoms in US survivors. N Engl J Med. 2015;373:2484–86. doi: 10.1056/NEJMc1506576. [DOI] [PubMed] [Google Scholar]
- 38.Qureshi AI, Chughtai M, Loua TO, et al. Study of Ebola virus disease survivors in Guinea. Clin Infect Dis. 2015;61:1035–42. doi: 10.1093/cid/civ453. [DOI] [PubMed] [Google Scholar]
- 39.Vetter P, Kaiser L, Schibler M, Ciglenecki I, Bausch DG. Sequelae of Ebola virus disease: the emergency within the emergency. Lancet Infect Dis. 2016;16:e82–91. doi: 10.1016/S1473-3099(16)00077-3. [DOI] [PubMed] [Google Scholar]
- 40.Sagui E, Janvier F, Baize S, et al. Severe Ebola virus infection with encephalopathy: evidence for direct virus involvement. Clin Infect Dis. 2015;61:1627–28. doi: 10.1093/cid/civ606. [DOI] [PubMed] [Google Scholar]
- 41.de Greslan T, Billhot M, Rousseau C, et al. Ebola virus-related encephalitis. Clin Infect Dis. 2016;63:1076–78. doi: 10.1093/cid/ciw469. [DOI] [PubMed] [Google Scholar]
- 42.Chertow DS, Nath A, Suffredini AF, et al. Severe meningoencephalitis in a case of Ebola virus disease: a case report. Ann Intern Med. 2016;165:301–04. doi: 10.7326/M15-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scott JT, Sesay FR, Massaquoi TA, Idriss BR, Sahr F, Semple MG. Post-Ebola syndrome, Sierra Leone. Emerg Infect Dis. 2016;22:641–46. doi: 10.3201/eid2204.151302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mattia JG, Vandy MJ, Chang JC, et al. Early clinical sequelae of Ebola virus disease in Sierra Leone: a cross-sectional study. Lancet Infect Dis. 2016;16:331–38. doi: 10.1016/S1473-3099(15)00489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cellarier G, Bordes J, De Greslan T, et al. Inappropriate bradycardia in Ebola virus disease. Med Sante Trop. 2016;26:283–86. doi: 10.1684/mst.2016.0586. [DOI] [PubMed] [Google Scholar]
- 46.Sueblinvong V, Johnson DW, Weinstein GL, et al. Critical care for multiple organ failure secondary to Ebola virus disease in the United States. Crit Care Med. 2015;43:2066–75. doi: 10.1097/CCM.0000000000001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chertow DS, Childs RW, Arai AE, Davey RT., Jr Cardiac MRI findings suggest myocarditis in severe Ebola virus disease. JACC Cardiovasc Imaging. 2016 doi: 10.1016/j.jcmg.2016.06.004. published online Aug 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolf T, Kann G, Becker S, et al. Severe Ebola virus disease with vascular leakage and multiorgan failure: treatment of a patient in intensive care. Lancet. 2015;385:1428–35. doi: 10.1016/S0140-6736(14)62384-9. [DOI] [PubMed] [Google Scholar]
- 49.de Wit E, Kramer S, Prescott J, et al. Clinical chemistry of patients with Ebola in Monrovia, Liberia. J Infect Dis. 2016;214(suppl 3):S303–07. doi: 10.1093/infdis/jiw187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sissoko D, Laouenan C, Folkesson E, et al. Experimental treatment with favipiravir for Ebola virus disease (the JIKI Trial): a historically controlled, single-arm proof-of-concept trial in Guinea. PLoS Med. 2016;13:e1001967. doi: 10.1371/journal.pmed.1001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Griensven J, Bah EI, Haba N, et al. Electrolyte and metabolic disturbances in Ebola patients during a clinical trial, Guinea, 2015. Emerg Infect Dis. 2016;22:2120–27. doi: 10.3201/eid2212.161136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richardson ET, Kelly JD, Barrie MB, et al. Minimally symptomatic infection in an Ebola ‘hotspot’: a cross-sectional serosurvey. PLoS Negl Trop Dis. 2016;10:e0005087. doi: 10.1371/journal.pntd.0005087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moyen N, Thirion L, Emmerich P, et al. Risk factors associated with Ebola and Marburg viruses seroprevalence in blood donors in the Republic of Congo. PLoS Negl Trop Dis. 2015;9:e0003833. doi: 10.1371/journal.pntd.0003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schoepp RJ, Rossi CA, Khan SH, Goba A, Fair JN. Undiagnosed acute viral febrile illnesses, Sierra Leone. Emerg Infect Dis. 2014;20:1176–82. doi: 10.3201/eid2007.131265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.WHO Ebola Response Team. Agua-Agum J, Allegranzi B, et al. After Ebola in west Africa—unpredictable risks, preventable epidemics. N Engl J Med. 2016;375:587–96. doi: 10.1056/NEJMsr1513109. [DOI] [PubMed] [Google Scholar]
- 56.Ansumana R, Jacobsen KH, Sahr F, et al. Ebola in Freetown area, Sierra Leone—a case study of 581 patients. N Engl J Med. 2015;372:587–88. doi: 10.1056/NEJMc1413685. [DOI] [PubMed] [Google Scholar]
- 57.Uyeki TM, Mehta AK, Davey RT, Jr, et al. Clinical management of Ebola virus disease in the United States and Europe. N Engl J Med. 2016;374:636–46. doi: 10.1056/NEJMoa1504874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet. 2011;377:849–62. doi: 10.1016/S0140-6736(10)60667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Falasca L, Agrati C, Petrosillo N, et al. Molecular mechanisms of Ebola virus pathogenesis: focus on cell death. Cell Death Differ. 2015;22:1250–59. doi: 10.1038/cdd.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martines RB, Ng DL, Greer PW, Rollin PE, Zaki SR. Tissue and cellular tropism, pathology and pathogenesis of Ebola and Marburg viruses. J Pathol. 2015;235:153–74. doi: 10.1002/path.4456. [DOI] [PubMed] [Google Scholar]
- 61.Bordes J, Janvier F, Aletti M, et al. Organ failures on admission in patients with Ebola virus disease. Intensive Care Med. 2015;41:1504–05. doi: 10.1007/s00134-015-3912-0. [DOI] [PubMed] [Google Scholar]
- 62.Cournac JM, Karkowski L, Bordes J, et al. Rhabdomyolysis in Ebola virus disease. Results of an observational study in a treatment center in Guinea. Clin Infect Dis. 2016;62:19–23. doi: 10.1093/cid/civ779. [DOI] [PubMed] [Google Scholar]
- 63.Janvier F, Gorbatch S, Queval L, et al. Difficulties of interpretation of Zaire Ebola Virus PCR results and implication in the field. J Clin Virol. 2015;67:36–37. doi: 10.1016/j.jcv.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 64.Zhang X, Rong Y, Sun L, et al. Prognostic analysis of patients with Ebola virus disease. PLoS Negl Trop Dis. 2015;9:e0004113. doi: 10.1371/journal.pntd.0004113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qin E, Bi J, Zhao M, et al. Clinical features of patients with Ebola virus disease in Sierra Leone. Clin Infect Dis. 2015;61:491–95. doi: 10.1093/cid/civ319. [DOI] [PubMed] [Google Scholar]
- 66.Petrosillo N, Nicastri E, Lanini S, et al. Ebola virus disease complicated with viral interstitial pneumonia: a case report. BMC Infect Dis. 2015;15:432. doi: 10.1186/s12879-015-1169-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chertow DS, Kleine C, Edwards JK, Scaini R, Giuliani R, Sprecher A. Ebola virus disease in west Africa—clinical manifestations and management. N Engl J Med. 2014;371:2054–57. doi: 10.1056/NEJMp1413084. [DOI] [PubMed] [Google Scholar]
- 68.Wilson AJ, Martin DS, Maddox V, et al. Thromboelastography in the management of coagulopathy associated with Ebola virus disease. Clin Infect Dis. 2016;62:610–12. doi: 10.1093/cid/civ977. [DOI] [PubMed] [Google Scholar]
- 69.Jacobs M, Rodger A, Bell DJ, et al. Late Ebola virus relapse causing meningoencephalitis: a case report. Lancet. 2016;388:498–503. doi: 10.1016/S0140-6736(16)30386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lanini S, Portella G, Vairo F, et al. Blood kinetics of Ebola virus in survivors and nonsurvivors. J Clin Invest. 2015;125:4692–98. doi: 10.1172/JCI83111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Griensven J, Edwards T, de Lamballerie X, et al. Evaluation of convalescent plasma for Ebola virus disease in Guinea. N Engl J Med. 2016;374:33–42. doi: 10.1056/NEJMoa1511812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li J, Duan HJ, Chen HY, et al. Age and Ebola viral load correlate with mortality and survival time in 288 Ebola virus disease patients. Int J Infect Dis. 2016;42:34–39. doi: 10.1016/j.ijid.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Faye O, Andronico A, Faye O, et al. Use of viremia to evaluate the baseline case fatality ratio of Ebola virus disease and inform treatment studies: a retrospective cohort study. PLoS Med. 2015;12:e1001908. doi: 10.1371/journal.pmed.1001908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baize S, Leroy EM, Georges AJ, et al. Inflammatory responses in Ebola virus-infected patients. Clin Exp Immunol. 2002;128:163–68. doi: 10.1046/j.1365-2249.2002.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McElroy AK, Erickson BR, Flietstra TD, et al. Biomarker correlates of survival in pediatric patients with Ebola virus disease. Emerg Infect Dis. 2014;20:1683–90. doi: 10.3201/eid2010.140430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McElroy AK, Harmon JR, Flietstra TD, et al. Kinetic analysis of biomarkers in a cohort of US patients with Ebola virus disease. Clin Infect Dis. 2016;63:460–47. doi: 10.1093/cid/ciw334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rosenke K, Adjemian J, Munster VJ, et al. Plasmodium parasitemia associated with increased survival in Ebola virus-infected patients. Clin Infect Dis. 2016;63:1026–33. doi: 10.1093/cid/ciw452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kerber R, Krumkamp R, Diallo B, et al. Analysis of diagnostic findings from the European mobile laboratory in Gueckedou, Guinea, March 2014 through March 2015. J Infect Dis. 2016;214(suppl 3):S250–57. doi: 10.1093/infdis/jiw269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schwarze-Zander C, Blackard JT, Rockstroh JK. Role of GB virus C in modulating HIV disease. Expert Rev Anti Infect Ther. 2012;10:563–72. doi: 10.1586/eri.12.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lamontagne F, Clément C, Fletcher T, Jacob ST, Fischer WA, 2nd, Fowler RA. Doing today’s work superbly well—treating Ebola with current tools. N Engl J Med. 2014;371:1565–66. doi: 10.1056/NEJMp1411310. [DOI] [PubMed] [Google Scholar]
- 81.WHO. Manual for the care and management of patients in Ebola care units/Community Care Centres: interim emergency guidance, January 2015. [accessed June 11, 2016];2015 http://apps.who.int/iris/handle/10665/149781.
- 82.Médécins Sans Frontières. Filovirus haemorrhagic fever guideline. [accessed June 12, 2016];2008 http://www.slamviweb.org/es/ebola/FHFfinal.pdf.
- 83.Roberts I, Perner A. Ebola virus disease: clinical care and patient-centred research. Lancet. 2014;384:2001–02. doi: 10.1016/S0140-6736(14)62316-3. [DOI] [PubMed] [Google Scholar]
- 84.Maitland K, Babiker A, Kiguli S, Molyneux E, FEAST Trial Group The FEAST trial of fluid bolus in African children with severe infection. Lancet. 2012;379:613–14. doi: 10.1016/S0140-6736(12)60260-8. [DOI] [PubMed] [Google Scholar]
- 85.Kreuels B, Addo MM, Schmiedel S. Severe Ebola virus infection complicated by Gram-negative septicemia. N Engl J Med. 2015;372:1377. doi: 10.1056/NEJMc1500455. [DOI] [PubMed] [Google Scholar]
- 86.Hellman J. Addressing the complications of Ebola and other viral hemorrhagic fever infections: using insights from bacterial and fungal sepsis. PLoS Pathog. 2015;11:e1005088. doi: 10.1371/journal.ppat.1005088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Trehan I, Kelly T, Marsh RH, George PM, Callahan CW. Moving towards a more aggressive and comprehensive model of care for children with Ebola. J Pediatr. 2016;170:28–33. doi: 10.1016/j.jpeds.2015.11.054. [DOI] [PubMed] [Google Scholar]
- 88.Clark DV, Kibuuka H, Millard M, et al. Long-term sequelae after Ebola virus disease in Bundibugyo, Uganda: a retrospective cohort study. Lancet Infect Dis. 2015;15:905–12. doi: 10.1016/S1473-3099(15)70152-0. [DOI] [PubMed] [Google Scholar]
- 89.Rowe AK, Bertolli J, Khan AS, et al. Clinical, virologic, and immunologic follow-up of convalescent Ebola hemorrhagic fever patients and their household contacts, Kikwit, Democratic Republic of the Congo. Commission de Lutte contre les Epidemies a Kikwit. J Infect Dis. 1999;179(suppl 1):S28–35. doi: 10.1086/514318. [DOI] [PubMed] [Google Scholar]
- 90.Mohammed A, Sheikh TL, Gidado S, et al. An evaluation of psychological distress and social support of survivors and contacts of Ebola virus disease infection and their relatives in Lagos, Nigeria: a cross sectional study— 2014. BMC Public Health. 2015;15:824. doi: 10.1186/s12889-015-2167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Varkey JB, Shantha JG, Crozier I, et al. Persistence of Ebola virus in ocular fluid during convalescence. N Engl J Med. 2015;372:2423–27. doi: 10.1056/NEJMoa1500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shantha JG, Crozier I, Varkey JB, et al. Long-term management of panuveitis and iris heterochromia in an Ebola survivor. Ophthalmology. 2016;123:2626–28. doi: 10.1016/j.ophtha.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bower H, Smout E, Bangura MS, et al. Deaths, late deaths, and role of infecting dose in Ebola virus disease in Sierra Leone: retrospective cohort study. BMJ. 2016;353:i2403. doi: 10.1136/bmj.i2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thorson A, Formenty P, Lofthouse C, Broutet N. Systematic review of the literature on viral persistence and sexual transmission from recovered Ebola survivors: evidence and recommendations. BMJ Open. 2016;6:e008859. doi: 10.1136/bmjopen-2015-008859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chughtai AA, Barnes M, Macintyre CR. Persistence of Ebola virus in various body fluids during convalescence: evidence and implications for disease transmission and control. Epidemiol Infect. 2016;144:1652–60. doi: 10.1017/S0950268816000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Soka MJ, Choi MJ, Baller A, et al. Prevention of sexual transmission of Ebola in Liberia through a national semen testing and counselling programme for survivors: an analysis of Ebola virus RNA results and behavioural data. Lancet Glob Health. 2016;4:e736–43. doi: 10.1016/S2214-109X(16)30175-9. [DOI] [PubMed] [Google Scholar]
- 97.Deen GF, Knust B, Broutet N, et al. Ebola RNA persistence in semen of Ebola virus disease survivors—preliminary report. N Engl J Med. 2015 doi: 10.1056/NEJMoa1511410. published online Oct 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baggi FM, Taybi A, Kurth A, et al. Management of pregnant women infected with Ebola virus in a treatment centre in Guinea, June 2014. Euro Surveill. 2014;19:1–4. doi: 10.2807/1560-7917.es2014.19.49.20983. [DOI] [PubMed] [Google Scholar]
- 99.Bower H, Grass JE, Veltus E, et al. Delivery of an Ebola virus-positive stillborn infant in a rural community health center, Sierra Leone, 2015. Am J Trop Med Hyg. 2016;94:417–19. doi: 10.4269/ajtmh.15-0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lyon GM, Mehta AK, Varkey JB, et al. Clinical care of two patients with Ebola virus disease in the United States. N Engl J Med. 2014;371:2402–09. doi: 10.1056/NEJMoa1409838. [DOI] [PubMed] [Google Scholar]
- 101.Bausch DG, Towner JS, Dowell SF, et al. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis. 2007;196(suppl 2):S142–47. doi: 10.1086/520545. [DOI] [PubMed] [Google Scholar]
- 102.Rodriguez LL, De Roo A, Guimard Y, et al. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179(suppl 1):S170–76. doi: 10.1086/514291. [DOI] [PubMed] [Google Scholar]
- 103.Diallo B, Sissoko D, Loman NJ, et al. Resurgence of Ebola virus disease in Guinea linked to a survivor with virus persistence in seminal fluid for more than 500 days. Clin Infect Dis. 2016;63:1353–56. doi: 10.1093/cid/ciw601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mate SE, Kugelman JR, Nyenswah TG, et al. Molecular evidence of sexual transmission of Ebola virus. N Engl J Med. 2015;373:2448–54. doi: 10.1056/NEJMoa1509773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fallah MP, Skrip LA, Dahn BT, et al. Pregnancy outcomes in Liberian women who conceived after recovery from Ebola virus disease. Lancet Glob Health. 2016;4:e678–79. doi: 10.1016/S2214-109X(16)30147-4. [DOI] [PubMed] [Google Scholar]
- 106.Sonnenberg P, Field N. Sexual and mother-to-child transmission of Ebola virus in the postconvalescent period. Clin Infect Dis. 2015;60:974–75. doi: 10.1093/cid/ciu981. [DOI] [PubMed] [Google Scholar]
- 107.Mupapa K, Massamba M, Kibadi K, et al. Treatment of Ebola hemorrhagic fever with blood transfusions from convalescent patients. International Scientific and Technical Committee. J Infect Dis. 1999;179(suppl 1):S18–23. doi: 10.1086/514298. [DOI] [PubMed] [Google Scholar]
- 108.PREVAIL II Writing Group, Multi-National PREVAIL II Study Team. A randomized, controlled trial of ZMapp for Ebola virus infection. N Engl J Med. 2016;375:1448–56. doi: 10.1056/NEJMoa1604330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dunning J, Sahr F, Rojek A, et al. Experimental treatment of Ebola virus disease with TKM-130803: a single-arm phase 2 clinical trial. PLoS Med. 2016;13:e1001997. doi: 10.1371/journal.pmed.1001997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dunning J, Kennedy SB, Antierens A, et al. Experimental treatment of Ebola virus disease with brincidofovir. PLoS One. 2016;11:e0162199. doi: 10.1371/journal.pone.0162199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.van Griensven J, Edwards T, Baize S, Ebola-Tx Consortium Efficacy of convalescent plasma in relation to dose of Ebola virus antibodies. N Engl J Med. 2016;375:2307–09. doi: 10.1056/NEJMc1609116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mora-Rillo M, Arsuaga M, Ramirez-Olivencia G, et al. Acute respiratory distress syndrome after convalescent plasma use: treatment of a patient with Ebola virus disease contracted in Madrid, Spain. Lancet Respir Med. 2015;3:554–62. doi: 10.1016/S2213-2600(15)00180-0. [DOI] [PubMed] [Google Scholar]
- 113.Schibler M, Vetter P, Cherpillod P, et al. Clinical features and viral kinetics in a rapidly cured patient with Ebola virus disease: a case report. Lancet Infect Dis. 2015;15:1034–40. doi: 10.1016/S1473-3099(15)00229-7. [DOI] [PubMed] [Google Scholar]
- 114.Bai CQ, Mu JS, Kargbo D, et al. Clinical and virological characteristics of Ebola virus disease patients treated with favipiravir (T-705)—Sierra Leone, 2014. Clin Infect Dis. 2016;63:1288–94. doi: 10.1093/cid/ciw571. [DOI] [PubMed] [Google Scholar]
- 115.WHO. Ebola vaccines, therapies, and diagnostics: questions and answers. [accessed Feb 1, 2016];2015 http://www.who.int/medicines/emp_ebola_q_as/en/
- 116.Fedson DS, Jacobson JR, Rordam OM, Opal SM. Treating the host response to Ebola virus disease with generic statins and angiotensin receptor blockers. MBio. 2015;6:e00716. doi: 10.1128/mBio.00716-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gignoux E, Azman AS, de Smet M, et al. Effect of artesunate–amodiaquine on mortality related to Ebola virus disease. N Engl J Med. 2016;374:23–32. doi: 10.1056/NEJMoa1504605. [DOI] [PubMed] [Google Scholar]
- 118.Madrid PB, Chopra S, Manger ID, et al. A systematic screen of FDA-approved drugs for inhibitors of biological threat agents. PLoS One. 2013;8:e60579. doi: 10.1371/journal.pone.0060579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Thi EP, Mire CE, Lee AC, et al. Lipid nanoparticle siRNA treatment of Ebola-virus-Makona-infected nonhuman primates. Nature. 2015;521:362–65. doi: 10.1038/nature14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Qiu X, Wong G, Audet J, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014;514:47–53. doi: 10.1038/nature13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mendoza EJ, Qiu X, Kobinger GP. Progression of Ebola therapeutics during the 2014–2015 outbreak. Trends Mol Med. 2016;22:164–73. doi: 10.1016/j.molmed.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 122.WHO. Ebola R&D landscape of clinical candidates and trials. [accessed Feb 1, 2016];2015 Oct; http://www.who.int/medicines/ebola-treatment/EbolaR_D_public-report_updt2015.pdf?ua=1.
- 123.Henao-Restrepo AM, Preziosi MP, Wood D, Moorthy V, Kieny MP, WHO Ebola Research Development Team On a path to accelerate access to Ebola vaccines: the WHO’s research and development efforts during the 2014–2016 Ebola epidemic in west Africa. Curr Opin Virol. 2016;17:138–44. doi: 10.1016/j.coviro.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Henao-Restrepo AM, Longini IM, Egger M, et al. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet. 2015;386:857–66. doi: 10.1016/S0140-6736(15)61117-5. [DOI] [PubMed] [Google Scholar]
- 125.WHO. International Clinical Trials Registry Platform—PACTR201503001057193. [accessed Oct 20, 2016];2016 http://apps.who.int/trialsearch/Trial2.aspx?TrialID=PACTR201503001057193.
- 126.Ebola ça Suffit Ring Vaccination Trial Consortium. The ring vaccination trial: a novel cluster randomised controlled trial design to evaluate vaccine efficacy and effectiveness during outbreaks, with special reference to Ebola. BMJ. 2015;351:h3740. doi: 10.1136/bmj.h3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Agnandji ST, Huttner A, Zinser ME, et al. Phase 1 Trials of rVSV Ebola vaccine in Africa and Europe. N Engl J Med. 2016;374:1647–60. doi: 10.1056/NEJMoa1502924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cnops L, Gerard M, Vandenberg O, et al. Risk of misinterpretation of Ebola virus PCR results after rVSV ZEBOV-GP vaccination. Clin Infect Dis. 2015;60:1725–26. doi: 10.1093/cid/civ131. [DOI] [PubMed] [Google Scholar]
- 129.Tapia MD, Sow SO, Lyke KE, et al. Use of ChAd3-EBO-Z Ebola virus vaccine in Malian and US adults, and boosting of Malian adults with MVA-BN-Filo: a phase 1, single-blind, randomised trial, a phase 1b, open-label and double-blind, dose-escalation trial, and a nested, randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2016;16:31–42. doi: 10.1016/S1473-3099(15)00362-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.De Santis O, Audran R, Pothin E, et al. Safety and immunogenicity of a chimpanzee adenovirus-vectored Ebola vaccine in healthy adults: a randomised, double-blind, placebo-controlled, dose-finding, phase 1/2a study. Lancet Infect Dis. 2016;16:311–20. doi: 10.1016/S1473-3099(15)00486-7. [DOI] [PubMed] [Google Scholar]
- 131.Ledgerwood JE, Sullivan NJ, Graham BS. Chimpanzee adenovirus vector Ebola vaccine—preliminary report. N Engl J Med. 2015;373:776. doi: 10.1056/NEJMc1505499. [DOI] [PubMed] [Google Scholar]
- 132.Ewer K, Rampling T, Venkatraman N, et al. A monovalent chimpanzee adenovirus Ebola vaccine boosted with MVA. N Engl J Med. 2016;374:1635–46. doi: 10.1056/NEJMoa1411627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Milligan ID, Gibani MM, Sewell R, et al. Safety and immunogenicity of novel adenovirus type 26- and modified Vaccinia Ankara-vectored Ebola vaccines: a randomized clinical trial. JAMA. 2016;315:1610–23. doi: 10.1001/jama.2016.4218. [DOI] [PubMed] [Google Scholar]
- 134.Bausch DG, Rojek A. West Africa 2013: re-examining Ebola. Microbiol Spectr. 2016;4 doi: 10.1128/microbiolspec.EI10-0022-2016. EI10-0022-2016. [DOI] [PubMed] [Google Scholar]
- 135.WHO. Meeting summary of the WHO consultation on potential Ebola therapies and vaccines. Geneva: World Health Organization; 2014. [Google Scholar]
- 136.Henao-Restrepo AM, Camacho A, Longini IM, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!) Lancet. 2016;389:505–518. doi: 10.1016/S0140-6736(16)32621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.