Abstract
The emerging diverse roles of ether (phospho)lipids in nervous system development and function in health and disease are currently attracting growing interest. Plasmalogens, a subgroup of ether lipids, are important membrane components involved in vesicle fusion and membrane raft composition. They store polyunsaturated fatty acids and may serve as antioxidants. Ether lipid metabolites act as precursors for the formation of glycosyl-phosphatidyl-inositol anchors; others, like platelet-activating factor, are implicated in signaling functions. Consolidating the available information, we attempt to provide molecular explanations for the dramatic neurological phenotype in ether lipid-deficient human patients and mice by linking individual functional properties of ether lipids with pathological features. Furthermore, recent publications have identified altered ether lipid levels in the context of many acquired neurological disorders including Alzheimer’s disease (AD) and autism. Finally, current efforts to restore ether lipids in peroxisomal disorders as well as AD are critically reviewed.
Keywords: autism, peroxisome, plasmalogen
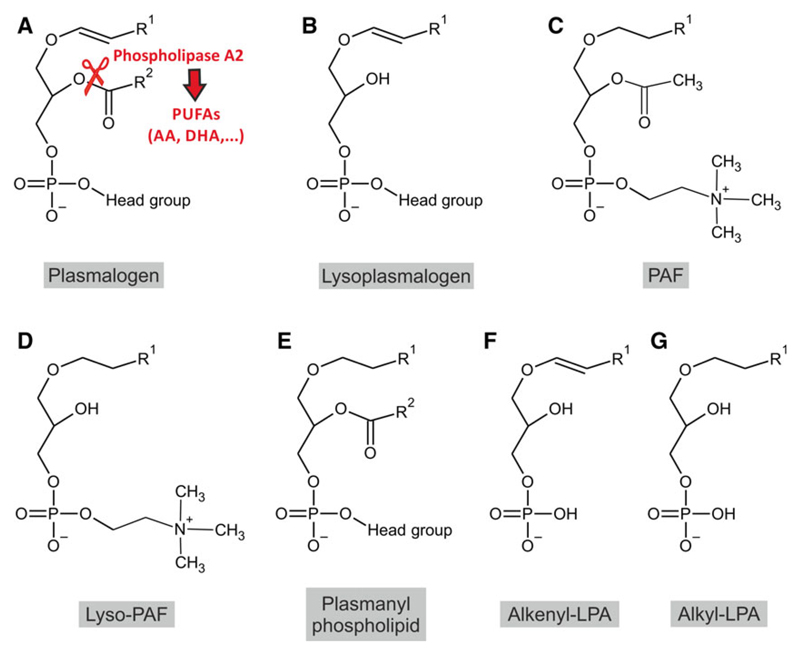
Ether (phospho)lipids are a particularly common subgroup of glycerophospholipids in the mammalian nervous system and diseases involving ether lipid deficiency have most drastic consequences in nervous tissue. The distinctive chemical feature of ether lipids is the ether bond at the sn-1 position of the glycerol backbone, where a fatty alcohol is attached as opposed to the more common diacyl phospholipids, in which fatty acids are esterified at both the sn-1 and sn-2 position. The biosynthesis of ether lipids requires the concerted action of different organelles. The first steps of the pathway take place in peroxisomes, where the sequential activities of dihydroxyacetone phosphate acyltransferase (DHAPAT; gene name: glycerone phosphate acyltransferase, GNPAT) and alkyl-dihydroxyacetone phosphate synthase (ADHAPS; gene name: alkylglycerone phosphate synthase, AGPS) are needed to generate the ether bond [1]. A fatty alcohol for the ADHAPS reaction is supplied by fatty acyl-CoA reductase (FAR), a peroxisomal tail-anchored protein [2], which catalyzes the step suggested to be rate limiting in ether lipid biosynthesis and whose activity is subject to feedback regulation by the cellular plasmalogen level [3,4]. The resulting precursor, alkyl-dihydroxyacetone phosphate (DHAP), is shuttled across the peroxisomal membrane by a currently unknown mechanism. Ether lipid biosynthesis proceeds at the outer (cytosolic) face of the peroxisomal membrane, where an acyl/alkyl-DHAP reductase (AADHAPR; alternative name: peroxisomal reductase activating PPARγ, PexRAP) reduces the ketone group at the sn-2 position [5]. This step also occurs in the biosynthesis of diacyl phospholipids and can be performed at both peroxisomal and endoplasmic reticulum (ER) membranes [6,7]. All subsequent enzymatic steps take place at other organelles, depending on the type of ether lipid. The most abundant ether lipids are the plasmalogens (Fig. 1A), which are characterized by a vinyl ether bond (a double bond adjacent to the ether bond) at sn-1 and mostly carry either ethanolamine or choline as head group thereby forming compounds termed plasmenylethanolamine1 (PlsEtn, ethanolamine plasmalogen) or plasmenylcholine (PlsCho, choline plasmalogen). Less abundant but also found in a variety of tissues, are the plasmanyl phospholipids (Fig. 1E), which differ from the plasmalogens by a simple ether bond at sn-1 instead of the vinyl ether. Other prominent types of ether lipids include the small inflammatory mediator platelet-activating factor (PAF, 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine; Fig. 1C) or the sulfoglycolipid seminolipid [8], which is found mainly in testis. For details on ether lipid structure and metabolism, we may refer the readers to previous reviews [1,9–11].
Fig. 1.
Structure of ether lipids with reported involvement in signaling processes. A prototypic plasmalogen (A), lysoplasmalogen (B), PAF (C), lyso-PAF (D), plasmanyl phospholipid (E), alkenyl-LPA (F), and alkyl-LPA (G) are drawn disregarding stereochemistry. R1 represents alkyl residues originating from the primary alcohols C16:0, C18:1, or C18:0; R2 designates a wider range of saturated and unsaturated fatty acyl residues. The cleavage site for plasmalogen-specific phospholipase A2 is indicated in (A). Phospholipase A2 activity releases PUFAs, which can be converted into a variety of additional signaling mediators.
Plasmalogens are especially enriched in the nervous system, where, in mammals, ethanolamine is the dominant head group with PlsCho present only in trace amounts. In man and most animals, brain tissue levels of plasmalogens are reported to constitute between 15 and 30 mol% of total phospholipids [1,12], depending on the method and brain region used for analysis. Although also neurons contain considerable levels of these lipids, they are particularly enriched in the myelin sheath, with more than 30 mol% of all phospholipids and the majority of ethanolamine phospholipids in the plasmalogen form [12,13]. In the human central nervous system (CNS), the levels of plasmalogens are relatively low at birth, but increase strongly during early development [14], concomitant with myelination [15]. Early studies implied that the amounts of plasmalogens in the human brain rise until the age of 30 years and then start to decline [16]. Analyses of plasmalogen levels in the peripheral nervous system (PNS) have mainly been restricted to the sciatic nerve, which, due to its extensive myelination, shows similarly high levels as brain white matter [12].
In the present review, we summarize the current knowledge on the biological roles of ether phospholipids in nervous tissue. Based on the phenotypic traits in the nervous system of human patients and animal models with ether lipid deficiency, we recapitulate the main cellular functions of these lipids in the search of explanations for the observed phenotypes. Lastly, we highlight potential roles of ether lipids in more common neurological diseases and discuss recent approaches to treat ether lipid deficiency.
Nervous system pathology in human ether lipid deficiency
In man, the inherited deficiency in ether lipid biosynthesis results in a rare (estimated incidence: 1 : 100 000) and fatal disease, rhizomelic chondrodysplasia punctata (RCDP). Currently, five types of RCDP, differing in the affected gene, are known. Type 1 (MIM #215100), the by far most common subtype, is caused by mutations in the peroxin (PEX) 7 gene [17–19], which encodes the cytosolic receptor for proteins containing a peroxisomal targeting signal (PTS) 2. Upon PEX7 deficiency, ADHAPS is not imported into the peroxisome leading to loss of its function. The other RCDP types are evoked by mutations in GNPAT (type 2, MIM #222765) [20], AGPS (type 3, MIM #600121) [21], FAR1 (type 4, MIM #616154) [22], and PEX5 (type 5, MIM #616716) [23]. The latter subtype represents a peculiarity, as it only affects the isoform PEX5L, a long splice variant of the PEX5 protein. PEX5L can also mediate peroxisomal import of PTS1-containing proteins [24,25] but is dispensable here providing the shorter PEX5 isoform is intact. However, it is essential as a coreceptor for PEX7 in the import of proteins relying on PTS2 [26] and, therefore, a defect in PEX5L has similar consequences as PEX7 deficiency.
Inborn ether lipid deficiency is also a secondary feature of peroxisome biogenesis disorders of the Zellweger syndrome spectrum, in which all peroxisomal functions are impaired due to deficient biogenesis of the whole organelle and whose nervous system phenotype has been reviewed extensively elsewhere [27]. Formally, RCDP types 1 and 5 belong to the peroxisome biogenesis disorders, as not only ether lipid biosynthesis but also peroxisomal α-oxidation [28] and, in some tissues, peroxisomal β-oxidation [29] are compromised by the dysfunctional PTS2 protein import. However, due to the comparatively late onset of symptoms in α-oxidation deficiency (Refsum disease), the phenotype of RCDP type 1 and 5 patients can be with some rare exceptions ascribed exclusively to the deficiency in ether lipids. RCDP types 2–4 belong to the group of peroxisomal single enzyme or transporter deficiencies and result in similar phenotypes. Merely, the few patients described to suffer from the recently discovered RCDP types 4 and 5 display a somewhat milder phenotype compared with types 1–3. The clinical hallmarks of RCDP are rhizomelia, a shortening of the proximal long bones, and chondrodysplasia punctata, a type of skeletal dysplasia. Other typical symptoms involve congenital cataracts and joint contractures as well as profound growth and developmental retardation. In general, disease severity shows good inverse correlation with the residual activity of the affected enzyme and, therefore, the plasmalogen levels. Although the severe form of RCDP is usually lethal within the first years of life, patients with a less severe (mild or intermediate) disease course may reach adolescence; these do not display the full spectrum of classical symptoms, and often have modestly reduced plasmalogen levels [30,31].
The nervous system is severely affected in RCDP patients with frequent intellectual disabilities and motor impairment. Developmental deficits manifest in microcephaly, and head growth ceases at 3 years of age in cases with particularly low plasmalogen levels [32]. A large proportion of patients develop epileptic seizures of varying type and frequency [33]. Main pathologic features commonly described in severe RCDP cases include myelination deficits, enlarged ventricles and subarachnoidal spaces, and cerebellar atrophy. White matter signal abnormalities, indicating myelin deficiency, have been detected by magnetic resonance imaging in different brain regions and often involve the parieto-occipital region [34–36]. The exact origin of impaired myelination is largely unclear. Cerebellar atrophy is usually progressive and presumably due to loss of Purkinje cells [37]. In individual cases, disturbances in neuronal migration have been reported resulting in polymicrogyria [38], pachygyria [39,40], agenesis of the corpus callosum [41], or dysplastic olivary bodies [42].
Spastic paresis is a frequent symptom in RCDP [43]. Hypotonia has been sporadically observed [22,44] but is not a common feature [43]. Peripheral neuropathy and retinitis pigmentosa were diagnosed in several atypical RCDP type 1 cases with a milder disease course that strikingly mimic Refsum disease, the disorder resulting from defects in peroxisomal α-oxidation [45–47]. Thus, the accumulation of phytanic acid, the main substrate for peroxisomal α-oxidation, is a likely contributor to the pathogenesis in these patients. In patients with the severe form of RCDP, stenosis of the cervical canal is prevalent [34,48]. Other commonly reported skeletal abnormalities of the spine include coronal clefts, likely caused by ossification defects, and scoliosis [22,49].
Lessons from animal models of ether lipid deficiency
Animal models are commonly used tools to unravel the pathomechanisms underlying human disorders. Here, we review the nervous system phenotype of animal models that are currently in use to study ether lipid deficiency.
Mouse models
Several widely used and well characterized mouse models of ether lipid deficiency have been generated by targeted inactivation of one of the genes encoding biosynthetic enzymes. Three different knockout (KO) mouse strains with complete ether lipid deficiency genetically mimic types 1, 2, and 3 of RCDP: Pex7 [50], Gnpat [51], and Agps [52] KO mice, respectively, of which the first two have been more extensively described. Whereas, similarly to the human disease, Pex7 mutations also affect α-oxidation (and potentially other pathways involving yet unknown PTS2-containing proteins), Gnpat and Agps KO mice represent models of isolated ether lipid deficiency.
The major pathological features in the CNS of RCDP patients are well reflected in these KO mouse models. Involvement of the cerebellum is typical of RCDP. Accordingly, investigations in Gnpat KO mice have revealed morphological alterations at early post-natal time points, particularly impaired cerebellar foliation and poorly developed fissures [53,54], which have been suggested to persist in some but not all animals over time [54]. The detailed results of these studies might depend on contributions from the genetic background of the mice used, as different strains vary considerably in their cerebellar foliation pattern [55]. The migration of granule cell precursors from the external granule layer is delayed in the Gnpat KO cerebellum [53]; a finding that is complemented by the observation of an increased number of apoptotic cells in the external granular layer [54]. Furthermore, Purkinje cells, the major output neurons of the cerebellar cortex, show an increased density of spines and structurally altered innervation by parallel fibers (originating from granule cells) and climbing fibers (projecting from the inferior olivary nucleus) in Gnpat KO mice. The Purkinje cell axon often features signs of neurodegeneration in the form of axonal swellings that are filled with smooth ER-like membranes of enigmatic origin [53].
Also another pathological hallmark of RCDP brains, hypo- and dysmyelination, has been well documented in the Gnpat KO brain, particularly in the neocortex, cerebellum, and corpus callosum as well as in optic nerve [53,55]; but in contrast to a conditional mouse model of peroxisome biogenesis disorders (Nestin-Pex5 KO), progressive demyelination was not observed [57]. In the cerebellum, hypomyelination ameliorates with age, but is still prominent, particularly in folium VI, by postnatal day 45 [53]. At the protein level, the amount of myelin basic protein was found to be reduced by 40–60% in affected areas [53,57].
Apparently, inflammatory processes do not play a major role in young ether lipid-deficient mice (up to 5 months), as indicated by the absence of microgliosis and only mild astrogliosis and axonal loss, when compared with the situation in the conditional Pex5 KO mice [57]. However, our own observations indicate marked microglia activation in white matter tracts of Gnpat KO mice at 7 months of age (S. Forss-Petter & J. Bauer, unpublished observation). Neuroinflammation in the CNS is much more pronounced upon combined defects in ether lipid biosynthesis and peroxisomal β-oxidation (Pex7/Abcd1 double KO) [58]. Recently, a thorough investigation of PNS (sciatic nerve) myelin in ether lipid-deficient (Gnpat and Pex7 KO) mice revealed reduced thickness of the myelin sheath and a defect in the process of radial sorting, by which axons destined for myelination are separated from larger axon bundles, at postnatal day 5 [59]. Even though myelin thickness catches up in young adult mice, plasmalogen-deficient myelin appears less stable and Schwann cells, the myelinating cells of the PNS, show a reduced ability to remyelinate axons after sciatic nerve injury. In the PNS of aged (1.5 years old) mice, axonal loss and demyelination are pronounced and accompanied by partly abnormal Schwann cell morphology [59]. The myelination phenotype in the CNS and PNS of ether lipid-deficient mice manifests in reduced conduction velocities in the corpus callosum [53] and in motor nerves [59,60], respectively.
Mild impairment of neuronal migration was observed in the neocortex of Pex7 KO embryos at embryonic day 18.5 (E18.5) [50], but could not be confirmed in Gnpat KO mice [54]. Next to background strain differences, additional defects in Pex7 mice (as opposed to the isolated ether lipid deficiency in Gnpat KO mice), related to peroxisomal α-oxidation or still unknown proteins depending on PEX7 for peroxisomal import [61], might play a role in these discrepant findings.
Furthermore, Pex7 KO mice have been described to suffer from hypotonia, a condition that may or may not have a neurologic cause. Hypotonia has not been reported in single Gnpat KO mice, but was severe in mice with combined deficiency of Gnpat and Hsd17b4 (encoding multifunctional protein 2, an enzyme involved in peroxisomal fatty acid β-oxidation) [54]. Recently, our own studies have unraveled a defect also at the level of the neuromuscular junction of Gnpat KO mice. In addition to abnormal nervous innervation of the diaphragm muscle, characterized by exaggerated phrenic nerve sprouting and widening of the endplate zone, we also identified alterations in neuromuscular transmission [62].
In terms of behavior, the combined CNS and PNS pathologies of Gnpat KO mice results in impaired motor performance and muscular strength [53,62] as well as hyperactivity in the open field paradigm (F. Dorninger, G. Zeitler, C. Pifl, S. Forss-Petter & J. Berger, unpublished observation). Further behavioral evaluation, for example, of cognitive function would be an interesting addition to these findings. However, reliable studies of behavior are hindered by the fact that the visual system of ether lipid-deficient mice is strongly impaired in all KO mouse models, including bilateral congenital cataracts. In addition, detailed studies in Gnpat KO mice have identified a variety of morphological lens abnormalities and developmental ocular defects, including microphthalmia, a persistent hyaloid artery (Bergmeister’s papilla), dysgenesis of the anterior eye chamber, and optic nerve hypoplasia [51,56], as well as abnormal angiogenesis resulting in an aberrant vascular network [63].
In addition to the KO mice with complete deficiency, several models of partial ether lipid deficiency exist. Mice with an inducible knockdown of AAD-HAPR (PexRAP-iKO mice) have been used for immunological studies [64], but their nervous system has not yet been characterized. Two further mouse models with residual plasmalogen levels represent the milder forms of RCDP: Pex7 hypomorphic mice [65] and bs2 mice (carrying a spontaneous hypomorphic mutation in the Agps gene) [66]. As expected due to the residual ether lipid levels, neuronal migration is normal in blind sterile 2 (bs2) mice. Both hypomorphic models, like the null mice, develop cataracts [65,66], but currently no additional information is available on the nervous system status of these animals. However, a careful characterization would be of major significance in order to obtain additional knowledge on how reduced, but not completely abolished, levels of ether lipids influence the CNS and PNS. Valuable insights into the role of ether phospholipids in the nervous system may also be gained from cell type- or brain region-specific KO or knockdown models, which have yet to be generated.
Other animal models
Animal models of isolated ether lipid deficiency have been largely restricted to mice. Purely ether lipid-deficient variants of Caenorhabditis elegans have been reported [67–69] but, so far, no information has been provided on the potential role of ether lipids in their nervous system. Strikingly, the capability of ether lipid-deficient worms to develop into adults differs remarkably between studies. Although earlier reports using an RNA interference (RNAi) strategy against Agps described an arrest mainly at the larval stage [69,70], another study did not find any major impairment of development and viability was only reduced upon lower temperatures after chemical mutagenesis of different genes involved in ether lipid biosynthesis (including Agps) [67]. Although this discrepancy might be associated with differences in background strain and the mode and time point of genetic intervention, the differences are striking and the molecular basis for this outcome would be of general interest.
Organisms with an inability to form peroxisomes cannot synthesize ether lipids, either. In recent years, models of generalized peroxisome deficiency have been generated in Mus musculus (mouse, for example [71,72]), Drosophila melanogaster (fruitfly [73,74]), C. elegans (roundworm [69,75]), and Danio rerio (zebrafish [76]). However, due to the multiplicity of other metabolic disturbances in these animals, it is impossible to assign an individual phenotypic alteration or pathology in the nervous system to the defect in ether lipid biosynthesis. Therefore, we refrain from discussing these models here and may refer the reader to another excellent review handling this issue [77].
Molecular basis and potential mechanisms underlying the phenotypic traits of ether lipid deficiency
The molecular mechanisms causing the nervous system pathology in ether lipid-deficient individuals are largely enigmatic. Therefore, we will next discuss the main biological functions that have been documented or proposed for ether lipids and try to envision how the loss of these functions and/or attempts of compensation might contribute to the nervous system abnormalities observed upon ether lipid deficiency.
The importance of plasmalogens as membrane constituents
Ether lipids, predominantly plasmalogens, are major components of the plasma membrane and various organellar membranes, in which they shape the physicochemical properties of the lipid bilayers. Plasmalogens exhibit biophysical properties, which distinguish them from other phospholipids. Model membranes composed of PlsEtn undergo the transition from gel to liquid-crystalline phase at lower temperatures than membranes made up of the corresponding diacyl phospholipids [78]. Fluorescence anisotropy studies indicate that the membranes of plasmalogen-deficient cells are more fluid and less ordered than those of control cells, implying that plasmalogens confer increased order and decrease lipid mobility [79]. Membranes consisting of plasmalogens had slightly reduced thickness in molecular dynamics simulations and the vinyl ether bond causes increased ordering and denser packing of the sn-1 and sn-2 chains as compared with diacyl phospholipids [80,81]. In addition to the vinyl ether bond, the fatty acyl chain at sn-2 by itself, with the increased occurrence of polyunsaturated fatty acids (PUFAs) in plasmalogens, has an impact on the biophysical role of these phospholipids and, thus, on membrane properties [82]. Another important aspect of plasmalogens in membrane biology lies in their ‘fusogenic’ activity. The hypothesis that these lipids favor membrane fusion and fission is derived from the fact that plasmalogens have an increased propensity to undergo the transition from a nonlamellar to a hexagonal phase [78,83]. Accordingly, plasmalogen-containing vesicles or membranes show an increased tendency toward fusion [84] and have reduced surface tension [85], implying an important role in various physiological processes, in particular, those relying on exo- and endocytosis [86]. Furthermore, it has been proposed that not only the plasmalogens themselves but also their lyso-derivatives are important players in membrane fusion and phase transition [87].
In line with the importance of plasmalogens for membrane properties, their deprivation strongly impairs cholesterol trafficking [88,89] resulting in accumulation of free cholesterol but reduced levels of esterified cholesterol in ether lipid-deficient mouse and human fibroblasts as well as Chinese hamster ovary (CHO) cells [44,51,88]. Interestingly, plasmalogen levels also seem to have a direct effect on cholesterol homeostasis, as recently shown by an inverse correlation between the amount of plasmalogen and the extent of cholesterol biosynthesis [90].
Moreover, plasmalogens have been described to be enriched in so-called lipid rafts (also termed membrane rafts) [91], which are small and heterogeneous membrane domains that are highly dynamic and compartmentalize cellular processes like, for example, signal transduction [92]. These domains were originally defined via their resistance to detergents [93]. Interestingly, detergent-resistant membranes isolated from the brains of ether lipid-deficient mice showed abnormal levels of marker proteins like flotillin and disturbed migration in a sucrose gradient [51]. Similarly, caveolae, small membrane compartments facilitating endo- and exocytosis, as well as clathrin-coated pits, are morphologically altered and reduced numerically in fibroblasts derived from ether lipid-deficient human patients in comparison with those of healthy controls [44]. Consistent with these results, also the rate of endocytosis proved decreased.
When considering how these biochemical findings apply to the nervous system, numerous processes come to mind that could be affected by the altered membrane lipid composition evoked by ether lipid deficiency. Basically, every membrane-associated event could be modulated in some way, which has prompted speculation on the molecular basis of the neurologic phenotype of ether lipid-deficient mice and men. However, aside from the recently reported impairment of AKT signaling due to deficient recruitment of AKT kinase to the plasma membrane (see The versatile functions of ether lipids in signaling), detailed experimental data on specific molecular mechanisms are lacking.
The synaptic vesicle cycle, which orchestrates the release of neurotransmitters from synaptic vesicles and allows communication between neurons, comprises a series of membrane-associated steps that have long been subject to hypotheses in the context of ether lipid deficiency. The lipid environment plays an important role in the synaptic vesicle cycle. Lipid bilayers and their interplay with the involved proteins provide the framework for endo- and exocytotic processes and the detailed molecular composition of membranes regulates the dynamics of fusion events [94]. Plasmalogens were shown to be a major constituent of the membranes of synapses [95] as well as synaptic vesicles [96], which—together with their proposed role in membrane fusion and constriction processes—has led to speculations about an essential role of plasmalogens in the synaptic vesicle cycle and, thus, in neurotransmission [10,56,97–99]. Another link between plasmalogens and the synaptic vesicle cycle involves lipid rafts. These plasmalogen-rich microdomains are presumed to be responsible for sorting and sequestration of the soluble N-ethylmaleimide-sensitive receptor attachment protein receptor (SNARE) proteins [100,101], which mediate synaptic vesicle exocytosis, and to provide the environment for many different postsynaptic receptors [102,103] and to play a role in synaptic plasticity [101,104]. Moreover, not only the biophysical properties of the plasmalogen backbone (i.e., the vinyl ether bond) but also their enrichment in PUFAs has to be considered. Arachidonic acid (AA), which is particularly enriched in nerve growth cones [105], and docosahexaenoic (DHA) are abundant in synaptic membranes, rendering these highly dynamic [94]. They modulate the endocannabinoid system, an important regulator of synaptic function, and, possibly, the assembly of SNARE proteins [106–108].
The impact of ether lipids, or the lack thereof, on neurotransmission in vivo has not yet been fully elucidated. A study by the group of Wilhelm Just using synaptosomes isolated from Gnpat-deficient mice has provided first clues: While calcium-dependent neurotransmitter release was decreased in ether lipid-deficient nerve terminals, calcium-independent transmitter outflow was increased [109], which may derive from a reversal in ion transport [110]. In addition, ether lipid-deficient synaptosomes exhibited decreases in the rate of respiration and ATP levels [109] probably impacting energy-dependent processes like the vesicular uptake of neurotransmitters.
Also, our electrophysiological studies on the neuromuscular junction in ether lipid-deficient mice support a role of ether lipids in vesicular exocytosis at the synapse [62]. Here, we identified a reduced spontaneous fusion rate of vesicles in Gnpat KO mice. Also in line with the hypothesis of reduced fusion capacity under conditions of ether lipid deficiency, the quantal content after electrical stimulation was decreased, indicating that a lower number of vesicles fuse in each release event. Furthermore, we discovered abnormal clustering of acetylcholine receptors in ether lipid-deficient myotubes differentiated in vitro, possibly associated with altered lipid raft properties evoked by ether lipid deficiency.
Plasmalogens have also been proposed to contribute to the structure of myelin, the highly specialized membrane of oligodendrocytes. This is supported by the relative enrichment of PlsEtn in white matter compared with gray matter [12]. Specifically, plasmalogens may ensure membrane compaction. This hypothesis is based on the rigidity and dense packing conferred by the vinyl ether bond [80,111] and the preferred esterification of saturated or monounsaturated fatty acids to the sn-2 position in white matter tissue [112,113].
Interestingly, also seminolipid-like sulfated glycoglycerolipids of the alkylacyl type (i.e., containing an ether bond) were detected in the brain of adult rats, but upon aging the levels decreased [114]. Based on their temporal occurrence during development, parallel to plasmalogens, a role of these lipids (along with sulfatides) in myelination was suggested but apparently never investigated in further detail.
The versatile functions of ether lipids in signaling
It has repeatedly been shown that PUFAs are enriched at the sn-2 position of plasmalogens [12,115,116]. In the nervous system, particularly gray matter plasmalogens are rich in PUFAs [97]. These fatty acids can be easily released from phospholipids by the action of phospholipase A2 (Fig. 1A), for which a plasmalogen-selective, calcium-independent subtype has been identified and purified [117]. Among the PUFAs, AA (ω-6) and DHA (ω-3) are of particular biological and physiological importance. In mammals, they can be biosynthesized only through the metabolization of essential precursors. Consequently, mammals rely on the dietary intake of AA and DHA or their precursors linoleic acid and α-linolenic acid, respectively. Both PUFAs are vital for brain development and, especially DHA, for normal neural functioning [118]. AA serves as a precursor for a variety of inflammatory mediators like leukotrienes, lipoxins, epoxyeicosatrienoic acids, thromboxanes, and prostaglandins. DHA in turn can be metabolized to resolvins, protectins, and maresins, all of which have immunomodulatory effects as well [119]. Generally, ω-6 and ω-3 PUFAs are often portrayed as antagonistic players with ω-6 and ω-3 PUFAs (and their metabolites) adopting proinflammatory and anti-inflammatory functions, respectively [120], although this view may be oversimplified.
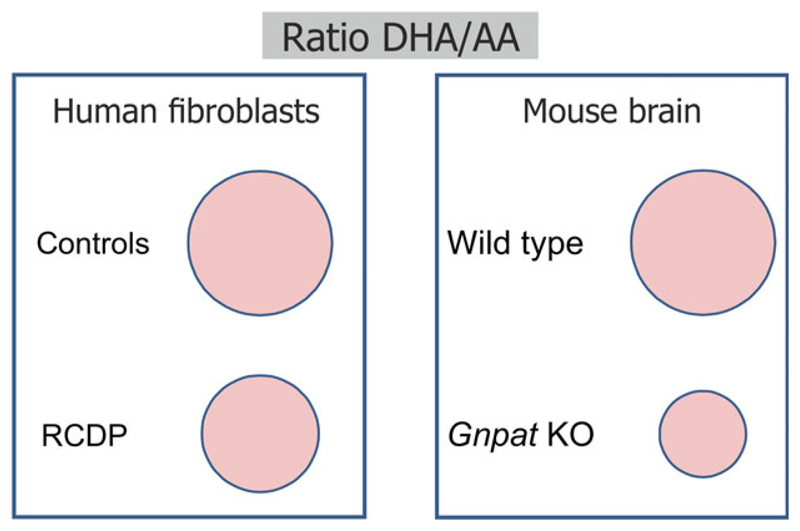
Gnpat KO mice show a defect in retinal vascularization, presumably due to erroneous astrocyte template formation [63]. Based on the similarity of this phenotype and that of a mouse model with inhibited calcium-independent phospholipase A2, the authors hypothesized that PUFAs released from plasmalogens are essential for proper development of the retinal vasculature [63,121]. In neocortex and hippocampus of Gnpat KO mice, however, ether lipid deficiency does not lead to a general loss of PUFAs. Instead, there is a shift from DHA- to AA-containing phospholipids resulting in reduced amounts of DHA in total ethanolamine phospholipids (Fig. 2) [51,122], which may have physiological consequences on its own. DHA is a highly versatile compound that has attracted considerable attention, particularly in the field of neuronal development and memory formation. A role of this PUFA has been suggested in various molecular events regulating neurite outgrowth, neuro-, and synaptogenesis, as well as synaptic plasticity and learning- and memory-related processes [123]. Based on the available data, the DHA depletion in ether lipid-deficient brains is relatively modest and has arguably less drastic effects than complete deprivation of DHA (or all ω-3 PUFAs; reviewed in ref. [124,125]), as described in many studies investigating the biological relevance of DHA. However, a moderate global DHA deficit and the loss of DHA-containing plasmalogens as a source of substrates for plasmalogen-specific phospholipase A2 may be additional puzzle pieces that modulate the pathogenic events under conditions of complete or partial ether lipid deficiency in the nervous system.
Fig. 2.
Shift from DHA- to AA-containing ethanolamine phospholipids under conditions of ether lipid deficiency. The ratio between DHA- and AA-containing ethanolamine phospholipids in human fibroblasts of healthy controls and RCDP patients (left) and in gray matter-enriched brain tissue of adult wild-type and ether lipid-deficient (Gnpat KO) mice (right) is indicated by circles drawn to scale. Upon ether lipid deficiency, the diminished levels of DHA-containing ethanolamine phospholipids are generally compensated by an increase in AA-containing species. The diagrams are based on the data published in ref. [122].
Increasing evidence indicates that several key signal transduction pathways are impacted by the lack of ether lipids. Recent reports have shown that phosphorylation of both AKT (protein kinase B) [59] and extracellular signal-regulated kinase (ERK; a mitogen-activated protein kinase, MAPK) [126] is reduced in nervous tissue of ether lipid-deficient mice. In Schwann cells, the defect in ether lipid biosynthesis was demonstrated to impair the recruitment of AKT to the plasma membrane thereby inhibiting its proper phosphorylation, which in turn causes aberrant phosphorylation and overt activation of the downstream kinase glycogen synthase kinase (GSK) 3β and finally impaired myelin formation and maintenance [59]. However, a recent study of mice with Schwann cell-selective KO of Pex5 did not reveal such abnormalities in myelination [127] suggesting that the inherent defect in Schwann cells is only one aspect of the myelination problem and that also interaction with other compartments (e.g., axons) with additional disturbances is involved in mice with global ether lipid deficiency. Another study found decreased phosphorylation of both AKT and ERK phosphorylation after injection of lentiviral shRNAs against the Gnpat mRNA into the cerebral cortex of mice [126]; while exogenous supplementation with plasmalogens in cultured neuronal cells was suggested to enhance AKT and ERK phosphorylation [128] via the induction of G protein-coupled receptors [129]. AKT as well as MAPK signaling play indispensable roles in nervous system development and function [130–133]. However, future studies will be required to establish to what extent different cell types are affected by the reported defects and the impact on specific downstream targets. Nevertheless, these findings open interesting new perspectives in plasmalogen research and may enable the dissection of crucial molecular networks underlying the phenotypes in ether lipid-deficient individuals.
Most of the phenotypic traits of ether lipid-deficient patients and animal models have been attributed to the lack of plasmalogens in recent literature. Still, particularly in the context of signal transduction, a variety of other ether lipid compounds may contribute to pathology. The release of the fatty acyl group from the sn-2 position of plasmalogens does not only produce free fatty acids, which can mediate signaling functions but also lysoplasmalogens (Fig. 1B), which can also serve as second messengers. Lysoplasmalogens with an ethanolamine head group have the potential to act as self antigens in the stimulation and maturation of semi-invariant natural killer cells [134]; those with a choline head group are able to induce increased adherence of neutrophils to human endothelial cells [135], possibly by activating a cAMP-dependent protein kinase [136]. The current knowledge on the functions of lysoplasmalogen in the nervous system is limited, but presumably some roles in signaling apply as well here.
The results of several studies have implicated an involvement of PAF (Fig. 1C) and its receptor in the processes of synaptic transmission and synaptic plasticity associated with long-term potentiation (LTP). In details, however, the reports are conflicting. PAF has been suggested to induce or enhance LTP by acting as a retrograde messenger that is released from postsynaptic neurons and enhances presynaptic release [137–140], likely through the activation of several protein kinases [141]. PAF is also able to stimulate ATP release from PC12 cells and causes influx of calcium in PC12 cells and hippocampal neurons in vitro [142,143]. These observations fit the finding that LTP is weakened in hippocampal slices from mice with a deficiency in the G protein-coupled PAF receptor [144]. In line with these data, the infusion of a PAF analog into several brain regions improved the performance of mice in different memory-related tasks; whereas conversely, application of a PAF antagonist impaired memory function [145]. In stark contrast, other authors found no consequence of PAF receptor deficiency for hippocampal LTP [146] or even a negative effect of PAF on LTP mediated by tyrosine kinases [147]. However, different models and different PAF concentrations were used in these studies, and might have caused the contradictory findings, with low concentrations having a stimulating effect on LTP and high concentrations being rather inhibitory [147].
Similar to its role in peripheral tissues and in the circulation, under pathological conditions, PAF promotes inflammatory responses in the nervous system [148] and may induce neuronal apoptosis in both PAF receptor-dependent [149] and -independent ways [150]. Accordingly, PAF receptor inhibition or deficiency protects from neuroinflammation in different disease models [151,152].
In addition, PAF has been ascribed a role in brain development. PAF receptor activation has the ability to inhibit neuronal migration in vitro [153], and mice with a heterozygous deletion in the β-subunit of PAF acetylhydrolase, the enzyme that releases the acetyl group of PAF to produce lyso-PAF (Fig. 1D), display aberrant neuronal layering in several brain regions [154]. In contrast, PAF receptor KO mice do not exhibit major brain malformations [155] suggesting that either PAF receptor-independent actions of PAF or additional, PAF-unrelated functions of the acetylhydrolase affect neuronal migration. However, subsequent, more detailed studies of PAF receptor KO mice revealed a thickened external granular layer in the cerebellum and decreased migration capability of cerebellar granule cells in vitro and in vivo [156]—a phenotype that is also reproduced in Gnpat KO mice [53]. The regulation of the levels of PAF and its downstream actions, thus, seem to be important modulators of neuronal development.
Also some less abundant ether-linked lipids have been described to mediate signaling. Plasmanyl phospholipids (containing an ether, but not a vinyl ether bond; Fig. 1E) serve as ligands for the nuclear peroxisome proliferator-activated receptor (PPAR) γ [157]. Alkenyl-lysophosphatidic acid (alkenyl-LPA; 1-O-cis-alk-1′-enyl-2-lyso-sn-glycero-3-phosphate; Fig. 1F) elicits activation of the MAPKs ERK1 and ERK2 in 3T3 mouse fibroblasts [158]; and alkyl-LPA (Fig. 1G) participates in the activation of semi-invariant natural killer cells (similarly as lysoplasmalogens) [134]. Alkyl-LPA has been detected in considerable amounts in rat brain [159] and appears to be able to activate microglia via the LPA5 receptor [160,161].
From a signaling perspective, ether lipid deficiency may, thus, have opposing effects on inflammatory processes in the nervous system (and elsewhere). On one hand, plasmalogen deficiency leads to an increased ratio between AA and DHA (Fig. 2), likely shifting the immunological balance in the proinflammatory direction [122]. Moreover, exogenously applied plasmalogens were reported to prevent serum starvation-induced neuronal apoptosis in cell culture experiments [128,162] and to ameliorate systemically induced neuroinflammation in mice, when coadministered intraperitoneally with lipopolysaccharide [163]. Conversely, plasmalogen deficiency may render neurons more vulnerable to stress conditions and inflammation. One study reported signs of neuroinflammation to be largely absent in the brain of younger (up to 5 months) Gnpat KO mice [57] but microglia activation may become apparent only with advancing age (S. Forss-Petter & J. Bauer, unpublished observation). However, we do not exclude that in constitutive KO models adaptive compensatory processes may occur, as we have previously argued in the case of neutropenia, which seems to be present in an inducible KO model of ether lipid deficiency but not in (constitutive) Gnpat KO mice [64,164]. Acute reduction in plasmalogen levels, as evaluated after in vivo knockdown of Gnpat, was described to promote microglia activation and upregulation of inflammatory cytokines in adult mice [126]. On the other hand, the lack of proinflammatory mediators like PAF or alkyl-LPA may dampen the immune response and protect neurons from death signaling in the ether lipid-deficient nervous system.
Taken together, signaling alterations are highly likely to play a crucial role in the nervous system phenotype elicited by ether lipid deficiency, but many of the underlying molecular mechanisms still need to be unraveled in detail. We want to emphasize that the perception of ether lipid deficiency should not be restricted only to plasmalogens but rather expanded to comprise also less common signaling mediators that have often been neglected in the discussion.
The enigmatic role of ether lipid components in GPI-anchored proteins
A relatively new addition to the proposed roles of ether lipids is emerging from the discovery of an ether-bonded lipid derivative in the glycosyl-phosphatidyl-inositol (GPI) anchor, a post-translational modification of membrane proteins that tethers these proteins to the outer leaflet of the plasma membrane [165]. In eukaryotes, about 0.5% of all proteins are GPI-anchored [166], including prominent examples in the nervous system like the prion protein or a particular isoform of acetylcholinesterase. The detailed structure of the GPI anchor differs across species, but a conserved core is formed by a lipid part derived from phosphatidylinositol, a sequence of sugar residues and ethanolamine phosphate linking the anchor to the C terminus of the protein [167]. Remarkably, although phosphatidylinositol occurs mostly in the diacyl form, the lipid part of several GPI-anchored proteins was described already decades ago to involve 1-alkyl-2-acylglycerol (alkylacylglycerol), an ether lipid [168–170]. Later, a systematic study confirmed these findings and identified alkylacylglycerol as the dominant lipid variant in mammals, but not in other species [171,172]. The reason for the differential lipid composition of mature GPI-anchored proteins and phosphatidylinositol appears to be a remodeling step, in which diacylglycerol is exchanged for an alkylacyl moiety. The latter is derived from a peroxisomal precursor, as it has been shown that alkyl group-bearing GPI anchors cannot be formed in the absence of peroxisomal ether lipid biosynthesis [173]. The biological significance of the ether bond in the GPI anchor has not yet been identified. In ether lipid-deficient CHO cells and human fibroblasts, the alkylacylglycerol moiety is replaced by a diacyl lipid. Remarkably, in these cells, the surface levels of the GPI-anchored protein urokinase-type plasminogen activator receptor were even drastically increased in comparison with control cells [174]. However, a functional evaluation of the shift from alkylacyl to diacyl moiety is still missing. Intriguingly, these observations have only scarcely been picked up by the scientific community, likely because there is no obvious impairment due to the absence of the alkylacyl moiety in the GPI anchor. However, already subtle modifications of GPI-anchored protein function could have drastic consequences for the nervous system. Several GPI-anchored proteins are highly expressed in the nervous system, for example, cell adhesion molecules, mostly members of the immunoglobulin superfamily (like contactin 1 and neural cell adhesion molecule) [175], which are critical for neuronal growth and nervous system development. Also, ephrinA proteins, GPI-anchored ligands for receptor tyrosine kinases of the EphA family, are crucial cell–cell signaling mediators and key players in neural development, regulating such essential processes as neuronal migration, axon guidance, and synaptogenesis [176]. So far, any contribution of GPI anchor dysfunction to the pathology in ether lipid-deficient organisms remains speculative, but this issue demands further attention and may bring some valuable insights.
The controversial function of plasmalogens as antioxidants
The defense against oxidative stress was initially proposed to be a major function of plasmalogens. Experiments carried out in vitro—by different research groups—provided evidence that the vinyl ether bond protects against various stress conditions, among others exposure to UV light [177] or hypoxia [178], in vitro. Plasmalogens act as scavengers of singlet oxygen and free radicals [179] and, thus, have the capability to protect PUFAs from oxidative modification [180,181]. It was repeatedly shown that this occurs at the cost of the plasmalogens themselves, which were preferentially destroyed by the different oxidative agents; and plasmalogen-deficient cells proved less resistant to stress conditions than control cells [182,183]. Also, the findings of decreased plasmalogen levels, mostly in the circulation, in several pathological conditions that are associated with enhanced oxidative stress (e.g., hyperlipidemia or sepsis) has been used as an argument in favor of the proposed antioxidative capacity of these compounds [184,185]. In line with these considerations, a recent study using myelin isolated from Pex7 KO mice showed that plasmalogen deficiency confers increased vulnerability of myelin to oxidative attack by hydroxyl radicals in vitro, possibly due to enhanced oxidative damage of myelin membrane proteins and/or lipids; the authors concluded that plasmalogens are able to protect myelin from oxidative stress [186].
However, the role of plasmalogens as antioxidants has been repeatedly challenged: Some opponents argue that a potential antioxidative effect might be derived from PUFAs esterified to the sn-2 position of plasmalogens, rather than from the vinyl ether bond [187,188]. No protective effect of plasmalogens could be determined in cultured astrocytes exposed to lactic acid stress [189]. Furthermore, a study using primary skin fibroblasts derived from human patients with plasmalogen deficiency did not detect any difference in sensitivity to reactive oxygen species [190]. Other adversaries contend that the oxidative degradation of plasmalogens produces compounds that could themselves be harmful for the organism [187,191,192]. For example, plasmalogens were identified as a preferred target of reactive chlorinating species produced by neutrophils thereby generating α-chloro fatty aldehydes, which might promote inflammation [193,194]. One study even reported a pro-oxidative effect of plasmalogens in a bulk lipid system [195].
Analyses of the nervous system in ether lipid-deficient mice have rather supported the hypothesis that plasmalogen deficiency does not necessarily cause enhanced oxidative stress. In the cerebellum of Gnpat KO mice, the oxidative stress markers 3-nitrotyrosine and 4-hydroxynonenal were consistently detected in earlier developmental stages (3 weeks), but only occasionally in advanced ones (12 weeks and 5 months) [57]. Another study found the overall antioxidative potential to be unaltered and even decreased levels of thiobarbiturate-reactive substances (markers of lipid peroxidation) in the cortex and cerebellum of Gnpat KO mice, thus contradicting the notion of increased vulnerability resulting from chronic lack of plasmalogens [109]. However, in the same report, primary fibroblasts derived from these mice were shown to be more susceptible to oxidative damage in vitro. These findings reinforce that caution is warranted when attempting to translate the data derived from in vitro experiments to the complex situation in vivo. The role of plasmalogens in oxidative defense remains controversial and the antioxidant capacity of these compounds appears to be strongly dependent on sn-2 composition and the cellular context [188]. Therefore, based on the current state of knowledge, there is no indication that oxidative stress would be a major inherent factor in the nervous system pathology of ether lipid-deficient mice or human patients. However, it is plausible that under certain circumstances, for example, upon exogenous oxidative attack triggered by additional toxic or pathogenic events, a lack of plasmalogens could be detrimental.
Potential involvement of ether lipids in more common neurologic diseases
In addition to the inborn deficiencies in ether lipid biosynthesis, many other, often more common, diseases have been associated with ether phospholipids, for example, due to abnormal levels or composition in certain tissues of patients or animal models. Table 1 provides an overview of neurological disorders with a reported link to ether lipids and specifies the nature of the proposed associations. Many of the effects were not anticipated and open novel perspectives that may reveal potential therapeutic targets. Although some of the reported alterations and connections must still be verified and/or their clinical relevance has to be established, the plethora of reports dealing with the role of ether lipids in various neurological diseases underscores their importance for the nervous system. However, we are also aware that changes in lipid levels, like those listed in Table 1, also have their drawbacks: The reported involvement of ether lipids in many cases may not be of causative nature, but rather secondary to other pathologic processes. This applies particularly to alterations of the levels of ether lipids in peripheral blood, which often allow only limited conclusions to be drawn on the pathological mechanisms underlying neurological diseases. In addition, lipid profiles are often dependent on the diet or other lifestyle parameters and, therefore, might be population-specific.
Table 1.
List of neurologic disorders with suggested involvement of ether lipids.
| Disease | Reported involvement of ether lipids | Reference |
|---|---|---|
| Alzheimer’s disease | Reduced PlsEtn levels in postmortem brain tissue of AD patients (including both gray and white matter) | [112,198–200] |
| Increased PlsEtn levels in postmortem brain tissue of AD patients | [205] | |
| Decreased PlsCho levels in prefrontal cortex autopsy tissue of patients | [201] | |
| Decreased PlsEtn levels in plasma of AD patients and in serum of patients with cognitive impairment | [207,208,210] | |
| Decreased PlsEtn levels in brain tissue derived from mouse models of the disease (APPswe/PS1-dE9 transgenic; APPswe/tauP301L transgenic) |
[203,204] | |
| Reduced ADHAPS protein levels (but increased AGPS mRNA levels) in brain tissue of patients; dysregulation of expression of ether lipid biosynthetic enzymes in brain and cultured cells of mouse models deprived of Aβ peptides (PS 1/2 KO, APP KO, APP/APLP 2 KO) | [215] | |
| Increased activity of plasmalogen-selective phospholipase A2 in several brain regions of AD patients | [214] | |
| Reduced activity of γ-secretase upon treatment with different PlsEtn or PlsCho species in vitro | [218] | |
| Accumulation of C16:0-PAF and C16:0-lyso-PAF in posterior/entorhinal cortex of AD patients and of an AD mouse model (TgCRND8; transgenic APPswe/ind double mutation) | [223] | |
| Impaired binding of PAF to platelets of patients with AD or multiinfarct dementia | [238] | |
| Parkinson’s disease | Reduced levels of plasmalogens with C16:0 at sn-1 in plasma of PD patients | [239] |
| Reduced levels of plasmalogens in lipid rafts isolated from cortical gray matter of PD patients | [240] | |
| Reduced PlsEtn levels in serum of MPTP-treated mice (a model of PD) | [241] | |
| Relief of dyskinetic side effects of levodopa in Parkinsonian monkeys by treatment with a plasmalogen precursor | [242] | |
| Down syndrome | Reduced PlsEtn levels in frontal cortex and cerebellum of patients (but associated with a general decrease in ethanolamine phospholipids) | [243] |
| Reduced plasmalogen levels in erythrocytes of patients | [244] | |
| Schizophrenia | Elevated levels of some PlsCho and PlsEtn species in frontal cortex of patients and of a mouse model of the disease (G72/G30 transgenic) | [245,246] |
| Reduced plasmalogen levels in plasma of first episode and recurrent patients | [247] | |
| Reduced PlsEtn levels in plasma and platelets of patients; decreased PlsCho levels in plasma, but increased levels in platelets of patients | [248] | |
| Reduced levels of several plasmalogen species in fibroblasts from patients (particularly under stress conditions) | [249] | |
| Lower PlsEtn levels in erythrocyte membranes of patients, particularly in individuals with low sphingomyelin levels | [250] | |
| Autism | Genetic association between mutations or polymorphisms in PEX7 and autism spectrum disorder | [225,226] |
| Reduced plasmalogen levels in plasma and in erythrocytes of patients | [228,229] | |
| Reduced PlsEtn levels in brain of propionic acid-infused rats (a model of autism) | [230] | |
| Depression/anxiety | Reduction of several ether-bonded phospholipid species in plasma of patients with major depressive disorder | [251] |
| Inverse correlation between symptoms of depression and anxiety and plasmanylcholine C36:4 levels | [252] | |
| Genetic overlap between levels of ether-linked AA-containing choline phospholipids and major depressive disorder | [253] | |
| Epilepsy | Increased levels of PAF in rat brain upon convulsive treatment | [254] |
| Increased levels of PAF and PAF receptor overactivation as cause for neuronal circuit dysfunction in rodent models of limbic epileptogenesis | [255] | |
| Multiple sclerosis (MS) | Reduction of PlsEtn levels in demyelinated areas of the cerebral cortex in MS patients | [256] |
| Reduction of the levels of DHA-containing PlsEtn in serum of MS patients | [257] | |
| Reduction of plasmalogen levels in brain of rats with experimental autoimmune encephalomyelitis (EAE), along with reduced DHAPAT activity in brain peroxisomes and reduced Gnpat mRNA levels in the spinal cord | [258] | |
| Pelizaeus–Merzbacher disease | Reduction of PlsEtn levels in oligodendrocytes of a mouse model of the disease and, of some species, in patient lymphocytes | [259] |
| X-linked adrenoleukodystrophy (X-ALD) | Reduction of PlsEtn levels in white matter of patients with cerebral ALD (not restricted to lesions), in brains of Abcd1 KO mice and in primary mouse astrocytes upon silencing of Abcd1 and Abcd2 | [260] |
| Prion diseases | PAF as suggested mediator of prion-induced toxicity in vitro | [221] |
| Head trauma/spinal cord trauma | Increased levels of ether-bonded choline and (by trend) ethanolamine phospholipids in the hippocampus; decreased levels of ether-bonded ethanolamine phospholipids in cortex and plasma and of ether-bonded choline phospholipids in cerebellum of a mouse model of traumatic brain injury | [261] |
| Increased levels of some ether-bonded species, particularly such with choline head groups in murine optic nerve after repetitive mild traumatic brain injury | [262] | |
| Reduced PlsEtn levels in spinal cord after laminectomy and spinal cord compression in a cat model | [263] | |
| Spinal cord ischemia | Reduction of PlsEtn levels (and other ethanolamine phospholipids) in spinal cord of rabbits after ischemia | [264] |
| Ischemic stroke | Reduction of PlsEtn levels in synaptosomes from rat striatum after injection of endothelin-1 (producing an ischemia-like lesion) | [265] |
| Increased levels of PAF in blood of patients | [266] | |
| Correlation between PAF binding to platelets and infarct volume as well as neurological outcome | [267] |
APLP, amyloid precursor protein-like protein; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridin; MS, multiple sclerosis; PS, presenilin
Alzheimer’s disease
More than in any other sporadic neurological disease, a role of plasmalogen deficiency in pathogenesis is discussed in the context of Alzheimer’s disease (AD), the most abundant neurodegenerative disease and cause of dementia worldwide. AD is estimated currently to affect more than 35 million people [196] with a strong rise in the number of patients predicted [197]. Several reports have stated considerably reduced levels of PlsEtn [112,198–200] and PlsCho [201,202] in postmortem brain samples of AD patients (see also Table 1). Similarly, moderate deficits in PlsEtn were also detected in the brain of mouse models of the disease [203,204]. Interestingly, also increased levels of PlsEtn in brains of AD patients were reported in one study, but this finding has never been confirmed [205]. Decreased plasmalogen levels are not a general feature of neurodegenerative diseases, as this was not detected in brain samples of patients with Parkinson’s disease (PD) or Huntington’s disease [206]. However, to a lesser extent, it is associated with the normal aging process [16]. In AD, the deficit is present in white matter, where it appears very early in the disease course (i.e., in subjects with a clinical dementia rating of 0.5) [112], as well as in gray matter, where it correlates with cognitive decline [97] and neuropathological staging [199].
Several reports also state a reduction in some plasmalogen species in the circulation of AD patients [207,208] and, consequently, their utility as a biomarker for cognitive decline has been suggested [209,210]. Taking into account that lipid levels in the blood are considerably influenced by external factors like diet, lifestyle, medications, or age and that the observed differences in plasmalogen levels are subtle, the putative use of plasmalogens as a single prognostic or diagnostic marker should be treated with caution. However, individual plasmalogen species may be valuable as components of a biomarker panel, as proposed recently [211].
The origin of plasmalogen depletion in AD is so far unresolved: A generalized dysfunction of peroxisomes evoked by aging in general or by AD pathology could lead to impaired plasmalogen biosynthesis [199,212,213]. In particular, the preferential degradation of these lipids under oxidative stress, a condition also found in AD [97], might account for the observed changes. Others suggest that amyloid-beta (Aβ) is responsible for the plasmalogen reduction, either by stimulation of plasmalogen-selective phospholipase A2 [214] or by destabilization of the ADHAPS protein, thus limiting plasmalogen synthesis [215]. Alternatively, based on the neuroinflammation present in AD [216] and results obtained in an AD mouse model, a recent report claims that the inflammatory response downregulates Gnpat expression via NF-κB signaling and c-Myc binding to the Gnpat promoter [126].
It is not clear whether the plasmalogen deficits represent a secondary phenomenon or play a causative role in the disease process and further aggravate the disease symptoms. In line with the proposed role of plasmalogens as radical scavengers, their depletion might amplify the oxidative stress burden in the AD brain. However, we may again emphasize the controversy around the role of plasmalogens in oxidative stress prevention and defense (see The controversial function of plasmalogens as antioxidants). Other authors hypothesize that the elevated activity of plasmalogen-selective phospholipase A2 stimulates the production of lysoplasmalogens, resulting in excessive vesicular fusion and eventually causing synaptic failure [104]. Furthermore, signaling defects resulting from plasmalogen reduction (like those discovered in the ERK and AKT pathways) may cause additional damage in AD pathology, as suggested based on experiments involving RNAi to downregulate Gnpat mRNA levels in the cortex of mice [126]. Intriguing, in this context, is the impaired phosphorylation of AKT and, therefore, loss of inhibitory phosphorylation of GSK 3β observed in the PNS of plasmalogen-deficient mice [59]. GSK 3β is one of the kinases implicated in tau phosphorylation [217]; and increased GSK 3β activity upon plasmalogen deficiency may well contribute to the characteristic hyperphosphorylation of tau protein in AD.
In addition, plasmalogens may have a direct effect on the production of Aβ peptides. A recent study showed that in purified membranes from neuronal cells, supplementation with plasmalogens stimulates the activity of α-secretase, which releases nontoxic fragments of the amyloid precursor protein (APP), and restricts the activity of γ-secretase, which—in concert with β-secretase—is responsible for the formation of the Aβ peptides that aggregate in amyloid plaques, a hallmark of AD brains [218]. Reduced levels of plasmalogens could therefore promote the formation of Aβ leading to the assembly of plaques in AD. However, our own results from the APPswe-PS1dE9 double transgenic AD mouse model on a Gnpat KO background show no increase in amyloidogenic Aβ plaque or peptide levels and, thus, do not support the concept that ether lipid deficiency amplifies Aβ production or deposition (S. Forss-Petter & J. Bauer, unpublished observation). Furthermore, cleavage of APP has been suggested to take place in lipid rafts [219]. Therefore, the potential disturbance of these membrane domains caused by the lack of plasmalogens may also influence the generation of Aβ peptides.
Docosahexaenoic acid deficits may play a role in AD, as elaborated in recent reviews [106,123]; and given that ether lipid deficiency also causes a decline in brain DHA levels in mice [51,122], it is tempting to speculate that the decrease in ether lipids in AD contributes to the pathogenic processes in part also via DHA depletion. However, considering the relatively modest plasmalogen reduction in AD (as compared with complete ether lipid deficiency), the modulatory effect on DHA levels due to plasmalogen deficits may not be of major relevance for the pathogenesis in AD.
Altogether, it appears plausible that plasmalogens are a modulating factor in AD pathology and play an important role in disease progression. However, in our opinion, it is safe to say that plasmalogen deficiency is not the main cause of disease in AD. Consequently, supplementation of plasmalogens alone as a treatment strategy for AD, as proposed recently [220], may not be of major benefit for affected patients (see also Overcoming ether lipid deficiency in the nervous system).
In addition to plasmalogens, PAF might also be involved in the etiology of AD. It has been suggested previously that PAF is a mediator of Aβ-induced neurotoxicity. PAF receptor antagonists are beneficial for neuronal survival after Aβ treatment in vitro [221,222]. This is in line with more recent findings showing increased levels of specific PAF and lyso-PAF species, the latter possibly generated in attempts to get rid of excessive PAF, in the brain of AD patients and transgenic mouse models of the disease [223]. The same study found PAF but not lyso-PAF to be upregulated by Aβ exposure in a human cell culture model of terminally differentiated neurons leading to increased neuronal death, which could be rescued by inhibition of PAF signaling. In addition, PAF induced the AD-characteristic hyperphosphorylation of tau, mediated by cyclin-dependent kinase 5 [223]. Exactly how the action of PAF fits into the sequence of pathologic events in AD still remains to be elucidated.
Neurodevelopmental disorders
Already in 1999, a case report first mentioned signs of autism, a neurodevelopmental disorder involving lack of social interaction, impaired verbal and nonverbal communication, and repetitive behavioral patterns, in a patient suffering from RCDP [224]. However, most likely due to the broad range of life-threatening symptoms particularly in severe RCDP cases, autistic features did not gain much attention as potential consequence of ether lipid deficiency until, recently, a whole exome sequencing study indicated a genetic link between PEX7 and autism spectrum disorders [225]. The consortium behind that study identified a mutation affecting the WD-40 domain of PEX7 in three affected children, but not in the nonaffected siblings, of consanguineous parents. This discovery supports the findings in an earlier paper, which proposed an association between single nucleotide polymorphisms in PEX7 and autism [226]. Furthermore, retrospective screening of the clinical records of previously reported RCDP patients [227] revealed that two patients with less severe disease course had later been diagnosed with a neurodevelopmental condition (autism and attention deficit hyperactivity disorder) [225]. An additional observation linking ether lipid deficiency and neurodevelopmental disorders is the hyperactive behavior displayed by Gnpat KO mice in their home cage as well as in paradigms specifically testing for hyperactivity like, for example, the open field test (F. Dorninger, G. Zeitler, C. Pifl, S. Forss-Petter & J. Berger, unpublished observation). Moreover, the total levels of plasmalogens were found reduced by about 15–20% in the plasma of autistic patients in two independent studies [228,229] and, similarly, ethanolamine plasmalogens were decreased by about 15% in the brain of a rat model of autism [230], further corroborating the hypothesis of an association between ether lipid deficiency and autism. How ether lipids and autism spectrum disorders are connected mechanistically, is still unclear. However, in the future, raised awareness of the topic may increase the attention of clinicians for potential autistic features in RCDP, particularly in patients affected by the milder forms.
Overcoming ether lipid deficiency in the nervous system
Treatment approaches targeting ether lipid deficiency, either in the inborn peroxisomal disorders or in one of the more common neurologic diseases, are limited. Currently, treatment of RCDP is mainly symptomatic and strategies to overcome the core of the problem, that is, ether lipid deficiency, are urgently needed. Based on the severe pathology in nervous tissue of patients with peroxisomal disorders (see Nervous system pathology in human ether lipid deficiency) and the involvement of reduced ether lipid levels in neurological disorders (see Potential involvement of ether lipids in more common neurologic diseases), the success of any treatment strategy is critically dependent on the ability to ameliorate ether lipid deficiency in the nervous system. This might be achieved either by restoring ether lipids themselves or by circumventing the consequences of their shortage.
For the first strategy, the replenishment of ether lipids, treatment with alkylglycerols is an obvious candidate, as these compounds are commonly believed to have the capability to restore the full spectrum of ether lipid species. Alkylglycerols already carry an ether bond at the sn-1 position and, therefore, the peroxisomal steps of ether lipid biosynthesis are not required. Their ability to restore plasmalogen levels is well documented in ether lipid-deficient cultured cells [122,231,232]. As a logical consequence, dietary supplementation of alkylglycerols, either in the form of chimyl alcohol (hexadecylglycerol; C16:0 chain at sn-1) or batyl alcohol (octadecylglycerol; C18:0 at sn-1), was proposed as potential treatment strategy for ether lipid deficiency already in the last century. In 2006, a registered trial was started in the Netherlands to evaluate the effects of batyl alcohol in humans (ISRCTN4 4820021), but the results have not yet been published. However, earlier studies in wild-type rats indicated that alkylglycerol replaces plasmalogens in various peripheral tissues but not in the brain [233,234]. This finding was later confirmed in a systematic preclinical trial in Pex7 KO mice, which demonstrated that, upon oral batyl alcohol treatment, the levels of C18:0 plasmalogens are restored in a variety of organs, but not in the nervous system [60]. Other groups have tried oral administration of different alkylglycerol-based ether lipid precursors with DHA attached to the sn-2 position and lipoic acid at sn-3, in the hypomorphic Pex7 mouse model but, again, incorporation into the brain was modest; the ability of these substances to alleviate ether lipid deficiency in fully deficient KO animals has not been tested [235].
Hence, apparently, plasmalogens and their precursors either cannot efficiently cross the blood–brain barrier (at least not in the form, in which they are present in the blood) or brain tissue relies exclusively on its own ether lipid biosynthesis and does not incorporate exogenous ether lipids. However, the observation that upon intracerebral injections of radioactively labeled chimyl alcohol, radioactive plasmalogens appeared in the brain in a time-dependent manner [236], does not support the latter possibility.
In light of the findings suggesting that ether lipids do not overcome the blood–brain barrier, a recent report that treatment with plasmalogens isolated from scallops improves cognitive performance in a subgroup of mild AD patients is particularly puzzling [220]. Currently, it is unclear how orally administered plasmalogens would affect brain function. Therefore, until this discrepancy is resolved, claims of cognitive improvement owing to plasmalogen treatment should be considered with caution.
Besides the therapeutics aimed at restoring ether lipid levels, drugs targeting the downstream pathways affected by ether lipid deficiency may offer viable alternatives. A seminal study for such an approach recently applied inhibitors of GSK 3β, mainly lithium chloride, to treat ether lipid-deficient mice [59]. By using this strategy, which may also be relevant for the CNS, the investigators were able to reverse the downstream effects of impaired AKT signaling (see The versatile functions of ether lipids in signaling) and ameliorate the dysmyelination of peripheral nerves in Gnpat KO mice. An obvious caveat of similar approaches is that they may not restore the whole range of functions covered by ether lipids including, for example, the shaping of membrane properties. Also, some concerns have been expressed about potential adverse effects of the long-term use of GSK 3β inhibitors [237]. Nevertheless, as lithium chloride (and other specific GSK 3β inhibitors) can cross the blood–brain barrier and is already in clinical use as a mood stabilizer, the results are exciting and might bring at least some relief for ether lipid-deficient patients. In particular, if therapeutic attempts to restore ether lipids in the CNS keep failing, inhibition of GSK 3β may be the most promising pharmacological target at the moment.
Concluding remarks and perspectives
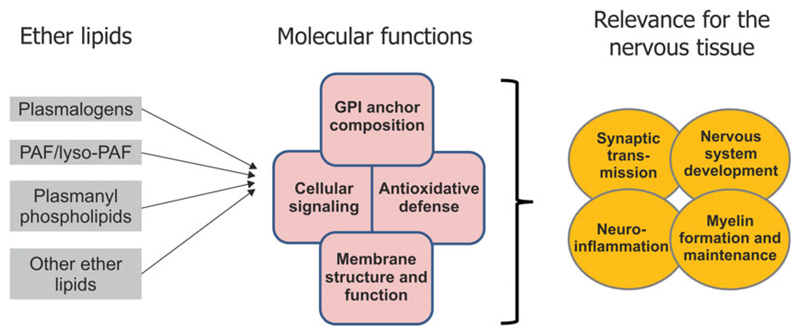
Ether lipids are a versatile group of lipids that fulfill a wide range of tasks and, thus, have the ability to shape development and function of a complex tissue like the nervous system in a number of ways (Fig. 3). As we have also pointed out in the present review, the diversity of ether lipid functions is granted by the vast number of subspecies of these lipids. Although previous research has mainly focused on plasmalogens, which certainly are of major importance for the mammalian nervous system, recent studies have demonstrated that also a number of other ether lipid species like PAF or alkenyl- and alkyl-LPAs fulfill biologically relevant tasks in the nervous system. Accumulating research has also overturned the view that ether lipids are merely antioxidants and shape membrane properties but has uncovered exciting new functions like those in crucial signaling pathways, for example, the AKT, ERK, or PPARγ pathways, or the assembly of GPI anchors.
Fig. 3.
The relevance of ether lipids for the nervous system. To the left, the main molecular functions proposed to involve ether lipids in the nervous system include: the fine-tuning of membrane properties and functions (see The importance of plasmalogens as membrane constituents), the mediation of signaling processes (see The versatile functions of ether lipids in signaling), the composition of GPI anchors (see The enigmatic role of ether lipid components in GPI-anchored proteins) and, presumably, the defense against oxidative damage (see The controversial function of plasmalogens as antioxidants). Thus, in a physiological context, ether lipids modulate crucial processes like nervous system development, myelin formation and maintenance, synaptic transmission, and neuroinflammation. As indicated by the overlap of circles or squares, these functions and activities are interdependent.
A remarkable development is the increased number of papers reporting alterations, most often reductions, of ether lipid levels in different neurological disorders, particularly in AD. Considering these findings, it will be of major importance to determine the impact of such modest changes in the levels of ether lipids. Although current lipidome analyses, which have focused mainly on plasmalogens, point to a generalized reduction of ether lipids, some ether lipid subspecies might be affected more severely. On the other hand, a slight reduction in certain species may have more drastic consequences than that of others.
Concerning the proposed association between ether lipid deficiency and neurodevelopmental disorders, subtle impairments in ether lipid synthesis that do not manifest in the severe symptoms seen in RCDP, might still influence brain circuitry and function. Complete ether lipid deficiency can cause neuronal migration defects or, at least in the mouse model, aberrant axonal branching at the neuromuscular junction. In the CNS, plasmalogen levels are relatively low at birth, but increase strongly during infancy. Thus, particularly during this early postnatal period of high demand for plasmalogen synthesis, a small reduction in these lipids might already increase the susceptibility for disturbances in postnatal fine-tuning and strengthening of neuronal interactions, thereby conferring a later risk for neurodevelopmental disorders. We expect that the progress in diagnostic techniques, like next-generation sequencing, will reveal new phenotypes evoked by moderate reductions in ether lipid levels, as this may enable the identification of patients with mutations affecting ether lipid biosynthesis that might be disregarded when using classical diagnostic strategies. Inevitably upcoming results of large-scale sequencing studies will show whether associations as described for PEX7 polymorphisms and autism will indeed be substantiated. Also, further characterization and generation of hypomorphic and cell type-specific animal models of ether lipid deficiency should provide valuable clues.
Drugs to restore ether lipid function in the brain are of utmost importance in the treatment of peroxisomal disorders and the observation that ether lipid levels are also decreased in more common disease like AD creates an additional interest in such treatment strategies. Currently, therapeutic success is still limited, but increased knowledge on lipid transport across the blood–brain barrier and lipid homeostasis in the nervous system as well as the development of novel delivery systems will hopefully pave the way to overcome ether lipid deficiency in the nervous system.
Acknowledgements
The authors are supported by the Austrian Science Fund (FWF, P24843-B24 and I2738-B26 to JB). The authors thank Markus Kunze and Christoph Wiesinger for helpful discussions.
Abbreviations
- AA
arachidonic acid
- AADHAPR
acyl/alkyl-dihydroxyacetone phosphate reductase
- AD
Alzheimer’s disease
- ADHAPS/AGPS
alkyl-dihydroxyacetone/alkylglycerone phosphate synthase
- CHO
Chinese hamster ovary
- CNS
central nervous system
- DHA
docosahexaenoic acid
- DHAP
dihydroxyacetone phosphate
- DHAPAT/GNPAT
dihydroxyacetone/glycerone phosphate acyltransferase
- ER
endoplasmic reticulum
- ERK
extracellular signal-regulated kinase
- FAR
fatty acyl-CoA reductase
- GPI
glycosyl-phosphatidyl-inositol
- GSK
glycogen synthase kinase
- KO
knockout
- LPA
lysophospatidic acid
- LTP
long-term potentiation
- MAPK
mitogen-activated protein kinase
- PAF
platelet-activating factor
- PEX
peroxin
- PexRAP
peroxisomal reductase activating PPARγ
- PlsCho
plasmenylcholine
- PlsEtn
plasmenylethanolamine
- PNS
peripheral nervous system
- PTS
peroxisomal targeting signal
- PUFA
polyunsaturated fatty acid
- RCDP
rhizomelic chondrodysplasia punctata
- RNAi
RNA interference
- SNARE
soluble N-ethylmaleimide-sensitive receptor attachment protein receptor
Footnotes
In the present review, we use the term ‘plasmalogens’, when referring to all plasmalogens irrespective of head group, and the term ‘plasmenylethanolamine’ or ‘plasmenylcholine’ for species with a specific head group.
Fabian Dorninger: 0000-0003-0852-1524
Sonja Forss-Petter: 0000-0002-1630-5422
Johannes Berger: 0000-0003-0182-2658
References
- 1.Braverman NE, Moser AB. Functions of plasmalogen lipids in health and disease. Biochim Biophys Acta. 2012;1822:1442–1452. doi: 10.1016/j.bbadis.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Honsho M, Asaoku S, Fukumoto K, Fujiki Y. Topogenesis and homeostasis of fatty acyl-CoA reductase 1. J Biol Chem. 2013;288:34588–34598. doi: 10.1074/jbc.M113.498345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Honsho M, Abe Y, Fujiki Y. Plasmalogen biosynthesis is spatiotemporally regulated by sensing plasmalogens in the inner leaflet of plasma membranes. Sci Rep. 2017;7:43936. doi: 10.1038/srep43936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Honsho M, Asaoku S, Fujiki Y. Posttranslational regulation of fatty acyl-CoA reductase 1, Far1, controls ether glycerophospholipid synthesis. J Biol Chem. 2010;285:8537–8542. doi: 10.1074/jbc.M109.083311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Datta SC, Ghosh MK, Hajra AK. Purification and properties of acyl/alkyl dihydroxyacetone-phosphate reductase from guinea pig liver peroxisomes. J Biol Chem. 1990;265:8268–8274. [PubMed] [Google Scholar]
- 6.James PF, Lake AC, Hajra AK, Larkins LK, Robinson M, Buchanan FG, Zoeller RA. An animal cell mutant with a deficiency in acyl/alkyl-dihydroxyacetone-phosphate reductase activity. Effects on the biosynthesis of ether-linked and diacyl glycerolipids. J Biol Chem. 1997;272:23540–23546. doi: 10.1074/jbc.272.38.23540. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh MK, Hajra AK. Subcellular distribution and properties of acyl/alkyl dihydroxyacetone phosphate reductase in rodent livers. Arch Biochem Biophys. 1986;245:523–530. doi: 10.1016/0003-9861(86)90245-6. [DOI] [PubMed] [Google Scholar]
- 8.Honke K. Biosynthesis and biological function of sulfoglycolipids. Proc Jpn Acad Ser B Phys Biol Sci. 2013;89:129–138. doi: 10.2183/pjab.89.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagan N, Zoeller RA. Plasmalogens: biosynthesis and functions. Prog Lipid Res. 2001;40:199–229. doi: 10.1016/s0163-7827(01)00003-0. [DOI] [PubMed] [Google Scholar]
- 10.Brites P, Waterham HR, Wanders RJ. Functions and biosynthesis of plasmalogens in health and disease. Biochim Biophys Acta. 2004;1636:219–231. doi: 10.1016/j.bbalip.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Watschinger K, Werner ER. Orphan enzymes in ether lipid metabolism. Biochimie. 2013;95:59–65. doi: 10.1016/j.biochi.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horrocks LA. Content, composition, and metabolism of mammalian and avian lipids that contain ether groups. In: Snyder F, editor. Ether Lipids: Chemistry and Biology. Academic Press; New York, NY: 1972. pp. 177–272. [Google Scholar]
- 13.Macala LJ, Yu RK, Ando S. Analysis of brain lipids by high performance thin-layer chromatography and densitometry. J Lipid Res. 1983;24:1243–1250. [PubMed] [Google Scholar]
- 14.Altrock K, Debuch H. Fatty acids and aldehydes of brain phospholipids during fetal and infant development in man. J Neurochem. 1968;15:1351–1359. doi: 10.1111/j.1471-4159.1968.tb05914.x. [DOI] [PubMed] [Google Scholar]
- 15.Balakrishnan S, Goodwin H, Cumings JN. The distribution of phosphorus-containing lipid compounds in the human brain. J Neurochem. 1961;8:276–284. doi: 10.1111/j.1471-4159.1961.tb13553.x. [DOI] [PubMed] [Google Scholar]
- 16.Rouser G, Yamamoto A. Curvilinear regression course of human brain lipid composition changes with age. Lipids. 1968;3:284–287. doi: 10.1007/BF02531202. [DOI] [PubMed] [Google Scholar]
- 17.Braverman N, Steel G, Obie C, Moser A, Moser H, Gould SJ, Valle D. Human PEX7 encodes the peroxisomal PTS2 receptor and is responsible for rhizomelic chondrodysplasia punctata. Nat Genet. 1997;15:369–376. doi: 10.1038/ng0497-369. [DOI] [PubMed] [Google Scholar]
- 18.Motley AM, Hettema EH, Hogenhout EM, Brites P, ten Asbroek AL, Wijburg FA, Baas F, Heijmans HS, Tabak HF, Wanders RJ, et al. Rhizomelic chondrodysplasia punctata is a peroxisomal protein targeting disease caused by a non-functional PTS2 receptor. Nat Genet. 1997;15:377–380. doi: 10.1038/ng0497-377. [DOI] [PubMed] [Google Scholar]
- 19.Purdue PE, Zhang JW, Skoneczny M, Lazarow PB. Rhizomelic chondrodysplasia punctata is caused by deficiency of human PEX7, a homologue of the yeast PTS2 receptor. Nat Genet. 1997;15:381–384. doi: 10.1038/ng0497-381. [DOI] [PubMed] [Google Scholar]
- 20.Wanders RJ, Schumacher H, Heikoop J, Schutgens RB, Tager JM. Human dihydroxyacetonephosphate acyltransferase deficiency: a new peroxisomal disorder. J Inherit Metab Dis. 1992;15:389–391. doi: 10.1007/BF02435984. [DOI] [PubMed] [Google Scholar]
- 21.Wanders RJ, Dekker C, Hovarth VA, Schutgens RB, Tager JM, Van Laer P, Lecoutere D. Human alkyldihydroxyacetonephosphate synthase deficiency: a new peroxisomal disorder. J Inherit Metab Dis. 1994;17:315–318. doi: 10.1007/BF00711817. [DOI] [PubMed] [Google Scholar]
- 22.Buchert R, Tawamie H, Smith C, Uebe S, Innes AM, Al Hallak B, Ekici AB, Sticht H, Schwarze B, Lamont RE, et al. A peroxisomal disorder of severe intellectual disability, epilepsy, and cataracts due to fatty acyl-CoA reductase 1 deficiency. Am J Hum Genet. 2014;95:602–610. doi: 10.1016/j.ajhg.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baroy T, Koster J, Stromme P, Ebberink MS, Misceo D, Ferdinandusse S, Holmgren A, Hughes T, Merckoll E, Westvik J, et al. A novel type of rhizomelic chondrodysplasia punctata, RCDP5, is caused by loss of the PEX5 long isoform. Hum Mol Genet. 2015;24:5845–5854. doi: 10.1093/hmg/ddv305. [DOI] [PubMed] [Google Scholar]
- 24.Braverman N, Dodt G, Gould SJ, Valle D. An isoform of pex5p, the human PTS1 receptor, is required for the import of PTS2 proteins into peroxisomes. Hum Mol Genet. 1998;7:1195–1205. doi: 10.1093/hmg/7.8.1195. [DOI] [PubMed] [Google Scholar]
- 25.Otera H, Okumoto K, Tateishi K, Ikoma Y, Matsuda E, Nishimura M, Tsukamoto T, Osumi T, Ohashi K, Higuchi O, et al. Peroxisome targeting signal type 1 (PTS1) receptor is involved in import of both PTS1 and PTS2: studies with PEX5-defective CHO cell mutants. Mol Cell Biol. 1998;18:388–399. doi: 10.1128/mcb.18.1.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunze M, Malkani N, Maurer-Stroh S, Wiesinger C, Schmid JA, Berger J. Mechanistic insights into PTS2-mediated peroxisomal protein import: the co-receptor PEX5L drastically increases the interaction strength between the cargo protein and the receptor PEX7. J Biol Chem. 2015;290:4928–4940. doi: 10.1074/jbc.M114.601575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berger J, Dorninger F, Forss-Petter S, Kunze M. Peroxisomes in brain development and function. Biochim Biophys Acta. 2016;1863:934–955. doi: 10.1016/j.bbamcr.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heymans HS, Oorthuys JW, Nelck G, Wanders RJ, Schutgens RB. Rhizomelic chondrodysplasia punctata: another peroxisomal disorder. New Engl J Med. 1985;313:187–188. [PubMed] [Google Scholar]
- 29.Schutgens RB, Bouman IW, Nijenhuis AA, Wanders RJ, Frumau ME. Profiles of very-long-chain fatty acids in plasma, fibroblasts, and blood cells in Zellweger syndrome, X-linked adrenoleukodystrophy, and rhizomelic chondrodysplasia punctata. Clin Chem. 1993;39:1632–1637. [PubMed] [Google Scholar]
- 30.White AL, Modaff P, Holland-Morris F, Pauli RM. Natural history of rhizomelic chondrodysplasia punctata. Am J Med Genet A. 2003;118A:332–342. doi: 10.1002/ajmg.a.20009. [DOI] [PubMed] [Google Scholar]
- 31.Motley AM, Brites P, Gerez L, Hogenhout E, Haasjes J, Benne R, Tabak HF, Wanders RJ, Waterham HR. Mutational spectrum in the PEX7 gene and functional analysis of mutant alleles in 78 patients with rhizomelic chondrodysplasia punctata type 1. Am J Hum Genet. 2002;70:612–624. doi: 10.1086/338998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duker AL, Niiler T, Eldridge G, Brereton NH, Braverman NE, Bober MB. Growth charts for individuals with rhizomelic chondrodysplasia punctata. Am J Med Genet A. 2017;173:108–113. doi: 10.1002/ajmg.a.37961. [DOI] [PubMed] [Google Scholar]
- 33.Bams-Mengerink AM, Koelman JH, Waterham H, Barth PG, Poll-The BT. The neurology of rhizomelic chondrodysplasia punctata. Orphanet J Rare Dis. 2013;8:174. doi: 10.1186/1750-1172-8-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bams-Mengerink AM, Majoie CB, Duran M, Wanders RJ, Van Hove J, Scheurer CD, Barth PG, Poll-The BT. MRI of the brain and cervical spinal cord in rhizomelic chondrodysplasia punctata. Neurology. 2006;66:798–803. doi: 10.1212/01.wnl.0000205594.34647.d0. discussion 789. [DOI] [PubMed] [Google Scholar]
- 35.Sztriha L, Al-Gazali LI, Wanders RJ, Ofman R, Nork M, Lestringant GG. Abnormal myelin formation in rhizomelic chondrodysplasia punctata type 2 (DHAPAT-deficiency) Dev Med Child Neurol. 2000;42:492–495. doi: 10.1017/s0012162200000918. [DOI] [PubMed] [Google Scholar]
- 36.Viola A, Confort-Gouny S, Ranjeva JP, Chabrol B, Raybaud C, Vintila F, Cozzone PJ. MR imaging and MR spectroscopy in rhizomelic chondrodysplasia punctata. AJNR Am J Neuroradiol. 2002;23:480–483. [PMC free article] [PubMed] [Google Scholar]
- 37.Powers JM, Kenjarski TP, Moser AB, Moser HW. Cerebellar atrophy in chronic rhizomelic chondrodysplasia punctata: a potential role for phytanic acid and calcium in the death of its Purkinje cells. Acta Neuropathol. 1999;98:129–134. doi: 10.1007/s004010051060. [DOI] [PubMed] [Google Scholar]
- 38.Goh S. Neuroimaging features in a neonate with rhizomelic chondrodysplasia punctata. Pediatr Neurol. 2007;37:382–384. doi: 10.1016/j.pediatrneurol.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Yakovac WC. Calcareous chondropathies in the newborn infant. AMA Arch Pathol. 1954;57:62–79. [PubMed] [Google Scholar]
- 40.Powers JM. The pathology of peroxisomal disorders with pathogenetic considerations. J Neuropathol Exp Neurol. 1995;54:710–719. [PubMed] [Google Scholar]
- 41.Itzkovitz B, Jiralerspong S, Nimmo G, Loscalzo M, Horovitz DD, Snowden A, Moser A, Steinberg S, Braverman N. Functional characterization of novel mutations in GNPAT and AGPS, causing rhizomelic chondrodysplasia punctata (RCDP) types 2 and 3. Hum Mutat. 2012;33:189–197. doi: 10.1002/humu.21623. [DOI] [PubMed] [Google Scholar]
- 42.Poulos A, Sheffield L, Sharp P, Sherwood G, Johnson D, Beckman K, Fellenberg AJ, Wraith JE, Chow CW, Usher S, et al. Rhizomelic chondrodysplasia punctata: clinical, pathologic, and biochemical findings in two patients. J Pediatr. 1988;113:685–690. doi: 10.1016/s0022-3476(88)80378-0. [DOI] [PubMed] [Google Scholar]
- 43.Klouwer FC, Huffnagel IC, Ferdinandusse S, Waterham HR, Wanders RJ, Engelen M, Poll-The BT. Clinical and biochemical pitfalls in the diagnosis of peroxisomal disorders. Neuropediatrics. 2016;47:205–220. doi: 10.1055/s-0036-1582140. [DOI] [PubMed] [Google Scholar]
- 44.Thai TP, Rodemer C, Jauch A, Hunziker A, Moser A, Gorgas K, Just WW. Impaired membrane traffic in defective ether lipid biosynthesis. Hum Mol Genet. 2001;10:127–136. doi: 10.1093/hmg/10.2.127. [DOI] [PubMed] [Google Scholar]
- 45.Nanetti L, Pensato V, Leoni V, Rizzetto M, Caccia C, Taroni F, Mariotti C, Gellera C. PEX7 mutations cause congenital cataract retinopathy and late-onset ataxia and cognitive impairment: report of two siblings and review of the literature. J Clin Neurol. 2015;11:197–199. doi: 10.3988/jcn.2015.11.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horn MA, van den Brink DM, Wanders RJ, Duran M, Poll-The BT, Tallaksen CM, Stokke OH, Moser H, Skjeldal OH. Phenotype of adult Refsum disease due to a defect in peroxin 7. Neurology. 2007;68:698–700. doi: 10.1212/01.wnl.0000255960.01644.39. [DOI] [PubMed] [Google Scholar]
- 47.van den Brink DM, Brites P, Haasjes J, Wierzbicki AS, Mitchell J, Lambert-Hamill M, de Belleroche J, Jansen GA, Waterham HR, Wanders RJ. Identification of PEX7 as the second gene involved in Refsum disease. Am J Hum Genet. 2003;72:471–477. doi: 10.1086/346093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khanna AJ, Braverman NE, Valle D, Sponseller PD. Cervical stenosis secondary to rhizomelic chondrodysplasia punctata. Am J Med Genet. 2001;99:63–66. doi: 10.1002/1096-8628(20010215)99:1<63::aid-ajmg1117>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 49.Wells TR, Landing BH, Bostwick FH. Studies of vertebral coronal cleft in rhizomelic chondrodysplasia punctata. Pediatr Pathol. 1992;12:593–600. doi: 10.3109/15513819209024210. [DOI] [PubMed] [Google Scholar]
- 50.Brites P, Motley AM, Gressens P, Mooyer PA, Ploegaert I, Everts V, Evrard P, Carmeliet P, Dewerchin M, Schoonjans L, et al. Impaired neuronal migration and endochondral ossification in Pex7 knockout mice: a model for rhizomelic chondrodysplasia punctata. Hum Mol Genet. 2003;12:2255–2267. doi: 10.1093/hmg/ddg236. [DOI] [PubMed] [Google Scholar]
- 51.Rodemer C, Thai TP, Brugger B, Kaercher T, Werner H, Nave KA, Wieland F, Gorgas K, Just WW. Inactivation of ether lipid biosynthesis causes male infertility, defects in eye development and optic nerve hypoplasia in mice. Hum Mol Genet. 2003;12:1881–1895. doi: 10.1093/hmg/ddg191. [DOI] [PubMed] [Google Scholar]
- 52.Liegel RP, Ronchetti A, Sidjanin DJ. Alkylglycerone phosphate synthase (AGPS) deficient mice: models for rhizomelic chondrodysplasia punctate type 3 (RCDP3) malformation syndrome. Mol Genet Metab Rep. 2014;1:299–311. doi: 10.1016/j.ymgmr.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teigler A, Komljenovic D, Draguhn A, Gorgas K, Just WW. Defects in myelination, paranode organization and Purkinje cell innervation in the ether lipid-deficient mouse cerebellum. Hum Mol Genet. 2009;18:1897–1908. doi: 10.1093/hmg/ddp110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krysko O, Bottelbergs A, Van Veldhoven P, Baes M. Combined deficiency of peroxisomal beta-oxidation and ether lipid synthesis in mice causes only minor cortical neuronal migration defects but severe hypotonia. Mol Genet Metab. 2010;100:71–76. doi: 10.1016/j.ymgme.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 55.Sillitoe RV, Joyner AL. Morphology, molecular codes, and circuitry produce the three-dimensional complexity of the cerebellum. Annu Rev Cell Dev Biol. 2007;23:549–577. doi: 10.1146/annurev.cellbio.23.090506.123237. [DOI] [PubMed] [Google Scholar]
- 56.Gorgas K, Teigler A, Komljenovic D, Just WW. The ether lipid-deficient mouse: tracking down plasmalogen functions. Biochim Biophys Acta. 2006;1763:1511–1526. doi: 10.1016/j.bbamcr.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 57.Bottelbergs A, Verheijden S, Van Veldhoven PP, Just W, Devos R, Baes M. Peroxisome deficiency but not the defect in ether lipid synthesis causes activation of the innate immune system and axonal loss in the central nervous system. J Neuroinflammation. 2012;9:61. doi: 10.1186/1742-2094-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brites P, Mooyer PA, El Mrabet L, Waterham HR, Wanders RJ. Plasmalogens participate in very-long-chain fatty acid-induced pathology. Brain. 2009;132:482–492. doi: 10.1093/brain/awn295. [DOI] [PubMed] [Google Scholar]
- 59.da Silva TF, Eira J, Lopes AT, Malheiro AR, Sousa V, Luoma A, Avila RL, Wanders RJ, Just WW, Kirschner DA, et al. Peripheral nervous system plasmalogens regulate Schwann cell differentiation and myelination. J Clin Invest. 2014;124:2560–2570. doi: 10.1172/JCI72063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brites P, Ferreira AS, Ferreira da Silva T, Sousa VF, Malheiro AR, Duran M, Waterham HR, Baes M, Wanders RJ. Alkyl-glycerol rescues plasmalogen levels and pathology of ether-phospholipid deficient mice. PLoS One. 2011;6:e28539. doi: 10.1371/journal.pone.0028539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.da Silva TF, Sousa VF, Malheiro AR, Brites P. The importance of ether-phospholipids: a view from the perspective of mouse models. Biochim Biophys Acta. 2012;1822:1501–1508. doi: 10.1016/j.bbadis.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 62.Dorninger F, Herbst R, Kravic B, Camurdanoglu BZ, Macinkovic I, Zeitler G, Forss-Petter S, Strack S, Khan MM, Waterham HR, et al. Reduced muscle strength in ether lipid-deficient mice is accompanied by altered development and function of the neuromuscular junction. J Neurochem. 2017 doi: 10.1111/jnc.14082. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saab S, Buteau B, Leclere L, Bron AM, Creuzot-Garcher CP, Bretillon L, Acar N. Involvement of plasmalogens in post-natal retinal vascular development. PLoS One. 2014;9:e101076. doi: 10.1371/journal.pone.0101076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lodhi IJ, Wei X, Yin L, Feng C, Adak S, Abou-Ezzi G, Hsu F, Link DC, Semenkovich CF. Peroxisomal lipid synthesis regulates inflammation by sustaining neutrophil membrane phospholipid composition and viability. Cell Metab. 2015;21:51–64. doi: 10.1016/j.cmet.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Braverman N, Zhang R, Chen L, Nimmo G, Scheper S, Tran T, Chaudhury R, Moser A, Steinberg S. A Pex7 hypomorphic mouse model for plasmalogen deficiency affecting the lens and skeleton. Mol Genet Metab. 2010;99:408–416. doi: 10.1016/j.ymgme.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liegel R, Chang B, Dubielzig R, Sidjanin DJ. Blind sterile 2 (bs2), a hypomorphic mutation in Agps, results in cataracts and male sterility in mice. Mol Genet Metab. 2011;103:51–59. doi: 10.1016/j.ymgme.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi X, Tarazona P, Brock TJ, Browse J, Feussner I, Watts JL. A Caenorhabditis elegans model for ether lipid biosynthesis and function. J Lipid Res. 2016;57:265–275. doi: 10.1194/jlr.M064808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Drechsler R, Chen SW, Dancy BC, Mehrabkhani L, Olsen CP. HPLC-based mass spectrometry characterizes the phospholipid alterations in ether-linked lipid deficiency models following oxidative stress. PLoS One. 2016;11:e0167229. doi: 10.1371/journal.pone.0167229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Petriv OI, Pilgrim DB, Rachubinski RA, Titorenko VI. RNA interference of peroxisome-related genes in C. elegans: a new model for human peroxisomal disorders. Physiol Genomics. 2002;10:79–91. doi: 10.1152/physiolgenomics.00044.2002. [DOI] [PubMed] [Google Scholar]
- 70.Motley AM, Hettema EH, Ketting R, Plasterk R, Tabak HF. Caenorhabditis elegans has a single pathway to target matrix proteins to peroxisomes. EMBO Rep. 2000;1:40–46. doi: 10.1093/embo-reports/kvd010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baes M, Gressens P, Baumgart E, Carmeliet P, Casteels M, Fransen M, Evrard P, Fahimi D, Declercq PE, Collen D, et al. A mouse model for Zellweger syndrome. Nat Genet. 1997;17:49–57. doi: 10.1038/ng0997-49. [DOI] [PubMed] [Google Scholar]
- 72.Faust PL, Hatten ME. Targeted deletion of the PEX2 peroxisome assembly gene in mice provides a model for Zellweger syndrome, a human neuronal migration disorder. J Cell Biol. 1997;139:1293–1305. doi: 10.1083/jcb.139.5.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen H, Liu Z, Huang X. Drosophila models of peroxisomal biogenesis disorder: peroxins are required for spermatogenesis and very-long-chain fatty acid metabolism. Hum Mol Genet. 2010;19:494–505. doi: 10.1093/hmg/ddp518. [DOI] [PubMed] [Google Scholar]
- 74.Mast FD, Li J, Virk MK, Hughes SC, Simmonds AJ, Rachubinski RA. A Drosophila model for the Zellweger spectrum of peroxisome biogenesis disorders. Dis Model Mech. 2011;4:659–672. doi: 10.1242/dmm.007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thieringer H, Moellers B, Dodt G, Kunau WH, Driscoll M. Modeling human peroxisome biogenesis disorders in the nematode Caenorhabditis elegans. J Cell Sci. 2003;116:1797–1804. doi: 10.1242/jcs.00380. [DOI] [PubMed] [Google Scholar]
- 76.Krysko O, Stevens M, Langenberg T, Fransen M, Espeel M, Baes M. Peroxisomes in zebrafish: distribution pattern and knockdown studies. Histochem Cell Biol. 2010;134:39–51. doi: 10.1007/s00418-010-0712-z. [DOI] [PubMed] [Google Scholar]
- 77.Van Veldhoven PP, Baes M. Peroxisome deficient invertebrate and vertebrate animal models. Front Physiol. 2013;4:335. doi: 10.3389/fphys.2013.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lohner K, Hermetter A, Paltauf F. Phase-behavior of ethanolamine plasmalogen. Chem Phys Lipids. 1984;34:163–170. [Google Scholar]
- 79.Hermetter A, Rainer B, Ivessa E, Kalb E, Loidl J, Roscher A, Paltauf F. Influence of plasmalogen deficiency on membrane fluidity of human skin fibroblasts: a fluorescence anisotropy study. Biochim Biophys Acta. 1989;978:151–157. doi: 10.1016/0005-2736(89)90510-5. [DOI] [PubMed] [Google Scholar]
- 80.Rog T, Koivuniemi A. The biophysical properties of ethanolamine plasmalogens revealed by atomistic molecular dynamics simulations. Biochim Biophys Acta. 2016;1858:97–103. doi: 10.1016/j.bbamem.2015.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Han XL, Gross RW. Plasmenylcholine and phosphatidylcholine membrane bilayers possess distinct conformational motifs. Biochemistry. 1990;29:4992–4996. doi: 10.1021/bi00472a032. [DOI] [PubMed] [Google Scholar]
- 82.Mitchell DC, Niu S-L, Litman BJ. DHA-rich phospholipids optimize G-protein–coupled signaling. J Pediatr. 2003;143:80–86. doi: 10.1067/s0022-3476(03)00405-0. [DOI] [PubMed] [Google Scholar]
- 83.Han X, Gross RW. Nonmonotonic alterations in the fluorescence anisotropy of polar head group labeled fluorophores during the lamellar to hexagonal phase transition of phospholipids. Biophys J. 1992;63:309–316. doi: 10.1016/S0006-3495(92)81616-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Glaser PE, Gross RW. Plasmenylethanolamine facilitates rapid membrane fusion: a stopped-flow kinetic investigation correlating the propensity of a major plasma membrane constituent to adopt an HII phase with its ability to promote membrane fusion. Biochemistry. 1994;33:5805–5812. doi: 10.1021/bi00185a019. [DOI] [PubMed] [Google Scholar]
- 85.Rudiger M, Kolleck I, Putz G, Wauer RR, Stevens P, Rustow B. Plasmalogens effectively reduce the surface tension of surfactant-like phospholipid mixtures. Am J Physiol. 1998;274:L143–L148. doi: 10.1152/ajplung.1998.274.1.L143. [DOI] [PubMed] [Google Scholar]
- 86.Lohner K. Is the high propensity of ethanolamine plasmalogens to form non-lamellar lipid structures manifested in the properties of biomembranes? Chem Phys Lipids. 1996;81:167–184. doi: 10.1016/0009-3084(96)02580-7. [DOI] [PubMed] [Google Scholar]
- 87.Han XL, Gross RW. Alterations in membrane dynamics elicited by amphiphilic compounds are augmented in plasmenylcholine bilayers. Biochim Biophys Acta. 1991;1069:37–45. doi: 10.1016/0005-2736(91)90101-d. [DOI] [PubMed] [Google Scholar]
- 88.Mankidy R, Ahiahonu PW, Ma H, Jayasinghe D, Ritchie SA, Khan MA, Su-Myat KK, Wood PL, Goodenowe DB. Membrane plasmalogen composition and cellular cholesterol regulation: a structure activity study. Lipids Health Dis. 2010;9:62. doi: 10.1186/1476-511X-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Munn NJ, Arnio E, Liu D, Zoeller RA, Liscum L. Deficiency in ethanolamine plasmalogen leads to altered cholesterol transport. J Lipid Res. 2003;44:182–192. doi: 10.1194/jlr.m200363-jlr200. [DOI] [PubMed] [Google Scholar]
- 90.Honsho M, Abe Y, Fujiki Y. Dysregulation of plasmalogen homeostasis impairs cholesterol biosynthesis. J Biol Chem. 2015;290:28822–28833. doi: 10.1074/jbc.M115.656983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pike LJ, Han X, Chung KN, Gross RW. Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: a quantitative electrospray ionization/mass spectrometric analysis. Biochemistry. 2002;41:2075–2088. doi: 10.1021/bi0156557. [DOI] [PubMed] [Google Scholar]
- 92.Pike LJ. Rafts defined: a report on the Keystone Symposium on lipid rafts and cell function. J Lipid Res. 2006;47:1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 93.Lingwood D, Simons K. Detergent resistance as a tool in membrane research. Nat Protoc. 2007;2:2159–2165. doi: 10.1038/nprot.2007.294. [DOI] [PubMed] [Google Scholar]
- 94.Davletov B, Montecucco C. Lipid function at synapses. Curr Opin Neurobiol. 2010;20:543–549. doi: 10.1016/j.conb.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 95.Hofteig JH, Noronha AB, Druse MJ, Keresztes-Nagy C. Synaptic membrane phospholipids: effects of maternal ethanol consumption. Exp Neurol. 1985;87:165–171. doi: 10.1016/0014-4886(85)90142-6. [DOI] [PubMed] [Google Scholar]
- 96.Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, Urlaub H, Schenck S, Brugger B, Ringler P, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 97.Han X. Lipid alterations in the earliest clinically recognizable stage of Alzheimer’s disease: implication of the role of lipids in the pathogenesis of Alzheimer’s disease. Curr Alzheimer Res. 2005;2:65–77. doi: 10.2174/1567205052772786. [DOI] [PubMed] [Google Scholar]
- 98.Farooqui AA, Horrocks LA. Plasmalogens: workhorse lipids of membranes in normal and injured neurons and glia. Neuroscientist. 2001;7:232–245. doi: 10.1177/107385840100700308. [DOI] [PubMed] [Google Scholar]
- 99.Farooqui AA, Rapoport SI, Horrocks LA. Membrane phospholipid alterations in Alzheimer’s disease: deficiency of ethanolamine plasmalogens. Neurochem Res. 1997;22:523–527. doi: 10.1023/a:1027380331807. [DOI] [PubMed] [Google Scholar]
- 100.Churchward MA, Coorssen JR. Cholesterol, regulated exocytosis and the physiological fusion machine. Biochem J. 2009;423:1–14. doi: 10.1042/BJ20090969. [DOI] [PubMed] [Google Scholar]
- 101.Sebastiao AM, Colino-Oliveira M, Assaife-Lopes N, Dias RB, Ribeiro JA. Lipid rafts, synaptic transmission and plasticity: impact in age-related neurodegenerative diseases. Neuropharmacology. 2013;64:97–107. doi: 10.1016/j.neuropharm.2012.06.053. [DOI] [PubMed] [Google Scholar]
- 102.Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci. 2007;8:128–140. doi: 10.1038/nrn2059. [DOI] [PubMed] [Google Scholar]
- 103.Hering H, Lin CC, Sheng M. Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability. J Neurosci. 2003;23:3262–3271. doi: 10.1523/JNEUROSCI.23-08-03262.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bennett SA, Valenzuela N, Xu H, Franko B, Fai S, Figeys D. Using neurolipidomics to identify phospholipid mediators of synaptic (dys)function in Alzheimer’s Disease. Front Physiol. 2013;4:168. doi: 10.3389/fphys.2013.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Negre-Aminou P, Pfenninger KH. Arachidonic acid turnover and phospholipase A2 activity in neuronal growth cones. J Neurochem. 1993;60:1126–1136. doi: 10.1111/j.1471-4159.1993.tb03263.x. [DOI] [PubMed] [Google Scholar]
- 106.Bazinet RP, Laye S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci. 2014;15:771–785. doi: 10.1038/nrn3820. [DOI] [PubMed] [Google Scholar]
- 107.Darios F, Davletov B. Omega-3 and omega-6 fatty acids stimulate cell membrane expansion by acting on syntaxin 3. Nature. 2006;440:813–817. doi: 10.1038/nature04598. [DOI] [PubMed] [Google Scholar]
- 108.Davletov B, Connell E, Darios F. Regulation of SNARE fusion machinery by fatty acids. Cell Mol Life Sci. 2007;64:1597–1608. doi: 10.1007/s00018-007-6557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brodde A, Teigler A, Brugger B, Lehmann WD, Wieland F, Berger J, Just WW. Impaired neurotransmission in ether lipid-deficient nerve terminals. Hum Mol Genet. 2012;21:2713–2724. doi: 10.1093/hmg/dds097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kauppinen RA, McMahon HT, Nicholls DG. Ca2+-dependent and Ca2+-independent glutamate release, energy status and cytosolic free Ca2+ concentration in isolated nerve terminals following metabolic inhibition: possible relevance to hypoglycaemia and anoxia. Neuroscience. 1988;27:175–182. doi: 10.1016/0306-4522(88)90228-x. [DOI] [PubMed] [Google Scholar]
- 111.Han XL, Chen X, Gross RW. Chemical and magnetic inequivalence of glycerol protons in individual subclasses of choline glycerophospholipids – implications for subclass-specific changes in membrane conformational states. J Am Chem Soc. 1991;113:7104–7109. [Google Scholar]
- 112.Han X, Holtzman DM, McKeel DW., Jr Plasmalogen deficiency in early Alzheimer’s disease subjects and in animal models: molecular characterization using electrospray ionization mass spectrometry. J Neurochem. 2001;77:1168–1180. doi: 10.1046/j.1471-4159.2001.00332.x. [DOI] [PubMed] [Google Scholar]
- 113.Han X. Multi-dimensional mass spectrometry-based shotgun lipidomics and the altered lipids at the mild cognitive impairment stage of Alzheimer’s disease. Biochim Biophys Acta. 2010;1801:774–783. doi: 10.1016/j.bbalip.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ishizuka I, Inomata M. Sulphated glycoglycerolipids in rat brain: decrease and disappearance after developmental age. J Neurochem. 1979;33:387–388. doi: 10.1111/j.1471-4159.1979.tb11749.x. [DOI] [PubMed] [Google Scholar]
- 115.Ford DA, Gross RW. Plasmenylethanolamine is the major storage depot for arachidonic acid in rabbit vascular smooth muscle and is rapidly hydrolyzed after angiotensin II stimulation. Proc Natl Acad Sci USA. 1989;86:3479–3483. doi: 10.1073/pnas.86.10.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Thomas SE, Byers DM, Palmer FB, Spence MW, Cook HW. Incorporation of polyunsaturated fatty acids into plasmalogens, compared to other phospholipids of cultured glioma cells, is more dependent on chain length than on selectivity between (n - 3) and (n - 6) families. Biochim Biophys Acta. 1990;1044:349–356. doi: 10.1016/0005-2760(90)90079-d. [DOI] [PubMed] [Google Scholar]
- 117.Hirashima Y, Farooqui AA, Mills JS, Horrocks LA. Identification and purification of calcium-independent phospholipase-A2 from bovine brain cytosol. J Neurochem. 1992;59:708–714. doi: 10.1111/j.1471-4159.1992.tb09426.x. [DOI] [PubMed] [Google Scholar]
- 118.Horrocks LA, Yeo YK. Health benefits of docosahexaenoic acid (DHA) Pharmacol Res. 1999;40:211–225. doi: 10.1006/phrs.1999.0495. [DOI] [PubMed] [Google Scholar]
- 119.Stables MJ, Gilroy DW. Old and new generation lipid mediators in acute inflammation and resolution. Prog Lipid Res. 2011;50:35–51. doi: 10.1016/j.plipres.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 120.Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res. 2008;47:147–155. doi: 10.1016/j.plipres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 121.Saab S, Mazzocco J, Creuzot-Garcher CP, Bron AM, Bretillon L, Acar N. Plasmalogens in the retina: from occurrence in retinal cell membranes to potential involvement in pathophysiology of retinal diseases. Biochimie. 2014;107(Pt A):58–65. doi: 10.1016/j.biochi.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 122.Dorninger F, Brodde A, Braverman NE, Moser AB, Just WW, Forss-Petter S, Brugger B, Berger J. Homeostasis of phospholipids – the level of phosphatidylethanolamine tightly adapts to changes in ethanolamine plasmalogens. Biochim Biophys Acta. 2015;1851:117–128. doi: 10.1016/j.bbalip.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dyall SC. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front Aging Neurosci. 2015;7:52. doi: 10.3389/fnagi.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McNamara RK, Carlson SE. Role of omega-3 fatty acids in brain development and function: potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fatty Acids. 2006;75:329–349. doi: 10.1016/j.plefa.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 125.Messamore E, Almeida DM, Jandacek RJ, McNamara RK. Polyunsaturated fatty acids and recurrent mood disorders: phenomenology, mechanisms, and clinical application. Prog Lipid Res. 2017;66:1–13. doi: 10.1016/j.plipres.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hossain MS, Abe Y, Ali F, Youssef M, Honsho M, Fujiki Y, Katafuchi T. Reduction of ether-type glycerophospholipids, plasmalogens, by NF-kappaB signal leading to microglial activation. J Neurosci. 2017;37:4074–4092. doi: 10.1523/JNEUROSCI.3941-15.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kleinecke S, Richert S, de Hoz L, Brugger B, Kungl T, Asadollahi E, Quintes S, Blanz J, McGonigal R, Naseri K, et al. Peroxisomal dysfunctions cause lysosomal storage and axonal Kv1 channel redistribution in peripheral neuropathy. Elife. 2017;6:e23332. doi: 10.7554/eLife.23332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hossain MS, Ifuku M, Take S, Kawamura J, Miake K, Katafuchi T. Plasmalogens rescue neuronal cell death through an activation of AKT and ERK survival signaling. PLoS One. 2013;8:e83508. doi: 10.1371/journal.pone.0083508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hossain MS, Mineno K, Katafuchi T. Neuronal orphan G-protein coupled receptor proteins mediate plasmalogens-induced activation of ERK and Akt signaling. PLoS One. 2016;11:e0150846. doi: 10.1371/journal.pone.0150846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang L, Zhou K, Fu Z, Yu D, Huang H, Zang X, Mo X. Brain development and Akt signaling: the crossroads of signaling pathway and neurodevelopmental diseases. J Mol Neurosci. 2017;61:379–384. doi: 10.1007/s12031-016-0872-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Samuels IS, Saitta SC, Landreth GE. MAP’ing CNS development and cognition: an ERKsome process. Neuron. 2009;61:160–167. doi: 10.1016/j.neuron.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pucilowska J, Puzerey PA, Karlo JC, Galan RF, Landreth GE. Disrupted ERK signaling during cortical development leads to abnormal progenitor proliferation, neuronal and network excitability and behavior, modeling human neuro-cardio-facial-cutaneous and related syndromes. J Neurosci. 2012;32:8663–8677. doi: 10.1523/JNEUROSCI.1107-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jansen LA, Mirzaa GM, Ishak GE, O’Roak BJ, Hiatt JB, Roden WH, Gunter SA, Christian SL, Collins S, Adams C, et al. PI3K/AKT pathway mutations cause a spectrum of brain malformations from megalencephaly to focal cortical dysplasia. Brain. 2015;138:1613–1628. doi: 10.1093/brain/awv045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Facciotti F, Ramanjaneyulu GS, Lepore M, Sansano S, Cavallari M, Kistowska M, Forss-Petter S, Ni G, Colone A, Singhal A, et al. Peroxisome-derived lipids are self antigens that stimulate invariant natural killer T cells in the thymus. Nat Immunol. 2012;13:474–480. doi: 10.1038/ni.2245. [DOI] [PubMed] [Google Scholar]
- 135.White MC, Rastogi P, McHowat J. Lysoplasmenylcholine increases neutrophil adherence to human coronary artery endothelial cells. Am J Physiol Cell Physiol. 2007;293:C1467–C1471. doi: 10.1152/ajpcell.00290.2007. [DOI] [PubMed] [Google Scholar]
- 136.Williams SD, Ford DA. Activation of myocardial cAMP-dependent protein kinase by lysoplasmenylcholine. FEBS Lett. 1997;420:33–38. doi: 10.1016/s0014-5793(97)01482-8. [DOI] [PubMed] [Google Scholar]
- 137.Kato K, Clark GD, Bazan NG, Zorumski CF. Platelet-activating factor as a potential retrograde messenger in CA1 hippocampal long-term potentiation. Nature. 1994;367:175–179. doi: 10.1038/367175a0. [DOI] [PubMed] [Google Scholar]
- 138.Wieraszko A, Li G, Kornecki E, Hogan MV, Ehrlich YH. Long-term potentiation in the hippocampus induced by platelet-activating factor. Neuron. 1993;10:553–557. doi: 10.1016/0896-6273(93)90342-o. [DOI] [PubMed] [Google Scholar]
- 139.Clark GD, Happel LT, Zorumski CF, Bazan NG. Enhancement of hippocampal excitatory synaptic transmission by platelet-activating factor. Neuron. 1992;9:1211–1216. doi: 10.1016/0896-6273(92)90078-r. [DOI] [PubMed] [Google Scholar]
- 140.Heusler P, Boehmer G. Platelet-activating factor contributes to the induction of long-term potentiation in the rat somatosensory cortex in vitro. Brain Res. 2007;1135:85–91. doi: 10.1016/j.brainres.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 141.Moriguchi S, Shioda N, Yamamoto Y, Fukunaga K. Platelet-activating factor-induced synaptic facilitation is associated with increased calcium/calmodulin-dependent protein kinase II, protein kinase C and extracellular signal-regulated kinase activities in the rat hippocampal CA1 region. Neuroscience. 2010;166:1158–1166. doi: 10.1016/j.neuroscience.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 142.Bito H, Nakamura M, Honda Z, Izumi T, Iwatsubo T, Seyama Y, Ogura A, Kudo Y, Shimizu T. Platelet-activating factor (PAF) receptor in rat brain: PAF mobilizes intracellular Ca2+ in hippocampal neurons. Neuron. 1992;9:285–294. doi: 10.1016/0896-6273(92)90167-c. [DOI] [PubMed] [Google Scholar]
- 143.Kornecki E, Ehrlich YH. Neuroregulatory and neuropathological actions of the ether-phospholipid platelet-activating factor. Science. 1988;240:1792–1794. doi: 10.1126/science.3381103. [DOI] [PubMed] [Google Scholar]
- 144.Chen C, Magee JC, Marcheselli V, Hardy M, Bazan NG. Attenuated LTP in hippocampal dentate gyrus neurons of mice deficient in the PAF receptor. J Neurophysiol. 2001;85:384–390. doi: 10.1152/jn.2001.85.1.384. [DOI] [PubMed] [Google Scholar]
- 145.Izquierdo I, Fin C, Schmitz PK, Da Silva RC, Jerusalinsky D, Quillfeldt JA, Ferreira MB, Medina JH, Bazan NG. Memory enhancement by intrahippocampal, intraamygdala, or intraentorhinal infusion of platelet-activating factor measured in an inhibitory avoidance task. Proc Natl Acad Sci USA. 1995;92:5047–5051. doi: 10.1073/pnas.92.11.5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kobayashi K, Ishii S, Kume K, Takahashi T, Shimizu T, Manabe T. Platelet-activating factor receptor is not required for long-term potentiation in the hippocampal CA1 region. Eur J Neurosci. 1999;11:1313–1316. doi: 10.1046/j.1460-9568.1999.00538.x. [DOI] [PubMed] [Google Scholar]
- 147.Reiner B, Wang W, Liu J, Xiong H. Platelet-activating factor attenuation of long-term potentiation in rat hippocampal slices via protein tyrosine kinase signaling. Neurosci Lett. 2016;615:83–87. doi: 10.1016/j.neulet.2016.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stafforini DM, McIntyre TM, Zimmerman GA, Prescott SM. Platelet-activating factor, a pleiotrophic mediator of physiological and pathological processes. Crit Rev Clin Lab Sci. 2003;40:643–672. doi: 10.1080/714037693. [DOI] [PubMed] [Google Scholar]
- 149.Perry SW, Hamilton JA, Tjoelker LW, Dbaibo G, Dzenko KA, Epstein LG, Hannun Y, Whittaker JS, Dewhurst S, Gelbard HA. Platelet-activating factor receptor activation. An initiator step in HIV-1 neuropathogenesis. J Biol Chem. 1998;273:17660–17664. doi: 10.1074/jbc.273.28.17660. [DOI] [PubMed] [Google Scholar]
- 150.Ryan SD, Harris CS, Mo F, Lee H, Hou ST, Bazan NG, Haddad PS, Arnason JT, Bennett SA. Platelet activating factor-induced neuronal apoptosis is initiated independently of its G-protein coupled PAF receptor and is inhibited by the benzoate orsellinic acid. J Neurochem. 2007;103:88–97. doi: 10.1111/j.1471-4159.2007.04740.x. [DOI] [PubMed] [Google Scholar]
- 151.Yin XJ, Chen ZY, Zhu XN, Hu JJ. Loss of PAFR prevents neuroinflammation and brain dysfunction after traumatic brain injury. Sci Rep. 2017;7:40614. doi: 10.1038/srep40614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Teather LA, Afonso VM, Wurtman RJ. Inhibition of platelet-activating factor receptors in hippocampal plasma membranes attenuates the inflammatory nociceptive response in rats. Brain Res. 2006;1097:230–233. doi: 10.1016/j.brainres.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 153.Bix GJ, Clark GD. Platelet-activating factor receptor stimulation disrupts neuronal migration in vitro. J Neurosci. 1998;18:307–318. doi: 10.1523/JNEUROSCI.18-01-00307.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hirotsune S, Fleck MW, Gambello MJ, Bix GJ, Chen A, Clark GD, Ledbetter DH, McBain CJ, Wynshaw-Boris A. Graded reduction of Pafah1b1 (Lis1) activity results in neuronal migration defects and early embryonic lethality. Nat Genet. 1998;19:333–339. doi: 10.1038/1221. [DOI] [PubMed] [Google Scholar]
- 155.Ishii S, Kuwaki T, Nagase T, Maki K, Tashiro F, Sunaga S, Cao WH, Kume K, Fukuchi Y, Ikuta K, et al. Impaired anaphylactic responses with intact sensitivity to endotoxin in mice lacking a platelet-activating factor receptor. J Exp Med. 1998;187:1779–1788. doi: 10.1084/jem.187.11.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tokuoka SM, Ishii S, Kawamura N, Satoh M, Shimada A, Sasaki S, Hirotsune S, Wynshaw-Boris A, Shimizu T. Involvement of platelet-activating factor and LIS1 in neuronal migration. Eur J Neurosci. 2003;18:563–570. doi: 10.1046/j.1460-9568.2003.02778.x. [DOI] [PubMed] [Google Scholar]
- 157.Davies SS, Pontsler AV, Marathe GK, Harrison KA, Murphy RC, Hinshaw JC, Prestwich GD, Hilaire AS, Prescott SM, Zimmerman GA, et al. Oxidized alkyl phospholipids are specific, high affinity peroxisome proliferator-activated receptor gamma ligands and agonists. J Biol Chem. 2001;276:16015–16023. doi: 10.1074/jbc.M100878200. [DOI] [PubMed] [Google Scholar]
- 158.Liliom K, Fischer DJ, Virag T, Sun G, Miller DD, Tseng JL, Desiderio DM, Seidel MC, Erickson JR, Tigyi G. Identification of a novel growth factor-like lipid, 1-O-cis-alk-1’-enyl-2-lyso-sn-glycero-3-phosphate (alkenyl-GP) that is present in commercial sphingolipid preparations. J Biol Chem. 1998;273:13461–13468. doi: 10.1074/jbc.273.22.13461. [DOI] [PubMed] [Google Scholar]
- 159.Sugiura T, Nakane S, Kishimoto S, Waku K, Yoshioka Y, Tokumura A, Hanahan DJ. Occurrence of lysophosphatidic acid and its alkyl ether-linked analog in rat brain and comparison of their biological activities toward cultured neural cells. Biochim Biophys Acta. 1999;1440:194–204. doi: 10.1016/s1388-1981(99)00127-4. [DOI] [PubMed] [Google Scholar]
- 160.Williams JR, Khandoga AL, Goyal P, Fells JI, Perygin DH, Siess W, Parrill AL, Tigyi G, Fujiwara Y. Unique ligand selectivity of the GPR92/LPA5 lysophosphatidate receptor indicates role in human platelet activation. J Biol Chem. 2009;284:17304–17319. doi: 10.1074/jbc.M109.003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kozian DH, von Haeften E, Joho S, Czechtizky W, Anumala UR, Roux P, Dudda A, Evers A, Nazare M. Modulation of hexadecyl-LPA-mediated activation of mast cells and microglia by a chemical probe for LPA5. ChemBioChem. 2016;17:861–865. doi: 10.1002/cbic.201500559. [DOI] [PubMed] [Google Scholar]
- 162.Yamashita S, Kanno S, Nakagawa K, Kinoshita M, Miyazawa T. Extrinsic plasmalogens suppress neuronal apoptosis in mouse neuroblastoma Neuro-2A cells: importance of plasmalogen molecular species. RSC Adv. 2015;5:61012–61020. [Google Scholar]
- 163.Ifuku M, Katafuchi T, Mawatari S, Noda M, Miake K, Sugiyama M, Fujino T. Anti-inflammatory/anti-amyloidogenic effects of plasmalogens in lipopolysaccharide-induced neuroinflammation in adult mice. J Neuroinflammation. 2012;9:197. doi: 10.1186/1742-2094-9-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dorninger F, Wiesinger C, Braverman NE, Forss-Petter S, Berger J. Ether lipid deficiency does not cause neutropenia or leukopenia in mice and men. Cell Metab. 2015;21:650–651. doi: 10.1016/j.cmet.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mayor S, Riezman H. Sorting GPI-anchored proteins. Nat Rev Mol Cell Biol. 2004;5:110–120. doi: 10.1038/nrm1309. [DOI] [PubMed] [Google Scholar]
- 166.Eisenhaber B, Bork P, Eisenhaber F. Post-translational GPI lipid anchor modification of proteins in kingdoms of life: analysis of protein sequence data from complete genomes. Protein Eng. 2001;14:17–25. doi: 10.1093/protein/14.1.17. [DOI] [PubMed] [Google Scholar]
- 167.Ikezawa H. Glycosylphosphatidylinositol (GPI)-anchored proteins. Biol Pharm Bull. 2002;25:409–417. doi: 10.1248/bpb.25.409. [DOI] [PubMed] [Google Scholar]
- 168.Redman CA, Thomas-Oates JE, Ogata S, Ikehara Y, Ferguson MA. Structure of the glycosylphosphatidylinositol membrane anchor of human placental alkaline phosphatase. Biochem J. 1994;302(Pt 3):861–865. doi: 10.1042/bj3020861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Roberts WL, Myher JJ, Kuksis A, Rosenberry TL. Alkylacylglycerol molecular species in the glycosylinositol phospholipid membrane anchor of bovine erythrocyte acetylcholinesterase. Biochem Biophys Res Commun. 1988;150:271–277. doi: 10.1016/0006-291x(88)90516-5. [DOI] [PubMed] [Google Scholar]
- 170.Rudd PM, Morgan BP, Wormald MR, Harvey DJ, van den Berg CW, Davis SJ, Ferguson MA, Dwek RA. The glycosylation of the complement regulatory protein, human erythrocyte CD59. J Biol Chem. 1997;272:7229–7244. doi: 10.1074/jbc.272.11.7229. [DOI] [PubMed] [Google Scholar]
- 171.Houjou T, Hayakawa J, Watanabe R, Tashima Y, Maeda Y, Kinoshita T, Taguchi R. Changes in molecular species profiles of glycosylphosphatidylinositol anchor precursors in early stages of biosynthesis. J Lipid Res. 2007;48:1599–1606. doi: 10.1194/jlr.M700095-JLR200. [DOI] [PubMed] [Google Scholar]
- 172.Fujita M, Kinoshita T. Structural remodeling of GPI anchors during biosynthesis and after attachment to proteins. FEBS Lett. 2010;584:1670–1677. doi: 10.1016/j.febslet.2009.10.079. [DOI] [PubMed] [Google Scholar]
- 173.Kanzawa N, Maeda Y, Ogiso H, Murakami Y, Taguchi R, Kinoshita T. Peroxisome dependency of alkyl-containing GPI-anchor biosynthesis in the endoplasmic reticulum. Proc Natl Acad Sci USA. 2009;106:17711–17716. doi: 10.1073/pnas.0904762106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kanzawa N, Shimozawa N, Wanders RJ, Ikeda K, Murakami Y, Waterham HR, Mukai S, Fujita M, Maeda Y, Taguchi R, et al. Defective lipid remodeling of GPI anchors in peroxisomal disorders, Zellweger syndrome, and rhizomelic chondrodysplasia punctata. J Lipid Res. 2012;53:653–663. doi: 10.1194/jlr.M021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rosen CL, Lisanti MP, Salzer JL. Expression of unique sets of GPI-linked proteins by different primary neurons in vitro. J Cell Biol. 1992;117:617–627. doi: 10.1083/jcb.117.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kania A, Klein R. Mechanisms of ephrin-Eph signalling in development, physiology and disease. Nat Rev Mol Cell Biol. 2016;17:240–256. doi: 10.1038/nrm.2015.16. [DOI] [PubMed] [Google Scholar]
- 177.Zoeller RA, Morand OH, Raetz CR. A possible role for plasmalogens in protecting animal cells against photosensitized killing. J Biol Chem. 1988;263:11590–11596. [PubMed] [Google Scholar]
- 178.Zoeller RA, Grazia TJ, LaCamera P, Park J, Gaposchkin DP, Farber HW. Increasing plasmalogen levels protects human endothelial cells during hypoxia. Am J Physiol Heart Circ Physiol. 2002;283:H671–H679. doi: 10.1152/ajpheart.00524.2001. [DOI] [PubMed] [Google Scholar]
- 179.Broniec A, Klosinski R, Pawlak A, Wrona-Krol M, Thompson D, Sarna T. Interactions of plasmalogens and their diacyl analogs with singlet oxygen in selected model systems. Free Radic Biol Med. 2011;50:892–898. doi: 10.1016/j.freeradbiomed.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sindelar PJ, Guan Z, Dallner G, Ernster L. The protective role of plasmalogens in iron-induced lipid peroxidation. Free Radic Biol Med. 1999;26:318–324. doi: 10.1016/s0891-5849(98)00221-4. [DOI] [PubMed] [Google Scholar]
- 181.Reiss D, Beyer K, Engelmann B. Delayed oxidative degradation of polyunsaturated diacyl phospholipids in the presence of plasmalogen phospholipids in vitro. Biochem J. 1997;323(Pt 3):807–814. doi: 10.1042/bj3230807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hoefler G, Paschke E, Hoefler S, Moser AB, Moser HW. Photosensitized killing of cultured fibroblasts from patients with peroxisomal disorders due to pyrene fatty acid-mediated ultraviolet damage. J Clin Invest. 1991;88:1873–1879. doi: 10.1172/JCI115509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zoeller RA, Lake AC, Nagan N, Gaposchkin DP, Legner MA, Lieberthal W. Plasmalogens as endogenous antioxidants: somatic cell mutants reveal the importance of the vinyl ether. Biochem J. 1999;338(Pt 3):769–776. [PMC free article] [PubMed] [Google Scholar]
- 184.Brosche T, Platt D. The biological significance of plasmalogens in defense against oxidative damage. Exp Gerontol. 1998;33:363–369. doi: 10.1016/s0531-5565(98)00014-x. [DOI] [PubMed] [Google Scholar]
- 185.Brosche T, Bertsch T, Sieber CC, Hoffmann U. Reduced plasmalogen concentration as a surrogate marker of oxidative stress in elderly septic patients. Arch Gerontol Geriatr. 2013;57:66–69. doi: 10.1016/j.archger.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 186.Luoma AM, Kuo F, Cakici O, Crowther MN, Denninger AR, Avila RL, Brites P, Kirschner DA. Plasmalogen phospholipids protect internodal myelin from oxidative damage. Free Radic Biol Med. 2015;84:296–310. doi: 10.1016/j.freeradbiomed.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 187.Lessig J, Fuchs B. Plasmalogens in biological systems: their role in oxidative processes in biological membranes, their contribution to pathological processes and aging and plasmalogen analysis. Curr Med Chem. 2009;16:2021–2041. doi: 10.2174/092986709788682164. [DOI] [PubMed] [Google Scholar]
- 188.Broniec A, Zadlo A, Pawlak A, Fuchs B, Klosinski R, Thompson D, Sarna T. Interaction of plasmenylcholine with free radicals in selected model systems. Free Radic Biol Med. 2017;106:368–378. doi: 10.1016/j.freeradbiomed.2017.02.029. [DOI] [PubMed] [Google Scholar]
- 189.Fauconneau B, Stadelmann-Ingrand S, Favreliere S, Baudouin J, Renaud L, Piriou A, Tallineau C. Evidence against a major role of plasmalogens in the resistance of astrocytes in lactic acid-induced oxidative stress in vitro. Arch Toxicol. 2001;74:695–701. doi: 10.1007/s002040000185. [DOI] [PubMed] [Google Scholar]
- 190.Jansen GA, Wanders RJ. Plasmalogens and oxidative stress: evidence against a major role of plasmalogens in protection against the superoxide anion radical. J Inherit Metab Dis. 1997;20:85–94. doi: 10.1023/a:1005321910248. [DOI] [PubMed] [Google Scholar]
- 191.Felde R, Spiteller G. Plasmalogen oxidation in human serum lipoproteins. Chem Phys Lipids. 1995;76:259–267. doi: 10.1016/0009-3084(94)02448-e. [DOI] [PubMed] [Google Scholar]
- 192.Loidl-Stahlhofen A, Hannemann K, Felde R, Spiteller G. Epoxidation of plasmalogens: source for long-chain alpha-hydroxyaldehydes in subcellular fractions of bovine liver. Biochem J. 1995;309(Pt 3):807–812. doi: 10.1042/bj3090807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Thukkani AK, Hsu FF, Crowley JR, Wysolmerski RB, Albert CJ, Ford DA. Reactive chlorinating species produced during neutrophil activation target tissue plasmalogens: production of the chemoattractant, 2-chlorohexadecanal. J Biol Chem. 2002;277:3842–3849. doi: 10.1074/jbc.M109489200. [DOI] [PubMed] [Google Scholar]
- 194.Ford DA. Lipid oxidation by hypochlorous acid: chlorinated lipids in atherosclerosis and myocardial ischemia. Clin Lipidol. 2010;5:835–852. doi: 10.2217/clp.10.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wang G, Wang T. The role of plasmalogen in the oxidative stability of neutral lipids and phospholipids. J Agric Food Chem. 2010;58:2554–2561. doi: 10.1021/jf903906e. [DOI] [PubMed] [Google Scholar]
- 196.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9:63–75 e2. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 197.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Guan ZZ, Wang YA, Cairns NJ, Lantos PL, Dallner G, Sindelar PJ. Decrease and structural modifications of phosphatidylethanolamine plasmalogen in the brain with Alzheimer disease. J Neuropathol Exp Neurol. 1999;58:740–747. doi: 10.1097/00005072-199907000-00008. [DOI] [PubMed] [Google Scholar]
- 199.Kou J, Kovacs GG, Hoftberger R, Kulik W, Brodde A, Forss-Petter S, Honigschnabl S, Gleiss A, Brugger B, Wanders R, et al. Peroxisomal alterations in Alzheimer’s disease. Acta Neuropathol. 2011;122:271–283. doi: 10.1007/s00401-011-0836-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wood PL, Barnette BL, Kaye JA, Quinn JF, Woltjer RL. Non-targeted lipidomics of CSF and frontal cortex grey and white matter in control, mild cognitive impairment, and Alzheimer’s disease subjects. Acta Neuropsychiatr. 2015;27:270–278. doi: 10.1017/neu.2015.18. [DOI] [PubMed] [Google Scholar]
- 201.Igarashi M, Ma K, Gao F, Kim HW, Rapoport SI, Rao JS. Disturbed choline plasmalogen and phospholipid fatty acid concentrations in Alzheimer’s disease prefrontal cortex. J Alzheimers Dis. 2011;24:507–517. doi: 10.3233/JAD-2011-101608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Grimm MO, Grosgen S, Riemenschneider M, Tanila H, Grimm HS, Hartmann T. From brain to food: analysis of phosphatidylcholins, lyso-phosphatidylcholins and phosphatidylcholin-plasmalogens derivates in Alzheimer’s disease human post mortem brains and mice model via mass spectrometry. J Chromatogr A. 2011;1218:7713–7722. doi: 10.1016/j.chroma.2011.07.073. [DOI] [PubMed] [Google Scholar]
- 203.Fabelo N, Martin V, Marin R, Santpere G, Aso E, Ferrer I, Diaz M. Evidence for premature lipid raft aging in APP/PS1 double-transgenic mice, a model of familial Alzheimer disease. J Neuropathol Exp Neurol. 2012;71:868–881. doi: 10.1097/NEN.0b013e31826be03c. [DOI] [PubMed] [Google Scholar]
- 204.Tajima Y, Ishikawa M, Maekawa K, Murayama M, Senoo Y, Nishimaki-Mogami T, Nakanishi H, Ikeda K, Arita M, Taguchi R, et al. Lipidomic analysis of brain tissues and plasma in a mouse model expressing mutated human amyloid precursor protein/tau for Alzheimer’s disease. Lipids Health Dis. 2013;12:68. doi: 10.1186/1476-511X-12-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Pettegrew JW, Panchalingam K, Hamilton RL, McClure RJ. Brain membrane phospholipid alterations in Alzheimer’s disease. Neurochem Res. 2001;26:771–782. doi: 10.1023/a:1011603916962. [DOI] [PubMed] [Google Scholar]
- 206.Ginsberg L, Rafique S, Xuereb JH, Rapoport SI, Gershfeld NL. Disease and anatomic specificity of ethanolamine plasmalogen deficiency in Alzheimer’s disease brain. Brain Res. 1995;698:223–226. doi: 10.1016/0006-8993(95)00931-f. [DOI] [PubMed] [Google Scholar]
- 207.Goodenowe DB, Cook LL, Liu J, Lu Y, Jayasinghe DA, Ahiahonu PW, Heath D, Yamazaki Y, Flax J, Krenitsky KF, et al. Peripheral ethanolamine plasmalogen deficiency: a logical causative factor in Alzheimer’s disease and dementia. J Lipid Res. 2007;48:2485–2498. doi: 10.1194/jlr.P700023-JLR200. [DOI] [PubMed] [Google Scholar]
- 208.Yamashita S, Kiko T, Fujiwara H, Hashimoto M, Nakagawa K, Kinoshita M, Furukawa K, Arai H, Miyazawa T. Alterations in the levels of amyloid-beta, phospholipid hydroperoxide, and plasmalogen in the blood of patients with Alzheimer’s disease: possible interactions between amyloid-beta and these lipids. J Alzheimers Dis. 2015;50:527–537. doi: 10.3233/JAD-150640. [DOI] [PubMed] [Google Scholar]
- 209.Wood PL, Mankidy R, Ritchie S, Heath D, Wood JA, Flax J, Goodenowe DB. Circulating plasmalogen levels and Alzheimer disease assessment scale-cognitive scores in Alzheimer patients. J Psychiatry Neurosci. 2010;35:59–62. doi: 10.1503/jpn.090059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Maeba R, Araki A, Ishii K, Ogawa K, Tamura Y, Yasunaga M, Minami U, Komori A, Okazaki T, Nishimukai M, et al. Serum ethanolamine plasmalogens improve detection of cognitive impairment among elderly with high excretion levels of urinary myo-inositol: a cross-sectional study. Clin Chim Acta. 2016;453:134–140. doi: 10.1016/j.cca.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 211.Mapstone M, Cheema AK, Fiandaca MS, Zhong X, Mhyre TR, MacArthur LH, Hall WJ, Fisher SG, Peterson DR, Haley JM, et al. Plasma phospholipids identify antecedent memory impairment in older adults. Nat Med. 2014;20:415–418. doi: 10.1038/nm.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Cimini A, Moreno S, D’Amelio M, Cristiano L, D’Angelo B, Falone S, Benedetti E, Carrara P, Fanelli F, Cecconi F, et al. Early biochemical and morphological modifications in the brain of a transgenic mouse model of Alzheimer’s disease: a role for peroxisomes. J Alzheimers Dis. 2009;18:935–952. doi: 10.3233/JAD-2009-1199. [DOI] [PubMed] [Google Scholar]
- 213.Lizard G, Rouaud O, Demarquoy J, Cherkaoui-Malki M, Iuliano L. Potential roles of peroxisomes in Alzheimer’s disease and in dementia of the Alzheimer’s type. J Alzheimers Dis. 2012;29:241–254. doi: 10.3233/JAD-2011-111163. [DOI] [PubMed] [Google Scholar]
- 214.Farooqui AA. Studies on plasmalogen-selective phospholipase A2 in brain. Mol Neurobiol. 2010;41:267–273. doi: 10.1007/s12035-009-8091-y. [DOI] [PubMed] [Google Scholar]
- 215.Grimm MO, Kuchenbecker J, Rothhaar TL, Grosgen S, Hundsdorfer B, Burg VK, Friess P, Muller U, Grimm HS, Riemenschneider M, et al. Plasmalogen synthesis is regulated via alkyl-dihydroxyacetonephosphate-synthase by amyloid precursor protein processing and is affected in Alzheimer’s disease. J Neurochem. 2011;116:916–925. doi: 10.1111/j.1471-4159.2010.07070.x. [DOI] [PubMed] [Google Scholar]
- 216.Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16:358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 217.Iqbal K, Alonso Adel C, Chen S, Chohan MO, El-Akkad E, Gong CX, Khatoon S, Li B, Liu F, Rahman A, et al. Tau pathology in Alzheimer disease and other tauopathies. Biochim Biophys Acta. 2005;1739:198–210. doi: 10.1016/j.bbadis.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 218.Rothhaar TL, Grosgen S, Haupenthal VJ, Burg VK, Hundsdorfer B, Mett J, Riemenschneider M, Grimm HS, Hartmann T, Grimm MO. Plasmalogens inhibit APP processing by directly affecting gamma-secretase activity in Alzheimer’s disease. ScientificWorldJournal. 2012;2012:141240. doi: 10.1100/2012/141240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ehehalt R, Keller P, Haass C, Thiele C, Simons K. Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J Cell Biol. 2003;160:113–123. doi: 10.1083/jcb.200207113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Fujino T, Yamada T, Asada T, Tsuboi Y, Wakana C, Mawatari S, Kono S. Efficacy and blood plasmalogen changes by oral administration of plasmalogen in patients with mild Alzheimer’s disease and mild cognitive impairment: a multicenter, randomized, double-blind, placebo-controlled trial. EBioMedicine. 2017;17:199–205. doi: 10.1016/j.ebiom.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Bate C, Salmona M, Williams A. The role of platelet activating factor in prion and amyloid-beta neurotoxicity. NeuroReport. 2004;15:509–513. doi: 10.1097/00001756-200403010-00025. [DOI] [PubMed] [Google Scholar]
- 222.Bate C, Kempster S, Williams A. Platelet-activating factor antagonists protect amyloid-beta damaged neurons from microglia-mediated death. Neuropharmacology. 2006;51:173–181. doi: 10.1016/j.neuropharm.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 223.Ryan SD, Whitehead SN, Swayne LA, Moffat TC, Hou W, Ethier M, Bourgeois AJ, Rashidian J, Blanchard AP, Fraser PE, et al. Amyloid-beta42 signals tau hyperphosphorylation and compromises neuronal viability by disrupting alkylacylglycerophosphocholine metabolism. Proc Natl Acad Sci USA. 2009;106:20936–20941. doi: 10.1073/pnas.0905654106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Moser HW. Genotype-phenotype correlations in disorders of peroxisome biogenesis. Mol Genet Metab. 1999;68:316–327. doi: 10.1006/mgme.1999.2926. [DOI] [PubMed] [Google Scholar]
- 225.Yu TW, Chahrour MH, Coulter ME, Jiralerspong S, Okamura-Ikeda K, Ataman B, Schmitz-Abe K, Harmin DA, Adli M, Malik AN, et al. Using whole-exome sequencing to identify inherited causes of autism. Neuron. 2013;77:259–273. doi: 10.1016/j.neuron.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ro M, Park J, Nam M, Bang HJ, Yang J, Choi KS, Kim SK, Chung JH, Kwack K. Association between peroxisomal biogenesis factor 7 and autism spectrum disorders in a Korean population. J Child Neurol. 2012;27:1270–1275. doi: 10.1177/0883073811435507. [DOI] [PubMed] [Google Scholar]
- 227.Braverman N, Chen L, Lin P, Obie C, Steel G, Douglas P, Chakraborty PK, Clarke JT, Boneh A, Moser A, et al. Mutation analysis of PEX7 in 60 probands with rhizomelic chondrodysplasia punctata and functional correlations of genotype with phenotype. Hum Mutat. 2002;20:284–297. doi: 10.1002/humu.10124. [DOI] [PubMed] [Google Scholar]
- 228.Wiest MM, German JB, Harvey DJ, Watkins SM, Hertz-Picciotto I. Plasma fatty acid profiles in autism: a case-control study. Prostaglandins Leukot Essent Fatty Acids. 2009;80:221–227. doi: 10.1016/j.plefa.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 229.Bell JG, MacKinlay EE, Dick JR, MacDonald DJ, Boyle RM, Glen AC. Essential fatty acids and phospholipase A2 in autistic spectrum disorders. Prostaglandins Leukot Essent Fatty Acids. 2004;71:201–204. doi: 10.1016/j.plefa.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 230.Thomas RH, Foley KA, Mepham JR, Tichenoff LJ, Possmayer F, MacFabe DF. Altered brain phospholipid and acylcarnitine profiles in propionic acid infused rodents: further development of a potential model of autism spectrum disorders. J Neurochem. 2010;113:515–529. doi: 10.1111/j.1471-4159.2010.06614.x. [DOI] [PubMed] [Google Scholar]
- 231.Schrakamp G, Schalkwijk CG, Schutgens RB, Wanders RJ, Tager JM, van den Bosch H. Plasmalogen biosynthesis in peroxisomal disorders: fatty alcohol versus alkylglycerol precursors. J Lipid Res. 1988;29:325–334. [PubMed] [Google Scholar]
- 232.Honsho M, Yagita Y, Kinoshita N, Fujiki Y. Isolation and characterization of mutant animal cell line defective in alkyl-dihydroxyacetonephosphate synthase: localization and transport of plasmalogens to post-Golgi compartments. Biochim Biophys Acta. 2008;1783:1857–1865. doi: 10.1016/j.bbamcr.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 233.Das AK, Hajra AK. High incorporation of dietary 1-O-heptadecyl glycerol into tissue plasmalogens of young rats. FEBS Lett. 1988;227:187–190. doi: 10.1016/0014-5793(88)80895-0. [DOI] [PubMed] [Google Scholar]
- 234.Das AK, Holmes RD, Wilson GN, Hajra AK. Dietary ether lipid incorporation into tissue plasmalogens of humans and rodents. Lipids. 1992;27:401–405. doi: 10.1007/BF02536379. [DOI] [PubMed] [Google Scholar]
- 235.Wood PL, Khan MA, Smith T, Ehrmantraut G, Jin W, Cui W, Braverman NE, Goodenowe DB. In vitro and in vivo plasmalogen replacement evaluations in rhizomelic chrondrodysplasia punctata and Pelizaeus-Merzbacher disease using PPI-1011, an ether lipid plasmalogen precursor. Lipids Health Dis. 2011;10:182. doi: 10.1186/1476-511X-10-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Bickerstaffe R, Mead JF. Metabolism of chimyl alcohol and phosphatidyl ethanolamine in the rat brain. Lipids. 1968;3:317–320. doi: 10.1007/BF02530931. [DOI] [PubMed] [Google Scholar]
- 237.Cohen P, Goedert M. GSK3 inhibitors: development and therapeutic potential. Nat Rev Drug Discov. 2004;3:479–487. doi: 10.1038/nrd1415. [DOI] [PubMed] [Google Scholar]
- 238.Hershkowitz M, Adunsky A. Binding of platelet-activating factor to platelets of Alzheimer’s disease and multiinfarct dementia patients. Neurobiol Aging. 1996;17:865–868. doi: 10.1016/s0197-4580(96)00073-5. [DOI] [PubMed] [Google Scholar]
- 239.Dragonas C, Bertsch T, Sieber CC, Brosche T. Plasmalogens as a marker of elevated systemic oxidative stress in Parkinson’s disease. Clin Chem Lab Med. 2009;47:894–897. doi: 10.1515/CCLM.2009.205. [DOI] [PubMed] [Google Scholar]
- 240.Fabelo N, Martin V, Santpere G, Marin R, Torrent L, Ferrer I, Diaz M. Severe alterations in lipid composition of frontal cortex lipid rafts from Parkinson’s disease and incidental Parkinson’s disease. Mol Med. 2011;17:1107–1118. doi: 10.2119/molmed.2011.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Miville-Godbout E, Bourque M, Morissette M, Al-Sweidi S, Smith T, Mochizuki A, Senanayake V, Jayasinghe D, Wang L, Goodenowe D, et al. Plasmalogen augmentation reverses striatal dopamine loss in MPTP mice. PLoS One. 2016;11:e0151020. doi: 10.1371/journal.pone.0151020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Gregoire L, Smith T, Senanayake V, Mochizuki A, Miville-Godbout E, Goodenowe D, Di Paolo T. Plasmalogen precursor analog treatment reduces levodopa-induced dyskinesias in parkinsonian monkeys. Behav Brain Res. 2015;286:328–337. doi: 10.1016/j.bbr.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 243.Murphy EJ, Schapiro MB, Rapoport SI, Shetty HU. Phospholipid composition and levels are altered in Down syndrome brain. Brain Res. 2000;867:9–18. doi: 10.1016/s0006-8993(00)02205-8. [DOI] [PubMed] [Google Scholar]
- 244.Bueno AA, Brand A, Neville MM, Lehane C, Brierley N, Crawford MA. Erythrocyte phospholipid molecular species and fatty acids of Down syndrome children compared with non-affected siblings. Br J Nutr. 2014;113:72–81. doi: 10.1017/S0007114514003298. [DOI] [PubMed] [Google Scholar]
- 245.Wood PL, Filiou MD, Otte DM, Zimmer A, Turck CW. Lipidomics reveals dysfunctional glycosynapses in schizophrenia and the G72/G30 transgenic mouse. Schizophr Res. 2014;159:365–369. doi: 10.1016/j.schres.2014.08.029. [DOI] [PubMed] [Google Scholar]
- 246.Wood PL, Holderman NR. Dysfunctional glycosynapses in schizophrenia: disease and regional specificity. Schizophr Res. 2015;166:235–237. doi: 10.1016/j.schres.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 247.Kaddurah-Daouk R, McEvoy J, Baillie R, Zhu H, Yao JK, Nimgaonkar VL, Buckley PF, Keshavan MS, Georgiades A, Nasrallah HA. Impaired plasmalogens in patients with schizophrenia. Psychiatry Res. 2012;198:347–352. doi: 10.1016/j.psychres.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 248.Wood PL, Unfried G, Whitehead W, Phillipps A, Wood JA. Dysfunctional plasmalogen dynamics in the plasma and platelets of patients with schizophrenia. Schizophr Res. 2015;161:506–510. doi: 10.1016/j.schres.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 249.Huang JH, Park H, Iaconelli J, Berkovitch SS, Watmuff B, McPhie D, Ongur D, Cohen BM, Clish CB, Karmacharya R. Unbiased metabolite profiling of schizophrenia fibroblasts under stressful perturbations reveals dysregulation of plasmalogens and phosphatidylcholines. J Proteome Res. 2017;16:481–493. doi: 10.1021/acs.jproteome.6b00628. [DOI] [PubMed] [Google Scholar]
- 250.Tessier C, Sweers K, Frajerman A, Bergaoui H, Ferreri F, Delva C, Lapidus N, Lamaziere A, Roiser JP, De Hert M, et al. Membrane lipidomics in schizophrenia patients: a correlational study with clinical and cognitive manifestations. Transl Psychiatry. 2016;6:e906. doi: 10.1038/tp.2016.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Liu X, Zheng P, Zhao X, Zhang Y, Hu C, Li J, Zhao J, Zhou J, Xie P, Xu G. Discovery and validation of plasma biomarkers for major depressive disorder classification based on liquid chromatography-mass spectrometry. J Proteome Res. 2015;14:2322–2330. doi: 10.1021/acs.jproteome.5b00144. [DOI] [PubMed] [Google Scholar]
- 252.Demirkan A, Isaacs A, Ugocsai P, Liebisch G, Struchalin M, Rudan I, Wilson JF, Pramstaller PP, Gyllensten U, Campbell H, et al. Plasma phosphatidylcholine and sphingomyelin concentrations are associated with depression and anxiety symptoms in a Dutch family-based lipidomics study. J Psychiatr Res. 2013;47:357–362. doi: 10.1016/j.jpsychires.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 253.Knowles EE, Huynh K, Meikle PJ, Goring HH, Olvera RL, Mathias SR, Duggirala R, Almasy L, Blangero J, Curran JE, et al. The lipidome in major depressive disorder: shared genetic influence for ether-phosphatidylcholines, a plasma-based phenotype related to inflammation, and disease risk. Eur Psychiatry. 2017;43:44–50. doi: 10.1016/j.eurpsy.2017.02.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kumar R, Harvey SA, Kester M, Hanahan DJ, Olson MS. Production and effects of platelet-activating factor in the rat brain. Biochim Biophys Acta. 1988;963:375–383. doi: 10.1016/0005-2760(88)90304-9. [DOI] [PubMed] [Google Scholar]
- 255.Musto AE, Rosencrans RF, Walker CP, Bhattacharjee S, Raulji CM, Belayev L, Fang Z, Gordon WC, Bazan NG. Dysfunctional epileptic neuronal circuits and dysmorphic dendritic spines are mitigated by platelet-activating factor receptor antagonism. Sci Rep. 2016;6:30298. doi: 10.1038/srep30298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Yanagihara T, Cumings JN. Alterations of phospholipids, particularly plasmalogens, in the demyelination of multiple sclerosis as compared with that of cerebral oedema. Brain. 1969;92:59–70. doi: 10.1093/brain/92.1.59. [DOI] [PubMed] [Google Scholar]
- 257.Senanayake VK, Jin W, Mochizuki A, Chitou B, Goodenowe DB. Metabolic dysfunctions in multiple sclerosis: implications as to causation, early detection, and treatment, a case control study. BMC Neurol. 2015;15:154. doi: 10.1186/s12883-015-0411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Singh I, Paintlia AS, Khan M, Stanislaus R, Paintlia MK, Haq E, Singh AK, Contreras MA. Impaired peroxisomal function in the central nervous system with inflammatory disease of experimental autoimmune encephalomyelitis animals and protection by lovastatin treatment. Brain Res. 2004;1022:1–11. doi: 10.1016/j.brainres.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 259.Wood PL, Smith T, Pelzer L, Goodenowe DB. Targeted metabolomic analyses of cellular models of pelizaeus-merzbacher disease reveal plasmalogen and myo-inositol solute carrier dysfunction. Lipids Health Dis. 2011;10:102. doi: 10.1186/1476-511X-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Khan M, Singh J, Singh I. Plasmalogen deficiency in cerebral adrenoleukodystrophy and its modulation by lovastatin. J Neurochem. 2008;106:1766–1779. doi: 10.1111/j.1471-4159.2008.05513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Abdullah L, Evans JE, Ferguson S, Mouzon B, Montague H, Reed J, Crynen G, Emmerich T, Crocker M, Pelot R, et al. Lipidomic analyses identify injury-specific phospholipid changes 3 mo after traumatic brain injury. FASEB J. 2014;28:5311–5321. doi: 10.1096/fj.14-258228. [DOI] [PubMed] [Google Scholar]
- 262.Tzekov R, Dawson C, Orlando M, Mouzon B, Reed J, Evans J, Crynen G, Mullan M, Crawford F. Sub-chronic neuropathological and biochemical changes in mouse visual system after repetitive mild traumatic brain injury. PLoS One. 2016;11:e0153608. doi: 10.1371/journal.pone.0153608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Demediuk P, Saunders RD, Anderson DK, Means ED, Horrocks LA. Membrane lipid changes in laminectomized and traumatized cat spinal cord. Proc Natl Acad Sci USA. 1985;82:7071–7075. doi: 10.1073/pnas.82.20.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Lukacova N, Halat G, Chavko M, Marsala J. Ischemia-reperfusion injury in the spinal cord of rabbits strongly enhances lipid peroxidation and modifies phospholipid profiles. Neurochem Res. 1996;21:869–873. doi: 10.1007/BF02532334. [DOI] [PubMed] [Google Scholar]
- 265.Viani P, Zini I, Cervato G, Biagini G, Agnati LF, Cestaro B. Effect of endothelin-1 induced ischemia on peroxidative damage and membrane properties in rat striatum synaptosomes. Neurochem Res. 1995;20:689–695. doi: 10.1007/BF01705537. [DOI] [PubMed] [Google Scholar]
- 266.Satoh K, Imaizumi T, Yoshida H, Hiramoto M, Takamatsu S. Increased levels of blood platelet-activating factor (PAF) and PAF-like lipids in patients with ischemic stroke. Acta Neurol Scand. 1992;85:122–127. doi: 10.1111/j.1600-0404.1992.tb04010.x. [DOI] [PubMed] [Google Scholar]
- 267.Adunsky A, Hershkowitz M, Atar E, Bakoun M, Poreh A. Infarct volume, neurological severity and PAF binding to platelets of patients with acute cerebral ischemic stroke. Neurol Res. 1999;21:645–648. doi: 10.1080/01616412.1999.11740990. [DOI] [PubMed] [Google Scholar]