Abstract
Antiangiogenic agents block the effects of tumor-derived angiogenic factors (paracrine factors), such as vascular endothelial growth factor (VEGF), on endothelial cells (EC), inhibiting the growth of solid tumors. However, whether inhibition of angiogenesis also may play a role in liquid tumors is not well established. We recently have shown that certain leukemias not only produce VEGF but also selectively express functional VEGF receptors (VEGFRs), such as VEGFR-2 (Flk-1, KDR) and VEGFR1 (Flt1), resulting in the generation of an autocrine loop. Here, we examined the relative contribution of paracrine (EC-dependent) and autocrine (EC-independent) VEGF/VEGFR signaling pathways, by using a human leukemia model, where autocrine and paracrine VEGF/VEGFR loops could be selectively inhibited by neutralizing mAbs specific for murine EC (paracrine pathway) or human tumor (autocrine) VEGFRs. Blocking either the paracrine or the autocrine VEGF/VEGFR-2 pathway delayed leukemic growth and engraftment in vivo, but failed to cure inoculated mice. Long-term remission with no evidence of disease was achieved only if mice were treated with mAbs against both murine and human VEGFR-2, whereas mAbs against human or murine VEGFR-1 had no effect on mice survival. Therefore, effective antiangiogenic therapies to treat VEGF-producing, VEGFR-expressing leukemias may require blocking both paracrine and autocrine VEGF/VEGFR-2 angiogenic loops to achieve remission and long-term cure.
Emerging data suggests that targeting tumor angiogenesis with agents that block endothelial proliferation, such as with neutralizing vascular endothelial growth factor receptor (VEGFR)-specific antibodies, results in delayed growth of solid tumor cell lines implanted into mice (1). However, the exact mechanism whereby inhibition of the VEGF-VEGFR axis results in the regression of tumor tissue is not well studied.
There is accumulating evidence that angiogenesis supports the growth of solid tumors in vitro and in vivo. It has been suggested that as the tumor endothelial mass expands, in response to tumor-derived angiogenic factors such as VEGF, it supports tumor growth in a paracrine fashion. However, demonstration that angiogenesis also plays a role in liquid tumors such as leukemia has not been rigorously examined. Nevertheless, there are numerous reports suggesting there is increased bone marrow angiogenesis in patients with different hematological malignancies such as acute lymphoblastic leukemias (2–6). Moreover, leukemic cell release of angiogenic growth factors such as VEGF portends poor clinical outcome and progression of the disease (5). Similar to solid tumor growth, in response to leukemia-derived angiogenic factors such as VEGF, it has been suggested that the proliferating bone marrow endothelial mass may release growth factors that support leukemic cell growth in a paracrine fashion (7). Therefore, blocking VEGF signaling on endothelial cells (EC) may reduce growth factor production, thereby retarding leukemic growth. However, thus far, studies in a clinical setting have failed to demonstrate whether blocking angiogenesis results in delayed human leukemic growth in vivo.
We recently have shown that certain leukemic cells not only produce VEGF, but have also acquired the capacity to express functional VEGFRs (such as VEGFR-2), which results in the generation of an endothelial-independent autocrine loop that supports leukemic survival and migration in vivo (8). Therefore, in VEGF-producing, VEGFR-expressing leukemias, generation of VEGF/VEGFR autocrine (endothelial-independent) and paracrine (endothelial-dependent) loops, may contribute toward leukemic growth. In the present report we assessed the relative contribution of paracrine and autocrine VEGF/VEGFR signaling pathways to the growth of human leukemias in vivo. This was achieved by using neutralizing mAbs specific for human or mouse VEGFRs. We demonstrate that targeting the paracrine (endothelial-dependent) or the autocrine (endothelial-independent) VEGF/VEGFR-2 pathway delays leukemic growth, but it is not sufficient to cure inoculated mice. Long-term remission with no evidence of leukemia was achieved only if mice were treated with mAbs against both murine and human VEGFR-2, whereas mAbs against murine or human VEGFR-1 had no effect. Therefore, effective antiangiogenic therapies against VEGF-producing, VEGFR-expressing human leukemias should target both endothelial-dependent and endothelial-independent signaling pathways to achieve remission.
Materials and Methods
All materials were obtained from Sigma, unless indicated otherwise.
Cell Culture.
HL-60 pro-myelomonocytic leukemia cells (obtained from American Type Tissue Culture Collection) were cultured in RPMI (Life Technologies, Grand Island, NY) medium, supplemented with 10% FBS, penicillin, and streptomycin. Primary leukemic samples were isolated from the peripheral blood of leukemic patients, and cultured overnight in serum-free RPMI. For each in vivo experiment, viable leukemic cells were counted and ressuspended in serum-free RPMI.
Human umbilical vein EC (HUVEC) and bone marrow-derived EC (BMEC) were cultured in complete endothelial medium as described (21). For leukemia proliferation experiments, EC were cultured in 6-well plates (Costar) in complete EC medium until the start of the experiment. Before starting the proliferation experiments, EC were placed in serum-free medium.
Endothelium/Leukemia Cocultures.
Leukemic cells were cultured alone, or in the presence of a confluent layer of HUVEC or BMEC in serum-free medium. To avoid direct cell/cell contact, 1 × 105 leukemic cells were placed on transwell (Costar) inserts with a pore size of 0.4 μm. Viable cells were counted by trypan blue exclusion, using a hemacytometer.
Cytokine ELISA.
Conditioned medium was collected from BMEC or HUVEC monolayers, cultured in serum-free conditions over a 48-h period. Granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-6 levels were measured by ELISA, following conventional protocols (Cytokine Core Laboratory, Baltimore).
Antibodies.
Neutralizing mAbs against human (clone IMC-1C11) or mouse (clone DC101) VEGFR-2 (KDR/Flk-1) and against human (clone 6.12) or mouse (clone mF-1) VEGFR-1 were provided by ImClone Systems. These antibodies are specific for one or the other VEGFR and have strict species specificity. A human Ig preparation (Bayer, Elkhart, IN) was used at the same dose as the neutralizing antibodies, as a negative control.
Leukemic Growth in Vivo.
Nonobese diabetic immunocompromised (NOD-SCID) mice, age-matched (6–8 weeks) and sex-matched, were used in all experiments. Briefly, mice were irradiated with 3.5 Gy from a 137Cs g-ray source at a dose rate of ≈0.90 Gy/min and intravenously inoculated with 1 × 107 HL-60 cells in 0.2 ml serum-free RPMI on day 0. Three days after inoculation, mice were divided into four groups of eight mice. One group was treated with 400 μg human IgG thrice weekly (control), one group received IMC-1C11 alone (400 μg/mouse thrice weekly), one was treated with DC101 alone (800 μg/mouse thrice weekly), and the last group was treated with IMC-1C11 and DC101 twice weekly, at the same dosage. Each experiment was done twice. Mice were evaluated for any signs of toxicity throughout the experiment, and survival was recorded. At days 7, 14, 21, 42, 60, and 120 after the start of the experiment, two mice from each group were killed, and their organs and peripheral blood were collected for histology and quantification of plasma VEGF levels and circulating leukemic cells, respectively.
Bone Marrow Leukemic Cell Content.
The presence of human leukemia cells in the bone marrow of inoculated mice was quantified by flow cytometry. Briefly, surgically removed tibias day 21 after the start of the experiment were flushed from their bone marrow content with a 19-g syringe, and the single cell suspension was stained for the presence of leukemic cells. Antibodies used for the FACS analysis were: mAb against human CD15 [phycoerythrin (PE)-labeled, clone DU-HL60–3, Sigma]; mAb against human VEGFR-2 (clone 6.64, ImClone Systems), with a secondary goat anti-mouse IgG PE-labeled antibody (Kirkegaard & Perry Laboratories); mAb against murine VEGFR-2 (FITC-labeled DC101) and an isotypic control, MSIgG1 (FITC/PE-labeled clone 2T8–2F5, Coulter). The percentage of human CD15+ VEGFR-2+ leukemic cells and murine VEGFR-2+ cells in mouse bone marrow samples was determined by using a Coulter Elite flow cytometer. These results are representative of three mice/treatment group. Each experiment was done twice.
VEGF Plasma Levels.
Human and murine-VEGF plasma levels were evaluated by using commercial ELISA kits (R&D Systems), according to the manufacturer's instructions. These assays had a sensitivity of 7.5 pg/ml. Each sample was analyzed in duplicate, and two samples (a total of six mice) were assayed per time point. The results shown are representative of three mice/time point and two separate experiments.
Statistical Analysis.
The FACS results are expressed as mean ± SEM. Statistical analyses were performed by using the unpaired two-tailed Student's t test. For survival analysis, the nonparametric one-tailed Mann–Whitney u test was used.
Results
EC Support Leukemic Cell Growth in a Paracrine Fashion.
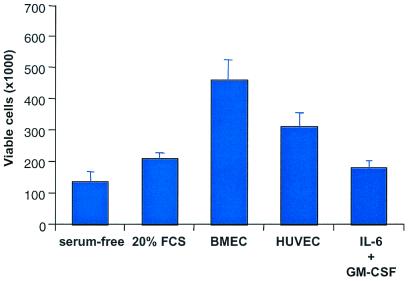
Freshly isolated leukemias, or their cell line counterpart, if cultured in serum-free condition survive and even show a modest increase in proliferation over a 48-h period (ref. 8 and Fig. 1). Earlier, we showed that leukemia survival in serum-free conditions is caused by the generation of an autocrine VEGF/VEGFR-2 loop (8). However, if cultured in the presence of bone marrow-derived or umbilical vein EC, leukemic cells proliferate significantly more (Fig. 1). This finding suggested that EC support leukemia growth and survival in vitro, perhaps by secreting specific growth factors into the cell culture supernatants. Therefore, we analyzed EC supernatants for the presence of growth factors that promote leukemic growth, such as GM-CSF, IL-6, or IL-8 (Table 1). In the present study, both endothelial sources released significant amounts of GM-CSF, IL-6, or IL-8 into the cell culture supernatants, as measured by ELISA (Table 1). This finding indicates that EC may support leukemia survival and proliferation in a paracrine fashion, by secreting specific growth factors that will subsequently act on the leukemic cells. Importantly, a constant source of growth factors appears to be critical for the survival and proliferation of the leukemic cells, because addition of exogenous GM-CSF or IL-6 to leukemic cultures was less effective at promoting leukemia proliferation (Fig. 1).
Figure 1.
Incubation of freshly isolated monocytic leukemic cells (M5) or cell lines (HL-60) in the presence of BMEC or HUVEC resulted in a remarkable increase in leukemic proliferation over a 48-h period (P < 0.05). As control, leukemic cells were incubated with IL-6 (10 ng/ml) and GM-CSF (10 ng/ml), complete medium or in serum-free conditions. Results shown represent the average leukemia proliferation with three primary leukemias. Each experiment was done in triplicate.
Table 1.
GM-CSF, IL-6, and IL-8 production
| BMEC | HUVEC | |
|---|---|---|
| GM-CSF | 373 ± 78.3 | 100.2 ± 2.8 |
| IL-6 | 292 ± 21. | 96.3 ± 3.9 |
| IL-8 | 440 ± 15 | 410 ± 22.3 |
GM-CSF, IL-6, and IL-8 production over a 48-h period, by confluent BMEC or HUVEC layers, as determined by ELISA. Each sample was measured in triplicate and is shown as pg/ml per 106 cells.
As they expand, the leukemic cells produce angiogenic growth factors such as VEGF and fibroblast growth factor, which stimulate both proliferation as well as growth factor production by the endothelial layer. Notably, activation of HUVEC or BMEC by angiogenic growth factors such as VEGF in vitro significantly increased the production of GM-CSF, IL-6, and IL-8, mimicking the coculture system described above (data not shown). Taken together, these results suggest that generation of such paracrine loops in vivo may be essential for leukemia engraftment and proliferation of a malignant clone.
Next, we examined the relative contribution of the paracrine (endothelial-derived) as well as autocrine (endothelial-independent) loops toward leukemic growth and engraftment in vivo.
Autocrine and Paracrine VEGF/VEGFR2 Signaling Pathways Are Essential for Leukemic Cell Growth.
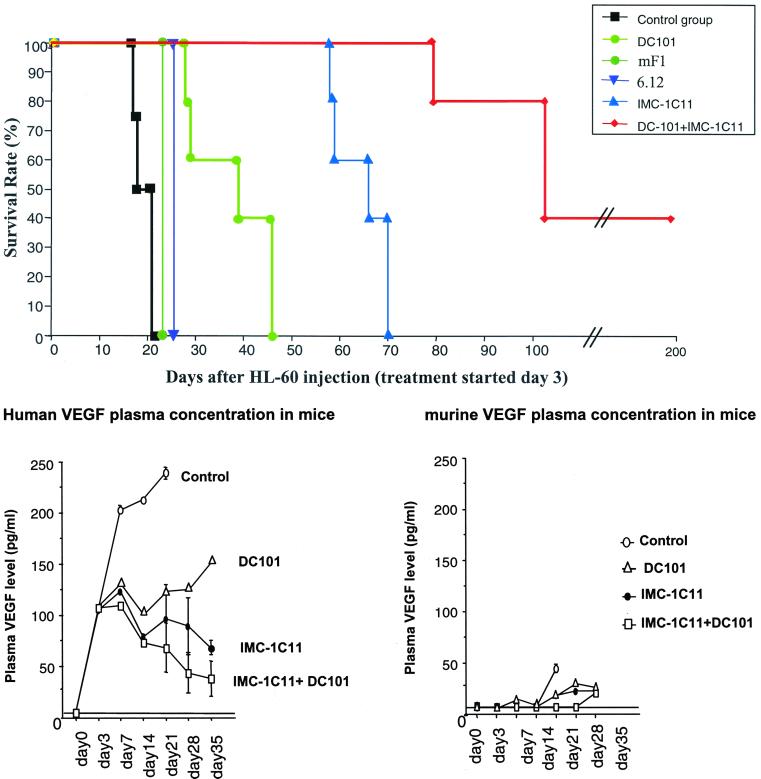
Sublethally irradiated NOD/SCID mice inoculated with HL-60 leukemic cells, if left untreated, or treated with an irrelevant human IgG, die within 2–3 weeks (Fig. 2A, mean survival time = 18.2 ± 1.3 days. In these mice, clinical signs and symptoms of acute leukemia, such as severe anemia, lymphadenopathy, and cachexia became apparent within 2 weeks. As with leukemic patients (3), clinical progression in inoculated mice is characterized by increased circulating human VEGF levels. As shown in Fig. 2B, human, but not murine, VEGF plasma levels increased in inoculated mice with disease progression.
Figure 2.
(A) Mouse survival (%) after i.v. injection of HL-60 leukemia cells. Neutralizing mAbs against human (clone IMC-1C11) or mouse (clone DC101) VEGFR-2 (KDR/Flk-1) and human (clone 6.12) and mouse (mF1) VEGFR-1 (FLT-1). Mice treated with DC101 survived two times more than control (P < 0.05), whereas those treated with IMC-1C11 survived three times more (P < 0.005). Mice treated with mF1 or 6.12 showed no increase in survival as compared with control mice. All mice treated with IMC-1C11 + DC101 survived up to day 100, and 40% survived beyond day 200. (B) HL-60 injection into NOD-SCID mice induces high levels of human but not murine VEGF in mouse plasma. Human and murine VEGF plasma levels were determined at different time points postinjection. Mice treated with IMC-1C11, DC101, or IMC-1C11 + DC101 had reduced circulating human VEGF levels up to day 35 (P < 0.01).
To investigate whether the growth of leukemia in vivo is angiogenesis-dependent, or is promoted solely via an autocrine VEGF/human VEGFR stimulation, we used neutralizing mAbs to murine (clone DC101) or human (clone IMC-1C11) VEGFR-2 and mAb to murine (clone mF1) or human (clone 6.12) VEGFR-1. These antibodies have strict species specificity and are capable of blocking VEGF-induced receptor phosphorylation and endothelial proliferation (9–11). In addition, we have previously shown that the HL-60 leukemic cells used in our studies express functional VEGFR-2, and therefore respond to treatment with IMC-1C11 (8). Moreover, DC101 (antimurine VEGFR-2) has been shown to delay tumor growth, through inhibition of angiogenesis, in several murine solid tumor models (10).
Treatment of leukemic mice with DC101, three times a week, at 800 μg/injection (a treatment schedule determined for other tumor models), prolonged survival by more than 2-fold (Fig. 2A, mean survival time = 36.8 ± 6.3 days, P < 0.05 compared with the control group), but the mice eventually died within 42 days. Treatment with IMC-1C11 (three times a week, at 400 μg/injection) prolonged survival by more than 3-fold (Fig. 2A, mean survival time = 70.3 ± 3.3 days, P < 0.01) compared with the human IgG-treated group but, again, all of the mice still died within 70–80 days. The results obtained by treating leukemic mice with DC101 show that blocking VEGF-induced angiogenesis in vivo, via interaction with murine VEGFR-2, delays leukemic growth, but is not sufficient to eradicate the disease. Targeting the autocrine VEGF/VEGFR-2 loop on leukemic cells with IMC-1C11 significantly blocked leukemic growth and invasiveness, but also failed to induce long-term remission of inoculated mice. Notably, administration of either mF1 or 6.12 to leukemia-inoculated mice, to target the paracrine or the autocrine VEGF/VEGFR-1 loops, respectively, had no effect on mouse survival. Mice treated with either mF1 or 6.12 died because of leukemia proliferation 23 and 25 days after the start of the experiment, similarly to those in the control group (Fig. 2A). These results demonstrate that the VEGF/VEGFR-2 loops are critical for leukemia proliferation in vivo, whereas VEGFR-1 appears to play a marginal role.
Coadministration of IMC-1C11 and DC101 had a synergistic effect on survival of leukemic mice. Eighty percent (10/12, and 11/12 in a total of two experiments, P < 0.001) of the mice treated with both antibodies survived beyond 100 days (Fig. 2A). Moreover, 40% of these mice achieved prolonged remission (up to 200 days, Fig. 2A) with no clinical symptoms or histological evidence of disease. Given the overall short life expectancy of NOD-SCID mice (≈10–12 months), this result is highly significant. As predicted, plasma levels of human VEGF remained lower in the DC101 + IMC-1C11-treated group, presumably reflecting slower tumor progression (Fig. 2B).
Targeting Autocrine and Paracrine VEGF/VEGFR-2 Angiogenic Pathways Blocks Leukemia Invasiveness.
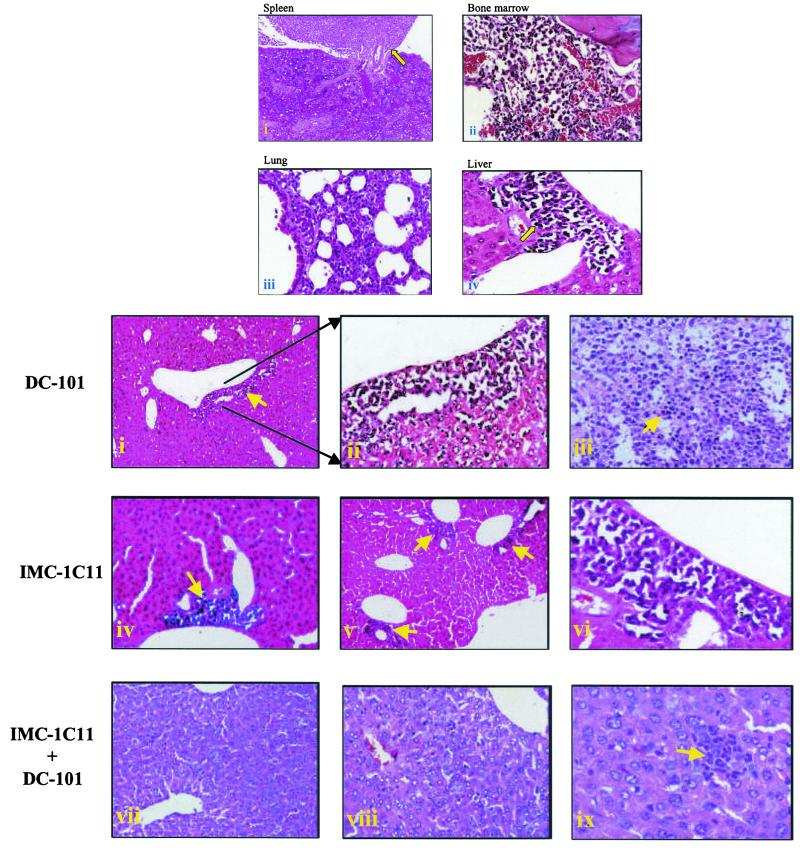
We determined whether treatment of leukemic mice with the anti-VEGFR-2 antibodies also blocked the dissemination of the disease. Gross inspection of control mice on days 7 and 14 revealed marked lymphadenopathy and infiltration of HL-60 cells into the bone marrow, spleen, liver, and lung. Infiltrating HL-60 cells also were found in the liver, spleen, bone marrow, and lungs of control mice on day 21 (Fig. 3A). In addition, bone marrow sections showed abundant vascularization, suggesting increased angiogenesis (Fig. 3A). Similarly, leukemia mice treated with mF1 or 6.12 also had evidence of leukemia proliferation in peripheral organs and bone marrow (data not shown), which correlated with the inability of these antibodies to delay leukemic growth. In contrast, all organs from IMC-1C11- or DC101-treated mice were free of HL-60 cells microscopically on days 7 and 14, and, even after 21 days, tumor infiltration was limited to the perivascular areas of the liver (Fig. 3B). DC101-treated mice did eventually develop lymphadenopathy and massive tumor infiltration of the spleen (Fig. 3B), at which point they succumbed to the proliferating leukemia. In mice treated with IMC-1C11, tumor infiltration was reduced and still limited to the perivascular area of the liver on day 42 (Fig. 3B). However, in mice treated with both antibodies, there was no evidence of disseminated leukemia until day 60 (Fig. 3B), at which time selected liver sections had evidence of micrometastases (Fig. 3B). All mice in this group survived beyond 100 days and, upon achieving remission, had no evidence of metastatic disease up to day 120 (last time point analyzed, data not shown).
Figure 3.
(A) Histology of spleen (i), bone marrow (ii), lung (iii), and liver (iv) from control HL-60-injected mice day 21 postleukemia inoculation. (i) Note a massive tumor infiltrate (arrow) in the spleen of these mice. The bone marrow (ii) has evidence of increased angiogenesis and is largely replaced by tumor cells. Finally, the lungs of these mice were largely replaced by tumor cells (iii), and the liver showed extensive areas of invading tumor cells (iv). (Magnifications: i–iii, ×; iv, ×400.) (B) Histology of livers (i, ii, and iv–ix) and spleen (iii) of HL-60-inoculated, antibody-treated mice. DC101-treated mice, day 21 postinoculation, had some sign of leukemic infiltrates in the liver (arrow, i and ii) and the spleen was largely replaced by tumor cells (iii). On the other hand, in IMC-1C11-treated mice tumor infiltration was localized to the perivascular area of the liver on days 21 and 42 (iv–vi). Finally, IMC-1C11 + DC101-treated mice had no evidence of metastatic disease on days 21 and 42 (vii and viii) but one small micrometastasis could be detected on day 60 (ix). (Magnifications: i, iii, iv, v, vii, viii, and ix, ×200; ii and vi, ×400.)
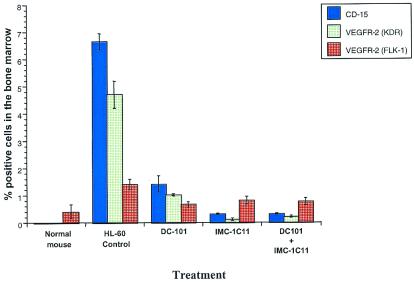
To determine the effects of anti-VEGFR-2 antibody therapy on the capacity of human leukemic cells to remain engrafted, we examined the bone marrow cells of leukemic mice by flow cytometric analysis, using human-specific antibodies to leukemic (CD15) and endothelial (VEGFR-2) markers. Twenty one days after the start of the experiment, there were large numbers of human-CD15+, VEGFR-2+ cells in the control group treated with human-IgG (Fig. 3, human CD15+, 7% of total bone marrow cells; VEGFR-2+, 5% of bone marrow cells). On the other hand, in IMC-1C11, DC101- or IMC-1C11 + DC101-treated mice, at the same time point, the number of leukemic cells in the bone marrow was significantly reduced, with human CD15+ VEGFR-2+ cells comprising less than 1% of the total bone marrow cells. Similar results were obtained in peripheral blood samples from the same mice (data not shown). In addition, to confirm that leukemia engraftment increases bone marrow angiogenesis, we analyzed bone marrow samples for the presence of VEGFR-2+ (Flk-1+) murine EC. Twenty one days after the start of the experiment, the percentage of murine VEGFR-2+ bone marrow cells increased from 0.4% in noninoculated normal mice (baseline levels), to over 1.4% in human IgG-treated, leukemia-inoculated mice (Fig. 4). This result correlated with the bone marrow histology (Fig. 2A) and suggests that bone marrow angiogenesis increases after inoculation with leukemic cells. The percentage of murine VEGFR-2+ cells in bone marrow aspirates was reduced to baseline levels in IMC-1C11-, DC101-, and IMC-1C11 + DC101-treated mice (Fig. 4). Similar results were obtained in mice treated with IMC-1C11 alone (up to day 60) or the combination of IMC-1C11 + DC101 up to day 120 (data not shown).
Figure 4.
Leukemic cell engraftment (%) in the bone marrow of normal (noninoculated) and HL-60 inoculated NOD-SCID mice. HL-60-injected mice had a 2-fold increase in the number of Flk-1-positive cells, compared with normal mice, reflecting an increase in bone marrow angiogenesis after leukemia inoculation. DC101-, IMC-1C11-, and DC101 + IMC-1C11-treated mice had a significantly lower percentage of human cells in the bone marrow than untreated mice (P < 0.001).
Discussion
It has been recently shown in murine models of human solid tumors that combining antiangiogenesis agents, such as the mAb to VEGFR-2 (DC101), with standard chemotherapy or radiotherapy increases therapeutic efficacy (12, 13). In a separate study, metronomic administration of chemotherapeutic drugs to mice bearing different tumors was shown to be more effective than bulk chemotherapy, because it produced antitumor as well as antiangiogenesis effects (14). These studies highlighted the importance of the autocrine (targeting tumor cells) and paracrine (targeting tumor angiogenesis) components of tumor growth, but have not identified any particular molecule(s) or factor(s) involved in this processes. In addition, thus far a clear demonstration that angiogenesis also plays an important role in the progression of liquid tumors such as acute leukemias has not been provided.
In the present report, we have shown, using an in vivo model of human leukemia, that blocking angiogenesis induced by the interaction of leukemia-derived VEGF with murine VEGFR-2 delays leukemic growth, but is not sufficient for its eradication from inoculated mice. These results demonstrate that targeting VEGF-induced angiogenesis may be at least partially effective at delaying leukemia progression. In addition, these in vivo results correlate with the ability of endothelial layers to support leukemia proliferation in the absence of any exogenous growth factors in vitro. The generation of such endothelial-leukemia paracrine loops may be of critical importance for the establishment of the disease. On the other hand, earlier we showed that subsets of leukemias survive and proliferate in serum-free conditions, in an endothelial-independent manner, because of the existence of a VEGF/VEGFR-2 autocrine loop (8).
In the present study, we evaluated the relative contribution of paracrine (endothelial-dependent) and autocrine (endothelial-independent) loops in supporting leukemic growth and engraftment in vivo. Notably, long-term remission with no evidence of the disease could be achieved only if mice were treated with neutralizing mAb against murine and human VEGFR-2, blocking the paracrine and autocrine VEGF/VEGFR-2 signaling pathways. On the other hand, mAbs against murine or human VEGFR-1 had no effect toward improving the survival of leukemic mice, suggesting that the VEGF/VEGFR-2 pathway is more important for the proliferation and engraftment of acute leukemias in vivo. However, it is also possible that certain leukemias may depend on VEGFR-1 signalling.
As suggested for solid tumors, our data suggest that therapeutic strategies directed against leukemic cells, such as standard chemotherapy, may be insufficient to completely eradicate a proliferating leukemic mass. On the other hand, antiangiogenesis therapies target only tumor-induced neo-vascularization, and a number of studies already have shown that this strategy results in delayed growth of solid tumor cell lines implanted into mice (15, 16). However, based on evidence shown here, antiangiogenesis therapy targeting the paracrine loop alone may be insufficient to completely eradicate proliferating, VEGF-producing and VEGFR-expressing leukemias. Interestingly, most solid tumors produce VEGF, and subsets of solid tumor cells express at least one of its receptors (17–19), suggesting that the observations described above may apply to other tumors as well.
Emerging data suggest that VEGF production by leukemia cells may reflect their ability to engraft into mice in vivo (20). Based on the results shown in the present report, we hypothesize that VEGF production and VEGFR coexpression by leukemic cells may identify a leukemic clone with the capacity to engraft and proliferate in vivo. VEGF production has been correlated with disease progression in patients with a variety of hematological malignancies (3–6), but the expression of VEGFRs on circulating leukemic blasts has not been evaluated. In this regard, it would be of interest to identify other proangiogenic or hematopoietic cytokines that regulate VEGFR expression on the leukemic cells. Also, VEGFR expression by leukemias may correlate with leukemia progression or resistance to conventional therapies. Effective therapeutic strategies against rapidly proliferating acute leukemias may therefore be required to regulate VEGF production and VEGFR expression by the expanding leukemic clone.
Taken together, the data presented here, underscoring the significance of autocrine and paracrine/angiogenic pathways, may lay the foundation for a different therapeutic approach for the treatment of VEGF-producing, VEGFR-expressing tumors, in particular acute leukemias. In addition, it also introduces additional paradigms in tumor angiogenesis that will facilitate tailoring future clinical anti-angiogenesis trials based on expression pattern of VEGFR-1 or VEGFR-2.
Acknowledgments
S.R. is supported by a Translational Research Award from The Leukemia and Lymphoma Society, National Heart, Lung, and Blood Institute Grants R01 HL-58707 and R01 HL-61849, Research Scholar Grant from American Cancer Society (RSG-01-091-01), the Dorothy Rodbell Foundation for Sarcoma Research, and the Lupin Foundation. M.A.S.M. is supported by National Heart, Lung, and Blood Institute Grant R01 HL-61401 and the Gar Reichman Fund of Cancer Research Institute. B.H. is the recipient of a fellowship from the Mildred Scheel Stiftung fur Krebsforschung (Bonn, Germany). K.H. is the recipient of a fellowship from the Uehara Memorial Foundation (Tokyo).
Abbreviations
- VEGF
vascular endothelial growth factor
- VEGFR
VEGF receptor
- EC
endothelial cells
- HUVEC
human umbilical vein EC
- BMEC
bone marrow-derived EC
- GM-CSF
granulocyte–macrophage colony-stimulating factor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Lu D, Kussie P, Pytowski B, Persaud K, Bohlen P, Witte L, Zhu Z. J Biol Chem. 2000;275:14321–14330. doi: 10.1074/jbc.275.19.14321. [DOI] [PubMed] [Google Scholar]
- 2.Perez-Atayde A R, Sallan S E, Tedrow U, Connors S, Allred E, Folkman J. Am J Pathol. 1997;150:815–821. [PMC free article] [PubMed] [Google Scholar]
- 3.Hussong J W, Rodgers G M, Shami P J. Blood. 2000;95:309–313. [PubMed] [Google Scholar]
- 4.Padro T, Ruiz S, Bieker R, Burger H, Steins M, Kienast J, Buchner T, Berdel W E, Mesters R M. Blood. 2000;95:2637–2644. [PubMed] [Google Scholar]
- 5.Aguayo A, Estey E, Kantarjian H, Mansouri T, Gidel C, Keating M, Giles F, Estrov Z, Barlogie B, Albitar M. Blood. 1999;94:3717–3721. [PubMed] [Google Scholar]
- 6.Aguayo A, Kantarjian H, Manshouri T, Gidel C, Estey E, Thomas D, Koller C, Estrov Z, O'Brien S, Keating M, et al. Blood. 2000;96:2240–2245. [PubMed] [Google Scholar]
- 7.Fiedler W, Graeven U, Ergun S, Verago S, Kilic N, Stockschlader M, Hossfeld D K. Blood. 1997;89:1870–1875. [PubMed] [Google Scholar]
- 8.Dias S, Hattori K, Zhu Z, Heissig B, Choy M, Lane W, Wu Y, Chadburn A, Hyjek E, Gill M, et al. J Clin Invest. 2000;106:511–521. doi: 10.1172/JCI8978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Z, Rockwell P, Lu D, Kotanides H, Pytowski B, Hicklin D J, Bohlen P, Witte L. Cancer Res. 1998;58:3209–3214. [PubMed] [Google Scholar]
- 10.Prewett M, Huber J, Li Y, Santiago A, O'Connor W, King K, Overholser J, Hooper A, Pytowski B, Witte L, et al. Cancer Res. 1999;59:5209–5218. [PubMed] [Google Scholar]
- 11.Zhu Z, Lu D, Kotanides H, Santiago A, Jimenez X, Simcox T, Hicklin D J, Bohlen P, Witte L. Cancer Lett. 1999;136:203–213. doi: 10.1016/s0304-3835(98)00324-3. [DOI] [PubMed] [Google Scholar]
- 12.Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin D J, Bohlen P, Kerbel R S. J Clin Invest. 2000;105:R15–R24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mauceri H J, Hanna N N, Beckett M A, Gorski D H, Staba M J, Stellato K A, Bigelow K, Heimann R, Gately S, Dhanabal M, et al. Nature (London) 1998;394:287–291. doi: 10.1038/28412. [DOI] [PubMed] [Google Scholar]
- 14.Browder T, Butterfield C E, Kraling B M, Shi B, Marshall B, O'Reilly M S, Folkman J. Cancer Res. 2000;60:1878–1886. [PubMed] [Google Scholar]
- 15.Folkman J. Semin Cancer Biol. 1992;3:65–71. [PubMed] [Google Scholar]
- 16.Folkman J. Exs. 1997;79:1–8. doi: 10.1007/978-3-0348-9006-9_1. [DOI] [PubMed] [Google Scholar]
- 17.Ferrer F A, Miller L J, Lindquist R, Kowalczyk P, Laudone V P, Albertsen P C, Kreutzer D L. Urology. 1999;54:567–572. doi: 10.1016/s0090-4295(99)00156-9. [DOI] [PubMed] [Google Scholar]
- 18.Herold-Mende C, Steiner H H, Andl T, Riede D, Buttler A, Reisser C, Fusenig N E, Mueller M M. Lab Invest. 1999;79:1573–1582. [PubMed] [Google Scholar]
- 19.Speirs V, Atkin S L. Br J Cancer. 1999;80:898–903. doi: 10.1038/sj.bjc.6690438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fusetti L, Pruneri G, Gobbi A, Rabascio C, Carboni N, Peccatori F, Martinelli G, Bertolini F. Cancer Res. 2000;60:2527–2534. [PubMed] [Google Scholar]
- 21.Mohle R, Bautz F, Rafii S, Moore M A, Brugger W, Kanz L. Ann NY Acad Sci. 1999;872:176–185. doi: 10.1111/j.1749-6632.1999.tb08463.x. [DOI] [PubMed] [Google Scholar]