Abstract
Atrial switch operations for D-Transposition of the great arteries (D-TGA) were performed until the late 20th century. These patients have substantial rates of re-operation, particularly for baffle related complications. This study sought to analyze the efficacy of percutaneous transcatheter intervention (PTI) for baffle leak and/or stenosis in adult atrial switch patients. Adult patients with a prior atrial switch operation who underwent heart catheterization (2002–2014) at a tertiary adult congenital heart disease referral center were retrospectively analyzed. In 58 adults (30 ± 8 years, 75% men, 14% New York Heart Association (NYHA) functional class ≥2) who underwent 79 catheterizations, PTI was attempted in 50 (baffle leak (n = 10, 20%), stenosis (n = 27, 54%), or both (n = 13, 26%)). PTI was successful in 45 and 5 were referred for surgery due to complex anatomy. A total of 40 bare metal stents, 18 covered stents, 16 occlusion devices, 2 angioplasties, and 1 endovascular graft were deployed. In isolated stenosis, there was improvement in NYHA functional class after PTI (8 vs. 0 patients were NYHA FC > 2, p = 0.004), which was matched by improvement in maximal oxygen consumption on exercise testing (VO2) (25.1 ± 5.4 mL/kg/min vs. 27.9 ± 9 mL/kg/min, p = 0.03). There were no procedure-related deaths or emergent surgeries in this cohort.
This single-center cohort is the largest reported series of adult atrial switch operation patients who have undergone PTI for baffle stenosis and/or leak. We demonstrate that PTI with an expert multi-disciplinary team is a safe and effective alternative to surgery in adult patients with an atrial switch operation.
Keywords: D-transposition of the great arteries, Mustard repair, Percutaneous intervention, Baffle leak, Baffle stenosis
1. Introduction
Cyanotic congenital heart patients have benefitted from cardiothoracic surgical innovation that occurred in the mid to late 20th century, without which, these conditions would not be survivable. Among this group, patients with dextro-transposition of the great arteries (D-TGA) are unique. Atrial switch operations, such as the Mustard [1] or Senning [2] procedures, became available in 1963 and 1957 respectively, and have subsequently been supplanted by the arterial switch (Jatene) operation [3]. Late re-operation is often required after Mustard/Senning repairs, and most frequently this is for baffle-related complications such as stenosis and/or leak. In an effort to avoid the morbidity and mortality associated with re-operation, percutaneous transcatheter interventions (PTI) aimed at treating baffle stenosis and leaks are often sought. To date, there is limited data describing the efficacy and outcomes with currently available percutaneous treatment options. We sought to analyze the efficacy of PTI in adult atrial switch patients undergoing PTI for baffle leak and/or stenosis at a large U.S. tertiary referral adult congenital heart disease center with expertise in both routine and high risk percutaneous interventions.
2. Methods
Adult patients with a prior atrial switch operation who underwent heart catheterization (2002–2014) at our center were retrospectively analyzed. We collected basic demographics, exercise test results, echo-cardiograms and advanced cardiac imaging (computed tomography or magnetic resonance imaging) when available, as well as the results of cardiac catheterization/PTI. More specifically, when collecting invasive hemodynamic results, we included baseline and post-PTI oxygen saturation, baffle diameter, and baffle gradient measurements. Information specific to the intervention performed, devices used, presence of post-angiographic leak, and major adverse events (death, need for emergent operation, bleeding requiring transfusion, need for urgent/emergent repeat percutaneous intervention) were also collected. In patients who received an electrophysiology study (EPS) or device (pacemaker or defibrillator), data specific to this procedure was reviewed: inducible arrhythmia, ablation, device placed, and lead extraction if required.
Given the retrospective nature of this study comprised of data from the previous 12 years, consent waiver was obtained, and the local institutional review board approved this study. Descriptive data analyzing baseline characteristics of the total population, those with isolated baffle stenosis, isolated baffle leak, and combination leak/stenosis was generated. Descriptive characteristics of devices placed, with respect to the underlying baffle abnormality (leak or stenosis), were also generated. When evaluating for differences between pre-PTI and post-PTI parameters, dependent T-tests were used to compare continuous variables and Fisher’s exact tests were used to analyze categorical and ordinal variables. All significance tests were evaluated with type I error rate of 5% (α = 0.05). All data are presented as means with standard deviation. The data were analyzed using SPSS (IBM Corp. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY).
3. Results
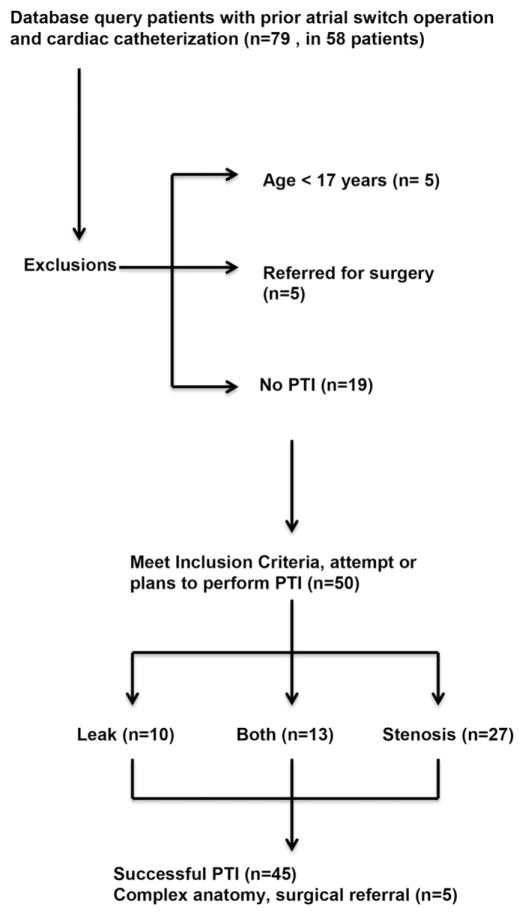
There were 58 adults (30 ± 8 years, 75% men) who underwent 79 catheterizations at our institution over 12 years. In those who underwent catheterization, PTI was attempted in 50 cases, 45 of which received successful PTI. Of these, there were 2 cases where combination PTI/surgery was done. Ultimately there were 5 cases where PTI was not performed, and the patient was referred for surgery due to complex anatomy. (Fig. 1) At baseline, a minority of patients had advanced New York Heart Association (NYHA) functional class defined as NYHAFC ≥2 (n = 11) or significant systemic (right) ventricular dysfunction (n = 23). In patients with an isolated baffle leak, the nadir oxygen saturation on VO2 testing was low (87 ± 6%). The remainder of baseline patient characteristics is outlined in Table 1.
Fig. 1.
Search methods.
Table 1.
Patient Characteristics.
| Overall Mean ± SD or Frequency (%) (n = 50) | Isolated Leak Mean ± SD or Frequency (%) (n = 10) | Isolated Stenosis Mean ± SD or Frequency (%) (n = 27) | Stenosis and Leak Mean ± SD or Frequency (%) (n = 13) | |
|---|---|---|---|---|
| Age | 30 ± 8 | 31 ± 6 | 31 ± 9 | 29 ± 7 |
| Age original surgery | 2 ± 3.3 | 3.3 ± 5.4 | 1.2 ± 2.7 | 1.4 ± 1.6 |
| Male | 37 (75) | 7 (70) | 19 (70) | 11 (84) |
| NYHA FC ≥ 2 | 11 (22) | 1 (10) | 8 (30) | 2 (15) |
| ≥Moderate RV dysfunction | 23(46) | 4 (40) | 13(48) | 6(46) |
| Ejection fraction by CT or MR | 39 ± 14 | 39 ± 11 | 37 ± 15 | 45 ± 12 |
| ≥Moderate tricuspid (SAVV) regurgitation | 2 (4) | 0 | 2 (7) | 0 |
| Baseline oxygen sat | 98 ± 2 | 97 ± 2 | 98 ± 2 | 100 ± 0 |
| Nadir oxygen sat (on VO2) | 93 ± 7 | 87 ± 6 | 97 ± 2 | 98 ± 0 |
| Average VO2 | 26 ± 5 | 24 ± 2 | 25 ± 5 | 29 ± 5 |
| Indication for catheterization | ||||
| DOE | 12 (24) | 1 (10) | 8 (30) | 3 (23) |
| Desaturation | 3 (6) | 2 (20) | 0 | 1 (8) |
| Symptoms of heart failure | 3 (6) | 0 | 3 (11) | 0 |
| Asymptomatic | 6 (12) | 0 | 4 (15) | 2 (15) |
| Fatigue | 6 (12) | 2 (20) | 4 (15) | 0 |
| Other | 20 (40) | 5(50) | 8 (30) | 7(54) |
CT = computed tomography scan, DOE = dyspnea on exertion, MR = magnetic resonance imaging, NYHA FC = New York Heart Association functional class, RV = right ventricle, SAVV = systemic atrioventricular valve, SD = standard deviation, VO2 = maximum oxygen consumption on exercise test.
Patients undergoing PTI had baffle leak (n = 10, 20%), stenosis (n = 27, 54%), or both (n = 13, 26%). A total of 40 bare metal stents, 18 covered stents, 16 occlusion devices, 2 angioplasties, and 1 endovascular graft were deployed. Covered stents were placed in 11 patients, and of those 8 patients received the device under compassionate use criteria and 3 under emergency use criteria. Stenoses were found in the superior limb of the systemic venous baffle (SVC) (n = 39), the inferior limb (IVC) (n = 13), and in the pulmonary venous baffle (n = 1). In this cohort average fluoroscopy time was 53 ± 33 min and average procedure length was 178 ± 84 min.
3.1. Isolated baffle stenosis
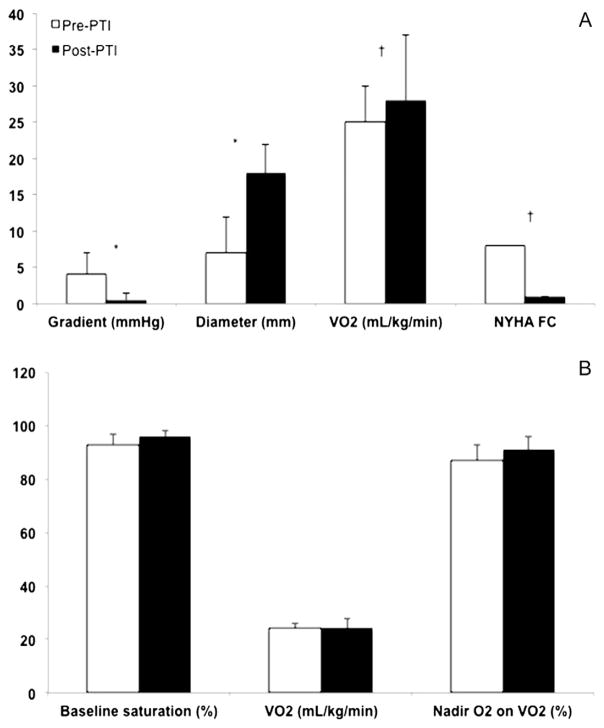
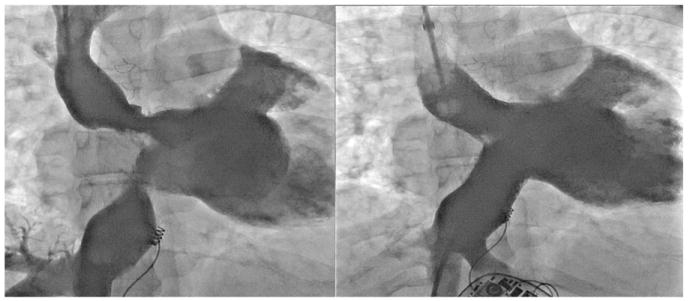
In patients with isolated baffle stenosis (n = 27), there was significant improvement in NYHA functional class after PTI, which was matched by improvement in VO2. In this group, the average pre-PTI mean gradient was 5 ± 11 mmHg and post-PTI was 0.6 ± 1.6 mmHg. Stenosis diameter went from 6 ± 5 mm to 18 ± 7 mm post-PTI (Fig. 2A). Of the 11 patients with a pre-existing device (pacemaker or implantable cardiac defibrillator – ICD), a total of 4 patients with isolated stenosis required lead extraction and re-implantation at the time of PTI. Three additional patients received a device at the time of PTI. Individual characteristics of each intervention are outlined in Table 2. Representative angiography from an example case which required lead extraction and intervention to the SVC and IVC limbs of the systemic venous baffle is demonstrated in Fig. 3.
Fig. 2.
Characteristics pre/post percutaneous intervention A. Isolated baffle stenosis cases: Mean pre and post-PTI gradient, diameters. Mean VO2 test results and NYHA functional class pre and post-PTI B. Isolated baffle leak cases: Pre and post-PTI baseline oxygen saturation, VO2, and nadir oxygen saturation on VO2 testing. * p < 0.0001. † p < 0.05. PTI: Percutaneous transcatheter intervention.
Table 2.
Characteristics of patients with isolated baffle stenosis.*
| ID | Age | Indication | Site | Intervention | Pre/post gradient | Pre/post diameter | EP Study |
|---|---|---|---|---|---|---|---|
| 2.1 | 48 | Other | SVC | Max LD stent 26 mm | 2/0 | 12/20 | NAFAT |
| 3.1 | 38 | Fatigue | IVC | Max LD stent 16 mm | 5/0 | 5/15 | No |
| 6.1 | 18 | Other | Both | No intervention; stenosis felt to be secondary to pulmonary venous atrial contraction | 2 | 5 | Prior CHB and pacemaker, AVNRT induced, no ablation |
| 9.1 | 24 | Asymptomatic | SVC | Max LD stent 18 mm | 8/0 | 0/18 | IART clinically – DCCV, No EP study |
| 15.1 | 30 | Desaturation | Both | SVC: Max LD stent 26 mm | 0/0 | SVC: 11/22 | No |
| IVC: Max LD stent 26 mm | 0/0 | IVC: 11/22 | |||||
| 17.1 | 22 | Asymptomatic | SVC | Max LD stent 36 mm | 3/0 | 9/18 | Prior ICD |
| 19.1 | 34 | DOE | Both | SVC: Balloon angioplasty | 3/0 | SVC 8/11 | No |
| IVC: Max LD stent 26 mm | IVC 10/24 | ||||||
| 22.1 | 40 | Heart failure | SVC | Max LD stent 26 mm | 0/0 | 2/20 | Prior ICD |
| 23.1 | 25 | Syncope | SVC | Max LD stent 36 mm | 5/0 | 0/20 | Prior ICD for CHB |
| 25.1 | 30 | Heart failure | Both | Max LD stent 26 mm × 2 | SVC: 2/0 | SVC: 14/26 | No |
| IVC: 3/0 | IVC: 15/26 | ||||||
| 26.1 | 44 | Fatigue | Both | Palmaz XL 20 mm × 2 | SVC: 4/0 | SVC: 9/20 | Prior ICD, VF, lead |
| IVC: 5/0 | IVC: 7/20 | extraction/re-implanted | |||||
| 28.2 | 18 | Arrhythmia | SVC | NuMed CP covered stent 50 mm | 13/0 | 0/8 | Lead extraction/re-implanted |
| 30.1 | 17 | Arrhythmia | SVC | Balloon angioplasty | 0/0 | IART, ablation done | |
| 32.1 | 24 | Asymptomatic | SVC | Palmaz XL18mm | 9/0 | 0/18 | Lead extraction/re-implantation |
| 34.1 | 37 | Fatigue | SVC | NuMed covered CP stent × 2, 45 mm, 39 mm | 13/18 | Pacemaker upgraded to ICD | |
| 35.1 | 29 | DOE | SVC | Max LD stent 26 mm | 0/2 | 8/25 | No |
| 37.2 | 43 | Fatigue | Both | SVC: Palmaz XL 20 mm | SVC: 8/0 | SVC: 8/20 | Lead extraction/re-implantation |
| IVC: Palmaz XL 24 mm | IVC: 3/0 | IVC: 15/24 | |||||
| 38.1 | 20 | Fatigue | SVC | Max LD stent 26 mm | 3/1 | 12/20 | NAFAT, NSVT with ICD placed |
| 39.1 | 32 | DOE | SVC | Max LD stent 36 mm × 2 | 8/1 | 2/14 | Upgrade ppm to ICD |
| Max LD stent 26 mm | |||||||
| 40.1 | 39 | DOE | SVC | Max LD stent 36 mm | 2/0 | 8/25 | No |
| 40.2 | 42 | DOE | SVC | Balloon expansion of stent to 20 mm | 0/0 | 16/20 | ICD placed |
| 42.1 | 30 | Fatigue | SVC & PVB | SVC: Genesis XD 39 mm SVC | 6/4 | 0/18 | AVNRT |
| PVB: Recommended surgery | |||||||
| 43.1 | 34 | Asymptomatic | SVC | Max LD stent 36 mm | 3/0 | 10/18 | No |
| Max LD stent 26 mm | |||||||
| 45.1 | 24 | Arrhythmia | SVC | Max LD stent 36 mm | 2/0 | 13/18 | ICD placed |
| 47.1 | 33 | DOE | SVC | Max LD stent 36 mm | 1/0 | 9/22 | No |
| 49.1 | 23 | DOE | Both | SVC: Max LD stent 26 mm × 2 | SVC: 2/0 | SVC: 10/18 | No |
| IVC: Max LD stent 26 mm | IVC: 3/0 | IVC: 11/20 | |||||
| 51.1 | 33 | DOE | SVC | Max LD stent 26 mm | 1/0 | 8/20 | Prior ICD |
“Both” refers to involvement of the SVC and IVC. CHB = complete heart block, DCCV = direct current cardioversion, DOE = dyspnea on exertion, EP = electrophysiology, IART = intra-atrial reentrant tachycardia, ICD = implantable cardiac defibrillator, IVC = inferior vena cava limb of systemic venous baffle, NAFAT = Non-automatic focal atrial tachycardia, PVB = pulmonary venous baffle, SVC = superior vena cava limb of systemic venous baffle, VT = ventricular tachycardia.
Fig. 3.
Multiple baffle stenoses Significant superior and inferior baffle stenoses that have been treated with bare metal stents. Lead extraction and re-implantation was required in this case.
3.2. Isolated baffle leak
In the 10 patients with isolated baffle leak, the resting O2 was 93 ± 4% and post-PTI was 96 ± 2%. Leaks were documented in the SVC (n = 16), IVC (n = 11), and pulmonary venous baffle (n = 2). A combination of covered stents, septal and patent foramen ovale (PFO) occluders, and endovascular stents were used to treat isolated leaks. Although the nadir O2 level on VO2 improved post-PTI, it was not significantly different than pre-PTI levels (Fig. 2B). In this group, 2 patients had a prior pacemaker or ICD, one of which required lead extraction and re-implantation at the time of PTI. Of the remainder, 2 patients had a new device placed at the time of catheterization. (Table 3).
Table 3.
Characteristics of patients with isolated baffle leak.*
| ID | Age | Indication | Leak site | Intervention | Baseline oxygen saturation (%) | Post-procedure oxygen saturation (%) | Post PTI Angio Leak | EP Study/Device |
|---|---|---|---|---|---|---|---|---|
| 3.2 | 38 | Fatigue | IVC | Palmaz XL stent + Zenith TX2 endovascular graft × 2 | 89 | 96 | Yes | No |
| 7.1 | 27 | DOE | SVC | NuMed CP covered stent * 2 | 95 | 99 | No | VF on EPS, ICD placed |
| 13.2 | 30 | Other | IVC | Amplatzer septal occluder × 2 (4 mm, 6 mm) | 95 | 95 | No | IART on EPS, Prior ICD |
| 14.2 | 21 | Other | IVC | Max LD stent | 99 | 99 | ICD placed for NSVT | |
| 21.1 | 25 | Desaturation | SVC | NuMED CP covered stent | No | No | ||
| 21.2 | 33 | Fatigue | IVC | Amplatzer septal occluder 32 mm placed – almost completely obstructed pulmonary venous chamber, removed, referred for surgical repair | 89 | 89 | Yes | No |
| 21.3 | 34 | Desaturation | IVC | Found CS draining into IVC which prevented safe placement of endovascular graft – referred for surgical repair | 96 | 96 | Yes | No |
| 24.1 | 40 | Fatigue | SVC | Amplatzer septal occluder 24 mm sizing balloon occluded the SVC with azygous decompression, referred for surgical repair | 91 | 92 | Yes | No |
| 50.1 | 23 | Chest pain | IVC SVC | IVC: Amplatzer septal occluder 34 mm SVC: Amplatzer septal occluder 4 mm × 2 | 97 | 97 | No | No |
| 55.2 | 37 | Arrhythmias | IVC PVB | IVC: Amplatzer septal occluder 4 mm PVB: Amplatzer septal occluder 5 mm | 93 | NA | No | Prior ICD, leads extracted/re-implanted at cath |
CHB = complete heart block, DCCV = direct current cardioversion, DOE = dyspnea on exertion, EP = electrophysiology, IART = intra-atrial reentrant tachycardia, ICD = implantable cardiac defibrillator, IVC = inferior vena cava limb of systemic venous baffle, NA = not available, NAFAT = Non-automatic focal atrial tachycardia, NSVT = non-sustained ventricular tachycardia, PVB = pulmonary venous baffle, SVC = superior vena cava limb of systemic venous baffle, VF = ventricular fibrillation, VT = ventricular tachycardia.
“Both” refers to involvement of the SVC and IVC.
3.3. Combined baffle leak and stenosis
Several patients had both baffle leak and stenosis (n = 13) and were treated with a combination of bare metal stents, covered stents, and septal/PFO occluders. There was no significant difference in pre and post-PTI oxygen saturation (94 ± 5% vs. 97 ± 2%, p = 0.07) and post-PTI gradients and diameters were similar to the isolated stenosis group. In these patients, 7 had a pre-existing pacemaker or ICD, 2 of which required lead extraction and replacement at the time of PTI. In addition, 2 new devices were placed at the time of PTI. Individual characteristics and results of PTI are reviewed in Table 4. Angiography from a case requiring covered stenting to treat both baffle leak and stenosis along with pacemaker removal and reimplantation by a combined EP-interventional catheterization team is illustrated in Fig. 4.
Table 4.
Characteristics of Patients with Both Baffle Stenosis and Leak.*
| ID | Age | Indication | Site | Intervention | Baseline O2 sat (%) | Post-procedure O2 sat (%) | Pre/post gradient (mmHg) | Pre/post diameter (mm) | Post PTI Angio Leak | EP Study/Device |
|---|---|---|---|---|---|---|---|---|---|---|
| 4.1 | 26 | Palpitations | SVC | Amplatzer septal occluder 4 mm | 98 | 98 | 3/0 | 7/35 | No | Prior ICD |
| 12.1 | 32 | DOE | Both | Amplatzer PFO occluder 25 mm, NuMed CP covered stent 55 mm | 88 | 98 | 6/1 | 5/18 | Yes | Prior pacemaker |
| 16.1 | 25 | Asymptomatic | SVC | Max LD stent 36 mm × 2 Max LD stent 26 mm | 93 | 96 | 10/1 | 2/20 | No | No |
| 20.1 | 37 | Desaturation | Both | SVC: Max LD stent 26 mm | 87 | 96 | 2/0 | 5/20 | Yes | No |
| SVC: Amplatzer septal occluder 8 mm | ||||||||||
| IVC: 2 small leaks, no treatment | ||||||||||
| 29.2 | 23 | ICD generator change | SVC | NuMed CP covered stent 45 mm | 88 | 100 | 2/0 | 10/22 | No | VF induced, ICD replaced |
| 31.3 | 37 | Desaturation | SVC | NuMed CP covered stent 45 mm | 93 | 97 | SVC: 2/0 | SVC: 11/18 | No | AVNRT induced, Prior ICD |
| IVC:3/0 | IVC: 13/23 | |||||||||
| 33.2 | 26 | DOE | SVC | NuMed CP covered stent × 4; 45 mm × 3, 39 mm × 1 | 97 | 98 | 2/0 | 0/22 | No | ICD placed for heart failure |
| 48.1 | 38 | DOE | SVC | Max LD 36 mm, Amplatzer septal occluder | 95 | 99 | 2/0 | 8/20 | No | No |
| 53.1 | 24 | Asymptomatic | Both | SVC: Max LD stent 26 mm | 95 | 97 | SVC: 4/1 | SVC: 4/20 | No | ICD placed for known VT |
| IVC: Max LD stent 16 mm, Amplatzer septal occluder 5 mm | IVC: 3/1 | IVC: 12/22 | ||||||||
| 56.2 | 18 | Palpitations | IVC | Amplatzer septal occluder × 2, 8 mm & 5 mm. No treatment for stenosis. | 100 | 100 | NA | NA | No | No |
| 56.3 | 25 | Asymptomatic | SVC | SVC: NuMed CP covered stent 34 mm | 100 | 100 | 5/0 | 0/18 | No | Pacemaker leads extracted, replaced |
| 57.1 | 37 | TIA | Both | SVC: NuMed CP covered stent 45 mm | 96 | 97 | 5/0 | 5/16 | No | Pacemaker leads extracted, replaced |
| IVC: Amplatzer septal occluder 12 mm | ||||||||||
| 58.1 | 28 | TIA | SVC | NuMed CP covered stent × 39 mm, 45 mm, 50 mm | NA | 95 | 2/0 | 10/20 | No | Prior ICD in place |
CHB = complete heart block, DCCV = direct current cardioversion, DOE = dyspnea on exertion, EP = electrophysiology, IART = intra-atrial reentrant tachycardia, ICD = implantable cardiac defibrillator, IVC = inferior vena cava limb of systemic venous baffle, NA = not applicable, NAFAT = Non-automatic focal atrial tachycardia, NSVT = non-sustained ventricular tachycardia, PVB = pulmonary venous baffle, O2 sat = oxygen saturation, SVC = superior vena cava limb of systemic venous baffle, TIA = transient ischemic attack, VF = ventricular fibrillation, VT = ventricular tachycardia.
“Both” refers to involvement of the SVC and IVC.
Fig. 4.
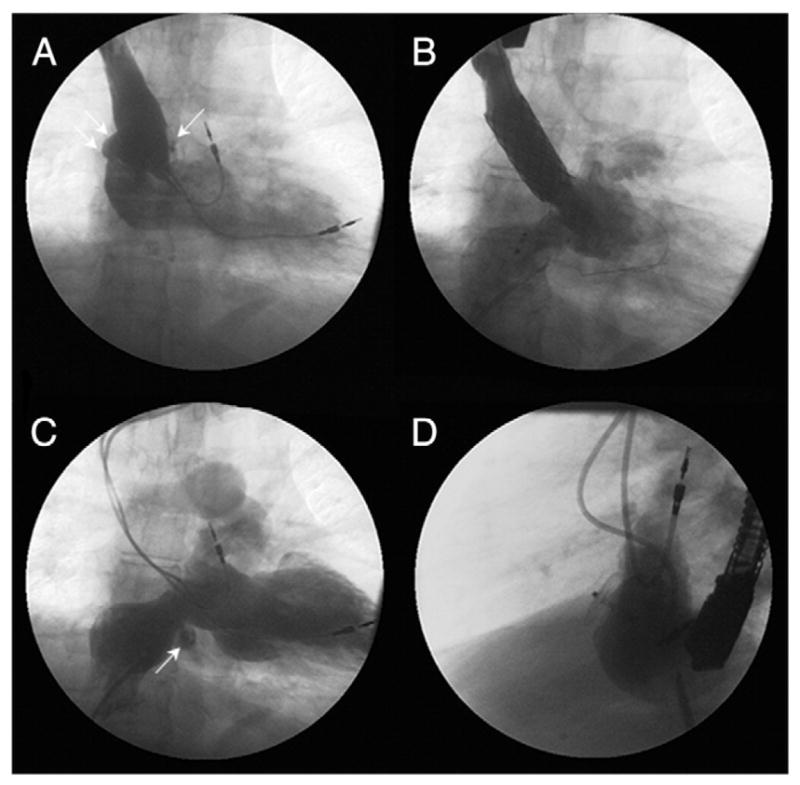
Combined baffle stenosis/leak Baffle stenosis and leak treated during a single procedure using a combined EP-interventional catheterization team. Superior baffle stenosis along with a large right to left shunt at the old right atrial appendage (arrow) with contrast seen entering the pulmonary venous atrium (double arrows) (A), intracardiac pacing leads removed and subsequent placement of a covered stent was implanted to treat both the superior baffle stenosis and leak (B). Small baffle leak (arrow) in the inferior baffle (C), interval placement of an Amplatzer PFO Occluder to close the inferior baffle leak and replacement of permanent pacing leads (D). EP: Electrophysiology PFO: Patent foramen ovale.
In the entire group (stenosis, leak, combination stenosis/leak), there were no major adverse events: death, need for emergent surgery/intervention, or blood loss requiring transfusion. On average, patients were observed overnight, and discharged from the hospital within 24 h after PTI.
4. Discussion
Patients with D-TGA had little hope for long-term survival prior to the Mustard/Senning procedures. For those that underwent an atrial switch operation, long-term survival is good, ranging from 71% to 94% at 10–20 years follow up [4–9] and 68–80% at >20 years follow up [10–12]. The study reporting the longest follow up was at 39 years where 68% survival was documented. [10] However, event-free survival and need for re-operation is substantial, [9,13–16] with many of these patients requiring re-operation to treat baffle stenosis, leak, or a combination of both. [4,17–20] Repeat surgeries in this group are common, occurring in 15–20%, and when required, over half are due to baffle-related complications (stenosis and/or leak). [10,21].
4.1. The history of PTI in baffle leak/stenosis
In the last 20 years, several cases of percutaneous baffle stenting and baffle leak closure have been reported. [22–36] Two case series have more recently demonstrated good short and medium-term outcomes with these procedures. [37,38] The first case series came out of Germany, where 12 of 13 patients with a median age of 20.8 years underwent 14 successful procedures including baffle stent and septal occluder placement without complication, baffle rupture, or hemorrhage. In this group, at 21 months of follow-up there was no recurrent stenosis or leak. [37] The second case series in 2012 included data from three U.S. institutions where 29 percutaneous interventions were completed in 22 patients at an average age of 32 ± 8 years. In this cohort, ventricular arrhythmia led to one death 2 days after angioplasty to the pulmonary venous baffle, and one patient had inferior systemic venous baffle obstruction that was treated with a stent. At 33 months of follow-up, there was no evidence of symptom recurrence related to baffle stenosis or leak. The authors concluded that stent placement for baffle obstruction is effective and likely safer than surgical intervention, and baffle leak occlusion is safely treated from a percutaneous approach, albeit the presence of residual leak in the short-term. [38].
4.2. Multi-disciplinary team approach
We report the largest overall, and single-center, description of PTI offered to adult patients with a prior atrial switch procedure and late baffle leak and/or stenosis. Our results demonstrate that PTI is a safe and effective alternative to surgery for the treatment of late baffle related stenosis and/or leak. In late D-TGA survivors with a prior atrial switch operation, we found that isolated baffle stenosis was more common than baffle leak or combination stenosis/leak. When isolated stenosis was present, patients were more likely to report symptoms, evidenced by higher NYHA functional class, and had objective evidence of improved exercise capacity after PTI (VO2). From a functional standpoint, this group benefitted the most from PTI, with bare metal stents comprising the majority of interventions performed.
Previous authors have published institutional criteria defining stenosis or leak in atrial switch patients. [39–41] However, it is our opinion that significant baffle stenosis cannot be solely defined by a specific gradient, because some patients will develop large decompressing veins that lower the mean gradient. In addition, some patients will have baffle narrowing that might be considered mild by gradient (mean gradient <3 mmHg) yet the neck of the stenotic area is significantly smaller than the maximal diameter. One must also consider that many of these patients will eventually require transvenous pacemaker lead placement, in particular whereby treating the SVC baffle narrowing, will allow device wires to be placed through a patent baffle and decrease the need for combined future interventional/electrophysiology procedures. Higher than usual fluoroscopy and procedure times were seen in this cohort, however, considering that all treatment was performed in a single procedure rather than up to 3 separate procedures, we believe slightly higher times are justified.
There were no adverse events including death, emergent surgery, or blood loss requiring transfusion, in this cohort. However, in order to offer safe and effective PTI in these complex patients a team approach is required, as is used at our institution. This team consists of adult congenital heart disease (ACHD) experts, a dedicated interventional cardiology and surgical service experienced in complex congenital heart disease, and a specialized adult/pediatric electrophysiology team. This team has to work together to perform combined interventions in the same patient, such as device lead extraction and baffle stenting, during the same procedure. In addition to the specific skills required by the interventional cardiology team, a wide variety of inventory, including but not limited to bare metal stents (BMS), covered stents (both balloon and self-expandable, and intracardiac closure devices) should be available. In patients where a covered stent was required, the majority was under compassionate use by U.S. Food and Drug Administration (FDA) as requested by the sponsor/manufacturer. This typically requires documentation including: physician request outlining the clinical indication for the device, a letter from a physician remote to the case (in our institution this was typically a cardiothoracic surgeon who was not involved with the case), patient consent with post-device deployment follow-up outlined, and IRB along with institutional approval for the procedure. The remainder were placed under emergency use, which required participation in a clinical trial or a pending compassionate use application. We underscore the importance of pre-procedural planning, as often the need for lead extraction, device placement, special equipment, and advanced imaging can be identified prior to the procedure. Meticulous planning ensures that necessary personnel and equipment are available in these complex cases.
4.3. Limitations
Referral and temporal bias are limitations of this study. The clinical review of this data identified adult patients in a cohort that were able to successfully undergo PTI for late baffle-related complications after atrial switch operations. However, inherent to the retrospective nature of this study, there is limited ability to prospectively predict outcomes at other institutions where local practice and expertise differ from the treatment team that performed complex interventions in this group.
5. Conclusions
This single-center cohort is the largest reported series of adult atrial switch operation patients who have undergone PTI for treatment of baffle stenosis and/or leak. We demonstrate that several percutaneous options are available to successfully treat late stenosis and leak in this group and emphasize the need for a skilled multi-disciplinary team approach to achieve optimal patient outcomes. Patients with isolated baffle stenosis had the most improvement in functional capacity after intervention, although subjectively nearly all patients noted some improvement in symptoms. We conclude that percutaneous transcatheter intervention with an expert multi-disciplinary team is a safe and effective alternative to surgery in adult patients with an atrial switch operation.
Footnotes
Financial/Other Disclosures/Conflict of Interest
Elisa Bradley, MD – none.
Amanda Cai, MD – none.Sharon Cheatham, PhD – NuMED, Inc. (Denton TX): consultant, proctor, and primary investigator.
Joanne Chisolm, MSN, RN – none. Tracey Sisk, RN – none.
Curt Daniels, MD – none.
John Cheatham, MD – NuMED, Inc. (Denton TX): consultant, proctor, and primary investigator.
Author Contributions
Elisa Bradley, MD – Concept and design, data collection, analysis and interpretation of data, table/figure preparation, manuscript preparation/review/revision/approval.
Amanda Cai, MD – Design, data collection, manuscript review/approval.
Sharon Cheatham, PhD – Design, data collection, manuscript review/approval.
Joanne Chisolm, MSN, RN – Design, data collection, manuscript review/approval.
Tracey Sisk, RN – Design, data collection, manuscript review/approval.
Curt Daniels, MD – Concept and design, manuscript review/approval.
John Cheatham, MD – Concept and design, manuscript review/approval.
References
- 1.Mustard WT. Successful two-stage correction of transposition of the great vessels. Surgery. 1964;55:469–472. [PubMed] [Google Scholar]
- 2.Senning A. Surgical correction of transposition of the great vessels. Surgery. 1959;45:966–80. [PubMed] [Google Scholar]
- 3.Jatene AD, Fontes VF, Paulista PP, Neto CA, Sousa JE. Anatomic correction of transposition of the great vessels. J Thorac Cardiovasc Surg. 1976;72:364–70. [PubMed] [Google Scholar]
- 4.Ashraf MH, Cortroneo J, DiMarco D, Subramanian S. Fate of long-term survivors of mustard procedure (inflow repair) for simple and complex transposition of the great arteries. Ann Thorac Surg. 1986;42:385–9. doi: 10.1016/s0003-4975(10)60541-3. [DOI] [PubMed] [Google Scholar]
- 5.Birnie D, Tometzki A, Curzio J, Houston A, Swan L, Doig W, Wilson N, Jamieson M, Pollock J, Hillis WS. Outcomes of transposition of the great arteries in the era of atrial inflow correction. Heart. 1998;80:170–3. doi: 10.1136/hrt.80.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gelatt M, Hamilton RM, McCrindle BW, Connelly M, Davis A, Harris L, Gow RM, Williams WG, Trusler GA, Freedom RM. Arrhythmia and mortality after the mustard procedure: a 30-year single-center experience. J Am Coll Cardiol. 1997;29:194–201. doi: 10.1016/s0735-1097(96)00424-x. [DOI] [PubMed] [Google Scholar]
- 7.Helbing WA, Hansen B, Ottenkamp J, Rohmer J, Chin JG, Brom AG, Quaegebeur JM. Long-term results of atrial correction for transposition of the great arteries. Comp Mustard Senning Oper. 1994;108:363–72. [PubMed] [Google Scholar]
- 8.Horer J, Herrmann F, Schreiber C, Cleuziou J, Prodan Z, Vogt M, Holper K, Lange R. How well are patients doing up to 30 years after a mustard Operation? Thorac Cardiovasc Surg. 2007;55:359–64. doi: 10.1055/s-2007-964847. [DOI] [PubMed] [Google Scholar]
- 9.Sarkar D, Bull C, Yates R, Wright D, Cullen S, Gewillig M, Clayton R, Tunstill A, Deanfield J. Comparison of long-term outcomes of atrial repair of simple transposition with implications for a late arterial switch strategy. Circulation. 1999;100:176–81. doi: 10.1161/01.cir.100.suppl_2.ii-176. [DOI] [PubMed] [Google Scholar]
- 10.Cuypers JA, Eindhoven JA, Slager MA, Opic P, Utens EM, Helbing WA, van den Bosch AEWM, Ouhlous M, van Domburg RT, Rizpoulos D, Meijboom FJ, Bogers AJ, Roos-Hesselink JW. The natural and unnatural history of the mustard procedure: long-term outcome up to 40 years. Eur Heart J. 2014;35:1666–74. doi: 10.1093/eurheartj/ehu102. [DOI] [PubMed] [Google Scholar]
- 11.Lange R, Horer J, Kostolny M, Cleuziou J, Vogt M, Busch R, Holper K, Meisner H, Hess J, Schreiber C. Presence of a ventricular septal defect and the mustard operation are risk factors for late mortality after the atrial switch operation: thirty years of follow-up in 417 patients at a single center. Circulation. 2006;114:1905–13. doi: 10.1161/CIRCULATIONAHA.105.606046. [DOI] [PubMed] [Google Scholar]
- 12.Wilson NJ, Clarkson PM, Barratt-Boyes BG, Calder AL, Whitlock RM, Easthope RN, Neutze JM. Long-term outcome after the mustard repair for simple transposition of the great arteries. 28-year follow-up. J Am Coll Cardiol. 1998;32:759–65. doi: 10.1016/s0735-1097(98)00309-x. [DOI] [PubMed] [Google Scholar]
- 13.Dos L, Teruel L, Ferreira IJ, Rodriguez-Larrea J, Miro L, Girona J, Albert DC, Goncalves A, Murtra M, Casaldaliga J. Late outcome of senning and mustard procedures for correction of transposition of the great arteries. Heart. 2005;91:652–6. doi: 10.1136/hrt.2003.029769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myridakis DJ, Ehlers KH, Engle MA. Late follow-up after venous switch operation (mustard procedure) for simple and complex transposition of the great arteries. Am J Cardiol. 1994;74:1030–6. doi: 10.1016/0002-9149(94)90854-0. [DOI] [PubMed] [Google Scholar]
- 15.Turley K, Hanley FL, Verrier ED, Merrick SH, Ebert PA. The mustard procedure in infants (less than 100 days of age). ten-year follow-up. J Thorac Cardiovasc Surg. 1988;96:849–53. [PubMed] [Google Scholar]
- 16.Wells WJ, Blackstone E. Intermediate outcome after Mustard and Senning procedures: A study by the Congenital Heart Surgeons Society. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2000;3:186–97. doi: 10.1053/tc.2000.6043. [DOI] [PubMed] [Google Scholar]
- 17.Meijboom F, Szatmari A, Deckers JW, Utens EM, Roelandt JR, Bos E, Hess J. Long-term follow-up (10–17 years) after mustard repair for transposition of the great arteries. J Thorac Cardiovasc Surg. 1996;111:1158–68. doi: 10.1016/s0022-5223(96)70217-9. [DOI] [PubMed] [Google Scholar]
- 18.Hucin B, Voriskova M, Hruda J, Marek J, Janousek J, Reic O, Skovranek J. Late complications and quality of life after atrial correction of transposition of the great arteries in 12 to 18 year follow up. J Cardiovasc Surg (Torino) 2000;41:233–9. [PubMed] [Google Scholar]
- 19.Patel S, Shah D, Chintala K, Karpawich PP. Atrial baffle problems following the Mustard operation in children and young adults with dextro-transposition of the great arteries: the need for improved clinical detection in the current era. Congenit Heart Dis. 6:466–474. doi: 10.1111/j.1747-0803.2011.00532.x. 201. [DOI] [PubMed] [Google Scholar]
- 20.Peters B, Abdul-Khaliq H, Lange PE. Late complications following early childhood atrial switch operations for d-transposition of the great arteries. Incidence, diagnosis and therapy. Dtsch Med Worchenschr. 2001;126:1037–42. doi: 10.1055/s-2001-17311. [DOI] [PubMed] [Google Scholar]
- 21.Khairy P, Landzberg MJ, Lambert J, O’Donnell CP. Long term outcomes after the atrial switch for surgical correction of transposition: a meta-analysis comparing the mustard and senning procedures. Cardiol Young. 2004;14:284–92. doi: 10.1017/S1047951104003063. [DOI] [PubMed] [Google Scholar]
- 22.Chatelain P, Meier B, Friedli B. Stenting of superior vena cava and inferior vena cava for symptomatic narrowing after repeated atrial surgery for D-transposition of the great vessels. Br Heart J. 1991;66:466–8. doi: 10.1136/hrt.66.6.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ward CJ, Mullins CE, Nihill MR, Grifka RG, Vick GW., III Use of intravascular stents in systemic venous and systemic venous baffle obstructions. Short-term follow-up results. Circulation. 1995;91:2948–54. doi: 10.1161/01.cir.91.12.2948. [DOI] [PubMed] [Google Scholar]
- 24.MacLellan-Tobert SG, Cetta F, Hagler DJ. Use of intravascular stents for superior vena caval obstruction after the mustard operation. Mayo Clin Proc. 1996;71:1071–6. doi: 10.4065/71.11.1071. [DOI] [PubMed] [Google Scholar]
- 25.Brown SC, Eyskens B, Mertens L, Stockx L, Dumoulin M, Gewillig M. Self expandable stents for relief of venous baffle obstruction after the mustard operation. Heart. 1998;79:230–3. doi: 10.1136/hrt.79.3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michel-Behnke I, Hagel KJ, Bauer J, Schranz D. Superior caval venous syndrome after atrial switch procedure: relief of complete venous obstruction by gradual angioplasty and placement of stents. Cardiol Young. 1998;8:443–8. doi: 10.1017/s1047951100007095. [DOI] [PubMed] [Google Scholar]
- 27.Santoro G, Ballerini L, Bialkowski J, Bermudez-Canete R. Stent implantation for post-mustard systemic venous obstruction. Eur J Cardiothorac Surg. 1998;14:332–4. doi: 10.1016/s1010-7940(98)00174-2. [DOI] [PubMed] [Google Scholar]
- 28.Apostolopoulou SC, Papagiannis J, Hausdorf G, Rammos S. Transcatheter occlusion of atrial baffle leak after mustard repair. Catheter Cardiovasc Interv. 2000;51:305–7. doi: 10.1002/1522-726x(200011)51:3<305::aid-ccd13>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 29.Mohsen AE, Rosenthal E, Qureshi SA, Tynan M. Stent implantation for superior vena cava occlusion after the mustard operation. Catheter Cardiovasc Interv. 2001;52:351–4. doi: 10.1002/ccd.1080. [DOI] [PubMed] [Google Scholar]
- 30.Villain E, Saliba Z, Bonhoeffer P, Iserin L, Bonnet D, Kachaner J. Stenosis of the superior caval canal after mustard and senning procedures: treatment by dilatation and stent implantation. Two case reports. Arch Mal Coeur Vaiss. 2001;94:139–43. [PubMed] [Google Scholar]
- 31.Sharaf E, Waight DJ, Hijazi ZM. Simultaneous transcatheter occlusion of two atrial baffle leaks and stent implantation for SVC obstruction in a patient after mustard repair. Catheter Cardiovasc Interv. 2001;64:72–6. doi: 10.1002/ccd.1242. [DOI] [PubMed] [Google Scholar]
- 32.Schneider DJ, Moore JW. Transcatheter treatment of IVC channel obstruction and baffle leak after mustard procedure for D-transposition of the great arteries using amplatzer ASD device and multiple stents. J Invasive Cardiol. 2001;13:306–9. [PubMed] [Google Scholar]
- 33.Balzer DT, Johnson M, Sharkey AM, Kort H. Transcatheter occlusion of baffle leaks following atrial switch procedures for transposition of the great vessels (D-TGV) Catheter Cardiovasc Interv. 2004;61:259–63. doi: 10.1002/ccd.10740. [DOI] [PubMed] [Google Scholar]
- 34.Dragulescu A, Sidibe N, Aubert F, Fraisse A. Successful use of covered stent to treat superior systemic baffle obstruction and leak after atrial switch procedure. Pediatr Cardiol. 2008;29:954–6. doi: 10.1007/s00246-007-9168-x. [DOI] [PubMed] [Google Scholar]
- 35.Farooqui K, Lopez L, Sutton NJ, Pass RH. Closure of a superior vena cava baffle leak in a patient with D-transposition after mustard palliation: importance of both angiography and echocardiography for the confirmation of closure. Pediatr Cardio. 2009;30:1046–7. doi: 10.1007/s00246-009-9517-z. [DOI] [PubMed] [Google Scholar]
- 36.Hill KD, Fudge JC, Rhodes JF. Complete resolution of systemic venous baffle obstruction and baffle leak using gore excluder covered stent in two patients with transposition of the great arteries and prior mustard procedure. Catheter Cardiovasc Interv. 2010;76:878–81. doi: 10.1002/ccd.22567. [DOI] [PubMed] [Google Scholar]
- 37.Daehnert I, Hennig B, Wiener M, Rotzsch C. Interventions in leaks and obstructions of the interatrial baffle late after mustard and senning correction for transposition of the great arteries. Catheter Cardiovasc Interv. 2005;66:400–7. doi: 10.1002/ccd.20504. [DOI] [PubMed] [Google Scholar]
- 38.Hill KD, Fleming G, Fudge JC, Albers EL, Doyle TP, Rhodes JF. Percutaneous interventions in high-risk patients following mustard repair of transposition of the great arteries. Catheter Cardiovasc Interv. 2012;80:905–14. doi: 10.1002/ccd.23470. [DOI] [PubMed] [Google Scholar]
- 39.Marx GR, Hougen TJ, Norwood WI, Fyler DC, Castaneda AR, Nadas AS. Transposition of the great arteries with intact ventricular septum: results of the mustard and senning operations in 123 consecutive patients. J Am Coll Cardiol. 1983;1:476–83. doi: 10.1016/s0735-1097(83)80076-x. [DOI] [PubMed] [Google Scholar]
- 40.Bender HW, Jr, Graham TP, Jr, Bouceck RJ, Jr, Walker WE, Boerth RG. Comparative operative results of the senning and mustard procedures for transposition of the great arteries. Circulation. 1980;62:I197–203. [PubMed] [Google Scholar]
- 41.Mahoney L, Turley K, Ebert P, Heymann MA. Long-term results after atrial repair of transposition of the great arteries in early infancy. Circulation. 1982;66:253–8. doi: 10.1161/01.cir.66.2.253. [DOI] [PubMed] [Google Scholar]