Abstract
Background
Our previous work revealed substantial heterogeneity in the cognitive Profile of bipolar disorder (BD) due to the presence of three underlying cognitive subgroups characterized as: globally impaired, selectively impaired, or cognitively intact. In an effort to determine whether these subgroups are differentially related to genetic risk for the illness, we investigated whether cognitive deficits were more pronounced in unaffected siblings (UAS) of BD probands within identified clusters.
Methods
Cluster analysis was used to identify cognitive clusters in BD (N = 60). UAS (N = 49) were classified into groups according to their proband sibling’s cluster assignment; comparisons were made across all clusters and healthy controls (HCs; N = 71).
Results
Three cognitive clusters in BD emerged: a globally impaired (36.7%), a selectively impaired (30%), and a cognitively intact cluster (33.3%). UAS showed a qualitatively similar pattern to their BD siblings; UAS of the globally impaired BD cluster showed verbal memory and general cognitive impairments relative to HCs. In contrast, UAS of the other two clusters did not differ from HCs.
Conclusions
This study corroborates findings from prior work regarding the presence of cognitive heterogeneity in BD. UAS of subjects in the globally impaired BD cluster presented with a qualitatively similar cognitive Profile to their siblings and performed worse than all other BD clusters and UAS groups. This suggests that inherited risk factors may be contributing to cognitive deficits more notably in one subgroup of patients with BD, pointing toward differential causes of cognitive deficits in discrete subgroups of patients with the disorder.
Keywords: Bipolar disorder, cognition, heterogeneity, unaffected sibling, verbal memory
Introduction
A core challenge to understanding the pathophysiology of bipolar disorder (BD) is the complexity of its architecture due to clinical and neurocognitive heterogeneity. BD diagnosis is based on phenomenological presentation; however, intra- and inter-diagnostic variability is prominent with regard to clinical variables, including illness subtype, predominant type of episode, and history of psychosis (American Psychiatric Association, 2013). deficits in neurocognitive functioning, now acknowledged as one of the core features of BD, occur not only during the acute phases of the illness but also during inter-episode remission (Torres et al. 2007; Arts et al. 2008). Cognitive deficits during euthymia range from 0.5 to 1.5 standard deviations (S.D.) below the mean of healthy controls (HCs), particularly in the domains of attention, verbal memory, and executive functions (Torres et al. 2007; Arts et al. 2008; Bora et al. 2009). The persistence of these cognitive deficits, along with subsyndromal affective symptoms, contributes directly to poor psychosocial functioning (overall quality of life, independent living, financial independence, and social relations) in BD (Altshuler et al. 2002; Bowie et al. 2010; Burdick et al. 2010; Van Rheenen & Rossell, 2014; Baş et al. 2015).
Although group-level data consistently show that cognitive deficits in BD are less severe than those reported in schizophrenia (SZ) (Daban et al. 2006; Burdick et al. 2011), increasing evidence points toward Significant cognitive heterogeneity in BD with data showing the presence of groups defined as cognitively-impaired versus cognitively intact (Altshuler et al. 2004; Martino et al. 2008; Reichenberg et al. 2009). Knowledge of this heterogeneity may provide new insights beyond group-wise analysis.
Recently, our group utilized an empirical approach to parse the cognitive heterogeneity in BD, by applying hierarchical clustering analysis (HCA) to a well-characterized cohort (N = 136) of affectively stable BD patients (Burdick et al. 2014). We found evidence of three discrete cognitive subgroups based on a full neurocognitive Profile: (1) a group with severe cognitive impairments across all cognitive domains (globally impaired cluster; 40% of subjects), which was similar in both its pattern and the extent of impairment commonly seen in SZ; (2) a group with mild-to-moderate deficits only on specific domains (selectively impaired cluster; 30% of subjects); and (3) a group with intact cognitive performance relative to HCs (cognitively intact cluster; 30% of subjects). This was the first study using this method of classification across a wide range of cognitive domains to identify discrete clusters within a group of BD patients. A very recent study reported similar results, which showed the presence of three discrete cognitive clusters: one defined by a global impairment (21.2%) present across all domains (i.e. working memory and executive functions, processing speed and verbal memory), another cluster with selective impairment (32%), and a final cluster (46%) with normal cognitive functioning (Jensen et al. 2016). Other recent studies that have applied cluster analytic approaches to psychotic disorders (including new-onset patients) have also found cognitive heterogeneity to be anchored by the presence of a neuropsychological normal cluster, a globally impaired cluster, and either one or two clusters with mixed cognitive functioning (Lewandowski et al. 2014; Reser et al. 2015). Similar to our previous data (Burdick et al. 2014), a three-cluster solution, including globally, selectively impaired and cognitively normal patients, emerged in a sample of euthymic BD type II (Solé et al. 2016). A very recent study investigating the presence of heterogeneity in executive functions and social cognition across SZ and BD patients detected four discrete groups, showing that, in the cluster defined by selective impairment on theory of mind measures, SZ and BD patients were equally represented, whereas the ‘neuropsychologically normal’ cluster consisted primarily of BD patients (Bora et al. 2016). Likewise, a three-cluster solution has been recently described in the largest sample to date, combining 1541 subjects suffering with SZ and BD, supporting the presence of cross-diagnostic, empirically-driven subgroups of patients with severe cognitive deficits, minimal impairment, and normal cognitive functioning (Van Rheenen et al. 2017).
These data are not only clinically relevant when considering the direct effect that cognition has on functional outcome (Burdick et al. 2010), they may also contribute to our understanding of underlying neurobiological and genetic risk factors associated with BD and a range of other neuropsychiatric disorders with apparent genetic overlap (Lichtenstein et al. 2009). Indeed, large-scale initiatives such as the Bipolar-Schizophrenia Network for Intermediate Phenotypes Study (BSNIP) have shown that variability in general cognitive performance in SZ and psychotic BD patients maps onto ‘cross-diagnostic biotypes’ derived from several brain-based biomarkers (Clementz et al. 2016). Since brain-based biomarkers are presumed to have at least partial genetic influences (Narayanan et al. 2015; Mokhtari et al. 2016), it is plausible that specific genetic factors contributing to BD co-vary with phenotypic heterogeneity. In the case of neurocognition, disparate phenotypic presentations may reflect disparate genetic architectures. These hypothetically differing architectures may be indirectly evident through the assessment of cognition in the family members of more homogenously defined BD subgroups.
Several family-based studies highlight the endophenotypic status of cognitive impairment in BD (Bora et al. 2009). However, progress toward understanding the genetic relevance of this has been hampered by a failure to account for the group-level cognitive heterogeneity that is present in probands and potentially in the relatives as well. We can begin to address this issue through the application of HCA approaches in discordant BD sibling pairs, which will allow us to determine whether the parsing of cognitive heterogeneity in BD maps onto biologically meaningful cognitive subgroups. Here we use this approach, in an effort to expand upon previous observations of neurocognitive subgroups within the broader diagnostic boundary of BD (Burdick et al. 2014), and to determine whether these subgroups can inform our understandings of etiological factors that contribute to cognitive impairment in the disorder with regard to genetic risk.
The primary aims of this study were threefold: firstly, we aimed to test for endophenotypes using a standard ‘group-level’ approach, by examining whether unaffected siblings (UAS) performed intermediate to their affected sibling and a sample of unrelated HCs across a cognitive battery tapping into attention/processing speed, verbal memory, and executive functions. Based upon prior work, we hypothesized that BD subjects would perform worse than both UAS and HC, and that UAS would perform worse than HCs on select measures (putative endophenotypes) that index verbal memory and executive functions. Secondly, we aimed to replicate our previous HCA outcomes in an independent sample of affectively stable BD subjects. We expected to find evidence of three distinct cognitive clusters. Finally, we aimed to assess the presence of endophenotypic markers within the derived cognitive subgroups after dividing the UAS sample into cognitive groups based upon their BD sibling’s cognitive cluster assignment. We hypothesized that while UAS would have qualitatively similar Profiles to their BD siblings regardless of cluster assignment, inherited risk factors would contribute to a greater degree to cognitive outcome in the families where the BD probands’ cognitive Profile overlapped most with that seen in SZ (globally impaired).
Methods
Participants
Data were collected from 180 subjects: 60 BD individuals, 49 UAS, and 71 HCs that were recruited from two sites: the Zucker Hillside Hospital (ZHH) – Northwell and the Icahn School of Medicine at Mount Sinai (ISMMS). This sample is completely independent from the prior sample reported on in Burdick et al. 2014. Patients were (1) diagnosed with BD I or BD II by the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (SCID-IV) (First et al. 2002); (2) 18–65 years old; (3) affectively stable [as indexed by a score of <12 on the Hamilton Rating Scale for Depression (HRSD) (Hamilton, 1967); (4) scored <8 on the Clinician Administered Rating Scale for Mania (CARS-M)] (Altman et al. 1994); and (5) had no history of central nervous system trauma, neurological disorder, attention deficit hyperactivity disorder (ADHD), recent substance abuse/dependence (past 3 months), or history of electroconvulsive therapy (ECT) in the past year.
UAS were free from any major Axis I disorder as determined by the SCID-IV. UAS were above the modal age of onset (>25 years old) and were at least 2 years older than the age of onset in their affected sibling. All unrelated HCs were free from lifetime Axis I diagnoses and did not have a first-degree relative with any Axis I disorder.
All procedures were approved by the local Institutional Review Board (IRB) committee and written informed consent was obtained from all participants.
Neurocognitive assessment
Data were available from a neurocognitive battery that assessed several domains of cognition relevant to BD: attention and processing speed – Trail Making Test (TMT)-A and Wechsler Adult Intelligence Scale (WAIS)-Digit symbol subtest (WAIS-III); verbal learning and memory – the California Verbal Learning Test (CVLT) total learning across trials (Trials 1–5) and the short-delay free recall; executive functions – TMT-B and the Wisconsin Card Sorting Test (WCST) categories completed and perseverative errors (Strauss et al. 2006). Estimated premorbid IQ was derived from the Wide Range Achievement Test– Third Edition (WRAT-3) Reading subtest (Wilkinson, 1993).
Analytic approach
Initially, BD, UAS and HC were compared on demographic/clinical characteristics and cognitive performance using analysis of variance (ANOVA) and χ2 tests as appropriate. HRSD and CARS-M scores differed by group and were thus included as covariates in all subsequent analyses. For ease of interpretation of cognitive scores, crude and adjusted (for mood scores) analysis results are reported where appropriate using z-scores (mean = 0, S.D. = 1; based on HC sample performance).
HCA was used to investigate the presence of cognitive clusters in the BD sample using the Squared Euclidian Distance and Ward’s Linkage methods. The following seven neurocognitive variables were entered into the model: TMT-A, Digit symbol, CVLT total learning, CVLT short-delay recall, TMT-B, and WCST categories and WCST perseverative errors. The appropriate number of clusters was determined by taking into consideration the inspection of the dendrogram and more objective criteria based on the agglomeration schedule. These criteria (also known as ‘elbow’ rule) consist of determining the optimal number of clusters to retain by calculating the difference between the total number of cases and the precise step (stage at which cases are merged in forming clusters), wherein the distance coefficient (value indicating the similarity between cases) becomes larger (i.e. increased heterogeneity between cases) than in the two previous steps (Yim & Ramdeen, 2015). Based upon this procedure, the model was determined and cluster membership was saved as a grouping variable. In order to determine the validity of the HCA, a discriminant function analysis (DFA) was then conducted and the predictive power of the cognitive tasks in determining subjects’ allocation into discrete cognitive groups was examined using the original classification score.
Multivariate analysis of covariance (MANCOVA) with post hoc least Significant difference (LSD) correction was carried out to compare cognitive functioning between the BD cognitive clusters for both specific cognitive measures and general cognitive performance (mean cognitive composite z-score). The 49 UAS subjects were then classified based upon the cognitive cluster of their BD sibling and the resulting groups (BD cognitive clusters v. UAS cognitive groups v. HCs) were compared using the same method as above. Magnitude of effects between groups was calculated, and reported where appropriate, using Cohen’s d (difference between means by the pooled S.D.). Estimates of cognitive decline, calculated as the difference between current general cognitive performance and premorbid IQ, was compared across BD clusters, UAS groups, and HC with ANOVA. For these analyses, only BD subjects with corresponding relatives were used (i.e. 49 BD-UAS pairs).
Results
Whole group descriptives
BD, UAS, and HC did not differ in terms of premorbid IQ and demographic features. Although still in the euthymic range, BD subjects had higher current mood ratings than both UAS and HC subjects (Table 1). Within the BD group, 52 (86.7%) were diagnosed with BD I and 8 (13.3%) with BD II; among the BD I subjects 47 (90.4%) and 1 (12.5%) of the BD II subjects had a positive history of psychosis (Table 1). There were no differences between BD I and BD II in terms of age (p = 0.472), sex (p = 0.707), race (p = 0.122), premorbid IQ (p = 0.658), or current symptomatology [CARS-M (p = 0.146); HRSD (p = 0.149)].
Table 1.
Comparison between bipolar disorder patients, unaffected siblings and healthy controls in terms of demographic and clinical characteristics
| Bipolar disorder patientsa (N = 60)
|
Unaffected siblingsb (N = 49)
|
Healthy controlsc (N = 71)
|
Significance
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | df | F or χ2 | p value | Group comparison | |
| Female | 40 | 66.7 | 34 | 69.4 | 37 | 52.1 | 2 | 4.621 | 0.10 | a = b = c |
| Caucasian | 34 | 56.7 | 26 | 53.1 | 26 | 36.6 | 2 | 6.00 | 0.050 | a = b = c |
| Bipolar disorder type I with psychotic features | 52 | 86.7 | – | – | – | – | – | – | – | |
| 47 | 90.4 | – | – | – | – | – | – | – | ||
| Bipolar disorder type II with psychotic features | 8 | 13.3 | – | – | – | – | – | – | – | |
| 1 | 12.5 | – | – | – | – | – | – | – | ||
| Mean | S.D. | Mean | S.D. | Mean | S.D. | |||||
| Age (in years) | 38.0 | 10.6 | 38.9 | 10.3 | 37.8 | 11.8 | 2179 | 0.18 | 0.839 | a = b = c |
| a=c | ||||||||||
| Premorbid IQ (WRAT-3) | 97.7 | 10.7 | 97.2 | 10.6 | 98.3 | 10.0 | 2167 | 0.15 | 0.864 | a = b = c |
| Depressive symptoms (HRSD) | 6.8 | 5.8 | 2.9 | 2.6 | 2.0 | 2.7 | 2179 | 24.65 | <0.001 | a > b = c* |
| a> c* | ||||||||||
| Manic symptoms (CARS-M) | 3.8 | 4.7 | 1.3 | 1.4 | 0.9 | 1.3 | 2179 | 17.95 | <0.001 | a > b = c* |
| a> c* | ||||||||||
WRAT-3, Wide Range Achievement Test–third edition; CARS-M, Clinician Administered Rating Scale for Mania.
The mean difference is significant at the 0.05 level using Least Significance Difference correction for multiple comparison
Cognitive comparisons by group prior to clustering
A Significant main effect of group on cognition emerged [F(14, 320) = 2.48; p = 0.002, partial eta square = 0.10]. Between-subjects effects were statistically Significant for digit symbol (p < 0.001), CVLT total learning and delayed recall (Significant at p = 0.001) as well as the cognitive composite score (p = 0.003). Post hoc testing revealed that BD patients performed worse on the digit symbol test compared with both UAS (p = 0.001) and HCs (p < 0.001); and worse than HC subjects on CVLT total learning and delayed recall (both p < 0.001; Fig. 1). The composite cognitive score was Significantly lower in the BD group compared with both UAS and HCs (p = 0.011 and 0.001, respectively). A lower performance of UAS compared with HCs emerged on CVLT total learning and delayed recall (p = 0.033 and 0.036, respectively).
Fig. 1.
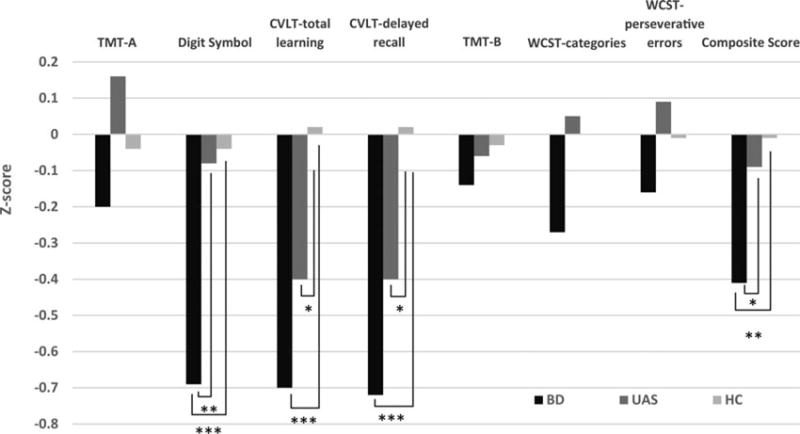
Comparison between bipolar disorder (BD) patients, unaffected siblings (UAS) and healthy controls (HC) across neurocognitive measures. Note: manic and depressive symptom ratings were used as covariates in the model. *Significant at p < 0.05 after Least Significance Difference correction for multiple comparison; **Significant at p < 0.002 after Least Significance Difference correction for multiple comparison; ***Significant at p < 0.001 after Least Significance Difference correction for multiple comparison.
Cognitive clustering
Consistent with our previous report (Burdick et al. 2014), HCA yielded three cognitive clusters. The optimal number of clusters was determined by the dendrogram (Supplementary Material 1), and corroborated using the elbow rule where the agglomeration indicated a larger difference (43.54 between steps 57 and 56) than that noted in the prior step (27.756 between steps 56 and 55). Using the nomenclature adopted in our previous study (Burdick et al. 2014), 36.7% (n = 22) of subjects were globally impaired, 30% (n = 18) were selectively impaired and 33.3% (n = 20) were cognitively intact v. HCs. Results from the DFA revealed the presence of two discriminant functions explaining 86.7% and 13.3% of the variance, respectively (Wilks’ λ = 0.11, χ2 (14) = 120.0, p < 0.001; after removal of the first function the Wilks’ λ = 0.60, χ2 (6) = 28.2, p < 0.001). The verbal memory total and delayed recall scores (correlation coefficients were 0.703 and 0.567, respectively) contributed more than the other cognitive tasks in discriminating subjects into clusters. All cases were correctly allocated into their newly emergent clusters (100% accuracy).
Comparisons between BD clusters and HCs
There were no Significant group differences between BD clusters and HCs for demographic or clinical features, with the exception of premorbid IQ [F(3,125) = 2.77; p = 0.045]; with lower premorbid IQ in the globally impaired cluster (93.5 ± 10.5) v. the cognitively intact cluster (102.9 ± 6.8; p = 0.005). Although the BD sample was affectively stable, depressive and manic symptoms were still Significantly higher in all BD clusters compared with the HC sample (Table 2). MANCOVA revealed a main effect of cluster on cognitive functioning [F(21, 342) = 6.25; partial eta square = 0.27, p < 0.001]. The globally impaired cluster performed Significantly worse than both the cognitively intact cluster and the HCs on all measures except the WCST perseverative errors (all p < 0.02; Table 2). The globally impaired cluster performed Significantly worse than the selectively impaired cluster across all measures except verbal learning, immediate recall, and WCST perseverative errors. The selectively impaired cluster performed worse than the cognitively intact cluster and HCs on learning, delayed recall (all p < 0.001), composite score (p = 0.001 and 0.011, respectively), and lower than just HC on TMT-A (p = 0.018).
Table 2.
Comparison between demographic, clinical features, and cognitive performance across BD clusters and the HC sample
| Demographic and clinical measures | Globally impaired BD cluster (a) | Selectively impaired BD cluster (b) | BD cognitively intact (c) | HC (d) | Significance
|
|||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Mean (S.D.) | Mean (S.D.) | Mean (S.D.) | Mean (S.D.) | Df | F or χ2 | p value | Group comparison | |
| Age (in years) | 42.10 (11.67) | 35.83 (7.43) | 35.35 (10.80) | 37.75 (11.79) | 3130 | 1.60 | N.S. | – |
| Premorbid IQ (WRAT-3) | 93.48 (10.55) | 97.71 (12.17) | 102.88 (6.78) | 98.28 (10.03) | 3125 | 2.77 | 0.045 | a < c |
| Depressive symptoms (HRSD) | 6.86 (5.23) | 5.43 (4.92) | 7.80 (6.97) | 2.02 (2.73) | 3130 | 13.58 | <0.001 | d <a=b=c |
| Manic symptoms (CARS-M) | 4.32 (5.65) | 3.54 (4.12) | 3.35 (4.04) | 0.87 (1.30) | 3130 | 8.63 | <0.001 | d <a = b = c |
| N (%) | N (%) | N (%) | N (%) | |||||
| Female | 18 (81.8) | 12 (66.7) | 10 (50.0) | 37 (52.1%) | 3 | 7.22 | N.S. | N.S. |
| Caucasian | 12 (54.5) | 11 (61.1) | 11 (55.0) | 26 (36.6) | 3 | 5.47 | N.S. | N.S. |
| Bipolar disorder type I | 20 (90.9) | 16 (88.9) | 16 (80.0) | – | – | – | – | – |
| Bipolar disorder type II | 2 (9.1) | 2 (11.1) | 4 (20.0) | – | ||||
| Positive history of psychosis | 18 (81.8) | 14 (77.8) | 16 (80.0) | N.S. | ||||
| Cognitive measures (Z-score) | Mean (S.D.) | Mean (S.D.) | Mean (S.D.) | Mean (S.D.) | Post hoc tests significance | |||
| TMT-A | −1.13 (0.21) | 0.50 (0.23) | 0.19 (0.21) | −0.05 (0.12) | a < b = c = d; p < 0.001 | |||
| Digit symbol | −1.40 (0.66) | −0.57 (1.09) | −0.01 (1.34) | −0.04 (1.00) | a < b; p = 0.007 | |||
| a< c; p < 0.001 | ||||||||
| a< d; p < 0.001 | ||||||||
| b< d; p = 0.018 | ||||||||
| CVLT – total learning | −1.38 (0.86) | −1.11 (0.68) | 0.42 (0.66) | 0.02 (1.01) | a < c = d;p < 0.001 | |||
| b<c = d; p < 0.001 | ||||||||
| CVLT – delayed recall | −1.30 (0.93) | −1.04 (0.69) | 0.23 (0.56) | 0.02 (1.03) | a < c = d;p < 0.001 | |||
| b<c = d; p < 0.001 | ||||||||
| TMT - B | −1.25 (0.78) | 0.41 (0.64) | 0.57 (0.45) | −0.03 (0.98) | a < b = c = d; p < 0.001 | |||
| WCST-categories | −0.77 (0.77) | −0.18 (0.60) | 0.20 (0.72) | 0.00 (1.00) | a < b; p = 0.039 | |||
| a< c; p < 0.001 | ||||||||
| a< d; p = 0.002 | ||||||||
| WCST-perseverative errors | −0.54 (0.91) | −0.09 (0.85) | 0.19 (0.53) | −0.01 (0.96) | a < c; p = 0.011 | |||
| Composite score | −1.11 (0.35) | −0.28 (0.37) | 0.25 (0.25) | −0.01 (0.54) | a < b; p < 0.001 a< c; p < 0.001 a< d; p < 0.001 b< c; p < 0.001 b< d; |
|||
|
| ||||||||
| p = 0.011 | ||||||||
BD, bipolar disorder; HC, healthy control; WRAT-3, Wide Range Achievement Test–third edition; CARS-M, Clinician Administered Rating Scale for Mania; TMT, Trail Making Test; CVLT, California Verbal Learning Test; WCST, Wisconsin Card Sorting Test.
Only results (adjusted for mood symptoms as measured by CARS-M and HRSD ratings) significant at Least Mean Difference p = 0.05 are reported.
Comparisons of BD clusters v. UAS groups and UAS groups v. HCs (Fig. 2)
Fig. 2.
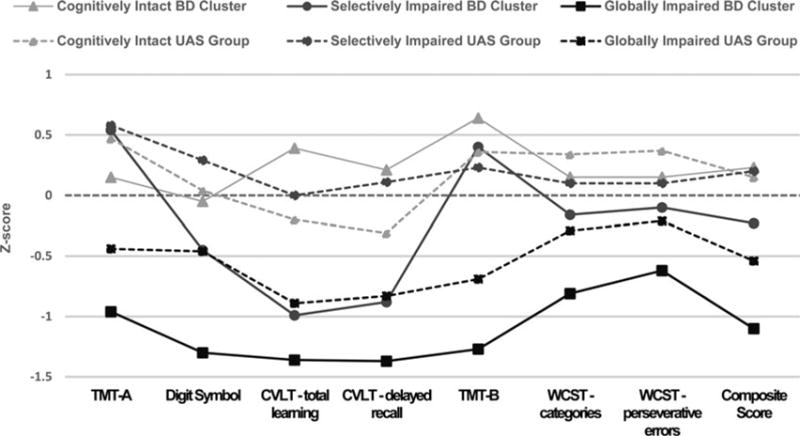
Profiles of the bipolar disorder (BD) clusters (continuous lines) and the unaffected sibling (UAS) groups (dotted lines) (49 pairs) based upon cognitive clusters. Note: manic (CARS-M) and depressive (HRSD) symptom ratings were used as covariates in the model. Dotted straight line represents the HC sample.
After the UAS subjects were classified into cognitive groups based upon their BD siblings’ cluster assignment, MANCOVA revealed a Significant overall main effect of group [F(42, 894) = 2.51 p < 0.001, partial eta squared = 0.11]. Post hoc tests (statistically Significant comparisons reported in Table 3) revealed that although the globally impaired BD cluster was more impaired than their siblings (the UAS globally impaired group) on digit symbol and the composite score (d = 1.20 and 1.01, respectively), they did not Significantly differ from them on any other measure and the UAS globally impaired group was impaired relative to HCs on the composite score and on individual tests of total verbal learning (d = 0.80) and delayed recall (d = 0.86), TMT-B (executive functioning; d = 0.50) – supporting these measures as likely endophenotypic markers, at least within this subset of patients. In addition, the UAS globally impaired group performed worse than the UAS cognitively intact group on total learning (d = 0.58), TMT-B (d = 0.80), WCST categories (d = 0.71), and the composite score (d = 1.13); and lower than the UAS selectively impaired group on all measures (d values ranging from 0.63 in TMT-B up to d = 1.12 in the composite score) but WCST perseverative errors and categories; and worse than the cognitively intact BD cluster on total verbal learning (d = 1.28) (Table 3).
Table 3.
Comparison between cognitive performance of the BD patient clusters, UAS groups, and the HC sample
| Globally impaired BD cluster | Selectively impaired BD cluster | Cognitively intact BD cluster | Globally impaired UAS group | selectively impaired UAs group | Cognitively intact UAs group | HC | ||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Mean (S.D.) | Mean (S.D.) | Mean (S.D.) | Mean (S.D.) | Mean (S.D.) | Mean (S.D.) | Mean (S.D.) | Post hoc tests significance* | |
| TMT-A | −0.96 (0.85) | 0.54 (0.59) | 0.15 (1.24) | −0.44 (1.28) | 0.58 (0.85) | 0.47 (0.69) | −0.05 (1.00) | BD global < BD selective p < 0.001 |
| BD global < BD intact p = 0.001 | ||||||||
| BD global < UAS selective p < 0.001 | ||||||||
| BD global < UAS intact p < 0.001 | ||||||||
| BD global < HC p = 0.001 | ||||||||
| BD selective < UAS global p = 0.008 | ||||||||
| UAS global < UAS selective p = 0.007 | ||||||||
| UAS global < UAS intact p = 0.008 | ||||||||
| UAS selective > HC p = 0.049 | ||||||||
| Digit symbol | −1.30 (0.66) | −0.45 (1.23) | −0.05 (1.37) | −0.46 (0.73) | 0.29 (0.89) | 0.04 (0.84) | −0.04 (1.00) | BD global < BD selective p = 0.008 |
| BD global < BD intact p < 0.001 | ||||||||
| BD global < UAS global p = 0.004 | ||||||||
| BD global < UAS selective p < 0.001 | ||||||||
| BD global < UAS intact p < 0.001 | ||||||||
| BD global < HC p < 0.001 | ||||||||
| UAS global < UAS selective p = 0.038 | ||||||||
| CVLT – total learning | −1.36 (0.88) | −0.99 (0.67) | 0.39 (0.66) | −0.89 (1.25) | 0.00 (1.15) | −0.20 (1.13) | 0.02 (1.01) | BD global < BD intact p < 0.001 |
| BD global < UAS selective p < 0.001 | ||||||||
| BD global < UAS intact p = 0.001 | ||||||||
| BD global < HC p < 0.001 | ||||||||
| BD selective < BD intact p = 0.001 | ||||||||
| BD selective < UAS selective p = 0.011 | ||||||||
| BD selective < UAS intact p = 0.027 | ||||||||
| BD selective < HC p = 0.001 | ||||||||
| BD intact > UAS global p < 0.001 | ||||||||
| UAS global < UAS selective p = 0.017 | ||||||||
| UAS global < UAS intact p = 0.041 | ||||||||
| UAS global < HC p = 0.001 | ||||||||
| CVLT – delayed recall | −1.37 (0.87) | −0.88 (0.63) | 0.21 (0.57) | −0.83 (0.95) | 0.11 (1.31) | −0.31 (1.09) | 0.02 (1.03) | BD global < BD intact p < 0.001 |
| BD global v. UAS selective p < 0.001 | ||||||||
| BD global < UAS intact p = 0.002 | ||||||||
| BD global < HC p < 0.001 | ||||||||
| BD selective < BD intact p = 0.006 | ||||||||
| BD selective < UAS selective p = 0.018 | ||||||||
| BD selective < HC p = 0.006 | ||||||||
| BD intact > UAS global p = 0.004 | ||||||||
| UAS global < UAS selective p = 0.011 | ||||||||
| UAS global < HC p = 0.001 | ||||||||
| TMT-B | −1.27 (0.84) | 0.40 (0.58) | 0.64 (0.35) | −0.69 1.60 | 0.23 (1.28) | 0.36 (0.92) | −0.03 (0.98) | BD global < BD selective p < 0.001 |
| BD global < BD intact p < 0.001 | ||||||||
| BD global < UAS selective p < 0.001 | ||||||||
| BD global < UAS intact p < 0.001 | ||||||||
| BD global < HC p < 0.001 | ||||||||
| BD selective > UAS global p = 0.006 | ||||||||
| BD intact > UAS global p < 0.001 | ||||||||
| UAS global < UAS selective p = 0.014 | ||||||||
| UAS global < UAS intact p = 0.002 | ||||||||
| UAS global < HC p = 0.013 | ||||||||
| WCST-categories | −0.81 0.77 | −0.16 (0.63) | 0.15 (0.71) | −0.29 (1.00) | 0.10 (0.84) | 0.34 (0.74) | 0.00 (1.00) | BD global < BD intact p = 0.001 |
| BD global < UAS selective p = 0.009 | ||||||||
| BD global < UAS intact p < 0.001 | ||||||||
| BD global < HC p = 0.002 | ||||||||
| UAS global < UAS intact p = 0.036 | ||||||||
| WCST-perseverative errors | −0.62 (0.92) | −0.10 (0.83) | 0.15 (0.52) | −0.21 (0.86) | 0.10 (0.76) | 0.37 (0.60) | −0.01 (0.96) | BD global < BD intact p = 0.006 |
| BD global < UAS intact p = 0.003 | ||||||||
| BD global < HC p = 0.045 | ||||||||
| Composite score | −1.10 (0.34) | −0.23 (0.39) | 0.23 (0.24) | −0.54 (0.70) | 0.20 (0.61) | 0.15 (0.50) | −0.01 (0.54) | BD global < BD selective p < 0.001 |
| BD global < BD intact p < 0.001 | ||||||||
| BD global < UAS global p = 0.001 | ||||||||
| BD global < UAS selective p < 0.001 | ||||||||
| BD global < UAS intact p < 0.001 | ||||||||
| BD global < HC p < 0.001 | ||||||||
| BD selective < BD intact p = 0.028 | ||||||||
| BD selective < UAS selective p = 0.035 | ||||||||
| BD selective < UAS intact p = 0.044 | ||||||||
| BD intact > UAS global p < 0.001 UAS | ||||||||
| global < UAS selective p < 0.001 UAS | ||||||||
| global < UAS intact p < 0.001 UAS | ||||||||
| global < HC p < 0.001 | ||||||||
BD, bipolar disorder; UAS, unaffected siblings; HC, healthy control; CARS-M, Clinician Administered Rating Scale for Mania; TMT, Trail Making Test; CVLT, California Verbal Learning Test; WCST, Wisconsin Card Sorting Test.
Only results [adjusted for mood symptoms as measured by CARS-M, Clinician Administered Rating Scale for Mania (CARS-M) and HRSD ratings] significant at Least Mean Difference p = 0.05 are reported.
The selectively impaired UAS group outperformed their BD siblings on total learning (d = 1.06) and delayed recall (d = 0.96), and composite score (d = 0.06), but did not differ on other measures. Apart from TMT-A, wherein UAS selectively impaired group performed better than the HCs (d = 0.60), there were no other differences between the two groups. Finally, the cognitively intact BD cluster did not differ from the UAS cognitively intact group or HCs on any measure (Table 3).
General cognitive performance (composite score) was lowest in the BD globally impaired cluster relative to all the other BD clusters, the UAS groups, and the HC sample. The UAS globally impaired group performed lower than the other UAS groups, the HC group, and the cognitively intact BD cluster (Table 3). Using a proxy measure of cognitive decline (the difference between current composite score and premorbid IQ based upon WRAT-3), a Significant difference in the degree of decline was evident [F(6, 147) = 4.206; p = 0.001]. Decline was noted in the BD globally impaired cluster (−0.61); but not in the BD selectively impaired cluster (0.02), or in the BD cognitively intact cluster (0.08). Likewise, in the UAS globally impaired group, decline was noted (−0.45) but not seen in the UAS selectively impaired group (0.67) nor in the UAS cognitively intact group (0.21). Post hoc analysis revealed that the globally impaired BD patients had Significantly greater ‘decline’ in general cognition compared with all other groups (all p < 0.043). Estimated decline in the UAS globally impaired group was also greater than in the UAS selectively impaired group, the BD cognitively intact cluster, and the HCs (p < 0.023). Finally, the UAS selectively impaired group showed an ‘improvement’ in cognitive functioning v. HCs (p = 0.030; Fig. 3).
Fig. 3.
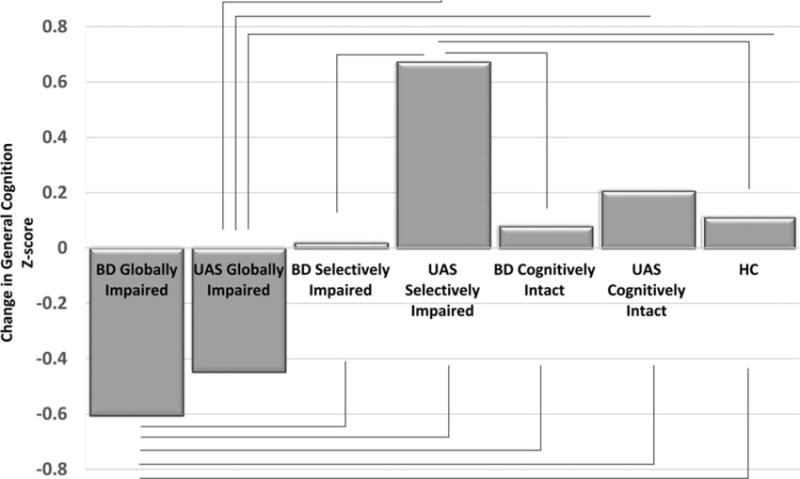
Change between premorbid IQ and current composite score across bipolar disorder (BD) clusters, unaffected siblings (UAS) groups and healthy controls (HC).
Discussion
In this study, we examined the neurocognitive performance of 60 affectively stable BD patients, 49 of their UAS, and 71 unrelated HCs with no family history of psychiatric illness. We tested for evidence of within-group cognitive heterogeneity by classifying BD patients using neurocognitive Profile data with cluster analysis. We then grouped the UAS according to the cognitive cluster assignment of their affected BD siblings and tested for group differences.
As a group, BD patients performed worse than both UAS and HCs on processing speed and composite score, and also on verbal memory compared with HC only. Corroborating our hypothesis and previous reports (Torres et al. 2007; Arts et al. 2008; Bora et al. 2009), we found that verbal memory is a valid endophenotype for the disorder, as the full sample of UAS performed Significantly worse than the unrelated HCs on verbal learning (both total learning and delayed recall).
Consistent with our previous work, three distinct cognitive subgroups within the BD cohort emerged: one with a global impairment defined by moderately severe deficits in general cognition (composite z = −1.11) and moderate-to-severe deficits across all cognitive measures; a second subgroup with general cognition falling within the normal range (composite z = −0.28), with a selective impairment in verbal memory (z = −1.11); and a third subgroup characterized by cognitively intact functioning (composite z = 0.20). The relative proportion of BD subjects allocated into the three subgroups were similar to our previous work (Burdick et al. 2014); however, in the current study, the cognitive performance of the subgroups was generally superior to that seen in our prior report where we used the MATRICS Consensus Cognitive Battery (MCCB) (Nuechterlein et al. 2008) to assess cognition. This may be due to the different test batteries employed or to differences in the two samples themselves. The present results are also in line with the cluster solution found by Jensen et al. (2016); however, it can be noted that the proportion of BD belonging to the more severe cognitive impaired cluster is larger in our study (36.7% v. 21.2%). This might be due, not only to the use of different cognitive tests, but also to same differences in the samples like age (our subjects are slightly older) and in BD type (a higher proportion of BD type I and a higher proportion of psychotic subtype in our sample).
After grouping the UAS based upon their affected sibling cluster assignment, we were able to compare performance within each of the resultant subgroups. Within these cognitive subgroups, we found compelling evidence to suggest that deficits in cognition may be differentially associated with genetic risk for the illness. This is of particular importance, as cognitive impairment has been consistently shown to be a viable endophenotype in patients with SZ; however, results in BD have been less convincing. The lack of consistent evidence for cognitive endophenotypes in BD may be at least in part explained by the profound cognitive heterogeneity in the disease and the presence of a substantial subset of patients who function in the normal range thereby ‘watering down’ the overall level of deficit noted in many samples. When not considering the cognitive subgroups separately, one might erroneously conclude that inherited risk factors do not contribute to the cognitive deficits seen in BD at all and that they are solely explained by the expression of the disease and its confounds. Our data suggest that both genetic and environmental factors are contributing to the impairments noted in the illness, a finding that is most clearly revealed after classifying patients into more homogeneous subgroups. Specifically, within those families characterized by a proband with a global cognitive impairment, the UAS performed Significantly worse than unrelated HCs in terms of verbal learning, executive functions (TMT-B), and general cognitive ability. In contrast, the UAS of the selectively impaired and cognitively intact BD probands performed well compared with HCs apart from processing speed, wherein the UAS selectively impaired group did actually better than HCs on all domains. These data suggest the presence of a specific subgroup of BD patients that is characterized by a more severe and global level of cognitive impairment that can be at least partially attributed to inherited risk factors.
The parsing of heterogeneity in BD using cognition as a classifying variable is an approach that is of critical importance for two reasons. First, it can help to account for the considerable variability between individuals with BD both in terms of the course of the illness and the disparate levels of functional disability (Sanchez-Moreno et al. 2009; Burdick et al. 2010; Bonnín et al. 2014). Second, it may provide insight into the way in which neurodevelopmental and/or genetic factors play a role in the etiology of cognitive impairments in BD. Indeed, the presence of a globally impaired subgroup in our data lends support to the concept of a dimensional continuum between BD and other severe psychiatric disorders such as SZ, at least with regard to cognitive factors. While a neurodevelopmental hypothesis has long been considered primary in SZ research, only recently has such a hypothesis been put forth in BD, particularly with regard to similarities in neurodevelopmental trajectories common to SZ (Arango et al. 2014; Bora & Pantelis, 2015). It has been hypothesized (Bora & Pantelis, 2015) that a subgroup of BD patients may exist with a mix of affective and psychotic features, who share more of the genetic risk factors with SZ than do other BD subtypes. Such a proposed subgroup would be more likely to share a similar cognitive phenotype with SZ, including a neurodevelopmental trajectory marked by the presence of premorbid cognitive deficits and reduced cognitive reserve (Burdick et al. 2014; Anaya et al. 2016). Longitudinal studies are clearly needed to test this hypothesis. Nonetheless, our work does support this idea, with evidence of a subgroup of BD patients (i.e. globally impaired cluster) that have lower premorbid IQ than other BD subgroups, consistent with Profiles seen in SZ. This was evident both in our prior work, and in the current investigation where the cognitive deficits and estimated decline in cognitive functioning of this subgroup appear to be associated with genetic risk for the illness. It is certainly plausible that this group of BD individuals is one in which lower cognitive reserve (proxied by premorbid IQ) results in greater susceptibility to cognitive insults.
Our findings should be considered in the context of several limitations. The cross-sectional nature of this study does not allow us to directly test our hypothesis that these cognitive subgroups may have differential neurodevelopmental trajectories; however, the evidence provided from the UAS in this study does provide support that a subset of BD patients are likely to have cognitive impairment that is more pronounced than other BD patients, which may be attributed to inherited risk factors. Our BD clustering sample was small, and despite assessing several domains of cognition of relevance to BD, the neurocognitive battery used here lacked measures of working memory, vigilance, and social cognition. The data collected in the current study preceded the release of the MCCB, thus the battery was inherently different. Whether a larger sample and broader battery would have yielded the same clusters in this BD cohort remains an empirical question. However, given that these results were consistent with our past work that used a larger sample and a more comprehensive cognitive battery, these factors are unlikely to have had a major effect on the clustering outcomes. This is particularly plausible given that both our current and previous results show evidence that the cluster solutions are largely driven by general cognitive ability resulting in high-, intermediate-, and low-performance groups. Nonetheless, a broader battery would be useful in asses-sing all tests/domains that have been identified as potential endophenotypes for BD, to ascertain whether the UAS globally impaired group’s results extend beyond the domains tested here. Our BD sample was also characterized by an unusually high rate of psychosis; therefore, these results may not generalize to all BD samples. Finally, we were unable to adequately control for medication effects in the BD sample; however, UAS were never medicated so are not confounded by this factor. Future work should endeavor to address this limitation in the BD patients themselves.
In conclusion, our findings suggest that different, and potentially biologically meaningful cognitive subgroups exist within BD. These subgroups support the validity of a dimensional continuum with SZ and other psychotic disorders. The insights provided by the cognitive performance of the UAS here highlight the relevance that cognitive subgroups have for molecular genetic studies across the range of psychotic disorders. As more is learned about the underlying causes of cognitive impairment in BD, we can begin to use this information to suggest differential strategies for optimizing intervention.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge patients and their relatives who took part in the research. This work was supported by grants from the National Institute of Mental Health (grant numbers: R03MH079995; K23MH077807; R01MH100125 to KEB) and the Veterans Health Administration (grant number: I01CX000995-03 to KEB).
Footnotes
Supplementary Material
The supplementary material for this article can be found at https://doi.org/10.1017/S003329171700143X
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
References
- Altman EG, Hedeker DR, Janicak PG, Peterson JL, Davis JM. The Clinician-Administered Rating Scale for Mania (CARS-M): development, reliability, and validity. Biological Psychiatry. 1994;36:124–134. doi: 10.1016/0006-3223(94)91193-2. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Gitlin MJ, Mintz J, Leight KL, Frye MA. Subsyndromal depression is associated with functional impairment in patients with bipolar disorder. Journal of Clinical Psychiatry. 2002;63:807–811. doi: 10.4088/jcp.v63n0910. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Ventura J, van Gorp WG, Green MF, Theberge DC, Mintz J. Neurocognitive function in clinically stable men with bipolar I disorder or schizophrenia and normal control subjects. Biological Psychiatry. 2004;56:560–569. doi: 10.1016/j.biopsych.2004.08.002. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th. American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- Anaya C, Torrent C, Caballero FF, Vieta E, Bonnin CDM, Ayuso-Mateos JL, CIBERSAM Functional Remediation Group Cognitive reserve in bipolar disorder: relation to cognition, psychosocial functioning and quality of life. Acta Psychiatrica Scandinavica. 2016;133:386–398. doi: 10.1111/acps.12535. [DOI] [PubMed] [Google Scholar]
- Arango C, Fraguas D, Parellada M. Differential neurodevelopmental trajectories in patients with early-onset bipolar and schizophrenia disorders. Schizophrenia Bulletin. 2014;40(Suppl 2):S138–S146. doi: 10.1093/schbul/sbt198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arts B, Jabben N, Krabbendam L, van Os J. Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychological Medicine. 2008;38:771–785. doi: 10.1017/S0033291707001675. [DOI] [PubMed] [Google Scholar]
- Baş TÖ, Poyraz CA, Baş A, Poyraz BÇ, Tosun M. The impact of cognitive impairment, neurological soft signs and subdepressive symptoms on functional outcome in bipolar disorder. Journal of Affective Disorders. 2015;174:336–341. doi: 10.1016/j.jad.2014.12.026. [DOI] [PubMed] [Google Scholar]
- del Mar Bonnín C, González-Pinto A, Solé B, Reinares M, González-Ortega I, Alberich S, Crespo JM, Salamero M, Vieta E, Martínez-Arán A, Torrent C, CIBERSAM Functional Remediation Group Verbal memory as a mediator in the relationship between subthreshold depressive symptoms and functional outcome in bipolar disorder. Journal of Affective Disorders. 2014;160:50–54. doi: 10.1016/j.jad.2014.02.034. [DOI] [PubMed] [Google Scholar]
- Bora E, Pantelis C. Meta-analysis of cognitive impairment in first-episode bipolar disorder: comparison with first-episode schizophrenia and healthy controls. Schizophrenia Bulletin. 2015;41:1095–1104. doi: 10.1093/schbul/sbu198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Veznedaroğlu B, Vahip S. Theory of mind and executive functions in schizophrenia and bipolar disorder: a cross-diagnostic latent class analysis for identification of neuropsychological subtypes. Schizophrenia Research. 2016;176:500–505. doi: 10.1016/j.schres.2016.06.007. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. Journal of Affective Disorders. 2009;113:1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Depp C, McGrath JA, Wolyniec P, Mausbach BT, Thornquist MH, Luke J, Patterson TL, Harvey PD, Pulver AE. Prediction of real-world functional disability in chronic mental disorders: a comparison of schizophrenia and bipolar disorder. American Journal of Psychiatry. 2010;167:1116–1124. doi: 10.1176/appi.ajp.2010.09101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick KE, Goldberg JF, Harrow M. Neurocognitive dysfunction and psychosocial outcome in patients with bipolar I disorder at 15-year follow-up. Acta Psychiatrica Scandinavica. 2010;122:499–506. doi: 10.1111/j.1600-0447.2010.01590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick KE, Goldberg TE, Cornblatt BA, Keefe RS, Gopin CB, Derosse P, Braga RJ, Malhotra AK. The MATRICS consensus cognitive battery in patients with bipolar I disorder. Neuropsychopharmacology. 2011;36:1587–1592. doi: 10.1038/npp.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick KE, Russo M, Frangou S, Mahon K, Braga RJ, Shanahan M, Malhotra AK. Empirical evidence for discrete neurocognitive subgroups in bipolar disorder: clinical implications. Psychological Medicine. 2014;44:3083–3096. doi: 10.1017/S0033291714000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, Keshavan MS, Tamminga CA. Identification of distinct psychosis biotypes using brain-based biomarkers. American Journal of Psychiatry. 2016;173:373–384. doi: 10.1176/appi.ajp.2015.14091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daban C, Martinez-Aran A, Torrent C, Tabarés-Seisdedos R, Balanzá-Martínez V, Salazar-Fraile J, Selva-Vera G, Vieta E. Specificity of cognitive deficits in bipolar disorder versus schizophrenia. A systematic review. Psychotherapy and Psychosomatics. 2006;75:72–84. doi: 10.1159/000090891. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, GIbbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York State Psychiatric Institute; New York: 2002. ((SCID-I/P) Biometric Research: New York). [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. British Journal of Social and Clinical Psychology. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Jensen JH, Knorr U, Vinberg M, Kessing LV, Miskowiak KW. Discrete neurocognitive subgroups in fully or partially remitted bipolar disorder: associations with functional abilities. Journal of Affective Disorders. 2016;205:378–386. doi: 10.1016/j.jad.2016.08.018. [DOI] [PubMed] [Google Scholar]
- Lewandowski KE, Sperry SH, Cohen BM, Ongür D. Cognitive variability in psychotic disorders: a cross-diagnostic cluster analysis. Psychological Medicine. 2014;44:3239–3248. doi: 10.1017/S0033291714000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Björk C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino DJ, Strejilevich SA, Scápola M, Igoa A, Marengo E, Ais ED, Perinot L. Heterogeneity in cognitive functioning among patients with bipolar disorder. Journal of Affective Disorders. 2008;109:149–156. doi: 10.1016/j.jad.2007.12.232. [DOI] [PubMed] [Google Scholar]
- Mokhtari M, Narayanan B, Hamm JP, Soh P, Calhoun VD, Ruaño G, Kocherla M, Windemuth A, Clementz BA, Tamminga CA, Sweeney JA, Keshavan MS, Pearlson GD. Multivariate genetic correlates of the auditory paired stimuli-based P2 event-related potential in the psychosis dimension from the BSNIP study. Schizophrenia Bulletin. 2016;42:851–862. doi: 10.1093/schbul/sbv147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan B, Ethridge LE, O’Neil K, Dunn S, Mathew I, Tandon N, Calhoun VD, Ruaño G, Kocherla M, Windemuth A, Clementz BA, Tamminga CA, Sweeney JA, Keshavan MS, Pearlson GD. Genetic sources of subcomponents of event-related potential in the dimension of psychosis analyzed from the B-SNIP study. American Journal of Psychiatry. 2015;172:466–478. doi: 10.1176/appi.ajp.2014.13101411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ, Gold JM, Goldberg T, Heaton RK, Keefe RSE, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR. The MATRICS consensus cognitive battery, part 1: test selection, reliability, and validity. American Journal of Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Harvey PD, Bowie CR, Mojtabai R, Rabinowitz J, Heaton RK, Bromet E. Neuropsychological function and dysfunction in schizophrenia and psychotic affective disorders. Schizophrenia Bulletin. 2009;35:1022–1029. doi: 10.1093/schbul/sbn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reser MP, Allott KA, Killackey E, Farhall J, Cotton SM. Exploring cognitive heterogeneity in first-episode psychosis: what cluster analysis can reveal. Psychiatry Research. 2015;229:819–827. doi: 10.1016/j.psychres.2015.07.084. [DOI] [PubMed] [Google Scholar]
- Sanchez-Moreno J, Martinez-Aran A, Tabarés-Seisdedos R, Torrent C, Vieta E, Ayuso-Mateos JL. Functioning and disability in bipolar disorder: an extensive review. Psychotherapy and Psychosomatics. 2009;78:285–297. doi: 10.1159/000228249. [DOI] [PubMed] [Google Scholar]
- Solé B, Jiménez E, Torrent C, Del Mar Bonnin C, Torres I, Reinares M, Priego Á, Salamero M, Colom F, Varo C, Vieta E, Martínez-Arán A. Cognitive variability in bipolar II disorder: who is cognitively impaired and who is preserved. Bipolar Disorders. 2016;18:288–299. doi: 10.1111/bdi.12385. [DOI] [PubMed] [Google Scholar]
- Strauss E, Sherman E, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Oxford University Press; USA: 2006. [Google Scholar]
- Torres IJ, Boudreau VG, Yatham LN. Neuropsychological functioning in euthymic bipolar disorder: a meta-analysis. Acta Psychiatrica Scandinavica. 2007:17–26. doi: 10.1111/j.1600-0447.2007.01055.x. Supplementum. [DOI] [PubMed] [Google Scholar]
- Van Rheenen TE, Lewandowski KE, Tan EJ, Ospina LH, Ongur D, Neill E, Gurvich C, Pantelis C, Malhotra AK, Rossell SL, Burdick KE. Characterizing cognitive heterogeneity on the schizophrenia-bipolar disorder spectrum. Psychological Medicine. 2017:1–17. doi: 10.1017/S0033291717000307. [DOI] [PubMed] [Google Scholar]
- Van Rheenen TE, Rossell SL. Objective and subjective psychosocial functioning in bipolar disorder: an investigation of the relative importance of neurocognition, social cognition and emotion regulation. Journal of Affective Disorders. 2014;162:134–141. doi: 10.1016/j.jad.2014.03.043. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS. WRAT-3: Wide Range Achievement Test Administration Manual. Wide Range, Inc; Wilmington, Del: 1993. [Google Scholar]
- Yim O, Ramdeen K. Hierarchical cluster analysis: comparison of three linkage measures and application to psychological data. The Quantitative Methods for Psychology. 2015;11:8–21. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


