Abstract
Aims
Mitral valve interstitial cells (MVIC) play an important role in the pathogenesis of degenerative mitral regurgitation (MR) due to mitral valve prolapse (MVP). Numerous clinical studies have observed serotonin (5HT) dysregulation in cardiac valvulopathies; however, the impact of 5HT-mediated signaling on MVIC activation and leaflet remodeling in MVP have been investigated to a limited extent. Here we test the hypothesis that 5HT receptors (5HTRs) signaling contributes to MVP pathophysiology.
Methods and Results
Diseased human MV leaflets were obtained during cardiac surgery for MVP; normal MV leaflets were obtained from heart transplants. MV RNA was used for microarray analysis of MVP patients versus control, highlighting genes that indicate the involvement of 5HTR pathways and extracellular matrix remodeling in MVP. Human MV leaflets were also studied in vitro and ex vivo with biomechanical testing to assess remodeling in the presence of a 5HTR2B antagonist (LY272015). MVP leaflets from Cavalier King Charles Spaniels were used as a naturally acquired in vivo model of MVP. These canine MVP leaflets (N = 5/group) showed 5HTR2B upregulation. This study also utilized CB57.1ML/6 mice in order to determine the effect of Angiotensin II infusion on MV remodeling. Histological analysis showed that MV thickening due to chronic Angiotensin II remodeling is mitigated by a 5HTR2B antagonist (LY272015) but not by 5HTR2A inhibitors.
Conclusion
In humans, MVP is associated with an upregulation in 5HTR2B expression and increased 5HT receptor signaling in the leaflets. Antagonism of 5HTR2B mitigates MVIC activation in vitro and MV remodeling in vivo. These observations support the view that 5HTR signaling is involved not only in previously reported 5HT-related valvulopathies, but it is also involved in the pathological remodeling of MVP.
Keywords: mitral valve, angiotensin, physiology, surgery, cardiovascular disease, serotonin
INTRODUCTION
Mitral Valve Prolapse (MVP) affects approximately 7.2 million individuals in the US, and over 144 million individuals worldwide[1,2]. MVP is defined echocardiographically as a single or bileaflet prolapse that is at least 2 mm above the annular plane in the long-axis view, with or without leaflet thickening[2,3]. In contrast to other types of mitral regurgitation (MR), such as ischemic MR – which is driven in large part by a ventricular remodeling – mitral insufficiency in the setting of MVP is primarily a disease of the valve leaflets. Severe MR associated with MVP is most commonly treated with surgery via repair or replacement of the mitral valve. In recent years, percutaneous alternatives have become available for patients who are deemed too high risk for surgical intervention[4].
The pathophysiology of MVP involves myxomatous degeneration, which is defined by the accumulation of mucopolysaccharides and other extracellular matrix components that are responsible for the thickening and “proliferative” aspect of the valve tissue. The center scallop of the posterior leaflet is more commonly involved, as is the area of coaptation between the two leaflets at maximum stress. Heart valve leaflets are dynamic, multi-layered structures that are actively remodeled by the activation of their main cellular component, the valve interstitial cells (VICs)[5–8]. In healthy adult valves, VICs are typically in a quiescent phenotype (qVICs); however these cells become active in response to altered biomechanical stimuli[6,9,10]. Notably, VIC activation is often associated with extracellular matrix (ECM) remodeling. We have previously described some of the biomechanical responses controlling VIC activation, typically with upregulation of alpha-smooth muscle cell actin (αSMA) during congenital and acquired valvulopathies[10–17]. Thus, switching the VIC phenotype with upregulation of αSMA is a key component in mediating leaflet tissue growth and the overall leaflet’s deformation[18,19].
Numerous clinical and experimental studies have reported serotonin (5HT) dysregulation as a possible element in the development of MR in humans and animals[20–25]. 5HT is associated with cardiac valvulopathy in several clinical settings, including: carcinoid heart disease due to 5HT secreting chromaffin tumors, which is characterized by fibrotic endocardial plaques and associated right-sided heart valve dysfunction[26]; and the diet drug combination Fenfluramine/Phentermine (Fen/Phen) - resulting in sustained 5HT activity based on Fen inhibition of the serotonin transporter (SERT) and 5HT receptor agonist, and sustained 5HT signaling due to monoamine oxidase inhibition (Phen) - which was withdrawn by the FDA in 1997 because of the relatively large number of valvulopathy cases that affected primarily the mitral and aortic valves[27–32]. The anti-Parkinson’s agent, Pergolide, and other related drugs primarily affect dopamine receptors, however off target effects related to 5HT are most likely responsible for the valvulopathy observed in a small, but significant number of patients[27,28,33]. 5HT-valvulopathy has been observed in transgenic mice deficient in the serotonin transporter (SERT), resulting in prolonged 5HT-receptors (5HTRs) signaling, resulting in thickened cardiac valves[20,34]. Valvulopathies have also been observed in rats which were administered high doses of 5HT[35,36]. All of these clinical and laboratory results indicate a pivotal role for 5HT homeostasis in normal valve physiology that can be disrupted by aberrant 5HTRs signaling activity. However, 5HTR mediated VIC activation and MV leaflet remodeling is not well understood. The present paper represents the first example of a mechanistic connection between serotonin receptor signaling and MVP, one of the most common heart valve diseases affecting millions of patients.
5HT receptors are encoded by 13 distinct genes and classified into 7 families including the Gq/11-coupled 5HT receptors (5HTR2), which are understood to mediate both the contractile and proliferative effects of 5HT on smooth muscle cells, fibroblasts, and valve interstitial cells[37,38]. In Xu J, et al., we dissected the role of 5HTR2 receptors in isolated valve cells[23]. Specifically, it was shown that 5HTR2 agonism activates the MAP kinase pathway via ERK[37,38]. In the present studies, we investigated the hypothesis that 5HT receptor (5HTR) signaling contributes to MVP pathophysiology. We will present evidence that 5HT signaling through 5HTR2B controls human-derived VIC activation in vitro (cell culture) and ex vivo (biomechanical assays). We will demonstrate 5HTR signaling in a canine model of MVP with data showing that a 5HT antagonist abrogates VIC activation via inhibition of ERK phosphorylation. Finally, we will show that 5HT-mediated MV remodeling is associated with a murine model using chronic infusion of Angiotensin II (Ang II), a mechanism described to generate heart valve remodeling via TGF-beta/ROS and was recently proposed as a model of MVP[39].
MATERIAL AND METHODS
Patient enrollment and classification
Patients requiring MV surgery were enrolled in this study with informed consent at the time of surgery in the Penn Cardiac Bioregistry (PCB) and the Valley Hospital Cardiothoracic Surgery Biobank, as approved by the IRB of the University of Pennsylvania Perelman School of Medicine (protocol #809349) and Valley Hospital (protocol #11.0009). All MVP patients had clinically indicated cardiac surgery. Each patient had a well-documented history of MR and presented with leaflet thickening and annular dilatation. Control MV were collected through the heart transplant and the Gift of Life program, or purchased from BioServe (Beltsville, MD) and were asymptomatic with no significant medical history. Analyses were conducted primarily on control-harvested hearts showing normal features and no signs of any pathological conditions upon echocardiographic evaluation. Exclusion criteria for this study included endocarditis, history of cancer, autoimmune diseases. MVP pathological explants do not show fibrotic endocardial plaques.
Microarray and Network Reconstruction
Microarray analysis was carried out on 4 control and 4 MVP patients as previously described[17]. Microarray analysis was performed using the Human Whole Genome OneArray™ (Phalanx Biotech, Palo Alto, CA). PANDA (Passing Attributes between Networks for Data Assimilation)[40] was used for genome-wide regulatory network reconstruction to integrate gene expression data with transcription factor motif and protein-interaction data and construct directed, genome-wide regulatory networks.
PANDA analysis
We obtained expression data for twenty-four total samples, twelve samples (three replicates on four subjects) each in control samples and MVP patients. We applied PANDA twice to separately integrate the MVP and control sets of expression data samples with the “prior” motif and transcription factor interaction data, resulting in an MVP-network and a C-network. We next defined sub-networks of edges based on the probability that an edge is both “supported” by the predicted network models, and is “different” between the MVP and control networks. To calculate the probability that an edge is “supported,” we applied the inverse cumulative distribution function to the z-score edge-weight reported by PANDA, thereby assigning a probability value between zero and one for each. Similarly, the probability that an edge is “different” between the networks was calculated by subtracting the z-score weight values estimated by PANDA for the MVP (“M”) and control (“C”) networks and then calculating the probability of this difference value based on the inverse cumulative distribution. To determine the probability that an edge is both “supported” and “different” we took the product of these two and selected edges for which this combined probability is greater than 95%. Specifically:
 |
Finally, based on these two sets of edges, one set assigned to the MVP-sub-network and the other assigned to the C-sub-network, we determined the significance of the association of each gene and transcription factors with the defined sub-networks. Specifically, we modeled the overlap between the edges in the MVP sub-network with those associated with a given gene or transcription factor (X) by the hypergeometric distribution as follows:
An equivalent formula exchanging “M” and “C” degree values were used to calculate the significance of association with the control subnetwork.
Morphology methods
Human and canine valve tissue were fixed in 10% formalin and embedded in paraffin for cross-sectioning. H&E and MOVAT staining were performed according to standard protocols. Microscopy images were taken at 20x magnification. MV thickening was quantified by Image J software.
Isolation of mitral valve interstitial cells (VICs)
Isolation of MVICs was performed using a modified method described by Branchetti et al[11]. Briefly, mitral leaflets were placed in 2 mg/ml type II collagenase (Worthington Biochemical Corp., Worthington, VA) in Dulbecco’s modified Eagle’s medium containing 1% Penicillin/Streptomycin solution and incubated in a shaker for 20 min at 37°C. Loosened endothelial layer was removed by wiping the leaflet surfaces with sterile cotton swabs. Tissues were then finely minced and dissociated in type II collagenase (1 mg/ml) and hyaluronidase (100 U/ml) for 4 h at 37°C. The resulting VICs were seeded in tissue culture plates in Advanced DMEM media and maintained at 37°C and 5% CO2. VICs growth medium contained Advanced DMEM supplemented with 10% Fetal Bovine Serum (Thermo Scientific, Hudson, NH), and 1% Penicillin/Streptomycin solution (Life Technologies, Carlsbad, CA). All the experiments were performed on cultured cells between their second and fourth passages. Isolated cells were banked in liquid nitrogen for further studies.
Biomechanical testing
A tension bioreactor was used for flexural stimulation of engineered valve tissue as previously described[41,42]. The bioreactor consists of two chambers, each with multiple media baths; each bath has stationary pins press-fit into the bottom for tissue anchorage. The opposing pins are fixed to an actuating arm that is attached to a cross-arm which is connected to a central motorized piston. Each leaflet was trimmed to form a tissue strip, measuring approximately 20 mm by 8 mm. The tissue strip was inserted into the tension bioreactor with 5 mL of complete Advanced DMEM medium ± 10mM of the 5HTR2B inhibitor, 100 μM LY272015, and subsequently changed every 2 days. At day 6, tissues were flash frozen for RNA isolation.
Animal model studies
Mitral valve specimens from 4 Cavalier King Charles Spaniels with severe MVP and MR and 4 normal controls were obtained at post-mortem (IACUC protocol #803348). A murine model of mitral valve remodeling used C57BL/6J male mice purchased from Jackson Laboratories (Bar Harbor, Maine). Experiments were conducted under the IACUC protocol #804440. Seven week old mice were fed a hypercholesterolaemic diet and infused with saline (n=10) or Angiotensin II (AngII) (n=10; 1000 ng/kg/min) using osmotic pumps (Alzet) for 28 days as previously described[43]. LY 272015 treatment (3 mg/kg in sterile PBS) was given twice/week over the 28 day AngII infusion by interperitoneal injection according to a previous publication[44]. Terguride and Ketansarin, 5HTR2A antagonists, were given at a dose of 0.03 mg/Kg and 1 mg/Kg, respectively.
Statistical analysis
The data from all microarrays in each experimental set were passed to Omicsoft Array Studio software, control and missing features were removed, and the remaining signals were normalized and transformed to log2 values. Hypothesis testing and generation of fold change values: testing was performed by combining technical replicates and performing a standard student’s t-test to calculate raw p-values. Adjusted p values were calculated using the Benjamini and Hochberg method with a false discovery rate alpha-value of 0.05. Fold change for the gene expression was calculated based on the mean values of the technical replicates for each probe. Data filtering and selection of affected genes: a subset of genes was generated based on an internal intensity stringency filter. Furthermore a set of ‘affected genes’ was established using a raw p-value cutoff of 0.05 and a two-fold change cutoff. Gene Ontology (GO): Omicsoft Array Studio was used to evaluate the Gene Ontology enrichment. In addition, p-values were calculated for each classification based on Fischer’s exact test using all the annotated probes on the array as a comparison set and the Benjamini and Hochberg method for p-value adjustment. The data were analyzed with the SPSS software using suitable methods for parametric and non-parametric analyses (Student’s t-test, ANOVA test, and Kruskal–Wallis test).
Quantitative PCR
mRNA was isolated from the posterior leaflet of MVP and control tissues using the RNeasy Fibrous Tissue kit (Qiagen) or from MVICs using the RNeasy Mini Kit (Qiagen). mRNA was reverse transcribed using the Applied Biosystems TaqMan Reverse Transcription reagents. Quantitative PCR was performed in triplicate with the following primers: 5HTR2A: 5′-ACTCCAGAACTAAGGCATTT-3′, 5′-AGCTAATTTGGCCCGTGTGCC-3′ and 5HTR2B: 5′-ACGTTCTCTTTTCAACCGCA-3′ and 5′-CCGGTGACGAGCAAGGTGTT- 3′ (Ng, et al, 2012), OPN, OCN, BMP-4, and SMA on the ARC 7500 Fast format and analyzed using Applied Biosystems 7500 Fast System SDS software.
Antibodies
Antibodies used include: anti-5HTR2A (EMD Millipore, Darmstadt, Germany), anti-5HTR2B (Novus Biologics, Littleton, CO), anti-αSMA and anti-OPN (Abcam, Cambridge, MA), anti-ERK and anti-phosphoERK (Cell Signaling, Beverly, MA), and HRP-linked secondary (Santa Cruz Biotechnology, Dallas, TX).
Immunohistochemistry
Paraffin embedded sections for human posterior MV, anterior canine MV, and mouse heart tissues were stained using the detection kit from DAKO ((Agilent Technologies, Santa Clara, CA). A standard protocol was followed. Primary antibody incubation was followed by a HRP-linked secondary (DAKO). After color development using DAK+ (DAKO), a hematoxylin stain (Vector Laboratories, Burlingame, CA) was applied. Stained slides were digitally imaged on the Aperio ImageScope software.
RESULTS
Microarray and Network Reconstruction analysis in MVP patients and controls reveals a role for the 5HT signaling pathway in MVP pathology
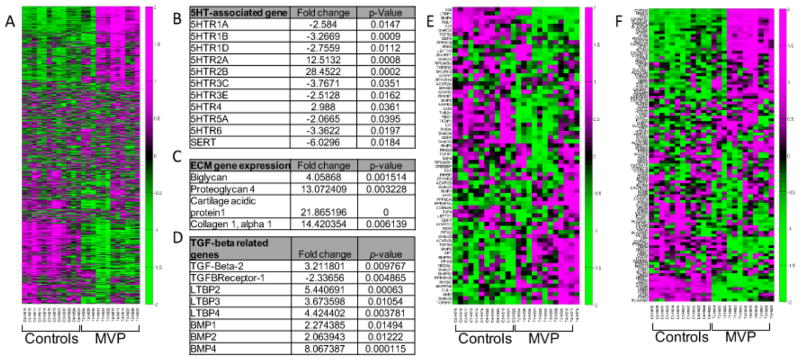
As reported in our previous work, we performed microarray analysis of 4 MVP patients and 4 controls[17]; however, our prior publication did not report results on 5HT related gene expression patterns. Among the transcriptional activities of 19,553 human sequences determined by use of an oligonucleotide microarray, we found a total of 1,883 probe sets which fulfilled the criteria for differential expression (GEO access number requested). These transcripts represent genes showing at least a two-fold change in MVP tissue vs. controls. Of the 1,883 transcripts considered, 1,033 were upregulated (54.8%) and 850 down regulated (45.2%). A reassessment with bio-informatics analysis was conducted on the 8 samples from our previously published paper. The results of these studies highlight the differential expression of genes that directly or indirectly indicate the involvement of 5HTR pathways and extracellular matrix remodeling in MVP (Figure 1A). Here we report significant increases in 5HTR2A (12.5 fold change, p= 0.0008) and 5HTR2B (28.4 fold change, p= 0.0002) respectively with decreases in other 5HTRs in patients with MVP as compared to control (Figure 1B). These results agree with other observations in canine MVP[45] where 5HTR2B expression is 3.9-fold higher in diseased valve compared to control tissue. Notably, canine MVP is the only naturally acquired MVP that resemble the human condition.
Figure 1. Human microarray analysis and identification of 5HT signaling pathways in MVP patients.
(A) Heat maps showing the relative expression levels of 1883 differentially-expressed transcripts from the MVP and control samples (N=4). Ordering of the genes and samples is based on a hierarchical clustering and the expression values across rows are Z-score normalized for visualization purposes only. Green indicates a positive fold change while purple indicates a negative fold change. (B) (C) and (D) Tables showing selected genes differentially expressed between MVP and Controls for 5HT, ECM, and TGFβ signaling, respectively. Fold changes and p value are indicated. (E) and (F) Heat maps showing the relative expression levels of genes from the MVP and control expression samples for the TGFB signaling pathway (hsa04350) or the serotonin pathways (hsa04726).
Increased expression of markers of MVIC activation and ECM synthesis (Figure 1C and E) is observed for a number of genes including Osteopontin, RUNX2, BMP4, Type 1 collagen, and glycosaminoglycan-associated proteins[17] in agreement with our prior results in sheep and porcine aortic VIC[24], and canine and human MVIC[21,24]. As per our previous work, microarray analysis also confirms differential expression of TGF-β-related signaling as a possible regulator of both 5HT metabolism and MV remodeling[17,22] (Figure 1D and F).
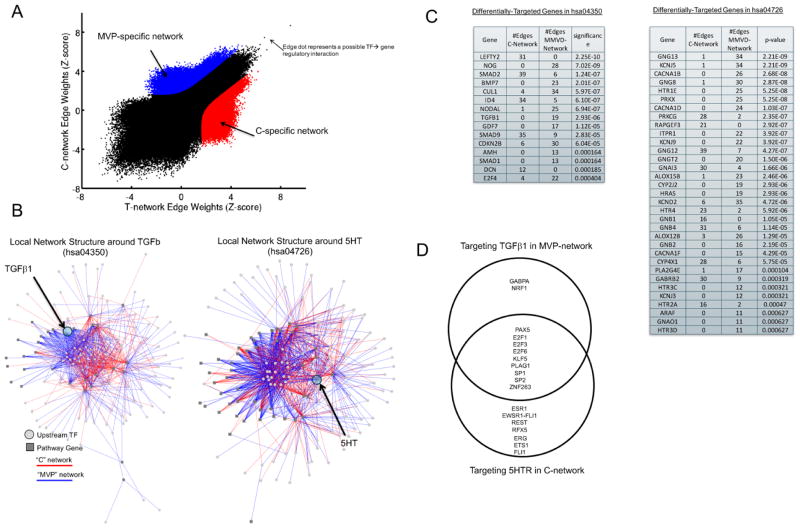
We next utilized PANDA (Passing Attributes between Networks for Data Assimilation) [40], a message-passing model using multiple sources of information to predict regulatory relationships, to integrate protein-protein interaction and gene expression, to reconstruct genome-wide, condition-specific regulatory networks (Figure 2A–C). First, we used this method to validate a well-known pathway involved in MVP: TGF-β signaling. We and others have reported that the pathological remodeling of the MV is associated with increased levels of TGF-β1[17,46,47]. We applied PANDA, constructing two directed, genome-wide regulatory networks, one for the control samples and the other for the MVP specimens. By comparing the predicted networks we identified regulatory relationships specific to either the MVP or control (“C”) samples (Figure 2A). We identified which of these MVP-network and C-network regulatory relationships included a member of either the TGF-β signaling pathway or the 5HT pathway, and show those regulatory relationships in Figure 2B–C. Interestingly, there is a high level of differential-targeting around several important genes in these pathways. For example, TGF-β1 is much more highly targeted (p=2.9e-6) in the control-network with 19 identified specific regulatory interactions, but none in the MVP network. Among genes belonging to the 5HT pathway, 5HTR2A is more highly targeted in the MVP-network compared to the control-network (p=4.7e-4) with 8 times as many regulatory relationships. Some of this differential-targeting of pathway genes may be in part mediated by differences in upstream transcription factors. A Venn-diagram of the transcription factors targeting TGF-β1 and 5HTR2A in either the identified C-network or MVP-network is shown in Figure 2D.
Figure 2. Regulatory Network reconstruction in MVP vs. Controls.
(A) A plot of the edge weights predicted by PANDA when reconstructing a regulatory network using the MVP or control expression data samples. On the plot, each point represents a potential transcription factor to target gene regulatory relationship. Regulatory edges that were identified as specific to either the MVP-network or the C-network are shown in red and blue, respectively. (B) Visualization of the MVP or C-specific sub-networks in which a member of either the TGFβ signaling pathway (hsa04350, left panel) or the serotonin pathway (hsa04726, right panel) are target genes. (C) The number of edges targeting members of the TGFβ signaling pathway or the serotonin signaling pathway in each of the two identified MVP-specific and C-specific sub-networks. The significance of any differential-targeting of these genes comparing the sub-networks is also shown. (D) A Venn diagram of the transcription factor regulators identified as targeting 5HT in the MVP-network and TGFβ in the C-network.
Human and canine expression of 5HTR-2B in MVP tissues and controls
We assessed the protein expression of 5HTR2A and 5HTR2B in isolated MV leaflets from both MVP and non-diseased patients. H&E analysis and MOVAT staining demonstrate the difference in the ECM deposition in the MVP tissue vs. controls (Figure 3A and B). qPCR data confirm increased RNA expression of both 5HTR2A and 2B, while IHC analysis only detects strong upregulation of 2B receptor. As shown in Figure 3C and D, 5HTR2A does not differs by IHC between controls and MVP leaflets, while 5HTR2B is upregulated in MVP specimens compared to controls, concomitant with an increase in expression of OPN and SMA (Figure 3E and F). Notably none of the histological analysis oh human explants show fibrotic endocardial plaques typical of previously reported 5HT-related valvulopathies.
Figure 3. MVP is associated with increased protein expression of 5HTR2B.
Representative immunohistochemistry staining of 4 μm thick cross sections of human MV leaflets surgically resected from patients with MR due to MVP and controls (N=4 group) using (A) H&E, (B) MOVAT staining. (C) Quantitative PCR analysis for 5HTRA and 5HTR2B expression in N=5 independent control and MVP MV specimens. *p=0.05. IHC with (D) anti-5HTR2A (E) anti-5HTR2B, (F) anti-αSMA, and (G) anti-OPN antibodies, respectively. Magnification, 63X.
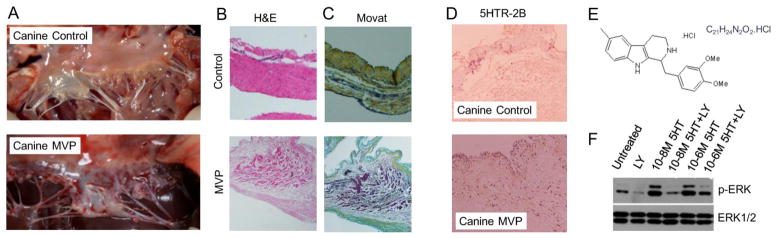
Canine non-diseased and MVP tissues were also analyzed for 5HTR2B expression. Figure 4A shows a post-mortem image of an intact healthy canine MV and a canine MV with severe MR related to MVP. Canine MVP shows similar patterns of H&E and MOVAT staining as human MVP (see Figure 3A and B) with thickened valve tissue and altered ECM deposition (Figure 4B and C). Immunohistochemical analysis shows that 5HTR2B expression is increased in canine MVP leaflet compared to non-diseased tissue (Figure 4D). It has been reported that the activation of 5HTR2B by 5HT results in the phosphorylation of ERK[21,46,48] indicating increased signal transduction. Control canine MVICs were exposed to 5HT stimulation in the presence or absence of 100nM LY 272015, a high affinity 5HTR2B receptor antagonist. Figure 4E and F shows a strong inhibitory effect of LY 272015 on ERK phosphorylation induced by 5HT. Thus, 5HT is mediating canine MVIC activation, which can be abrogated by 5HTR2B inhibition.
Figure 4. Canine MV disease shows a marked increase in 5HTR2B expression.
(A) Representative intra-op images of normal canine MV and canine valve with MVP presentations. Representative (B) H&E staining and (C) Modified Movat Pentachrome staining of control and MVP canine tissue (N=4) (Magnification 40x). (D) Immunohistochemistry staining of 4 μm thick cross sections of canine MV leaflets surgically resected from MVP and controls (N=4 group) using an anti-5HTR2B antibody. Magnification 40X. (E) LY 272015 and (F) Isolated canine MVICs were serum -starved overnight and pre-treated for 30 minutes with vehicle or 100 nM in serum free Advanced DMEM media, followed by treatment with the indicated dose of 5HT for 5 minutes. Protein lysates were analyzed by immunoblot analysis using anti-phospho-ERK and anti-ERK antibodies, respectively.
The impact of 5HTR2B (LY 272015) antagonists and MV remodeling under biomechanical stimulation
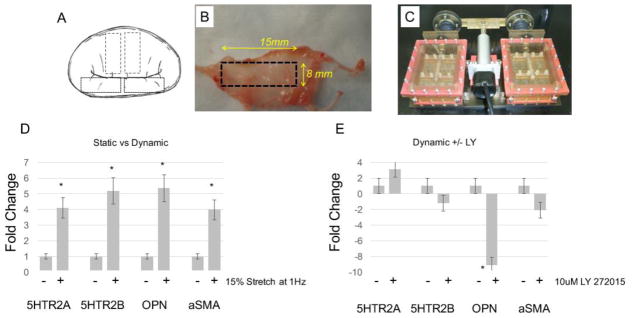
We used biomechanical stimulation to induce VIC activation ex vivo on explanted MV tissues using an established bioreactor method[10,12]. MV strips from anatomically normal MV, as controls, were isolated from either the anterior or posterior leaflets and exposed for 6 days at 15% stretch and 1 Hz (Figure 5A, B, and C) or maintained in static conditions. Figure 5D shows that biomechanical stimulation alone (dynamic conditions) induces both 5HTR2A and 5HTR2B expression (4.1 fold, p=0.04, and 5.18 fold, p= 0.003) as well as MVIC activation measured by expression levels of αSMA and OPN (5.3 fold, p<0,005, and 3.9 fold, p<0.01). Treatment of control tissue under dynamic conditions with the 5HTR2B antagonist LY272015 (100 μM) reverses αSMA and OPN upregulation (Figure 5E).
Figure 5. Ex vivo mechanical stimulation induces 5HTR expression.
(A–C) Processing of human MV tissue and the uniaxial bioreactor (D) Control MV tissues were loaded into the BioReactor and maintained in static or dynamic (1Hz, 15% stretch) conditions for 6 days. RNA was isolated from the tissue, reverse transcribed, and qPCR analysis was performed using primers specific for 5HTR2A, 5HTR2B, OPN and αSMA. Control MV tissues were exposed to (E) 10 μM 5HT +/− 100 μM LY 272015 for 6 days under controlled biomechanical stimulation (1Hz, 15% stretch). RNA was isolated from the tissue, reverse transcribed, and qPCR analysis was performed using primers specific for 5HTR2A, 5HTR2B, OPN, and αSMA, *p=0.05, **p=0.01.
Angiotensin II (ANGII) infusion provokes remodeling of mitral valve tissue that can be partially inhibited by 5HTR2B inhibition in mice
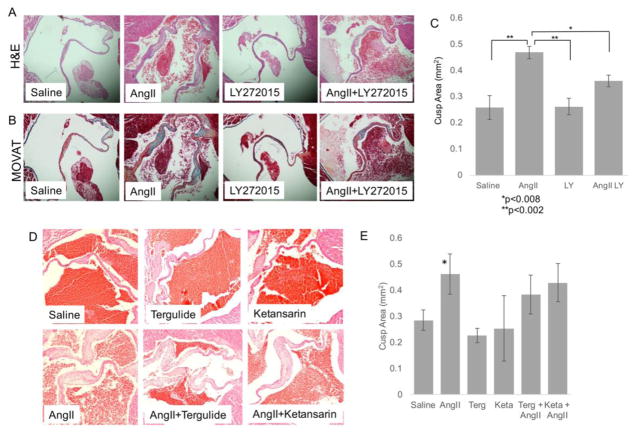
We next tested whether 5HTR2A or 5HTR2B inhibition would have an effect on MVIC activation and tissue remodeling in vivo. A recent work suggests that in vivo treatment with AngII partially recaptures molecular and phenotypic features of human MVP in murine mitral valves[39]. In addition, our work shows that this model is associated with VIC activation based on expression of activation markers including αSMA, OPN, OCN, and BMP4 as is seen in human heart valve diseases[12]. The chronic infusion of AngII (N=10/group, 1000 ng/kg/min, using osmotic pumps (Alzet) for 28 days) on CB57.BL/6 mice results in MV remodeling as seen by morphometric analyses revealing increased MV cusp thickness and ECM deposition in AngII treated compared to saline infused mice (Figure 6A and B). Mice chronically infused with AngII were treated with 10μM LY 272015, with Terguride (0.03 mg/Kg) or with Ketansarin (1 mg/Kg). Post mortem analysis show that only LY 272015 prevents the remodeling of the MV architecture in vivo with reduced valve volume and thickness compared to the AngII treated mice (Figure 6A and B). Figure 6C illustrates the valve leaflet area with each treatment, demonstrating a partial but significant decrease in valve leaflet area in AngII + LY 272015 mice compared to AngII alone (approximately 50% reduction). 5HTR2A antagonists Terguride and Ketansarin at the dose and timing indicated do not significantly reduce Ang II-mediated MV remodeling (Figure 6D and E).
Figure 6. Angiotensin II infusion promotes remodeling of the mitral valve tissue in mice which can be partially blocked by 5HTR2B inhibition.
(A) Representative H&E and (B) Modified Movat Pentachrome staining of cross sections of MV from mice hearts harvested 28 days after saline or Ang II chronic infusion with or without treatment of 10 μM LY 272015 (Magnification 20x). (C) Quantitative analysis of the average MV leaflet section area for each treatment group +/− SE. *p<0.008; **p<0.002. (D) Representative H&E of MV from mice hearts harvested 28 days after saline or Ang II chronic infusion with or without treatment Terguride or Ketansarin. (E) Quantitative analysis of the average MV leaflet section area for each treatment group indicated in D +/− SE. *p<0.002.
DISCUSSION
5HT-related mechanisms have been previously reported in specific valvulopathies such as those related to 5HT secreting carcinoid tumors, serotonergic agents, such as dexfenfluramine, and ergot-derived dopaminergic agents used to treat Parkinson’s Disease, such as carbergoline and pergolide. The present paper represents the first example of a mechanistic connection between serotonin receptor signaling and one of the most common heart valve diseases, MVP.
This work highlights the important role of 5HTR signaling in MVP. Our microarray data and immunohistological analysis show that MVP is associated with an increase in 5HTRs expression. Our data, and those of others, have shown that enhanced 5HTR2 signaling is associated with both increased VIC mitogenesis and increased production of ECM proteins. Our murine and canine animal models of MVP demonstrate the commonality of in-vivo 5HTR2 signaling in pathological conditions that are either a result of interstitial cell activation (murine model) or age-dependent myxomatous degeneration (canine model). It should be noted that MVP in the canine model is the only naturally occurring MVP that histopathologically resembles human MVP. Many additional 5HT-related processing molecules are controlling 5HT activity including the serotonin transporter (SERT)[23]. According to our microarray data, SERT is significantly down regulated in MVP, which could hypothetically result in sustained 5HTR signaling due to the slower processing of 5HT through SERT in diseased valves. SERT expression and physiology is not addressed in this manuscript because of its the highly complex regulation, which would include SERT gene-linked polymorphic region (5-HTTLPR) analysis, the variable number of tandem repeats in the second intron (VNTR-2), and the SNPs associated with its expression[49].
Our results, along with other published studies, indicate the need of investigating therapeutic opportunities around 5HT-related metabolism in the setting of MVP. We show that LY 272015, a 5HTR2B inhibitor, mitigated downstream cellular effects of 5HT signaling in MVICs and tissue. Other 5HTRs inhibitors tested here were not equally as effective. In general, 5HTR antagonists have been developed for central nervous system effects and poor blood brain barrier access was considered counter-productive. In the potential treatment of MVP using a 5HTR antagonism approach, blood brain barrier penetration is not needed, nor is it desirable. In addition, drugs that could be potentially active against relevant 5HTRs, such as atypical antipsychotic agents, often have major central nervous system side effects. If these agents are of potential interest for patients with MVP, chemical modifications could be studied that would limit their blood brain barrier access, but retain their 5HTR antagonist activity. Using computational biology approaches, additional targets related to the 5HT signaling pathway could potentially emerge. In the present study, we utilized PANDA, a message-passing model using multiple sources of information to predict regulatory relationships, to integrate protein-protein interaction, gene expression, and sequence motif data and reconstruct genome-wide, condition-specific regulatory networks. Our analysis shows crosstalk and differential regulation between 5HT and TGF-β1 in MVP and control patients.
There are several important limitations associated with this study. Given the difficulties in obtaining control MV tissue and the cost associated with genome-wide studies, network reconstruction was obtained on a relatively small group of patients (N=4 group), therefore a larger sample size is needed to obtain more powerful network analysis. IHC analysis was conducted on paraffin embedded tissue obtained at the time of surgery for the human study and murine and canine models; therefore, they are lacking a longitudinal component. Angiotensin II is not specific for mitral valve remodeling, but also induces remodeling of different cardiac and vascular tissues[10,12,43]. Furthermore, the reported direct link between Ang II and TGF-beta expression and signaling represents a possible mechanistic target for 5HT-related valvulopathies. 5HTR signaling in mitral valve leaflet cells, resulting in both increased mitogenesis and higher levels of TGF-beta-1, could promote ECM production and contribute to the pathophysiology of progressive MVP in these subjects. The biomechanical data were obtained using a uniaxial test, rather than a more physiological biaxial system, but this is an intrinsic limitation of the instrument. Uniaxial testing of canine and murine tissue are not feasible with the current set up, due to the size of the tissues.
While many patients diagnosed with some level of MVP do not progress to symptomatic MR, requiring surgery; a subgroup of MVP patients develop excessive tissue growth, most commonly involving the central scallop of the posterior leaflet, resulting in severe MR. It would be of great clinical interest whether this subset of patients have a higher level of circulating or locally produced 5HT compared to patients that do not progress to a surgical diagnosis. There is also a subset of patients that demonstrate recurrence of MR after their surgical repair. While a portion of these recurrences may be associated with suboptimal repair in the initial surgery, it could be tested whether these patients are more susceptible to 5HT signaling leading to further tissue remodeling based on their expression profile of 5HTRs.
Overall, this work supports the hypothesis that 5HT signaling plays an important role in the pathological development of MVP. The 5HT signaling pathway may be an important therapeutic target for the treatment of early stage MR to prevent or slow down the progression to disease state requiring surgical intervention.
Highlights.
Microarray and Network Reconstruction analysis in MVP patients and controls reveals a role for the 5HT signaling pathway in myxomatous mitral valve pathology;
5HTR2B is upregulated in human MVP leaflets when compared to control as well as in a canine model of myxomatous mitral regurgitation;
A 5HTR2B antagonist (LY 272015) reduces MVICs activation under biomechanical stimulation ex vivo;
LY 272015 prevents Angiotensin II-mediated heart valve thickening in vivo;
We reported for the first time a mechanistic connection between serotonin receptor signaling and one of the most common heart valve diseases, MVP, a disorder affecting millions of patients.
Acknowledgments
This research was supported in part by the following research grants and funds: NIH R01-HL131872 (GF and RJL), R01-HL122805 (GF), AHA GRANT 24810002 (GF), The Kibel Fund for Aortic Valve Research (GF and RJL), and The Valley Hospital Foundation “Marjorie C Bunnel” charitable fund (GF and JG), NIH T32-HL007954 (KHD), NIH T32-HL007915 (RJL and KHD), Barth Memorial Mitral Valve Disease Fund (MAO, GF, EB), and both Erin’s Fund and the William J. Rashkind Endowment of the Children’s Hospital of Philadelphia (RJL).
ABBREVIATIONS
- Ang II
Angiotensin II
- 5HT
Serotonin
- ERK
extracellular-signal-regulated kinase
- SERT
serotonin transporter
- MVICs
mitral valve interstitial cells
- 5HTR
serotonin receptor
- MV
mitral valve
- MR
mitral regurgitation
- MVP
mitral valve prolapse
- ECM
extracellular matrix
- qVIC
quiescent valve interstitial cells
- Fen/Phen
Fenfluramine/Phentermine
- OPN
Osteopontin
- BMP-4
Bone Morphogenic Protein-4
- ONC
Osteonectin
- αSMA
alpha smooth muscle actin
Footnotes
DISCLOSURES
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sainger R, Grau JB, Branchetti E, Poggio P, Seefried WF, Field BC, et al. Human myxomatous mitral valve prolapse: role of bone morphogenetic protein 4 in valvular interstitial cell activation. J Cell Physiol. 2012;227:2595–2604. doi: 10.1002/jcp.22999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guy TS, Hill AC. Mitral valve prolapse. Annu Rev Med. 2012;63:277–292. doi: 10.1146/annurev-med-022811-091602. [DOI] [PubMed] [Google Scholar]
- 3.Freed LA, Levy D, Levine RA, Evans JC, Larson MG, Fuller DL, et al. Mitral valve prolapse and atrial septal aneurysm: an evaluation in the Framingham Heart Study. Am J Cardiol. 2002;89:1326–1329. doi: 10.1016/s0002-9149(02)02340-8. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2438–2488. doi: 10.1016/j.jacc.2014.02.537. [DOI] [PubMed] [Google Scholar]
- 5.Salhiyyah K, Yacoub MH, Chester AH. Cellular mechanisms in mitral valve disease. J Cardiovasc Transl Res. 2011;4:702–709. doi: 10.1007/s12265-011-9318-7. [DOI] [PubMed] [Google Scholar]
- 6.Hinton RB, Yutzey KE. Heart valve structure and function in development and disease. Annu Rev Physiol. 2011;73:29–46. doi: 10.1146/annurev-physiol-012110-142145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathieu P, Boulanger MC, Bouchareb R. Molecular biology of calcific aortic valve disease: towards new pharmacological therapies. Expert Rev Cardiovasc Ther. 2014;12:851–862. doi: 10.1586/14779072.2014.923756. [DOI] [PubMed] [Google Scholar]
- 8.Hutcheson JD, Aikawa E, Merryman WD. Potential drug targets for calcific aortic valve disease. Nature Publishing Group. 2014;11:218–231. doi: 10.1038/nrcardio.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, et al. Calcific aortic valve disease: not simply a degenerative process: a review and agenda for research from the national heart and lung and blood institute aortic stenosis working group * executive summary: calcific aortic valve disease - 2011 update. Circulation. 2011;124:1783–1791. doi: 10.1161/CIRCULATIONAHA.110.006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poggio P, Sainger R, Branchetti E, Grau JB, Lai EK, Gorman RC, et al. Noggin attenuates the osteogenic activation of human valve interstitial cells in aortic valve sclerosis. Cardiovasc Res. 2013;98:402–410. doi: 10.1093/cvr/cvt055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Branchetti E, Sainger R, Poggio P, Grau JB, Patterson-Fortin J, Bavaria JE, et al. Antioxidant Enzymes Reduce DNA Damage and Early Activation of Valvular Interstitial Cells in Aortic Valve Sclerosis. Arterioscler Thromb Vasc Biol. 2012;33:e66–e74. doi: 10.1161/ATVBAHA.112.300177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poggio P, Branchetti E, Grau JB, Lai EK, Gorman RC, Gorman JH, et al. Osteopontin-CD44v6 Interaction Mediates Calcium Deposition via Phospho-Akt in Valve Interstitial Cells From Patients With Noncalcified Aortic Valve Sclerosis. Arterioscler Thromb Vasc Biol. 2014 doi: 10.1161/ATVBAHA.113.303017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poggio P, Grau JB, Field BC, Sainger R, Seefried WF, Rizzolio F, et al. Osteopontin controls endothelial cell migration in vitro and in excised human valvular tissue from patients with calcific aortic stenosis and controls. J Cell Physiol. 2011;226:2139–2149. doi: 10.1002/jcp.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grau JB, Poggio P, Sainger R, Vernick WJ, Seefried WF, Branchetti E, et al. Analysis of osteopontin levels for the identification of asymptomatic patients with calcific aortic valve disease. Ann Thorac Surg. 2012;93:79–86. doi: 10.1016/j.athoracsur.2011.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aggarwal A, Ferrari G, Joyce E, Daniels MJ, Sainger R, Gorman JH, 3, et al. Architectural Trends in the Human Normal and Bicuspid Aortic Valve Leaflet and Its Relevance to Valve Disease. Ann Biomed Eng. 2014;42:986–998. doi: 10.1007/s10439-014-0973-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Branchetti E, Bavaria JE, Grau JB, Shaw RE, Poggio P, Lai EK, et al. Circulating soluble receptor for advanced glycation end product identifies patients with bicuspid aortic valve and associated aortopathies. Arterioscler Thromb Vasc Biol. 2014;34:2349–2357. doi: 10.1161/ATVBAHA.114.303784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sainger R, Grau JB, Branchetti E, Poggio P, Seefried WF, Field BC, et al. Human myxomatous mitral valve prolapse: role of bone morphogenetic protein 4 in valvular interstitial cell activation. J Cell Physiol. 2012;227:2595–2604. doi: 10.1002/jcp.22999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sacks MS, Enomoto Y, Graybill JR, Merryman WD, Zeeshan A, Yoganathan AP, et al. In-Vivo Dynamic Deformation of the Mitral Valve Anterior Leaflet. Ann Thorac Surg. 2006;82:1369–1377. doi: 10.1016/j.athoracsur.2006.03.117. [DOI] [PubMed] [Google Scholar]
- 19.Lee CH, Carruthers CA, Ayoub S, Gorman RC, Gorman JH, Sacks MS. Quantification and simulation of layer-specific mitral valve interstitial cells deformation under physiological loading. J Theor Biol. 2015;373:26–39. doi: 10.1016/j.jtbi.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy RJ. Serotonin transporter mechanisms and cardiac disease. Circulation. 2006;113:2–4. doi: 10.1161/CIRCULATIONAHA.105.593459. [DOI] [PubMed] [Google Scholar]
- 21.Oyama MA, Levy RJ. Insights into serotonin signaling mechanisms associated with canine degenerative mitral valve disease. J Vet Intern Med. 2010;24:27–36. doi: 10.1111/j.1939-1676.2009.0411.x. [DOI] [PubMed] [Google Scholar]
- 22.Jian B, Xu J, Connolly J, Savani RC, Narula N, Liang B, et al. Serotonin mechanisms in heart valve disease I: serotonin-induced up-regulation of transforming growth factor-beta1 via G-protein signal transduction in aortic valve interstitial cells. Am J Pathol. 2002;161:2111–2121. doi: 10.1016/s0002-9440(10)64489-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu J, Jian B, Chu R, Lu Z, Li Q, Dunlop J, et al. Serotonin mechanisms in heart valve disease II: the 5-HT2 receptor and its signaling pathway in aortic valve interstitial cells. Am J Pathol. 2002;161:2209–2218. doi: 10.1016/S0002-9440(10)64497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Connolly JM, Bakay MA, Fulmer JT, Gorman RC, Gorman JH, Oyama MA, et al. Fenfluramine disrupts the mitral valve interstitial cell response to serotonin. Am J Pathol. 2009;175:988–997. doi: 10.2353/ajpath.2009.081101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arndt JW, Reynolds CA, Singletary GE, Connolly JM, Levy RJ, Oyama MA. Serum serotonin concentrations in dogs with degenerative mitral valve disease. J Vet Intern Med. 2009;23:1208–1213. doi: 10.1111/j.1939-1676.2009.0378.x. [DOI] [PubMed] [Google Scholar]
- 26.Bernheim AM, Connolly HM, Hobday TJ, Abel MD, Pellikka PA. Carcinoid heart disease. Prog Cardiovasc Dis. 2007;49:439–451. doi: 10.1016/j.pcad.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Bhattacharyya S, Schapira AH, Mikhailidis DP, Davar J. Drug-induced fibrotic valvular heart disease. Lancet. 2009;374:577–585. doi: 10.1016/S0140-6736(09)60252-X. [DOI] [PubMed] [Google Scholar]
- 28.Elangbam CS, Lightfoot RM, Yoon LW, Creech DR, Geske RS, Crumbley CW, et al. 5-Hydroxytryptamine (5HT) receptors in the heart valves of cynomolgus monkeys and Sprague-Dawley rats. J Histochem Cytochem. 2005;53:671–677. doi: 10.1369/jhc.4A6500.2005. [DOI] [PubMed] [Google Scholar]
- 29.Baumann MH, Rothman RB. Neural and cardiac toxicities associated with 3,4-methylenedioxymethamphetamine (MDMA) Int Rev Neurobiol. 2009;88:257–296. doi: 10.1016/S0074-7742(09)88010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothman RB. Anorexogen-related cardiac valvulopathy. Ann Intern Med. 2002;136:779. doi: 10.7326/0003-4819-136-10-200205210-00016. [DOI] [PubMed] [Google Scholar]
- 31.Rothman RB, Baumann MH, Savage JE, Rauser L, McBride A, Hufeisen SJ, et al. Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation. 2000;102:2836–2841. doi: 10.1161/01.cir.102.23.2836. [DOI] [PubMed] [Google Scholar]
- 32.Setola V, Dukat M, Glennon RA, Roth BL. Molecular determinants for the interaction of the valvulopathic anorexigen norfenfluramine with the 5-HT2B receptor. Mol Pharmacol. 2005;68:20–33. doi: 10.1124/mol.104.009266. [DOI] [PubMed] [Google Scholar]
- 33.Elangbam CS, Job LE, Zadrozny LM, Barton JC, Yoon LW, Gates LD, et al. 5-hydroxytryptamine (5HT)-induced valvulopathy: compositional valvular alterations are associated with 5HT2B receptor and 5HT transporter transcript changes in Sprague-Dawley rats. Exp Toxicol Pathol. 2008;60:253–262. doi: 10.1016/j.etp.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Mekontso-Dessap A, Brouri F, Pascal O, Lechat P, Hanoun N, Lanfumey L, et al. Deficiency of the 5-hydroxytryptamine transporter gene leads to cardiac fibrosis and valvulopathy in mice. Circulation. 2006;113:81–89. doi: 10.1161/CIRCULATIONAHA.105.554667. [DOI] [PubMed] [Google Scholar]
- 35.Gustafsson BI, Tømmerås K, Nordrum I, Loennechen JP, Brunsvik A, Solligård E, et al. Long-term serotonin administration induces heart valve disease in rats. Circulation. 2005;111:1517–1522. doi: 10.1161/01.CIR.0000159356.42064.48. [DOI] [PubMed] [Google Scholar]
- 36.Hauso Ø, Gustafsson BI, Loennechen JP, Stunes AK, Nordrum I, Waldum HL. Long-term serotonin effects in the rat are prevented by terguride. Regul Pept. 2007;143:39–46. doi: 10.1016/j.regpep.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 37.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 38.Nichols DE, Nichols CD. Serotonin receptors. Chem Rev. 2008;108:1614–1641. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- 39.Thalji NM, Hagler MA, Zhang H, Casaclang-Verzosa G, Nair AA, Suri RM, et al. Non-Biased Molecular Screening Identifies Novel Molecular Regulators of Fibrogenic and Proliferative Signaling in Myxomatous Mitral Valve Disease, Circulation: Cardiovascular Genetics. CIRCGENETICS. 2015 doi: 10.1161/CIRCGENETICS.114.000921. 114.000921. [DOI] [PMC free article] [PubMed]
- 40.Kimberly G, Huttenhower C, Quackenbush J, Yuan G-C. Passing messages between biological networks to refine predicted interactions. PLoS ONE. 2013:1–39. doi: 10.1371/journal.pone.0064832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merryman WD, Youn I, Lukoff HD, Krueger PM, Guilak F, Hopkins RA, et al. Correlation between heart valve interstitial cell stiffness and transvalvular pressure: implications for collagen biosynthesis. Am J Physiol Heart Circ Physiol. 2006;290:H224–31. doi: 10.1152/ajpheart.00521.2005. [DOI] [PubMed] [Google Scholar]
- 42.Merryman WD, Liao J, Parekh A, Candiello JE, Lin H, Sacks MS. Differences in tissue-remodeling potential of aortic and pulmonary heart valve interstitial cells. Tissue Eng. 2007;13:2281–2289. doi: 10.1089/ten.2006.0324. [DOI] [PubMed] [Google Scholar]
- 43.Branchetti E, Poggio P, Sainger R, Shang E, Grau JB, Jackson BM, et al. Oxidative stress modulates vascular smooth muscle cell phenotype via CTGF in thoracic aortic aneurysm. Cardiovasc Res. 2013;100:316–324. doi: 10.1093/cvr/cvt205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen ML, Schenck KW, Mabry TE, Nelson DL, Audia JE. LY272015, A potentm selective and orally active 5HT2B receptor antagonist. Journal of Serotonin Research. 1996;3:131–144. [Google Scholar]
- 45.Oyama MA, Chittur SV. Genomic expression patterns of mitral valve tissues from dogs with degenerative mitral valve disease. Am J Vet Res. 2006;67:1307–1318. doi: 10.2460/ajvr.67.8.1307. [DOI] [PubMed] [Google Scholar]
- 46.Disatian S, Orton EC. Autocrine serotonin and transforming growth factor beta 1 signaling mediates spontaneous myxomatous mitral valve disease. J Heart Valve Dis. 2009;18:44–51. [PubMed] [Google Scholar]
- 47.Geirsson A, Singh M, Ali R, Abbas H, Li W, Sanchez JA, et al. Modulation of transforming growth factor-β signaling and extracellular matrix production in myxomatous mitral valves by angiotensin II receptor blockers. Circulation. 2012;126:S189–97. doi: 10.1161/CIRCULATIONAHA.111.082610. [DOI] [PubMed] [Google Scholar]
- 48.Lu CC, Liu MM, Culshaw G, Clinton M, Argyle DJ, Corcoran BM. Gene network and canonical pathway analysis in canine myxomatous mitral valve disease: a microarray study. Vet J. 2015;204:23–31. doi: 10.1016/j.tvjl.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 49.Daws LC, Gould GG. Ontogeny and regulation of the serotonin transporter: providing insights into human disorders. Pharmacology and Therapeutics. 2011;131:61–79. doi: 10.1016/j.pharmthera.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]