Abstract
Introduction
Animals exposed to anesthetics during specific age periods of brain development experience neurotoxicity, with neurodevelopmental changes subsequently observed during adulthood. The corresponding vulnerable age in children however is unknown.
Methods
An observational cohort study was performed using a longitudinal dataset constructed by linking individual-level Medicaid claims from Texas and New York from 1999 to 2010. This dataset was evaluated to determine whether the timing of exposure to anesthesia under age 5 years for a single common procedure (pyloromyotomy, inguinal hernia, circumcision outside the perinatal period, or tonsillectomy and/or adenoidectomy) is associated with increased subsequent risk of diagnoses for any mental disorder, or specifically developmental delay (DD) such as reading and language disorders, and attention deficit hyperactivity disorder (ADHD). Exposure to anesthesia and surgery was evaluated in 11 separate age at exposure categories: ≤28 days old, >28 days and ≤6 months, >6 months and ≤1 year, and 6 month age intervals between >1 year old and ≤5 years old. For each exposed child, five children matched on propensity score calculated using sociodemographic and clinical covariates were selected for comparison. Cox proportional hazards models were used to measure the hazard ratio of a mental disorder diagnosis associated with exposure to surgery and anesthesia.
Results
A total of 38,493 children with a single exposure and 192,465 propensity score matched children unexposed before age 5 were included in the analysis. Increased risk of mental disorder diagnosis was observed at all ages at exposure with an overall hazard ratio of 1.26 (95% confidence interval [CI], 1.22–1.30), which did not vary significantly with the timing of exposure. Analysis of DD and ADHD showed similar results, with elevated hazard ratios distributed evenly across all ages, and overall hazard ratios of 1.26 (95% CI, 1.20–1.32) for DD and 1.31 (95% CI, 1.25–1.37) for ADHD.
Conclusions
Children who undergo minor surgery requiring anesthesia under age 5 have a small but statistically significant increased risk of mental disorder diagnoses, and DD and ADHD diagnoses, but the timing of the surgical procedure does not alter the elevated risks. Based on these findings, there is little support for the concept of delaying a minor procedure to reduce long-term neurodevelopmental risks of anesthesia in children. In evaluating the influence of age at exposure, the types of procedures included may need to be considered, as some procedures are associated with specific comorbid conditions and are only performed at certain ages.
Introduction
In animal models, exposure to commonly used anesthetic medications lead to dose-dependent neuronal cell death in immature brains, with neurodevelopmental changes subsequently observed in adulthood.(1–3) While the mechanisms behind neurotoxicity are still being examined, in rodents, a distinct window of vulnerability to apoptosis has been well described, with peak sensitivity after exposure between postnatal day (PD) 7 and PD10, reduced sensitivity at later ages, and insensitivity when exposed as adults at PD60.(4)
Differences in brain development between humans and rodents have made it difficult to determine a similar age of vulnerability in children. Currently the suspected window of vulnerability is from birth to ages 2 to 4 years old based on synaptogenesis peaking in humans at that time.(5) However, given the uncertainty regarding this vulnerable period, clinical studies of neurotoxicity have evaluated children exposed from as young as under 6 months old to as old as 7 years old with variable results.(6–22) While other studies have evaluated the impact of age at exposure of surgery and anesthesia, all have included children undergoing broad ranges of surgical procedures.(12–14,22,23) Evaluation of diverse groups of procedures however makes it difficult to determine if neurodevelopmental differences seen in children exposed at different ages are actually due to the age at exposure, or differences in the distributions of medical conditions and surgeries performed at different ages. Despite the limitations of the published studies, the Food and Drug Administration recently released a new warning regarding the use of anesthetics in children before age 3 years.(24) Since delaying necessary procedures may have unintended harmful consequences, whether delaying a procedure has any long-term clinical benefit is an important question to answer.
In the present observational study, a Medicaid cohort was used to compare children exposed to anesthesia for common pediatric procedures at varying ages under age 5 years old with propensity score matched children unexposed to surgery and anesthesia. The purpose of the study is to determine the impact of the age at exposure on the risk of a subsequent mental disorder diagnosis at up to nearly 12 years after exposure.
Materials and Methods
The study was approved by the Institutional Review Board at Columbia University Medical Center (New York, NY) as exempt from requiring written/informed consent.
Generating the Birth Cohort Dataset
The dataset was generated using Medicaid Analytic eXtract (MAX) files from the Centers for Medicare and Medicaid Services (CMS) and included children enrolled in Texas (TX) and New York (NY) Medicaid from 1999 to 2010. Using records from these children, a longitudinal dataset was created by linking demographic information with inpatient and outpatient claims data composed of International Classification of Diseases, Ninth Revision Clinical Modification (ICD-9) and Current Procedural Terminology 4th edition (CPT-4) codes for diagnoses and procedures. For additional details regarding the creation of this dataset, please see the Supplemental Content Methods.
Identifying the Anesthetic and Surgical Exposure
Exposure was defined as the presence of any one of four specific commonly performed ICD-9 or CPT-4 coded procedures for young children: pyloromyotomy, inguinal hernia repair, circumcisions outside the perinatal period, and tonsillectomy and/or adenoidectomy (T&A). (ICD-9 and CPT-4 codes are listed in the Supplemental Content, Tables 1 and 2) These procedure codes were selected because explicit ICD-9 codes for anesthesia do not exist, and it is not possible to perform these surgical procedures without administration of anesthesia. One exception is circumcision, which when performed during the inpatient birth admission are typically performed without general anesthesia and as a result, these patients were excluded. Boys receiving circumcisions outside the perinatal period however were included, as 91% have been reported to require general anesthesia.(25) Exposure to anesthesia and surgery was evaluated in 11 separate age at exposure categories: the neonatal period defined as the time between discharge from the birth admission and ≤28 days old, >28 days and ≤6 months, >6 months and ≤1 year, and 6 month age intervals between >1 year old and ≤5 years old. Children with more than one instance of the four surgical procedures of interest were excluded. Children were also excluded if they received prior to 5 years of age, any other CPT-4 or ICD-9 procedure code for a surgical procedure that required the operating room, as defined by the Agency for Healthcare Research and Quality (AHRQ) Healthcare Utilization Project (HCUP) Surgery Flags.(26)
Identifying ICD-9 Coded Mental Disorders
As a primary outcome, we evaluated the presence of any ICD-9 coded mental disorder diagnosis occurring in children. These included diagnoses for schizophrenia, bipolar disorder, depressive disorders, anxiety disorders, conduct disorders, autism, attention deficit hyperactivity disorder, intellectual disability, developmental delay, and other miscellaneous mental health conditions including: delusional disorders, dissociative and somatoform disorders, personality disorders, and adjustment disorders.(27) (ICD-9 codes listed in the Supplemental Content, Table 3) Study patients were classified with respect to the age at which a mental disorder was first diagnosed. Mental disorders diagnosed before or at the time of surgical exposure were considered to be pre-existing in nature and not due to the anesthetic exposure. The effects of anesthetic neurotoxicity are also unlikely to manifest immediately, while post-operative maladaptive behaviors, which are present in one third of children 2 weeks after surgery, may resemble a variety of different mental disorders.(28) In order to avoid capturing pre-existing diagnoses and cases of transient post-operative maladaptive behaviors, as well as reduce ascertainment bias due to increased medical visits in children immediately after surgery, a latency period of at least 6 months was imposed on the definition of anesthetic related incident mental disorders. Therefore, all exposed children with a mental disorder diagnosed prior to surgery, or in the first 6 months after the surgical procedure were excluded from analysis.
Identifying Children Unexposed to Anesthesia and Surgery
Children were considered to be unexposed if they did not have any of the four procedures of interest or any additional CPT-4 or ICD-9 procedure code for a surgical procedure prior to 5 years of age.(26) For the exposed children in each age at exposure category, the median age at exposure was calculated. Unexposed children were only eligible to be chosen as matched controls for exposed children in each age at exposure category if they did not have a mental disorder within 6 months after that calculated median age at exposure for each age category.(9) This accounts for the potential bias introduced if only exposed children who had mental disorder diagnoses before exposure were excluded, as well as for the latency period applied to the exposed children.
Generating the Propensity Score Matched Cohort
Propensity scores were calculated for all eligible exposed and unexposed children, with scores for each of the four individual procedures of interest independently calculated for children in each age at exposure category, and calculated separately for TX and NY. Fifty variables were used in the propensity score calculation and included sociodemographic characteristics (sex, race, and income), prior comorbidity (ICD-9 coded prematurity, congenital anomalies, and acute and chronic medical conditions), and the number of inpatient stays and outpatient visits prior to surgery. (Details in Supplemental Content Methods with variables listed in Supplemental Content, Tables 5–12) Given the differences in the Medicaid populations in TX and NY, each exposed child in each age at exposure category within each state was matched without replacement to five unexposed children from the same state using nearest neighbor propensity score matching. While controls were matched without replacement within each age at exposure category, they could be chosen more than once if matched in a separate age at exposure category. In order to ensure close matches, the estimated log-odds scores predicting surgical exposure were required to be within 0.2 standard deviation units between the exposed and matched controls, which eliminated approximately 99% of the bias due to measured confounding variables.(29)
Statistical Analysis
To check for appropriate balance of potential confounding variables between exposed and controls in each age category, standardized differences for all covariates used in performing the propensity score matching were evaluated. There is no clear consensus on an absolute standardized difference criterion for assessing balance in propensity score matching, however acceptable standardized differences have ranged from <0.1 to <0.25.(30,31) Survival was defined as the absence of a mental disorder diagnosis. Survival time was defined as the time in days, from the date of surgery (or for unexposed control patients, the median age at which surgery was performed in each age at exposure category) until a mental disorder diagnosis, with censoring at the end of the subject’s Medicaid eligibility or the end of the year 2010. The Kaplan-Meier method was used to estimate survival in each age at exposure category with a log-rank test used to test the statistical significance of observed survivorship between exposed and unexposed children. The a priori P value was set to P <0.05. Cox proportional hazards models were then used to evaluate the relative risk of a mental disorder diagnosis following exposure to anesthesia and surgery, with separate models used for each age category. Hazard ratios were calculated with combined data from TX and NY in each age category with state of residence as a covariate. To account for the matched nature of the sample, a robust variance estimator accounting for clustering within matched sets was used.(32) Several survival models were calculated: one overall model that used matched exposed cases and unexposed controls for all four surgical procedures, with exposure interpreted as the effect of receiving any one of the four common procedures; and then procedure-specific models for matched exposed cases and unexposed controls for each separate procedure in order to evaluate specific procedure effects. As a sensitivity analysis for the primary outcome, we also explored the effect of a potential unmeasured confounder and its ability to account for observed differences in mental disorder diagnosis between exposed and unexposed children.(33,34)
Given that language and higher-level cognition were hypothesized to be the most commonly affected neurodevelopmental domains by anesthesia, secondary analyses evaluating the risk of a developmental delay (DD [ICD-9: 315]) such as language and reading disorders, and ADHD and related disorders (ADHD [ICD-9: 314]) were also performed.(18,35) For assessment of DD and ADHD, the same procedures for propensity score matched cohort selection and survival analysis were repeated. Since exposed children with diagnoses for mental disorder, DD, or ADHD prior to surgery or in the 6 months after the surgical procedure were excluded from analysis, there were minor differences in the numbers of exposed children based on the outcome being evaluated. For example, in the analysis evaluating the risk of DD, a child with DD prior to surgery would be excluded, but not a child with ADHD.
Results
Patient Characteristics
Using Medicaid MAX data from 1999 to 2010, we built a birth cohort dataset of nearly 2 million children born in TX and NY with follow-up within the Medicaid system. Of the children in TX, we identified 22,965 with a single ICD-9 coded surgical exposure to one of the four commonly performed procedures of interest, no other procedures prior to age 5, and no mental disorders prior to or anytime up to 6 months after surgery. An additional 15,528 children were identified in NY under the same criteria, for a total combined exposed cohort of 38,493 children.
Exposed cases and unexposed controls in each age at exposure category had similar distributions on the fifty variables used to calculate the propensity score. Aggregated summary data with a few select variables are displayed in Table 1 where good balance between exposed and unexposed children can be seen. Detailed state and procedure specific tables showing the distribution of all variables are available in the Supplemental Content, Tables 5–12 confirming good balance by state and procedure. Standardized differences were calculated for each variable in the matched cohorts for evaluation of any mental disorder, with 96% of the variables having standardized differences <0.1 and 99.9% of variables having standardized differences <0.25, confirming good balance between exposed cases and controls (Supplemental Content, Tables 13–20).
Table 1.
Characteristics of Combined TX and NY Matched Cohorts by Aggregated Ages of Exposure
| >Birth – ≤6 m | >6 m – ≤1 yr | >1 yr – ≤2 yr | >2 yr – ≤3 yr | >3 yr – ≤4 yr | >4 yr – ≤5 yr | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||
| Exposure | Exposure | Exposure | Exposure | Exposure | Exposure | ||||||||
| No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | ||
| n(%) | n(%) | n(%) | n(%) | n(%) | n(%) | n(%) | n(%) | n(%) | n(%) | n(%) | n(%) | ||
| Sex |
Female
|
6292 (12.4) | 1288 (12.7) | 1366 (7.2) | 271 (7.2) | 7628 (20) | 1497 (19.6) | 10191 (31.5) | 2062 (31.9) | 11368 (38.8) | 2281 (38.9) | 9788 (42.9) | 1957 (42.9) |
| Male | 44633 (87.6) | 8897 (87.4) | 17529 (92.8) | 3508 (92.8) | 30552 (80) | 6139 (80.4) | 22184 (68.5) | 4413 (68.2) | 17927 (61.2) | 3578 (61.1) | 13007 (57.1) | 2602 (57.1) | |
|
| |||||||||||||
| Race |
White
|
14069 (27.6) | 2854 (28) | 5235 (27.7) | 1035 (27.4) | 11573 (30.3) | 2305 (30.2) | 9930 (30.7) | 1993 (30.8) | 8389 (28.6) | 1649 (28.1) | 6613 (29) | 1319 (28.9) |
|
Black
|
10134 (19.9) | 1949 (19.1) | 5948 (31.5) | 1125 (29.8) | 9292 (24.3) | 1847 (24.2) | 5854 (18.1) | 1165 (18) | 4348 (14.8) | 902 (15.4) | 3309 (14.5) | 672 (14.7) | |
|
Hispanic
|
21550 (42.3) | 4346 (42.7) | 5700 (30.2) | 1193 (31.6) | 13396 (35.1) | 2679 (35.1) | 13905 (43) | 2761 (42.6) | 14248 (48.6) | 2839 (48.5) | 11363 (49.9) | 2251 (49.4) | |
| Other | 5172 (10.2) | 1036 (10.2) | 2012 (10.7) | 426 (11.3) | 3919 (10.3) | 805 (10.5) | 2686 (8.3) | 556 (8.6) | 2310 (7.9) | 469 (8) | 1510 (6.6) | 317 (7) | |
|
| |||||||||||||
| Income based on ZIP code of residence (Quartile) |
Quartile 1
|
13368 (26.3) | 2688 (26.4) | 5022 (26.6) | 1000 (26.5) | 10725 (28.1) | 2111 (27.7) | 9158 (28.3) | 1800 (27.8) | 8455 (28.9) | 1666 (28.4) | 6501 (28.5) | 1293 (28.4) |
|
Quartile 2
|
13056 (25.6) | 2612 (25.7) | 4503 (23.8) | 917 (24.3) | 9998 (26.2) | 1993 (26.1) | 8649 (26.7) | 1718 (26.5) | 7894 (27) | 1597 (27.3) | 6397 (28.1) | 1290 (28.3) | |
|
Quartile 3
|
12236 (24) | 2437 (23.9) | 4568 (24.2) | 904 (23.9) | 9213 (24.1) | 1863 (24.4) | 7974 (24.6) | 1600 (24.7) | 7132 (24.4) | 1402 (23.9) | 5759 (25.3) | 1141 (25) | |
| Quartile 4 | 12265 (24.1) | 2448 (24) | 4802 (25.4) | 958 (25.4) | 8244 (21.6) | 1669 (21.9) | 6594 (20.4) | 1357 (21) | 5814 (19.9) | 1194 (20.4) | 4138 (18.2) | 835 (18.3) | |
|
| |||||||||||||
| Number of outpatient visits (Quartile) |
Quartile 1
|
5633 (11.1) | 1099 (10.8) | 1968 (10.4) | 390 (10.3) | 2977 (7.8) | 621 (8.1) | 2513 (7.8) | 525 (8.1) | 2359 (8.1) | 490 (8.4) | 1677 (7.4) | 360 (7.9) |
|
Quartile 2
|
13750 (27) | 2717 (26.7) | 3279 (17.4) | 662 (17.5) | 6574 (17.2) | 1325 (17.4) | 5391 (16.7) | 1056 (16.3) | 4850 (16.6) | 974 (16.6) | 4116 (18.1) | 821 (18) | |
|
Quartile 3
|
11074 (21.8) | 2173 (21.3) | 5542 (29.3) | 1120 (29.6) | 9539 (25) | 1881 (24.6) | 8000 (24.7) | 1593 (24.6) | 7607 (26) | 1544 (26.4) | 6477 (28.4) | 1275 (28) | |
| Quartile 4 | 20468 (40.2) | 4196 (41.2) | 8106 (42.9) | 1607 (42.5) | 19090 (50) | 3809 (49.9) | 16471 (50.9) | 3301 (51) | 14479 (49.4) | 2851 (48.7) | 10525 (46.2) | 2103 (46.1) | |
|
| |||||||||||||
| Total number of inpatient stays including the birth admission (n) |
0
|
0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
|
1
|
46873 (92) | 9335 (91.7) | 15503 (82.1) | 3055 (80.8) | 29057 (76.1) | 5755 (75.4) | 23711 (73.2) | 4674 (72.2) | 21020 (71.8) | 4140 (70.7) | 15990 (70.2) | 3161 (69.3) | |
|
2
|
3412 (6.7) | 712 (7) | 2668 (14.1) | 561 (14.9) | 6503 (17) | 1320 (17.3) | 6164 (19) | 1259 (19.4) | 5573 (19) | 1152 (19.7) | 4464 (19.6) | 921 (20.2) | |
| 3 or more | 640 (1.3) | 138 (1.4) | 724 (3.8) | 163 (4.3) | 2620 (6.9) | 561 (7.4) | 2500 (7.7) | 542 (8.4) | 2702 (9.2) | 567 (9.7) | 2341 (10.3) | 477 (10.5) | |
|
| |||||||||||||
| <36 weeks gestational age |
No
|
45241 (88.8) | 9045 (88.8) | 16435 (87) | 3258 (86.2) | 34795 (91.1) | 6906 (90.4) | 30606 (94.5) | 6095 (94.1) | 27798 (94.9) | 5541 (94.6) | 21887 (96) | 4366 (95.8) |
| Yes | 5684 (11.2) | 1140 (11.2) | 2460 (13) | 521 (13.8) | 3385 (8.9) | 730 (9.6) | 1769 (5.5) | 380 (5.9) | 1497 (5.1) | 318 (5.4) | 908 (4) | 193 (4.2) | |
|
| |||||||||||||
| Low birth weight (<2500g and ≥1500g) |
No
|
46312 (90.9) | 9298 (91.3) | 16820 (89) | 3369 (89.2) | 35374 (92.7) | 7048 (92.3) | 30790 (95.1) | 6130 (94.7) | 27874 (95.2) | 5569 (95.1) | 21778 (95.5) | 4342 (95.2) |
| Yes | 4613 (9.1) | 887 (8.7) | 2075 (11) | 410 (10.9) | 2806 (7.4) | 588 (7.7) | 1585 (4.9) | 345 (5.3) | 1421 (4.9) | 290 (5) | 1017 (4.5) | 217 (4.8) | |
|
| |||||||||||||
| Very low birth weight (<1500g and ≥1000g) |
No
|
49599 (97.4) | 9886 (97.1) | 18411 (97.4) | 3669 (97.1) | 37663 (98.7) | 7522 (98.5) | 32046 (99) | 6405 (98.9) | 29037 (99.1) | 5806 (99.1) | 22592 (99.1) | 4519 (99.1) |
| Yes | 1326 (2.6) | 299 (2.9) | 484 (2.6) | 110 (2.9) | 517 (1.4) | 114 (1.5) | 329 (1) | 70 (1.1) | 258 (0.9) | 53 (0.9) | 203 (0.9) | 40 (0.9) | |
|
| |||||||||||||
| Extremely low birth weight (<1000g) |
No
|
50444 (99.1) | 10058 (98.8) | 18618 (98.5) | 3714 (98.3) | 37934 (99.4) | 7578 (99.2) | 32251 (99.6) | 6447 (99.6) | 29130 (99.4) | 5825 (99.4) | 22691 (99.5) | 4538 (99.5) |
| Yes | 481 (0.9) | 127 (1.3) | 277 (1.5) | 65 (1.7) | 246 (0.6) | 58 (0.8) | 124 (0.4) | 28 (0.4) | 165 (0.6) | 34 (0.6) | 104 (0.5) | 21 (0.5) | |
|
| |||||||||||||
| Endocrine, nutritional, metabolic, and immunity disorders (Chronic) |
No
|
49707 (97.6) | 9919 (97.4) | 18251 (96.6) | 3632 (96.1) | 36622 (95.9) | 7294 (95.5) | 30851 (95.3) | 6129 (94.7) | 27742 (94.7) | 5524 (94.3) | 21463 (94.2) | 4279 (93.9) |
| Yes | 1218 (2.4) | 266 (2.6) | 644 (3.4) | 147 (3.9) | 1558 (4.1) | 342 (4.5) | 1524 (4.7) | 346 (5.3) | 1553 (5.3) | 335 (5.7) | 1332 (5.8) | 280 (6.1) | |
|
| |||||||||||||
| Diseases of blood and blood-forming organs (Chronic) |
No
|
50422 (99) | 10073 (98.9) | 18477 (97.8) | 3684 (97.5) | 36955 (96.8) | 7351 (96.3) | 31054 (95.9) | 6196 (95.7) | 27941 (95.4) | 5585 (95.3) | 21642 (94.9) | 4321 (94.8) |
| Yes | 503 (1) | 112 (1.1) | 418 (2.2) | 95 (2.5) | 1225 (3.2) | 285 (3.7) | 1321 (4.1) | 279 (4.3) | 1354 (4.6) | 274 (4.7) | 1153 (5.1) | 238 (5.2) | |
|
| |||||||||||||
| Diseases of the nervous system and sense organs (Chronic) |
No
|
48485 (95.2) | 9647 (94.7) | 16597 (87.8) | 3315 (87.7) | 28135 (73.7) | 5605 (73.4) | 23373 (72.2) | 4635 (71.6) | 21560 (73.6) | 4280 (73.1) | 16521 (72.5) | 3261 (71.5) |
| Yes | 2440 (4.8) | 538 (5.3) | 2298 (12.2) | 464 (12.3) | 10045 (26.3) | 2031 (26.6) | 9002 (27.8) | 1840 (28.4) | 7735 (26.4) | 1579 (27) | 6274 (27.5) | 1298 (28.5) | |
|
| |||||||||||||
| Diseases of the circulatory system (Chronic) |
No
|
49985 (98.2) | 9972 (97.9) | 18270 (96.7) | 3640 (96.3) | 36901 (96.7) | 7364 (96.4) | 31256 (96.5) | 6242 (96.4) | 28177 (96.2) | 5621 (95.9) | 21907 (96.1) | 4367 (95.8) |
| Yes | 940 (1.9) | 213 (2.1) | 625 (3.3) | 139 (3.7) | 1279 (3.4) | 272 (3.6) | 1119 (3.5) | 233 (3.6) | 1118 (3.8) | 238 (4.1) | 888 (3.9) | 192 (4.2) | |
|
| |||||||||||||
| Diseases of the respiratory system (Chronic) |
No
|
49424 (97.1) | 9853 (96.7) | 15320 (81.1) | 3071 (81.3) | 23683 (62) | 4718 (61.8) | 15204 (47) | 3070 (47.4) | 11954 (40.8) | 2404 (41) | 8406 (36.9) | 1718 (37.7) |
| Yes | 1501 (3) | 332 (3.3) | 3575 (18.9) | 708 (18.7) | 14497 (38) | 2918 (38.2) | 17171 (53) | 3405 (52.6) | 17341 (59.2) | 3455 (59) | 14389 (63.1) | 2841 (62.3) | |
|
| |||||||||||||
| Diseases of the digestive system (Chronic) |
No
|
45600 (89.5) | 9092 (89.3) | 16449 (87.1) | 3266 (86.4) | 32625 (85.5) | 6479 (84.9) | 28043 (86.6) | 5570 (86) | 25752 (87.9) | 5120 (87.4) | 20324 (89.2) | 4024 (88.3) |
| Yes | 5325 (10.5) | 1093 (10.7) | 2446 (13) | 513 (13.6) | 5555 (14.6) | 1157 (15.2) | 4332 (13.4) | 905 (14) | 3543 (12.1) | 739 (12.6) | 2471 (10.8) | 535 (11.7) | |
This table contains aggregated summary data displaying representative variables. For the purposes of this table, children from Texas and New York were combined. Children from the >Birth – ≤28 days and >28 days – ≤6 months, >1 yr – ≤1.5 yrs and >1.5 yrs – ≤2 yrs, >2 yrs – ≤2.5 yrs and >2.5 yrs – ≤3 yrs, >3 yrs – ≤3.5 yrs and >3.5 yrs – ≤4 yrs, and >4 yrs – ≤4.5 yrs and >4.5yrs – ≤5 yrs categories were also combined. For state and procedure specific tables with non-aggregated age categories and all variables used, refer to Supplemental Content Tables 5–12.
Risk of Mental Disorder Associated with Age at Exposure
The incidence rates of mental disorder diagnosis after a single exposure to any of the four procedures of interest in each age at exposure category were slightly higher in TX than NY. In TX they ranged from 3.5 to 5.4 diagnoses per 100 person-years in the controls and 4.4 to 6.8 diagnoses per 100 person-years in the exposed cases, while in NY it ranged from 2.3 to 3.5 diagnoses per 100 person-years in the controls and 3.0 to 5.3 diagnoses per 100 person-years in the exposed cases. The most common reasons for initial diagnosis of a mental disorder were DD and ADHD. (Table 2)
Table 2.
Incidence Rate of Each Type of Mental Disorder at Initial Diagnosis
|
|
||
|---|---|---|
| Unexposed | Exposed | |
|
|
||
| Diagnoses per 100 person-years | Diagnoses per 100 person-years | |
| Schizophrenia | 0 | 0.002 |
| Bipolar Disorder | 0.018 | 0.029 |
| Depression | 0.041 | 0.046 |
| Anxiety Disorder | 0.122 | 0.132 |
| Conduct Disorder | 0.45 | 0.557 |
| Autism | 0.079 | 0.101 |
| Attention Deficit and Hyperactivity Disorder | 1.016 | 1.271 |
| Intellectual Disability | 0.01 | 0.012 |
| Developmental Delay | 1.488 | 1.897 |
| Miscellaneous | 0.775 | 0.922 |
|
| ||
| Any Mental Disorder Diagnosis* | 3.915 | 4.869 |
Children may have more than one mental disorder at the time of diagnosis
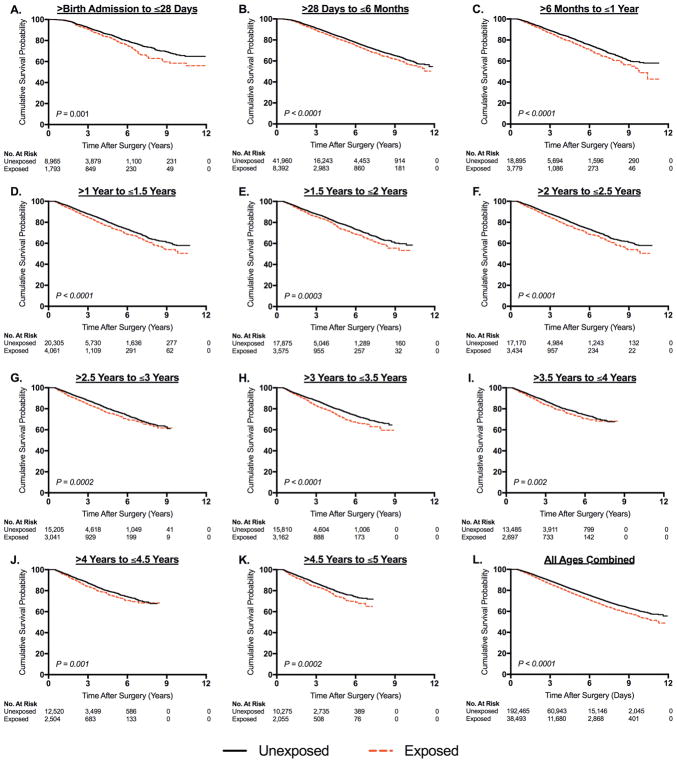
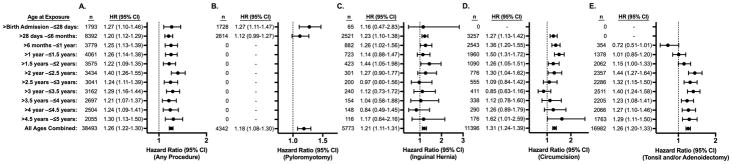
Significantly increased risk of mental disorder diagnosis was found at every age at exposure category and was also observed when evaluating all ages combined. (Figure 1) This difference was sustained at up to nearly 12 years after exposure. Cox proportional hazards modeling revealed that children exposed to any of the four surgical procedures had a significantly increased risk of receiving a mental disorder diagnosis at all ages of exposure, with hazard ratios (HR) ranging from 1.2 to 1.4, but with risk distributed evenly between ages. (Figure 2, Panel A) The overall HR for all ages combined in any of the four procedures evaluated was 1.26 (95% confidence interval [CI], 1.22–1.30). Among these children, 1793 had a procedure in the neonatal period, 8392 had a procedure between the neonatal period and the first 6 months of life, and between 2000 to 4100 children had a procedure in each 6-month age category thereafter. The majority of procedures in the neonatal period however were pyloromyotomies, a surgery that is generally not performed after 6 months of age. (Figure 2, Panel B) Inguinal hernia repairs were most commonly performed in the age category between >28 days and ≤6 months and were less commonly performed at later ages in early childhood. (Figure 2, Panel C) Circumcisions were also most commonly performed in infants, with decreased numbers in later ages. (Figure 2, Panel D) T&As, while less frequently performed in infants, were more common in older children and became the predominant procedure in children aged 2 through 5 years of age. (Figure 2, Panel E) While the risk of developing a mental disorder was evenly distributed between age at exposure categories in the combined cohort of any of the four procedures, (Figure 2, Panel A) when evaluating children undergoing individual procedures, some variation in the risk of developing a mental disorder based on age at exposure was seen. (Figure 2, Panels B–E) Specifically, children with circumcision at younger ages appeared to have a higher risk of mental disorder, while conversely a higher risk was seen in children who had T&A at older ages.
Figure 1. Kaplan-Meier Analysis of Mental Disorder Diagnosis after a Single Exposure to Surgery and Anesthesia in TX and NY.
For the exposed children, data in Panels A through K show the length of time after surgical procedure until diagnosis of a mental disorder. In each age at exposure category, the median age of surgery was calculated for the exposed children and applied to the unexposed children. For the unexposed children, Panels A through K show the length of time from this median age of surgery until diagnosis of a mental disorder. Panel L shows results from all age at exposure categories combined.
Figure 2. Hazard of an ICD-9 coded Mental Disorder Diagnosis after a Single Exposure to Surgery and Anesthesia in TX and NY.
Hazard ratios (HR) for diagnosis of mental disorder are displayed for exposed vs. matched unexposed children. The number (n) of exposed children in each age at exposure group is also displayed. Panel A shows hazard ratios for children who had any of the four procedures of interest vs. unexposed children. Panels B, C, D, and E show hazard ratios for children who had pyloromyotomy, inguinal hernia repair, circumcision, and tonsillectomy and/or adenoidectomy respectively, compared to unexposed children.
Effect of Unmeasured Confounding
The estimated hazard ratio for all procedures and all ages combined for mental disorder diagnosis was 1.26 (95% CI, 1.22–1.30). In order for a single unmeasured confounder to account for the observed increased risk of a mental disorder diagnosis with exposure (i.e., to shift the lower 95% confidence interval from 1.22 to 1.00), this unmeasured risk factor would need to be present in 10% of the patients in the unexposed group and 20%, 40%, or 60% or the patients in the exposed group and have an associated hazard ratio of 4.26, 1.89, and 1.52, respectively. Similarly, if an unmeasured risk factor was present in 20% of the patients in the unexposed group and in 30%, 50%, or 70% of the patients in the exposed group, then the hazard ratio that would be required for an unmeasured risk factor to account for the observed increased risk with exposure would be 5.85, 1.98, and 1.55, respectively.
Risk of DD or ADHD Associated with Age at Exposure
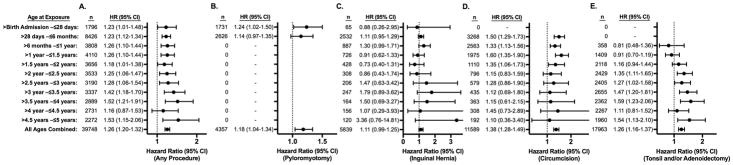
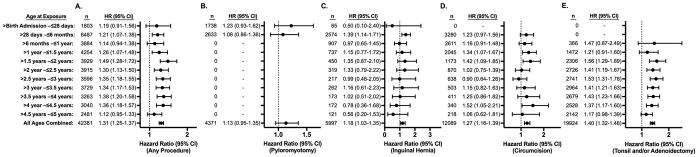
The matched cohorts for the DD and ADHD risk analyses also demonstrated good balance between cases and controls with 97.5% of the variables having standardized differences <0.1 and 99.9% of variables having standardized differences <0.25 for DD, and 97.7% of the variables having standardized differences <0.1 and 99.9% of variables having standardized differences <0.25 for ADHD. Survival analysis evaluating DD and ADHD also showed results that resembled the overall mental disorder analyses. In evaluating exposure to any of the four surgical procedures, a small increased risk was found at nearly all ages, with HRs for DD ranging from 1.16 to 1.53 and an overall HR at all ages of exposure of 1.26 (95% CI, 1.20–1.32). (Figure 3, Panel A) For ADHD, HRs ranged from 1.12 to 1.49, with an overall HR at all ages of 1.31 (95% CI, 1.25–1.37). (Figure 4, Panel A). The risk of developing DD or ADHD was evenly distributed between age at exposure categories in the combined cohort of any of the four procedures. In evaluating the individual procedures, the risk for DD and ADHD was similar across different procedures, but like the analysis of any mental disorder diagnosis, some minor procedure specific variation based on age at exposure was seen. (Figures 3 and 4, Panels B–E)
Figure 3. Hazard of an ICD-9 coded Developmental Delay Diagnosis after a Single Exposure to Surgery and Anesthesia in TX and NY.
Hazard ratios (HR) for diagnosis of a developmental delay are displayed for exposed vs. matched unexposed children. The number (n) of exposed children in each age at exposure group is also displayed. Panel A shows hazard ratios for children who had any of the four procedures of interest vs. unexposed children. Panels B, C, D, and E show hazard ratios for children who had pyloromyotomy, inguinal hernia repair, circumcision, and tonsillectomy and/or adenoidectomy respectively, compared to unexposed children.
Figure 4. Hazard of an ICD-9 coded Attention Deficit Hyperactivity Disorder Diagnosis after a Single Exposure to Surgery and Anesthesia in TX and NY.
Hazard ratios (HR) for diagnosis of an attention deficit hyperactivity disorder are displayed for exposed vs. matched unexposed children. The number (n) of exposed children in each age at exposure group is also displayed. Panel A shows hazard ratios for children who had any of the four procedures of interest vs. unexposed children. Panels B, C, D, and E show hazard ratios for children who had pyloromyotomy, inguinal hernia repair, circumcision, and tonsillectomy and/or adenoidectomy respectively, compared to unexposed children.
Discussion
Common minor surgical procedures were found to be associated with small but statistically significant long-term increased risks of any mental disorder diagnosis as well as specifically DD and ADHD diagnoses. The finding of a small but statistically significant increased risk of neurodevelopmental deficit associated with exposure to anesthesia and surgery is consistent with results from other population-based cohort studies.(7–14) However, while a window of vulnerability is seen in preclinical studies, our results show that the risks tended to be distributed evenly across children who received anesthesia and surgery at various ages over the first five years of life. Based on these findings, there is little support for the concept of delaying a minor procedure to reduce any potential long-term neurodevelopmental risks of anesthesia in children.
Recently, three large population-based cohort studies assessing children in Canada and Sweden found small decreases in teacher evaluation and academic performance scores associated with early anesthetic exposure, even with a single exposure.(12–14) Secondary analyses in these studies assessed the influence of the age at exposure with all three finding increased deficit in children exposed at older ages compared to those exposed at younger ages. Conversely, in a study of a cohort from Australia, an increased risk of language and cognitive deficit was identified in children exposed under age 3, but not in those exposed over age 3.(23) All of the above studies however evaluated children undergoing a broad range of surgical procedures. In one of these population-based studies, Graham et al. discussed the types of procedures performed, with dental procedures accounting for more than half of the anesthetics in older children, and a much smaller percentage in those who were younger.(13) In children, certain procedures are often performed at specific ages, and the types of procedures included may affect the results of the study, as children who require general anesthesia to tolerate dental procedures may differ from those who do not need it. The results from the above studies differ from those reported in the present study, where age at exposure had little impact on subsequent mental disorder, DD, or ADHD diagnoses. The present study evaluated four common minor procedures, finding that the age related risks of mental disorder for these procedures were similar. One variation observed in our results was that children receiving circumcisions at younger ages have a slightly higher risk of subsequent mental disorder diagnoses while children receiving T&A at older ages have a higher risk. On further evaluation, children who had T&A at an older age typically had both tonsillectomies and adenoidectomies while those exposed at under age 2 years commonly had adenoidectomies alone. Whether this accounts for the higher risk in the older T&A children however is unclear. Some procedure specific age related variation may also be due to smaller sample sizes in some categories.
ICD-9 coded clinical diagnoses and academic performance scores are less sensitive than directly assessed neuropsychological tests, but the benefit is that these outcomes are available in large population-based studies.(35) Recent prospective and ambidirectional studies used neuropsychological tests, but did not find a difference in children exposed to general anesthesia.(36,37) One potential reason for differences between these studies and population-based studies may be differences in the patients, as parents who agree to participate in prospective studies tend to come from higher socioeconomic strata, and growing up in a higher income household may mask some potential effects of neurotoxic exposures.(38,39) Although approximately 40% of TX and NY children are enrolled in Medicaid, the socioeconomic differences between children in prospective studies and those enrolled in Medicaid may be particularly stark.(40) Evaluation of a Medicaid population may be uniquely suited to evaluating long-term neurodevelopmental effects of anesthetics, as children in lower socioeconomic strata are also particularly vulnerable to other neurotoxic agents such as lead.(41)
Despite this, prospective clinical trials are still the gold standard, but randomizing a child to receive anesthesia and surgery at a specific age faces strong ethical and logistical constraints. As a result, large population-based observational study designs may be the most effective methods for evaluating questions like the age of vulnerability to anesthesia in children. However, as with any observational study, there will be some residual confounding, and our methods may not completely account for unmeasured differences in mental disorder risk between children with and without surgical procedures. Since there is currently no validated method of predicting neurodevelopmental deficits based on medical comorbidity, the propensity score matching method used in the present study is likely one of the more robust methods of comparing exposed and unexposed children with similar levels of medical illness. Data regarding inpatient stays and outpatient doctor’s visits were also included in the propensity score as frequent physician encounters may be associated with increased incidence of psychiatric diagnosis. Our results showing an overall 26% increased risk of a mental disorder diagnosis in TX and NY is lower than that found in prior studies of NY Medicaid data, likely due to improved adjustment for comorbidity using our propensity score matched model.(8,9) While T&A patients specifically may have comorbidities associated with cognitive dysfunction, after propensity score matching, the increased risk of a mental disorder diagnosis was similar to the other 3 procedures, which argues against a large unaccounted for effect of comorbidities specific to T&A patients. In order to further address the question of unmeasured confounding, we performed a sensitivity analysis to determine the required strength of an unmeasured confounder to negate our primary result finding a difference in mental disorder diagnosis risk between exposed and unexposed children receiving any procedure at all ages. Based this analysis, the required strength of a single unmeasured confounder would need to range from a HR or 1.52 to 5.85 based on the prevalence. To put this in perspective, the covariate sex, with a prevalence of being a male child of 76% in our exposed cohort and 50% in our unmatched unexposed cohort, would require a HR of 2.80 to negate our results. Since the calculated HR of male gender on mental disorder diagnosis is 1.39, the strength of this theoretical unmeasured confounder would need to be more than twice that of male gender in order to negate our results. As a second example, the covariate preterm (<36 weeks gestational age) is present in 8.5% of our exposed cohort and 5% of our unexposed cohort, which would require it to have a HR of 11.82 to negate our results. The calculated HR of being preterm was 1.41, so to negate our results, the strength of the unmeasured confounder would need to over 8 times that of preterm birth. Based on this sensitivity analysis, a relatively strong unmeasured confounder would be required to negate our primary findings.
Besides unmeasured confounding, which is a limitation of all observational studies, the present study also has a number limitations specific to this study. First, we are unable to distinguish the effects of the anesthetic from those of the surgery, or any perioperative complications. Second, the mental disorders are only detected in the Medicaid administrative data if the disorders are diagnosed and coded. While there may be misclassification in the ICD-9 codes, there is no reason to believe that it varies differentially with exposure status. Therefore the differences found between exposed and unexposed children cannot be attributed to coding inaccuracy.(42) Conversely, the results may be biased toward a null effect, potentially yielding a conservative estimate. Third, most of the mental disorders under study typically onset later in life, raising the possibility that stronger signals might have been detected in older cohorts or after longer follow-up periods. Fourth, the population was restricted to children who were continuously enrolled in Medicaid. Different results might have been observed in privately insured or uninsured children. A major strength of this study however is the large sample size, which allowed for matching on an extensive array of variables, and independent evaluation of each surgical procedure performed at a range of ages. Finally, many factors are involved in perioperative management including the types and doses of anesthetic and non-anesthetic medications given, and whether any perioperative complications occurred. Since this study used administrative data, these details were not available.
The inability to identify neurodevelopmental differences based on age at exposure runs counter to preclinical findings showing increased neuronal apoptosis in specific vulnerable age periods in rodents. One explanation may be that given the uncertainty of extrapolating neurodevelopmental periods between humans and animals, an increased or decreased risk in children following exposure to anesthesia and surgery may exist beyond age 5 years and a different pattern of risk might have been observed with a larger age window. One preclinical study postulates that vulnerability may lie with the age of the neuron, not the organism, which may suggest a larger window of vulnerability than previously suspected.(43) Alternatively, it is also possible that the vulnerable period seen in animals is simply not present in humans.
Whether neurotoxicity exists in children is still unclear. However, knowledge of a window of vulnerability to anesthesia is important as it directly impacts clinical decision-making, and delaying surgery can have harmful consequences. In this study, children exposed to anesthesia for a single minor surgical procedure under age 5 have an increased risk of any mental disorder, as well as DD, and ADHD diagnoses, but the risk is evenly distributed regardless of the age at exposure. These findings suggest that in children receiving a single minor procedure, delaying surgery and performing the procedure at an older age has little impact on altering any potential long-term neurodevelopmental risks of anesthesia.
Supplementary Material
Acknowledgments
Support: Dr. Caleb Ing is supported by the Agency for Healthcare Research and Quality (AHRQ) under award number K08HS022941. The content is solely the responsibility of the authors and does not necessarily represent the official views of the AHRQ.
We acknowledge Moira Rynn, MD, Professor of Psychiatry at Columbia University College of Physicians and Surgeons, New York, NY for her advice during the planning of the study.
Footnotes
Conflicts of Interest: None.
Authors’ contributions:
Study conception and design: C.I., M.S, M.O., C.J.D, L.S.S, M.M.W, G.L.
Data analysis, interpretation, or both: C.I., M.S, M.O., M.M.W, G.L.
Writing and revising the manuscript: C.I., M.S, M.O., C.J.D, L.S.S, M.M.W, G.L.
Final approval: C.I., M.S, M.O., C.J.D, L.S.S, M.M.W, G.L.
Agreement to be accountable for all aspects of the work: C.I., M.S, M.O., C.J.D, L.S.S, M.M.W, G.L.
Contributor Information
Caleb Ing, Departments of Anesthesiology and Epidemiology, Columbia University College of Physicians and Surgeons and Mailman School of Public Health, New York, NY.
Ming Sun, Departments of Anesthesiology and Biostatistics, Columbia University College of Physicians and Surgeons and Mailman School of Public Health, New York, NY.
Mark Olfson, Department of Psychiatry, Columbia University College of Physicians and Surgeons, New York, NY.
Charles J. DiMaggio, Department of Surgery, New York University School of Medicine, New York, NY.
Lena S. Sun, Departments of Anesthesiology and Pediatrics, Columbia University College of Physicians and Surgeons, New York, NY.
Melanie M. Wall, Departments of Psychiatry and Biostatistics, Columbia University College of Physicians and Surgeons and Mailman School of Public Health, New York, NY.
Guohua Li, Departments of Anesthesiology and Epidemiology, Columbia University College of Physicians and Surgeons and Mailman School of Public Health, New York, NY.
References
- 1.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. Journal of Neuroscience. 2003;23:876–82. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zou X, Liu F, Zhang X, Patterson TA, Callicott R, Liu S, Hanig JP, Paule MG, Slikker W, Jr, Wang C. Inhalation anesthetic-induced neuronal damage in the developing rhesus monkey. Neurotoxicol Teratol. 2011;33:592–7. doi: 10.1016/j.ntt.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Loepke AW, Soriano SG. An assessment of the effects of general anesthetics on developing brain structure and neurocognitive function. Anesth Analg. 2008;106:1681–707. doi: 10.1213/ane.0b013e318167ad77. [DOI] [PubMed] [Google Scholar]
- 4.Disma N, Mondardini MC, Terrando N, Absalom AR, Bilotta F. A systematic review of methodology applied during preclinical anesthetic neurotoxicity studies: important issues and lessons relevant to the design of future clinical research. Paediatr Anaesth. 2016;26:6–36. doi: 10.1111/pan.12786. [DOI] [PubMed] [Google Scholar]
- 5.Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci. 2005;9:104–10. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Sprung J, Flick RP, Wilder RT, Katusic SK, Pike TL, Dingli M, Gleich SJ, Schroeder DR, Barbaresi WJ, Hanson AC, Warner DO. Anesthesia for cesarean delivery and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;111:302–10. doi: 10.1097/ALN.0b013e3181adf481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL, Warner DO. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiMaggio C, Sun LS, Kakavouli A, Byrne MW, Li G. A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. J Neurosurg Anesthesiol. 2009;21:286–91. doi: 10.1097/ANA.0b013e3181a71f11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiMaggio C, Sun LS, Li G. Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth Analg. 2011;113:1143–51. doi: 10.1213/ANE.0b013e3182147f42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flick RP, Katusic SK, Colligan RC, Wilder RT, Voigt RG, Olson MD, Sprung J, Weaver AL, Schroeder DR, Warner DO. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011;128:e1053–61. doi: 10.1542/peds.2011-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ing C, Dimaggio C, Whitehouse A, Hegarty MK, Brady J, von Ungern-Sternberg BS, Davidson A, Wood AJ, Li G, Sun LS. Long-term Differences in Language and Cognitive Function After Childhood Exposure to Anesthesia. Pediatrics. 2012;130:e476–85. doi: 10.1542/peds.2011-3822. [DOI] [PubMed] [Google Scholar]
- 12.Glatz P, Sandin RH, Pedersen NL, Bonamy AK, Eriksson LI, Granath F. Association of Anesthesia and Surgery During Childhood With Long-term Academic Performance. JAMA Pediatr. 2016:e163470. doi: 10.1001/jamapediatrics.2016.3470. [DOI] [PubMed] [Google Scholar]
- 13.Graham MR, Brownell M, Chateau DG, Dragan RD, Burchill C, Fransoo RR. Neurodevelopmental Assessment in Kindergarten in Children Exposed to General Anesthesia before the Age of 4 Years: A Retrospective Matched Cohort Study. Anesthesiology. 2016;125:667–77. doi: 10.1097/ALN.0000000000001245. [DOI] [PubMed] [Google Scholar]
- 14.O’Leary JD, Janus M, Duku E, Wijeysundera DN, To T, Li P, Maynes JT, Crawford MW. A Population-based Study Evaluating the Association between Surgery in Early Life and Child Development at Primary School Entry. Anesthesiology. 2016;125:272–9. doi: 10.1097/ALN.0000000000001200. [DOI] [PubMed] [Google Scholar]
- 15.Bartels M, Althoff RR, Boomsma DI. Anesthesia and cognitive performance in children: no evidence for a causal relationship. Twin Res Hum Genet. 2009;12:246–53. doi: 10.1375/twin.12.3.246. [DOI] [PubMed] [Google Scholar]
- 16.Kalkman CJ, Peelen L, Moons KG, Veenhuizen M, Bruens M, Sinnema G, de Jong TP. Behavior and development in children and age at the time of first anesthetic exposure. Anesthesiology. 2009;110:805–12. doi: 10.1097/ALN.0b013e31819c7124. [DOI] [PubMed] [Google Scholar]
- 17.Hansen TG, Pedersen JK, Henneberg SW, Pedersen DA, Murray JC, Morton NS, Christensen K. Academic performance in adolescence after inguinal hernia repair in infancy: a nationwide cohort study. Anesthesiology. 2011;114:1076–85. doi: 10.1097/ALN.0b013e31820e77a0. [DOI] [PubMed] [Google Scholar]
- 18.Sprung J, Flick RP, Katusic SK, Colligan RC, Barbaresi WJ, Bojanic K, Welch TL, Olson MD, Hanson AC, Schroeder DR, Wilder RT, Warner DO. Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc. 2012;87:120–9. doi: 10.1016/j.mayocp.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Block RI, Thomas JJ, Bayman EO, Choi JY, Kimble KK, Todd MM. Are anesthesia and surgery during infancy associated with altered academic performance during childhood? Anesthesiology. 2012;117:494–503. doi: 10.1097/ALN.0b013e3182644684. [DOI] [PubMed] [Google Scholar]
- 20.Fan Q, Cai Y, Chen K, Li W. Prognostic study of sevoflurane-based general anesthesia on cognitive function in children. J Anesth. 2013;27:493–9. doi: 10.1007/s00540-013-1566-z. [DOI] [PubMed] [Google Scholar]
- 21.Backeljauw B, Holland SK, Altaye M, Loepke AW. Cognition and Brain Structure Following Early Childhood Surgery With Anesthesia. Pediatrics. 2015;136:e1–12. doi: 10.1542/peds.2014-3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ko WR, Liaw YP, Huang JY, Zhao DH, Chang HC, Ko PC, Jan SR, Nfor ON, Chiang YC, Lin LY. Exposure to general anesthesia in early life and the risk of attention deficit/hyperactivity disorder development: a nationwide, retrospective matched-cohort study. Paediatr Anaesth. 2014;24:741–8. doi: 10.1111/pan.12371. [DOI] [PubMed] [Google Scholar]
- 23.Ing CH, DiMaggio CJ, Whitehouse AJ, Hegarty MK, Sun M, von Ungern-Sternberg BS, Davidson AJ, Wall MM, Li G, Sun LS. Neurodevelopmental outcomes after initial childhood anesthetic exposure between ages 3 and 10 years. J Neurosurg Anesthesiol. 2014;26:377–86. doi: 10.1097/ANA.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 24.U.S. Food & Drug Administration. FDA Drug Safety Communication: FDA review results in new warnings about using general anesthetics and sedation drugs in young children and pregnant women. 2016 http://www.fda.gov/Drugs/DrugSafety/ucm532356.htm?source=govdelivery&utm_medium=email&utm_source=govdelivery.
- 25.Wiswell TE, Tencer HL, Welch CA, Chamberlain JL. Circumcision in children beyond the neonatal period. Pediatrics. 1993;92:791–3. [PubMed] [Google Scholar]
- 26.Agency for Healthcare Research and Quality (AHRQ) Healthcare Cost and Utilization Project. Surgery Flag Software. 2014 https://www.hcup-us.ahrq.gov/toolssoftware/surgflags/surgeryflags.jsp.
- 27.Matone M, Localio R, Huang YS, dosReis S, Feudtner C, Rubin D. The relationship between mental health diagnosis and treatment with second-generation antipsychotics over time: a national study of U.S. Medicaid-enrolled children. Health Serv Res. 2012;47:1836–60. doi: 10.1111/j.1475-6773.2012.01461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fortier MA, Del Rosario AM, Rosenbaum A, Kain ZN. Beyond pain: predictors of postoperative maladaptive behavior change in children. Paediatr Anaesth. 2010;20:445–53. doi: 10.1111/j.1460-9592.2010.03281.x. [DOI] [PubMed] [Google Scholar]
- 29.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–61. doi: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harder VS, Stuart EA, Anthony JC. Propensity Score Techniques and the Assessment of Measured Covariate Balance to Test Causal Associations in Psychological Research. Psychol Methods. 2010;15:234–49. doi: 10.1037/a0019623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin DY, Wei LJ. The Robust Inference for the Cox Proportional Hazards Model. J Am Stat Assoc. 1989;84:1074–8. [Google Scholar]
- 33.Weintraub WS, Grau-Sepulveda MV, Weiss JM, O’Brien SM, Peterson ED, Kolm P, Zhang Z, Klein LW, Shaw RE, McKay C, Ritzenthaler LL, Popma JJ, Messenger JC, Shahian DM, Grover FL, Mayer JE, Shewan CM, Garratt KN, Moussa ID, Dangas GD, Edwards FH. Comparative effectiveness of revascularization strategies. N Engl J Med. 2012;366:1467–76. doi: 10.1056/NEJMoa1110717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin DY, Psaty BM, Kronmal RA. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics. 1998;54:948–63. [PubMed] [Google Scholar]
- 35.Ing CH, DiMaggio CJ, Malacova E, Whitehouse AJ, Hegarty MK, Feng T, Brady JE, von Ungern-Sternberg BS, Davidson AJ, Wall MM, Wood AJ, Li G, Sun LS. Comparative analysis of outcome measures used in examining neurodevelopmental effects of early childhood anesthesia exposure. Anesthesiology. 2014;120:1319–32. doi: 10.1097/ALN.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 36.Davidson AJ, Disma N, de Graaff JC, Withington DE, Dorris L, Bell G, Stargatt R, Bellinger DC, Schuster T, Arnup SJ, Hardy P, Hunt RW, Takagi MJ, Giribaldi G, Hartmann PL, Salvo I, Morton NS, von Ungern Sternberg BS, Locatelli BG, Wilton N, Lynn A, Thomas JJ, Polaner D, Bagshaw O, Szmuk P, Absalom AR, Frawley G, Berde C, Ormond GD, Marmor J, McCann ME consortium GAS. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet. 2016;387:239–50. doi: 10.1016/S0140-6736(15)00608-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun LS, Li G, Miller TL, Salorio C, Byrne MW, Bellinger DC, Ing C, Park R, Radcliffe J, Hays SR, DiMaggio CJ, Cooper TJ, Rauh V, Maxwell LG, Youn A, McGowan FX. Association Between a Single General Anesthesia Exposure Before Age 36 Months and Neurocognitive Outcomes in Later Childhood. JAMA. 2016;315:2312–20. doi: 10.1001/jama.2016.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guilarte TR, Toscano CD, McGlothan JL, Weaver SA. Environmental enrichment reverses cognitive and molecular deficits induced by developmental lead exposure. Ann Neurol. 2003;53:50–6. doi: 10.1002/ana.10399. [DOI] [PubMed] [Google Scholar]
- 39.Shih J, May LD, Gonzalez HE, Lee EW, Alvi RS, Sall JW, Rau V, Bickler PE, Lalchandani GR, Yusupova M, Woodward E, Kang H, Wilk AJ, Carlston CM, Mendoza MV, Guggenheim JN, Schaefer M, Rowe AM, Stratmann G. Delayed environmental enrichment reverses sevoflurane-induced memory impairment in rats. Anesthesiology. 2012;116:586–602. doi: 10.1097/ALN.0b013e318247564d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.State Heath Facts. The Henry J. Kaiser Family Foundation; 2012. http://www.statehealthfacts.org/medicaid.jsp. [Google Scholar]
- 41.Bellinger DC. Lead neurotoxicity and socioeconomic status: conceptual and analytical issues. Neurotoxicology. 2008;29:828–32. doi: 10.1016/j.neuro.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodgers A, MacMahon S. Systematic underestimation of treatment effects as a result of diagnostic test inaccuracy: implications for the interpretation and design of thromboprophylaxis trials. Thromb Haemost. 1995;73:167–71. [PubMed] [Google Scholar]
- 43.Deng M, Hofacer RD, Jiang C, Joseph B, Hughes EA, Jia B, Danzer SC, Loepke AW. Brain regional vulnerability to anaesthesia-induced neuroapoptosis shifts with age at exposure and extends into adulthood for some regions. Br J Anaesth. 2014 doi: 10.1093/bja/aet469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.