Abstract
Persons who inject drugs, most of whom are opioid dependent, comprise the majority of the HCV infected in the United States. As the national opioid epidemic unfolds, increasing numbers of people are entering the medical system to access treatment for opioid use disorder, specifically with buprenorphine. Yet little is known about HCV care in patients accessing buprenorphine-based opioid treatment. We sought to determine the HCV prevalence, cascade of care, and the association between patient characteristics and completion of HCV cascade of care milestones for patients initiating buprenorphine treatment. We reviewed electronic health records of all patients who initiated buprenorphine treatment at a primary-care clinic in the Bronx, NY between January 2009-January 2014. Of the 390 patients who initiated buprenorphine treatment, 123 were confirmed to have chronic HCV infection. The only patient characteristic associated with achieving HCV care milestones was retention in opioid treatment. Patients retained (vs. not retained) in buprenorphine treatment were more likely to be referred for HCV specialty care (63.1% vs. 34.0%, p<0.01), achieve an HCV-specific evaluation (40.8% vs. 21.3%, p<0.05), be offered HCV treatment (22.4% vs. 8.5%, p<0.05), and initiate HCV treatment (9.2% vs. 6.4%, p=0.6). Given the current opioid epidemic in the US and the growing number of people receiving buprenorphine treatment, there is an unprecedented opportunity to access and treat persons with HCV, reducing HCV transmission, morbidity and mortality. Retention in opioid treatment may improve linkage and retention in HCV care; innovative models of care that integrate opioid drug treatment with HCV treatment are essential.
Keywords: Buprenorphine, Cascade of Care, Hepatitis C, Medication Assisted Treatment, Opioid Use Disorder
1. INTRODUCTION
An estimated 4 million Americans are chronically infected with hepatitis C virus (HCV) (Armstrong et al., 2006; Kanwal et al., 2011) and the true prevalence is likely to be even higher (Chak, Talal, Sherman, Schiff, & Saab, 2011). In the U.S., HCV is the leading cause of end-stage liver disease and hepatocellular carcinoma, and the most common indication for liver transplantation(Kanwal et al., 2011; Verna & Brown, 2006). Without imminent action, mortality from HCV-related disease is projected to triple over the next decade(Davis, Alter, El-Serag, Poynard, & Jennings, 2010; Rein et al., 2011) and HCV-related deaths have already surpassed deaths related to HIV(Ly et al., 2012).
Throughout the US, the overwhelming majority of people infected with HCV are persons who use drugs (PWUD)(Alter, 1999; Klevens, Hu, Jiles, & Holmberg, 2012), and 70–90% of PWUDs are HCV-infected(Hagan, Pouget, Des Jarlais, & Lelutiu-Weinberger, 2008; Hagan et al., 2010; Page et al., 2009). Heroin use is currently on the rise in the United States. From 2002 through 2013, past-year heroin use increased by 63% overall and by 103% in persons aged 18–25(Jones, Logan, Gladden, & Bohm, 2015). In communities affected most by this heroin epidemic, there has been a simultaneous increase in HCV diagnoses(Zibbell et al., 2015). Unfortunately, PWUDs have had significant barriers receiving HCV care. Less than 33% of PWUDs appear for HCV evaluation at specialty clinics, and less than 10% of those who are evaluated ever initiate HCV antiviral therapy(Grebely et al., 2007; Hellard, Sacks-Davis, & Gold, 2009; Mehta et al., 2008; Treloar, Hull, Dore, & Grebely, 2012). Despite new DAA (direct acting antiviral) medications, these drop-outs along the HCV cascade of care reduce the effectiveness of HCV therapies by as much as 75% and prevent patients from ever realizing the benefits of these highly efficacious medications(Linas et al., 2014).
Medication assisted treatment (methadone and buprenorphine) is an effective therapy for heroin (and other opioid) dependence. Though methadone has been most widely used, buprenorphine is becoming increasingly available and in 2012, 9.3 million buprenorphine prescriptions were filled ("Drug Enforcement Administration (DEA). Buprenorphine .", 2013). One potential benefit of buprenorphine is the ability for physicians, including those with expertise in treating HCV infection, to prescribe this medication in an office-based setting. Given the current heroin epidemic in the US, the Centers for Disease Control and Prevention (CDC) and the federal government have encouraged local governments, medical, and insurance institutions to expand access to treatment for opioid dependence, specifically with buprenorphine(Jones et al., 2015; "White House Office of the Press Secretary," 2016).
As more persons receive buprenorphine treatment, it is important to understand the relationship between HCV and buprenorphine based opioid treatment. Currently, there is a paucity of literature on HCV care among individuals receiving buprenorphine. Therefore, we examined HCV care and treatment outcomes among patients enrolled in primary care-based buprenorphine treatment in the Bronx, NY. We explored patient characteristics associated with successful completion of HCV care milestones along the HCV cascade of care, and specifically investigated if retention in buprenorphine treatment improved HCV care in this population.
2. METHODS
We conducted a retrospective cohort study, reviewing electronic health records (EHR) of all patients with opioid use disorders who initiated buprenorphine treatment at a primary-care clinic in the Bronx, NY between January 2009-January 2014. This study was determined to be IRB exempt by Albert Einstein College of Medicine Institutional Review Board.
2.1 Setting
The buprenorphine treatment program is co-located within a federally qualified health center (FQHC) that is affiliated with an academic medical center in The Bronx, NY(Cunningham et al., 2008). The health center is located in one of the poorest districts in the country; over one-third of people live below the poverty line (15). Patients are referred to the treatment program from various sources: healthcare providers within and outside the institution, community-based organizations, or self-referral. A clinical pharmacist completes an initial intake assessment and then assigns the patient to one of thirteen general internists (primary care physicians) to provide buprenorphine treatment. Approximately 60% of patients receiving buprenorphine treatment in this clinic have self-reported injection drug use(Cunningham et al., 2008; Whitley et al., 2010). Study patients, as with all patients attending this FQHC (including those not on buprenorphine), would be referred for HCV specialty care to an affiliated hepatology clinic. This hepatology clinic is located approximately 5 miles from the FQHC in which the buprenorphine treatment program exists and is accessible by public transportation. The hepatology clinic shares an EHR with the FQHC, and therefore all notes and labs were available for data extraction. The buprenorphine treatment program has no standard protocol for HCV assessment, referral, or treatment. The overall prevalence of HCV infection in this clinic is estimated to be 7.7%(Southern et al., 2011).
2.2 Participants
All opioid dependent adult patients who received at least one buprenorphine prescription between January 2009-January 2014, identified from the buprenorphine treatment program registry, were included in the analysis.
2.3 Data Collection
We reviewed the EHR of all patients who received buprenorphine treatment to determine if HCV antibody tests were performed (e.g. screening for HCV infection). Among patients who had positive antibodies for HCV, we then reviewed the EHR to determine if confirmatory HCV viral load testing was performed. Among patients with chronic HCV infection (positive HCV viral loads), using a standardized data collection tool, we then extracted detailed clinical and sociodemographic data, including HCV cascade of care milestones. EHR data were extracted from January 2009-July 2014, which allowed all patients at least six months to complete HCV cascade of care milestones.
2.4 HCV Cascade of Care Milestones
We defined the steps of the HCV care cascade as: 1) positive HCV antibody; 2) confirmation of chronic HCV infection (HCV RNA > 75 IU/mL); 3) referral for specialty HCV care (documentation of referral by the primary care provider or presence of a referral form); 4) HCV-specific evaluation (an HCV specialist visit note); 5) offering HCV treatment (documentation that HCV treatment was offered in the HCV provider note; 6) initiation of HCV treatment (at least one prescription for HCV medications); 7) achievement of cure (undetectable HCV viral load at least 12 weeks after treatment completion). The plasma HCV RNA was measured with the COBAS Taqman HCV/HPS v2.0 assay (Roche, Molecular Diagnostics, Pleasanton, California; lower limit of quantification 25 IU/mL; limit of detection 15 IU/mL). If there was evidence that a more distal milestone was achieved, then it was presumed that the more proximal outcomes were also achieved. We only collected data for those HCV cascade of care milestones that occurred after the initial visit for buprenorphine treatment.
2.5 Patient Key Variables
The exposure variable of interest was buprenorphine treatment retention, defined as having an active buprenorphine prescription for ≥ 6 months after the initial buprenorphine visit. This was measured using the date of the first and last electronic prescription accounting for multiple prescriptions and refills. We also collected variables that included patient demographic information (age, race/ethnicity, and gender), and clinical characteristics (HIV status, significant fibrosis/cirrhosis (yes/no), psychiatric disorder, substance use). Significant fibrosis/cirrhosis was defined as an AST to Platelet Ratio Index (APRI) score of ≥ 1.5 using the patients’ first AST and platelet measurements upon initiating buprenorphine treatment. Psychiatric disorder was defined as a disorder of depression, anxiety, bipolar, or schizophrenia in the patients problem list. Substance use was defined as a positive urine toxicology test for cocaine or canniboids at baseline. For this population, substances such as phencyclidine (PCP) and amphetamine in the Bronx are extremely rare, and therefore we have not included these in our consideration of substance use here or in prior publications(Cunningham et al., 2008; Whitley et al., 2010).” Alcohol use was defined as any patient indication of alcohol intake in the past 30 days, which was part of the standardized buprenorphine intake form.
2.6 Data Analysis
HCV antibody testing (screening), HCV viral load testing (determination of chronic HCV), and patient characteristics were reported in frequencies and percentages. To determine HCV cascade of care milestones among patients with chronic HCV, we first determined the frequency of each of the milestones of the HCV cascade (described above). Then, to examine associations between patient characteristics and HCV outcomes we conducted chi-square or Fisher exact tests for categorical variables and t-tests for continuous variables for each HCV milestones.
3. RESULTS
3.1 HCV screening
Of the 390 patients who initiated buprenorphine treatment between January 2009 and January 2014, 363 (93.1%) were screened for HCV. Of these 363 people screened, 192 (52.9%) were HCV antibody positive (Figure 1). Of these 192 patients with positive antibodies to HCV, 184 (95.8%) had confirmatory HCV viral load testing. Overall, 123 patients were confirmed to have chronic HCV infection.
Figure 1.
HCV Screening and HCV Cascade of Care Milestones for Patients Initiating Buprenorphine Treatment
Abbreviations: VL, viral load; HCV, hepatitis c virus; Tx, treatment; SVR, sustained virologic response
3.2 Characteristics of patients with chronic HCV infection
Among the 123 patients with chronic HCV infection, the median age was 47 years (IQR 38,54) and the majority were men (80.5%), Latino (64.2%) and had comorbid psychiatric disorders (63.4%) (Table 1). Many had active polysubstance use [cocaine (21.7%), marijuana (25.5%), and alcohol (49.6%)]. At 6 months, 61.8% were retained in buprenorphine treatment.
Table 1.
Characteristics of Patients with Chronic HCV
| Patient Characteristics (n=123) |
N (%) |
|---|---|
| Male | 99 (80.5) |
|
| |
| Age (median years, IQR) | 47 (38, 54) |
|
| |
| Race/Ethnicity (n=103) | |
| Hispanic/Latino | 79 (64.2) |
| Non Hispanic Black | 17 (13.8) |
| Non Hispanic White | 7 (5.7) |
| Unknown | 20 (16.3) |
|
| |
| Cirrhosis | |
| Yes | 19 (15.4) |
| No | 97 (78.9) |
| Unknown | 7 (5.7) |
|
| |
| Psychiatric Disorder | 78 (63.4) |
|
| |
| Substance Use* | |
| Baseline Cocaine Use | 23 (21.7) |
| Baseline Marijuana Use | 27 (25.5) |
|
| |
| Baseline Alcohol Use | |
| Yes | 61 (49.6) |
| No | 57 (46.3) |
| Unknown | 5 (4.1) |
|
| |
| HIV positivity | 25 (20.3) |
|
| |
| Retained in Buprenorphine Treatment ≥6 months | 76 (61.8) |
Baseline Urine Toxicologies were only available for 106 participants
3.3 HCV cascade of care milestones among patients with chronic HCV infection
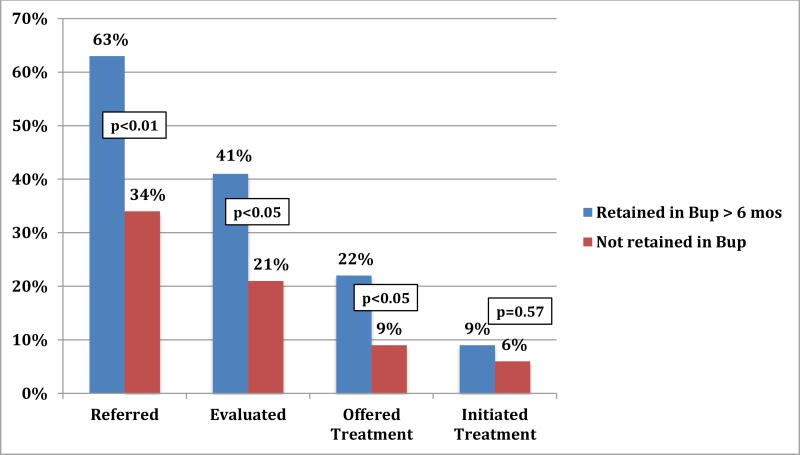
Among patients with chronic HCV infection, 52.0% were referred for HCV specialty care, 33.3% had an HCV-specific evaluation, 17.1% were offered HCV treatment, and 8.1% initiated HCV treatment (Figure 1). Of the 10 patients who initiated HCV treatment, 7 achieved cure. The only patient characteristic associated with completion of HCV care milestones was retention in buprenorphine treatment. Patients retained (vs. not retained) in buprenorphine treatment were more likely to be referred for HCV specialty care (63.1% vs. 34.0%, p<0.01), achieve an HCV-specific evaluation (40.8% vs. 21.3%, p<0.05), and be offered HCV treatment (22.4% vs. 8.5%, p<0.05)(Figure 2). Persons retained were also more likely to initiate HCV treatment (9.2% vs. 6.4%) but this was not significant (p=0.6).
Figure 2.
Retention in Buprenorphine Treatment is Associated with Improved HCV Outcomes
Abbreviations: Bup, buprenorphine
4. DISCUSSION
Among patients receiving buprenorphine treatment at a Bronx primary care clinic, HCV screening and confirmatory viral load testing were nearly universal, and HCV infection was highly prevalent. Nonetheless, there was a steep drop-off in the HCV care cascade for patients completing HCV-specific referrals, evaluations, and treatment. Importantly, the only patient characteristic associated with completing HCV cascade of care milestones was retention in buprenorphine treatment, highlighting the importance of integrating treatment for opioid use disorders with treatment for HCV infection.
Heroin use and HCV infection are overlapping epidemics in the United States. In this study of patients receiving buprenorphine treatment for opioid use disorders, 53% were infected with HCV, and 34% were confirmed to have chronic HCV infection. Our findings are similar to others’ findings in which HCV infection among patients receiving opioid agonist treatment has ranged from 30–90%(Hallinan, Byrne, Amin, & Dore, 2005; Murphy, Dweik, McPherson, & Roll, 2015; Taylor et al., 2012). Currently, heroin use is on the rise in the US, with unprecedented increases in almost every demographic group over the last decade(Jones et al., 2015). Furthermore, the incidence of acute HCV infection has simultaneously increased 150% from 2010 to 2013, particularly in communities of young PWUD("Centers for Disease Control and Prevention," 2103; Zibbell et al., 2015). As the heroin epidemic continues, public funds have increasingly been allocated to expand access to treatment for opioid use disorder(Jones et al., 2015; "White House Office of the Press Secretary," 2016). With more PWUDs receiving buprenorphine treatment, we will inevitably identify an increasing number of HCV infected patients that are accessing the health care system.
In our study, near universal HCV screening and HCV viral load testing demonstrates that identification of HCV was part of the initial evaluation for patients receiving buprenorphine treatment. In spite of screening, less than half were referred for HCV specialty care, only one third completed an HCV specific evaluation, and very few initiated HCV treatment. PWUDs have often been excluded from HCV care due to perceptions of poor candidacy, concerns about treatment adherence, or unsubstantiated beliefs in high rates of re-infection (Astone, Strauss, Hagan, & Des Jarlais, 2004; Davis & Rodrigue, 2001; Edlin et al., 2001; Treloar, Newland, Rance, & Hopwood, 2010); however, due to new DAA medications with high cure rates, low side effects, and short durations of therapy PWUDs are now more likely than ever to achieve HCV cure(Dore G, 2015; Lalezari et al., 2015; Norton et al., 2016). Despite this, linking and retaining PWUDs in HCV care so that they may benefit from these life-saving HCV medications will continue to be a challenge. Despite the availability of DAAs, only 39% of PWUD enrolled in an NYC linkage to care program in 2014 were ever referred to care, and much fewer initiated treatment("Hepatitis B and C Annual Report," 2016). The program sighted barriers such as competing patient psychosocial priorities as a limitation to the programs achievement of linkage to care goals. Innovative linkage-to-care strategies are needed for this population. Findings from our study, in which retention in buprenorphine treatment was the only patient characteristic associated with improved HCV cascade of care milestones, demonstrates the importance of concurrently treating HCV and substance use disorder. In the era of DAAs, linking persons on opioid agonist treatment to HCV care and treatment may prove to be even more advantageous given the ease in new treatment regimens and the lack of side effects leading to greater adherence and success in cure. Strategies may include early referral to HCV care once a patient initiates opioid drug treatment or co-locating treatment for substance use disorders with care for HCV infection. The implications of our findings are consistent with other studies conducted in methadone programs, in which HCV care was integrated into those settings and, even in the era of interferon, PWUDs were able to be successfully linked to HCV care, initiate treatment, and even achieve HCV cure (Bonkovsky et al., 2008; Litwin et al., 2009; Litwin et al., 2015; Stein et al., 2012). Additionally, co-location of HIV and buprenorphine treatment has also been associated with improved outcomes for both HIV and opioid use (Altice et al., 2011; Fiellin et al., 2011). Given the ease of the new, all oral HCV regimens, we have an unprecedented opportunity to treat PWUDs within medical settings that they are already accessing, that are culturally appropriate, and that also provide simultaneous treatment for opioid use disorder. The key to successful HCV treatment in PWUDs appears to be a multidisciplinary approach that includes treatment of substance use (Bruggmann & Litwin, 2013; Robaeys et al., 2013); and co-location of care for HCV and opioid dependence, such as buprenorphine treatment, may be a powerful intervention to halt the HCV epidemic.
There are several limitations to our study. Due to the retrospective nature of this study, our data are limited to data available in the EHR and the buprenorphine intake form; therefore, we may not have captured all potential confounders that are specific to HCV. Also, we did not collect reasons for lack of HCV care initiation, which may have been helpful to further understand the barriers to care in this population. Finally, this study was conducted in the interferon-era of HCV treatment; therefore, HCV cascade of care milestones for patients receiving buprenorphine may be better now. That said, barriers to linkage to HCV care have persisted despite DAAs, and interventions to retain PWUDs in medical care, such as early referral after drug treatment initiation or co-located treatment for opioid use disorders and HCV should be implemented.
With the advent of new, oral curative therapy, HCV eradication may be possible; however, this can only be achieved by focusing on HCV prevention and treatment among PWUDs, key drivers of the HCV epidemic. Given the current heroin epidemic in the US and the growing number of people receiving buprenorphine treatment, there is an unprecedented opportunity to access and treat persons with HCV, with substantial implications for reducing HCV transmission, morbidity and mortality. Successful HCV treatment and cure in this crucial population depends on innovative models of care that integrate opioid drug treatment with HCV treatment.
HIGHLIGHTS.
HCV prevalence was high (53%) among patients initiating buprenorphine treatment
Overall, completion of HCV cascade of care milestones was poor
Retention in buprenorphine treatment was the only patient characteristic associated with improved HCV care outcomes
Models of care that integrate opioid drug treatment with HCV treatment are essential
Acknowledgments
Funding Sources: This study was funded in part by NIH, National Institute on Drug Abuse K23DA039060, K24DA036955, R25DA02302, and R01 DA03408.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alter MJ. Hepatitis C virus infection in the United States. J Hepatol. 1999;31(Suppl 1):88–91. doi: 10.1016/s0168-8278(99)80381-x. [DOI] [PubMed] [Google Scholar]
- Altice FL, Bruce RD, Lucas GM, Lum PJ, Korthuis PT, Flanigan TP. HIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: results from a multisite study. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):S22–32. doi: 10.1097/QAI.0b013e318209751e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- Astone JM, Strauss SM, Hagan H, Des Jarlais DC. Outpatient drug treatment program directors' hepatitis C-related beliefs and their relationship to the provision of HCV services. Am J Drug Alcohol Abuse. 2004;30(4):783–797. doi: 10.1081/ada-200037544. [DOI] [PubMed] [Google Scholar]
- Bonkovsky HL, Tice AD, Yapp RG, Bodenheimer HC, Jr, Monto A, Rossi SJ, Sulkowski MS. Efficacy and safety of peginterferon alfa-2a/ribavirin in methadone maintenance patients: randomized comparison of direct observed therapy and self-administration. Am J Gastroenterol. 2008;103(11):2757–2765. doi: 10.1111/j.1572-0241.2008.02065.x. [DOI] [PubMed] [Google Scholar]
- Bruggmann P, Litwin AH. Models of care for the management of hepatitis C virus among people who inject drugs: one size does not fit all. Clin Infect Dis. 2013;57(Suppl 2):S56–61. doi: 10.1093/cid/cit271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. [Accessed 24.01.2017];Surveillance for Viral Hepatitis 2013. 2103 http://www.cdc.gov/hepatitis/statistics/2013surveillance/pdfs/2013hepsurveillancerpt.pdf.
- Chak E, Talal AH, Sherman KE, Schiff ER, Saab S. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31(8):1090–1101. doi: 10.1111/j.1478-3231.2011.02494.x. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Giovanniello A, Sacajiu G, Whitley S, Mund P, Beil R, Sohler N. Buprenorphine treatment in an urban community health center: what to expect. Fam Med. 2008;40(7):500–506. [PMC free article] [PubMed] [Google Scholar]
- Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138(2):513–521. 521, e511–516. doi: 10.1053/j.gastro.2009.09.067. S0016-5085(09)01885-X [pii] [DOI] [PubMed] [Google Scholar]
- Davis GL, Rodrigue JR. Treatment of chronic hepatitis C in active drug users. N Engl J Med. 2001;345(3):215–217. doi: 10.1056/NEJM200107193450312. [DOI] [PubMed] [Google Scholar]
- Dore G AF, Litwin AH, et al. C-EDGE CO-STAR: Efficacy of Grazoprevir and Elbasvir in Persons who Inject Drugs (PWID) Receiving Opioid Agonist Therapy; Paper presented at the AASLD Liver Meeting; San Francisco. 2015. [Google Scholar]
- Drug Enforcement Administration (DEA). Buprenorphine. Drug Enforcement Administration Office of Diversion Control. 2013. [Google Scholar]
- Edlin BR, Seal KH, Lorvick J, Kral AH, Ciccarone DH, Moore LD, Lo B. Is it justifiable to withhold treatment for hepatitis C from illicit-drug users? N Engl J Med. 2001;345(3):211–215. doi: 10.1056/NEJM200107193450311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiellin DA, Weiss L, Botsko M, Egan JE, Altice FL, Bazerman LB. Drug treatment outcomes among HIV-infected opioid-dependent patients receiving buprenorphine/naloxone. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):S33–38. doi: 10.1097/QAI.0b013e3182097537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebely J, Genoway K, Khara M, Duncan F, Viljoen M, Elliott D, Conway B. Treatment uptake and outcomes among current and former injection drug users receiving directly observed therapy within a multidisciplinary group model for the treatment of hepatitis C virus infection. Int J Drug Policy. 2007;18(5):437–443. doi: 10.1016/j.drugpo.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Hagan H, Pouget ER, Des Jarlais DC, Lelutiu-Weinberger C. Meta-regression of hepatitis C virus infection in relation to time since onset of illicit drug injection: the influence of time and place. Am J Epidemiol. 2008;168(10):1099–1109. doi: 10.1093/aje/kwn237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan H, Pouget ER, Williams IT, Garfein RL, Strathdee SA, Hudson SM, Ouellet LJ. Attribution of hepatitis C virus seroconversion risk in young injection drug users in 5 US cities. J Infect Dis. 2010;201(3):378–385. doi: 10.1086/649783. [DOI] [PubMed] [Google Scholar]
- Hallinan R, Byrne A, Amin J, Dore GJ. Hepatitis C virus prevalence and outcomes among injecting drug users on opioid replacement therapy. J Gastroenterol Hepatol. 2005;20(7):1082–1086. doi: 10.1111/j.1440-1746.2005.03882.x. [DOI] [PubMed] [Google Scholar]
- Hellard M, Sacks-Davis R, Gold J. Hepatitis C treatment for injection drug users: a review of the available evidence. Clin Infect Dis. 2009;49(4):561–573. doi: 10.1086/600304. [DOI] [PubMed] [Google Scholar]
- Hepatitis B and C Annual Report. New York City Department of Health and Mental Hygiene. 2016. [Google Scholar]
- Jones CM, Logan J, Gladden RM, Bohm MK. Vital Signs: Demographic and Substance Use Trends Among Heroin Users - United States, 2002–2013. MMWR Morb Mortal Wkly Rep. 2015;64(26):719–725. [PMC free article] [PubMed] [Google Scholar]
- Kanwal F, Hoang T, Kramer JR, Asch SM, Goetz MB, Zeringue A, El-Serag HB. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology. 2011;140(4):1182–1188.e1181. doi: 10.1053/j.gastro.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevens RM, Hu DJ, Jiles R, Holmberg SD. Evolving epidemiology of hepatitis C virus in the United States. Clin Infect Dis. 2012;55(Suppl 1):S3–9. doi: 10.1093/cid/cis393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalezari J, Sullivan JG, Varunok P, Galen E, Kowdley KV, Rustgi V, Cohen DE. Ombitasvir/paritaprevir/r and dasabuvir plus ribavirin in HCV genotype 1-infected patients on methadone or buprenorphine. J Hepatol. 2015;63(2):364–369. doi: 10.1016/j.jhep.2015.03.029. [DOI] [PubMed] [Google Scholar]
- Linas BP, Barter DM, Leff JA, Assoumou SA, Salomon JA, Weinstein MC, Schackman BR. The hepatitis C cascade of care: identifying priorities to improve clinical outcomes. PLoS One. 2014;9(5):e97317. doi: 10.1371/journal.pone.0097317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin AH, Harris KA, Jr, Nahvi S, Zamor PJ, Soloway IJ, Tenore PL, Arnsten JH. Successful treatment of chronic hepatitis C with pegylated interferon in combination with ribavirin in a methadone maintenance treatment program. J Subst Abuse Treat. 2009;37(1):32–40. doi: 10.1016/j.jsat.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin AH, Soloway IJ, Cockerham-Colas L, Reynoso S, Heo M, Tenore C, Roose RJ. Successful treatment of chronic hepatitis C with triple therapy in an opioid agonist treatment program. Int J Drug Policy. 2015;26(10):1014–1019. doi: 10.1016/j.drugpo.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156(4):271–278. doi: 10.1059/0003-4819-156-4-201202210-00004. 156/4/271 [pii] [DOI] [PubMed] [Google Scholar]
- Mehta SH, Genberg BL, Astemborski J, Kavasery R, Kirk GD, Vlahov D, Thomas DL. Limited uptake of hepatitis C treatment among injection drug users. J Community Health. 2008;33(3):126–133. doi: 10.1007/s10900-007-9083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SM, Dweik D, McPherson S, Roll JM. Association between hepatitis C virus and opioid use while in buprenorphine treatment: preliminary findings. Am J Drug Alcohol Abuse. 2015;41(1):88–92. doi: 10.3109/00952990.2014.983274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton BL, Fleming J, Steinman M, Yu K, Deluca JP, Cunningham CO, Litwin AH. High HCV Cure Rates for Drug Users Treated With DAAs at an Urban Primary Care Clinic; Paper presented at the CROI; Boston, MA. 2016. [Google Scholar]
- Page K, Hahn JA, Evans J, Shiboski S, Lum P, Delwart E, Busch MP. Acute hepatitis C virus infection in young adult injection drug users: a prospective study of incident infection, resolution, and reinfection. J Infect Dis. 2009;200(8):1216–1226. doi: 10.1086/605947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein DB, Wittenborn JS, Weinbaum CM, Sabin M, Smith BD, Lesesne SB. Forecasting the morbidity and mortality associated with prevalent cases of pre-cirrhotic chronic hepatitis C in the United States. Dig Liver Dis. 2011;43(1):66–72. doi: 10.1016/j.dld.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Robaeys G, Grebely J, Mauss S, Bruggmann P, Moussalli J, De Gottardi A, Dore GJ. Recommendations for the management of hepatitis C virus infection among people who inject drugs. Clin Infect Dis. 2013;57(Suppl 2):S129–137. doi: 10.1093/cid/cit302. [DOI] [PubMed] [Google Scholar]
- Southern WN, Drainoni ML, Smith BD, Christiansen CL, McKee D, Gifford AL, Litwin AH. Hepatitis C testing practices and prevalence in a high-risk urban ambulatory care setting. J Viral Hepat. 2011;18(7):474–481. doi: 10.1111/j.1365-2893.2010.01327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MR, Soloway IJ, Jefferson KS, Roose RJ, Arnsten JH, Litwin AH. Concurrent group treatment for hepatitis C: implementation and outcomes in a methadone maintenance treatment program. J Subst Abuse Treat. 2012;43(4):424–432. doi: 10.1016/j.jsat.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor LE, Maynard MA, Friedmann PD, Macleod CJ, Rich JD, Flanigan TP, Sylvestre DL. Buprenorphine for human immunodeficiency virus/hepatitis C virus-coinfected patients: does it serve as a bridge to hepatitis C virus therapy? J Addict Med. 2012;6(3):179–185. doi: 10.1097/ADM.0b013e318257377f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treloar C, Hull P, Dore GJ, Grebely J. Knowledge and barriers associated with assessment and treatment for hepatitis C virus infection among people who inject drugs. Drug Alcohol Rev. 2012;31(7):918–924. doi: 10.1111/j.1465-3362.2012.00468.x. [DOI] [PubMed] [Google Scholar]
- Treloar C, Newland J, Rance J, Hopwood M. Uptake and delivery of hepatitis C treatment in opiate substitution treatment: perceptions of clients and health professionals. J Viral Hepat. 2010;17(12):839–844. doi: 10.1111/j.1365-2893.2009.01250.x. [DOI] [PubMed] [Google Scholar]
- Verna EC, Brown RS., Jr Hepatitis C virus and liver transplantation. Clin Liver Dis. 2006;10(4):919–940. doi: 10.1016/j.cld.2006.08.012. [DOI] [PubMed] [Google Scholar]
- White House Office of the Press Secretary. [Accessed 13.10.2016];President Obama Proposes $1.1 Billion in New Funding to Address the Prescription Opioid Abuse and Heroin Use Epidemic. 2016 doi: 10.3109/15360288.2016.1173760. https:// http://www.whitehouse.gov/the-press-office/2016/02/02/president-obama-proposes-11-billion-new-funding-address-prescription. [DOI] [PubMed]
- Whitley SD, Sohler NL, Kunins HV, Giovanniello A, Li X, Sacajiu G, Cunningham CO. Factors associated with complicated buprenorphine inductions. J Subst Abuse Treat. 2010;39(1):51–57. doi: 10.1016/j.jsat.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zibbell JE, Iqbal K, Patel RC, Suryaprasad A, Sanders KJ, Moore-Moravian L, Prevention Increases in hepatitis C virus infection related to injection drug use among persons aged </=30 years - Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. MMWR Morb Mortal Wkly Rep. 2015;64(17):453–458. [PMC free article] [PubMed] [Google Scholar]