Significance
The mechanisms that underlie social touch analgesia are largely unknown. Here, we apply a hyperscanning approach with real-life interaction of dyads to examine the association between brain-to-brain coupling and pain relief. Our findings indicate that hand-holding during pain increases the brain-to-brain coupling network that correlates with the magnitude of the analgesia and the observer’s empathic accuracy. These findings make a unique contribution to our understanding of physiological mechanisms of touch-related analgesia.
Keywords: hyperscanning, social touch, empathy, pain, EEG
Abstract
The mechanisms underlying analgesia related to social touch are not clear. While recent research highlights the role of the empathy of the observer to pain relief in the target, the contribution of social interaction to analgesia is unknown. The current study examines brain-to-brain coupling during pain with interpersonal touch and tests the involvement of interbrain synchrony in pain alleviation. Romantic partners were assigned the roles of target (pain receiver) and observer (pain observer) under pain–no-pain and touch–no-touch conditions concurrent with EEG recording. Brain-to-brain coupling in alpha–mu band (8–12 Hz) was estimated by a three-step multilevel analysis procedure based on running window circular correlation coefficient and post hoc power of the findings was calculated using simulations. Our findings indicate that hand-holding during pain administration increases brain-to-brain coupling in a network that mainly involves the central regions of the pain target and the right hemisphere of the pain observer. Moreover, brain-to-brain coupling in this network was found to correlate with analgesia magnitude and observer’s empathic accuracy. These findings indicate that brain-to-brain coupling may be involved in touch-related analgesia.
Until recently, research on the sense of touch focused mainly on discriminative input to the brain and investigated sensory and perceptual effects caused by stimulation of mechanoreceptors located in the skin and joints. However, increasing evidence shows that touch has a critical social value and plays an important role in interpersonal communication (1–4), affecting our perception (5–9) and well-being (10, 11). For example, research has shown that social touch can affect emotions, reduce distress and pain in humans (12–19), and have an analgesic effect through social grooming in the animal kingdom (20, 21). Specifically for human beings, researchers have reported that skin-to-skin touch may have an analgesic effect on human babies undergoing minor medical procedures (16) and a therapeutic effect in reducing pain in cancer (14, 22) and chronic pain patients (11).
The mechanisms that underlie social touch analgesia are not totally understood (23, 24). While earlier research showed that tactile stimulation can interrupt pain input at the spinal cord (25–27), recent studies, using stimulation of Aβ afferents, have demonstrated that cortical and subcortical neural circuitry also modulate the analgesic effect of tactile stimulation (17, 28, 29). Some of these brain areas are also involved in the analgesic effect of touch applied to a distant area (28). Moreover, affective touch seems to affect the conscious perception of pain, thus expressing socio-cognitive factors (30). In line with this finding, research has shown that holding a partner’s hand decreases anxiety and blood pressure reactivity to stress, thus implying the involvement of emotional factors during tactile analgesia (12, 31, 32).
Considering that emotional factors may affect touch-induced analgesia, it is possible that the toucher’s empathy may contribute to pain reduction. Indeed, empathy—our ability to understand someone else’s emotional experience or state—plays a key role in social touch (23) and pain reduction (33). There is widespread consensus that empathy for pain recruits brain structures that are also involved in the firsthand experience of the pain for which the empathy is being shown. Indeed, research has repeatedly demonstrated that pain and empathy for pain activate the bilateral anterior insular and anterior midcingulate cortex (34–36), triggering emotional resonance in the observer. In line with this, recent electroencephalogram studies reveal that alpha rhythms particularly over frontocentral underlie both self and other pain (37, 38). These studies imply that shared neural networks are activated in the target and the observer, suggesting that brain-to-brain coupling should occur during empathy for pain.
However, although traditional research on shared pain implies that the target of the pain and the observer undergo simultaneous activation, research to date has been based on a “single-person” approach. This approach involves an artificial environment in which a single isolated human response is simplified and analyzed, but it does not consider the additional element involved in social interaction per se and therefore does not allow testing real-time brain coupling between target and observer (39). Researchers have increasingly acknowledged that pain is affected by multidimensional factors. The biopsychosocial model posits that a physical illness such as pain can be explained by a dynamic interaction between physiologic, psychological, and social factors (40). According to the biosocial model, communication of social understanding and empathetic responses to a person in pain (41) may reduce negative affect (42). Exploring brain-to-brain coupling during empathy and examining its contribution to pain relief would require simultaneously examining brain activity in the target and the toucher. Indeed, the study on touch-induced analgesia to date has largely focused on either the target of pain or the observer, limiting our understanding of the interactive nature of analgesia. Neural evidence has so far lacked the necessary specificity to build a detailed model of touch analgesia, owing to the difficulty of examining social interactions involving real touch using traditional neuroimaging approaches.
Recently, an approach known as hyperscanning has facilitated simultaneous monitoring of the brain activity of several persons taking part in an interpersonal mutual exchange (43–46). Considering the high temporal resolution and low sensitivity to motion artifacts of EEG, hyperscanning studies with EEG have provided compelling evidence of interbrain synchronization during various cognitive tasks, such as during conversations (47), spontaneous gestural imitation (48, 49), prisoner’s dilemma game (50), guitar playing (51, 52), and rhythmic finger movement (53). Furthermore, reports indicate that the neurohormone oxytocin, which has also been implicated in affective touch (54), enhances interpersonal brain coupling (55). This simultaneous monitoring of the brain activity of several persons provides an excellent ecological framework for studying the neurodynamics of social interactions. To date, however, no study has tested real-time brain-to-brain dynamics during touch and pain or the association between brain-to-brain coupling and pain relief.
One possibility is that since empathy has evolved to promote helping behaviors in social animals (56), an observer’s empathic response may have an effect on regulating a target’s distress. Indeed, being in a matched emotional state with the emotions of an observer has been shown to lead to positive feelings toward the observer and to activate the reward circuitry in the brain (57). Therefore, behavioral and neural coupling during touch may be related to the understanding an observer exhibits toward a target’s distress, thus blurring the boundaries between self and others (58, 59) and promoting analgesia for the target. In line with this notion, research has suggested that the feeling of being understood activates parts of the reward circuitry, including the ventral striatum (60). Furthermore, recent studies show that synchrony is enjoyable (61, 62) and that the reward circuitry, including the striatum, is activated when individuals experience synchrony (63). This suggests that brain-to-brain coupling between an observer and a target experiencing pain may signal social understanding, which in turn may be rewarding and promote analgesia. Thus, social touch may be a means of communicating social understanding between target and observer (9), which in turn may regulate pain by increasing reward. Indeed, a recent study has shown that partner touch enhances analgesia and that trait empathy predicts level of analgesia (64). Moreover, touch-enhanced interpersonal coupling of heart rate and respiration during the pain, along with high partner empathy and high levels of analgesia enhanced coupling during partner touch (65).
Here, we hypothesize that a partner’s touch will increase interpartner brain coupling during pain and that the level of coupling will be associated with analgesia magnitude and degree of touch empathy. The experiment consisted of six conditions in which romantic partners were instructed to hold hands or to sit together with no physical contact or to sit in separate rooms during the pain vs. no-pain conditions.
Considering the intersubject variability of hyperscanning and to decrease the impact of gender differences, the roles assigned to the male and female partners remained constant throughout the experiment. Since women are known to benefit from social support more than men (66–69), they were selected for the role of pain target.
Although fMRI studies show that deep-brain structures are involved in empathy for pain, recent EEG studies reveal that alpha rhythms underlie empathy for pain (37, 38) particularly over frontocentral regions. In line with this, in a recent study it has been shown that alpha and beta oscillations underlie the sensory qualities of others’ pain (70).
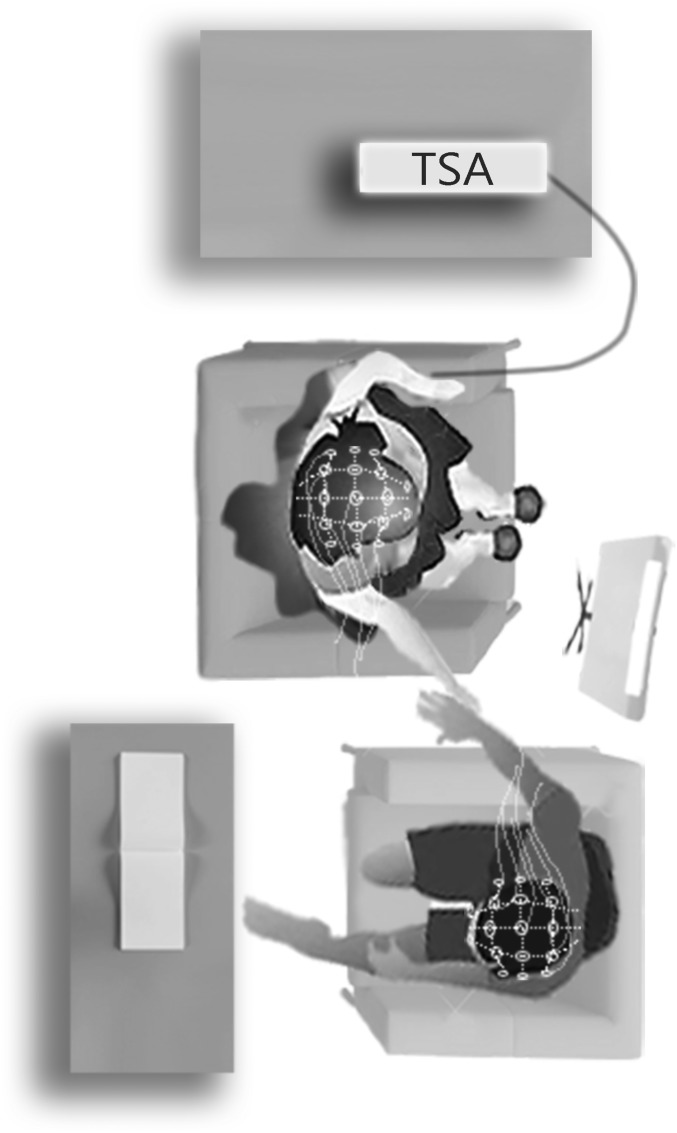
Throughout the experiment, the neural activity of both partners was simultaneously recorded (Fig. 1). We focused on coupling in the alpha–mu band (8–12 Hz), as previous research demonstrated that the alpha–mu band is related to pain perception and plays a significant role in empathy for pain (71–73). Moreover, research has shown that the alpha–mu band is involved in interbrain synchronization (49, 74, 75) during nonverbal social interaction and that it is the most robust band for brain-to-brain coupling (49). In addition, findings from different hyperscanning studies have confirmed the robustness of alpha-band implication (49, 75, 76). While most hyperscanning studies focus on the alpha–mu band, several studies reported interbrain coupling in the beta band (77–79); therefore, this band was analyzed as well. We hypothesized that coupling in the alpha–mu and beta bands in a pain-related interpartner network would increase during touch, compared with either the pain without touch condition or the conditions without pain. Finally, we expected that the level of brain-to-brain coupling would be associated with analgesia in the target of pain and empathic accuracy in the observer during the partner’s touch.
Fig. 1.
Experimental setting.
Results
Behavioral Analysis.
Empathic accuracy in the partner touch–pain [mean (M) = 0.24, SD = 0.27] condition was higher than in the no-touch–pain (M = 0.41, SD = 0.50) condition (Mdiff = −0.17 [−0.07, −0.27], P = 0.003), indicating that touch increases empathic accuracy between the partners. The ratings of the target of pain in the partner touch–pain [M = 25.03, SD = 20.32] condition were significantly lower than in the partner no-touch–pain (M = 37.74, SD = 24.82) condition (Mdiff = −12.71 [−1.97, −23.45], P = 0.021) and in the pain-alone (M = 52.41, SD = 29.41) condition (Mdiff = −27.38 [−16.21, −38.53], P < 0.001), confirming that touch had an analgesic effect.
Alpha Band: Interbrain Analysis.
In the first stage of the analysis, we filtered out electrode combinations with null effect, that is, electrode pairs with zero interpersonal coupling in all conditions of interest (partner touch–no-pain, partner no-touch–no-pain, partner touch–pain, and partner no-touch–pain). This analysis revealed 63 combinations of interbrain electrodes where the coupling in at least one of the conditions of interest exceeded the baseline no-pain-alone condition. Detailed findings are shown in Table S1.
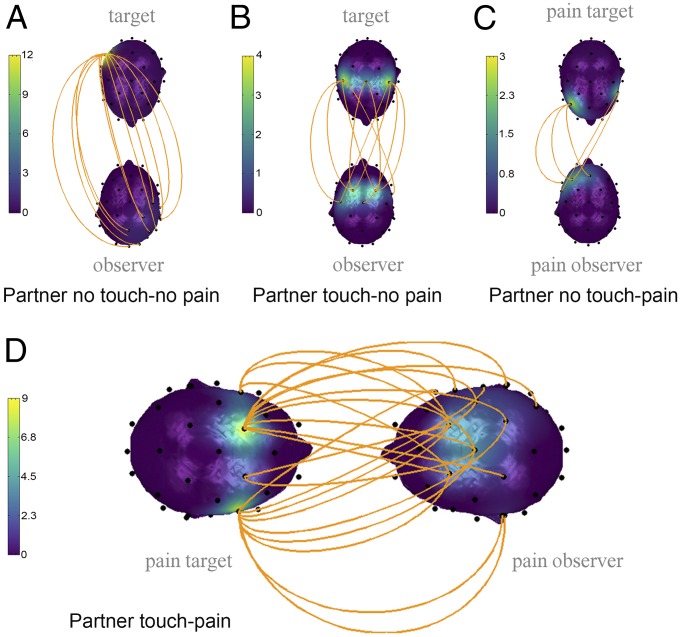
Fig. 2 A–C depict the interbrain coupling networks for the second step of the analysis, demonstrating brain-to-brain coupling networks for the conditions of partner touch–no-pain, partner no-touch–no-pain, and partner no-touch–pain compared with the no-pain-alone condition. As shown in the figures, the no-touch–no-pain condition demonstrated an interbrain coupling pattern, mostly between the right parietal regions of the female partner and the right parieto-occipito-temporal areas of the male partner. The partner touch–no-pain condition showed an interbrain coupling pattern between central regions of the female partner and fronto-central regions of the male partner. Last, the partner no-touch–pain condition demonstrated interbrain coupling between the right frontal regions of the female partner and the left central-frontal regions of the male partner, and between the left central-frontal areas of the female partner and the left central-frontal areas of the male partner. Detailed findings of step 2, including calculated post hoc power, are given in Table S2.
Fig. 2.
Interpartner EEG coupling in (A) partner no-touch–no-pain vs. pain-alone conditions (12 links); (B) partner touch–no-pain vs. pain-alone conditions (10 links); (C) partner no-touch–pain vs. pain-alone conditions (5 links); (D) partner touch–pain vs. all other conditions (22 links). The upper head represents the female partner (the pain target), and the lower head represents the male partner (the toucher). The orange lines represent statistically significant coupling links between corresponding areas in the male and female brains. The head color reflects the number of links.
Our main analysis, depicted in Fig. 2D, shows an interbrain coupling network for the partner touch–pain condition compared with all of the other conditions (step 3). The analysis revealed the largest interpersonal network comprising 22 links, mostly between the left and right central-frontal female regions and the right frontal-parietal-occipital male regions. Detailed findings of step 3, including calculated post hoc power, are given in Table S3.
Alpha Band: Clustered Interbrain Links.
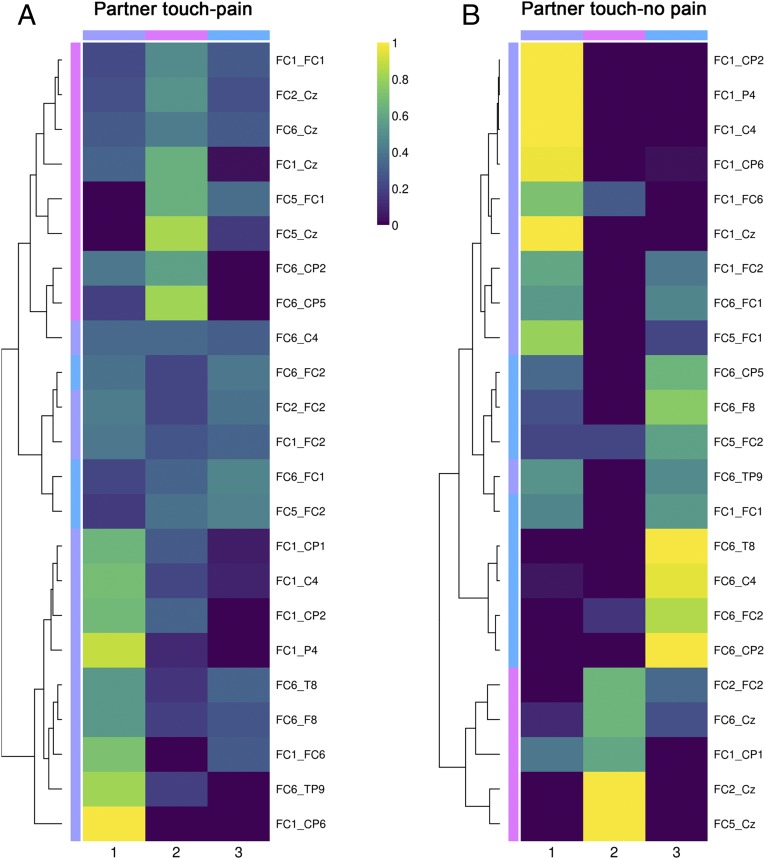
The interpartner links describing the unique interpartner network during pain and touch were clustered using NMF (Statistical Analysis). As a control, the same links were clustered in the partner no-touch–pain condition. The three-cluster solution showed the best fit in both cases (Fig. 3).
Fig. 3.
Heatmaps of the three-cluster solution for significant interbrain coupling links during pain: (A) partner touch–pain condition; (B) partner no-touch–pain condition. The colors reflect coupling link loadings for each cluster. The tree diagram (dendograms in the left part of the plots) illustrates the arrangement of the clusters produced by hierarchical clustering. The first and second electrode names separated by an underscore (right side of each heatmap) represent the target and observer EEG channels that take part in the brain-to-brain coupling.
Alpha Band: Correlations Between Brain-to-Brain Coupling and the Behavioral Data.
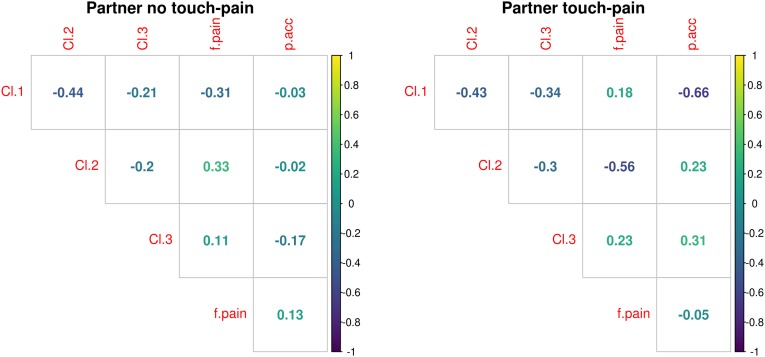
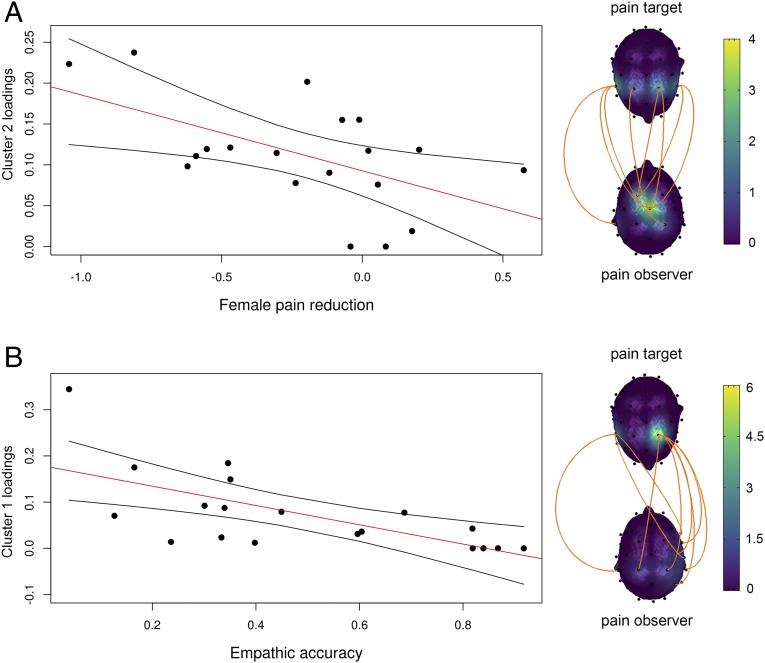
Cluster 2 showed significant correlation with female pain reduction during touch (r = −0.56 [−0.14, −0.81], P = 0.012, power = 0.82) (Fig. 4), that is, an increase in interpartner coupling as represented by cluster 2 that is associated with enhancement in touch-related analgesia in the female partner (see Fig. 5A). Cluster 1 (r = 0.18 [−0.29, 0.59], P = 0.35) and cluster 3 (r = 0.23 [−0.25, 0.61], P = 0.45) were not correlated with pain reduction in the female partner. In addition, in the partner pain–no-touch conditions, clusters 1–3 were not correlated with the female partner’s pain (Fig. 4).
Fig. 4.
Correlation matrix that includes interpartner coupling clusters (Cl.1–Cl.3), female analgesia (f.pain), and partner’s empathic accuracy (p.acc) in the touch–pain and no-touch–pain conditions.
Fig. 5.
Interpartner coupling predicts (A) touch-related analgesia in the target and (B) touch-related empathic accuracy of the observer. The brain-to-brain links on the right side show the pattern of coupling in clusters 2 (A) and 1 (B). The figures include a regression line with a 95% confidence interval. The y axis represents interpartner coupling loadings on cluster 2 (A) and cluster 1 (B). Empathic accuracy was defined as the absolute difference between the partners’ pain ratings divided by the sum of both partners’ pain ratings. A small discrepancy between the partners’ ratings corresponds to high empathic accuracy. Analgesia was calculated as the percentage difference between each woman’s final rating in the no-touch–pain and touch–pain conditions and her rating in the pain-alone condition.
Notably, in the pain–touch condition, cluster 1 correlated significantly with the empathic accuracy of the partner (r = −0.66 [−0.28, −0.86], P = 0.002, power = 0.95), indicating that an increase in interpartner coupling is associated with enhancement of the corresponding empathic accuracy (Fig. 5B). Cluster 2 (r = 0.23 [−0.25, 0.62], P = 0.35) and cluster 3 (r = 0.31 [−0.17, 67], P = 0.20) were unrelated to empathic accuracy. In addition, in the partner pain–no-touch conditions clusters 1–3 were not correlated with empathic accuracy (Fig. 4).
Beta-Band Analysis.
The first step of the algorithm revealed no findings after false-discovery rate (FDR) correction for the beta band.
Discussion
The aim of the present study was to test (i) whether interpersonal touch during pain enhances brain-to-brain real-time coupling between the target of the pain and the observer, and (ii) whether brain-to-brain coupling is associated with touch-related analgesia and with the partner’s empathic accuracy. In line with our previous report (64), the behavioral data demonstrated an analgesic effect of partner touch. Overall, all study conditions (i.e., partner touch–no-pain, partner no-touch–no-pain, partner no-touch–pain, and partner touch–pain) showed a nonzero pattern of brain-to-brain coupling in the alpha–mu band only but not in the beta band, indicating that social interactions are associated with brain-to-brain coupling in the alpha band. These results cannot be explained by spurious coupling because the interbrain coupling analyses were adjusted for the baseline condition in which we synchronously recorded EEG for the noninteracting partners (49, 80). Moreover, the main findings of the study are based on brain-to-brain circular correlation coefficients (CCorrs) that showed the lowest sensitivity to spurious couplings of EEG hyperscanning data (81). Furthermore, we carried out post hoc power analysis using simulations and found strong support for the validity of our findings.
To examine the touch–pain condition, this study was designed with four control conditions, one to rule out suspicious random brain-to-brain coupling (no-pain-alone) and three others: only interpersonal interaction (no-touch–no-pain), only touch (touch–no-pain), and only pain (no-touch–pain). These multiple control conditions were designed to decrease the risk of alternative explanations for our findings.
First, we found a coupling network in the partner touch–pain condition above and beyond all other conditions. This network consisted of coupling between the central regions of the female partners’ brains and mainly the right hemisphere of the male partners’ brains. Compared with the coupling observed in the other three conditions (i.e., partner touch–no-pain, partner no-touch–no-pain, and partner no-touch–pain), this coupling pattern is the strongest. Although the number of links in the coupling network is not necessarily associated with the power of the coupling, the links may represent a secondary marker for the interbrain coupling, providing good simplification of the coupling patterns. In this condition, the target of the pain processes both the pain and the partner’s touch, resulting in a more widespread interpersonal coupling pattern.
As the parietal lobe integrates sensory information of tactile and visual modalities (82), the increased involvement of the observers’ parietal areas in the coupling may represent multimodal integration of information, ranging from perception of the situation to the empathic reaction via touch. In support of these findings, tactile-induced analgesia (28) has been found to correlate with activations in brain areas related to multimodal neural activity (83) and emotional processes (84–88). Moreover, in the partner touch–pain condition, the coupling observed near the temporoparietal junction replicates findings from a joint attention paradigm (49). Previous neuroimaging studies showed that the right temporoparietal region is consistently activated in social cognitive processing involving attention orientation, self–other discrimination, and perspective-taking (89, 90). Nonetheless, the target of the pain may also integrate somatosensory information (touch and vicarious pain) that may explain the involvement of central regions in interpersonal coupling. Thus, the coupling in the partner touch–pain condition could be a result of integration of sensory information from tactile, visual, and nociceptive inputs.
Unlike the touch–pain condition, the no-touch–no-pain condition demonstrated a relatively weaker interbrain coupling pattern, mostly between the right parietal regions of the female partner and the right parieto-occipito-temporal areas of the male partner. In other words, the interpartner brain-to-brain coupling pattern in this control condition may represent a basic interpersonal interaction and therefore may also constitute part of the coupling during pain and touch. These results are consistent with the findings of Dumas et al. (49) who reported alpha–mu interpersonal coupling between the model and the imitator during a spontaneous imitation condition mostly between the right centro-parietal regions, both in the model and the imitator. While the alpha–mu band has been associated with the mirror neuron system (91), such activity in the right centro-parietal area has even been proposed as a neuromarker of social coordination (74). Thus, the simple coupling pattern in the no-touch–no-pain condition replicated previous hyperscanning findings of interpersonal cooperation. Since this pattern resembles part of the complex network in the partner touch–pain condition, it could be assumed that the pain and touch coupling network partially involves the general interpersonal interaction network. In support of this notion, researchers have demonstrated that, in addition to brain coupling, mere copresence can result in autonomic physiological coupling (92, 93). Notably, although this condition involves seemingly symmetric roles for both the target and the observer, the pattern of interbrain coupling is not entirely symmetric. It involves mostly right centro-parietal regions in both pain target and pain observer; however, the spatial distribution of the interbrain coupling links is higher for the observer. This asymmetric pattern of coupling could be explained by the differential roles of the partners during the interaction, which was based on the initial division into targets and observers at the beginning of the study: (i) The participants may have had different expectations, based on the assigned roles; (ii) pain targets underwent a pain calibration procedure before the main study began.
The partner touch–no-pain condition also demonstrated an interbrain coupling pattern that constitutes parts of a partner touch–pain coupling network. This network links central regions of the female partner with fronto-central regions of the male partner that are very close to somatosensory regions. Research indicates that social touch activates somatosensory areas, as expressed in the alpha–mu band (94–96) and the P45 somatosensory-evoked potentials amplitude (24). Moreover, touch was found to increase the coupling of electrodermal activity, which was found to be correlated with the somatosensory activation (63). Thus, this interbrain network during the partner touch–no-pain condition reflects how social touch increases interpersonal coupling in somatosensory regions.
The partner no-touch–pain condition exhibited the weakest interbrain coupling between the right frontal regions of the female partner and the left central-frontal regions of the male partner, and between the left central-frontal areas of the female partner and the left central-frontal areas of the male partner. In line with these findings, frontal alpha modulation correlates with both vicarious pain (97) and empathy for pain (37, 71). Moreover, research has repeatedly demonstrated that both pain and empathy for pain activate frontal brain areas (35).
Generally, the obtained patterns exhibited a consistent relationship at both the topographic and the intensity levels. For the no-touch–no-pain condition, the coupling between occipital areas could be explained by resting visual alpha activations. As expected, interpersonal touch results in a shift from occipital to central regions. The no-touch–pain condition yielded the weakest interbrain coupling, resulting in a pattern that was not additive to other conditions, thus emphasizing the nonlinearity of brain-to-brain coupling patterns. Nevertheless, the pattern of coupling in the no-touch–pain condition is in line with previous findings of disruption in autonomic coupling under similar conditions (65). In line with fMRI studies showing that empathy for pain recruits brain structures that are also involved in the firsthand experience of pain (34–36), one might argue that, during pain processing, the target is focused on his/her own pain. This focus may interfere with brain-to-brain coupling. Even if the observer’s empathy attempts to mirror this state (as shown in previous fMRI results), the lack of touch makes it difficult to synchronize the two states along the EEG scale. In addition, the observer may express empathy for the partner’s pain, but without touch this empathy cannot be communicated to the partner. Furthermore, in contrast to the notion of common brain activation for pain targets and pain observers, recent studies applying machine-learning techniques have shown that empathy for pain and the experience of pain result in activation of distinct brain pathways (98). These initial findings are in line with the pattern of brain-to-brain coupling observed in the present study.
Nonetheless, the partner no-touch–pain coupling was related neither to analgesia in the target of pain nor to the partner’s empathic accuracy. Thus, the mere presence of the partner seems to be insufficient for pain reduction (64). In line with these findings, a reduction in heart rate and respiration interpersonal coupling has been demonstrated as resulting from pain manipulation without touch (65).
One possible assumption when comparing pain conditions with and without partner’s touch is that the analgesic effect of touch can be explained by the well-known effect of distraction (99). Previous research has shown that the condition of a stranger’s touch did not yield an analgesic effect (12, 64), suggesting that touch by itself is not sufficient to induce analgesia. Moreover, in the touch conditions, partner’s empathy predicted analgesia in the target, indicating that touch can serve as a tool for communicating empathy, as has been shown for different emotions (9).
In summary, the patterns of interpersonal coupling during partner copresence, touch-only and pain-only conditions may reflect distinct parts of the brain-to-brain coupling found during the partner touch–pain condition. However, these three coupling patterns have no shared parts and are correspondently related to brain areas of general cooperation, somatosensory regions and more cognitive areas, thus representing different components of coupling in the partner touch–pain condition.
Notably, we found that the power of partner touch–pain coupling is related to the degree of pain analgesia in the target and to the partner’s empathic accuracy. We previously demonstrated the existence of such touch-related analgesia that can be predicted by observer empathy (64). Other studies have reported correlations between empathy and activations in affective (35, 100–103) and sensory (104, 105) parts of the pain matrix. Moreover, P50 amplitude (event-related potential occurring ∼50 ms after presentation of a stimulus) correlates with the perspective-taking component of empathy during both touch and pain (106). Thus, a touch-related brain-to-brain coupling network during pain may reflect expressions of empathy in the observer and pain analgesia in the target. It is interesting to note that interbrain links related to pain analgesia in the target mostly involved the central zone of the observer’s brain (represented by cluster 2) close to the somatosensory cortices. In contrast, the observer’s empathic accuracy correlated with links that partially involved the observer’s central zone and right parietal-occipital-temporal zone (cluster 1), which are related to self–other discrimination and the perspective-taking aspect of empathy (89, 90).
The analgesic effect of touch may thus be explained by two possible frameworks. One possibility is that observer touch enhances coupling, which increases the tendency of the target to feel understood, which in turn activates reward mechanisms (60). In line with this, recent research demonstrated that the reward circuitry is activated when humans experience synchrony (63). Thus, brain-to-brain coupling between an observer and a target experiencing pain may be affected by social understanding that can be rewarding and that may result in analgesia. Moreover, social touch may communicate empathy between target and observer (9) and social understanding may take part in pain management by increasing reward. Research supporting this idea has shown that partner touch enhances analgesia and that trait empathy predicts level of analgesia (64).
Another possibility is that interpersonal touch may blur the borders between self and other. Multiple studies have shown similar brain activation for both pain target and observer. In addition, researchers have demonstrated that the perspectives of self and other show similar patterns of physiological activation for painful and pleasurable virtual reality scenarios (107) and of shared brain activations for pleasant and unpleasant touch (108). Thus, interpersonal touch may increase empathic sharing, assisting the observer in feeling the target’s pain as well as in transmitting emotional support to the pain target, resulting in analgesia and expressed in interpersonal physiological coupling.
It is important to note that the intradyadic relationship can play an important role in brain-to-brain coupling. Research has shown that interpersonal coupling between same-sex unrelated dyads is evident when dyads perform a computer-based cooperation task. This effect is diminished in mixed-sex unrelated dyads (109). However, recent research indicates that a cooperation task between members of romantic dyads elicited increased coupling compared with dyads comprising friends and strangers, and this increased coupling also correlated with their task performance (110). However, the directional coupling from females to males differed from that of male-to-female, suggesting different roles for females and males during cooperation.
Although the current study used a controlled design with several balanced conditions, it has several limitations that need to be acknowledged. First, only the female participants underwent pain stimulation while the male participants did not. Therefore, the generalizability of our results is restricted to the male-to-female direction, while the opposite direction should be considered with caution. Future research is warranted to test the effect of touch and pain in both men and women as well as in homosexual and heterosexual participants. Second, since EEG recordings provide limited precision regarding brain locations, our interpretations regarding the brain locations should be considered with caution and future neuroimaging studies should help reveal more precise locations of the brain-to-brain coupling reported here. Third, we used Ccorr as a measurement of interbrain coupling because it was least sensitive to spurious couplings. However, future studies should investigate reference-free measures of coupling (111) for hyperscanning data. Fourth, this study does not explain the exact mechanism of interbrain coupling by pain and touch. Hence, causality between touch-related analgesia, partner’s empathy, and brain-to-brain coupling should be considered with caution. Finally, one might argue that the sound cue that appeared before each condition produced spurious synchronized activity. However, it should be noted that the sound cue served as a signal to the partners regarding when a condition started and was present during all conditions. Thus, although the sound cue may have triggered coupling, it cannot explain the differences between the coupling patterns observed in the different conditions.
Our findings support the theoretical framework of a biopsychosocial model of pain that suggests a dynamic interaction between biological, psychological, and social factors affecting pain perception (40, 112–114). Partner touch (social factor) may help in empathy sharing (psychological factor) with the target of the pain, resulting in analgesia and accompanied by interpersonal central neurophysiological coupling (biological factor). To conclude, this study demonstrates that interpersonal coupling plays a key role in analgesia during social touch. In contrast with the traditional approach that examines one part of the interaction, here we investigated neurophysiological response using a paradigm that also considers social contexts and social dynamics. Since physiological resonance has important evolutionary significance, investigation of interpersonal coupling provides an interesting opportunity to understand human behavior in a natural social environment.
Materials and Methods
Procedure.
The participants were recruited through fliers posted on campus and were informed that they would be participating in a study investigating brain mechanisms of pain perception. They were given no information regarding the effects of hand-holding. Upon arrival, the partners were escorted to different rooms and asked not to communicate verbally with each other until the experiment was over. Participants then underwent pain familiarization and pain calibration using the pain-60 approach (see below). This was followed by six counterbalanced conditions: no-pain-alone, pain-alone, partner touch–no-pain, partner no-touch–no-pain, partner touch–pain, and partner no-touch–pain, with only female participants receiving pain stimuli. The women were asked to rate their pain intensity 2 s before the end of each condition using the numerical pain scale (NPS). Concurrently, the male partners were instructed to rate their partners’ level of pain. Both partners wrote the number on a small piece of paper not visible to the other member of the couple. A 10-min break separated successive conditions.
The neural activity of both partners was simultaneously recorded with a dual-EEG recording system using a 64-channel Brainamp MR amplifier manufactured by Brain Products GmbH. The system was equipped with two Acticap helmets with 32 active electrodes, arranged according to the international 10/20 system and connected to two synchronized amplifiers to guarantee millisecond-range synchrony between the two EEG recordings. In the pain-alone condition, only the female partner was recorded using 32 channels (see details below in Experimental Conditions). This condition was used as a baseline and for calculation of the analgesic effect. Signals were analog filtered between 0.16 and 250 Hz, amplified and digitalized at 500 Hz with a 16-bit vertical resolution in the range of ±3.2 mV. The impedances were maintained below 10 kΩ.
Experimental Conditions.
The experiment consisted of six conditions within one session, each lasting 120 s. The pain-alone condition included pain stimulation applied to the woman’s left forearm at a temperature individually tailored to induce an NPS score of 60, while the partner sat in an adjacent room. During the partner touch–no-pain condition, the participants sat facing each other holding their dominant hands, while during the partner no-touch–no pain condition the partner was present without making any physical contact. During the partner touch–pain condition, the pain stimulus was administered to the female participant, while her partner held her dominant hand (Fig. 1). In the partner no-touch–pain condition, the female participant was administered the same pain stimulus, but her partner was only present without making any physical contact. In the no-touch conditions, participants were instructed to hold the handles of their armchair. During the no-pain-alone condition, the partners sat in the same room, separated by a nontransparent curtain. All of the pain conditions consisted of one continuous trial that lasted 120 s. With the exception of the no-pain-alone condition, the participants could see each other. In all conditions, the participants heard a sound cue that signaled the beginning of the trial. For the conditions without pain stimuli, the thermode was connected to the hand of the pain target, but no stimuli were administrated.
For the validation of the hand-holding procedure, we completed the following. Preparing the paradigm, we conducted a study in which 27 participants were presented with 10 pictures depicting various painful stimuli and were required to select a touch (presented in a 3-s clip) they estimates would diminish their pain out of three types of stimuli: a clip showing slow stroking (3 cm/s) on the arm, a clip showing slow stroking on the palm and static hand-holding. Remarkably, hand-holding was selected 75% (SD = 31.28) of the time as the preferred type of touch for pain reduction, indicating that although stroking is pleasurable, hand-holding may have a more powerful effect on pain relief. Therefore, static hand-holding was using in this study.
To examine whether a differential eye-gaze pattern occurred during the pain–no-pain, touch–no-touch condition, we conducted an additional experiment with 18 romantic couples between the ages of 18 and 45 to examine whether the eye-gaze pattern differs between the pain–no-pain, touch–no touch conditions. Each participant was administered 120 s of heat stimuli at the intensity of pain-60 under the same touch–no-touch conditions as in the current study. The pain interactions were videotaped. Objective coders evaluated the total duration of the mutual gazing and the number of mutual gazes. Data analysis of permutation tests showed no differences between the conditions in either the total duration of the mutual gazing (Diffmedian = 0.05, P = 0.99) or in the number of mutual gazes (Diffmedian = 0.51, P = 0.33). These findings indicate that interpersonal touch is not associated with differential patterns of eye-gaze compared with in the no-touch condition.
Participants.
Forty-four heterosexual (as confirmed by a sexual orientation scale) participants (22 couples) were recruited for this study (physiological data of this sample is reported in ref. 65). Four couples were married. Participants ranged in age from 23 to 32 y (mean and SD for men: 26.4 ± 2.27 y; for women: 25.6 ± 1.9 y), had no children, were in long-term relationships (3.46 ± 2.25 y), and had completed an average of 13 y of education (years of education for men: 13.3 ± 1.5; and for women: 13.6 ± 1.3). Only 9% of the couples were married. Two couples were dropped from the analysis because of unsuccessful physiological recording.
Couples were screened by a phone interview based on the following criteria: (i) right-handed and between the ages of 22 and 40; (ii) no chronic or acute pain; (iii) no medication use (except for oral contraceptives); (iv) no history of neurological disorders, psychiatric problems, or other problems relevant to the study; (v) not pregnant; (vi) in a heterosexual romantic relationship. All participants provided informed consent, and the study was approved by the Faculty of Social Welfare and Health Sciences Ethics Committee, University of Haifa.
Pain Familiarization and Pain-60 Calibration.
All contact heat stimuli in this experiment were applied to the left volar forearm using a 3-cm2 computer-controlled Peltier-type thermode (TSA-2001; Medoc). During the pain familiarization procedure, participants were exposed to three short contact-heat stimuli (43, 45, and 47 °C), each administered for 7 s in a semirandomized order with a break of 10 s. Participants were asked to report pain intensity using the NPS, ranging from 0, denoting “no pain,” to 100, denoting “the worst pain imaginable.” Thereafter, the stimulus intensity was adjusted to each participant to evoke a peak pain magnitude of 60/100 (pain-60) on the NPS, using the algorithm described by Granot et al. (115). The procedure was previously validated for this paradigm by Goldstein et al. (64). Pain-60 intensities ranged from 43.7 to 48.5, with an average of 46.11 and SD of 1.43.
Behavioral Measures.
The following measures were evaluated for each of the pain conditions.
Measure of empathy.
The measure of empathic accuracy was defined as the absolute difference between the partners’ pain ratings (each male partner’s pain ratings minus his female partner’s pain ratings) divided by the sum of both partners’ pain ratings. A small discrepancy between the partners’ ratings corresponds to high empathic accuracy (116).
Measure of analgesia.
The reduction in the female partner’s pain (i.e., woman’s analgesia) was calculated as the percentage difference between each woman’s final rating in the no-touch–pain and touch–pain conditions and her rating in the pain-alone condition.
EEG Preprocessing and Statistical Analysis.
The preprocessing was conducted using Matlab R2009b (The MathWorks) with Fieldtrip toolbox (117). EEG data were rereferenced off-line to an average of the left and right mastoid and filtered with a bandpass ranging from 1 to 45 Hz. Principal-component analysis (PCA) was used for artifact edification (118, 119) because PCA has been shown to be very effective in reducing artifacts with minimal effect on spectral distortion (119). PCA-dominant components that showed a noncortical source (eyeblinks or movement artifacts) were removed. The converted EEG signal was then calculated by using the inverse solution of the PCA. Finally, about 3% of the data were removed after visual examination of the obtained data.
The data were filtered into the 8- to 12-Hz frequency bands using Butterworth filters of order four, divided into consecutive epochs of 1,000 ms, and the instantaneous phase was estimated using the Hilbert transform. Data from the first 2 s and the last 2 s were excluded from the analysis because of multiple artifacts. Then CCorr (120) was calculated for each 1,000-ms window over every possible combination of interpartner EEG electrodes (total of 1,024) in each study condition. Simulations showed that CCorr has the lowest sensitivity to spurious couplings of EEG hyperscanning data (82). Afterward, calculated CCorr was normalized by Fisher’s Z transformation. The distribution of the transformed coupling data are shown in Fig. S1.
It is important to stress that, in the current statistical analysis, the partner’s touch–pain conditions were emphasized adjusting the related findings by the other study conditions. Since the coupling data are nested within conditions and participants, statistical analysis was based on the multilevel modeling (MLM) approach, taking into account the nested data structure and removing linear trends. We used the following algorithm to reduce the number of statistical tests. In the first step, complex contrast was used to test hypothesis H1 that at least a single condition of interest (partner touch–no-pain, partner no-touch–no-pain, partner touch–pain and partner no-touch–pain) shows higher interpartner coupling than the baseline no-pain-alone condition for each combination of interpartner electrodes. This step allowed us to filter out a major number of electrode combinations with null effect more effectively than classical ANOVA, which includes comparisons between all possible pairwise conditions. Based on the first step, only significant electrode combinations were analyzed in the second step, in which each of the four conditions of interest was compared separately to the baseline no-pain-alone condition. This was done to examine the interpartner connectivity network for interpartner touch with/without pain as well as the network for the partners’ copresence with/without pain. The third step was planned to identify a unique interpartner connectivity network for interpartner touch during pain. Therefore, three contrasts were tested to compare the partner touch–pain condition with the other three conditions of interest only for those combinations of electrodes that showed significant coupling for partner touch–pain conditions in the second step. Since the tests conducted within the three steps are organized hierarchically, the hierarchical FDR controlling procedure proposed by Yekutieli (121) and implemented mostly on genomic data (122, 123) was applied to control the findings for the multiple testing problem.
In the next stage, the interpartner networks during pain–no pain and touch were clustered using nonnegative matrix factorization (NMF) (124) with 1,000 runs to achieve stable results. Factorization rank was estimated by considering the smallest value at which the decrease in the residual sum of squares (RSS) is lower than the decrease in the RSS obtained from random (reshuffled) data (125). The “Brunet” version of NMF, which is based on Kullback–Leibler divergence and uses simple multiplicative updates, was applied (126). After that, the MLM approach was used to test the difference in the relationship between the pain-related interpartner network and the reduction in the female partner’s pain/empathic accuracy with and without touch.
Correlation analysis of pain-related outcomes with clustered interpartner coupling networks was adjusted by simulation-based multiple-tests correction (127) and includes a report of 95% confidence intervals. Last, bootstrapped paired t test was used for behavioral analysis. In all analyses, type 1 error was set to 0.05. The power of the significant findings was calculated by simulation study. Synthetic data were generated 500 times using the obtained parameter estimates and the initial analysis was conducted. The power was computed by comparing P values from the simulated data to the corresponding FDR criterion in initial analysis. All statistical analyses were performed in R 2.14.2 using the lme4 and NMF packages.
Supplementary Material
Acknowledgments
This research was supported by the Binational Science Foundation Grant 2015068.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1703643115/-/DCSupplemental.
References
- 1.Suvilehto JT, Glerean E, Dunbar RIM, Hari R, Nummenmaa L. Topography of social touching depends on emotional bonds between humans. Proc Natl Acad Sci USA. 2015;112:13811–13816. doi: 10.1073/pnas.1519231112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrison I, Löken LS, Olausson H. The skin as a social organ. Exp Brain Res. 2010;204:305–314. doi: 10.1007/s00221-009-2007-y. [DOI] [PubMed] [Google Scholar]
- 3.McGlone F, Wessberg J, Olausson H. Discriminative and affective touch: Sensing and feeling. Neuron. 2014;82:737–755. doi: 10.1016/j.neuron.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Feldman R, Singer M, Zagoory O. Touch attenuates infants’ physiological reactivity to stress. Dev Sci. 2010;13:271–278. doi: 10.1111/j.1467-7687.2009.00890.x. [DOI] [PubMed] [Google Scholar]
- 5.Gentsch A, Panagiotopoulou E, Fotopoulou A. Active interpersonal touch gives rise to the social softness illusion. Curr Biol. 2015;25:2392–2397. doi: 10.1016/j.cub.2015.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francis S, et al. The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. Neuroreport. 1999;10:453–459. doi: 10.1097/00001756-199902250-00003. [DOI] [PubMed] [Google Scholar]
- 7.Krämer HH, et al. Activation of the cortical pain network by soft tactile stimulation after injection of sumatriptan. Pain. 2007;133:72–78. doi: 10.1016/j.pain.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- 9.Hertenstein MJ, Keltner D, App B, Bulleit BA, Jaskolka AR. Touch communicates distinct emotions. Emotion. 2006;6:528–533. doi: 10.1037/1528-3542.6.3.528. [DOI] [PubMed] [Google Scholar]
- 10.Field T. Touch for socioemotional and physical well-being: A review. Dev Rev. 2010;30:367–383. [Google Scholar]
- 11.Smith AA, Kimmel SR, Milz SA. Effects of therapeutic touch on pain, function and well being in persons with osteo-arthritis of the knee: A pilot study. Internet J Adv Nurs Pract. 2010;10:2. [Google Scholar]
- 12.Coan JA, Schaefer HS, Davidson RJ. Lending a hand: Social regulation of the neural response to threat. Psychol Sci. 2006;17:1032–1039. doi: 10.1111/j.1467-9280.2006.01832.x. [DOI] [PubMed] [Google Scholar]
- 13.Field T. Massage therapy for infants and children. J Dev Behav Pediatr. 1995;16:105–111. [PubMed] [Google Scholar]
- 14.Fleisher KA, et al. Integrative Reiki for cancer patients: A program evaluation. Integr Cancer Ther. 2014;13:62–67. doi: 10.1177/1534735413503547. [DOI] [PubMed] [Google Scholar]
- 15.Gallace A, Spence C. The science of interpersonal touch: An overview. Neurosci Biobehav Rev. 2010;34:246–259. doi: 10.1016/j.neubiorev.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Gray L, Watt L, Blass EM. Skin-to-skin contact is analgesic in healthy newborns. Pediatrics. 2000;105:e14. doi: 10.1542/peds.105.1.e14. [DOI] [PubMed] [Google Scholar]
- 17.Inui K, Tsuji T, Kakigi R. Temporal analysis of cortical mechanisms for pain relief by tactile stimuli in humans. Cereb Cortex. 2006;16:355–365. doi: 10.1093/cercor/bhi114. [DOI] [PubMed] [Google Scholar]
- 18.Younger J, Aron A, Parke S, Chatterjee N, Mackey S. Viewing pictures of a romantic partner reduces experimental pain: Involvement of neural reward systems. PLoS One. 2010;5:e13309. doi: 10.1371/journal.pone.0013309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tarr B, Launay J, Cohen E, Dunbar R. Synchrony and exertion during dance independently raise pain threshold and encourage social bonding. Biol Lett. 2015;11:11. doi: 10.1098/rsbl.2015.0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunbar RI. The social role of touch in humans and primates: Behavioural function and neurobiological mechanisms. Neurosci Biobehav Rev. 2010;34:260–268. doi: 10.1016/j.neubiorev.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Niesink RJM, Van Ree JM. Involvement of opioid and dopaminergic systems in isolation-induced pinning and social grooming of young rats. Neuropharmacology. 1989;28:411–418. doi: 10.1016/0028-3908(89)90038-5. [DOI] [PubMed] [Google Scholar]
- 22.Post-White J, et al. Therapeutic massage and healing touch improve symptoms in cancer. Integr Cancer Ther. 2003;2:332–344. doi: 10.1177/1534735403259064. [DOI] [PubMed] [Google Scholar]
- 23.Bufalari I, Ionta S. The social and personality neuroscience of empathy for pain and touch. Front Hum Neurosci. 2013;7:393. doi: 10.3389/fnhum.2013.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bufalari I, Aprile T, Avenanti A, Di Russo F, Aglioti SM. Empathy for pain and touch in the human somatosensory cortex. Cereb Cortex. 2007;17:2553–2561. doi: 10.1093/cercor/bhl161. [DOI] [PubMed] [Google Scholar]
- 25.Braz J, Solorzano C, Wang X, Basbaum AI. Transmitting pain and itch messages: A contemporary view of the spinal cord circuits that generate gate control. Neuron. 2014;82:522–536. doi: 10.1016/j.neuron.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salter MW, Henry JL. Differential responses of nociceptive vs. non-nociceptive spinal dorsal horn neurones to cutaneously applied vibration in the cat. Pain. 1990;40:311–322. doi: 10.1016/0304-3959(90)91128-6. [DOI] [PubMed] [Google Scholar]
- 27.Salter MW, Henry JL. Physiological characteristics of responses of wide dynamic range spinal neurones to cutaneously applied vibration in the cat. Brain Res. 1990;507:69–84. doi: 10.1016/0006-8993(90)90524-f. [DOI] [PubMed] [Google Scholar]
- 28.Mancini F, Beaumont A-L, Hu L, Haggard P, Iannetti GD. Touch inhibits subcortical and cortical nociceptive responses. Pain. 2015;156:1936–1944, and erratum (2015) 156:2636–2637. doi: 10.1097/j.pain.0000000000000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mogil JS. Animal models of pain: Progress and challenges. Nat Rev Neurosci. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- 30.Krahé C, Drabek MM, Paloyelis Y, Fotopoulou A. Affective touch and attachment style modulate pain: A laser-evoked potentials study. Philos Trans R Soc Lond B Biol Sci. 2016;371:20160009. doi: 10.1098/rstb.2016.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langford DJ, et al. Social modulation of pain as evidence for empathy in mice. Science. 2006;312:1967–1970. doi: 10.1126/science.1128322. [DOI] [PubMed] [Google Scholar]
- 32.Grewen KM, Anderson BJ, Girdler SS, Light KC. Warm partner contact is related to lower cardiovascular reactivity. Behav Med. 2003;29:123–130. doi: 10.1080/08964280309596065. [DOI] [PubMed] [Google Scholar]
- 33.Tait RC. Empathy: Necessary for effective pain management? Curr Pain Headache Rep. 2008;12:108–112. doi: 10.1007/s11916-008-0021-6. [DOI] [PubMed] [Google Scholar]
- 34.Ellingsen D-M, et al. Placebo improves pleasure and pain through opposite modulation of sensory processing. Proc Natl Acad Sci USA. 2013;110:17993–17998. doi: 10.1073/pnas.1305050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 36.Lamm C, Majdandžić J. The role of shared neural activations, mirror neurons, and morality in empathy—a critical comment. Neurosci Res. 2015;90:15–24. doi: 10.1016/j.neures.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Perry A, Bentin S, Bartal IB-A, Lamm C, Decety J. “Feeling” the pain of those who are different from us: Modulation of EEG in the mu/alpha range. Cogn Affect Behav Neurosci. 2010;10:493–504. doi: 10.3758/CABN.10.4.493. [DOI] [PubMed] [Google Scholar]
- 38.Peled L. Empathy during consoling touch is modulated by mu-rhythm: An EEG study. Neuropsychologia. April 22, 2017 doi: 10.1016/j.neuropsychologia.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 39.Hari R, Sams M, Nummenmaa L. Attending to and neglecting people: Bridging neuroscience, psychology and sociology. Philos Trans R Soc Lond B Biol Sci. 2016;371:20150365. doi: 10.1098/rstb.2015.0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol Bull. 2007;133:581–624. doi: 10.1037/0033-2909.133.4.581. [DOI] [PubMed] [Google Scholar]
- 41.Cano A, Barterian JA, Heller JB. Empathic and nonempathic interaction in chronic pain couples. Clin J Pain. 2008;24:678–684. doi: 10.1097/AJP.0b013e31816753d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edmond SN, Keefe FJ. Validating pain communication: Current state of the science. Pain. 2015;156:215–219. doi: 10.1097/01.j.pain.0000460301.18207.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Babiloni F, Astolfi L. Social neuroscience and hyperscanning techniques: Past, present and future. Neurosci Biobehav Rev. 2014;44:76–93. doi: 10.1016/j.neubiorev.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dumas G, Lachat F, Martinerie J, Nadel J, George N. From social behaviour to brain synchronization: Review and perspectives in hyperscanning. IRBM. 2011;32:48–53. [Google Scholar]
- 45.Liu T, Pelowski M. Clarifying the interaction types in two-person neuroscience research. Front Hum Neurosci. 2014;8:276. doi: 10.3389/fnhum.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dumas G. Towards a two-body neuroscience. Commun Integr Biol. 2011;4:349–352. doi: 10.4161/cib.4.3.15110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stolk A, et al. Cerebral coherence between communicators marks the emergence of meaning. Proc Natl Acad Sci USA. 2014;111:18183–18188. doi: 10.1073/pnas.1414886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schippers MB, Roebroeck A, Renken R, Nanetti L, Keysers C. Mapping the information flow from one brain to another during gestural communication. Proc Natl Acad Sci USA. 2010;107:9388–9393. doi: 10.1073/pnas.1001791107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dumas G, Nadel J, Soussignan R, Martinerie J, Garnero L. Inter-brain synchronization during social interaction. PLoS One. 2010;5:e12166. doi: 10.1371/journal.pone.0012166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Vico Fallani F, et al. Defecting or not defecting: How to “read” human behavior during cooperative games by EEG measurements. PLoS One. 2010;5:e14187. doi: 10.1371/journal.pone.0014187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lindenberger U, Li S-C, Gruber W, Müller V. Brains swinging in concert: Cortical phase synchronization while playing guitar. BMC Neurosci. 2009;10:22. doi: 10.1186/1471-2202-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Müller V, Sänger J, Lindenberger U. Intra- and inter-brain synchronization during musical improvisation on the guitar. PLoS One. 2013;8:e73852. doi: 10.1371/journal.pone.0073852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Konvalinka I, Vuust P, Roepstorff A, Frith CD. Follow you, follow me: Continuous mutual prediction and adaptation in joint tapping. Q J Exp Psychol (Hove) 2010;63:2220–2230. doi: 10.1080/17470218.2010.497843. [DOI] [PubMed] [Google Scholar]
- 54.Ellingsen DM, et al. The neurobiology shaping affective touch: Expectation, motivation, and meaning in the multisensory context. Front Psychol. 2016;6:1986. doi: 10.3389/fpsyg.2015.01986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mu Y, Guo C, Han S. Oxytocin enhances inter-brain synchrony during social coordination in male adults. Soc Cogn Affect Neurosci. 2016;11:1882–1893. doi: 10.1093/scan/nsw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Waal FBM. Putting the altruism back into altruism: The evolution of empathy. Annu Rev Psychol. 2008;59:279–300. doi: 10.1146/annurev.psych.59.103006.093625. [DOI] [PubMed] [Google Scholar]
- 57.Kühn S, et al. Why do I like you when you behave like me? Neural mechanisms mediating positive consequences of observing someone being imitated. Soc Neurosci. 2010;5:384–392. doi: 10.1080/17470911003633750. [DOI] [PubMed] [Google Scholar]
- 58.Yun K, Watanabe K, Shimojo S. Interpersonal body and neural synchronization as a marker of implicit social interaction. Sci Rep. 2012;2:959. doi: 10.1038/srep00959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mogan R, Fischer R, Bulbulia JA. To be in synchrony or not? A meta-analysis of synchrony’s effects on behavior, perception, cognition and affect. J Exp Soc Psychol. 2017;72:13–20. [Google Scholar]
- 60.Morelli SA, Torre JB, Eisenberger NI. The neural bases of feeling understood and not understood. Soc Cogn Affect Neurosci. 2014;9:1890–1896. doi: 10.1093/scan/nst191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Noy L, Levit-Binun N, Golland Y. Being in the zone: Physiological markers of togetherness in joint improvisation. Front Hum Neurosci. 2015;9:187. doi: 10.3389/fnhum.2015.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kokal I, Engel A, Kirschner S, Keysers C. Synchronized drumming enhances activity in the caudate and facilitates prosocial commitment—if the rhythm comes easily. PLoS One. 2011;6:e27272. doi: 10.1371/journal.pone.0027272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chatel-Goldman J, Congedo M, Jutten C, Schwartz J-L. Touch increases autonomic coupling between romantic partners. Front Behav Neurosci. 2014;8:95. doi: 10.3389/fnbeh.2014.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goldstein P, Shamay-Tsoory SG, Yellinek S, Weissman-Fogel I. Empathy predicts an experimental pain reduction during touch. J Pain. 2016;17:1049–1057. doi: 10.1016/j.jpain.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 65.Goldstein P, Weissman-Fogel I, Shamay-Tsoory SG. The role of touch in regulating inter-partner physiological coupling during empathy for pain. Sci Rep. 2017;7:3252. doi: 10.1038/s41598-017-03627-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walen HR, Lachman ME. Social support and strain from partner, family, and friends: Costs and benefits for men and women in adulthood. J Soc Pers Relat. 2000;17:5–30. [Google Scholar]
- 67.Acitelli LK, Antonucci TC. Gender differences in the link between marital support and satisfaction in older couples. J Pers Soc Psychol. 1994;67:688–698. doi: 10.1037//0022-3514.67.4.688. [DOI] [PubMed] [Google Scholar]
- 68.Antonucci TC, Akiyama H. An examination of sex differences in social support among older men and women. Sex Roles. 1987;17:737–749. [Google Scholar]
- 69.Shumaker SA, Hill DR. Gender differences in social support and physical health. Health Psychol. 1991;10:102–111. doi: 10.1037//0278-6133.10.2.102. [DOI] [PubMed] [Google Scholar]
- 70.Motoyama Y, Ogata K, Hoka S, Tobimatsu S. Frequency-dependent changes in sensorimotor and pain affective systems induced by empathy for pain. J Pain Res. 2017;10:1317–1326. doi: 10.2147/JPR.S129791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mu Y, Fan Y, Mao L, Han S. Event-related theta and alpha oscillations mediate empathy for pain. Brain Res. 2008;1234:128–136. doi: 10.1016/j.brainres.2008.07.113. [DOI] [PubMed] [Google Scholar]
- 72.Whitmarsh S, Nieuwenhuis ILC, Barendregt HP, Jensen O. Sensorimotor alpha activity is modulated in response to the observation of pain in others. Front Hum Neurosci. 2011;5:91. doi: 10.3389/fnhum.2011.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perry A, Bentin S, Bartal IB, Lamm C, Decety J. “Feeling” the pain of those who are different from us: Modulation of EEG in the mu/alpha range. Cogn Affect Behav Neurosci. 2010;10:493–504. doi: 10.3758/CABN.10.4.493. [DOI] [PubMed] [Google Scholar]
- 74.Tognoli E, Lagarde J, DeGuzman GC, Kelso JAS. The phi complex as a neuromarker of human social coordination. Proc Natl Acad Sci USA. 2007;104:8190–8195. doi: 10.1073/pnas.0611453104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Astolfi L, et al. Neuroelectrical hyperscanning measures simultaneous brain activity in humans. Brain Topogr. 2010;23:243–256. doi: 10.1007/s10548-010-0147-9. [DOI] [PubMed] [Google Scholar]
- 76.Konvalinka I, et al. Frontal alpha oscillations distinguish leaders from followers: Multivariate decoding of mutually interacting brains. Neuroimage. 2014;94:79–88. doi: 10.1016/j.neuroimage.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 77.Astolfi L, et al. Investigating the neural basis of cooperative joint action. An EEG hyperscanning study. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:4896–4899. doi: 10.1109/EMBC.2014.6944721. [DOI] [PubMed] [Google Scholar]
- 78.Astolfi L, et al. Study of the functional hyperconnectivity between couples of pilots during flight simulation: An EEG hyperscanning study. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:2338–2341. doi: 10.1109/IEMBS.2011.6090654. [DOI] [PubMed] [Google Scholar]
- 79.Acquadro MAS, Congedo M, De Riddeer D. Music performance as an experimental approach to hyperscanning studies. Front Hum Neurosci. 2016;10:242. doi: 10.3389/fnhum.2016.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dumas G, Chavez M, Nadel J, Martinerie J. Anatomical connectivity influences both intra- and inter-brain synchronizations. PLoS One. 2012;7:e36414. doi: 10.1371/journal.pone.0036414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burgess AP. On the interpretation of synchronization in EEG hyperscanning studies: A cautionary note. Front Hum Neurosci. 2013;7:881. doi: 10.3389/fnhum.2013.00881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stein BE, Stanford TR. Multisensory integration: Current issues from the perspective of the single neuron. Nat Rev Neurosci. 2008;9:255–266. doi: 10.1038/nrn2331. [DOI] [PubMed] [Google Scholar]
- 83.Mouraux A, Iannetti GD. Nociceptive laser-evoked brain potentials do not reflect nociceptive-specific neural activity. J Neurophysiol. 2009;101:3258–3269. doi: 10.1152/jn.91181.2008. [DOI] [PubMed] [Google Scholar]
- 84.Kanske P, Kotz SA. Concreteness in emotional words: ERP evidence from a hemifield study. Brain Res. 2007;1148:138–148. doi: 10.1016/j.brainres.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 85.Kissler J, Assadollahi R, Herbert C. Emotional and semantic networks in visual word processing: Insights from ERP studies. Prog Brain Res. 2006;156:147–183. doi: 10.1016/S0079-6123(06)56008-X. [DOI] [PubMed] [Google Scholar]
- 86.Schindler S, Wegrzyn M, Steppacher I, Kissler J. Perceived communicative context and emotional content amplify visual word processing in the fusiform gyrus. J Neurosci. 2015;35:6010–6019. doi: 10.1523/JNEUROSCI.3346-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Raz S, Dan O, Zysberg L. Neural correlates of emotional intelligence in a visual emotional oddball task: An ERP study. Brain Cogn. 2014;91:79–86. doi: 10.1016/j.bandc.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 88.Ring C, Kavussanu M, Willoughby AR. Emotional modulation of pain-related evoked potentials. Biol Psychol. 2013;93:373–376. doi: 10.1016/j.biopsycho.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: How low-level computational processes contribute to meta-cognition. Neuroscientist. 2007;13:580–593. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- 90.Van Overwalle F. Social cognition and the brain: A meta-analysis. Hum Brain Mapp. 2009;30:829–858. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pfurtscheller G, Woertz M, Supp G, Lopes da Silva FH. Early onset of post-movement beta electroencephalogram synchronization in the supplementary motor area during self-paced finger movement in man. Neurosci Lett. 2003;339:111–114. doi: 10.1016/s0304-3940(02)01479-9. [DOI] [PubMed] [Google Scholar]
- 92.Golland Y, Arzouan Y, Levit-Binnun N. The mere co-presence: Synchronization of autonomic signals and emotional responses across co-present individuals not engaged in direct interaction. PLoS One. 2015;10:e0125804. doi: 10.1371/journal.pone.0125804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Helm JL, Sbarra D, Ferrer E. Assessing cross-partner associations in physiological responses via coupled oscillator models. Emotion. 2012;12:748–762. doi: 10.1037/a0025036. [DOI] [PubMed] [Google Scholar]
- 94.Singh H, et al. The brain’s response to pleasant touch: An EEG investigation of tactile caressing. Front Hum Neurosci. 2014;8:893. doi: 10.3389/fnhum.2014.00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peled-Avron L, Levy-Gigi E, Richter-Levin G, Korem N, Shamay-Tsoory SG. The role of empathy in the neural responses to observed human social touch. Cogn Affect Behav Neurosci. 2016;16:802–813. doi: 10.3758/s13415-016-0432-5. [DOI] [PubMed] [Google Scholar]
- 96.Heed T. Somatosensation: Putting touch on the map. Curr Biol. 2014;24:R119–R120. doi: 10.1016/j.cub.2013.12.037. [DOI] [PubMed] [Google Scholar]
- 97.Babiloni C, et al. Anticipatory electroencephalography alpha rhythm predicts subjective perception of pain intensity. J Pain. 2006;7:709–717. doi: 10.1016/j.jpain.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 98.Krishnan A, et al. Somatic and vicarious pain are represented by dissociable multivariate brain patterns. Elife. 2016;5:e15166. doi: 10.7554/eLife.15166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Birnie KA, et al. Systematic review and meta-analysis of distraction and hypnosis for needle-related pain and distress in children and adolescents. J Pediatr Psychol. 2014;39:783–808. doi: 10.1093/jpepsy/jsu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lamm C, Porges EC, Cacioppo JT, Decety J. Perspective taking is associated with specific facial responses during empathy for pain. Brain Res. 2008;1227:153–161. doi: 10.1016/j.brainres.2008.06.066. [DOI] [PubMed] [Google Scholar]
- 101.Singer T, et al. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 102.Lawrence EJ, et al. The role of “shared representations” in social perception and empathy: An fMRI study. Neuroimage. 2006;29:1173–1184. doi: 10.1016/j.neuroimage.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 103.Lang S, Yu T, Markl A, Müller F, Kotchoubey B. Hearing others’ pain: Neural activity related to empathy. Cogn Affect Behav Neurosci. 2011;11:386–395. doi: 10.3758/s13415-011-0035-0. [DOI] [PubMed] [Google Scholar]
- 104.Yang C-Y, Decety J, Lee S, Chen C, Cheng Y. Gender differences in the mu rhythm during empathy for pain: An electroencephalographic study. Brain Res. 2009;1251:176–184. doi: 10.1016/j.brainres.2008.11.062. [DOI] [PubMed] [Google Scholar]
- 105.Banissy MJ, Kanai R, Walsh V, Rees G. Inter-individual differences in empathy are reflected in human brain structure. Neuroimage. 2012;62:2034–2039. doi: 10.1016/j.neuroimage.2012.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Martínez-Jauand M, et al. Somatosensory activity modulation during observation of other’s pain and touch. Brain Res. 2012;1467:48–55. doi: 10.1016/j.brainres.2012.05.055. [DOI] [PubMed] [Google Scholar]
- 107.Fusaro M, Tieri G, Aglioti SM. Seeing pain and pleasure on self and others: Behavioral and psychophysiological reactivity in immersive virtual reality. J Neurophysiol. 2016;116:2656–2662. doi: 10.1152/jn.00489.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lamm C, Silani G, Singer T. Distinct neural networks underlying empathy for pleasant and unpleasant touch. Cortex. 2015;70:79–89. doi: 10.1016/j.cortex.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 109.Baker JM, et al. 2016. Sex differences in neural and behavioral signatures of cooperation revealed by fNIRS hyperscanning. Sci Rep 6, 10.1038/srep26492, and erratum (2016) 6, 10.1038/srep30512.
- 110.Pan Y, Cheng X, Zhang Z, Li X, Hu Y. Cooperation in lovers: An fNIRS-based hyperscanning study. Hum Brain Mapp. 2017;38:831–841. doi: 10.1002/hbm.23421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Koenig T, et al. Decreased functional connectivity of EEG theta-frequency activity in first-episode, neuroleptic-naıve patients with schizophrenia: Preliminary results. Schizophr Res. 2001;50:55–60. doi: 10.1016/s0920-9964(00)00154-7. [DOI] [PubMed] [Google Scholar]
- 112.Roth RS, Geisser ME, Williams DA. Interventional pain medicine: Retreat from the biopsychosocial model of pain. Transl Behav Med. 2012;2:106–116. doi: 10.1007/s13142-011-0090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lumley MA, et al. Pain and emotion: A biopsychosocial review of recent research. J Clin Psychol. 2011;67:942–968. doi: 10.1002/jclp.20816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hadjistavropoulos T, et al. A biopsychosocial formulation of pain communication. Psychol Bull. 2011;137:910–939. doi: 10.1037/a0023876. [DOI] [PubMed] [Google Scholar]
- 115.Granot M, et al. Determinants of endogenous analgesia magnitude in a diffuse noxious inhibitory control (DNIC) paradigm: Do conditioning stimulus painfulness, gender and personality variables matter? Pain. 2008;136:142–149. doi: 10.1016/j.pain.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 116.Gauthier N, Thibault P, Sullivan MJL. Individual and relational correlates of pain-related empathic accuracy in spouses of chronic pain patients. Clin J Pain. 2008;24:669–677. doi: 10.1097/AJP.0b013e318173c28f. [DOI] [PubMed] [Google Scholar]
- 117.Oostenveld R, Fries P, Maris E, Schoffelen J-M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jung T-P, et al. Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clin Neurophysiol. 2000;111:1745–1758. doi: 10.1016/s1388-2457(00)00386-2. [DOI] [PubMed] [Google Scholar]
- 119.Wallstrom GL, Kass RE, Miller A, Cohn JF, Fox NA. Automatic correction of ocular artifacts in the EEG: A comparison of regression-based and component-based methods. Int J Psychophysiol. 2004;53:105–119. doi: 10.1016/j.ijpsycho.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 120.Jammalamadaka SR, SenGupta A. Topics in Circular Statistics. World Scientific Publishing; Singapore: 2000. [Google Scholar]
- 121.Yekutieli D. Hierarchical false discovery rate–controlling methodology. J Am Stat Assoc. 2008;103:309–316. [Google Scholar]
- 122.Reiner-Benaim A, et al. Associating quantitative behavioral traits with gene expression in the brain: Searching for diamonds in the hay. Bioinformatics. 2007;23:2239–2246. doi: 10.1093/bioinformatics/btm300. [DOI] [PubMed] [Google Scholar]
- 123.Goldstein P, Korol AB, Reiner-Benaim A. Two-stage genome-wide search for epistasis with implementation to recombinant inbred lines (RIL) populations. PLoS One. 2014;9:e115680. doi: 10.1371/journal.pone.0115680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gaujoux R, Seoighe C. A flexible R package for nonnegative matrix factorization. BMC Bioinformatics. 2010;11:367. doi: 10.1186/1471-2105-11-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Frigyesi A, Höglund M. Non-negative matrix factorization for the analysis of complex gene expression data: Identification of clinically relevant tumor subtypes. Cancer Inform. 2008;6:275–292. doi: 10.4137/cin.s606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brunet J-P, Tamayo P, Golub TR, Mesirov JP. Metagenes and molecular pattern discovery using matrix factorization. Proc Natl Acad Sci USA. 2004;101:4164–4169. doi: 10.1073/pnas.0308531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Edwards D, Berry JJ. The efficiency of simulation-based multiple comparisons. Biometrics. 1987;43:913–928. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.