Through evolution, glycans have been selected as a class of biomolecules tasked with facilitating information exchange at the cellular boundary (1). Presented as attachments on membrane proteins or linked directly to lipids embedded in the plasma membrane of cells, glycans serve as recognition elements for glycan binding proteins, such as lectins or antibodies, and mediate essential biological processes, ranging from cell–cell adhesion and migration to more complex events associated with organismal development and function. Not surprisingly, glycans also contribute to disease development and progression and have been linked to a large number of pathophysiological processes, including infectious diseases, cancer, or autoimmune disorders (2). However, glycans and their cognate lectin counter-receptors are still rarely considered as suitable drug targets (3).
In PNAS, Xiao et al. (4) employ cell-surface mimetic models to dissect how glycan receptor organization in cellular membranes influences functional association with galectins, a family of human lectins (5). Among their many functions, galectins have been identified to serve as important extracellular modulators of immune responses and have been positively correlated with increased aggressiveness of tumors (6). As such, galectins are certainly enticing targets for medical intervention (7); however, the broad functional polymorphism observed for the galectin family members necessitates that principles governing their interactions with glycan receptors be established first to guide the design of selective galectin-targeting therapeutics.
The structures of glycans define their molecular interactions with lectins and all members of the galectin family generally recognize glycans containing β-galactose residues (8), with modifications such as sialylation (9) or sulfation (10) providing addition affinity. As is typical for most lectins, galectins show weak (high micromolar to low millimolar) affinity for simple soluble β-galactosides, such as lactose. The much higher-affinity interactions (submicromolar) observed for galectins in biological settings are the result of their ability to engage ensembles of glycans at surfaces of cells and in the extracellular matrix in a multivalent fashion (11). This behavior, characteristic to glycans and referred to as the “glycoside cluster effect” (12), provides a mechanism for enhancing the overall affinity and selectivity of glycan recognition by lectins, while also enabling the setting of thresholds for triggering signaling responses. Modulating biological functions can thus be achieved not only by altering the structures of glycans but also by varying the density and spatial organization of glycan determinants within the cellular glycocalyx (13).
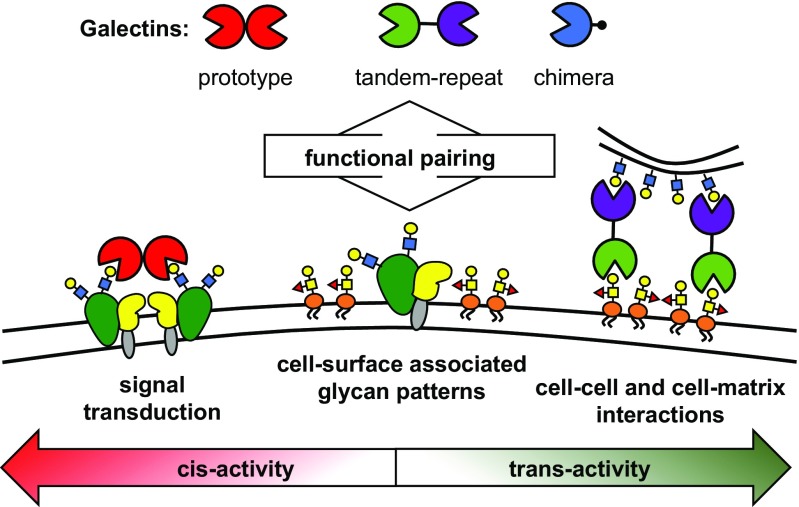
The multivalent presentation of glycans on surfaces of cells is mirrored in the architecture of lectins, which are often organized to present several glycan recognition domains (CRDs). This is also true for galectins, which can be classified into three distinct groups, according to the organization of their CRDs, as prototypical, chimeric, and tandem-repeat (Fig. 1) (8). The prototypical galectins contain a single CRD and associate into homodimers. The chimeric galectins contain at least two CRDs embedded within a single polypeptide chain separated by a short peptide linker. Finally, the chimeric galectins contain a single CRD and a large N-terminal domain, which facilitates self-aggregation into higher-order oligomers. Their architecture makes galectins well suited to serve as a bridging element between glycoonjugates populating the cell surface (cis-activity) and even between glycan receptors on opposing cells and in the extracellular matrix (trans-activity). While all galectins recognize a common β-galactoside receptor structure, their different abilities to bridge glycans are believed to be responsible for the distinct biological activity profiles observed for the individual members of the galectin family.
Fig. 1.
Galectins, β-galactoside binding mammalian lectins, act as regulators of intracellular trafficking, cell adhesion, and cell–cell signaling. The unique activity profiles observed for each member of the galectin family are associated with their ability to bridge glycan receptors. Whether galectins engage glycan partners presented on a single cell (cis-activity) or on two opposing cells or in the extracellular matrix (trans-activity) determines their biological functions. Both the structural identity of the glycan determinants and their spatial organization at the cellular boundary are likely to instruct the functional paring of galectins with their glycan receptors.
In their study, Xiao et al. (4) report a simple chemical system for dissecting the functional consequences of glycan presentation and spatial organization on their ability to induce trans-activity in galectins. The authors used self-assembling glycosylated amphiphilic dendrimers, termed Janus glycodendrimers, to generate glycodendrimersome (GDS) nanoparticles displaying lectin receptors in a manner that may resemble their presentation on glycolipids in cellular membranes (14). The self-assembly process allowed for modular display of unique glycan determinants of galectin binding (e.g., the canonical galectin ligand, lactose, or the higher affinity ligand, 3′-O-sulfated lactose) and their combinations at the nanoparticle surface, with good control over glycan composition and density. The GDS nanoparticles provided a platform to rapidly analyze the ability of unique glycan displays to promote cross-linking activity in galectins, which was detected as an increase in turbidity due to particle aggregation.
Synthetic glycomaterials, such as glycopolymers and glycodendrimers, have long been used (15) as soluble probes with controlled glycan presentations to provide mechanistic insights into how glycan valency (16) and glycoconjugate scaffold architecture (17) influence binding by oligomeric lectins. These tools were also instrumental in revealing the roles of cell-surface glycoconjugates in signaling receptor organization and modulation of signal transduction (18). While having many useful attributes, soluble glycoconjugates are limited in their ability to emulate the larger scale glycan displays found on cellular membranes, such as glycolipid patches. The GDS system addressed this limitation and provided new insights into how trans-activity of galectins may be regulated through dynamic changes in cell surface glycan presentation.
To obtain a fuller picture of the mutual relationships between cell surface glycan presentation and galectin recognition, Xiao et al. (4) also generated an extended set of galectin structures, including members of the prototype (Gal1), tandem-repeat (Gal4 and Gal8), and chimeric (Gal3) galectin subgroups, as well as newly engineered galectins to probe the effects of CRD organization and spacing on receptor cross-linking. The combination of both GDS and lectin toolkits yielded several interesting observations with implications for our understanding of how galectin activity might be regulated in cellular microenvironments.
While glycan ligands with increased affinity for their lectin partners would normally be expected to elicit a stronger recognition event, this may not necessarily translate into a better ability to induce receptor bridging. As Xiao et al. (4) report, presentation of 3-O-sulfated lactose (a more potent inhibitor of galectin binding compared with lactose) on the GDS particle did, indeed, yield enhancements in cross-linking by galectins 4 and 8 of the tandem-repeat class; however, it produced the opposite effect with the prototypical galectin 1. The programmability of the GDS surface also allowed for variations in the surface density of the active epitope by inclusion of spectator mannoside glycans, which are not natural receptors for galectins. This modification revealed greatly increased sensitivity of galectin 8 toward variations in 3-O-sulfated lactose displays compared with galectin 4. This suggests that dynamic alterations in glycosylation patterns may provide a functional switch for the interactions of galectins at the cell surface. Interestingly, the GDS system also provided evidence for the ability of galectins to cooperate in stabilizing nascent cross-linked networks.
Although alternative splicing in tandem-repeat galectins to produce protein variants with variable linker regions connecting
Combined, the findings of the Xiao et al. study begin to expose the intricate mechanisms through which galectins may achieve their function in the complex setting of the cellular glycocalyx.
the respective CRD domains is common, the functional consequences of altering the linker length have not been well-defined. By testing several naturally occurring as well as engineered variants of galectins 4 and 8 in the GDS platform, Xiao et al. (4) discovered significant effects of linker length variations on aggregation capacity of the lectins. This points to the possible functions of the linker region, which unlike the CRD regions shows little sequence homology across galectin classes, in regulating the activity of tandem-repeat galectins.
Combined, the findings of the Xiao et al. study (4) begin to expose the intricate mechanisms through which galectins may achieve their function in the complex setting of the cellular glycocalyx. Complete mapping of the galectin interactome will require considerable effort and will necessitate a better understanding of the contributions from not only simple galactoside-bearing glycolipids, but also larger galectin receptor structures embedded within the extended glycocalyx, particularly complex N-glycans and poly-N-acetyllactosamine chains (19). The programmable GDSs reported by Xiao et al. (4) are well positioned to aid in this quest by defining the broader principles that govern galectin engagement in biological settings. A more detailed view of the functional pairing between glycan displays and galectin function will help guide the design of glycomaterials capable of modulating galectin activity in vivo. Such efforts are already underway, as synthetic neoglycoproteins (20) or self-assembling glycopeptide nanoparticles (21) and nanofibers (22), which generate biomimetic glycan displays, are being explored as selective ligands for galectins or as biomaterials for modulating galectin activity.
Acknowledgments
My research is supported by the National Institutes of Health via the Director’s New Innovator Award (1DP2HD087954-01) and via a grant from the National institute of Allergy and Infectious Diseases (5R21AI129894-02). Significant support was also provided by the Alfred P. Sloan Foundation, the Research Corporation for Science Advancement via its Cottrell Scholar award, and the Programs of Excellence in Glycosciences (National Heart, Lung, and Blood Institute: 5P01HL107150-07).
Footnotes
The author declares no conflict of interest.
See companion article on page E2509.
References
- 1.Varki A, Lowe JB. 2017. Biological roles of glycans. Essentials of Glycobiology, Chapter 6, eds Varki A, et al. (Cold Spring Harbor Lab Press, Cold Spring Harbor, NY), 3rd Ed.
- 2.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 3.Hudak JE, Bertozzi CR. Glycotherapy: New advances inspire a reemergence of glycans in medicine. Chem Biol. 2014;21:16–37. doi: 10.1016/j.chembiol.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao Q, et al. Exploring functional pairing between surface glycoconjugates and human galectins using programmable glycodendrimersomes. Proc Natl Acad Sci USA. 2018;115:E2509–E2518. doi: 10.1073/pnas.1720055115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings RD, Liu F-T, Vasta GR. 2017. Galectins. Essentials of Glycobiology, Chapter 36, eds Varki A, et al. (Cold Spring Harbor Labo Press, Cold Spring Harbor, NY), 3rd Ed.
- 6.Kaltner H, et al. Galectins: Their network and roles in immunity/tumor growth control. Histochem Cell Biol. 2017;147:239–256. doi: 10.1007/s00418-016-1522-8. [DOI] [PubMed] [Google Scholar]
- 7.Yang RY, Rabinovich GA, Liu FT. Galectins: Structure, function and therapeutic potential. Expert Rev Mol Med. 2008;10:e17. doi: 10.1017/S1462399408000719. [DOI] [PubMed] [Google Scholar]
- 8.Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994;269:20807–20810. [PubMed] [Google Scholar]
- 9.Allen HJ, Ahmed H, Matta KL. Binding of synthetic sulfated ligands by human splenic galectin 1, a beta-galactoside-binding lectin. Glycoconj J. 1998;15:691–695. doi: 10.1023/a:1006988515346. [DOI] [PubMed] [Google Scholar]
- 10.Stowell SR, et al. Galectin-1, -2, and -3 exhibit differential recognition of sialylated glycans and blood group antigens. J Biol Chem. 2008;283:10109–10123. doi: 10.1074/jbc.M709545200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dam TK, Gerken TA, Brewer CF. Thermodynamics of multivalent carbohydrate-lectin cross-linking interactions: Importance of entropy in the bind and jump mechanism. Biochemistry. 2009;48:3822–3827. doi: 10.1021/bi9002919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lundquist JJ, Toone EJ. The cluster glycoside effect. Chem Rev. 2002;102:555–578. doi: 10.1021/cr000418f. [DOI] [PubMed] [Google Scholar]
- 13.Brewer CF, Miceli MC, Baum LG. Clusters, bundles, arrays and lattices: Novel mechanisms for lectin-saccharide-mediated cellular interactions. Curr Opin Struct Biol. 2002;12:616–623. doi: 10.1016/s0959-440x(02)00364-0. [DOI] [PubMed] [Google Scholar]
- 14.Percec V, et al. Modular synthesis of amphiphilic Janus glycodendrimers and their self-assembly into glycodendrimersomes and other complex architectures with bioactivity to biomedically relevant lectins. J Am Chem Soc. 2013;135:9055–9077. doi: 10.1021/ja403323y. [DOI] [PubMed] [Google Scholar]
- 15.Huang ML, Godula K. Nanoscale materials for probing the biological functions of the glycocalyx. Glycobiology. 2016;26:797–803. doi: 10.1093/glycob/cww022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mammen M, Choi S-K, Whitesides GM. Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors. Angew Chem Int Ed. 1998;37:2754–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Gestwicki JE, Cairo CW, Strong LE, Oetjen KA, Kiessling LL. Influencing receptor-ligand binding mechanisms with multivalent ligand architecture. J Am Chem Soc. 2002;124:14922–14933. doi: 10.1021/ja027184x. [DOI] [PubMed] [Google Scholar]
- 18.Kiessling LL, Grim JC. Glycopolymer probes of signal transduction. Chem Soc Rev. 2013;42:4476–4491. doi: 10.1039/c3cs60097a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patnaik SK, et al. Complex N-glycans are the major ligands for galectin-1, -3, and -8 on Chinese hamster ovary cells. Glycobiology. 2006;16:305–317. doi: 10.1093/glycob/cwj063. [DOI] [PubMed] [Google Scholar]
- 20.Laaf D, Bojarová P, Pelantová H, Křen V, Elling L. Tailored multivalent neo-glycoproteins: Synthesis, evaluation, and application of a library of galectin-3-binding glycan ligands. Bioconjug Chem. 2017;28:2832–2840. doi: 10.1021/acs.bioconjchem.7b00520. [DOI] [PubMed] [Google Scholar]
- 21.Bonduelle C, et al. Multivalent effect of glycopolypeptide based nanoparticles for galectin binding. Chem Commun (Camb) 2016;52:11251–11254. doi: 10.1039/c6cc06437j. [DOI] [PubMed] [Google Scholar]
- 22.Restuccia A, Tian YF, Collier JH, Hudalla GA. Self-assembled glycopeptide nanofibers as modulators of galectin-1 bioactivity. Cell Mol Bioeng. 2015;8:471–487. doi: 10.1007/s12195-015-0399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]