Abstract
Targeted prostate biopsy using magnetic resonance imaging (MRI) guidance is improving accuracy of prostate cancer (CaP) diagnosis. This new biopsy technology is especially important for men undergoing active surveillance, improving patient selection for enrollment and enabling precise longitudinal monitoring. MRI/US fusion biopsy allows for three functions not previously possible with US-guided biopsy: targeting of suspicious regions, template-mapping for systematic sampling, and tracking of cancer foci over time. This article reviews the evolving role of the new biopsy methods in active surveillance, including the UCLA Active Surveillance pathway, which has incorporated MRI/US fusion biopsy from program inception, as a possible model.
Keywords: Prostate cancer, active surveillance, fusion biopsy, prostate MRI, prostate biopsy
Introduction
Active surveillance (A.S.) is a management strategy for men with low risk prostate cancer (CaP). The aim of this strategy, which was introduced in the mid-1990s (Hopkins, Toronto), is to reduce over-treatment of indolent cancers while, at the same time, allow for the recognition of potentially serious cancers in time for curative intervention1,2. Use of A.S. is the most rapidly-growing approach to new cases of CaP today. Up to 40% of eligible men now select this management strategy, compared to very few a decade ago3. Thus, regarding newly-diagnosed CaP, the present time period may fairly be called an era of Active Surveillance.
A.S. relies primarily on biopsy information to determine whether or not the patient is appropriate for A.S. entry and to subsequently determine whether or not disease progression is found at follow-up. The original and most referenced biopsy standards for A.S. are the “Epstein criteria” developed in 1994: (1) absence of any Gleason pattern 4 or 5; (2) no more than 2 cores involved; and (3) no more than 50% tumor involvement in any core1. These 3 findings on needle biopsy, when combined with limits on PSA and PSA density, predicted ‘insignificant’ prostate cancer in the prostatectomy specimens of 157 men (all T1c) with overall accuracy of 73%. More than 20 years later, variations of the Epstein criteria are still widely used standards for entry into A.S.
However, the prostate biopsy of today is capable of characterizing true prostate pathology better than the prostate biopsy of the early 1990's. During that earlier time period (and to a large extent today), most active surveillance programs employed transrectal ultrasound (TRUS)-guided biopsy, as first described in the mid-1980s. Often, however, TRUS fails to visualize tumors, and the biopsy is essentially a blind sampling of the organ. Newer biopsy methods, particularly targeted biopsies using MRI-guidance, have led to improved detection and risk stratification of prostate cancer. Targeted biopsy techniques involving MRI/US fusion devices also allow for precise tracking of cancerous sites to detect possible progression. The American Urological Association has taken a position in favor of MRI-guided biopsy in the repeat-biopsy scenario4. In this review, we highlight the advances in MRI-guided prostate biopsy as applied to men undergoing A.S. for prostate cancer.
Active Surveillance (A.S.): Origins with US-guided Biopsy
Researchers at Johns Hopkins University and the University of Toronto were two of the earliest proponents of A.S.1,2 Programs started at these institutions in the mid-1990s are now among the largest in the world. From that time until very recently, both groups have relied primarily on US-guided biopsy for patient selection. Twenty-year follow-up from the Toronto cohort of nearly 1000 men revealed a 1.5% prostate cancer-specific mortality and 1.3% metastasis rate, with almost a 10-fold greater risk of mortality from other causes during the time interval5. Similarly, the Hopkins group of almost 1300 men with up to 18 years follow-up encountered a 0.4% metastasis rate and 0.15% cancer-specific mortality rate6. Thus, whether entry criteria were strict, as in the Hopkins program, or more liberal, as in the Toronto program, disease progression to incurability is not common in men participating in large A.S. programs employing conventional biopsy methods. Similar outcomes were recently reported in the PIVOT trial after 20 years of follow-up, in which US-guided biopsy was also used7.
However, using conventional US-guided biopsy, the initial findings are often later found to under-estimate the actual pathology, resulting in a delay of appropriate treatment. A SEER database analysis of over 10,000 patients revealed that 44% of ‘low-risk’ patients actually have at least intermediate-risk CaP at prostatectomy8. In another study using US-guided biopsy, 27% of men qualifying for A.S. (strict criteria) who underwent an immediate confirmatory biopsy were found to harbor more aggressive disease than found on initial biopsy9. Among 626 men in France who underwent prostatectomy despite fulfilling strict criteria for A.S. on US-guided biopsy, final pathology revealed “unfavourable disease” (stage >T2 and/or Gleason score > 6) in 50%10. In a recent report, where men found to have low-intermediate risk lesions based on conventional biopsy elected immediate prostatectomy, unfavorable pathology was found in 25% of men; a few had positive lymph nodes or seminal vesical involvement11. Thus, conventional biopsy often fails to reveal pathologic findings which may disqualify a man from A.S.
The recent ProtecT trial from England provides additional evidence of the benefits of A.S. but also of the limitations of conventional biopsy12. In this landmark trial, 1500 men were randomized to active monitoring, radical prostatectomy, or radiotherapy. Active monitoring in this study involved checking serum PSA levels every 3 months for the first year and every 6-12 months thereafter, with no role for repeat prostate biopsy. 75% of the study participants had T1c, Gleason 6 disease based on standard TRUS biopsy. 10-year cancer specific mortality (10%) and all-cause mortality (1%) were similar across all three arms, but incidence of metastasis (6% vs 2-3%) and clinical progression (23% vs 9%) were higher in the active monitoring cohort compared to the definitive treatment arms. This study did not include traditional active surveillance, with prostate biopsies at regular intervals for histopathologic assessment for disease progression. The addition of regular repeat prostate biopsies to active monitoring arm in this study may have mitigated the observed risk of metastasis and progression. Taken altogether, the above studies provide convincing evidence that A.S. is generally an excellent management strategy, but when based on conventional biopsy, the actual risk of ‘low-risk’ disease is often under-estimated.
Prostate MRI and MRI-guided Biopsy
Prostate MRI, which has become increasingly refined over the past decade, allows visualization of tumors and biopsy guidance not previously available. Attesting to the value of MRI-guided biopsy, two prospective studies of more than 1000 men each have been published in the past few years13,14. Using two different MRI/US fusion devices, both groups compared within patients the yield of systematic biopsies vs biopsies targeting MRI-visible lesions. The authors reached similar conclusions: targeting allows detection of more clinically significant cancers than systematic sampling alone. In both studies, targeted biopsy yield was directly related to MRI grade of the region of interest. The similarities and differences of these two large studies were clarified by Filson, et al14.
More than 80% of important prostate cancers may be visualized using MRI, whereas most PSA-detected cancers are not visible on US15,16. In 2016, the American Urological Association issued a joint ‘White Paper’ with the Society of Abdominal Radiology endorsing MRI-guided biopsy in the repeat biopsy setting4. Unlike any other imaging modality currently available, MRI is able to reveal prostate cancers not otherwise visible, providing greatly improved biopsy guidance.
MRI in Active Surveillance
Over the past two decades, MRI to study the prostate and A.S. to manage prostate cancer have evolved in parallel. MRI has been shown to supplement clinical data, helping to correctly identify men most suitable for A.S.17 MRI-visibility of tumors is associated with biopsy pathology that may render a patient unsuitable for active surveillance, and a negative MRI correlates with reduced risk of adverse biopsy pathology18. In a meta-analysis, Schoots and colleagues found that the presence of a prostatic lesion on MRI increased the likelihood of Gleason score upgrading in radical prostatectomy specimens from 27% to 43%19. Physicians monitoring A.S. patients assume responsibility for early detection of a potentially lethal cancer; thus, the best possible characterization of whole-organ pathology---initially and during follow-up--- is highly desirable.
Targeted Biopsy Before A.S. Enrollment
Up until very recently, biopsy of men entering A.S. has been performed using ultrasound-guidance. However, patient selection for A.S. using US guidance alone is often incorrect. Approximately 30% of men entering A.S. programs based on conventional prostate biopsy later undergo active treatment2,20. Explanations for the switch are numerous, but discovery of more severe cancer than originally diagnosed is medically the most important9,11. Anxiety, caused at least partly by uncertainty of biopsy accuracy, is another force that may drive men to active treatment21. When men, who apparently qualify for A.S. based on conventional biopsy, later undergo targeted biopsy, the rate of exclusion from A.S. is considerable: 36% in the UCLA experience22. When men conventionally biopsied are found to meet Epstein criteria but still select surgery, the upgrade rate at whole-organ pathology, excluding men from A.S., is reported to be as high as 40%11,23. Targeting of MRI-visible lesions helps to reduce sampling error and improve concordance with whole-organ pathology15. At the same time, that targeting often results in more positive cores and longer cancer core lengths than would be found by conventional biopsy. As the use of MRI-guided biopsies grows, A.S. enrollment criteria are expected to change, thus avoiding inappropriate exclusion of men from A.S. based on criteria established decades ago.
Targeted Biopsy to Confirm Low Risk
When low-risk CaP is first diagnosed (‘initial diagnostic biopsy’), the next biopsy following is called the confirmatory biopsy, usually performed within 6-12 months of the first. When low-risk is re-affirmed, A.S. can proceed with more assuredness than without such confirmation.
In the Table, results are summarized from 8 published studies (N>100 each), where MRI-guided biopsies were used in A.S. after the initial diagnostic biopsy and are considered ‘confirmatory’24–31. All originate in the past few years, and all are from academic medical centers in the U.S. or Europe. More than 1900 men are included. Men with low, very low, and intermediate-risk CaP, as defined by each author, were included; initial biopsy method was US-guided, except for studies by Frye24 and Chang31, who used MRI/US fusion for initial and subsequent biopsy. Average interval between biopsies was 1-2 years. Seven of the eight authors employed an MRI/US fusion device, and one used direct in-bore biopsy28. The majority of patients (N=1323) had an MRI-visible lesion (i.e., PI-RADS ≥3); median PSA levels ranged from 4.9 to 7.8 ng/ml and PSA density from 0.08 to 0.18 ng/ml/cc.
Table. Published Studies of MRI-guided Biopsies in Men Undergoing Active Surveillance of Prostate cancer*.
| Study | N | Risk Category | Patients with MRI targets (PI-RADS ≥3) | Baseline PSA, ng/ml, median (IQR) | Baseline PSAD, median, ng/ml/cc (IQR) | Upgrade ≥3+4, N (%) | Upgrade ≥4+3, N | Upgrade on MRI TB only, N | Upgrade on SB only, N |
|---|---|---|---|---|---|---|---|---|---|
| Frye (NCI) 24 [UroNav™, Invivo] | 166 | Low risk (N=128)a | 126g | 5.69 (±4.19)j | 0.12 (±0.09)j | 37 (29%) | NR | 14 | 12 |
| Intermediate risk (N=38)b | 6.16 (±3.54)j | 0.13 (±0.08)j | NA | 12 | 8 | 3 | |||
| Recabal (MSKCC) 25 [UroStation™, Koelis] | 206 | Low riska | 135h | 5.2 (3.8-7.4) | 0.13 (0.08-0.19) | 72 (35%) | NR | NR | 25 |
| Tran (UCSF) 26 [UroNav™, Invivo] | 207 | Low and intermediate riskc | 202 | 5.9 (4.3-8.8) | 0.15 (0.09-0.21) | 83 (40%) | 25 | 30 | 49 |
| Alberts (EUMC)27 [UroStation™, Koelis] | 210 | Low riskd | 134 | 7.8 (5.4-11.7) | 0.18 (0.11-0.28) | 55 (26%) | 17 | 51 | 4 |
| Venderink (RUMC)28 [DynaTRIM™, Invivo] | 224 | Low riska | 224 | 7.8 (5.7-11.0) | 0.17 (0.11-0.23) | 95 (42%) | 40 | 95 | NP |
| Ma (Hopkins) 29 [UroNav™, Invivo] | 256 | Very low risk and low riske | 103 | 5.4 (3.2-7.4) | 0.08 (0.06-0.13) | 25 (10%) | NR | 4 | 18 |
| Ouzzane (UL/UP)30 [UroStation™, Koelis and MyLab70™, Esaote] | 281 | Very low riskf | 163h | 6.0 (5.0-7.0) | 0.11 (0.08-0.15) | 28 (10%)k | 4 | 28 | NP |
| Chang (UCLA)31 [Artemis™, Eigen] | 352 | Low risk (N=268)a | 236i | 4.9 (2.8-6.6) | 0.108 (±0.078)j | 72 (27%) | 18 | 13 | 43 |
| Intermediate risk (N=84)b | 6.0 (4.4-8.8) | 0.164 (±0.109)j | NA | 19 | 7 | 10 |
Only studies of >100 men are included.
TB=Targeted Biopsy; SB=Systematic Biopsy; NA=Not Applicable; NR=Not Reported; NP=Not Performed
ISUP Group 1;
ISUP Group 2;
CAPRA;
PRIAS Criteria or Gleason 3+3;
NCCN Guidelines;
PSA < 10 ng/ml, Gleason 3+3, ≤ 5 mm CCL, ≤ 2 positive biopsy cores;
NIH ≥ moderate;
Likert ≥ 3;
UCLA or PI-RADS ≥ 3;
Mean (± SD);
Gleason ≥3+4 or Cancer Core Length > 5 mm
A synthesis of the table is difficult, because of the different methods used. However, clearly both targeted and systematic biopsies were needed to detect upgrades, highlighting the contribution of targeting to overall accuracy. Aside from the very low risk studies (Ma & Ouzzane), the rate of upgrading found with MRI-guided biopsy was 26%-42% which is substantially higher than the 2.5%-28% range reported when US-guided biopsy is used32. In men entering with defined intermediate-risk lesions, upgrading to GS>4+3 was found in 19/84 (23%) in the Chang study and 12/38 (32%) in the Frye study. The appreciable upgrade rates using MRI-guided biopsy most likely indicate improved sensitivity of the new method.
Tracking Biopsy in Active Surveillance
An advantage of MR/US fusion devices is the ability to track the location of biopsy cores, whether within or apart from MRI-visible lesions, for precise identification of biopsy core location. Tracking enables subsequent re-biopsy of the site of a prior positive biopsy, accurate to within a few mm33. Longitudinal assessment of specific cancerous sites in the prostate appears to increase detection of important cancers in men on active surveillance.
Chang et al studied the value of tracking biopsies in 352 men who underwent MRI/US fusion biopsy and were found to have low or intermediate risk CaP; the men entered A.S. and had another fusion biopsy approximately one year later31. All cancerous sites were re-biopsied by returning to the original positive sites during the second session; systematic non-tracking biopsy was then also performed. Of the 91 men who exhibited GS upgrading, 48 (53%) were detected only by tracking. As indicated in the Table, 23% of men with intermediate risk lesions were found to have >4+3 disease in follow-up session using tracking technology. These men were thus disqualified from active surveillance because of the finding of high-risk disease (Figure 1). Tracking of cancer foci enhances follow-up in active surveillance and allows increased detection of men for whom A.S. is no longer appropriate.
Figure 1.
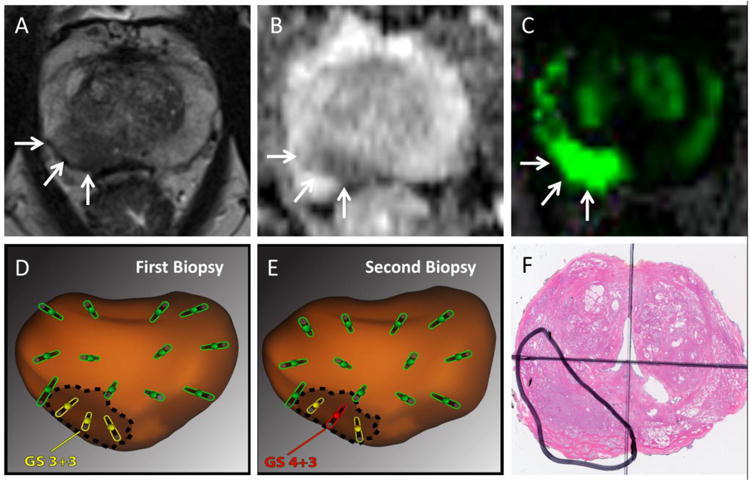
Value of Targeted and Tracking Biopsy in Active Surveillance (Example Case). A 69 year-old male with a rising PSA underwent a mpMRI. A suspicious region of interest (PI-RADS 4, arrows) was found in T2-weighted (A), diffusion-weighted (B), and dynamic contrast-enhanced images (C). At first biopsy (D) systematic (green) and targeted (yellow) cores were obtained using an MR/US fusion device (Artemis; Eigen, Grass Valley, California). Biopsy locations were stored in the device. Low-risk prostate cancer (Gleason Score 3+3) was detected in the region of interest. At one year follow-up (E), systematic and targeted biopsy cores were taken, using tracking technology to re-biopsy known cancer site31. The second biopsy revealed re-classification to Gleason score 4+3 cancer (red), disqualifying this patient from further active surveillance. Radical prostatectomy was performed (F), revealing Gleason 4+3 cancer (encircled) consistent in grade and location with that detected on targeted tracking biopsy. More than half of all GS upgrades in the UCLA A.S. program were detected only by tracking biopsy31.
The precision of tracking, as used in the above study, was confirmed by Palapattu and colleagues using sophisticated molecular analysis34. Of 26 men undergoing tracking biopsy one year after identification of a low-grade lesion, the same clone of cells was re-identified at the 2nd biopsy in 96% of men. Thus, tracking allows subsequent re-sampling of a specific cancer focus, which is another advantage of MRI/US fusion systems for men participating in A.S.
Tracking of cancerous sites requires an image-fusion device. So-called ‘cognitive’ image fusion, i.e., visual estimation on US of where an MRI-visible lesion is located, may be adequate for targeted biopsy of larger lesions, but provides no means for tracking biopsy35.
Can MRI replace biopsy?
If an MRI without lesions could exclude cancer, many men in A.S. could be spared biopsy. If tissue sampling restricted to MRI-visible lesions would suffice to accurately characterize whole-organ pathology, the number of samples could be reduced. And if a clearly positive MRI were always histologically positive for cancer, biopsy would be unnecessary to begin active treatment. None of these scenarios is completely tenable at this time. Several studies prove the existence of clinically-significant cancers that are not visible on MRI or that are located in parts of the prostate away from MRI-visible lesions36–38. Even a Grade 5 lesion on MRI may harbor no clinically significant cancer. Thus, at least initially for men entering A.S., both systematic and targeted biopsies should be obtained, and when otherwise indicated clinically---palpable abnormality, increased PSA density, racial or family history---a negative MRI should not preclude systematic biopsy39. However, in men undergoing A.S. with low-risk lesions diagnosed initially via MRI-guided biopsies, the risk of later upgrading appears very low if repeat MRI remains negative40. Further, with tracking biopsy as described above, repeat MRI is unnecessary in the near term. Prostate MRI is in an evolutionary state and should at this time be generally regarded as providing supplemental, rather than definitive information.
A.S. for Intermediate-Risk Prostate Cancer
Inclusion of men with intermediate-risk CaP in A.S. is consistent with recent clinical guidelines of the American Urological Association, Society of Urologic Oncology, American Society for Clinical Oncology, and National Comprehensive Cancer Network. Accordingly, patients with low-volume Gleason 3+4 disease may be considered for active surveillance 41,42. In support of these guidelines, Cooperberg et al reported in 2011 the inclusion of men with GS3+4, followed in A.S. for up to 4 years with no more upgrading than men with GS3+343.
Appearing to contradict the above is a recent study by Patel and colleagues in which 608 men diagnosed with low-volume intermediate-risk CaP underwent immediate radical prostatectomy. Final pathology revealed adverse findings in 25% of these men11, leading the authors to conclude that A.S. entails increased risk for men with GS3+4 CaP. Musunuru and colleagues at the University of Toronto similarly expressed concern about including men with intermediate risk (PSA 10-20 ng/ml and/or GS 3+4) disease in active surveillance, citing 10-year metastasis free survival and cancer-specific survival of 91% and 97%, respectively, in the intermediate risk cohort compared to 96% and 98%, respectively, in the low risk cohort44. 15-year metastasis free survival and cancer-specific survival in the intermediate risk group dropped to 82% and 89%, respectively, compared to the low risk group with 95% and 97%, respectively.
The implication is that men with a component of GS4 CaP should undergo active treatment. However, in the referenced studies, biopsy was only US-guided, which may fail to detect actual pathology in approximately one-third of cases22. In the Patel study, confirmatory biopsy, which is a key element necessary to help exclude high-risk individuals and used in all A.S. programs (see above), was not performed11. Moreover, of Patel's 150 men with “adverse pathology”, nearly all were intra-capsular Gleason score advances; disease outside the prostate (incurability) was found in only 3.6% of cases. Of further interest, the 25% upgrading rate is similar to the upgrading rate seen with MRI-guided confirmatory biopsy, suggesting that the fraction of men who upgraded might have been detected by the new biopsy method and withdrawn from A.S. in time for cure45.
Taken together, these data stand as justification for further study of confirmatory MRI-guided biopsy to identify potentially aggressive CaP. While men with GS3+4 lesions may be at higher risk for CaP progression than men with GS3+3 lesions, most remain stable and may remain in A.S., albeit with increased vigilance. Age at diagnosis and anticipated years of follow-up are also important considerations when undertaking A.S. for men with GS 3+4 lesions. Further stratification of GS3+4 risk using molecular markers and quantification of the GS4 component will likely help define eligibility for A.S. in the near future46,47.
An A.S. Program Based on MRI-Guided Biopsy
A structured A.S. program was started at UCLA in 2009 and now includes nearly 650 men. The UCLA program differs from most others in that from the start, all men enrolled in this IRB-approved registry underwent MRI-guided biopsy for confirmation and follow-up; many were also diagnosed initially with MRI/US fusion, allowing tracking and re-biopsy of tumorous sites from first detection of cancer. An image-fusion device (Artemis™; Eigen, Grass Valley, California) was used to obtain systematic samples following a 12-site template and, when an MRI-visible lesion was present, targeted samples were obtained from the lesion. When biopsy was confirmatory or in follow-up, all known cancerous sites were re-sampled by tracking biopsy, and all MRI-visible regions of interest were re-sampled. Men were accepted into the registry with low- and low-intermediate risk cancers, including some men with GS3+4 lesions. Some results from the UCLA A.S. registry have been published31,34,36,48.
The protocol currently in place for A.S. at UCLA is shown in Figure 2. This protocol incorporates MRI guidance at confirmatory biopsy, at follow-up, and at initial diagnostic biopsy, if available. For men initially diagnosed with 3+3 disease, an MRI-guided confirmatory biopsy is performed at 6-12 months. For men initially diagnosed with 3+4 disease, confirmatory biopsy is performed within 6 months. Tracking of cancerous sites is routinely performed if prior fusion biopsy was obtained. Results from the confirmatory biopsy dictate follow-up, as shown in the Figure. Men with 3+4 disease, a suspicious prostate MRI lesion (PI-RADS 4-5), or worrisome PSA density (≥0.15 ng/mL/cc) are followed with increased vigilance. For men who remain at the lowest risk, follow-up biopsy is ultimately performed every 2 years, and even this frequency is under review.
Figure 2.
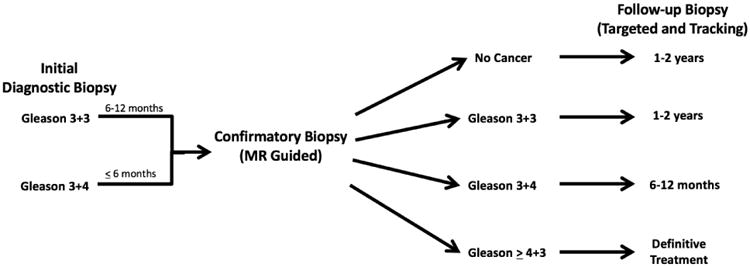
Inclusion of MR-guided biopsy in UCLA pathway for management of men undergoing active surveillance (A.S.) of prostate cancer. Initial diagnostic biopsy is performed via ultrasound (outside referrals) or MRI/US fusion guidance. Confirmatory biopsy is guided by MRI/US fusion and includes targeting, mapping, and when possible, tracking cores (see text and figure 1); following pathway is directed by findings at confirmatory biopsy. Follow-up biopsy should employ tracking of prior positive sites. Repeat MRI is generally not necessary within first year of follow-up. Beyond the first follow-up biopsy, timing of further MRI and biopsies is individualized. In this pathway, formal enrollment in A.S. occurs following the confirmatory biopsy. A PI-RADS 5 lesion or increased PSA density should increase vigilance. See text for discussion of inclusion of men with Gleason score 3+4 in A.S.
Whither the Epstein Criteria?
Men who are appropriate for A.S. may, because of the accuracy of targeting, have biopsy findings that exceed the Epstein criteria, i.e., cancer core lengths may be longer than 50% and numbers of positive cores may be greater than two, depending on how many “shots on target” are taken. Along with PSA density and in some cases patient age, indicators for increased vigilance are MRI grade and tumor volume. However, even with the PI-RADS scoring system, MRI grading is not completely objective, and MRI measures of tumor volume are often inaccurate16,49. Thus, while waiting for molecular characteristics of risk to become routinely available, Gleason score >3+4 remains the most important determinant of A.S. exclusion. Of Epstein's original tissue characteristics of ‘clinical significance,’ only the Gleason Score has survived the advent of MRI-guided biopsy. A replacement for the Epstein criteria, based on MRI-guided biopsy, has not yet been suggested.
MRI-Guided Biopsy: In-Bore or In-Clinic?
MRI-guided biopsy was first performed by radiologists, working within the bore of an MRI tube, i.e., in-bore50. The in-bore approach has the theoretical advantage of direct targeting of suspicious lesions. However, in-bore biopsy is time-consuming, resource- and labor-intensive, and expensive. In-bore biopsy does not include template mapping, and site-specific tracking of cancerous lesions is not possible. Because of the economic advantages and the added utilities (tracking, mapping) of performing the procedure with a fusion device, MRI-guided biopsy has become primarily a urological intervention. When a fusion device is not available or when trans-rectal access is not possible, in-bore biopsy is an alternative.
Conclusion
MRI-guided prostate biopsy provides accuracy of whole-organ characterization better than biopsy guidance only by ultrasound. For men undergoing active surveillance, three functions of the new biopsy method, not possible with conventional ultrasound guidance, make the new method valuable: (1) targeting of suspicious regions of interest, (2) template mapping of the organ, and (3) subsequent tracking of known tumor sites, within or apart from regions of interest, at confirmatory and follow-up biopsy. Use of the new biopsy methods leads to enhanced detection of men not suitable for entry or continuation of active surveillance. In men who enter active surveillance with an intermediate-risk component, i.e., Gleason 3+4=7 pathology, increased vigilance is required, and management in the future may be aided with molecular biomarkers and quantification of Gleason 4 elements.
Acknowledgments
This work was supported in part by the National Cancer Institute (R01CA158627), UCLA CTSI (UL1TR000124), the Jean Perkins Foundation, the Kent Kresa Family Foundation, and the Steven C. Gordon Family Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA J Am Med Assoc. 1994;271(5):368–374. doi: 10.1001/jama.271.5.368. [DOI] [PubMed] [Google Scholar]
- 2.Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28(1):126–131. doi: 10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 3.Cooperberg MR, Carroll PR. Trends in Management for Patients With Localized Prostate Cancer, 1990-2013. JAMA. 2015;314(1):80. doi: 10.1001/jama.2015.6036. [DOI] [PubMed] [Google Scholar]
- 4.Rosenkrantz AB, Verma S, Choyke PL, et al. Prostate MRI and MRI-Targeted Biopsy in Patients with Prior Negative Biopsy. Am Urol Assoc - Guidel. 2016 doi: 10.1016/j.juro.2016.06.079. http://www.auanet.org/guidelines/prostate-mri-and-mri-targeted-biopsy. [DOI] [PMC free article] [PubMed]
- 5.Klotz L, Vesprini D, Sethukavalan P, et al. Long-Term Follow-Up of a Large Active Surveillance Cohort of Patients With Prostate Cancer. J Clin Oncol. 2015;33(3):272–277. doi: 10.1200/JCO.2014.55.1192. [DOI] [PubMed] [Google Scholar]
- 6.Tosoian JJ, Mamawala M, Epstein JI, et al. Intermediate and Longer-Term Outcomes From a Prospective Active-Surveillance Program for Favorable-Risk Prostate Cancer. J Clin Oncol. 2015;33(30):3379–3385. doi: 10.1200/JCO.2015.62.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of Prostatectomy versus Observation for Early Prostate Cancer. N Engl J Med. 2017;377(2):132–142. doi: 10.1056/NEJMoa1615869. [DOI] [PubMed] [Google Scholar]
- 8.Dinh KT, Mahal BA, Ziehr DR, et al. Incidence and Predictors of Upgrading and Up Staging among 10,000 Contemporary Patients with Low Risk Prostate Cancer. J Urol. 2015;194(2):343–349. doi: 10.1016/j.juro.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Berglund RK, Masterson TA, Vora KC, Eggener SE, Eastham JA, Guillonneau BD. Pathological upgrading and up staging with immediate repeat biopsy in patients eligible for active surveillance. J Urol. 2008;180(5):1964-7–8. doi: 10.1016/j.juro.2008.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Hajj A, Ploussard G, de la Taille A, et al. Analysis of outcomes after radical prostatectomy in patients eligible for active surveillance (PRIAS) BJU Int. 2013;111(1):53–59. doi: 10.1111/j.1464-410X.2012.11276.x. [DOI] [PubMed] [Google Scholar]
- 11.Patel HD, Tosoian JJ, Carter HB, Epstein JI, J S, J H. Adverse Pathologic Findings for Men Electing Immediate Radical Prostatectomy. JAMA Oncol. 2017;70(5):760–766. doi: 10.1001/jamaoncol.2017.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamdy FC, Donovan JL, Lane JA, et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med. 2016;375(15):1415–1424. doi: 10.1056/NEJMoa1606220. [DOI] [PubMed] [Google Scholar]
- 13.Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/Ultrasound Fusion–Guided Biopsy With Ultrasound-Guided Biopsy for the Diagnosis of Prostate Cancer. JAMA. 2015;313(4):390. doi: 10.1001/jama.2014.17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filson CP, Natarajan S, Margolis DJA, et al. Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: The role of systematic and targeted biopsies. Cancer. 2016;122(6):884–892. doi: 10.1002/cncr.29874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le JD, Stephenson S, Brugger M, et al. Magnetic Resonance Imaging-Ultrasound Fusion Biopsy for Prediction of Final Prostate Pathology. J Urol. 2014;192(5):1367–1373. doi: 10.1016/j.juro.2014.04.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Priester A, Natarajan S, Khoshnoodi P, et al. Magnetic Resonance Imaging Underestimation of Prostate Cancer Geometry: Use of Patient Specific Molds to Correlate Images with Whole Mount Pathology. J Urol. 2016 doi: 10.1016/j.juro.2016.07.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turkbey B, Mani H, Aras O, et al. Prostate Cancer: Can Multiparametric MR Imaging Help Identify Patients Who Are Candidates for Active Surveillance? Radiology. 2013;268(1):144–152. doi: 10.1148/radiol.13121325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dianat SS, Carter HB, Pienta KJ, et al. Magnetic Resonance–invisible Versus Magnetic Resonance–visible Prostate Cancer in Active Surveillance: A Preliminary Report on Disease Outcomes. Urology. 2015;85(1):147–154. doi: 10.1016/j.urology.2014.06.085. [DOI] [PubMed] [Google Scholar]
- 19.Schoots IG, Petrides N, Giganti F, et al. Magnetic Resonance Imaging in Active Surveillance of Prostate Cancer: A Systematic Review. Eur Urol. 2015;67(4):627–636. doi: 10.1016/j.eururo.2014.10.050. [DOI] [PubMed] [Google Scholar]
- 20.van den Bergh RCN, Roemeling S, Roobol MJ, Roobol W, Schröder FH, Bangma CH. Prospective Validation of Active Surveillance in Prostate Cancer: The PRIAS Study. Eur Urol. 2007;52(6):1560–1563. doi: 10.1016/j.eururo.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Tan HJ, Marks LS, Hoyt MA, et al. The Relationship between Intolerance of Uncertainty and Anxiety in Men on Active Surveillance for Prostate Cancer. J Urol. 2016;195(6):1724–1730. doi: 10.1016/j.juro.2016.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu JC, Chang E, Natarajan S, et al. Targeted Prostate Biopsy to Select Men for Active Surveillance: Do the Epstein Criteria Still Apply? J Urol. 2014;192:385–390. doi: 10.1016/j.juro.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee MC, Dong F, Stephenson AJ, Jones JS, Magi-Galluzzi C, Klein EA. The Epstein Criteria Predict for Organ-Confined But Not Insignificant Disease and a High Likelihood of Cure at Radical Prostatectomy. Eur Urol. 2010;58(1):90–95. doi: 10.1016/j.eururo.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 24.Frye TP, George AK, Kilchevsky A, et al. Magnetic Resonance Imaging-Transrectal Ultrasound Guided Fusion Biopsy to Detect Progression in Patients with Existing Lesions on Active Surveillance for Low and Intermediate Risk Prostate Cancer. J Urol. 2017;197(3):640–646. doi: 10.1016/j.juro.2016.08.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Recabal P, Assel M, Sjoberg DD, et al. The Efficacy of Multiparametric Magnetic Resonance Imaging and Magnetic Resonance Imaging Targeted Biopsy in Risk Classification for Patients with Prostate Cancer on Active Surveillance. J Urol. 2016;196(2):374–381. doi: 10.1016/j.juro.2016.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tran GN, Leapman MS, Nguyen HG, et al. Magnetic Resonance Imaging–Ultrasound Fusion Biopsy During Prostate Cancer Active Surveillance. Eur Urol. 2016 doi: 10.1016/j.eururo.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 27.Alberts AR, Roobol MJ, Drost FJH, et al. Risk-stratification based on magnetic resonance imaging and prostate-specific antigen density may reduce unnecessary follow-up biopsy procedures in men on active surveillance for low-risk prostate cancer. BJU Int. Apr 2017; doi: 10.1111/bju.13836. [DOI] [PubMed] [Google Scholar]
- 28.Venderink W, van Luijtelaar A, Bomers JGR, et al. Results of Targeted Biopsy in Men with Magnetic Resonance Imaging Lesions Classified Equivocal, Likely or Highly Likely to Be Clinically Significant Prostate Cancer. Eur Urol. 2017 Feb; doi: 10.1016/j.eururo.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 29.Ma TM, Tosoian JJ, Schaeffer EM, et al. The Role of Multiparametric Magnetic Resonance Imaging/Ultrasound Fusion Biopsy in Active Surveillance. Eur Urol. 2017 doi: 10.1016/j.eururo.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 30.Ouzzane A, Renard-Penna R, Marliere FO, et al. Magnetic Resonance Imaging Targeted Biopsy Improves Selection of Patients Considered for Active Surveillance for Clinically Low Risk Prostate Cancer Based on Systematic Biopsies. J Urol. 2015;194:350–356. doi: 10.1016/j.juro.2015.02.2938. [DOI] [PubMed] [Google Scholar]
- 31.Chang E, Jones TA, Natarajan S, et al. Value of Tracking Biopsy in Men Undergoing Active Surveillance of Prostate Cancer. J Urol. 2017;0(0) doi: 10.1016/j.juro.2017.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dall'Era MA, Albertsen PC, Bangma C, et al. Active Surveillance for Prostate Cancer: A Systematic Review of the Literature. Eur Urol. 2012;62(6):976–983. doi: 10.1016/j.eururo.2012.05.072. [DOI] [PubMed] [Google Scholar]
- 33.Natarajan S, Marks LS, Margolis DJA, et al. Clinical application of a 3D ultrasound-guided prostate biopsy system. Urol Oncol Semin Orig Investig. 2011;29(3):334–342. doi: 10.1016/j.urolonc.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palapattu GS, Salami SS, Cani AK, et al. Molecular Profiling to Determine Clonality of Serial Magnetic Resonance Imaging/Ultrasound Fusion Biopsies from Men on Active Surveillance for Low-Risk Prostate Cancer. [Accessed June 11, 2017];Clin Cancer Res. 2017 23(4) doi: 10.1158/1078-0432.CCR-16-1454. http://clincancerres.aacrjournals.org/content/23/4/985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wysock JS, Rosenkrantz AB, Huang WC, et al. A Prospective, Blinded Comparison of Magnetic Resonance (MR) Imaging–Ultrasound Fusion and Visual Estimation in the Performance of MR-targeted Prostate Biopsy: The PROFUS Trial. Eur Urol. 2014;66(2):343–351. doi: 10.1016/j.eururo.2013.10.048. [DOI] [PubMed] [Google Scholar]
- 36.Sonn GA, Chang E, Natarajan S, et al. Value of Targeted Prostate Biopsy Using Magnetic Resonance–Ultrasound Fusion in Men with Prior Negative Biopsy and Elevated Prostate-specific Antigen. Eur Urol. 2014;65(4):809–815. doi: 10.1016/j.eururo.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arsov C, Rabenalt R, Blondin D, et al. Prospective Randomized Trial Comparing Magnetic Resonance Imaging (MRI)-guided In-bore Biopsy to MRI-ultrasound Fusion and Transrectal Ultrasound-guided Prostate Biopsy in Patients with Prior Negative Biopsies. Eur Urol. 2015;68(4):713–720. doi: 10.1016/j.eururo.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Vargas HA, Hötker AM, Goldman DA, et al. Updated prostate imaging reporting and data system (PIRADS v2) recommendations for the detection of clinically significant prostate cancer using multiparametric MRI: critical evaluation using whole-mount pathology as standard of reference. Eur Radiol. 2016;26(6):1606–1612. doi: 10.1007/s00330-015-4015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marks LS. Some prostate cancers are invisible to magnetic resonance imaging! BJU Int. 2016;118(4):492–493. doi: 10.1111/bju.13440. [DOI] [PubMed] [Google Scholar]
- 40.Simmons LAM, Kanthabalan A, Arya M, et al. The PICTURE study: diagnostic accuracy of multiparametric MRI in men requiring a repeat prostate biopsy. Br J Cancer. 2017;116(9):1159–1165. doi: 10.1038/bjc.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen RC, Rumble RB, Loblaw DA, et al. Active Surveillance for the Management of Localized Prostate Cancer (Cancer Care Ontario Guideline): American Society of Clinical Oncology Clinical Practice Guideline Endorsement. J Clin Oncol. 2016;34(18):2182–2190. doi: 10.1200/JCO.2015.65.7759. [DOI] [PubMed] [Google Scholar]
- 42.Cher ML, Dhir A, Auffenberg GB, et al. Appropriateness Criteria for Active Surveillance of Prostate Cancer. J Urol. 2017;197(1):67–74. doi: 10.1016/j.juro.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 43.Cooperberg MR, Cowan JE, Hilton JF, et al. Outcomes of Active Surveillance for Men With Intermediate-Risk Prostate Cancer. J Clin Oncol. 2011;29(2):228–234. doi: 10.1200/JCO.2010.31.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Musunuru HB, Yamamoto T, Klotz L, et al. Active Surveillance for Intermediate Risk Prostate Cancer: Survival Outcomes in the Sunnybrook Experience. J Urol. 2016;196(6):1651–1658. doi: 10.1016/j.juro.2016.06.102. [DOI] [PubMed] [Google Scholar]
- 45.Nassiri N, Margolis DJ, Natarajan S, et al. Targeted Biopsy to Detect Gleason Score Upgrading during Active Surveillance for Men with Low versus Intermediate Risk Prostate Cancer. J Urol. 2017;197(3):632–639. doi: 10.1016/j.juro.2016.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guedes LB, Tosoian JJ, Hicks J, Ross AE, Lotan TL. PTEN Loss in Gleason Score 3 + 4 = 7 Prostate Biopsies is Associated with Nonorgan Confined Disease at Radical Prostatectomy. J Urol. 2017;197(4):1054–1059. doi: 10.1016/j.juro.2016.09.084. [DOI] [PubMed] [Google Scholar]
- 47.Sauter G, Steurer S, Clauditz TS, et al. Clinical Utility of Quantitative Gleason Grading in Prostate Biopsies and Prostatectomy Specimens. Eur Urol. 2016;69(4):592–598. doi: 10.1016/j.eururo.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 48.Felker ER, Wu J, Natarajan S, et al. Serial Magnetic Resonance Imaging in Active Surveillance of Prostate Cancer: Incremental Value. J Urol. 2016;195(5):1421–1427. doi: 10.1016/j.juro.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenkrantz AB, Ginocchio LA, Cornfeld D, et al. Interobserver Reproducibility of the PI-RADS Version 2 Lexicon: A Multicenter Study of Six Experienced Prostate Radiologists. Radiology. 2016;280(3):793–804. doi: 10.1148/radiol.2016152542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.D'amico AV, Tempany CM, Cormack R, et al. Transperineal Magnetic Resonance Image Guided Prostate Biopsy. J Urol. 2000;164(2):385–387. doi: 10.1016/S0022-5347(05)67366-1. [DOI] [PubMed] [Google Scholar]


